Abstract
Objectives
To evaluate the in vitro antibacterial activity of cefiderocol, a siderophore cephalosporin against MBL-producing clinical isolates.
Methods
MBL-producing strains were selected from clinical isolates of Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii complex collected in North America and Europe in five consecutive annual multinational SIDERO-WT surveillance studies from 2014 to 2019. MICs of cefiderocol and comparator agents were determined by the broth microdilution method according to the CLSI guideline.
Results
A total of 452 MBL-producing strains consisting of 200 Enterobacterales, 227 P. aeruginosa and 25 A. baumannii complex were identified. The highest number of MBL-producing Enterobacterales strains were detected in Greece. MBL-producing strains of both P. aeruginosa and A. baumannii complex were isolated most frequently in Russia. For Enterobacterales, 91.5% or 67.5% of MBL-producing strains had cefiderocol MIC values ≤4 mg/L (CLSI susceptibility breakpoint) or ≤2 mg/L (EUCAST susceptibility breakpoint), respectively. All MIC values of cefiderocol for MBL-producing P. aeruginosa strains were ≤4 mg/L (CLSI susceptibility breakpoint), and 97.4% of them had cefiderocol MIC values ≤2 mg/L (EUCAST susceptibility breakpoint). For A. baumannii complex, 60.0% or 44.0% of MBL-producing strains had cefiderocol MIC values ≤4 mg/L (CLSI susceptibility breakpoint) or ≤2 mg/L (EUCAST pharmacokinetic-pharmacodynamic susceptibility breakpoint), respectively. Against all types of MBL-producing strains, MIC distribution curves of cefiderocol were located in the lowest numerical values, compared with other β-lactams and β-lactam/β-lactamase inhibitor combinations tested and ciprofloxacin.
Conclusions
Although the types of MBL-producing strains isolated by country varied, cefiderocol showed potent in vitro activity against all types of MBL-producing Gram-negative bacteria regardless of the bacterial species.
Introduction
MBLs (e.g. IMP, VIM, NDM and GIM) cause serious clinical problems because they can inactivate most of the commonly used β-lactam antibiotics, including carbapenems.1–3 Recently approved β-lactam/β-lactamase inhibitor combinations are not active against MBL-producing clinical isolates.4
Cefiderocol is a siderophore cephalosporin with activity against a wide variety of Gram-negative bacteria including carbapenem-resistant isolates and was approved in the USA in 2019 for the treatment of patients with complicated urinary tract infections, and in 2020 for nosocomial pneumonia (hospital-acquired bacterial pneumonia [HABP]/ventilator-associated bacterial pneumonia [VABP]) caused by Gram-negative bacteria.5 In Europe, cefiderocol was approved in 2020 for the treatment of infections due to Gram-negative pathogens with limited treatment options.6 Cefiderocol is stable against a wide variety of carbapenemases including MBLs such as IMP, VIM and NDM.7–9 We conducted annual surveillance studies (SIDERO-WT) over five consecutive years, with approximately 46 000 Gram-negative clinical isolates collected in North America and Europe between 2014 and 2019.10 Cefiderocol has shown potent antibacterial activity against clinical isolates of Enterobacterales and non-fermenters in these surveillance studies. In this study, we evaluated the antibacterial activity of cefiderocol and comparator agents against MBL-producing strains of Gram-negative bacteria collected in these five surveillance studies.
Materials and methods
Susceptibility testing
Susceptibility testing of cefiderocol (0.03–64 mg/L), ceftazidime/avibactam (0.12/4–16/4 mg/L), ceftolozane/tazobactam (0.12/4–16/4 mg/L), meropenem (0.06–16 mg/L), cefepime (0.12–16 mg/L), ciprofloxacin (0.12–8 mg/L) and colistin (0.25–8 mg/L) was conducted against clinical isolates collected from North America and 11 European countries in annual surveillance studies (SIDERO-WT; 2014–2019) conducted over five consecutive years. MICs of these agents were determined by the broth microdilution method according to the CLSI guidelines.11 For MIC determination of cefiderocol, iron-depleted cation-adjusted Mueller–Hinton broth medium was used. All MIC measurements were conducted at International Health Management Associates, Inc. (IHMA, Schaumburg, IL, USA).10
Bacterial strains
In this study, Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii complex were targeted, and strains to be analysed were selected from the isolates collected in the SIDERO-WT surveillance studies (2014–2019). Bacterial identification was performed using MALDI-TOF MS (Bruker Daltonics, Billerica, MA, USA).10 For the Enterobacterales, a total of 908 molecularly characterized meropenem-resistant strains (meropenem MIC ≥4 mg/L) (32 Citrobacter freundii complex, 1 Citrobacter amalonaticus, 2 Citrobacter koseri, 83 Enterobacter cloacae complex, 2 Enterobacter bugandensis, 33 Escherichia coli, 15 Klebsiella aerogenes, 16 Klebsiella oxytoca, 645 Klebsiella pneumoniae, 2 Klebsiella variicola, 2 Proteus mirabilis, 7 Providencia rettgeri and 68 Serratia marcescens) were selected from a total of 30 258 strains. For P. aeruginosa, a total of 1759 molecularly characterized meropenem-non-susceptible strains (meropenem MIC ≥ 4 mg/L) were selected from a total of 7700 strains. For A. baumannii complex, a total of 2809 molecularly characterized meropenem-non-susceptible strains (meropenem MIC ≥ 4 mg/L) were selected from a total of 5226 strains.
Detection of MBL genes
Most meropenem-non-susceptible strains were screened for the carriage of genes encoding MBLs (IMP, VIM, NDM and GIM) by multiplex PCR using published primers followed by Sanger sequencing.12 For all MBL genes sequenced, the deduced amino acid sequence was compared with NCBI databases (www.ncbi.nlm.nih.gov) to identify those variants. Isolates with elevated cefiderocol MICs [≥4 mg/L (in 2018) and ≥8 mg/L (in 2019)] were subjected to WGS instead of PCR and Sanger sequencing.12 Genomic DNA from each bacterial sample was extracted using the DNeasy Ultraclean Microbial extraction kit (Qiagen) and WGS was performed on an Illumina HiSeq system as described previously.12 All analyses were carried out using the CLC Genomics Workbench v. 20 (Qiagen). If the MBL genes had a match rate of less than 100% with a known nucleotide reference, those were translated to their deduced amino acid sequence and BLASTP search was performed against the Refseq database in Genbank dedicated to β-lactamase nomenclature (Bioproject 313047) to identify those variants.
Geographical analysis
MBL-producing strains were aggregated by MBL type and bacterial species to provide an epidemiological profile for each country. The percentages of MBL-producing strains among all isolated strains were calculated by country for Enterobacterales, P. aeruginosa and A. baumannii complex. In addition, the percentage of MBL-producing strains among meropenem-resistant Enterobacterales, meropenem-non-susceptible P. aeruginosa and meropenem-non-susceptible A. baumannii complex was also calculated.
Results
Identification of MBL-producing strains
Among 908 meropenem-resistant Enterobacterales strains (3.00% of all 30 258 strains), a total of 200 MBL-producing strains (0.66% of all 30 258 strains) consisting of 1 C. amalonaticus, 17 C. freundii complex, 42 E. cloacae complex, 7 E. coli, 5 K. oxytoca, 110 K. pneumoniae, 2 P. mirabilis, 1 P. rettgeri and 15 S. marcescens were identified (Table 1). Of the 200 strains of MBL-producing Enterobacterales, VIM-producing strains (n = 104) were the most common, with VIM-1 (n = 63) as the most prevalent variant, followed by NDM-producing strains (n = 94), with NDM-1 as the most common variant (n = 81); there were only two IMP-producing strains (Table 1 and Table S1, available as Supplementary data at JAC Online). Of the NDM-producing Enterobacterales strains, 77.7% (73/94) were K. pneumoniae. VIM was encountered in various Enterobacterales species such as K. pneumoniae, E. cloacae complex, C. freundii complex and S. marcescens.
Table 1.
Number of MBL-producing strains among meropenem-resistant Enterobacterales, meropenem-non-susceptible P. aeruginosa and A. baumannii complex strains collected in the SIDERO-WT surveillance programmes
| Number of strains | ||||||
|---|---|---|---|---|---|---|
| MEM-Ra or MEM-NSb | MBL producers | |||||
| Total | IMP | VIM | NDM | GIM | ||
| Enterobacterales | 908 | 200 | 2 | 104 | 94 | 0 |
| Citrobacter amalonaticus | 1 | 1 | 0 | 1 | 0 | 0 |
| Citrobacter freundii complex | 32 | 17 | 0 | 14 | 3 | 0 |
| Citrobacter koseri | 2 | 0 | 0 | 0 | 0 | 0 |
| Enterobacter cloacae complex | 83 | 42 | 0 | 33 | 9 | 0 |
| Enterobacter bugandensis | 2 | 0 | 0 | 0 | 0 | 0 |
| Escherichia coli | 33 | 7 | 0 | 2 | 5 | 0 |
| Klebsiella aerogenes | 15 | 0 | 0 | 0 | 0 | 0 |
| Klebsiella oxytoca | 16 | 5 | 0 | 5 | 0 | 0 |
| Klebsiella pneumoniae | 645 | 110 | 0 | 37 | 73 | 0 |
| Klebsiella variicola | 2 | 0 | 0 | 0 | 0 | 0 |
| Proteus mirabilis | 2 | 2 | 1 | 1 | 0 | 0 |
| Providencia rettgeri | 7 | 1 | 1 | 0 | 0 | 0 |
| Serratia marcescens | 68 | 15 | 0 | 11 | 4 | 0 |
| Non-fermenters | ||||||
| Pseudomonas aeruginosa | 1759 | 227 | 25 | 200 | 2 | 0 |
| Acinetobacter baumannii complex | 2809 | 25 | 2 | 0 | 21 | 2 |
MEM-R, meropenem-resistant. For Enterobacterales, numbers of meropenem-resistant strains are shown.
MEM-NS, meropenem-non-susceptible. For A. baumannii complex and P. aeruginosa, numbers of meropenem-non-susceptible strains are shown.
Among 1759 meropenem-non-susceptible P. aeruginosa strains (22.8% of all 7700 strains), a total of 227 MBL-producing P. aeruginosa strains (2.95% of all 7700 strains) were identified (Table 1). Of these, VIM-producing strains (n = 200) were the most common, with VIM-2 (n = 158) as the most prevalent variant; of the IMP-producing strains (n = 25), IMP-7 (n = 22) was the most common variant; and there were two NDM-1-producing strains (Table 1 and Table S2).
Among 2809 meropenem-non-susceptible A. baumannii complex strains (53.8% of all 5226 strains), a total of 25 MBL-producing A. baumannii complex strains (0.48% of all 5226 strains) were identified (Table 1). Of these 25 strains, NDM-producing strains (n = 21; all NDM-1) were the most common. In addition, two IMP-61- and GIM-1-producing strains were detected (Table 1 and Table S3).
Geographical characteristics
For Enterobacterales, the percentage of MBL-producing strains among all isolates and meropenem-resistant strains in all regions was 0.66% and 22.2%, respectively (Table 2). MBL-producing strains were predominantly isolated in Greece (n = 52), Russia (n = 38), Italy (n = 35) and Turkey (n = 26). No MBL-producing Enterobacterales were detected in the Czech Republic, France and Sweden. The percentage of MBL-producing strains among all isolates was highest in Greece (3.64%), followed by Russia (2.21%), Turkey (1.95%) and Italy (1.86%). In Canada and Hungary, although the number of isolated MBL-producing strains was small, the percentage of MBL-producing strains amongst meropenem-resistant strains was high, at 54.6% and 41.7%, respectively (Table 2). Both strains of IMP-producing Enterobacterales were isolated from North America (Canada: n = 1, USA: n = 1). Greece (n = 38) had the highest number of VIM-producing Enterobacterales strains isolated, followed by Italy (n = 34) and Spain (n = 18) (Table 2). Of the 38 strains of VIM-producing Enterobacterales isolated in Greece, more than half, 24 strains, were K. pneumoniae, followed by E. cloacae complex (n = 12), and these two bacterial species accounted for 94.7% (36/38) of the total (Table S4). On the other hand, in Italy, the isolation of E. cloacae complex was the most common, accounting for 41.2% (14/34) of the total, but multiple isolates of other bacterial species such as C. freundii, K. pneumoniae and S. marcescens were also included (Table S4). For NDM-producing strains, the most common isolates were from Russia (n = 38), followed by Turkey (n = 22) and Greece (n = 14) (Table 2). All MBL-producing Enterobacterales isolated in Russia were NDM-producing strains. NDM-producing Enterobacterales from Russia, Turkey and Greece had cefiderocol MICs ranging from 0.25 to >64 mg/L, 0.25 to 2 mg/L, and 0.12 to 8 mg/L, respectively (data not shown). Three cefiderocol-resistant NDM-producing Enterobacterales strains have been isolated from Russia, and the MICs of cefiderocol against these strains were 16, >64 and >64 mg/L. Both strains with cefiderocol MICs >64 mg/L produced NDM-5 (Table S1).
Table 2.
Number of MBL-producing strains and percentage of MBL-producing strains among all and meropenem-resistant Enterobacterales strains by country
| Region | Country | Number of strains | MBL (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | MEM-R | MBL producers | ||||||||
| Total | IMP | VIM | NDM | GIM | For all |
For MEM-R |
||||
| North America | Canada | 1967 | 11 | 6 | 1 | 0 | 5 | 0 | 0.31 | 54.5 |
| USA | 12 243 | 175 | 6 | 1 | 2 | 3 | 0 | 0.05 | 3.43 | |
| Europe | Czech Republic | 1296 | 6 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| France | 1575 | 4 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | |
| Germany | 1822 | 17 | 6 | 0 | 1 | 5 | 0 | 0.33 | 35.3 | |
| Greece | 1428 | 170 | 52 | 0 | 38 | 14 | 0 | 3.64 | 30.6 | |
| Hungary | 1050 | 12 | 5 | 0 | 5 | 0 | 0 | 0.48 | 41.7 | |
| Italy | 1883 | 198 | 35 | 0 | 34 | 1 | 0 | 1.86 | 17.7 | |
| Russia | 1719 | 131 | 38 | 0 | 0 | 38 | 0 | 2.21 | 29.0 | |
| Spain | 1865 | 59 | 19 | 0 | 18 | 1 | 0 | 1.02 | 32.2 | |
| Sweden | 580 | 1 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | |
| Turkey | 1334 | 104 | 26 | 0 | 4 | 22 | 0 | 1.95 | 25.0 | |
| UK | 1496 | 20 | 7 | 0 | 2 | 5 | 0 | 0.47 | 35.0 | |
| Total | 30 258 | 902 | 200 | 2 | 104 | 94 | 0 | 0.66 | 22.2 | |
MEM-R, meropenem-resistant.
For P. aeruginosa, the percentages of MBL-producing strains in all isolates and meropenem-non-susceptible strains in all regions were 2.95% and 12.9%, respectively (Table 3). MBL-producing strains were predominantly isolated in Russia (n = 106), Greece (n = 30), Czech Republic (n = 21) and Spain (n = 21). Countries with a high percentage of MBL-producing strains among all isolates collected and among meropenem-non-susceptible strains were similar to those with a high number of MBL-producing strains, with Russia having the highest percentage (20.4% for all, 50.0% for meropenem-non-susceptible), followed by Greece (9.87% for all, 44.1% for meropenem-non-susceptible), Czech Republic (6.29% for all, 21.0% for meropenem-non-susceptible) and Spain (4.36% for all, 17.1% for meropenem-non-susceptible) (Table 3). Russia (n = 106) had the highest number of VIM-producing P. aeruginosa strains isolated, followed by Greece (n = 30) and Spain (n = 21). Twenty-five IMP-producing strains were found, most of which were isolated in the Czech Republic (n = 21). Two NDM-producing strains were isolated in Canada and Turkey.
Table 3.
Number of MBL-producing strains and percentage of MBL-producing strains among all and meropenem-non-susceptible P. aeruginosa strains by country
| Region | Country | Number of strains | MBL (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | MEM-NS | MBL producers | ||||||||
| Total | IMP | VIM | NDM | GIM | For all |
For MEM-NS |
||||
| North America | Canada | 461 | 87 | 2 | 1 | 0 | 1 | 0 | 0.43 | 2.30 |
| USA | 3087 | 595 | 3 | 1 | 2 | 0 | 0 | 0.10 | 0.50 | |
| Europe | Czech Republic | 334 | 100 | 21 | 21 | 0 | 0 | 0 | 6.29 | 21.0 |
| France | 419 | 59 | 3 | 0 | 3 | 0 | 0 | 0.72 | 5.08 | |
| Germany | 473 | 110 | 6 | 1 | 5 | 0 | 0 | 1.27 | 5.45 | |
| Greece | 304 | 68 | 30 | 0 | 30 | 0 | 0 | 9.87 | 44.1 | |
| Hungary | 278 | 122 | 9 | 0 | 9 | 0 | 0 | 3.24 | 7.38 | |
| Italy | 463 | 118 | 14 | 1 | 13 | 0 | 0 | 3.02 | 11.9 | |
| Russia | 520 | 212 | 106 | 0 | 106 | 0 | 0 | 20.4 | 50.0 | |
| Spain | 482 | 123 | 21 | 0 | 21 | 0 | 0 | 4.36 | 17.1 | |
| Sweden | 124 | 10 | 1 | 0 | 1 | 0 | 0 | 0.81 | 10.0 | |
| Turkey | 369 | 103 | 10 | 0 | 9 | 1 | 0 | 2.71 | 9.71 | |
| UK | 386 | 52 | 1 | 0 | 1 | 0 | 0 | 0.26 | 1.92 | |
| Total | 7700 | 1759 | 227 | 25 | 200 | 2 | 0 | 2.95 | 12.9 | |
MEM-NS, meropenem-non-susceptible.
For A. baumannii complex, the percentage of MBL-producing strains in all isolates and in meropenem-resistant strains in all regions was 0.48% and 0.89%, respectively (Table 4). The number of isolates of MBL-producing strains was considerably lower than that of Enterobacterales and P. aeruginosa. Countries with relatively high numbers of MBL-producing strains isolated were Russia (n = 7), Germany (n = 4) and Italy (n = 4). The percentage of MBL-producing strains among all isolates and meropenem-non-susceptible strains was the highest in the UK (2.75% for all, 15.0% for meropenem-nonsusceptible) although it should be noted that the number of MBL-producing strains in the UK was rather small (n = 3). Two IMP-producing strains and 2 GIM-producing strains were found, all of which were isolated in Germany (Table 4). NDM-producing strains were isolated in multiple countries, most in Russia (n = 7), followed by Italy (n = 4) and the UK (n = 3).
Table 4.
Number of MBL-producing strains and percentage of MBL-producing strains among all and meropenem-non-susceptible A. baumannii complex strains by country
| Region | Country | Number of strains | MBL (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | MEM-NS | MBL producers | ||||||||
| Total | IMP | VIM | NDM | GIM | For all |
For MEM-NS |
||||
| North America | Canada | 243 | 20 | 1 | 0 | 0 | 1 | 0 | 0.41 | 5.00 |
| USA | 1757 | 678 | 2 | 0 | 0 | 2 | 0 | 0.11 | 0.29 | |
| Europe | Czech Republic | 213 | 28 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| France | 289 | 40 | 1 | 0 | 0 | 1 | 0 | 0.35 | 2.50 | |
| Germany | 265 | 51 | 4 | 2 | 0 | 0 | 2 | 1.51 | 7.84 | |
| Greece | 420 | 407 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | |
| Hungary | 210 | 151 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | |
| Italy | 505 | 432 | 4 | 0 | 0 | 4 | 0 | 0.79 | 0.93 | |
| Russia | 437 | 347 | 7 | 0 | 0 | 7 | 0 | 1.60 | 2.02 | |
| Spain | 336 | 238 | 1 | 0 | 0 | 1 | 0 | 0.30 | 0.42 | |
| Sweden | 10 | 6 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | |
| Turkey | 432 | 391 | 2 | 0 | 0 | 2 | 0 | 0.46 | 0.51 | |
| UK | 109 | 20 | 3 | 0 | 0 | 3 | 0 | 2.75 | 15.0 | |
| Total | 5226 | 2809 | 25 | 2 | 0 | 21 | 2 | 0.48 | 0.89 | |
MEM-NS, meropenem-non-susceptible.
In vitro activity against MBL-producing strains
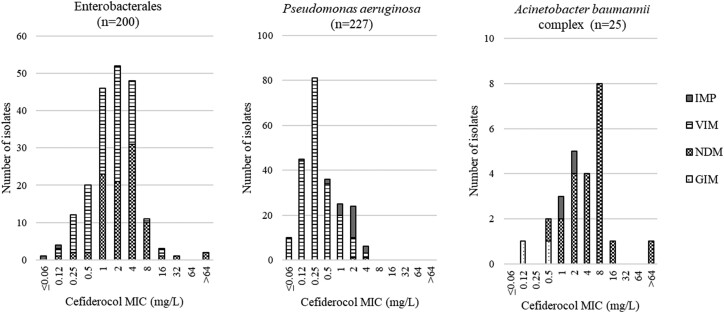
MIC distributions of cefiderocol against types of MBL-producing strains isolated in five SIDERO-WT surveillance studies (2014–2019) are shown in Figure 1. For Enterobacterales, 91.5% of MBL-producing strains showed MIC values ≤4 mg/L, which is the CLSI susceptibility breakpoint of cefiderocol, and 67.5% of MBL-producing strains showed MIC values ≤2 mg/L, which is the EUCAST susceptibility breakpoint of cefiderocol.13,14 It should be noted that 73.0% of strains showed cefiderocol MICs of 1–4 mg/L, with a peak of 2 mg/L. Of the 17 cefiderocol-non-susceptible strains by CLSI criteria (>4 mg/L), 11 strains had an MIC of 8 mg/L (CLSI: intermediate) and the remaining 6 strains were resistant. Of the six strains that showed resistance to cefiderocol, one strain was an NDM-1 producer, three were NDM-5 producers, and two were VIM-1 producers (Figure 1). Activity of cefiderocol against isolates expressing different MBL variants was similar (e.g. MIC ranges against VIM-1-, VIM-2- and NDM-1-producing strains were 0.12–16, 0.25–4 and 0.12–16 mg/L, respectively; MIC90 values against VIM-1-, VIM-2- and NDM-1-producing strains were 4, 4 and 8 mg/L, respectively), with the possible exception of isolates that produced VIM-26, which showed lower MIC values (range 0.25–2 mg/L; MIC90 1 mg/L), and NDM-5 producers, which showed higher MIC values (range 1 to >64 mg/L; MIC90 >64 mg/L) (Table S1). However, of all 104 strains producing VIM, only 10 strains produced VIM-26. Further studies are needed to confirm the susceptibility characteristics of cefiderocol against VIM-26-producing strains.
Figure 1.
MIC distribution of cefiderocol for MBL-producing strains by MBL type.
For P. aeruginosa, the MIC values of cefiderocol for all MBL-producing P. aeruginosa strains were ≤4 mg/L, which is the CLSI susceptibility breakpoint of cefiderocol, and 97.4% of MBL-producing P. aeruginosa strains showed MIC values ≤2 mg/L, which is the EUCAST susceptibility breakpoint of cefiderocol.13,14 It should be noted that 71.4% of strains showed cefiderocol MICs of 1–4 mg/L, with a peak of 0.25 mg/L. IMP-producing P. aeruginosa strains tended to have relatively higher cefiderocol MIC values (range 0.12–4 mg/L; MIC90 4 mg/L) than VIM-producing strains (range ≤0.06 to 4 mg/L; MIC90 1 mg/L) (Figure 1). Strains producing VIM-2 and VIM-4 tended to have lower MICs (MIC90 values against VIM-2- and VIM-4-producing strains were both 1 mg/L) than strains producing other MBL variants (MIC90 values against IMP-7-producing strains and VIM-1-producing strains were 4 and 2 mg/L, respectively). Although only two NDM-producing strains were isolated, cefiderocol showed activity against these two strains, with MICs of 2 and 4 mg/L (Table S2).
For A. baumannii complex, 60.0% of MBL-producing strains showed MIC values ≤4 mg/L, which is the CLSI susceptibility breakpoint of cefiderocol, and 44.0% of MBL-producing strains showed MIC values ≤2 mg/L, which is the EUCAST pharmacokinetic-pharmacodynamic (PK-PD) susceptibility breakpoint of cefiderocol.13,14 It should be noted that 72.0% of strains showed cefiderocol MICs of 1–8 mg/L, and the MIC value with the highest distribution number of strains was 8 mg/L. Of the 10 cefiderocol-non-susceptible strains, 8 had an MIC of 8 mg/L (CLSI: intermediate) whereas the remaining 2 were resistant. All cefiderocol-non-susceptible strains were NDM-1 producers (Figure 1). IMP- and GIM-producing A. baumannii complex strains showed lower MIC values (≤2 and ≤0.5 mg/L, respectively) compared with NDM-producing strains (range 0.5 to >64 mg/L), although the number of isolated IMP- and GIM-producing strains was small so it cannot be judged that there is a clear trend.
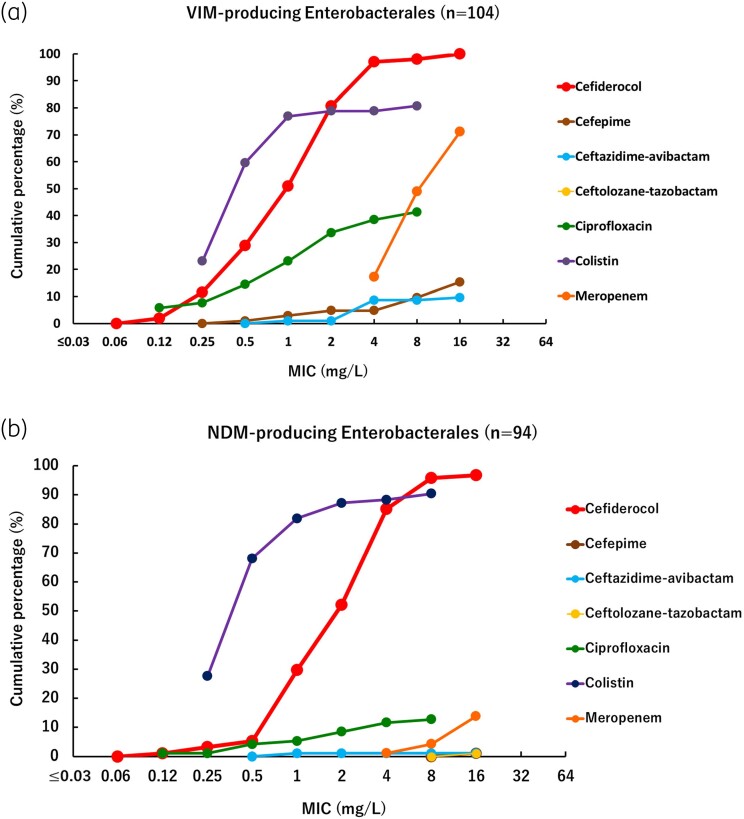
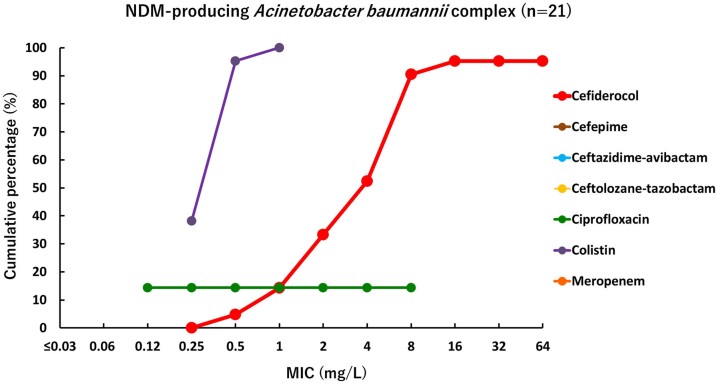
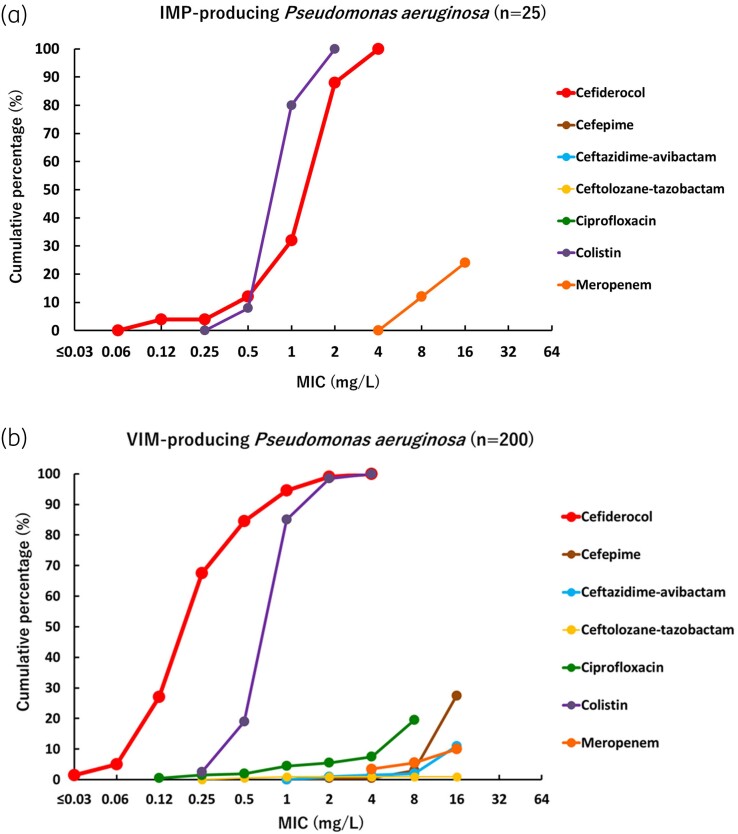
Cumulative percentage MIC distributions were plotted to compare the activity of cefiderocol and comparator agents against different types of MBL-producing strains (Figures 2–4). The MICs of cefiderocol were more distributed in the lower values, compared with other β-lactams and β-lactam/β-lactamase inhibitor combinations tested (cefepime, meropenem, ceftazidime/avibactam and ceftolozane/tazobactam) and ciprofloxacin. Colistin was the only other agent that showed relatively good activity against MBL-producing strains, although some Enterobacterales strains demonstrated high MIC values (>2 mg/L). These results confirm that MBL-producing strains are MDR, for which only very few treatment options remain available.
Figure 2.
Cumulative percentage MIC distributions of cefiderocol and comparator agents against MBL-producing Enterobacterales strains. (a) VIM-producing Enterobacterales (n = 104). Data for strains above the upper end of the MIC measurement range are not plotted. Ceftolozane/tazobactam MIC values were >16 mg/L against all strains, so not plotted on graph. In the ciprofloxacin plot, the MIC value of 0.12 mg/L means ≤0.12 mg/L. In the colistin plot, the MIC value of 0.25 mg/L means ≤0.25 mg/L. VIM-producing Enterobacterales were screened among Enterobacterales with meropenem MICs ≥ 4 mg/L, so meropenem MICs are not plotted below 4 mg/L. (b) NDM-producing Enterobacterales (n = 94). Data for strains above the upper end of the MIC measurement range are not plotted. In the ciprofloxacin plot, the MIC value of 0.12 mg/L means ≤0.12 mg/L. In the colistin plot, the MIC value of 0.25 mg/L means ≤0.25 mg/L. NDM-producing Enterobacterales were screened among Enterobacterales with meropenem MICs ≥ 4 mg/L, so meropenem MICs are not plotted below 4 mg/L.
Figure 4.
Cumulative percentage MIC distributions of cefiderocol and comparator agents against MBL-producing A. baumannii complex strains. Data for strains above the upper end of the MIC measurement range are not plotted. Cefepime MIC values were >16 mg/L against all strains, so not plotted on graph. Ceftazidime/avibactam MIC values were >16 mg/L against all strains, so not plotted on graph. Ceftolozane/tazobactam MIC values were >16 mg/L against all strains, so not plotted on graph. In the ciprofloxacin plot, the MIC value of 0.12 mg/L means ≤0.12 mg/L. In the colistin plot, the MIC value of 0.25 mg/L means ≤0.25 mg/L. NDM-producing A. baumannii complex were screened among A. baumannii complex with meropenem MICs ≥ 4 mg/L and meropenem MIC values were >16 mg/L against all strains, so not plotted on graph.
Figure 3.
Cumulative percentage MIC distributions of cefiderocol and comparator agents against MBL-producing P. aeruginosa strains. (a) IMP-producing P. aeruginosa (n = 25). Data for strains above the upper end of the MIC measurement range are not plotted. Cefepime MIC values were >16 mg/L against all strains, so not plotted on graph. Ceftazidime/avibactam MIC values were >16 mg/L against all strains, so not plotted on graph. Ceftolozane/tazobactam MIC values were >16 mg/L against all strains, so not plotted on graph. Ciprofloxacin MIC values were >8 mg/L against all strains, so not plotted on graph. In the colistin plot, the MIC value of 0.25 mg/L means ≤0.25 mg/L. IMP-producing P. aeruginosa were screened among P. aeruginosa with meropenem MICs ≥ 4 mg/L, so meropenem MICs are not plotted below 4 mg/L. (b) VIM-producing P. aeruginosa (n = 200). Data for strains above the upper end of the MIC measurement range are not plotted. In the ciprofloxacin plot, the MIC value of 0.12 mg/L means ≤0.12 mg/L. In the colistin plot, the MIC value of 0.25 mg/L means ≤0.25 mg/L. VIM-producing P. aeruginosa were screened among P. aeruginosa with meropenem MICs ≥ 4 mg/L, so meropenem MICs are not plotted below 4 mg/L.
Discussion
Cefiderocol is a parenteral siderophore cephalosporin that has a catechol moiety and behaves like a siderophore by chelating ferric iron, so it is efficiently taken up into the intracellular space of bacteria.15 Cefiderocol is also characterized by its stability against a variety of β-lactamases, including MBLs.7–9,16 This report describes the analysis of in vitro activity of cefiderocol against clinical isolates of MBL-producing Gram-negative bacteria collected in North America and European countries between 2014 and 2019 in SIDERO-WT surveillance studies (2014–2019).
MBLs are categorized into Ambler's class B and require Zn2+ metal as a cofactor.3 MBL-producing strains have spread globally and can inactivate most commonly used β-lactam antibiotics, including carbapenems, as well as β-lactam/serine-type β-lactamase inhibitor combinations, such as ceftazidime/avibactam and ceftolozane/tazobactam.1–3,17–20 The polymyxin antibiotics (colistin and polymyxin B) remain active against MBL-producing strains, with 92.6% of MBL-producing Enterobacterales reported as susceptible to colistin using the EUCAST breakpoint (MIC ≤2 mg/L).21 In the analysis we conducted here, only 82.0% of the MBL-producing Enterobacterales had a colistin MIC ≤2 mg/L. In addition, although polymyxin antibiotics can still be used as a therapeutic agent for infections caused by MBL-producing strains, there is grave concern about the development of nephrotoxicity, necessitating the need for safer and better agents to combat MBL-producing bacteria.22
In our analysis 100% or 97.4% of MBL-producing P. aeruginosa were susceptible to cefiderocol when using CLSI (≤4 mg/L) or EUCAST (≤2 mg/L) breakpoints, respectively, confirming high susceptibility for cefiderocol against clinical MBL-producing isolates of this species. Activity against MBL-producing clinical isolates of Enterobacterales was lower, with susceptibility at 91.5% or 67.5%, using the CLSI or EUCAST breakpoints, respectively. The large difference in susceptibility percentages using EUCAST and CLSI breakpoints is the result of the high percentage of strains with MIC values of 4 mg/L among MBL-producing Enterobacterales (Figure 1). For MBL-producing A. baumannii complex, 60.0% or 44.0% of MBL-producing strains were susceptible to cefiderocol using CLSI or EUCAST PK-PD breakpoints, respectively. The lower susceptibility percentages for Enterobacterales and A. baumannii complex compared with P. aeruginosa may be due to the high ratio of NDM-producing strains to MBL-producing strains in those species. Cefiderocol showed relatively higher MIC values against a subset of NDM-producing strains compared with other subsets of MBL-producing strains. For example, MIC90 values of cefiderocol against VIM-producing and NDM-producing Enterobacterales were 4 and 8 mg/L, respectively (Figure 2), and the MIC values of IMP- and GIM-producing A. baumannii complex strains were much lower (≤2 mg/L; Figure 1) compared with the NDM-producing isolates (MIC90 = 8 mg/L; Figure 2b) although only two of each were collected. It has been previously reported that some NDM-producing strains are highly resistant to cefiderocol, and the results of this analysis are consistent with previous reports.23–25 Considering that the MIC90 values of cefiderocol against all Enterobacterales, P. aeruginosa and A. baumannii complex strains isolated in SIDERO-WT studies were 1, 0.5 and 1 mg/L, respectively, MBL-producing strains, and NDM-producing isolates in particular, showed higher cefiderocol MIC values.10 NDM-5-producing E. coli isolated in China have been reported to exhibit high resistance to cefiderocol, though these NDM-5 producers also had a four-amino acid insertion into penicillin-binding protein 3 (PBP3) and mutations in the siderophore receptor gene cirA.26
The largest MBL-producing subset in this analysis was VIM-producing P. aeruginosa (n = 200), which were detected in all countries except Canada and the Czech Republic (Tables 1 and 3). Our analysis revealed that more than half (106/200) of the VIM-producing P. aeruginosa were isolated in Russia and all of them were VIM-2-producing strains (data not shown). In Russia, VIM-2-producing ST235 P. aeruginosa has been reported to spread rapidly, and according to a report by Edelstein et al.,27 96.5% of MBL-positive P. aeruginosa strains isolated in Russia between 2002 and 2010 were ST235, and 99.6% of them were VIM-2-producing strains. Cefiderocol showed high activity against VIM-producing P. aeruginosa, regardless of VIM variants, and showed MIC values ≤4 mg/L against all VIM-producing P. aeruginosa strains. Interestingly, 84.0% (21/25) of IMP-producing P. aeruginosa were isolated in the Czech Republic (Table 3). All of them were IMP-7-producing strains (data not shown). In another surveillance study of P. aeruginosa strains isolated in the Czech Republic in 2015, among 136 carbapenemase-producing P. aeruginosa strains, 117 IMP-producing strains [IMP-7 (n = 116) and IMP-1 (n = 1)] were found. One hundred and fifteen of the 116 IMP-7-producing strains were ST357, which was the most dominant ST among carbapenemase-producing P. aeruginosa.28
Although there are still few reports on the use of cefiderocol for MBL-producing strains in clinical practice, in the Phase 3 studies of cefiderocol (CREDIBLE-CR and APEKS-NP), there were multiple cases of use in patients infected with MBL-producing strains, and a higher clinical cure rate was observed in the cefiderocol group than in the best available therapy group and high-dose meropenem group.29 The most frequently isolated MBL in CREDIBLE-CR and APEKS-NP was NDM (15 NDM-1 and 1 NDM-5), with cefiderocol MICs of 4 mg/L reported in 9 of 22 cases.29
These clinical data and non-clinical data obtained by our analysis suggest that cefiderocol has potential for the treatment of infections caused by MBL-producing strains.
Supplementary Material
Acknowledgements
We thank all members of Shionogi & Co., Ltd, Shionogi TechnoAdvance Research & Co., Ltd and IHMA Inc. who were involved in obtaining the data.
Contributor Information
Miki Takemura, Laboratory for Drug Discovery and Disease Research, Shionogi & Co., Ltd., Osaka, Japan.
Mark G Wise, International Health Management Associates, Inc., Schaumburg, IL, USA.
Meredith A Hackel, International Health Management Associates, Inc., Schaumburg, IL, USA.
Daniel F Sahm, International Health Management Associates, Inc., Schaumburg, IL, USA.
Yoshinori Yamano, Laboratory for Drug Discovery and Disease Research, Shionogi & Co., Ltd., Osaka, Japan.
Funding
This study was supported by internal funding from Shionogi & Co., Ltd.
Transparency declarations
All authors have no declarations other than those provided below. M.T. and Y.Y. report being employed by and owning stock in Shionogi & Co., Ltd. M.G.W., M.A.H. and D.F.S. are employees of IHMA Inc.
Supplementary data
Tables S1–S4 are available as Supplementary data at JAC Online.
References
- 1. Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011; 17: 1791–8. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mojica MF, Rossi MA, Vila AJ et al. The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. Lancet Infect Dis 2022; 22: e28–34. 10.1016/S1473-3099(20)30868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd SE, Livermore DM, Hooper DC et al. Metallo-β-lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob Agents Chemother 2020; 64: e00397-20. 10.1128/AAC.00397-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yahav D, Giske CG, Grāmatniece A et al. New β-lactam-β-lactamase inhibitor combinations. Clin Microbiol Rev 2020; 34: e00115-20. 10.1128/CMR.00115-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shionogi Inc . Fetroja (cefiderocol) for injection, for intravenous use. Prescribing information. Shionogi Inc., 2019. [Google Scholar]
- 6. Shionogi BV . Fetcroja (cefiderocol). 1 g powder for concentrate for solution for infusion. Summary of product characteristics. Shionogi BV, 2020. [Google Scholar]
- 7. Ito-Horiyama T, Ishii Y, Ito A et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60: 4384–6. 10.1128/AAC.03098-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poirel L, Kieffer N, Nordmann P. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int J Antimicrob Agents 2018; 52: 866–7. 10.1016/j.ijantimicag.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 9. Zhanel GG, Golden AR, Zelenitsky S et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant gram-negative bacilli. Drugs 2019; 79: 271–89. 10.1007/s40265-019-1055-2 [DOI] [PubMed] [Google Scholar]
- 10. Karlowsky JA, Hackel MA, Takemura M et al. In vitro susceptibility of gram-negative pathogens to cefiderocol in five consecutive annual multinational SIDERO-WT surveillance studies, 2014 to 2019. Antimicrob Agents Chemother 2022; 66: e0199021. 10.1128/AAC.01990-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018. [Google Scholar]
- 12. Wise MG, Karlowsky JA, Hackel MA et al. In vitro activity of cefiderocol against meropenem-nonsusceptible gram-negative bacilli with defined β-lactamase carriage: SIDERO-WT surveillance studies, 2014–2019. Microb Drug Resist 2023. 10.1089/mdr.2022.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Second Edition: M100. 2022. [Google Scholar]
- 14. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 12.0, 2022.
- 15. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019; 69: S538–43. 10.1093/cid/ciz826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important gram-negative bacteria. Clin Infect Dis 2019; 69: S544–51. 10.1093/cid/ciz827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan X, Kim HS, Baugh K et al. Therapeutic options for metallo-β-lactamase-producing enterobacterales. Infect Drug Resist 2021; 14: 125–42. 10.2147/IDR.S246174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoon EJ, Jeong SH. Mobile carbapenemase genes in Pseudomonas aeruginosa. Front Microbiol 2021; 12: 614058. 10.3389/fmicb.2021.614058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsch EB, Brigman HV, Zucchi PC et al. Ceftolozane-tazobactam and ceftazidime-avibactam activity against β-lactam-resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing enterobacterales clinical isolates from U.S. medical centres. J Glob Antimicrob Resist 2020; 22: 689–94. 10.1016/j.jgar.2020.04.017 [DOI] [PubMed] [Google Scholar]
- 20. Abboud MI, Damblon C, Brem J et al. Interaction of avibactam with class B metallo-β-lactamases. Antimicrob Agents Chemother 2016; 60: 5655–62. 10.1128/AAC.00897-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradford PA, Kazmierczak KM, Biedenbach DJ et al. Correlation of β-lactamase production and colistin resistance among Enterobacteriaceae isolates from a global surveillance program. Antimicrob Agents Chemother 2015; 60: 1385–92. 10.1128/AAC.01870-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Javan AO, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol 2015; 71: 801–10. 10.1007/s00228-015-1865-4 [DOI] [PubMed] [Google Scholar]
- 23. Poirel L, de la Rosa JM O, Sakaoglu Z et al. NDM-35-producing ST167 Escherichia coli highly resistant to β-lactams including cefiderocol. Antimicrob Agents Chemother 2022; 66: e0031122. 10.1128/aac.00311-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coppi M, Antonelli A, Niccolai C et al. Nosocomial outbreak by NDM-1-producing Klebsiella pneumoniae highly resistant to cefiderocol, Florence, Italy, August 2021 to June 2022. Euro Surveill 2022; 27: 2200795. 10.2807/1560-7917.ES.2022.27.43.2200795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mushtaq S, Sadouki Z, Vickers A et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant gram-negative bacteria. Antimicrob Agents Chemother 2020; 64: e01582-20. 10.1128/AAC.01582-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Q, Jin L, Sun S et al. Occurrence of high levels of cefiderocol resistance in carbapenem-resistant Escherichia coli before its approval in China: a report from China CRE-network. Microbiol Spectr 2022; 10: e0267021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edelstein MV, Skleenova EN, Shevchenko OV et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect Dis 2013; 13: 867–76. 10.1016/S1473-3099(13)70168-3 [DOI] [PubMed] [Google Scholar]
- 28. Papagiannitsis CC, Medvecky M, Chudejova K et al. Molecular characterization of carbapenemase-producing Pseudomonas aeruginosa of Czech origin and evidence for clonal spread of extensively resistant sequence type 357 expressing IMP-7 metallo-β-lactamase. Antimicrob Agents Chemother 2017; 61: e01811-17. 10.1128/AAC.01811-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Timsit JF, Paul M, Shields RK et al. Cefiderocol for the treatment of infections due to metallo-B-lactamase-producing pathogens in the CREDIBLE-CR and APEKS-NP phase 3 randomized studies. Clin Infect Dis 2022; 75: 1081–4. 10.1093/cid/ciac078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.