Abstract
Background
The use of oral antimicrobial agents in patients with short bowel syndrome (SBS) is challenging due to the changes in gastrointestinal anatomy that may result in diminished absorption and altered drug bioavailability. Prospective studies evaluating bioavailability of antimicrobial agents after oral administration in SBS patients are lacking.
Objectives
To determine the bioavailability of orally administered antimicrobial agents commonly used for treatment in SBS patients to guide clinical decision making when faced with infections.
Methods
We performed an explorative, clinical study investigating the pharmacokinetics (PK) of clindamycin, ciprofloxacin, flucloxacillin and fluconazole in SBS patients with intestinal failure. Participants received a combination of two antimicrobial agents simultaneously. To determine the oral bioavailability, participants received a single oral and IV dose of both agents on two occasions, after which they underwent intensive PK sampling on six predefined time points up to 12 hours after administration. Primary outcome was the oral bioavailability of these antimicrobial agents. Secondary outcomes were intravenous PK characteristics following non-compartmental analysis.
Results
Eighteen SBS patients were included: the mean (SD) age was 59 (17) years and 61% of participants were female. The median observed (IQR) bioavailability of ciprofloxacin, clindamycin, flucloxacillin and fluconazole were 36% (24–50), 93% (56–106), 50% (32–76) and 98% (61–107), respectively.
Conclusion
The bioavailability of selected antimicrobial agents in certain patients with SBS appeared to be better than expected, providing a feasible treatment option. Due to the large observed differences between patients, therapeutic drug monitoring should be part of the treatment to safeguard adequate exposure in all patients.
Trial registration
Registered in the Dutch Trial Register (NL7796) and EudraCT number 2019-002587-28
Introduction
Short bowel syndrome (SBS) is a rare condition usually caused by partial removal of the small intestine due to various underlying conditions and is defined as having a functional small bowel length shorter than 200 cm.1 SBS leads to intestinal failure (IF) in case the remaining gut function is below the minimum to maintain or improve health without intravenous (IV) support of nutrients and/or fluids. IF patients require life-long home parenteral nutrition (HPN) support, which is administered via a central venous access device (CVAD), mostly a catheter. HPN is a complex, time-consuming treatment associated with life-threatening CVAD-related complications, mainly catheter-related bloodstream infections (CRBSIs). Nearly 70% of hospital admissions in HPN patients result from a CRBSI requiring prolonged IV antibiotic treatment.2 In most clinical settings, once a patient is recovering, or when the infection is considered mild, it is advised to rapidly switch to oral antibiotics since this results in a reduced length of stay, lowers costs, and limits potential complications of IV access.3 In addition, the ‘IV-to-oral switch therapy’ is a key quality-of-care indicator for evaluating appropriate antibiotic use.4 However, this option of switching to oral therapy may not apply to SBS patients in whom changes in the anatomy of the gastrointestinal tract may result in significant loss of absorptive surfaces and presumed impaired drug uptake. Therefore, the American Gastroenterological Association advises prolonged IV therapy in SBS patients.5 Besides bowel length, other factors influencing drug absorption and metabolism in patients with SBS comprise mucosal integrity, intestinal motility, site of drug absorption, drug formulation, presence of co-morbidities, pH of the gastric and intestinal lumen, and parenteral nutrition-associated metabolic changes.6–8 Following resection, the remaining intestinal tissue undergoes morphologic and functional changes to compensate for the lost function of the resected bowel over a prolonged period that may take months to years to complete. This process is known as intestinal adaptation and probably enhances drug absorption.8 Despite the described changes in bowel function in SBS patients, successful treatment with orally administered antimicrobial agents has only been reported in selected, mostly paediatric cases.9–13 The present investigation was fuelled by recent findings when we evaluated enteral absorption by measuring blood plasma concentrations of orally administered antibiotics in three SBS patients: two of these seemed to have more or less adequate enteral absorptive capacity.14
Unfortunately, well-designed studies evaluating bioavailability and other pharmacokinetic (PK) parameters of antimicrobial agents in SBS patients on HPN support are lacking.7 Thus, based on both the available literature and the expected process of intestinal adaptation following resection, we hypothesized that enteral absorption of antimicrobial agents may still be substantial in stable adult patients with SBS. To explore this, we included four frequently prescribed antimicrobial agents (clindamycin, ciprofloxacin, flucloxacillin and fluconazole) in our analysis.
Methods
Study design
This single-centre explorative study in SBS patients was conducted from July 2020 to January 2022 at the Radboud university medical center (Radboudumc), Nijmegen, the Netherlands. The study was designed to determine the oral bioavailability of ciprofloxacin, clindamycin, flucloxacillin and fluconazole in patients with SBS. The trial (Dutch Trial Registry: NL7796) was approved by the review board of the Radboudumc (reference number 2019-5561) and conducted according to the declaration of Helsinki. Written informed consent was obtained from all participants. The CONSORT guidelines were followed to report this study.15
Participants
Patients were eligible for inclusion if they were aged ≥18 years, received previous or current long-term HPN (>3 consecutive months) and in case they met the criteria for (functional) SBS.16 The remaining bowel length was estimated from imaging studies or as reported in surgical records. Exclusion criteria were: signs of infection (e.g. chills, fever), active vomiting, worsening or new diarrhoea, a history of allergies or hypersensitivity to the study drugs, potential toxicity or interfering co-medication, making it impossible to include the patient in one of the two treatment groups, impaired renal function (creatinine clearance <30 mL/min/1.73 m2), pregnancy and morbid obesity (BMI > 35).
Study drugs and dosing
On two occasions, participants received a combination of two antimicrobial drugs administered successively as a single dose: orally and IV. Patients received either the combination of ciprofloxacin and clindamycin (CC group) or the combination of flucloxacillin and fluconazole (FF group). Group allocation (eight per group) depended on individual patient characteristics (e.g. known allergies, renal impairment, intolerances and interacting co-medication). When a patient could not receive the combination of CC or FF, another combination was considered.
Participants of the CC group received ciprofloxacin (750 mg) followed by clindamycin (600 mg) (tablet or suspension) on study day one and an IV dose of clindamycin 600 mg and ciprofloxacin 400 mg on study day two. FF-group participants received flucloxacillin (1000 mg) next to fluconazole (400 mg) (tablet or suspension) on day one and an IV dose of flucloxacillin 1000 mg and fluconazole 400 mg on study day two. We gave the antimicrobial agents successively since administering two antimicrobial drugs together was not always compatible. A minimal washout period of 24 hours was chosen; for fluconazole, this was preferably at least 48 hours due to its long half-life.
Pharmacokinetic sampling
Blood samples were collected at baseline (before antimicrobial agent administration) and preferably at 1, 2, 4, 8 and 12 hours after administration of the study drugs to determine the blood concentration (μg/mL). The time of antimicrobial agent administration at the start (oral and IV) and end of infusion (IV only), and blood withdrawal procedures at various time points were documented.
A peripheral venous catheter for the purpose of drawing blood samples was placed. Blood samples of ciprofloxacin, clindamycin and flucloxacillin were collected in EDTA 3.0-mL tubes and blood samples for fluconazole analysis in Lithium Heparin 3.0-mL tubes. The blood samples were centrifuged at 1900g for 5 minutes and stored at −40°C until analysis.
Plasma concentrations of ciprofloxacin, clindamycin, fluconazole and flucloxacillin were assessed at the Department of Pharmacy of the Radboudumc, Nijmegen, using a UPLC–MS (Waters Corporation, Milford, MA, USA) method that was fully validated according to EMA guidelines.17 All analyses per group were analysed as a single batch on the same day to reduce measurement variation, i.e. all analyses were performed after the inclusion of all participants.
Biochemical analysis
The following blood parameters and plasma concentrations were analysed at the Radboudumc clinical chemistry laboratory on the day of admission: albumin, glucose, haemoglobin, white blood cell count and differentiation, alanine aminotransaminase, total protein, creatinine and citrulline. Citrulline is a non-protein amino acid produced by the intestine and is a marker of the absorptive function of the small bowel.18,19
Data collection
The following data were collected: patient characteristics (sex, age, weight, height, underlying disease leading to SBS, estimated length of remaining small bowel after surgical intervention, sites of resection, year of last bowel resection and presence of gastroparesis), HPN characteristics (type of infusion and the number of infusions per week), stoma output, chronic kidney disease epidemiology collaboration estimated glomerular filtration rate (CKD-EPI eGFR) and any allergies or (serious) adverse events.
Pharmacokinetic analysis
PK parameters for ciprofloxacin, clindamycin, flucloxacillin and fluconazole were calculated by non-compartmental methods using WinNonLin® software package (v.6.4; Pharsight, Mountain View, CA, USA) and the log-linear trapezoidal rule. On the basis of the individual plasma concentration–time data the following PK parameters were assessed: the AUC from zero to tau (AUC0-tau dose interval, 12 h for ciprofloxacin, 8 h for clindamycin, 6 h for flucloxacillin and 24 h for fluconazole), the maximum plasma concentration of the drug (Cmax; in mg per litre), the time to reach Cmax (Tmax; in hours) and the average concentration (Cavg; in mg per litre). Bioavailability was determined by the ratio of AUC after oral and IV administration per patient, after correction for differences in dose (AUC0-tau PO/AUC0-tau IV).
In the case of sampling anomalies (e.g. collecting a PK sample from an IV line used to deliver the drug dose), samples were excluded from the analysis. A minimum of three samples per patient in the terminal elimination phase was deemed acceptable to estimate the AUC correctly.
Safety
Vital signs (temperature, pulse saturation and blood pressure) were measured before and during the administration of the antimicrobial agents. (Serious) adverse events were recorded along with the degree of severity, timing and onset.
Statistical analysis
Due to the explorative nature of this study and because the literature on enteral drug absorption in SBS is lacking, we have refrained from performing a formal power calculation.
Baseline characteristics were summarized using descriptive statistical methods. Continuous variables were presented as means with standard deviations or, if not normally distributed, as medians and IQRs.
Possible correlations between bioavailability and variables of interest were analysed in an exploratory analysis by making scatter plots of bioavailability versus variable. Variables analysed were: remnant small bowel length, presence of gastropareses, number of infusions per week, citrulline, presence of stoma and kidney function, and ingestion of oral antimicrobial agent in suspension or tablet, on the basis of a recent systematic review.7 A Spearman’s correlation test was performed if a monotonic relation between bioavailability and variable was found.
All data analyses were performed using the software package GraphPad Prism®, v.9, for Windows (GraphPad Software, San Diego, CA, USA) or IBM SPSS Statistics for Windows, v.27.0 (IBM Corp. Armonk, NY, USA). Statistical significance was defined as a P value of <0.05 (two-tailed).
Results
Patient demographics and baseline characteristics
Between July 2020 and January 2022, 237 patients were treated in the Radboudumc for the management of long-term HPN. Of these, 103 (43%) were diagnosed with SBS, of whom 79 patients were assessed as eligible by their treating physician. In total, 18 SBS patients were included in the study. A flowchart of the enrolment sequence is shown in Figure S1 (available as Supplementary data at JAC Online). The mean (SD) age of enrolled patients was 59 (17) years, and 61% of participants were female. Baseline characteristics of the participants are shown in Table 1 and Table S1. Both the CC and FF groups included eight participants. Due to allergies, group allocation was not possible in two participants; therefore, these individuals received a combination of fluconazole and clindamycin. Participant nine had a creatinine clearance >30 mL/min at screening; however, at the start of the study, the CKD-EPI eGFR had decreased to 28 mL/min.
Table 1.
Baseline characteristics of the participants included in the study
| Participant | Group | Age (years) |
Sex | Weight (kg) |
Height (m) |
CKD-EPI eGFR (mL/min/1.73 m2) |
Cause of intestinal failure | Remnant small bowel length (m) | Stoma | Year since last bowel resection | Gastroparesis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CC | 25 | F | 62 | 1.7 | 90 | Necrotizing enterocolitis | 1.7 | No | 2015 | No |
| 2 | CC | 48 | F | 87 | 1.65 | 70 | Adhesions | >2 | Ileostomy | 2016 | Yes |
| 3 | CF | 52 | F | 46 | 1.73 | 81 | Volvulus | >2 | Ileostomy | 2004 | Yes |
| 4 | CC | 41 | M | 65 | 1.72 | 90 | Necrotizing enterocolitis | 1 | No | 1982 | No |
| 5 | CC | 74 | M | 81 | 1.72 | 78 | Adhesions | 1.2 | Ileostomy | 2017 | No |
| 6 | CC | 76 | M | 87 | 1.72 | 34 | Crohn’s disease | >2 | Ileostomy | 2015 | No |
| 7 | CC | 76 | M | 74 | 1.62 | 59 | Adhesions | 2 | Ileostomy | 2012 | No |
| 8 | FF | 68 | F | 59 | 1.65 | 89 | Crohn’s disease | 2 | Ileostomy | 2001 | No |
| 9 | FF | 65 | F | 59 | 1.78 | 28 | Other | >2 | Colostomy | 1996 | No |
| 10 | FF | 61 | F | 83 | 1.68 | 90 | Mesenteric ischaemia | 1.8 | Jejunostomy | 2020 | No |
| 11 | CC | 59 | F | 75 | 1.68 | 46 | Crohn’s disease | >2 | Ileostomy | 1992 | No |
| 12 | FF | 40 | F | 62 | 1.63 | 90 | Cancer | 0.05 | No | 2012 | Yes |
| 13 | FF | 69 | M | 84 | 1.93 | 67 | Other | >2 | No | NA | No |
| 14 | CF | 31 | F | 55 | 1.66 | 90 | Crohn’s disease | 1.4 | Ileostomy | 2010 | No |
| 15 | FF | 80 | F | 51 | 1.71 | 55 | Cancer | 1.4 | Ileostomy | 2016 | No |
| 16 | FF | 49 | F | 73 | 1.80 | 90 | Ulcerative colitis | >2 | Ileostomy | 2010 | Yes |
| 17 | FF | 73 | M | 84 | 1.88 | 75 | Mesenteric ischaemia | 0.07 | No | 2014 | No |
| 18 | CC | 73 | M | 84 | 1.81 | 71 | Crohn’s disease | 1–1.5 | Jejunostomy | 2009 | No |
CF, ciprofloxacin and fluconazole; CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; F, female; M, male; NA, not applicable.
Pharmacokinetics
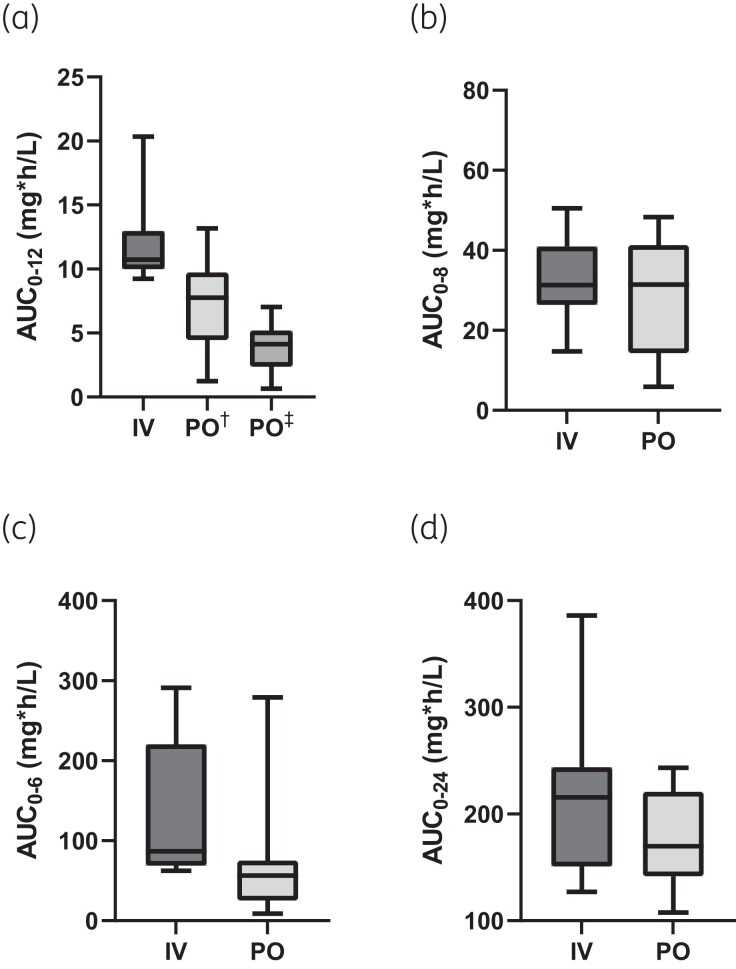
A total of 432 samples were planned for calculating the PK, however, six were missing due to clerical errors. Thus, a total of 426 samples were used to calculate PK parameters. The median PK parameters of ciprofloxacin, clindamycin, flucloxacillin and fluconazole are described in Table 2. Boxplots of ciprofloxacin, clindamycin, flucloxacillin and fluconazole AUC0-tau are presented in Figure 1. A list of all PK parameters per participant can be found in Table S2. Fifteen samples were excluded after analysis due to sampling anomalies.
Table 2.
Median (IQR) pharmacokinetic parameters of ciprofloxacin, clindamycin, flucloxacillin and fluconazole
| Ciprofloxacin† n = 8 |
Clindamycin n = 10 |
Flucloxacillin n = 8 |
Fluconazole n = 10 |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | IV | PO | IV | PO | IV | PO | IV | PO |
| AUC0-tau (mg × h/L) |
10.7 (10–13) |
4.1 (2.4–5.2) |
31.3 (26–41) |
31.5 (14–41) |
86.8 (69–221) |
56.4 (25–75) |
216 (151–244) |
170 (142–221) |
|
F
(%) |
100 | 35.8 (24–50) |
100 | 92.6 (56–106) |
100 | 49.9 (32–76) |
100 | 98.4 (61–107) |
|
C
max
(mg/L) |
2.1 (1.8–2.6) |
0.5 (0.3–1.0) |
7.0 (5.9–10) |
5.3 (3.8–8.2) |
40.1 (35–91) |
17.5 (6.7–21) |
12.5 (10–16) |
9.4 (7.9–12) |
|
C
avg
(mg/L) |
0.9 (0.8–1.1) |
0.6 (0.4–0.8) |
3.9 (3.3–5.1) |
3.9 (1.8–5.2) |
14.5 (11.5–36.8) |
9.4 (4.2–12.5) |
9.0 (6.3–10.2) |
6.7 (5.9–9.2) |
AUC0-tau, AUC from zero to tau (dose interval, 12 h for ciprofloxacin, 8 h for clindamycin, 6 h for flucloxacillin and 24 h for fluconazole); Cavg, average concentration; Cmax, peak plasma concentration; F, bioavailability; IV, intravenous; PO, per os. † Ciprofloxacin oral dose is normalized to 400 mg, to demonstrate oral bioavailability.
Figure 1.
Boxplot of ciprofloxacin (a), clindamycin (b), flucloxacillin (c) and fluconazole (d) AUC0-tau. Bottom and top edges of the boxplots represent 25th and 75th percentiles (difference is IQR), the horizontal line is the median and the whiskers indicate the 5th and 95th percentiles. AUC0-tau, AUC from zero to tau (dose interval, 12 h for ciprofloxacin, 8 h for clindamycin, 6 h for flucloxacillin and 24 h for fluconazole). † 750 mg, dose investigated; ‡ 400 mg, dose normalized to demonstrate oral bioavailability.
Correlation analysis
We analysed variables that might influence antimicrobial agent penetration in an exploratory analysis. We found a non-monotonic association between bioavailability and all variables of interest. Therefore, we did not perform Spearman’s correlation test. See Figure S2 for the scatter plots.
Safety
All antimicrobial agents were well tolerated, and none of the participants experienced adverse effects related to the drug administration. During the study, one female participant developed severe inguinal pain. The diagnosis was a psoas hematoma following a fall a few days before while having a dysregulated (high) anticoagulant (warfarin) level. This serious adverse event was classified as non-study related.
Discussion
Although SBS patients suffering from IF frequently require treatment for (catheter-related) infections, only anecdotal information is available on the absorption of oral antimicrobial agents in this group. Since such data are key, and even more so in this heterogeneous group of patients, we report here on the PK of frequently used antimicrobial agents (ciprofloxacin, clindamycin, flucloxacillin and fluconazole) that were administered as a single oral and IV dose. Our findings indicate that enteral absorption of oral antimicrobial agents in patients with SBS may be better than expected and that individual profiling of enteral absorption may safeguard treatment with oral antimicrobial agents for less severe infections. Unfortunately, but not unexpectedly, it proved not possible to predict absorption using other easier-to-obtain patient characteristics, such as remnant (small) bowel length.
When looking at specific antibiotics in more detail, ciprofloxacin is commonly used to treat several bacterial infections, given its broad spectrum of action against especially Gram-negative bacteria. It is often administered orally in a dosage of 500–750 mg twice daily and is well absorbed from the gastrointestinal (GI) tract in healthy volunteers with a bioavailability of approximately 70%.20,21 In comparison, we found a bioavailability of 36% (IQR 24–50) after correction for differences in dose (Table 2). To compensate for this loss in exposure, we advise prescribing ciprofloxacin in a higher dosage (750 mg BD) in SBS patients with normal kidney function.
Clindamycin is an antibiotic with a broad spectrum of activity against Staphylococcus spp., Streptococcus spp. and anaerobic bacteria. It is usually dosed at 600 mg three times daily. In the general population, it is rapidly and almost completely (bioavailability 90%) absorbed when administered orally.22,23 The median bioavailability of 93% (IQR 56–106) that we report is comparable to that of healthy individuals (Table 2). Hence, oral clindamycin seems a reliable oral antibiotic option in most SBS patients.
Flucloxacillin is indicated primarily for treating various skin and soft tissue infections caused by Gram-positive bacteria, like Staphylococcus aureus. It is dosed three to four times daily and is absorbed for approximately 55% after oral administration on an empty stomach.24,25 Flucloxacillin exhibits substantial interindividual variability in PK.26 The median bioavailability of 50% (IQR 32–76) that we found corresponds with that in healthy individuals (Table 2).24,25 However, also in line with the healthy population, a broad interindividual range between patients was observed; thus, therapeutic drug monitoring (TDM) is advised as part of future oral treatment in SBS patients with mild infections and oral step down therapy for severe infections to safeguard adequate exposure at the individual level.
Fluconazole is an antifungal agent active against most Candida species and is typically dosed once daily due to its long plasma half-life. The drug is rapidly and almost completely absorbed from the GI tract; oral bioavailability exceeds 90% in healthy adults.27,28 This matches our median bioavailability of 98 (IQR 61–107). Thus, fluconazole can be safely administered in most SBS patients.
It is unclear why ciprofloxacin displayed a diminished absorption and the other antimicrobial agents displayed bioavailability compared to the bioavailability described in the literature in the general population.
We analysed several variables that may influence antimicrobial agent absorption, including remnant small bowel length, presence of gastropareses, number of parenteral infusions per week, plasma citrulline concentration as a measure for functional enterocyte mass, presence of ostomies, ingestion of oral antimicrobial agent in suspension or tablet and kidney function. We could not demonstrate any clear correlation between oral bioavailability and these mentioned variables (Figure S1). This lacking correlation results from our modest sample size in combination with confounding factors and the heterogeneity of the SBS population in general.
This study comes with strengths and limitations. Due to its explorative nature, we only have a modest sample size. However, it should be realized here that our sample size matches that of studies in renal failure and liver failure patients.29–31
Also, the perfect timing of sample collection was hampered by the fact that infusion times varied per antimicrobial agent while collection time points were fixed for all drugs at baseline and 1, 2, 4, 8 and 12 hours after administration. We decided to take a sample 1 hour after the first antimicrobial agent infusion period, which may have been too late in some instances to pick up maximum plasma concentrations. Our primary outcome was not affected by this sampling design.
A few individual findings related to patient characteristics are worth mentioning. For instance, in one female participant, the oral bioavailability of ciprofloxacin was only 3%, which was not unexpected given that she underwent a major duodenal resection, which is the main absorption site for ciprofloxacin.32 Even though we could not demonstrate that certain ‘logical’ patient characteristics were predictive, knowledge regarding anatomy can be helpful in some cases to decide whether oral antimicrobial agents are safe or whether TDM is mandatory.
For two participants with ultra-SBS (remaining small bowel length of 5 and 7 cm), the bioavailability of flucloxacillin (43% and 14%, respectively) and fluconazole (65% and 49%, respectively) was, as expected, below that of healthy individuals (flucloxacillin: 55% and fluconazole: 90%).7,33
Concerning the strength of our study, this is the first prospective investigation evaluating (inter) individual bioavailability after oral administration of antimicrobial agents in SBS patients at a high inclusion rate since only a few patients were eligible for inclusion, and most of these were willing to participate. The latter emphasizes that these patients consider this an important treatment issue.
In conclusion, our study shows that oral clindamycin and fluconazole are likely possible treatment options in many SBS patients. Ciprofloxacin should probably be used at a higher dose. Due to substantial interindividual differences, TDM is advised as part of the treatment with flucloxacillin or in patients with duodenal resection and/or ultra-short remaining bowel. Future clinical studies should explore the feasibility of oral antimicrobial agent therapy in SBS patients with mild infections, with the ultimate goal of providing an effective, safe, cost-saving and minimally invasive treatment.
Supplementary Material
Acknowledgements
We thank the patients who participated and the nursing staff who cared for them.
Contributor Information
Julia W Korzilius, Department of Gastroenterology and Hepatology, Radboud university medical center, Nijmegen, the Netherlands.
Michelle Gompelman, Department of Gastroenterology and Hepatology, Radboud university medical center, Nijmegen, the Netherlands.
Guus T J Wezendonk, Department of Gastroenterology and Hepatology, Radboud university medical center, Nijmegen, the Netherlands.
Nynke G L Jager, Department of Pharmacy, Radboud Institute for Medical Innovation, Radboud university medical center, Nijmegen, the Netherlands.
Chantal P Rovers, Department of Internal Medicine, Division of Infectious Diseases, Radboud university medical center, Nijmegen, the Netherlands.
Roger J M Brüggemann, Department of Pharmacy, Radboud Institute for Medical Innovation, Radboud university medical center, Nijmegen, the Netherlands.
Geert J A Wanten, Department of Gastroenterology and Hepatology, Radboud university medical center, Nijmegen, the Netherlands.
Funding
This work was supported by the Dutch Governmental Organization for Health Research and Development (ZonMw; reference number 848015009). The funder had no involvement in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Transparency declarations
The authors have nothing to disclose with respect to funding and no conflicts of interest with respect to this paper.
Author contributions
Specific author contributions: M.G., R.B. and G.J.W. designed the research study; G.J.W. assessed the patients for eligibility; J.K., M.G. and G.W. performed the research; J.K. and R.B. collected and analysed the data; J.K. wrote the original draft; M.G., G.W., N.J., C.R., R.B. and G.J.W. reviewed and edited the manuscript.
Supplementary data
Figures S1 and S2 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Pironi L, Arends J, Bozzetti F et al. ESPEN Guidelines on chronic intestinal failure in adults. Clin Nutr 2016; 35: 247–307. 10.1016/j.clnu.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 2. O’Keefe SJ, Burnes JU, Thompson RL. Recurrent sepsis in home parenteral nutrition patients: an analysis of risk factors. JPEN J Parenter Enteral Nutr 1994; 18: 256–63. 10.1177/0148607194018003256 [DOI] [PubMed] [Google Scholar]
- 3. Buyle FM, Metz-Gercek S, Mechtler R et al. Prospective multicentre feasibility study of a quality of care indicator for intravenous to oral switch therapy with highly bioavailable antibiotics. J Antimicrob Chemother 2012; 67: 2043–6. 10.1093/jac/dks145 [DOI] [PubMed] [Google Scholar]
- 4. Kallen MC, Prins JM. A systematic review of quality indicators for appropriate antibiotic use in hospitalized adult patients. Infect Dis Rep 2017; 9: 6821. 10.4081/idr.2017.6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Gastroenterological Association . American Gastroenterological Association medical position statement: short bowel syndrome and intestinal transplantation. Gastroenterology 2003; 124: 1105–10. 10.1053/gast.2003.50139 [DOI] [PubMed] [Google Scholar]
- 6. Ward N. The impact of intestinal failure on oral drug absorption: a review. J Gastrointest Surg 2010; 14: 1045–51. 10.1007/s11605-009-1151-9 [DOI] [PubMed] [Google Scholar]
- 7. Hong WB, Tan WK, Law LS et al. Changes of drug pharmacokinetics in patients with short bowel syndrome: a systematic review. Eur J Drug Metab Pharmacokinet 2021; 46: 465–78. 10.1007/s13318-021-00696-y [DOI] [PubMed] [Google Scholar]
- 8. Severijnen R, Bayat N, Bakker H et al. Enteral drug absorption in patients with short small bowel: a review. Clin Pharmacokinet 2004; 43: 951–62. 10.2165/00003088-200443140-00001 [DOI] [PubMed] [Google Scholar]
- 9. Parsons RL. Drug absorption in gastrointestinal disease with particular reference to malabsorption syndromes. Clin Pharmacokinet 1977; 2: 45–60. 10.2165/00003088-197702010-00004 [DOI] [PubMed] [Google Scholar]
- 10. Menardi G, Guggenbichler JP. Bioavailability of oral antibiotics in children with short-bowel syndrome. J Pediatr Surg 1984; 19: 84–6. 10.1016/S0022-3468(84)80023-8 [DOI] [PubMed] [Google Scholar]
- 11. Dressman JB, Bass P, Ritschel WA et al. Gastrointestinal parameters that influence oral medications. J Pharm Sci 1993; 82: 857–72. 10.1002/jps.2600820902 [DOI] [PubMed] [Google Scholar]
- 12. Iacono G, Carroccio A, Montalto G et al. Extreme short bowel syndrome: a case for reviewing the guidelines for predicting survival. J Pediatr Gastroenterol Nutr 1993; 16: 216–9. 10.1097/00005176-199302000-00023 [DOI] [PubMed] [Google Scholar]
- 13. Joe LA, Jacobs RA, Guglielmo BJ. Systemic absorption of oral fluconazole after gastrointestinal resection. J Antimicrob Chemother 1994; 33: 1070. 10.1093/jac/33.5.1070 [DOI] [PubMed] [Google Scholar]
- 14. Gompelman M, Jager NGL, Wallenburg E et al. Oral antibiotics in patients with short bowel syndrome: do or don’t? Eur J Drug Metab Pharmacokinet 2021; 46: 821–3. 10.1007/s13318-021-00715-y [DOI] [PubMed] [Google Scholar]
- 15. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol 2016; 30: 173–85. 10.1016/j.bpg.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 17. Agency EM . Guideline on Bioanalytical Method Validation. 2011.
- 18. Koelfat KVK, Huijbers A, Schaap FG et al. Low circulating concentrations of citrulline and FGF19 predict chronic cholestasis and poor survival in adult patients with chronic intestinal failure: development of a model for end-stage intestinal failure (MESIF risk score). Am J Clin Nutr 2019; 109: 1620–9. 10.1093/ajcn/nqz036 [DOI] [PubMed] [Google Scholar]
- 19. Crenn P, Coudray-Lucas C, Thuillier F et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000; 119: 1496–505. 10.1053/gast.2000.20227 [DOI] [PubMed] [Google Scholar]
- 20. Vance-Bryan K, Guay DR, Rotschafer JC. Clinical pharmacokinetics of ciprofloxacin. Clin Pharmacokinet 1990; 19: 434–61. 10.2165/00003088-199019060-00003 [DOI] [PubMed] [Google Scholar]
- 21. Cipro(R) Oral Tablets, Oral Suspension, Ciprofloxacin Hydrochloride Oral Tablets, Oral Suspension [Package Insert]. Bayer HealthCare Pharmaceuticals Inc (per FDA), 2019. [Google Scholar]
- 22. CLEOCIN HCl(R) Oral Capsules, Clindamycin HCl Oral Capsules [Package Insert]. Pharmacia & Upjohn Co (per FDA), 2018. [Google Scholar]
- 23. Mazur D, Schug BS, Evers G et al. Bioavailability and selected pharmacokinetic parameters of clindamycin hydrochloride after administration of a new 600 mg tablet formulation. Int J Clin Pharmacol Ther 1999; 37: 386–92. [PubMed] [Google Scholar]
- 24. Gath J, Charles B, Sampson J et al. Pharmacokinetics and bioavailability of flucloxacillin in elderly hospitalized patients. J Clin Pharmacol 1995; 35: 31–6. 10.1002/j.1552-4604.1995.tb04742.x [DOI] [PubMed] [Google Scholar]
- 25. Nauta EH, Mattie H. Pharmacokinetics of flucloxacillin and cloxacillin in healthy subjects and patients on chronic intermittent haemodialysis. Br J Clin Pharmacol 1975; 2: 111–21. 10.1111/j.1365-2125.1975.tb01566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dijkmans AC, Kweekel DM, Balmforth C et al. The simplified oral flucloxacillin absorption test: an accurate method to identify patients with inadequate oral flucloxacillin absorption. Neth J Med 2019; 77: 255–60. [PubMed] [Google Scholar]
- 27. Brammer KW, Farrow PR, Faulkner JK. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis 1990; 12: S318–26. 10.1093/clinids/12.Supplement_3.S318 [DOI] [PubMed] [Google Scholar]
- 28. DIFLUCAN(R) Oral Tablets, IV Injection, Oral Suspension, Fluconazole Oral Tablets, IV Injection, Oral Suspension. Pfizer Inc., 2008. [Google Scholar]
- 29. de Vroom SL, van Hest RM, van Daalen FV et al. Pharmacokinetic/pharmacodynamic target attainment of ciprofloxacin in adult patients on general wards with adequate and impaired renal function. Int J Antimicrob Agents 2020; 56: 106166. 10.1016/j.ijantimicag.2020.106166 [DOI] [PubMed] [Google Scholar]
- 30. Valles J, Artigas R, Bertolotti M et al. Single and repeated dose pharmacokinetics of dexketoprofen trometamol in patients with impaired liver function. Methods Find Exp Clin Pharmacol 2006; 28: 29–36. [PubMed] [Google Scholar]
- 31. Esposito S, Miniero M, Barba D et al. Pharmacokinetics of ciprofloxacin in impaired liver function. Int J Clin Pharmacol Res 1989; 9: 37–41. [PubMed] [Google Scholar]
- 32. Staib AH, Beermann D, Harder S et al. Absorption differences of ciprofloxacin along the human gastrointestinal tract determined using a remote-control drug delivery device (HF-capsule). Am J Med 1989; 87: 66S–9S. 10.1016/0002-9343(89)90026-0 [DOI] [PubMed] [Google Scholar]
- 33. Cheung YW, Barco S, Mathôt RAA et al. Pharmacokinetics of dabigatran etexilate and rivaroxaban in patients with short bowel syndrome requiring parenteral nutrition: the PDER PAN study. Thromb Res 2017; 160: 76–82. 10.1016/j.thromres.2017.10.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.