Abstract
目的
阐明未分化型甲状腺癌(ATC)的分子病理状态,并挖掘其潜在的干预策略。
方法
利用GEO数据库联合R语言分析ATC组织与正常甲状腺组织差异表达的基因;利用京都基因与基因组百科全书(KEGG)通路数据库和基因本体(GO)数据库对差异表达的基因进行富集和功能注释;基于STRING数据库及Cytoscape软件构建蛋白质相互作用网络,分析其关键网络节点和基因簇;最后采用L1000CDS 2数据库预测ATC的潜在治疗药物。
结果
共获得2087个差异表达基因。与正常甲状腺组织相比,ATC组织细胞内信号通路及肿瘤微环境均发生显著改变,包括PI3K-Akt信号的持续激活、p53通路的激活、炎症反应、细胞外基质重塑等。蛋白质相互作用网络提示存在3个重要的基因簇和9个关键节点。将差异表达基因与L1000CDS 2数据库进行比对后发现22个能够逆转ATC病理状态的潜在化合物。
结论
本研究揭示了ATC发病机制中的关键节点,为ATC的治疗提供了潜在的靶点。
Abstract
Objective
To identify hub genes and key pathways associated with anaplastic thyroid carcinoma (ATC), and to explore possible intervention strategy.
Methods
The differentially expressed genes (DEGs) in ATC were identified by Gene Expression Omnibus (GEO) combined with using R language; the pathway enrichment of DEGs were performed by using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO). The protein-protein interaction (PPI) network of DEGs was constructed by STRING database and visualized by Cytoscape. Furthermore, the hub genes and key nodes were calculated by MCODE. Finally, the drug repurposing was performed by L1000CDS 2.
Results
A total of 2087 DEGs were identified. The DEGs were clustered based on functions and pathways with significant enrichment analysis, among which PI3K-Akt signaling pathway, p53 signaling pathway, inflammatory response, extracellular matrix organization were significantly upregulated. The PPI network was constructed and the most significant three modules and nine genes were filtered. Twenty-two potential compounds were repurposed for ATC treatment.
Conclusion
Using integrated bioinformatics analysis, we have identified hub genes and key pathways in ATC, and provide novel strategy for the treatment of ATC.
Keywords: Thyroid neoplasms/physiopathology, Computational biology, Gene expression, Signal transduction, Proteins, Gene regulatory networks
甲状腺癌是常见的内分泌系统恶性肿瘤,根据2017年全球癌症统计,甲状腺癌已位列女性恶性肿瘤的第五位,仅次于乳腺癌、肺癌、直肠癌和宫颈癌 [ 1] 。我国2015年甲状腺癌发病达9万例,发病率较10年前上升约5倍,严重威胁国民健康 [ 2] 。未分化型甲状腺癌(anaplastic thyroid carcinoma,ATC)是恶性程度最高的甲状腺癌,患者生存期仅为3~7个月,远低于分化型甲状腺癌患者的平均生存期。ATC是一类难治性的恶性甲状腺癌,病情进展快且缺乏有效的治疗靶点。ATC不仅对放射性碘抵抗,而且采用传统化学药物治疗和放射治疗均不能取得理想效果,患者常死于局部肿瘤扩张导致的窒息或肿瘤远处转移 [ 3- 4] 。因此,寻找ATC的发生、发展机制及潜在治疗靶点是目前研究的热点与难点。
目前关于ATC的机制研究主要聚焦于肿瘤内分子和基因的变化,包括驱动基因的筛选。ATC具有较高的突变负荷,其中 BRAF及 RAS被认为是ATC主要的驱动基因,同时,TP53突变(>50%)、端粒逆转录酶(TERT)启动子突变(33%~50%)、丝裂原活化蛋白激酶(MAPK)通路改变及人第10号染色体缺失的磷酸酶及张力蛋白同源基因( PTEN)突变引起的磷脂酰肌醇3-激酶(PI3K)-蛋白激酶B(Akt)-哺乳动物霉帕霉素靶蛋白(mTOR)通路异常激活可导致其基因组不稳定,而 CTNNB1突变(25%~60%)所致Wnt通路持续激活则是ATC发生上皮间质转化的重要因素 [ 5- 7] 。然而,ATC的分子病理状态仍未完全明确。因此,构建ATC发病的分子机制网络,并挖掘其关键节点,将为ATC发生、发展机制研究及寻找治疗靶点提供有利证据。
基于上述背景,我们利用生物信息学技术挖掘ATC相关基因芯片,分析其差异表达基因并构建蛋白相互作用网络,进而挖掘关键网络节点,以寻找其关键病理机制。最后,基于基因特征导向检索引擎(L1000CDS 2)预测ATC潜在的治疗药物或化合物,为ATC的治疗提供新的思路。
利用美国国立生物技术信息中心(NCBI)平台下的基因表达综合数据库(Gene Expression Omnibus,GEO)检索含有人源ATC样本的芯片,选取含有ATC样本最多且通过芯片质量要求的基因芯片GSE65144,芯片质量采用R语言()affyPLM数据包对数据集进行回归分析判断,并进行归一化处理。芯片信息:Affymetrix Human Genome U133 Plus 2.0 Array,平台为GPL570,共包含13个正常甲状腺组织样本,12个ATC样本。
采用R语言Limma数据包对ATC与正常组织进行差异表达基因筛选,筛选标准为 P < 0.01,log 2|倍数变化|≥1.5,并将探针名转化为标准基因名。
基于前述所得差异表达基因,利用京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)通路数据库进行信号通路的富集。同时通过DAVID在线软件( https://david.ncifcrf.gov),依据基因本体(Gene Ontology,GO)数据库对差异表达基因进行生物学功能注释。
基于STRING数据库构建蛋白质相互作用网络,并应用Cytoscape 3.5.1进行可视化分析。通过MCODE(Version 1.4.2,Bader Lab, University of Toronto)对蛋白质相互作用网络进行关联度分析,获得蛋白质相互作用网络中关键蛋白质簇和节点蛋白,并在Cytoscape中进行可视化显示。关键节点筛选标准为节点度(node degree)大于10。
基于L1000CDS 2数据库,根据小分子化合物引起的细胞基因谱变化与肿瘤差异表达基因的相似性,推测小分子逆转肿瘤病理状态的可能性,并基于C- SPADE()在线工具对小分子化合物进行聚类分析。
共获得2087个差异表达基因,其中变化差异最大的30个基因(15个上调,15个下调)见 图 1。差异表达基因的聚类结果显示,12个ATC样本和13个正常甲状腺组织样本分别聚为一类,且ATC组织甲状腺功能特异标志物促甲状腺激素受体(thyrotropin receptor,TSHR)、甲状腺过氧化物酶(thyroid peroxidase,TPO)、甲状腺球蛋白(thyroglobulin,TG)等表达均显著降低,提示ATC失分化状态。
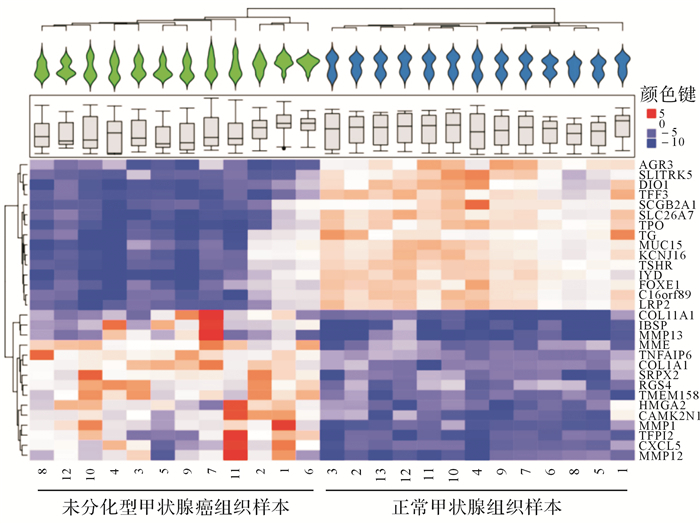
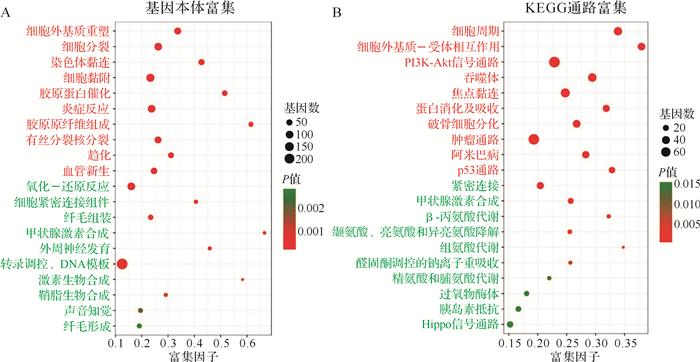
通过分析差异表达基因本体的生物进程(biological process)发现,ATC组织上调基因主要富集于细胞外基质重塑、胶原蛋白催化、细胞分裂、细胞黏附、炎症反应、血管新生、染色体黏连、趋化等过程,而下调的基因主要富集于氧化-还原反应、甲状腺激素合成、外周神经发育、纤毛组装、纤毛形成、声音知觉、鞘脂生物合成、细胞紧密连接组件等生物过程( 图 2A)。进一步对差异表达基因进行KEGG通路富集发现,ATC组织上调基因主要富集于细胞周期、细胞外基质-受体相互作用、PI3K-Akt信号通路、吞噬体、焦点黏连、蛋白消化及吸收、肿瘤通路、p53通路、破骨细胞分化、阿米巴病等通路; 下调基因则主要富集于紧密连接, 甲状腺激素合成, β-丙氨酸代谢, 缬氨酸、亮氨酸和异亮氨酸降解, 组氨酸代谢, 醛固酮调控的钠离子重吸收, 精氨酸和脯氨酸代谢, 过氧物酶体, 胰岛素抵抗, Hippo信号通路( 图 2B)。
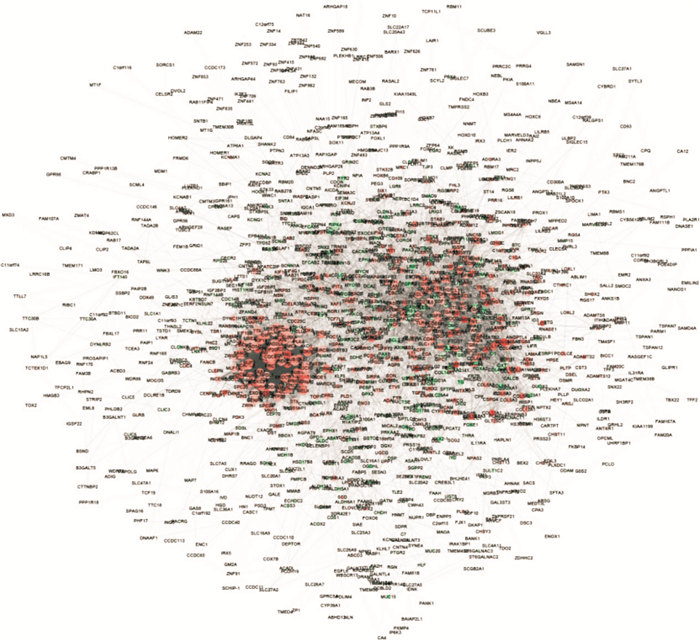
为明确ATC的分子病理状态,研究利用差异表达基因构建其潜在的蛋白质相互作用网络,其中共存在1295个网络节点,基因上调以红色表示,下调以青色表示;与其他蛋白质相互作用越多,节点越大;作用越强,连线越粗( 图 3)。
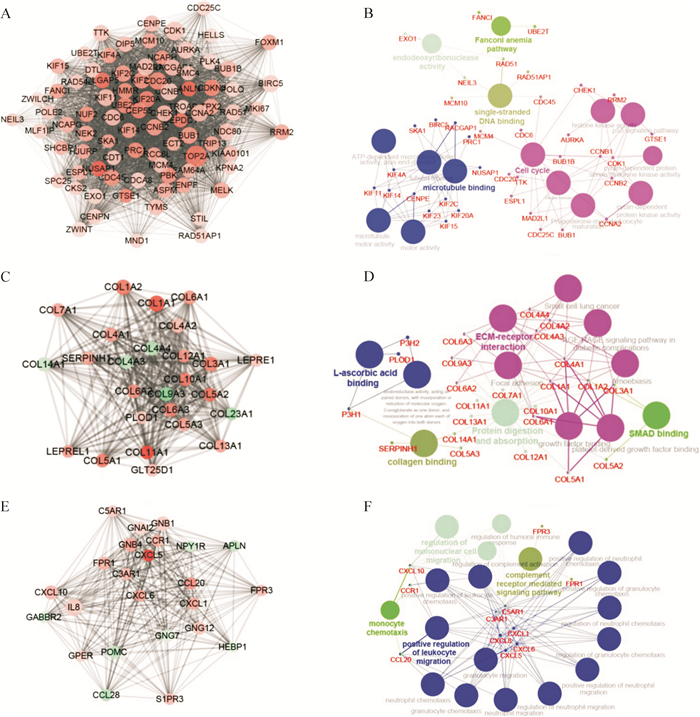
进一步分析网络中的关键节点和基因簇,结果显示,蛋白质相互作用网络中存在三个比较重要的基因簇。第一个基因簇关键节点为 TOP2A、 CDK1和 CCNB1,分别与蛋白质相互作用网络中204、161、141个基因存在相互作用( 图 4A),该基因簇生物功能主要富集于微管蛋白结合、细胞周期、单链DNA结合、范可尼贫血通路及脱氧核糖核酸内切酶活性等通路( 图 4B);第二个基因簇关键节点为 COL1A1、 COL1A2和 COL3A1 ( 图 4C),该基因簇功能主要富集于细胞外基质-受体相互作用、SMAD结合、蛋白消化及吸收、胶原结合及抗坏血酸结合( 图 4D);第三个基因簇关键节点为 IL-8、 GNB1和 CXCL10 ( 图 4E),该基因簇主要与补体受体通路、单核细胞趋化及招募及白细胞迁移调控相关( 图 4F)。
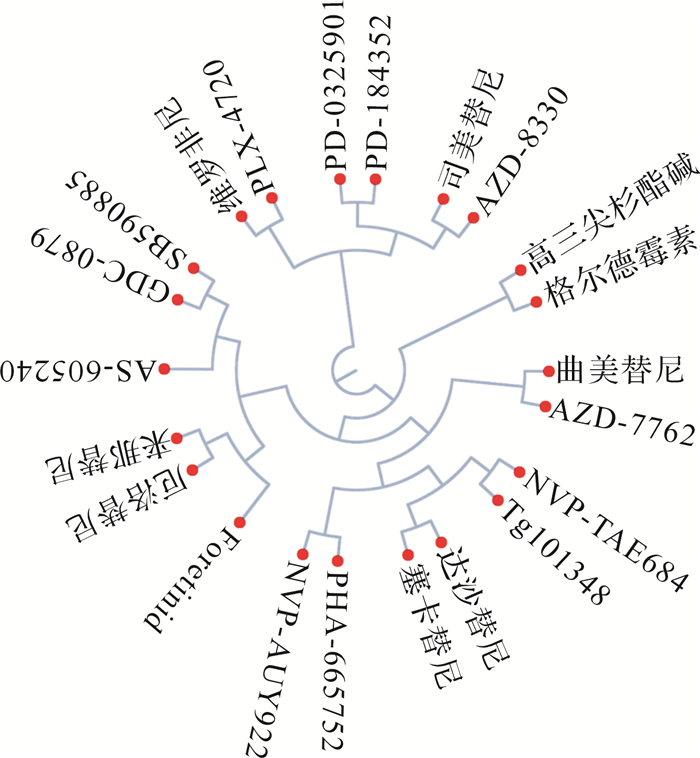
将差异表达基因与L1000CDS 2数据库进行比对后发现22个能够逆转ATC病理状态的潜在化合物( 图 5),其中主要包括酪氨酸激酶抑制剂、表皮生长因子受体抑制剂、MEK抑制剂、RAF抑制剂等。这些药物较传统化学药物具有更明确的靶向性,有利于精准治疗。
ATC的失分化状态不仅导致其肿瘤细胞的恶性化程度更高,而且对现有放射治疗和化学治疗药物均不敏感。因此,研究ATC的发病机制、寻找其关键基因并实现靶向治疗临床意义重大。
本研究发现,ATC组织不仅细胞内发生通路的改变,且肿瘤微环境也与正常甲状腺组织不同。与正常甲状腺组织相比,ATC组织中细胞周期、PI3K-Akt信号通路、吞噬体、蛋白消化及吸收、肿瘤通路、p53通路显著上调,提示ATC组织中调控肿瘤的经典通路被激活,细胞增殖旺盛,且可通过对非正常折叠蛋白进行清除而避免凋亡信号激活。与本研究结果相一致,ATC测序结果显示,ATC存在 TP53突变(>50%)和 PTEN突变引起的PI3K-Akt-mTOR通路异常激活 [ 5, 8] 。而在肿瘤微环境方面,ATC可显著进行细胞外基质重塑,且发生炎症反应及趋化行为,并存在血管新生。这些基因的功能富集结果与肿瘤的进展以及侵袭转移密切相关 [ 9] ,提示ATC细胞的恶性状态。
进一步进行基因簇及关键网络节点挖掘显示,ATC的疾病状态网络存在三个关键基因簇,分别与细胞周期、脱氧核糖核酸内切酶活性、细胞外基质-受体相互作用、蛋白消化及吸收、胶原结合、补体受体通路、单核细胞趋化及招募及白细胞迁移调控相关。其中,网络关键节点 TOP2A是调控DNA拓扑结构的拓扑异构酶,与细胞分裂、DNA损伤修复有关,目前, TOP2A已被作为抗肿瘤靶点进行研究 [ 10- 12] ,其临床试验主要集中于利用 TOP2A抑制剂表柔比星治疗乳腺癌患者。 COL1A1、 COL1A2和 COL3A1属于胶原家族,其活性与细胞增殖及肿瘤迁移能力密切相关 [ 13] 。 COL1A1、 COL1A2和 COL3A1的表达是多数肿瘤的不良预后因素 [ 14] 。另一网络关键节点 IL-8是恶性肿瘤分泌的炎症信号,可促进胃癌的转移 [ 15] ,且血清中IL-8水平与肿瘤负荷相关,是一个潜在检测肿瘤负荷标志物 [ 16] 。Kobawala等 [ 17] 研究发现ATC患者血清IL-8水平较健康人显著上升。
如何针对肿瘤特定基因特征进行药物干预是目前研究的热点。基于基因特征导向的检索引擎可为肿瘤治疗提供潜在的干预策略。L1000CDS 2数据库利用2000多种小分子化合物对六株细胞进行干预,进而通过基因芯片获取对应基因的变化,通过将肿瘤的差异表达基因与该数据库比对,即可获得一系列潜在可逆转肿瘤基因状态的化合物。本研究通过该方法获得了22个治疗ATC的潜在化合物,主要为酪氨酸激酶抑制剂、表皮生长因子受体抑制剂、MEK抑制剂、RAF抑制剂等与临床肿瘤治疗关系比较密切的靶向药物。
本研究通过系统地分析ATC的发病机制及其干预策略,在一定程度上揭示了ATC的发病机制,为ATC的治疗靶点研究提供了理论依据。但通过基因组层面改变到蛋白组学层面的体现,中间需要甲基化组学、微RNA组学、转录组组学等多个过程,需要多组学技术联用、进行多层面的共同分析,才能系统地揭示肿瘤的发生、发展机制,为肿瘤精准治疗提供依据。
Funding Statement
浙江省基础公益研究计划(LQ18H160017);浙江省医药卫生科技计划(2017192848,2018245206);杭州市卫生科技计划(2017B56)
References
- 1.SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(1):7-30.] [DOI] [PubMed] [Google Scholar]
- 2.CHEN W, ZHENG R, BAADE P D, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [CHEN W, ZHENG R, BAADE P D, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2):115-132.] [DOI] [PubMed] [Google Scholar]
- 3.FAGIN J A, WELLS S A. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375(11):1054–1067. doi: 10.1056/NEJMra1501993. [FAGIN J A, WELLS S A. Biologic and clinical perspectives on thyroid cancer[J]. N Engl J Med, 2016, 375(11):1054-1067.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WISEMAN S M, MASOUDI H, NIBLOCK P, et al. Anaplastic thyroid carcinoma:expression profile of targets for therapy offers new insights for disease treatment. Ann Surg Oncol. 2007;14(2):719–729. doi: 10.1245/s10434-006-9178-6. [WISEMAN S M, MASOUDI H, NIBLOCK P, et al. Anaplastic thyroid carcinoma:expression profile of targets for therapy offers new insights for disease treatment[J]. Ann Surg Oncol, 2007, 14(2):719-729.] [DOI] [PubMed] [Google Scholar]
- 5.LANDA I, IBRAHIMPASIC T, BOUCAI L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. doi: 10.1172/JCI85271. [LANDA I, IBRAHIMPASIC T, BOUCAI L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers[J]. J Clin Invest, 2016, 126(3):1052-1066.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SANTARPIA L, EL-NAGGAR A K, COTEG J, et al. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008;93(1):278–284. doi: 10.1210/jc.2007-1076. [SANTARPIA L, EL-NAGGAR A K, COTEG J, et al. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer[J]. J Clin Endocrinol Metab, 2008, 93(1):278-284.] [DOI] [PubMed] [Google Scholar]
- 7.LANDA I, GANLY I, CHANT A, et al. Frequent somatic TERT promoter mutations in thyroid cancer:higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98(9):E1562–E1566. doi: 10.1210/jc.2013-2383. [LANDA I, GANLY I, CHANT A, et al. Frequent somatic TERT promoter mutations in thyroid cancer:higher prevalence in advanced forms of the disease[J]. J Clin Endocrinol Metab, 2013, 98(9):E1562-E1566.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HOU P, LIU D, SHAN Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13(4):1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [HOU P, LIU D, SHAN Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer[J]. Clin Cancer Res, 2007, 13(4):1161-1170.] [DOI] [PubMed] [Google Scholar]
- 9.HUSAIN A, HU N, SADOW P M, et al. Expression of angiogenic switch, cachexia and inflammation factors at the crossroad in undifferentiated thyroid carcinoma with BRAF(V600E) Cancer Lett. 2016;380(2):577–585. doi: 10.1016/j.canlet.2015.07.012. [HUSAIN A, HU N, SADOW P M, et al. Expression of angiogenic switch, cachexia and inflammation factors at the crossroad in undifferentiated thyroid carcinoma with BRAF(V600E)[J]. Cancer Lett, 2016, 380(2):577-585.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EJLERTSEN B, TUXEN M K, JAKOBSEN E H, et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A-normal breast cancer:DBCG 07-READ, an open-label, phase Ⅲ, randomized trial. J Clin Oncol. 2017;35(23):2639–2646. doi: 10.1200/JCO.2017.72.3494. [EJLERTSEN B, TUXEN M K, JAKOBSEN E H, et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A-normal breast cancer:DBCG 07-READ, an open-label, phase Ⅲ, randomized trial[J]. J Clin Oncol, 2017, 35(23):2639-2646.] [DOI] [PubMed] [Google Scholar]
- 11.TURNER J G, DAWSON J L, GRANT S, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase Ⅱ inhibitors. J Hematol Oncol. 2016;9(1):73. doi: 10.1186/s13045-016-0304-z. [TURNER J G, DAWSON J L, GRANT S, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase Ⅱ inhibitors[J]. J Hematol Oncol, 2016, 9(1):73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TARPGAARD L S, QVORTRUP C, NYGRD S B, et al. A phase Ⅱ study of epirubicin in oxaliplatin-resistant patients with metastatic colorectal cancer and TOP2A gene amplification. BMC Cancer. 2016;16:91. doi: 10.1186/s12885-016-2124-5. [TARPGAARD L S, QVORTRUP C, NYGRD S B, et al. A phase Ⅱ study of epirubicin in oxaliplatin-resistant patients with metastatic colorectal cancer and TOP2A gene amplification[J]. BMC Cancer, 2016, 16:91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CHRISTNER P J, AYITEY S. Extracellular matrix containing mutated fibrillin-1(Fbn1) down regulates Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2 mRNA levels in Tsk/+ and Tsk/Tsk embryonic fibroblasts. Amino Acids. 2006;30(4):445–451. doi: 10.1007/s00726-005-0265-y. [CHRISTNER P J, AYITEY S. Extracellular matrix containing mutated fibrillin-1(Fbn1) down regulates Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2 mRNA levels in Tsk/+ and Tsk/Tsk embryonic fibroblasts[J]. Amino Acids, 2006, 30(4):445-451.] [DOI] [PubMed] [Google Scholar]
- 14.BARCUS C E, O'LEARY K A, BROCKMAN J L, et al. Elevated collagen-Ⅰ augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017;19(1):9. doi: 10.1186/s13058-017-0801-1. [BARCUS C E, O'LEARY K A, BROCKMAN J L, et al. Elevated collagen-Ⅰ augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells[J]. Breast Cancer Res, 2017, 19(1):9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CHEN L, MIN L, WANG X, et al. Loss of RACK1 promotes metastasis of gastric cancer by inducing a miR-302c/IL8 signaling loop. Cancer Res. 2015;75(18):3832–3841. doi: 10.1158/0008-5472.CAN-14-3690. [CHEN L, MIN L, WANG X, et al. Loss of RACK1 promotes metastasis of gastric cancer by inducing a miR-302c/IL8 signaling loop[J]. Cancer Res, 2015, 75(18):3832-3841.] [DOI] [PubMed] [Google Scholar]
- 16.SANMAMED M F, CARRANZA-RUA O, ALFARO C, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20(22):5697–5707. doi: 10.1158/1078-0432.CCR-13-3203. [SANMAMED M F, CARRANZA-RUA O, ALFARO C, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins[J]. Clin Cancer Res, 2014, 20(22):5697-5707.] [DOI] [PubMed] [Google Scholar]
- 17.KOBAWALA T P, PATEL G H, GAJJAR D R, et al. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. http://www.ncbi.nlm.nih.gov/pubmed/21461397. J Thyroid Res. 2011;2011:270149. doi: 10.4061/2011/270149. [KOBAWALA T P, PATEL G H, GAJJAR D R, et al. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases[J]. J Thyroid Res, 2011, 2011:270149.] [DOI] [PMC free article] [PubMed] [Google Scholar]


