Abstract
Background
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a genetic disorder caused by a structural abnormality in the enzyme. G6PD deficiency is most prevalent among African, Asian, and Mediterranean people. This study aimed to investigate how prevalent G6PD deficiency is in African neonates with jaundice.
Methods
The public sources, such as PubMed, Science Direct, Google Scholar, and Africa Journal Online were searched for articles that reported the prevalence of G6PD deficiency published before March 21st, 2022. The Joanna Briggs Institute’s (JBI) critical assessment checklist was used to evaluate the quality of individual studies. STATA-17 was used to do the statistical analysis. The pooled prevalence of G6PD deficiency in neonates with jaundice in Africa was calculated using a forest plot and a random effects model. I2 statistics and Galbraith plots were used to assess heterogeneity. Publication bias was assessed using a funnel plot and Egger’s statistical test.
Results
Ten studies involving 1555 neonates with jaundice were involved in the study. G6PD deficiency was prevalent in 24.60% of African neonates with jaundice (95% CI:12.47–36.74) with considerable heterogeneity (I2 = 100%). Nigerian neonates with jaundice had the highest G6PD deficiency (49.67%), whereas South Africans had the lowest (3.14%).
Conclusion
G6PD deficiency has been implicated in a significant portion of African neonates with jaundice, notwithstanding the need for greater research on predisposing variables from other countries. Therefore, it should be thought of performing screening and diagnostic laboratory tests for G6PD deficiency.
Keywords: Africa, Glucose-6-phosphate dehydrogenase deficiency, Hyperbilirubinemia, Meta-analysis, Neonates, Jaundice, Systematic review
1. Introduction
Around 80% of neonates worldwide have some degree of hyperbilirubinemia and jaundice. Severe cases of hyperbilirubinemia eventually result in kernicterus, leading to permanent developmental disorders. Several risk factors contribute to hyperbilirubinemia and kernicterus, including Glucose-6-phosphate dehydrogenase (G6PD) deficiency [1]. G6PD deficiency is the most prevalent enzyme deficiency in humans, affecting about 400 million people globally, with a high occurrence in African, Asian, and Mediterranean descent populations [2].
G6PD insufficiency is a genetic disorder caused by a structural flaw in G6PD, a “housekeeping” enzyme that is critical for red blood cell survival and the ability to respond to oxidative stress [3]. Single nucleotide polymorphisms (SNPs) in the protein G6PD cause single amino acid alterations, resulting in the enzyme deficiency. G6PD insufficiency has been linked to over 400 SNPs, resulting in 160 distinct amino acid alterations [4].
G6PD catalyzes the oxidation of glucose-6-phosphate to 6-phosphogluconate while reducing NADP+ to NADPH, making it the rate-limiting enzyme in the pentose phosphate pathway. The regeneration of reduced glutathione (GSH) is then fueled by NADPH, which neutralizes reactive oxygen species (ROS) [4,5]. G6PD is the principal generator of NADPH in erythrocytes, which lack mitochondria, and hence plays a critical role in ROS defense [6,7].
The deficiency of G6PD will induce an accelerated rate of hemolysis that causes serum bilirubin to be generated faster than it can be conjugated or diffuse into the skin and other bodily tissues; during this spike, bilirubin crosses the blood-brain barrier and enters brain cells [8]. Because bilirubin is lipophilic and concentrated in membrane compartments, damage occurs at the cell membranes. This causes lipid peroxidation and hinders membrane-bound proteins like ATPases from performing their tasks. Similarly, bilirubin attacks mitochondrial membranes, disrupting the electron transport chain and membrane-bound proteins, causing mitochondrial enlargement, membrane permeability, depolarization, cytochrome c release, and cell death by apoptosis and necrosis [[8], [9], [10], [11]]. High bilirubin causes behavioral and neurological damage (Neurotoxicity or Kernicterus) even in term neonates [[12], [13], [14]].
Every year, about 1.1 million babies worldwide acquire severe hyperbilirubinemia with or without bilirubin encephalopathy, with Sub-Saharan Africa and South Asia accounting for the majority [15]. According to the global burden of neonatal jaundice study, the African region has the greatest rate of severe neonatal jaundice per 1000 live births (667.8–738.5), followed by Southeast Asia (251.3–473.2) and the Americas and European regions (4.4 and 3.7, respectively) [16].
From a recent study, the pooled magnitude of neonatal hyperbilirubinemia in sub-Saharan Africa was reported about 28.08% [17]. Neonatal jaundice is frequently associated with sepsis in Africa, which is a leading cause of neonatal death. In developing Sub-Saharan African nations, newborn morbidity and mortality are still common, and neonatal hyperbilirubinemia is one of the main causes of morbidity and mortality, with G6PD deficiency being one of the linked factors [17,18]. Therefore, the aim of this systematic review and meta-analysis was to estimate the pooled prevalence of G6PD deficiency among African neonates with jaundice.
2. Methods
2.1. Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-2020) guideline was followed for this systematic review and meta-analysis [19]. Published and unpublished studies reporting the prevalence of G6PD deficiency among neonates with jaundice were found in online public sources such as PubMed, Science Direct, African Journals Online, and Google Scholar up until March 21, 2022.
The search terms were used in agreement with the Medical Subject Heading (MeSH) using the arrangement of key words which were used to select related research articles. The search approach used to retrieve related articles was as follows: (Prevalence AND Glucose-6-phosphate dehydrogenase OR G6PD AND “newborn with jaundice” OR “icterus newborn” AND Africa). EndNote 20 citation and referencing manager was used to arrange references and remove duplicates.
2.2. Eligibility criteria
All original research articles published in English and containing basic information concerning sample size and prevalence of G6PD deficiency among jaundiced neonates ((0–28 days old) (age indicated at time of diagnosis)) in different parts of Africa were included. Duplicated articles, articles unable to access the full text, and abstracts were removed from the review. Moreover, studies conducted among all non-jaundiced neonates, children, unknown sample size, and lack clear figure about cases were excluded from this review.
2.3. Selection process and quality assessment
Two authors (MS and WK) individually conducted a search in PubMed, Science Direct, African Journals Online, and Google Scholar using keywords. The searched articles were screened by title and abstract to transfer the articles in the full-text review. Two authors (AA and AT) independently assessed the quality of the journals included in this review using JBI-critical appraisal checklist. The differences in the inclusion and quality of individual articles between the two authors were resolved by discussion with the third author (MS).
2.4. Outcome variable
The outcome of interest for this systematic review was G6PD deficiency prevalence among African neonates with jaundice.
2.5. Data extraction
Data extraction protocol consisting of names of the first author, year of publication, study area (country), design, sample size, and prevalence of G6PD deficiency was developed by two authors (MS and WK) and evaluated by AT and AA. The extracted data were entered in to Excel.
2.6. Data analysis
Eligible primary studies were extracted, entered into Microsoft Excel, and then exported to STATA version 17. Descriptive characteristics of the studies included were presented in tables. Forest plots were used to estimate the pooled effect size and effect of each study with their confidence interval (CI) and to provide a visual image of the data. The degree of heterogeneity between the included studies was evaluated by the inverse of variance (I2) and Galbraith plot. Publication bias across studies were evaluated by the funnel plot and Egger’s regression test objectively.
3. Result
3.1. Identification of included studies
We found 267 papers by searching Google Scholar, African Journals Online, PubMed/MEDLINE, and Science Direct, with 210, 3, 50, and 4 articles from each database, respectively. After removing the duplications, there were 260 articles left. After reading the titles, 80 articles were retained, eliminating 180. After removing 56 articles based on abstract reading, 24 remained for full article review. Finally, the pooled prevalence of G6PD deficiency among neonates with jaundice was determined using 10 papers that met the inclusion criteria (Fig. 1).
Fig. 1.
PRISMA flow diagram showing search results for the inclusion of studies focusing on G6PD deficiency among neonates with jaundice in Africa published to March 21, 2022.
3.2. Characteristics of studies included
Nine of the studies included were cross-sectional, while one was a case series. The sample sizes in the included studies ranged from 21 to 400. A total of 1555 neonates with jaundice were included in this meta-analysis. The studies were reported from five African countries, with Egypt and Nigeria contributing the most (Table 1).
Table 1.
Characteristics of studies reporting G6PD deficiency among neonates with jaundice in Africa published up to March 21, 2022.
| First Author name, Publication year | Country | Study design | Studied population (N) | G6PD deficiency Prevalence (%) | Age range in days | Mean age in days | G6PD deficiency screening/diagnosis method | Reference |
|---|---|---|---|---|---|---|---|---|
| Mostefa M. et al., 2014 | Egypt | CS* | 300 | 6.0 | 2–10 | 4.18 | Quantitative Enzyme assay (Not specified) | [20] |
| Mohammed AF. et al., 2010 | Egypt | Case series | 69 | 14.4 | <28 | 5.87 | Qualitative screening (Methemoglobin reduction test) | [21] |
| Zehra M. et al., 2013 | Egypt | CS | 21 | 42.0 | <28 | 4.66 | Qualitative screening (Methemoglobin reduction test) | [22] |
| Waffa MM et al., 2016 | Egypt | CS | 202 | 8.9 | 1–13 | 3.75 | Quantitative Spectrophotometric assay (Cutt-off<4.6 U/g Hb¥) | [23] |
| Bienzle U et al., 1976 | Nigeria | CS | 70 | 51.0 | <28 | NS** | Qualitative screening (Methemoglobin reduction test) | [24] |
| Farouk ZLet al. 2017 | Nigeria | CS | 100 | 46.0 | 1–21 | 4.6 | Quantitative Spectrophotometric assay (Cutt-off<4.6 U/g Hb¥) | [25] |
| George IO et al., 2011 | Nigeria | CS | 400 | 52.0 | <28 | NS** | Qualitative screening test (Methaemoglobin reduction test) | [26] |
| Levin Se et al., 1964 | South Africa | CS | 159 | 3.14 | <14 | 6.6 | Qualitative screening test (Motulsky-Campbell technique) | [27] |
| Ahmed et al., 2015 | Sudan | CS | 80 | 3.75 | <28 | NS** | [28] | |
| Rym D et al., 2020 | Tunisia | CS | 154 | 18.83 | <28 | 4.5 | Quantitative colorimetric assay (cut-off <7.0 IU/g Hb¥) | [29] |
*Cross-sectional study.
** Not specified.
¥ Unit of G6PD activity per gram of hemoglobin (G6PD/hemoglobin).
3.3. Pooled estimates of G6PD deficiency among neonates with jaundice in Africa
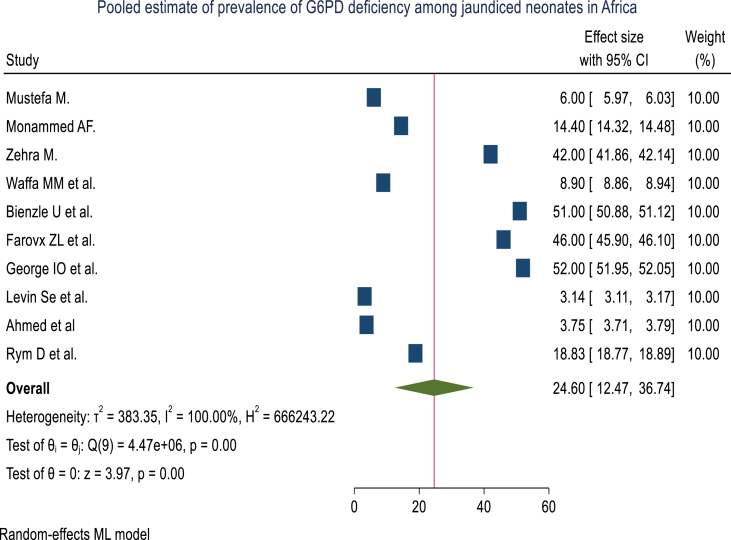
The pooled prevalence of G6PD deficiency among jaundiced neonates in Africa was 24.60% (95% CI: 12.47, 36.74%) with considerable heterogeneity between studies (I2 = 100%%) (Fig. 2) however they fall in 95% confidence interval on the Galbraith plot for qualitative heterogeneity of studies (Fig. 3).
Fig. 2.
Forest plot showing the prevalence of G6PD deficiency among neonates with jaundice in Africa from studies published up to March 21, 2022.
Fig. 3.
Assessment of the qualitative variability of studies reporting a G6PD deficiency among neonates with jaundice in Africa in Africa published up to March 21, 2022, using the Galbraith plot.
3.4. Sub group analysis by country and design
The test of no difference between the groups (countries) is rejected, with chi-square test statistics of 397.04 and a p-value of less than 0.01. Subgroup analysis by county showed that the lowest G6PD deficiency was found among South African neonates with jaundice (3.14%) followed by Sudan neonates with jaundice (3.75%) while the highest was recorded in Nigeria (49.67% (Fig. 4).
Fig. 4.
Subgroup analysis of the proportion of G6PD deficiency among neonates with jaundice in Africa from studies published up to March 21, 2022.
3.5. Publication bias assessment
Funnel plot shows some studies are missing both in the right and left portion of the funnel plot, which makes it look asymmetrical. Heterogeneity may be the reason for the asymmetry of the plot. To test the asymmetry statistically, a small study effect in the meta-analysis was tested using Egger test under the random effects model and found that there was small study effect (z = 2.88, p = 0.004) (Fig. 5).
Fig. 5.
Funnel plot showing publication bias among studies reporting the prevalence of G6PD deficiency among neonates with jaundice in Africa from studies published up to March 21, 2022.
4. Discussion
Glucose-6-phosphate dehydrogenase (G6PD) enzyme is involved in the hexose monophosphate pathway, producing NADPH, which maintains reduced glutathione that maintains the oxidative state of red blood cells [30]. G6PD deficiency makes red blood cells (RBCs) vulnerable to oxidative stress, resulting in hemolysis. Clinical, biochemical, and molecular variability, as well as a wide range of prevalence, describe G6PD deficiency [[31], [32], [33], [34]]. G6PD deficiency is the most prevalent cause of neonatal jaundice, according to reports from throughout the world [17,[35], [36], [37]]. Neonatal jaundice, if left untreated, can cause neurological issues such as poor neurocognitive development, cerebral palsy, neuropathy, deafness, and death [38].
The pooled prevalence of G6PD deficiency among neonates with jaundice in Africa was 24.60% (95% CI: 12.47–36.74%) in the current study. This incidence is consistent with research conducted in Egypt (14.4%), Iraq (16%), and Tunisia (18.83%) [21,29,39], while it was higher than a global estimation for Africa (7.5%) [2] and studies done in Dubai (10.5%), Southern Brazil (4.6%), India (1.8%), Tehran (2.1%), China (8.36) and Iran (7%) [30,[40], [41], [42], [43], [44]]. However, the pooled prevalence of this study was lower than the prevalence of G6PD deficiency among Nigeria (51.0%) and Egypt (42.2%) [22,24]. These differences can be explained by the fact that different ethnic groups have different versions of the G6PD enzyme, resulting in variable degrees of severity among those who are affected. G6PD deficiency is more common in places where people have been exposed to endemic malaria in the past, such as Africa, Mediterranean Europe, Southeast Asia, and Latin America. Enzyme deficiency primarily affects African and Mediterranean-descent groups in the United States [45].
Even though there are numerical variations with in a wide range, when we compare the combined prevalence of G6PD deficiency among neonates with jaundice in Africa to the national or large population estimates of the participating countries, it was greater than Egypt (4.9%) and Sudan (5.5%) [46,47] but comparable Nigeria (24.6%) [48]. However, we couldn’t retrieve a representative estimate for Tunisia and South Africa.
More than 200 mutations have been documented for the highly polymorphic G6PD gene worldwide. However, only a small percentage of mutant G6PD variants lead to a functionally defective G6PD enzyme [49]. The geographical distribution of G6PD mutation variants in Africa is heterogenous across nations and even with in a population. The two most common G6PD variants of clinical significance on the African continent are the African-type G6PD A-variant, which is primarily found in sub-Saharan Africa, and the Mediterranean-type G6PD B- variant, which is primarily found in North Africa [50]. In addition, there are also ethnogeography specific variants reported from different parts of the continent. For example, G6PD Cairo, G6PD Aures and G6PD Chatham reported from Egypt and Tunisia [22,51]. This can result in significant differences in the prevalence of G6PD deficiency between different regions and populations as the degree of disease severity and triggering factors vary from place to place [52].
As individuals with silent manifestations will not complain and frequent investigations will not be undertaken, the incidence of G6PD deficiency may be comparatively higher in locations where variants can cause severe hemolytic anemia. Therefore, the prevalence by country level was subjected to subgroup analysis. The subgroup analysis showed that Nigerian neonates with jaundice (49.67%), Tunisians (18.83%), and Egyptians (17.82%) had higher rates of G6PD deficiency, while South African and Sudanese jaundiced neonates (3.14% and 3.75%, respectively) had lower rates of G6PD deficiency. The increased prevalence of G6PD deficiency among Nigerian neonates with jaundice could be attributed to malaria endemicity, ethnicity, and sample size differences, whereas Arab descent may contribute to the increase in Tunisia and Egypt [53,54].
In G6PD-deficient neonates, environmental variables such as maternal exposure to oxidant medications, herbal medicines, and clothes containing naphthalene may trigger or worsen neonatal jaundice [55]. Other stressors including infections, oxidative drugs, immaturity, hypoxia and fava beans can lead to acute hemolytic anemia and hyperbilirubinemia in G6PD deficient individuals include [17,56,57].
Several researchers recently concluded that hemolysis is not the cause of jaundice in G6PD-deficient neonates [58,59]. Indeed, the neonate liver’s lower ability to conjugate bilirubin appears to be the most important component, especially when G6PD deficiency is co-inherited with mutations in UDP-glucuronosyltransferase-1, which appear to be the other variable involved in newborn jaundice [60].
In addition to phototherapy and exchange transfusion for jaundiced neonates, long-lasting treatment approaches for G6PD deficient neonates are directed toward avoiding these and other stressors, which can cause acute hemolytic anemia and hyperbilirubinemia in G6PD deficient individualism [57,61].
From this analysis, we learned that the overall magnitude of G6PD deficiency is high and more frequently observed around the Northern part of the continent.
4.1. Limitations of the study
This analysis may have limitations due to the small number of publications from which full papers could be obtained and the significant heterogeneity of the studies included.
4.2. Conclusion and recommendations
This study has shown that nearly one out of four neonates with jaundice have a chance to be deficient for G6PD enzyme activity. As G6PD deficiency is a genetic mutation, the current clinical treatment approaches can only alleviate the suffering and complications. However, G6PD deficiency not only cause hemolytic anemia and hyperbilirubinemia but also sever neurological consequences like kernicterus, increased infection susceptibility and predispose to some forms of chronic disease like diabetes. Therefore, in order to provide evidence-based treatment, routine screening for G6PD activity in all neonates with jaundice could be necessary to reduce the complication of G6PD deficiency and better to incorporate it to the health care system of Africa, particularly Nigeria.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Contributor Information
Woldeteklehaymanot Kassahun, Email: teklu1142@gmail.com, wolde.t@wldu.edu.et.
Abayneh Tunta, Email: abaynehtunta@gmail.com.
Atitegeb Abera, Email: kidiemihiret@gmail.com.
Mulu Shiferaw, Email: mulushi9804@gmail.com.
List of abbreviations and acronyms
- ATP
Adenosine Triphosphate
- G6PD
Glucose 6 phosphate Dehydrogenase
- NADH
Reduced Nicotinamide Adenine Dinucleotide
- NADPH
Reduced Nicotinamide Adenine Dinucleotide Phosphate
- ROS
Reactive Oxygen Species
- SNPs
Single Nucleotide Polymorphisms
- UDP
Uridine Diphosphate
References
- 1.Cunningham A.D., Hwang S., Mochly-Rosen D. Glucose-6-Phosphate dehydrogenase deficiency and the need for a novel treatment to prevent kernicterus. Clin. Perinatol. 2016;43(2):341–354. doi: 10.1016/j.clp.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nkhoma E.T., Poole C., Vannappagari V., Hall S.A., Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cell Mol. Dis. 2009;42(3):267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Srikanth Nagalla M. Glucose-6-Phosphate dehydrogenase (G6PD) deficiency. 2021. https://emedicine.medscape.com/article/200390-overview [Available from:
- 4.Verrelli B.C., McDonald J.H., Argyropoulos G., Destro-Bisol G., Froment A., Drousiotou A., et al. Evidence for balancing selection from nucleotide sequence analyses of human G6PD. Am. J. Hum. Genet. 2002;71(5):1112–1128. doi: 10.1086/344345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton R. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanton R.C. Glucose-6-Phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64(5) doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martich-Kriss V., Kollias S.S., Ball W.S., Jr. MR findings in kernicterus. AJNR: Am. J. Neuroradiol. 1995;16(4):819. [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan M., Bromiker R., Hammerman C., editors. Hyperbilirubinemia, Hemolysis, and Increased Bilirubin Neurotoxicity. Elsevier; 2014. (Seminars in Perinatology). [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues C.M., Solá S., Castro R.E., Laires P.A., Brites D., Moura J.J. Perturbation of membrane dynamics in nerve cells as an early event during bilirubin-induced apoptosis. J. Lipid Res. 2002;43(6):885–894. [PubMed] [Google Scholar]
- 10.Keshavan P., Schwemberger S.J., Smith D.L., Babcock G.F., Zucker S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer. 2004;112(3):433–445. doi: 10.1002/ijc.20418. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues C.M., Solá S., Silva R., Brites D. Bilirubin and amyloid-β peptide induce cytochrome c release through mitochondrial membrane permeabilization. Mol. Med. 2000;6(11):936–946. [PMC free article] [PubMed] [Google Scholar]
- 12.Paludetto R., Mansi G., Raimondi F., Romano A., Crivaro V., Bussi M., et al. Moderate hyperbilirubinemia induces a transient alteration of neonatal behavior. Pediatrics. 2002;110(4):e50. doi: 10.1542/peds.110.4.e50. [DOI] [PubMed] [Google Scholar]
- 13.Boo N.Y., Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. J. Paediatr. Child Health. 2007;43(4):297–302. doi: 10.1111/j.1440-1754.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 14.Nass R., Frank Y. Oxford University Press; 2010. Cognitive and Behavioral Abnormalities of Pediatric Diseases. [Google Scholar]
- 15.Olusanya B., Osibanjo F., Mabogunje C., Slusher T., Olowe S. The burden and management of neonatal jaundice in Nigeria: a scoping review of the literature. Niger. J. Clin. Pract. 2016;19(1):1–17. doi: 10.4103/1119-3077.173703. [DOI] [PubMed] [Google Scholar]
- 16.Olusanya B., Slusher T. Infants at risk of significant hyperbilirubinemia in poorly-resourced countries: evidence from a scoping review. World Journal of Pediatrics. 2015;11:293–299. doi: 10.1007/s12519-015-0037-z. [DOI] [PubMed] [Google Scholar]
- 17.Aynalem Y.A., Mulu G.B., Akalu T.Y., Shiferaw W.S. Prevalence of neonatal hyperbilirubinaemia and its association with glucose-6-phosphate dehydrogenase deficiency and blood-type incompatibility in sub-Saharan Africa: a systematic review and meta-analysis. BMJ Paediatrics Open. 2020;4(1) doi: 10.1136/bmjpo-2020-000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassa R., Gudeta H., Assen Z., Demlew T., Teshome G. Neonatal hyperbilirubinemia: magnitude and associated etiologic factors among neonates admitted at Tikur Anbessa specialized Hospital, Ethiopia. J. Pregn. Child Health. 2018;5 doi: 10.4172/2376-127X.1000384. [DOI] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abosdera M.M., Almasry A.E., editors. Prevalence of Glucose 6 Ph O Sphate Dehydrogenase Among Hyperbilrubinemic Neonates in Sohag Governorate , Egypt. 2014. [Google Scholar]
- 21.Abdel Fattah M., Abdel Ghany E., Adel A., Mosallam D., Kamal S. Glucose-6-phosphate dehydrogenase and red cell pyruvate kinase deficiency in neonatal jaundice cases in Egypt. Pediatr. Hematol. Oncol. 2010;27(4):262–271. doi: 10.3109/08880011003639986. [DOI] [PubMed] [Google Scholar]
- 22.Ezz El-Deen Z.M., Hussin N.F., Abdel Hamid T.A., Abdel Migeed O.R., Samy R.M. G6PD deficiency and G6PD (mediterranean and silent) polymorphisms in Egyptian infants with neonatal hyperbilirubinemia. Lab. Med. 2013;44(3):228–234. doi: 10.1309/lmqosc1ry6ectdu2. [DOI] [Google Scholar]
- 23.Abo M., El Fotoh W.M., Rizk M.S. Prevalence of glucose-6-phosphate dehydrogenase deficiency in jaundiced Egyptian neonates. J. Matern. Fetal Neonatal Med. 2016;29(23):3834–3837. doi: 10.3109/14767058.2016.1148133. [DOI] [PubMed] [Google Scholar]
- 24.Bienzle U., Effiong C., Luzzatto L. Erythrocyte glucose 6-phosphate dehydrogenase deficiency (G6PD type A-) and neonatal jaundice. Acta Paediatr. Scand. 1976;65(6):701–703. doi: 10.1111/j.1651-2227.1976.tb18006.x. [DOI] [PubMed] [Google Scholar]
- 25.Farouk Z.L.I.M., Ogala W.N. Glucose-6-phosphate dehydrogenase deficiency; the single most important cause of neonatal hyperbilirubinaemia in Kano, Nigeria. Niger. J. Paediatr. 2017;44:44–49. doi: 10.4314/njp.v44i2.1. [DOI] [Google Scholar]
- 26.Akani IOGaNA Evaluation of Glucose- 6- phosphate dehydrogenase deficiency in icteric newborns in Nigeria. Am. J. Trop. Med. Publ. Health. 2011;1(3) [Google Scholar]
- 27.Levin S.E., Charton R.W., Freiman I. Glucose-6-phosphate dehydrogenase deficiency and neonatal jaundice in South African Bantu infants. J. Pediatr. 1964;65(5):757–764. doi: 10.1016/S0022-3476(64)80162-1. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed A., editor. The Level of Glucose-6-Phosphate Dehydrogenase in Neonates with Hyperbilirubinaemia in Gezira State, Sudan. 2015. [Google Scholar]
- 29.Dabboubi R., Amri Y., Hamdi S., Jouini H., Sahli C., Fredj S.H., et al. Glucose-6-phosphate dehydrogenase deficiency in Tunisian jaundiced neonates. Ann. Biol. Clin. 2020;78(4):411–416. doi: 10.1684/abc.2020.1558. [DOI] [PubMed] [Google Scholar]
- 30.Javadi M., Deravi S., Zarei S., Mahdavi N., Ranjbaran M. Prevalence of G6PD deficiency in Iranian neonates with jaundice: a systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2021:1–8. doi: 10.1080/14767058.2021.1895738. [DOI] [PubMed] [Google Scholar]
- 31.Chan T. University of Hong Kong; 2013. Glucose-6-phosphate Dehydrogenase Deficiency: A Review Glucose-6-Phosphate Dehydrogenase. [Google Scholar]
- 32.Bhutani V.K., Kaplan M., Glader B., Cotten M., Kleinert J., Pamula V. Point-of-care quantitative measure of glucose-6-phosphate dehydrogenase enzyme deficiency. Pediatrics. 2015;136(5):e1268–e1275. doi: 10.1542/peds.2015-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hameed N.N., Vilms R., Bhutani V.K. Severe neonatal hyperbilirubinemia and adverse short-term consequences in Baghdad, Iraq. Neonatology. 2011;100(1):57–63. doi: 10.1159/000321990. [DOI] [PubMed] [Google Scholar]
- 34.Iranpour R., Hashemipour M., Talaei S.-M., Soroshnia M., Amini A. Newborn screening for glucose-6-phosphate dehydrogenase deficiency in Isfahan, Iran: a quantitative assay. J. Med. Screen. 2008;15(2):62–64. doi: 10.1258/jms.2008.008027. [DOI] [PubMed] [Google Scholar]
- 35.Olusanya B.O., Emokpae A.A., Zamora T.G., Slusher T.M. Addressing the burden of neonatal hyperbilirubinaemia in countries with significant glucose‐6‐phosphate dehydrogenase deficiency. Acta Paediatr. 2014;103(11):1102–1109. doi: 10.1111/apa.12735. [DOI] [PubMed] [Google Scholar]
- 36.Nasserullah Z., Alshammari A., Al Abbas M., Abu-Khamsseen Y., Qadri M., Al Jafer S., et al. Regional experience with newborn screening for sickle cell disease, other hemoglobinopathies and G6PD deficiency. Ann. Saudi Med. 2003;23(6):354–357. doi: 10.5144/0256-4947.2003.354. [DOI] [PubMed] [Google Scholar]
- 37.Haloui S., Laouini N., Sahli C.A., Daboubi R., Becher M., Jouini L., et al., editors. Molecular Identification of Gd A-And Gd B-G6pd Deficient Variants by ARMS-PCR in a Tunisian Population. Annales de biologie clinique; 2016. [DOI] [PubMed] [Google Scholar]
- 38.Mwaniki M.K., Atieno M., Lawn J.E., Newton C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379(9814):445–452. doi: 10.1016/s0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eissa A.A., Haji B.A., Al-Doski A.A. G6PD deficiency prevalence as a cause of neonatal jaundice in a neonatal ward in Dohuk, Iraq. Am. J. Perinatol. 2021;38(6):575–580. doi: 10.1055/s-0039-1700854. [DOI] [PubMed] [Google Scholar]
- 40.Saleh Elhalik M. Incidence of G6PD deficiency among neonates with hyperbilirubinemia requiring phototherapy at postnatal wards of a tertiary care perinatal center, Dubai, UAE: a longitudinal cross-sectional study. J. Pediatr. Neonatal Care. 2020;10(4) doi: 10.15406/jpnc.2020.10.00425. [DOI] [Google Scholar]
- 41.Carvalho C.G., Castro S.M., Santin A.P., Zaleski C., Carvalho F.G., Giugliani R. Glucose-6-phosphate-dehydrogenase deficiency and its correlation with other risk factors in jaundiced newborns in Southern Brazil. Asian Pac. J. Trop. Biomed. 2011;1(2):110–113. doi: 10.1016/s2221-1691(11)60006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deshmukh Prabhakar D. Prevalence of G6PD deficiency and its association with neonatal jaundice in babies born at Tertiary Care Hospital. Int. Archiv. BioMed. Clin. Res. 2020;6(4) [Google Scholar]
- 43.Abolghasemi H., Mehrani H., Amid A. An update on the prevalence of glucose-6-phosphate dehydrogenase deficiency and neonatal jaundice in Tehran neonates. Clin. Biochem. 2004;37:241–244. doi: 10.1016/j.clinbiochem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Xu J.-X., Lin F., Chen Z.-K., Luo Z.-Y., Zhan X.-F., Wu J.-R., et al. Co-inheritance of G6PD deficiency and 211 G to a variation of UGT1A1 in neonates with hyperbilirubinemia in eastern Guangdong. BMC Pediatr. 2021;21(1):564. doi: 10.1186/s12887-021-03010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nkhoma E.T., Poole C., Vannappagari V., Hall S.A., Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol. Dis. 2009;42(3):267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Elella S.A., Tawfik M., Barseem N., Moustafa W. Prevalence of glucose-6-phosphate dehydrogenase deficiency in neonates in Egypt. Ann. Saudi Med. 2017;37(5):362–365. doi: 10.5144/0256-4947.2017.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali Albsheer M.M., Lover A.A., Eltom S.B., Omereltinai L., Mohamed N., Muneer M.S., et al. Prevalence of glucose-6-phosphate dehydrogenase deficiency (G6PDd), CareStart qualitative rapid diagnostic test performance, and genetic variants in two malaria-endemic areas in Sudan. PLoS Neglected Trop. Dis. 2021;15(10) doi: 10.1371/journal.pntd.0009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidavalur R., Ezeaka V.C., Bhutani V.K. Estimated disease burden and lost economic productivity due to glucose-6-phosphate dehydrogenase deficiency in Nigerian newborns. Semin. Perinatol. 2021;45(1) doi: 10.1016/j.semperi.2020.151360. [DOI] [PubMed] [Google Scholar]
- 49.Koromina M., Pandi M.T., van der Spek P.J., Patrinos G.P., Lauschke V.M. The ethnogeographic variability of genetic factors underlying G6PD deficiency. Pharmacol. Res. 2021;173 doi: 10.1016/j.phrs.2021.105904. [DOI] [PubMed] [Google Scholar]
- 50.Djigo O.K.M., Ould Ahmedou Salem M.S., Diallo S.M., Bollahi M.A., Boushab B.M., Garre A., et al. Molecular epidemiology of G6PD genotypes in different ethnic groups residing in Saharan and Sahelian Zones of Mauritania. Pathogens. 2021;10(8):931. doi: 10.3390/pathogens10080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben Mansour I., Moradkhani K., Moumni I., Hafsia R., Ghanem A., Abbes S., et al. Two new class III G6PD variants [G6PD Tunis (c.920A > C: p.307Gln > Pro) and G6PD Nefza (c.968T > C: p.323 Leu > Pro)] and overview of the spectrum of mutations in Tunisia. Blood Cell Mol. Dis. 2012;50 doi: 10.1016/j.bcmd.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Howes R.E., Piel F.B., Patil A.P., Nyangiri O.A., Gething P.W., Dewi M., et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9(11) doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO Malaria. 2022. https://www.who.int/news-room/fact-sheets/detail/malaria#:~:text=Children%20under%205%20years%20of%20age%20accounted%20for%20about%2080,%25)%20and%20Mozambique%20(3.8%25) [updated 06 April 2022. Available from:
- 54.Doss C.G.P., Alasmar D.R., Bux R.I., Sneha P., Bakhsh F.D., Al-Azwani I., et al. Genetic epidemiology of glucose-6-phosphate dehydrogenase deficiency in the Arab world. Sci. Rep. 2016;6(1) doi: 10.1038/srep37284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luzzatto L. Glucose 6-phosphate dehydrogenase deficiency: from genotype to phenotype. Haematologica. 2006;91(10):1303–1306. [PubMed] [Google Scholar]
- 56.Kaplan M., Muraca M., Vreman H.J., Hammerman C., Vilei M.T., Rubaltelli F.F., et al. Neonatal bilirubin production-conjugation imbalance: effect of glucose-6-phosphate dehydrogenase deficiency and borderline prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2005;90(2):F123–F127. doi: 10.1136/adc.2004.058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank J.E. Diagnosis and management of G6PD deficiency. Am. Fam. Physician. 2005;72(7):1277–1282. [PubMed] [Google Scholar]
- 58.Jalloh S., Van Rostenberghe H., Yusoff N.M., Ghazali S., Nik Ismail N.Z., Matsuo M., et al. Poor correlation between hemolysis and jaundice in glucose 6-phosphate dehydrogenase-deficient babies. Pediatr. Int. 2005;47(3):258–261. doi: 10.1111/j.1442-200x.2005.02052.x. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan M., Herschel M., Hammerman C., Hoyer J.D., Stevenson D.K. Hyperbilirubinemia among African American, glucose-6-phosphate dehydrogenase-deficient neonates. Pediatrics. 2004;114(2):e213–e219. doi: 10.1542/peds.114.2.e213. [DOI] [PubMed] [Google Scholar]
- 60.Herschel M., Beutler E. Low glucose-6-phosphate dehydrogenase enzyme activity level at the time of hemolysis in a male neonate with the African type of deficiency. Blood Cells Mol. Dis. 2001;27(5):918–923. doi: 10.1006/bcmd.2001.0467. [DOI] [PubMed] [Google Scholar]
- 61.Kemper A.R., Newman T.B., Slaughter J.L., Maisels M.J., Watchko J.F., Downs S.M., et al. Clinical practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2022;150(3) doi: 10.1542/peds.2022-058859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.