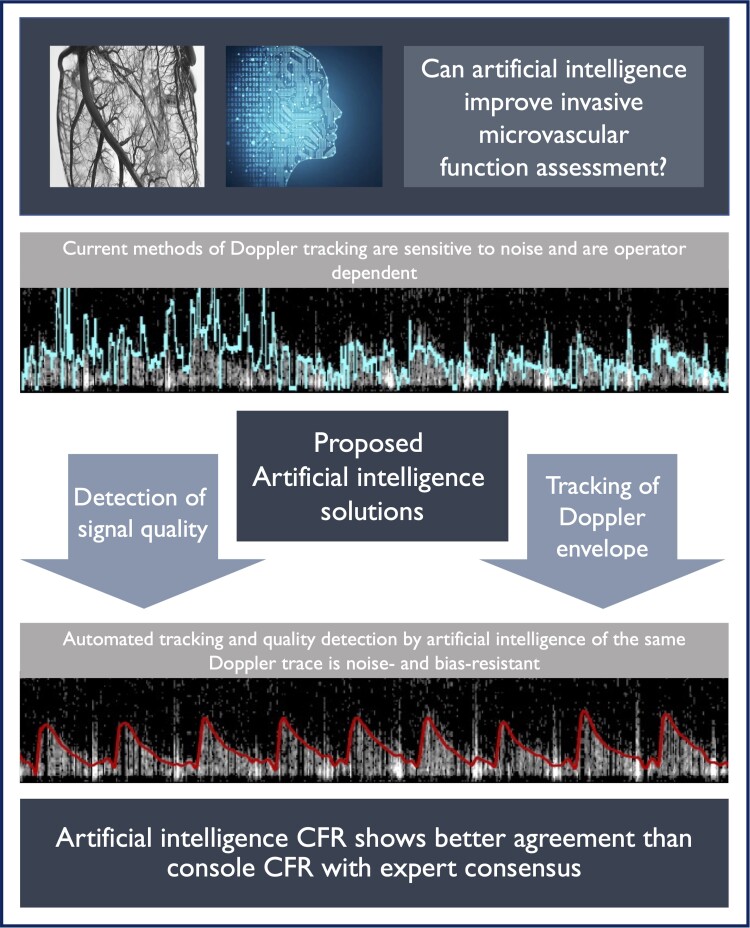
Abstract
Aims
Coronary flow reserve (CFR) assessment has proven clinical utility, but Doppler-based methods are sensitive to noise and operator bias, limiting their clinical applicability. The objective of the study is to expand the adoption of invasive Doppler CFR, through the development of artificial intelligence (AI) algorithms to automatically quantify coronary Doppler quality and track flow velocity.
Methods and results
A neural network was trained on images extracted from coronary Doppler flow recordings to score signal quality and derive values for coronary flow velocity and CFR. The outputs were independently validated against expert consensus. Artificial intelligence successfully quantified Doppler signal quality, with high agreement with expert consensus (Spearman’s rho: 0.94), and within individual experts. Artificial intelligence automatically tracked flow velocity with superior numerical agreement against experts, when compared with the current console algorithm [AI flow vs. expert flow bias −1.68 cm/s, 95% confidence interval (CI) −2.13 to −1.23 cm/s, P < 0.001 with limits of agreement (LOA) −4.03 to 0.68 cm/s; console flow vs. expert flow bias −2.63 cm/s, 95% CI −3.74 to −1.52, P < 0.001, 95% LOA −8.45 to −3.19 cm/s]. Artificial intelligence yielded more precise CFR values [median absolute difference (MAD) against expert CFR: 4.0% for AI and 7.4% for console]. Artificial intelligence tracked lower-quality Doppler signals with lower variability (MAD against expert CFR 8.3% for AI and 16.7% for console).
Conclusion
An AI-based system, trained by experts and independently validated, could assign a quality score to Doppler traces and derive coronary flow velocity and CFR. By making Doppler CFR more automated, precise, and operator-independent, AI could expand the clinical applicability of coronary microvascular assessment.
Keywords: Coronary, Doppler, Invasive, Artificial intelligence, Angina, Diagnosis
Graphical Abstract
Graphical Abstract.
Introduction
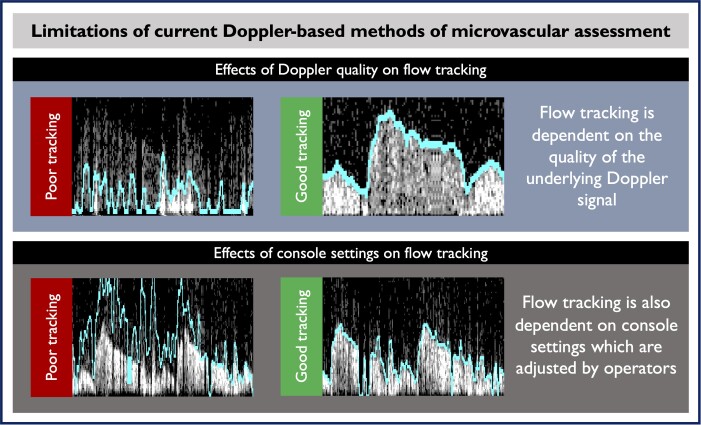
Coronary microvascular dysfunction is known to significantly affect patients’ quality of life,1 burden healthcare systems, and reduce life expectancy.2–5 Therefore, a clinical assessment of microvascular function with coronary flow reserve (CFR) is recommended by international clinical guidelines as a means of guiding therapy and stratifying cardiovascular risk.6–8 Compared with other invasive approaches, like coronary thermodilution, intracoronary Doppler allows a continuous assessment of flow velocity, thus allowing coronary flow measurements during vasodilator stress and other diagnostic paradigms including during exercise.9,10 Doppler has demonstrated better correlation with positron emission tomography-based CFR than thermodilution.11 Despite this, adoption of invasive microvascular function assessment with Doppler-derived CFR is limited in routine clinical practice. This low adoption is largely due to the limitations of current Doppler-based CFR diagnostics12,13 (Figure 1), which are (i) sensitive to Doppler’s intrinsic noisy signals and (ii) profoundly expert-operator-dependent, as flow detection and digitization requires constant optimization during measurements (Graphical Abstract; the upper trace shows tracking of noisy trace with optimization).
Figure 1.
Examples of Doppler recordings from the coronary arteries during microvascular assessment. The current console algorithm is used to trace the flow velocity from Doppler spectral signals. The digitized flow is used to derive flow reserve (coronary flow reserve) values and guide clinical decisions. In the upper left panel, the Doppler flow signal is weak and so the line tracks only noise. In the upper right panel, the flow signal is stronger and so the line tracks it well. In the lower left panel, the Doppler flow signal is strong and well formed, but the flow tracking line is measuring only noise due to the parameter set by the operator. In the right lower panel, the settings are optimized and the line is following the Doppler waveform more precisely.
In this study, we present the development and validation of an artificial intelligence (AI)-based methodology, designed to expand adoption of microvascular assessment via improvements in the precision of invasive CFR measurements. Firstly, we present a method to objectively quantify the quality of coronary Doppler data. Secondly, we develop a tool that consistently extracts reliable coronary flow and CFR from Doppler. We compare the performance of our AI methodology with the currently available flow tracking algorithm embedded in clinical consoles and validate it against the consensus of international experts in the field of invasive CFR measurement.
Methods
Doppler recordings
This study used recordings from four clinical studies of which two have already been published,9,14 in which CFR was measured using a pressure and Doppler flow sensor-tipped coronary guide wire (Combowire, Philips, Eindhoven, The Netherlands). Recordings were made using the Combomap console (Philips). All subjects gave written informed consent to take part including for their anonymized data to be used in future analysis. All studies received appropriate ethical approval (see Supplementary material online, Table S1). All experts whose discriminations were used to train and validate the neural network were practising cardiologists with international reputation in the field of invasive coronary physiology and had undertaken doctoral or post-doctoral level research in the field of coronary flow and coronary physiology.
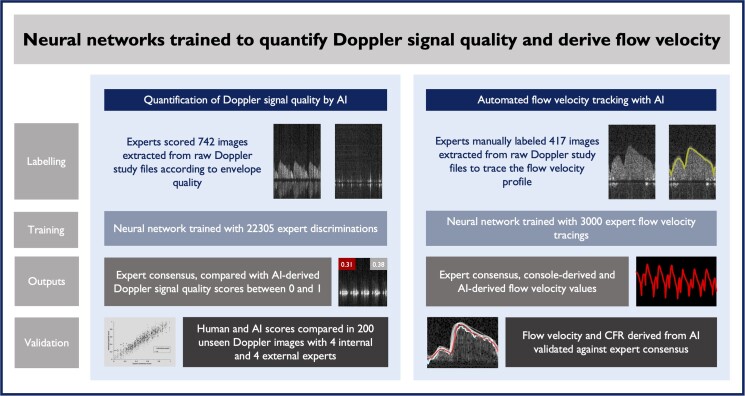
Images were anonymously extracted from recordings taken from four previous invasive studies, and therefore, the overall protocol for timing and duration of acquisitions varied. Despite this, a central method of recording was followed. Subjects were laid on the catheter lab table. Arterial access was obtained with a 6 Fr sheath. A 6 Fr coronary-guiding catheter was advanced to the coronary ostium. Aortic pressure on the haemodynamic system and the Combomap terminal was calibrated at the zero point. The patient was given intravenous heparin and intracoronary isosorbide di-nitrate. The Combowire was advanced to the tip of the guiding catheter and then the pressure transducers were normalized. The wire was advanced to the appropriate position within the coronary artery, and the signal was optimized by withdrawing the needle introducer and gently manipulating the wire position until an appropriate signal was obtained. The visual display and sound of the Doppler signal guided operators to obtain good-quality data. Although good signal quality was the goal of the acquiring operators on each individual study, both good- and poor-quality Doppler envelopes were inevitably recorded. We deliberately included a wide range of Doppler signal qualities into this study data set to expose the network to a diverse sample. Raw Doppler envelopes with a wide range of qualities were deliberately included (from black screens with no signal up to perfectly formed and clear envelopes) to expose the network to the variety of signals encountered during CFR measurement in routine clinical practice.
Raw spectral Doppler images were extracted from .SDY files exported from the console (see Figure 2). Images from three studies from one centre were used to form the development data set and images from one study from another centre were used to form the external validation data set. From the development data set, 742 images (∼7 beats per image) were used to train the network in the quality task and 417 images (∼7 beats per image) were used to train the network in the tracking task. The external validation data set comprised 200 images (∼4 beats per image) to assess performance on the quality task and 30 images (30 beats) were used for the tracking task. The development data set was further subdivided into tuning and internal validation data sets for the building and initial testing of the network and then monitoring of training progress.
Figure 2.
Summary of study design and methodology. AI, artificial intelligence; CFR, coronary flow reserve.
Experts
Two groups of experts in coronary spectral Doppler interpretation were formed. The first group of 10 experts collaboratively labelled the development data set. The second group of nine experts individually labelled the external validation data set.
Labelling
A purpose-built labelling platform was used by the experts to label the flow images for the two tasks.15 There is no established metric for the assessment of spectral Doppler signal quality. We therefore used a technique for converting subjective imaging judgement into a global score using an adapted version of the Glicko2 algorithm.16 Experts were presented with all images in the data set in different groupings, and the resulting scores were normalized into an index between 0 and 1 for each image. Over time, the distribution of scores converged on a steady state with maximum correlation between individual experts and the pooled consensus of experts.
In the velocity-tracing task, experts were presented with individual Doppler images and asked to place markers, connected by Bezier curves, along the boundary of the Doppler envelope until the curves accurately followed the peak of the signal corresponding to the instantaneous peak velocity (‘flow’; see Supplementary material online, Figure S1).
Neural networks
For the quality task, we used the publicly available ResNet 3417 with a single output neuron. Training data were augmented using random scaling, translation, and Gamma correction. For the velocity-tracing task, we used HighHRNet-W3218 optimized for pixel level segmentation. The expert tracing of the envelope of Doppler spectrum was converted into a heat map with a Gaussian distribution with a standard deviation of two pixels. Both networks were trained using batches of 16 images split across 4 NVIDIA Titan RTX graphical processing units (NVIDIA Corporation, DE, USA) using the Adam optimizer19 and the mean-squared error loss function. They were trained for 200 and 400 epochs, respectively. Initial learning rates were set and then modulated using the ‘OneCycle’ learning rate scheduler.20
Evaluation
For the quality task, the performance of the first neural network was assessed using an independent data set of 200 Doppler images. The network generated a raw score for each image and the same images were then scored by nine independent human experts. The AI generated score for each image was then compared with the score from each of the experts as well as the pooled expert score (‘expert consensus’). The primary outcome measure was the degree of correlation and numerical agreement between the different methods across the range of scores in the validation data set when compared with the expert consensus. In the tracking task, the performance of the second neural network was assessed using an independent data set of 30 images of coronary Doppler taken from 15 patients in the form of paired resting and hyperaemic recordings. The network generated curves for each image, and the same images were then traced by nine independent human experts with internationally recognized expertise in coronary Doppler. Experts were instructed to label approximately two cardiac cycles per image to ensure that at least one cardiac cycle per image was labelled by all experts. When experts did not extend their curves sufficiently to be analysed, their tracings were excluded. Both AI- and expert-generated curves were extracted as instantaneous peak flow velocity values and then used to derive average resting and hyperaemic flow velocity [average peak velocity (APV)] and CFR values (the ratio of paired hyperaemic APV and resting APV values) for the purposes of validation. The median APV and CFR values for each image were taken as the expert consensus. The primary outcome measure was the error between the APV and CFR values from the different methods (AI, embedded console algorithm and individual experts) and the expert consensus.
Statistical analysis
Results are presented as mean and standard deviation unless otherwise specified. Spearman’s rank correlation coefficient was used to assess the correlation between experts and thus monitor the progress of training in the quality task and was also used to demonstrate final agreement between methods. A one-way analysis of variance was used to compare values between the three tracking methods. Median values were used to determine the expert consensus from human scores. Correlations were reported using Spearman’s rho and agreement was assessed with a Bland and Altman analysis. Statistical analysis was done in ‘R Studio’ using the ‘tidyverse’ package.21
The ratio of APV during hyperaemia to that during resting conditions is the coronary flow velocity reserve (CFVR). For convenience and ease of understanding, and because vessel diameter changes little between rest and hyperaemia, we used the term CFR as an equivalent to CFVR throughout the manuscript.
Results
Automated artificial intelligence–based index of Doppler signal quality
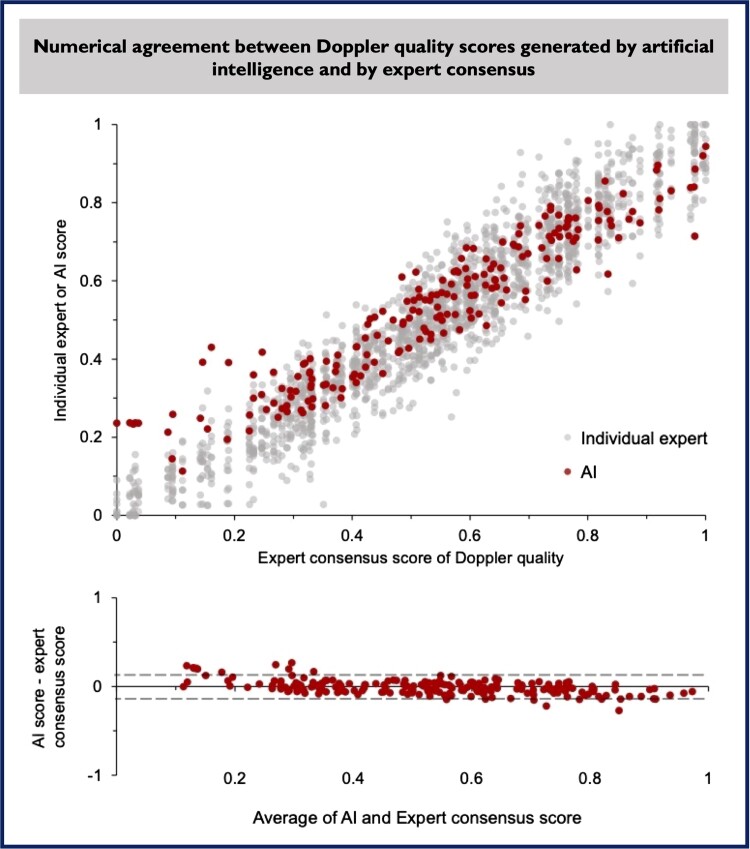
Artificial intelligence–derived Doppler signal quality scores were compared with those derived from the consensus of nine experts. The Spearman’s correlation coefficient between AI quality score and consensus expert quality score was 0.94 (P < 0.001), similar to the correlations observed between each of the individual experts’ scores and their consensus scores, which ranged from 0.93 and 0.98 m, mean 0.97 (Figure 3). Numerical agreement between AI score and expert consensus score was high. Using the methods of Bland and Altman,22 the bias between AI score and expert consensus was minimal [−0.006, 95% confidence interval (CI) −0.017 to 0.006, P = 0.316], with good agreement [95% limits of agreement (LOA) −0.16 to 0.15, Figure 3, bottom panel]. Examples of raw Doppler signals with a wide range of AI and expert indices of quality are shown in Figure 4.
Figure 3.
Correlation between quality scores generated by artificial intelligence and individual human experts is shown in top panel. Each dot represents a single score for a single image from nine experts and artificial intelligence. A Bland–Altman plot (lower panel) shows there is no systematic bias between artificial intelligence and expert consensus and a high numerical agreement. AI, artificial intelligence.
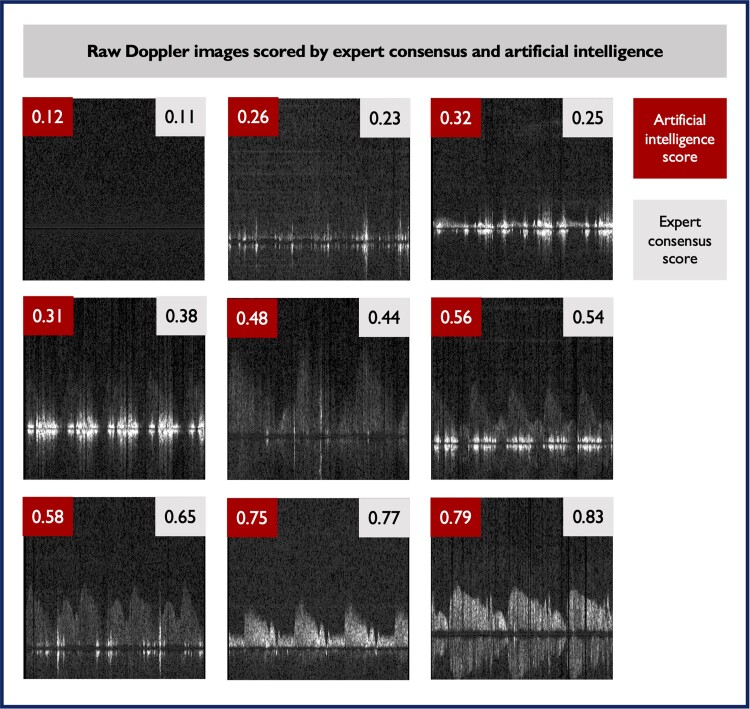
Figure 4.
Examples of Doppler signals (extracted from coronary arteries during microvascular assessment) are shown in increasing order of quality from top left to bottom right. Quality scores generated by artificial intelligence and expert consensus are displayed (0 being the lowest possible quality and 1 the highest possible quality). A good agreement between scores can be visually appreciated.
Artificial intelligence–derived flow velocity tracking and coronary flow reserve calculation
Following Doppler quality quantification, our tool was set to automatically trace Doppler envelopes and derive flow velocity data using AI (‘AI flow’). Artificial intelligence flow was compared with the consensus of multiple experts who had manually traced the same envelopes (expert consensus flow) as well as with the original flow velocity tracking obtained from the clinical Combomap console (‘console flow’). Paired values of hyperaemic and resting velocity data were used to calculate flow reserves (‘AI CFR’, ‘expert consensus CFR', and ‘console CFR’).
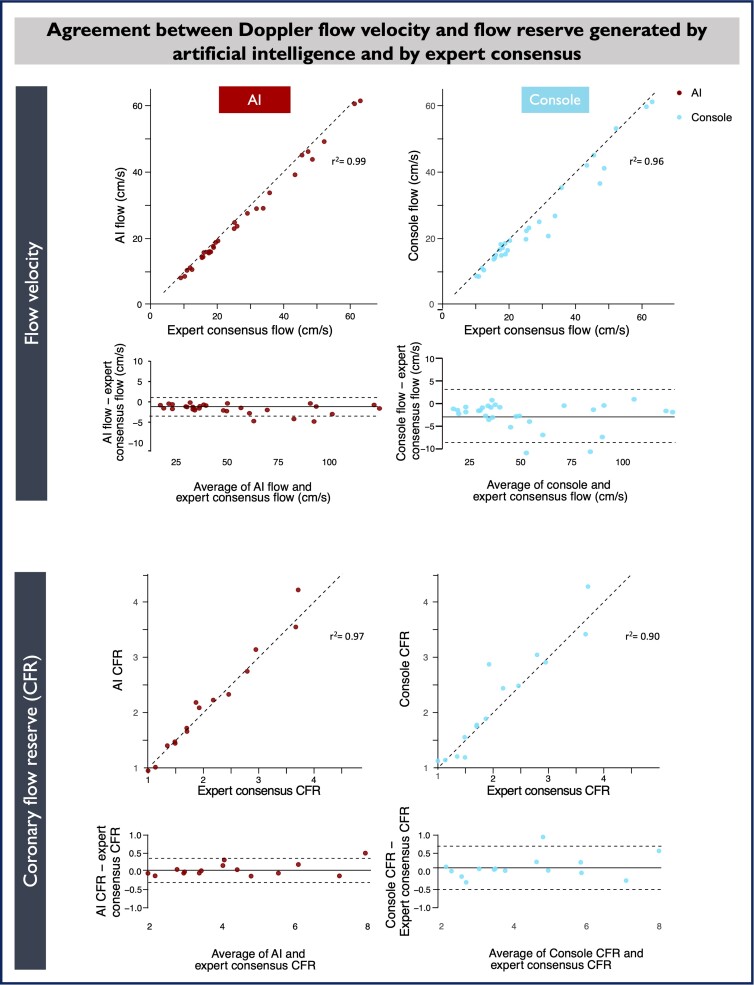
Mean flow velocities in the validation data set were similar (although statistically different) among all three methods (mean expert consensus flow 27.3 ± 15.6 cm/s, mean AI flow 25.6 ± 15.2 cm/s, and mean console flow 24.6 ± 15.1 cm/s, respectively, F = 18.1, P < 0.001). However, AI flow showed closer numerical agreement than console flow, against expert consensus flow (Figure 5), reflecting better tracking of the Doppler envelope (Figure 6). Using the method of Bland and Altman, there was minimal bias between AI flow and expert consensus flow (−1.68 cm/s, 95% CI −2.13 to −1.23 cm/s, P < 0.001) with good agreement (95% LOA −4.03 to 0.68 cm/s). The bias between console flow and expert consensus flow was higher (−2.63 cm/s, 95% CI −3.74 to −1.52, P < 0.001), also with lower agreement (95% LOA −8.45 and −3.19 cm/s; Figure 5). The median absolute difference (MAD) between AI flow and expert consensus flow was 7.9%. Between console flow and expert consensus flow, MAD was 11.8%. For the difference between AI flow and console flow, P = 0.013.
Figure 5.
Numerical relationships between artificial intelligence–derived, console-derived, and expert consensus flow and flow reserve values. Bland–Altman plots show that, relative to expert consensus flow and coronary flow reserve, artificial intelligence showed better agreement and less bias than the console algorithm. AI, artificial intelligence.
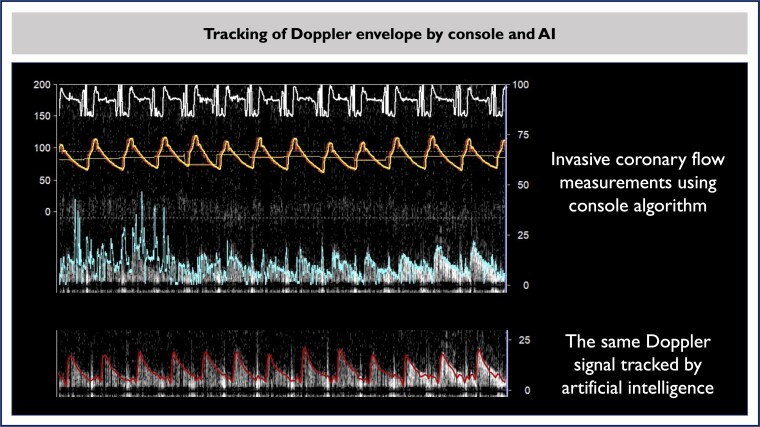
Figure 6.
The same raw Doppler recording is shown as seen in the console with tracking by the console algorithm and artificial intelligence. AI, artificial intelligence; CFR, coronary flow reserve.
Paired resting and hyperaemic values for the patients who made up the validation data set were combined to generate a CFR value (hyperaemic flow velocity divided by resting flow velocity). Using the method of Bland and Altman, there was minimal bias between AI CFR and expert consensus CFR (0.05, 95% CI −0.05 to 0.15, P = 0.312) with good agreement (95% LOA −0.33 to 0.43; Figure 5).
When console CFR is compared with expert consensus CFR, there is greater bias (0.11, 95% CI −0.06 to 0.28, P = 0.19). The 95% LOA are −0.56 to 0.78. The MAD (error) was 4.0% between AI CFR and expert consensus CFR and 7.4% between console CFR and expert consensus CFR. For the difference between AI and console CFR, P = 0.229.
Flow tracking accuracy and underlying Doppler quality
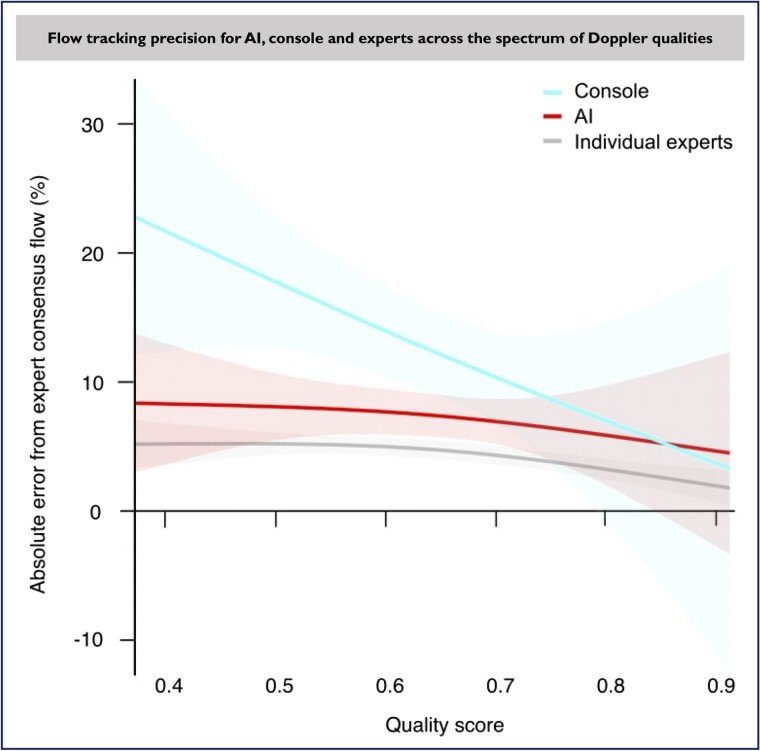
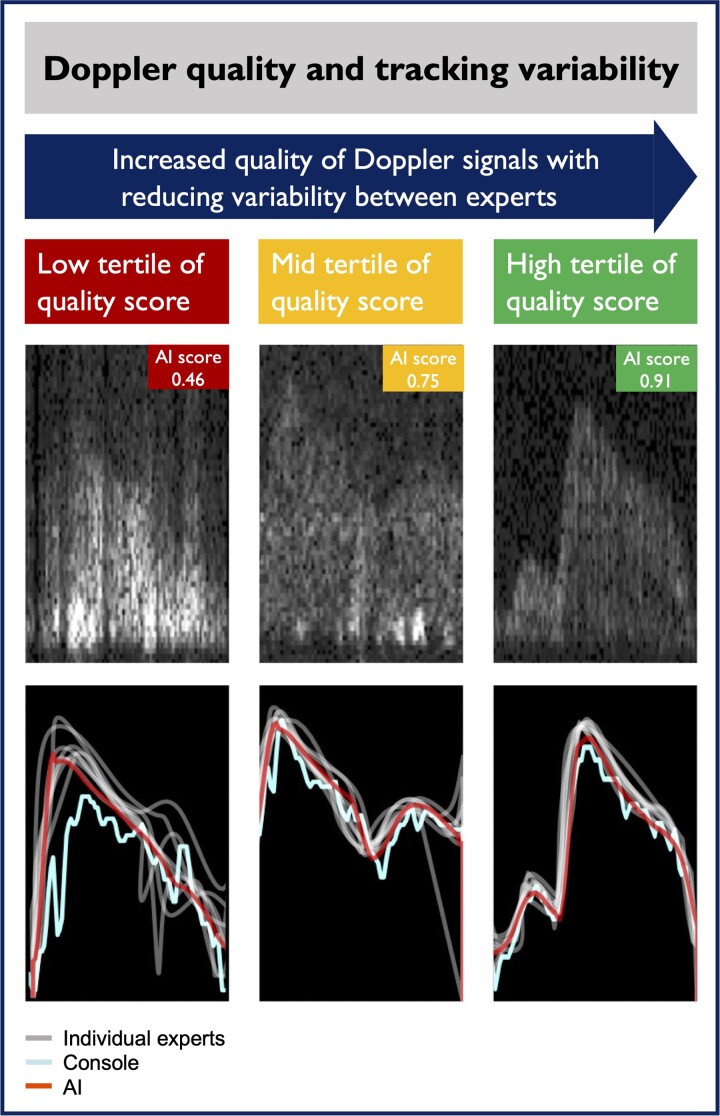
We tested the interaction between Doppler signal quality and the precisions of AI and console flow tracking. We found that, when compared with expert consensus flow, median absolute error of AI flow remained largely the same across the Doppler quality spectrum, while console flow error (measurement variability) increased significantly in low-quality Doppler envelopes. While the measurement variability increases for all methods in low-quality Doppler signals, including between-expert variability, AI flow error against expert consensus flow remains stable and predictable unlike that of console flow (median absolute error at the low, mid, and high tertiles of Doppler AI quality score were 8.3, 8.0, and 6.9% for AI flow and 16.7, 13.0, and 8.3% for console flow, respectively, P = 0.014; Figures 7 and 8). The AI was able to track even Doppler envelopes of poor quality, down to scores of 0.4 (40th percentile) as can be visually appreciated in Figure 7.
Figure 7.
The mean percentage error (with spline regression of the 95% confidence interval) between each of the tracking methods (console, artificial intelligence, and individual experts) is shown across the spectrum of Doppler signal qualities (judged using artificial intelligence scores). AI, artificial intelligence.
Figure 8.
Examples of flow tracking methods acting over Doppler envelops of different qualities. Higher individual variability among experts and increased noise from console-derived flow can be visually appreciated for lower quality Doppler signals. Artificial intelligence–derived flow is less sensitive to Doppler flow quality. AI, artificial intelligence.
There was no significant difference in the expert consensus quality scores for baseline and hyperaemic images (0.64 ± 0.14 vs. 0.67 ± 0.15, P = 0.21). The R2 for correlation between flow velocity and AI quality score was 0.0231.
Discussion
In this study, we developed and validated a methodology, based on AI, which was able to (i) quantify Doppler signal quality as accurately as international experts, (ii) track flow velocity from Doppler signals with greater precision than currently available clinical consoles, and as a result, (iii) derive CFR values with better numerical agreement with expert consensus. This method was operator independent and particularly precise when tracking poorer quality Doppler signals, therefore presenting a solution to the limitations of Doppler-based systems, such as signal noise and console setting dependency. Implementation of this AI approach in commercially available systems may improve the precision of Doppler-based coronary metrics, allowing expansion of microvascular function interrogation in clinical practice by less expert physicians. The AI algorithm may also be applied to previously acquired Doppler flow data to improve the precision of flow measurements, an aspect that may prove valuable for research purposes.
Automated quantification of Doppler signal quality
One of the major limitations of the use of Doppler-derived CFR calculation in assessing coronary artery disease is the variability of Doppler signal quality observed (i) across patients and also, (ii) within the same measurement in a single patient. Measurement of flow velocities and CFR relies on the tracking of Doppler envelopes with software embedded in clinical consoles, variability in underlying signal quality directly affects CFR output values. It takes great experience from operators to identify suitable Doppler envelopes and adjust console tracking settings to ensure velocities output are accurate (Figure 1 and Graphical Abstract). This ‘expert operator dependency’ of Doppler quality assessment significantly restricts the adoption of coronary microvascular assessment in clinical practice by the vast majority of physicians.
Using a novel machine-learning-based index of Doppler quality, which was developed and validated by international experts in invasive flow measurements, we have demonstrated independent and automated expert-level assessment of flow quality during CFR measurements in clinical practice. As Part 1 of a two-step approach (with automated flow tracking being the second), this could allow for only higher quality Doppler signals to be used for CFR calculation, reducing noise and improving measurement precision. As can be seen in Figures 7 and 8, measurement precision deteriorates with poor Doppler quality, reaching a threshold below which flow measurements should perhaps be avoided.
Operator-independent, high-precision Doppler flow tracking
Current invasive CFR calculation relies on capturing flow velocities, using a tracking algorithm embedded in clinical consoles, which relies on a clear signal separation between Doppler envelopes and the background. While this is achievable with acceptable precision when Doppler signals are clear, this approach struggles with underlying poor-quality, noisy Doppler signals. Current tracking is also entirely dependent on operator-adjusted settings, which means that even for the same underlying Doppler, flow velocities can be adjusted to output different values (Figure 1). Because CFR is a ratio of two flow velocity values, errors and noise can be magnified, reducing its reliability as a clinical tool.
Our AI tracking tool was designed to improve upon both such limitations. After many years of training, human experts become able to visualize Doppler envelopes partially obscured by noise even when Doppler quality is poor. While standard computer algorithms cannot easily replicate such higher order cognition of discerning signal from noise in a grey-scale image, neural networks can be trained to capture such expertise. And our results demonstrate this principle. Artificial intelligence–based flow tracking is more precise than console tracking particularly in poor Doppler traces, reducing error by half in the lowest tertile of AI quality scores (16.7 vs. 8.3%, P = 0.014; Figure 7). As a result, numerical agreement between AI CFR and expert consensus CFR was higher than what can be achieved by operators using console algorithm (Figure 5). Furthermore, our AI tracking of flow velocities is entirely automated, without the need for operator adjustments during measurements (Figures 1 and 6, and Graphical Abstract).
Clinical implications
We envisage that this method, once applied to clinical CFR consoles, will greatly facilitate Doppler CFR measurements and lead to increased adoption of coronary microvascular assessment in routine clinical practice, a process that should directly benefit patients with angina and non-obstructive coronary disease, who often remain undiagnosed and without appropriately tailored treatment. Considering the low adoption of the current method among general physicians who are not experts in coronary physiology, a tool that improves test reliability could lead to a significant expansion of coronary microvascular testing in clinical practice and expand its use to diagnostic scenarios in which continuous flow measurement is needed,10 or post processing of signals is important to assess the status of the microcirculation.
Future larger studies using prospectively collected Doppler data will need to demonstrate the clinical usefulness of our AI approaches to CFR measurements during routine clinical coronary microvascular assessment.
Applications to research and to other Doppler-based methods
Previous studies had used computer algorithms to automate and optimize Doppler signal processing in a mouse model and in transthoracic Doppler sonography of the human coronary artery.23–25 These studies tended to focus on the use of computer algorithm–based automation rather than machine learning. Although these methods are potentially useful, they do not benefit from the advantages that neural networks offer in terms of approximating human expert discrimination in both image quality and envelope tracking.
Our technique can be retrospectively applied to previously collected Doppler data (saved as .SDY files from clinical consoles). This could help coronary physiology researchers to improve flow velocity tracking precision of previously collected data, particularly if data collection is known to have been challenging, with resulting poor-quality signals. Also, because of reduced beat-to-beat noise, we believe our approach would be particularly useful in more complex types of analysis, such as wave intensity analysis and derivation of pressure and flow loops. We will make this tool immediately available offline for researchers who wish to use our approach on previously collected Doppler data. We would encourage them to contact us so that we can discuss collaborations. Our aim is for the methods outlined here to be used on clinically available consoles, a step that will need further work on software and hardware integration.
A new generation of Doppler wires and software is expected in the next few years. We expect such an upgrade to improve upon some of the limitations of current devices, although we also envisage that some challenges will continue to exist as coronary flow is intrinsically a biologically complex entity to measure. We believe that, although our current AI developments were based on existing Doppler technology, the principles of imaging quality quantification and envelope tracking are largely translatable to future Doppler displays and, if required, retraining of the network would be easily achievable with an appropriate data set.
Finally, although our neural network tool was trained using coronary Doppler images collected invasively, our results demonstrate a potential for AI-derived Doppler tracking to be applied in other fields such as echocardiography or other vascular studies.
Limitations
The AI approaches presented in this study do not improve raw Doppler signal quality per se, but instead offer a method to improve digitization and quality control of Doppler signals even when acquisition is challenging, augmenting CFR calculation precision. Although this study included a sizeable data set of images recorded by multiple operators in multiple centres, it is likely that the performance of the neural network would increase if a larger data set were used with continuous training. However, the excellent agreement with expert consensus that was achieved suggests that the diversity of input is sufficient to demonstrate the principle of improved precision with AI. In its current form, the system does not automatically detect the windows for resting and hyperaemic flow that would allow us to consider it a truly automated tool. This is a task which would be suited to AI and we plan to include this in future iterations of the development. It also does not allow for making adjustments to the tracking threshold, which might be a feature attractive to more expert users. This is an iteration which could be developed in the future if there is clinical or academic interest. This tool has not been tested prospectively in a real-time setting, and this should be the focus of future work during microvascular assessment in clinical practice. Finally, our proposition does not improve raw Doppler signal quality, but instead offers a tool which improves CFR data extracted from what current Doppler technology can offer. We are currently working on methods to improve Doppler signal quality and aim to use our AI approach to validate such developments.
Conclusions
An AI-based coronary Doppler flow tracking tool increases CFR measurement precision and shows high agreement with international experts. Artificial intelligence CFR outperforms algorithm-based CFR, particularly when Doppler signals are poor. If implemented on measurement consoles, AI CFR has the potential to simplify coronary microvascular assessment and expand its utilization in clinical practice.
Supplementary Material
Contributor Information
Henry Seligman, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Royal Brompton and Harefield Hospitals, Guy's and St Thomas' NHS Foundation Trust, London, UK.
Sapna B Patel, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK.
Anissa Alloula, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK.
James P Howard, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Christopher M Cook, Essex Cardiothoracic Centre, Basildon, Essex, UK; Anglia Ruskin University, Chelmsford, UK.
Yousif Ahmad, Yale School of Medicine, Yale University, New Haven, Connecticut, USA.
Guus A de Waard, Heart Centre, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Mauro Echavarría Pinto, Hospital General ISSSTE Queretaro, Faculty of Medicine, Autonomous University of Queretaro, Querétaro, Mexico.
Tim P van de Hoef, Heart Centre, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Haseeb Rahman, The British Heart Foundation Centre of Excellence and the National Institute for Health and Care Research Biomedical Research Centre at the School of Cardiovascular Medicine and Sciences, Kings College Medical School, St Thomas Hospital, London, UK.
Mihir A Kelshiker, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Christopher A Rajkumar, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Michael Foley, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Alexandra N Nowbar, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK.
Samay Mehta, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK.
Mathieu Toulemonde, Department of Engineering, Imperial College London, London, UK.
Meng-Xing Tang, Department of Engineering, Imperial College London, London, UK.
Rasha Al-Lamee, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Sayan Sen, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Graham Cole, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Sukhjinder Nijjer, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Javier Escaned, Hospital Clínico San Carlos IDISSC and Universidad Complutense de Madrid, Madrid, Spain.
Niels Van Royen, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Darrel P Francis, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Matthew J Shun-Shin, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Ricardo Petraco, National Heart and Lung Institute, Imperial College London, B Block, Hammersmith Hospital, London W12 0HS, UK; Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Funding
C.A.R. (MR/S021108/1) and M.F. (MR/V001620/1) are supported by the Medical Research Council (United Kingdom). A.N.N. was supported by the NIHR Academy. R.P. (FS/11/46/28861), D.P.F. (FS 04/079), J.P.H. (FS/ICRF/22/26039) and M.J.S.-S. (FS/14/27/30752) were supported by the British Heart Foundation. H.S. was supported by the Imperial NIHR Biomedical Research Centre (PA3065). The authors are grateful to the Imperial NIHR Biomedical Research Centre for infrastructural support.
Data availability
The data that support the findings of this study are available from the corresponding author (R.P.) upon reasonable request.
References
- 1. Schumann CL, Mathew RC, Dean J-HL, Yang Y, Balfour PC, Shaw PW, et al. Functional and economic impact of INOCA and influence of coronary microvascular dysfunction. JACC Cardiovasc Imaging 2021;14:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mileva N, Nagumo S, Mizukami T, Sonck J, Berry C, Gallinoro E, et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and meta-analysis. J Am Heart Assoc 2022;11:e023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelshiker MA, Seligman H, Howard JP, Rahman H, Foley M, Nowbar AN, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J 2021;43:1582–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimokawa H, Suda A, Takahashi J, Berry C, Camici PG, Crea F, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) group. Eur Heart J 2021;42:4592–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heggie R, Briggs A, Stanley B, Good R, Rocchiccioli P, McEntegart M, et al. Stratified medicine using invasive coronary function testing in angina: a cost-effectiveness analysis of the British Heart Foundation CorMicA trial. Int J Cardiol 2021;337:44–51. [DOI] [PubMed] [Google Scholar]
- 6. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 7. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021;144:e368–e454. [DOI] [PubMed] [Google Scholar]
- 9. Cook CM, Ahmad Y, Howard JP, Shun-Shin MJ, Sethi A, Clesham GJ, et al. Impact of percutaneous revascularization on exercise hemodynamics in patients with stable coronary disease. J Am Coll Cardiol 2018;72:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation 2019;140:1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Everaars H, de Waard GA, Driessen RS, Danad I, van de Ven PM, Raijmakers PG, et al. Doppler flow velocity and thermodilution to assess coronary flow reserve: a head-to-head comparison with [15O]H2O PET. JACC Cardiovasc Interv 2018;11:2044–2054. [DOI] [PubMed] [Google Scholar]
- 12. Philips . Combomap product details [Internet]. Combomap pressure and flow system.https://www.usa.philips.com/healthcare/product/HCIGTDCMAP/combomap-pressure-and-flow-system(5 June 2022).
- 13. Demir OM, Schrieken C, Curio J, Rahman H. Behavioural determinants impacting the adoption rate of coronary physiology. Int J Cardiol 2021;330:12–14. [DOI] [PubMed] [Google Scholar]
- 14. Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol 2012;59:1392–1402. [DOI] [PubMed] [Google Scholar]
- 15. Howard JP, Stowell CC, Cole GD, Ananthan K, Demetrescu CD, Pearce K, et al. Automated left ventricular dimension assessment using artificial intelligence developed and validated by a UK-wide collaborative. Circ Cardiovasc Imaging 2021;14:e011951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glickman ME. Example of the Glicko-2 system [Internet]. p1–6.http://www.glicko.net/;http://www.glicko.net/glicko/glicko2.pdf(1 December 2022).
- 17. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: IEEE Conference on Computer Vision and Pattern Recognition (CVPR). New York City: Computer Vision Foundation; 2016, p770–778.
- 18. Cheng B, Xiao B, Wang J, Shi H, Huang TS, Zhang L. HigherHRNet: scale-aware representation learning for bottom-up human pose estimation. In: 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR). New York City: Computer Vision Foundation; 2020, p5385–5394.
- 19.Liu L, Jiang H, He P, Chen W, Liu X, Gao J, et al. On the variance of the adaptive learning rate and beyond. arXiv 2019. [DOI]
- 20. Howard J, Gugger S. Fastai: a layered API for deep learning. Information 2020;11:108. [Google Scholar]
- 21. R Development Core Team . R: A Language and Environment for Statistical Computing. New Zealand: University of Auckland; 2017. [Google Scholar]
- 22. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 23. Bossenbroek J, Ueyama Y, McCallinhart PE, Bartlett CW, Ray WC, Trask AJ. Improvement of automated analysis of coronary Doppler echocardiograms. Sci Rep 2022;12:7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sunyecz IL, McCallinhart PE, Patel KU, McDermott MR, Trask AJ. Defining coronary flow patterns: comprehensive automation of transthoracic Doppler coronary blood flow. Sci Rep 2018;8:17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magagnin V, Delfino L, Cerutti S, Turiel M, Caiani EG. Nearly automated analysis of coronary Doppler flow velocity from transthoracic ultrasound images: validation with manual tracings. Med Biol Eng Comput 2007;45:483–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (R.P.) upon reasonable request.