Abstract
目的
观察姜黄素对帕金森病细胞模型中多巴胺能神经元的保护作用并探讨其作用机制。
方法
人神经母细胞瘤SH-SY5Y细胞采用1-甲基-4-苯基-四氢吡啶离子(MPTP)处理建立帕金森病细胞模型,进一步设立姜黄素干预、自噬抑制剂3-甲基腺嘌呤(3-MA)干预以及姜黄素和3-MA同时干预组。各组细胞在药物处理48 h后分别进行酪氨酸羟化酶(TH)免疫荧光染色观察多巴胺能神经元存活数;蛋白质印迹法检测α-突触核蛋白(α-Syn)、转录因子EB(TFEB)、自噬相关蛋白多克隆抗溶酶体相关膜蛋白2A(LAMP2A)和微管相关蛋白1轻链3-Ⅱ(LC3-Ⅱ)的蛋白表达;RT-PCR检测α-Syn的mRNA表达。
结果
与模型对照组比较,姜黄素组多巴胺能神经元存活数增加( P < 0.01),α-Syn蛋白及mRNA表达减少(均 P < 0.01),TFEB以及自噬蛋白LAMP2A和LC3-Ⅱ表达上调(均 P < 0.01);3-MA和姜黄素同时干预组多巴胺能神经元存活数增加( P < 0.05),α-Syn蛋白及mRNA表达减少( P < 0.05或 P < 0.01),TFEB、LAMP2A和LC3-Ⅱ蛋白表达上调(均 P < 0.01)。与姜黄素组比较,姜黄素和3-MA同时干预组多巴胺能神经元存活数减少,LC3-Ⅱ和LAMP2A蛋白表达减少(均 P < 0.05)。
结论
姜黄素可激活细胞自噬功能促进α-Syn自噬性清除,从而减轻MPTP所致的多巴胺能神经元损伤。
Abstract
Objective
To investigate the effect of curcumin on dopamine neurons in Parkinson's disease (PD) and its mechanism.
Methods
SH-SY5Y human neuroblastoma cells were treated with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) to establish the PD cell model. The model cells were treated with curcumin and/or autophagy inhibitor 3-MA. After 48 h of drug treatment, the number of surviving dopamine neurons was detected by tyrosine hydroxylase immunofluorescence method. Western blotting was used to detect protein expression of α-Synuclein (α-Syn), transcription factor EB (TFEB) and autophagy-related proteins lysosome-associated membrane protein 2A (LAMP2A) and microtubule-associated protein 1 light chain 3-Ⅱ(LC3-Ⅱ); RT-PCR was used to detect mRNA expression of α-Syn.
Results
Compared with MPTP model group, curcumin increased the number of surviving dopamine neurons( P < 0.01), decreased both protein expression and mRNA expression of α-Syn (all P < 0.01), and increased protein expression of TFEB, LAMP2A and LC3-Ⅱ (all P < 0.01). When curcumin and 3-MA were given concurrently, the number of surviving dopamine neurons, protein expression of TFEB, LAMP2A and LC3-Ⅱ increased ( P < 0.05 or P < 0.01), and both protein expression and mRNA expression of α-Syn decreased ( P < 0.05 or P < 0.01) compared with MPTP model group; but the number of surviving dopamine neurons and protein expression of LAMP2A and LC3-Ⅱ decreased compared with curcumin group (all P < 0.05).
Conclusion
Curcumin exerts protective effect on dopamine neurons in PD, which may be associated with enhancing autophagy and promoting the clearance of α-Syn.
Keywords: Curcumin/pharmacology; Parkinson disease/physiopathology; Neurons/metabolism; α-Synuclein; Transcription factors; Autophagy; Disease models, animal
帕金森病是目前中老年人最常见的神经系统变性疾病之一。路易小体中α-突触核蛋白(α-Synuclein, α-Syn)异常聚集与帕金森病密切相关 [ 1] ,而细胞自噬功能障碍是导致α-Syn清除障碍和异常聚集的重要因素 [ 2- 3] 。姜黄素是从姜黄根茎中提取的一种多酚类物质,具有诱导自噬激活的作用,近年来研究发现其对帕金森病具有保护作用 [ 4- 5] ,但具体机制尚未明确。本研究诱导SH-SY5Y细胞建立帕金森病细胞模型,评价姜黄素对多巴胺能神经元、α-Syn、转录因子EB(transcription factor EB,TFEB)及自噬相关蛋白的影响,探讨姜黄素保护多巴胺能神经元的作用机制。
人神经母细胞瘤SH-SY5Y细胞购于中国科学院典型培养物保藏委员会细胞库;1-甲基- 4-苯基-四氢吡啶(1-methyl- 4-phenyl-1, 2, 3, 6-tetrahydropyridine,MPTP)、姜黄素、自噬抑制剂3-甲基腺嘌呤(3-methyladenine,3-MA)和鼠抗酪氨酸羟化酶(tyrosine hydroxylase,TH)单克隆抗体为美国Sigma公司产品;兔多克隆抗微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3,LC3)抗体为英国Abcam公司产品;兔多克隆α-Syn抗体为美国Santa公司产品;兔多克隆抗溶酶体相关膜蛋白2A(lysosome-associated membrane protein 2A,LAMP2A)抗体为美国Proteintech公司产品;鼠抗TFEB单克隆抗体为美国CST公司产品;核蛋白试剂盒为上海碧云天生物技术有限公司产品;超纯RNA提取试剂盒为北京康为世纪生物科技有限公司产品;PrimeScript TM RT Master Mix试剂盒和Premix Taq TM试剂盒为日本TaKaRa公司产品。
人神经母细胞瘤SH-SY5Y细胞于DMEM高糖培养液中,单层细胞汇合至80 %~90 %时使用。取对数生长期的SH-SY5Y细胞,培养基中加入MPTP(10 μmol/L)诱导48 h建模。以正常SH-SY5Y细胞为空白对照组,另设模型对照组、姜黄素组、姜黄素+3-MA组和3-MA组。姜黄素组、姜黄素+3-MA组和3-MA组在MPTP处理的同时分别将姜黄素(40 μmol/L)、姜黄素(40 μmol/L)+ 3-MA(4 mmol/L)、3-MA(4 mmol/L)加入培养基中孵育48 h。各组分别在MPTP和药物诱导后去除培养基,收集细胞用于各项指标测定。
细胞经药物处理后将细胞悬液滴加至盖玻片,4 %多聚甲醛固定30 min,PBS洗三次,加破膜工作液室温孵育10 min进行细胞破膜,牛血清白蛋白封闭;去除封闭液后滴加小鼠TH单克隆抗体(1: 500),4 ℃过夜,荧光二抗室温孵育,4', 6-二脒基-2-苯基吲哚(DAPI)复染细胞核,PBS洗涤后封片;切片于荧光显微镜下观察,采用Case Viewer切片扫描软件采集图像,在高倍视野下每个标本随机取10个视野,其中TH阳性为存活的细胞。
收集处理好的细胞,加入蛋白裂解液冰上裂解,4 ℃离心取上清液。测定蛋白浓度,提取总蛋白,然后取蛋白提取液,100 ℃变性5 min,制胶,加样,SDS-PAGE后恒压转至硝酸纤维素膜(NC膜),5 %脱脂奶粉室温封闭1 h,分别加入封闭液稀释的α-Syn抗体(1: 200)、TFEB抗体(1: 200)、LC3抗体(1: 2000)、LAMP2A抗体(1: 500)和内参蛋白,4 ℃孵育过夜,将一抗孵育后的膜放入二抗中,室温孵育1 h。膜于荧光检测试剂中反应2 min后在凝胶成像系统中观察、拍照。
按照超纯RNA提取试剂盒说明书提取RNA,按照PrimeScript TM RT Master Mix试剂盒说明书配制反应液,轻柔混匀后进行逆转录反应,条件如下:37 ℃ 15 min,85 ℃ 5 s,4 ℃。α-Syn引物序列为:上游引物5'-ATGGATGTATTCATGAAAGGACT-3',下游引物5'-CTACATAGAGAACACCCTCTTT-3'。按照Premix Taq TM试剂盒反应体系加入试剂和引物,进行PCR,反应条件为:95 ℃预变性2 min;95 ℃变性15 s,55 ℃退火15 s,72 ℃延伸15 s,35个循环;72 ℃延伸10 min。取反应产物5 μL于琼脂糖凝胶电泳130 V、25 min,凝胶成像分析系统中观察DNA条带并拍照保存,采用ImageJ2x软件分析图片条带灰度值。
所有实验独立重复6次。采用SPSS 20.0软件进行统计分析,计量资料用均数±标准差( x ± s)表示,组间比较采用单因素方差分析, P<0.05为差异有统计学意义。
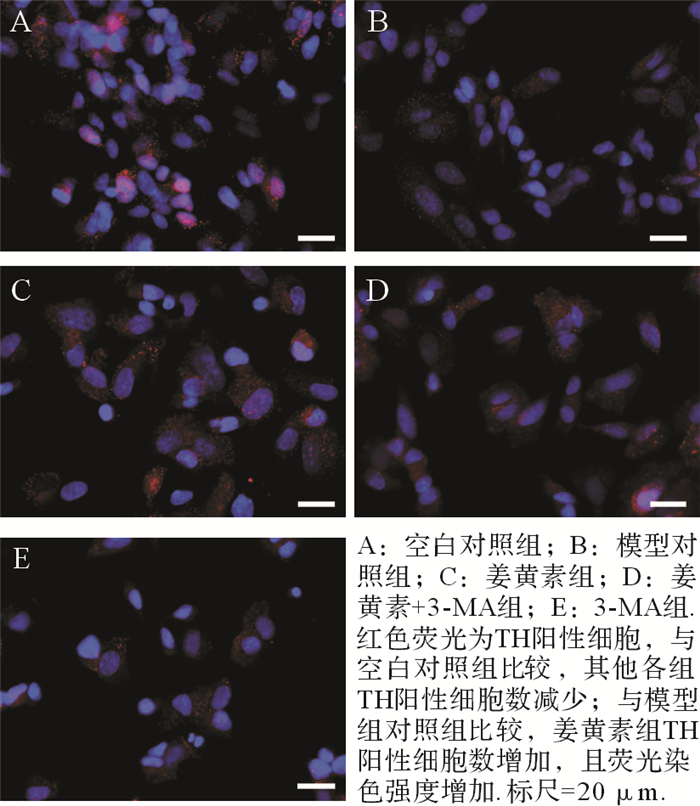
各组TH免疫荧光染色结果见 图 1。空白对照组、模型对照组、姜黄素组、姜黄素+3-MA组和3-MA组TH阳性细胞数分别为(25.9±3.8)、(14.3±2.3)、(19.0±2.7)、(16.6±2.3)和(11.7±1.6)×800/每高倍视野。与空白对照组比较,模型对照组、姜黄素组、姜黄素+3-MA组和3-MA组TH阳性细胞数减少(均 P<0.01);与模型对照组比较,姜黄素组TH阳性细胞数增加( P<0.01),荧光染色强度增加,且细胞排列紧密,而3-MA组TH阳性细胞数减少( P<0.05);姜黄素+3-MA组TH阳性细胞数也较模型对照组增加( P<0.05),但较姜黄素组少( P<0.05)。结果提示,姜黄素可减轻MPTP对神经元的毒性,增加多巴胺能神经元的存活数。
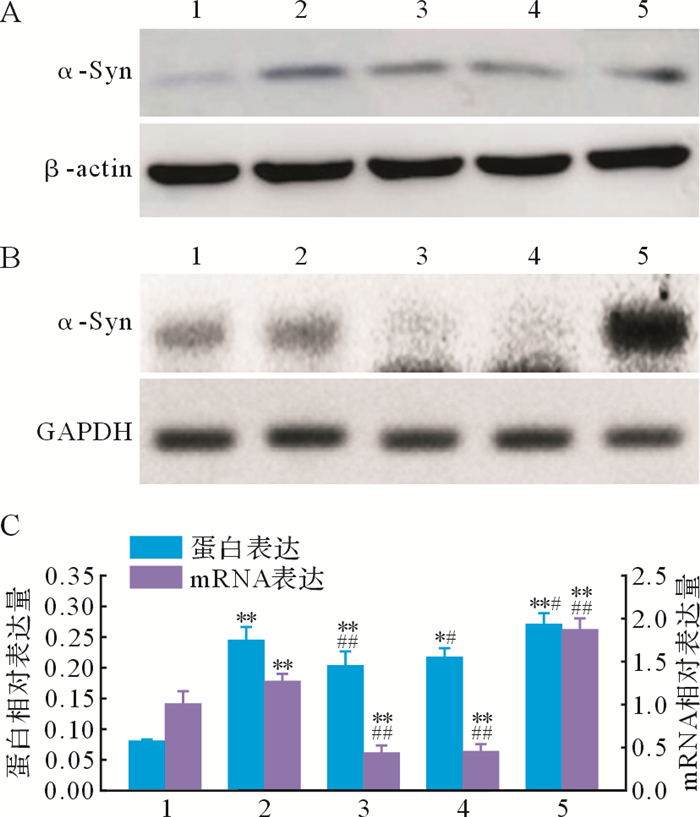
与空白对照组比较,模型对照组、姜黄素组、姜黄素+3-MA组和3-MA组α-Syn蛋白和mRNA表达增加(均 P<0.01);与模型对照组比较,姜黄素组α-Syn蛋白和mRNA表达减少( P<0.01),而3-MA组α-Syn蛋白和mRNA表达增加( P<0.05);姜黄素+3-MA组α-Syn蛋白和mRNA表达也较模型对照组减少( P<0.05),与姜黄素组差异无统计学意义( P>0.05),见 图 2。结果提示,在帕金森病细胞模型中,姜黄素可促进细胞α-Syn清除,减少α-Syn表达和聚集。
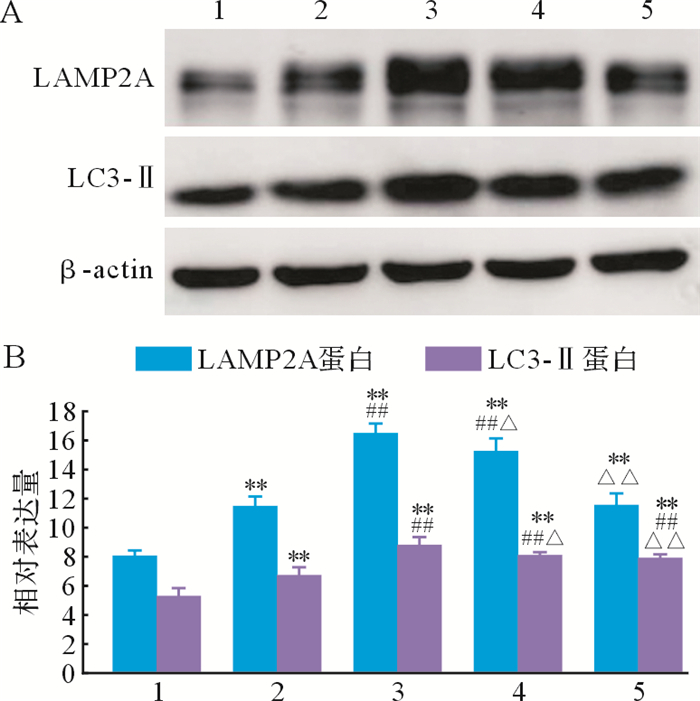
与空白对照组比较,模型对照组、姜黄素组、姜黄素+3-MA组和3-MA组LAMP2A和LC3-Ⅱ蛋白表达增加(均 P<0.01);与模型对照组比较,姜黄素组LAMP2A和LC3-Ⅱ蛋白表达增加(均 P<0.01);姜黄素+3-MA组LAMP2A和LC3-Ⅱ表达较模型对照组增加(均 P<0.01),但较姜黄素组减少(均 P<0.05);3-MA组LAMP2A表达较姜黄素组及姜黄素+3-MA组均减少(均 P<0.01),LC3-Ⅱ表达也较姜黄素组减少( P<0.01),但与姜黄素+3-MA组差异无统计学意义( P>0.05),见 图 3。结果提示,在帕金森病细胞模型中,姜黄素干预具有激活细胞自噬的作用。
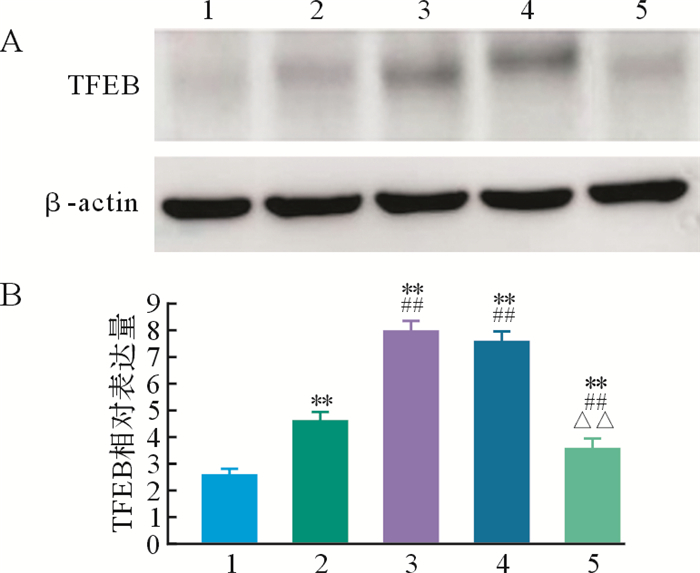
与空白对照组比较,模型对照组、姜黄素组、姜黄素+3-MA组和3-MA组TFEB蛋白表达均增加(均 P<0.01);与模型对照组比较,姜黄素组和姜黄素+3-MA组TFEB蛋白表达增加(均 P<0.01),而3-MA组TFEB蛋白表达减少( P<0.01),见 图 4。结果提示,在帕金森病细胞模型中,姜黄素可能通过增加TFEB表达来调节自噬。
帕金森病是一种以黑质多巴胺能神经元变性坏死为主要病理特征的常见神经系统退行性疾病,其发病率和致残率高。目前,帕金森病治疗以药物为主,中药在帕金森病治疗中具有独特作用。姜黄素具有抗肿瘤、抗氧化、抗炎症及器官保护等多种药理作用 [ 6] ,近年来逐渐用于帕金森病治疗。研究表明,姜黄素在多种帕金森病小鼠模型中表现出抗炎、抗氧化等作用,可减少氧化应激和线粒体损伤,改善小鼠运动障碍,发挥神经保护作用 [ 7- 13] 。在帕金森病细胞模型中姜黄素也表现出抗氧化应激、减少线粒体损伤、调节细胞内钙离子释放等作用,从而保护多巴胺能神经元、减少细胞死亡 [ 14- 17] 。本研究采用MPTP诱导建立帕金森病细胞模型,TH免疫荧光结果显示姜黄素可增加多巴胺能神经元存活数,减少MPTP所致的神经毒性作用。
帕金森病的发病与多种机制相关,其中细胞自噬功能障碍致α-Syn清除受损在其中发挥重要作用,因此能够激活自噬的药物可通过促进α-Syn自噬性清除来治疗帕金森病 [ 18] 。经典的自噬激活剂雷帕霉素在帕金森病细胞和动物模型中对多巴胺能神经元具有保护作用 [ 19] ,证实了上述观点。研究表明,姜黄素具有诱导自噬的功能,在肺癌、肝癌、恶性胶质细胞瘤等多种肿瘤细胞模型中可诱导自噬从而抑制肿瘤细胞生长 [ 20- 22] ; 阿尔茨海默病动物模型证实,姜黄素可通过下调PI3K/Akt/mTOR信号途径诱导自噬、抑制β淀粉样蛋白形成,发挥神经保护作用 [ 23] 。因此我们推测姜黄素在帕金森病中同样具有诱导自噬的功能,并可能通过此功能发挥神经保护作用。
LC3-Ⅱ作为哺乳动物细胞中自噬体的标志物之一,可反映自噬活性的高低。但也有观点认为,LC3-Ⅱ蛋白表达增加也可能是由于溶酶体功能缺失而并非自噬活性增加。当溶酶体功能缺陷导致自噬途径阻断时,自噬标志物蛋白会因为降解减少而积聚,但这种因积聚而增多的自噬蛋白并不能提高细胞内自噬活性 [ 24] 。因此本实验还测定了LAMP2A蛋白的表达。LAMP2A是一种重要的溶酶体膜蛋白,主要定位于溶酶体膜上,可作为溶酶体的标志物。研究结果显示,姜黄素可以增加帕金森病细胞中LC3-Ⅱ和LAMP2A的表达,提示姜黄素具有激活细胞自噬的作用。姜黄素还可以减少细胞α-Syn的蛋白及mRNA表达,说明其激活的自噬可促进细胞中α-Syn清除,可见细胞自噬激活促进α-Syn自噬性清除是姜黄素对帕金森病发挥神经保护作用的机制之一。自噬抑制剂3-MA和姜黄素同时干预则部分消除了姜黄素对LC3-Ⅱ和LAMP2A的影响,且多巴胺能神经元存活数也相应减少,进一步验证了上述结论。但两者同时干预仍使得多巴胺能神经元存活数较模型对照组增加,α-Syn蛋白及mRNA表达减少,提示激活细胞自噬并不是姜黄素促进α-Syn清除的唯一机制,姜黄素还可能通过其他途径清除α-Syn发挥多巴胺能神经元保护作用。本研究还观察到模型对照组较空白对照组LAMP2A和LC3-Ⅱ表达增加,提示自噬激活;但与此同时α-Syn蛋白表达增加,TH阳性细胞减少,提示MPTP激活的自噬并没有促进α-Syn的降解,这可能是一种自噬应激现象,蛋白积聚加重导致自噬性细胞死亡,需要今后更深入的研究。
TFEB是细胞自噬的重要调控蛋白。在过表达α-Syn的帕金森病动物模型中,TFEB功能受损可增加多巴胺能神经元对α-Syn毒性的易感性,TFEB过表达则可增强细胞自噬功能,促进α-Syn清除,还有研究发现在帕金森病患者中脑的多巴胺能神经元细胞核内TFEB表达减少 [ 25- 26] 。Song等 [ 27] 发现一种合成的姜黄素衍生物可与TFEB特异性结合,促进TFEB核转运,从而增强自噬和溶酶体功能,但并未抑制雷帕霉素靶蛋白活性。Zhang等 [ 28] 研究发现,姜黄素在激活细胞自噬的同时增加了TFEB蛋白表达,进一步证实TFEB表达增加可能是姜黄素激活自噬的重要途径。3-MA是磷脂酰肌醇3激酶的抑制剂,可特异性阻断自噬体的形成,广泛用作自噬抑制剂。3-MA与姜黄素同时干预并未消除姜黄素对TFEB的影响,可能与两者对自噬的作用途径不同、药物存在相互作用等相关。
综上所述,姜黄素可保护MPTP所致的多巴胺能神经元损伤,激活细胞自噬功能、促进α-Syn自噬性清除可能是作用机制之一;同时,TFEB在细胞自噬过程中发挥重要作用,可能是姜黄素激活自噬的重要途径。此外,姜黄素还可能通过激活AMPK信号通路、抑制PI3K/Akt/mTOR信号通路、增加转铁蛋白受体1和铁调节蛋白1的表达、磷酸化细胞外信号调节激酶1/2信号通路等多种途径激活自噬功能 [ 29- 32] 。因此,姜黄素是帕金森病治疗的一个非常有潜力的中药,但也有学者认为姜黄素药物峰浓度低、口服吸收差、生物利用度低、体内的化学性质不稳定、生物学活性较弱,可能导致临床应用无效 [ 33- 35] 。因此,姜黄素在帕金森病治疗中的作用、机制及其在临床应用的可行性、有效性和安全性等问题需要更多、更深入的研究加以探讨。
Funding Statement
浙江省自然科学基金(LQ17H280003);浙江省中医药科技计划(2015ZQ017);浙江省医药卫生科技计划(2014KYB181)
References
- 1.BENSKEY M J, PEREZ R G, MANFREDSSON F P. The contribution of alpha synuclein to neuronal survival and function-implications for Parkinson's disease. J Neurochem. 2016;137(3):331–359. doi: 10.1111/jnc.2016.137.issue-3. [BENSKEY M J, PEREZ R G, MANFREDSSON F P. The contribution of alpha synuclein to neuronal survival and function-implications for Parkinson's disease[J]. J Neurochem, 2016, 137(3):331-359.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LEHRI-BOUFALA S, OUIDJA M O, BARBIER-CHASSEFIÈRE V, et al. New roles of glycosaminoglycans in α-synuclein aggregation in a cellular model of Parkinson disease[J/OL]. PLoS One, 2015, 10(1): e0116641. 10.1371/journal.pone.0116641. . [DOI] [PMC free article] [PubMed]
- 3.SALA G, MARINIG D, AROSIO A, et al. Role of chaperone-mediated autophagy dysfunctions in the pathogenesis of Parkinson's disease. http://pubmed.cn/28066181. Front Mol Neurosci. 2016;9:157. doi: 10.3389/fnmol.2016.00157. [SALA G, MARINIG D, AROSIO A, et al. Role of chaperone-mediated autophagy dysfunctions in the pathogenesis of Parkinson's disease[J]. Front Mol Neurosci, 2016, 9:157.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KHUWAJA G, KHAN M M, ISHRAT T, et al. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats:behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011;1368:254–263. doi: 10.1016/j.brainres.2010.10.023. [KHUWAJA G, KHAN M M, ISHRAT T, et al. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats:behavioral, neurochemical and immunohistochemical studies[J]. Brain Res, 2011, 1368:254-263.] [DOI] [PubMed] [Google Scholar]
- 5.PANDAREESH M D, SHRIVASH M K, NAVEEN KUMAR H N, et al. Curcumin monoglucoside shows improved bioavailability and mitigates rotenone induced neurotoxicity in cell and drosophila models of Parkinson's disease. Neurochem Res. 2016;41(11):3113–3128. doi: 10.1007/s11064-016-2034-6. [PANDAREESH M D, SHRIVASH M K, NAVEEN KUMAR H N, et al. Curcumin monoglucoside shows improved bioavailability and mitigates rotenone induced neurotoxicity in cell and drosophila models of Parkinson's disease[J]. Neurochem Res, 2016, 41(11):3113-3128.] [DOI] [PubMed] [Google Scholar]
- 6.AQQARWAL B B, HARIKUMAR K B. Potential therapeutic effect of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, meta-bolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [AQQARWAL B B, HARIKUMAR K B. Potential therapeutic effect of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, meta-bolic, autoimmune and neoplastic diseases[J]. Int J Biochem Cell Biol, 2009, 41(1):40-59.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HE X J, UCHIDA K, MEGUMI C, et al. Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice. J Toxicol Pathol. 2015;28(4):197–206. doi: 10.1293/tox.2015-0020. [HE X J, UCHIDA K, MEGUMI C, et al. Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice[J]. J Toxicol Pathol, 2015, 28(4):197-206.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SPINELLI K J, OSTERBERG V R, MESHUL C K, et al. Curcumin treatment improves motor behavior in α-synuclein transgenic mice[J/OL]. PLoS One, 2015, 10(6): e0128510. 10.1371/journal.pone.0128510. . [DOI] [PMC free article] [PubMed]
- 9.MANSOURI Z, SABETKASAEI M, MORADI F, et al. Curcumin has neuroprotection effect on homocysteine rat model of Parkinson. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=99776f5282437144b8bc41a86125755b. J Mol Neurosci. 2012;47(2):234–242. doi: 10.1007/s12031-012-9727-3. [MANSOURI Z, SABETKASAEI M, MORADI F, et al. Curcumin has neuroprotection effect on homocysteine rat model of Parkinson[J]. J Mol Neurosci, 2012, 47(2):234-242.] [DOI] [PubMed] [Google Scholar]
- 10.SONG S, NIE Q, LI Z, et al. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol Res Pract. 2016;212(4):247–251. doi: 10.1016/j.prp.2015.11.012. [SONG S, NIE Q, LI Z, et al. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats[J]. Pathol Res Pract, 2016, 212(4):247-251.] [DOI] [PubMed] [Google Scholar]
- 11.KHATRI D K, JUVEKAR A R. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2016;150-151:39–47. doi: 10.1016/j.pbb.2016.09.002. [KHATRI D K, JUVEKAR A R. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson's disease[J]. Pharmacol Biochem Behav, 2016, 150-151:39-47.] [DOI] [PubMed] [Google Scholar]
- 12.CUI Q, LI X, ZHU H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol Med Rep. 2016;13(2):1381–1388. doi: 10.3892/mmr.2015.4657. [CUI Q, LI X, ZHU H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway[J]. Mol Med Rep, 2016, 13(2):1381-1388.] [DOI] [PubMed] [Google Scholar]
- 13.SIDDIQUE Y H, NAZ F, JYOTI S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson's disease. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ab72b0edb5843cab1fbcf9d565de6b57. Biomed Res Int. 2014;2014:606928. doi: 10.1155/2014/606928. [SIDDIQUE Y H, NAZ F, JYOTI S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson's disease[J]. Biomed Res Int, 2014, 2014:606928.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VAN DER MERWE C, VAN DYK H C, ENGELBRECHT L, et al. Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson's disease from mitochondrial dysfunction and cell death. Mol Neurobiol. 2017;54(4):2752–2762. doi: 10.1007/s12035-016-9843-0. [VAN DER MERWE C, VAN DYK H C, ENGELBRECHT L, et al. Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson's disease from mitochondrial dysfunction and cell death[J]. Mol Neurobiol, 2017, 54(4):2752-2762.] [DOI] [PubMed] [Google Scholar]
- 15.UǦUZ A C, ÖZ A, NAZIROǦLU M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J Recept Signal Transduct Res. 2016;36(4):395–401. doi: 10.3109/10799893.2015.1108337. [UǦUZ A C, ÖZ A, NAZIROǦLU M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells[J]. J Recept Signal Transduct Res, 2016, 36(4):395-401.] [DOI] [PubMed] [Google Scholar]
- 16.JAISIN Y, THAMPITHAK A, MEESARAPEE B, et al. CurcuminⅠ protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci Lett. 2011;489(3):192–196. doi: 10.1016/j.neulet.2010.12.014. [JAISIN Y, THAMPITHAK A, MEESARAPEE B, et al. CurcuminⅠ protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis[J]. Neurosci Lett, 2011, 489(3):192-196.] [DOI] [PubMed] [Google Scholar]
- 17.BOLLIMPELLI V S, KUMAR P, KUMARI S, et al. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem Int. 2016;95:37–45. doi: 10.1016/j.neuint.2016.01.006. [BOLLIMPELLI V S, KUMAR P, KUMARI S, et al. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity[J]. Neurochem Int, 2016, 95:37-45.] [DOI] [PubMed] [Google Scholar]
- 18.HARRIS H, RUBINSZTEIN D C. Control of autophagy as a therapy for neurodegenerative disease. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3541300. Nat Rev Neurol. 2011;8(2):108–117. doi: 10.1038/nrneurol.2011.200. [HARRIS H, RUBINSZTEIN D C. Control of autophagy as a therapy for neurodegenerative disease[J]. Nat Rev Neurol, 2011, 8(2):108-117.] [DOI] [PubMed] [Google Scholar]
- 19.MALAGELADA C, JIN Z H, JACKSON-LEWIS V, et al. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease . J Neurosci. 2010;30(3):1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [MALAGELADA C, JIN Z H, JACKSON-LEWIS V, et al. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease[J]. J Neurosci, 2010, 30(3):1166-1175. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.QIAN H, YANG Y, WANG X. Curcumin enhanced Adriamycin-induced human liver-derived hepatoma G2 cell death through activation of mitochondria-mediated apoptosis and autophagy. Eur J Pharm Sci. 2011;43(3):125–131. doi: 10.1016/j.ejps.2011.04.002. [QIAN H, YANG Y, WANG X. Curcumin enhanced Adriamycin-induced human liver-derived hepatoma G2 cell death through activation of mitochondria-mediated apoptosis and autophagy[J]. Eur J Pharm Sci, 2011, 43(3):125-131.] [DOI] [PubMed] [Google Scholar]
- 21.THAYYULLATHIL F, RAHMAN A, PALLICHANKANDY S, et al. ROS-dependent prostate apoptosis response-4(Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio. 2014;4:763–776. doi: 10.1016/j.fob.2014.08.005. [THAYYULLATHIL F, RAHMAN A, PALLICHANKANDY S, et al. ROS-dependent prostate apoptosis response-4(Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma[J]. FEBS Open Bio, 2014, 4:763-776.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JAROONWITCHAWAN T, CHAICHAROENAU-DOMRUNG N, NAMKAEW J, et al. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy. Neurosci Lett. 2017;636:40–47. doi: 10.1016/j.neulet.2016.10.050. [JAROONWITCHAWAN T, CHAICHAROENAU-DOMRUNG N, NAMKAEW J, et al. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy[J]. Neurosci Lett, 2017, 636:40-47.] [DOI] [PubMed] [Google Scholar]
- 23.KLIONSKY D J, ABDELMOHSEN K, ABE A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy(3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [KLIONSKY D J, ABDELMOHSEN K, ABE A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy(3rd edition)[J]. Autophagy, 2016, 12(1):1-222.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WANG C, ZHANG X, TENG Z, et al. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. doi: 10.1016/j.ejphar.2014.06.051. [WANG C, ZHANG X, TENG Z, et al. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice[J]. Eur J Pharmacol, 2014, 740:312-320.] [DOI] [PubMed] [Google Scholar]
- 25.EBRAHIMI-FAKHARI D, WAHLSTER L. Restoring impaired protein metabolism in Parkinson's disease-TFEB-mediated autophagy as a novel therapeutic target. Mov Disord. 2013;28(10):1346. doi: 10.1002/mds.25601. [EBRAHIMI-FAKHARI D, WAHLSTER L. Restoring impaired protein metabolism in Parkinson's disease-TFEB-mediated autophagy as a novel therapeutic target[J]. Mov Disord, 2013, 28(10):1346.] [DOI] [PubMed] [Google Scholar]
- 26.DECRESSAC M, BJÖRKLUND A. TFEB:Pathogenic role and therapeutic target in Parkinson disease. Autophagy. 2013;9(8):1244–1246. doi: 10.4161/auto.25044. [DECRESSAC M, BJÖRKLUND A. TFEB:Pathogenic role and therapeutic target in Parkinson disease[J]. Autophagy, 2013, 9(8):1244-1246.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SONG J X, SUN Y R, PELUSO I, et al. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition . Autophagy. 2016;12(8):1372–1389. doi: 10.1080/15548627.2016.1179404. [SONG J X, SUN Y R, PELUSO I, et al. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition[J]. Autophagy, 2016, 12(8):1372-1389. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ZHANG J, WANG J, XU J, et al. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. http://www.oncotarget.com/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=12318. Oncotarget. 2016;7(46):75659–75671. doi: 10.18632/oncotarget.12318. [ZHANG J, WANG J, XU J, et al. Curcumin targets the TFEB-lysosome pathway for induction of autophagy[J]. Oncotarget, 2016, 7(46):75659-75671.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.XIAO K, JIANG J, GUAN C, et al. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J Pharmacol Sci. 2013;123(2):102–109. doi: 10.1254/jphs.13085FP. [XIAO K, JIANG J, GUAN C, et al. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells[J]. J Pharmacol Sci, 2013, 123(2):102-109.] [DOI] [PubMed] [Google Scholar]
- 30.LI W, ZHOU Y, YANG J, et al. Curcumin induces apoptotic cell death and protective autophagy in human gastric cancer cells. http://pubmed.cn/28498433. Oncol Rep. 2017;37(6):2459–3466. doi: 10.3892/or.2017.5637. [LI W, ZHOU Y, YANG J, et al. Curcumin induces apoptotic cell death and protective autophagy in human gastric cancer cells[J]. Oncol Rep, 2017, 37(6):2459-3466.] [DOI] [PubMed] [Google Scholar]
- 31.YANG C, MA X, WANG Z, et al. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des Devel Ther. 2017;11:431–439. doi: 10.2147/DDDT. [YANG C, MA X, WANG Z, et al. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation[J]. Drug Des Devel Ther, 2017, 11:431-439.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LI X, FENG K, LI J, et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy[J/OL]. Nutrients, 2017, 9(4): E414. http://www.ncbi.nlm.nih.gov/pubmed/28430129. . [DOI] [PMC free article] [PubMed]
- 33.HEGER M, VAN GOLEN R F, BROEKGAARDEN M, et al. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f30c9a85743a661e60070cd8c21def40. Pharmacol Rev. 2013;66(1):222–307. doi: 10.1124/pr.110.004044. [HEGER M, VAN GOLEN R F, BROEKGAARDEN M, et al. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer[J]. Pharmacol Rev, 2013, 66(1):222-307.] [DOI] [PubMed] [Google Scholar]
- 34.SCHNEIDER C, GORDON O N, EDWARDS R L, et al. Degradation of curcumin:from mechanism to biological implications. J Agric Food Chem. 2015;63(35):7606–7614. doi: 10.1021/acs.jafc.5b00244. [SCHNEIDER C, GORDON O N, EDWARDS R L, et al. Degradation of curcumin:from mechanism to biological implications[J]. J Agric Food Chem, 2015, 63(35):7606-7614.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NELSON K M, DAHLIN J L, BISSON J, et al. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [NELSON K M, DAHLIN J L, BISSON J, et al. The essential medicinal chemistry of curcumin[J]. J Med Chem, 2017, 60(5):1620-1637.] [DOI] [PMC free article] [PubMed] [Google Scholar]


