Abstract
Fibrillary glomerulonephritis (FGN), a rare disease is pathologically characterized by glomerular fibril accumulation ranging from 12 to 24 nm in diameter with negative Congo red staining. Recently, the identification of DnaJ homolog subfamily B member 9 (DNAJB9) as a highly sensitive and specific marker for FGN has revolutionized diagnosis of this disease. However, few recent studies have reported DNAJB9-negative glomerulonephritis with fibrillar deposits. As such, it remains unclear whether DNAJB9-negative cases can be considered equivalent to FGN. Here, we report the case of a 70-year-old woman who developed renal impairment and nephrotic-range proteinuria. Renal biopsy and pathological examination revealed focal glomerulonephritis with fibrocellular crescents. Immunofluorescence microscopy showed IgA-dominant deposition of polytypic IgG in the glomerulus. Electron microscopy revealed hump-like subepithelial electron dense deposits with fibrils of 15–25 nm in diameter. These findings were consistent with FGN; thus, Congo red and direct fast scarlet (DFS) staining, and immunohistochemistry for DNAJB9 were performed. In addition to negative Congo red/DFS/DNAJB9 staining, laser microdissection (LMD) and liquid chromatography-tandem mass spectrometry (LC–MS/MS) resulted negative for DNAJB9, which is a highly sensitive and specific marker for FGN. The patient’s renal function further declined, prompting administration of rituximab weekly for 2 weeks, similar to the treatment for FGN. This is a unique case of IgA-dominant glomerulonephritis with DNAJB9-negative fibrillar polytypic immunoglobulin deposits in the subepithelium, unlike previous DNAJB9-negative cases. Thus, DNAJB9-negative cases diagnosed based on accurate electron microscopic evaluation must be gathered, and LMD and LC–MS/MS must be used to analyze the organized fibrillar deposits to reveal the disease entity.
Keywords: Fibrillar glomerulonephritis, DNAJB9, IgA-dominant deposits, Subepithelial deposits, Kidney biopsy, Mass spectrometry
Introduction
Fibrillary glomerulonephritis (FGN) is a rare disorder defined as an immune complex-mediated glomerulonephritis (GN) with Congo red-negative, organized, randomly arranged fibrils with a diameter ranging from 12 to 24 nm. Generally idiopathic, FGN can be associated with hepatitis C, autoimmune diseases, and, to a lesser extent, malignancies [1]. The prognosis of FGN is poor, with 50% of patients progressing to end stage kidney disease (ESKD) within several years of diagnosis [2]. There are no established therapeutic regimens, although the administration of rituximab has been reported to stabilize disease progression in some patients [3]. Recently, the identification of DnaJ homolog subfamily B member 9 (DNAJB9) as a highly sensitive and specific marker for FGN has revolutionized FGN diagnosis [4–6]. DNAJB9 detection in biopsies using immunohistochemistry, laser microdissection, and liquid chromatography-tandem mass spectrometry (LMD and LC–MS/MS) is proven to be reliable in the diagnosis of FGN. However, a few recent studies have reported rare cases of GN with DNAJB9-negative fibrillar Immunoglobulin (Ig) G deposits [1, 7]. It is unclear whether DNAJB9-negative cases can be included and classified as a disease similar to FGN. Here, we report a case of IgA-dominant GN with DNAJB9-negative fibrillar polytypic Ig deposits in the subepithelium, resembling FGN.
Case report
A 70-year-old woman was referred to our department with decreased renal function, nephrotic-range proteinuria, and creatinine levels from 0.75 to 0.95 mg/dL [estimated glomerular filtration rate (eGFR) from 58 to 40 mL/min/1.73 m2] over the past five months. The patient had diabetes with simple diabetic retinopathy and hyperlipidemia, managed with oral medication. Prior to admission, the patient was recently diagnosed with recurrent uveitis. This was due to frequent redness and pain in both eyes with no obvious infection or autoimmune disease, such as sarcoidosis, Bechet disease, or vasculitis. The patient had no obvious family history. The patient presented with hypertension, blood pressure of 160/107 mmHg, though other vital signs were unremarkable. Physical examination revealed no abnormalities. There were no systemic symptoms suggestive of an obvious bacterial infection, including staphylococcal infection. Clinical laboratory analyses revealed an elevated serum creatinine (Cr) of 1.0 mg/dL (eGFR: 38 mL/min/1.73 m2), blood urea nitrogen of 19.9 mg/dL, decreased albumin of 3.3 g/dL, and total protein of 6.0 g/dL. Glycated hemoglobin (HbA1c) was slightly raised at 6.9% (normal range 4.8–6.1%). Dipstick urinalysis revealed the presence of 3+ blood and 3+ protein. Urine microscopy showed 20–29 red blood cell count (RBC) per high-power-field (HPF), and urinary protein was 11.9 g/gCr. Further workup, including C3, C4, anti-nuclear antibodies (ANA), anti-neutrophil cytoplasmic antibody, anti-glomerular basement membrane antibody, cryoglobulin, and protein electrophoresis, were all within normal limits. The patient tested negative for hepatitis B and C viruses.
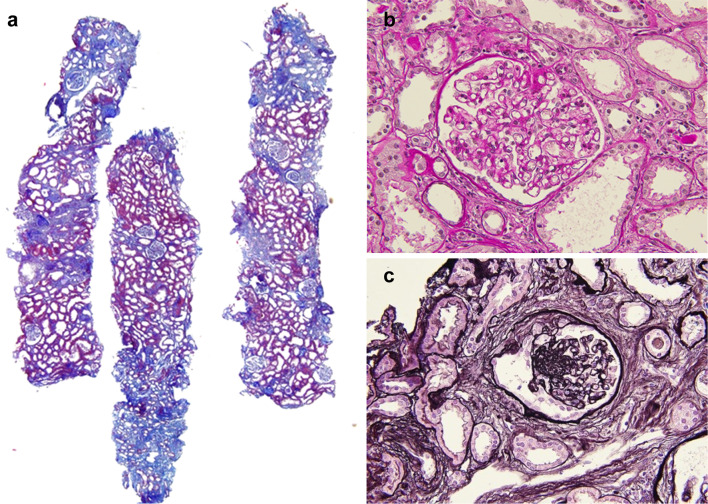
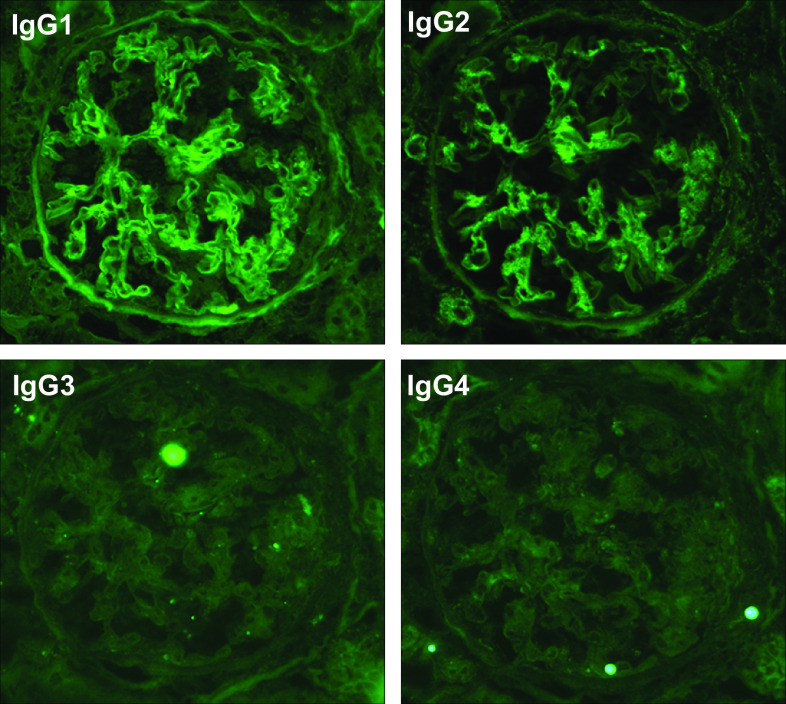
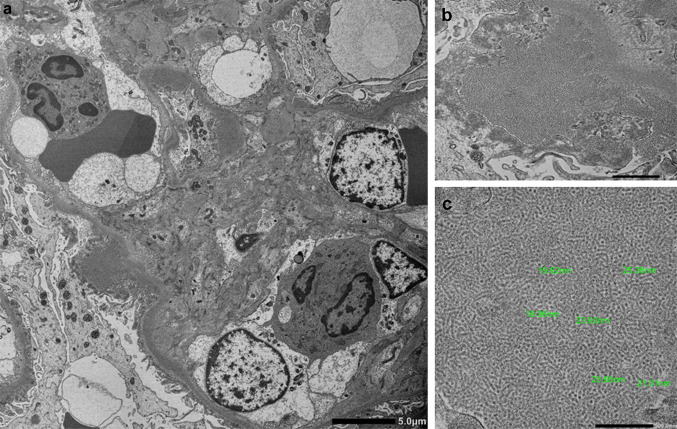
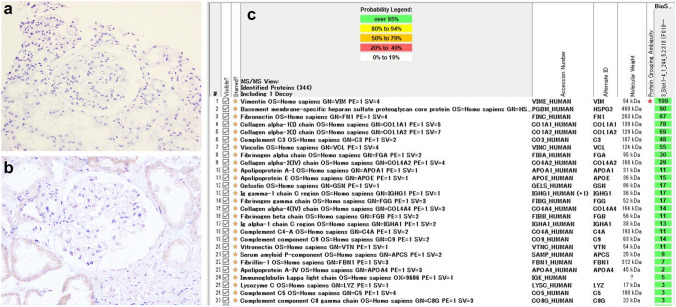
Subsequently, the patient was admitted for renal biopsy to elucidate the cause of renal impairment with nephrotic-range proteinuria. Light microscopy showed moderate interstitial fibrosis and tubular atrophy involving 30% of sampled cortical areas (Fig. 1a). Glomeruli showed mild mesangial expansion (Fig. 1b), and one of the 14 glomeruli showed segmental sclerosis with fibrocellular crescents and segmental endocapillary hypercellularity (Fig. 1c). There was one other glomerulus with a small fibrocellular crescent. Immunofluorescent (IF) staining was positive for IgG (1+), IgA (3+), C3 (2+), κ (1+), and λ (1+), showing a granular pattern of deposition along the segmental capillary wall and in the mesangial region (Fig. 2). IgG subclasses showed IgG1 and IgG2 were also present in the same region (Fig. 3). Electron microscopy revealed electron dense deposits (EDD) of various sizes including hump-like deposits in the subepithelial areas, but not in the mesangial areas (Fig. 4a). All the subepithelial EDD contained randomly arranged fibrillar structures without hollow cores ranging from 15 to 25 nm in diameter (Fig. 4b–c). These findings were consistent with FGN. As such, Congo red and direct fast scarlet (DFS; data not shown) staining, and immunohistochemistry for DNAJB9 were performed. In addition to negative Congo red/DFS/DNAJB9 staining (Fig. 5a and b), DNAJB9 was negative even after laser microdissection (LMD) and liquid chromatography-tandem mass spectrometry (LC–MS/MS). A specific protein could not be identified using LMD and LC–MS/MS (Fig. 5c).
Fig. 1.
Kidney biopsy analysis using light microscopy. Kidney sections under light microscopy show mild interstitial fibrosis and tubular atrophy (a Masson’s trichrome staining, original magnification× 40). The glomeruli show mild mesangial expansion (b Periodic acid-Schiff staining, original magnification × 400). One of the 14 glomeruli showed segmental sclerosis with fibrocellular crescents and segmental endocapillary hypercellularity. (c Periodic acid-Schiff staining, original magnification × 400)
Fig. 2.
Immunofluorescence staining of the kidney biopsy: positive immunofluorescence staining for IgG, IgA, IgM, C3, κ, and λ along the segmental capillary walls
Fig. 3.
Immunofluorescence staining for IgG subclasses: immunofluorescence staining for IgG subclasses showed that IgG1 and IgG2 were present along the segmental capillary wall and in the mesangial region
Fig. 4.
Electron microscopy analysis of the kidney biopsy: electron microscopy showing electron-dense deposits (EDD) in the subepithelial areas (a original magnification × 3000). The EDD shows randomly arranged fibrillar structures (b original magnification × 20,000). The diameter of the fibrils ranged from 15 to 25 nm (c original magnification × 50,000)
Fig. 5.
Immunohistochemistry and mass spectrometry findings of kidney biopsy: Negative Congo red staining (a original magnification × 400) and DnaJ homolog subfamily B member 9 (DNAJB9) staining (b original magnification × 400). Result of laser microdissection (LMD) and liquid chromatography-tandem mass spectrometry (LC–MS/MS) (c). A specific protein was not identified using LMD and LC–MS/MS
The possibility of FGN was initially considered based on negative DFS staining and the size and shape of the organized structure. However, immunohistochemistry and LMD and LC–MS/MS did not detect DNAJB9, a highly sensitive and specific marker for FGN. In addition, IgA-dominant and hump-like subepithelial polytypic Ig deposits and negative IgG4 staining are atypical features of FGN. Therefore, we speculate that this case might have been an unidentified disease that mimics FGN. In 6 months, the patient’s creatinine level increased from 1.0 to 1.58 mg/dL, prompting administration of rituximab at 375 mg/m2 of body surface area, once weekly for 2 weeks.
Discussion
Herein, we report a case of IgA-dominant GN with DNAJB9-negative fibrillar polytypic Ig deposits in the subepithelium. The morphology of the organized structures was similar to that of FGN; however, negative DNAJB9 staining and IgA-dominant subepithelial deposits were not consistent with typical FGN.
In glomerulopathy with organized deposits including FGN, a negative Congo red (or DFS) staining is required to distinguish it from renal amyloidosis. However, Congo red-positive FGN has been reported as a subtype of atypical FGN. Analysis of 18 Congophilic FGN cases showed that no proteomic signature of amyloid was detected with mass spectrometry, whereas DNAJB9 was detected in all cases [8]. In these cases, while Congo red staining is not useful to distinguish FGN from renal amyloidosis, diagnosis with mass spectrometry and DNAJB9 staining is more reliable. Thus, mass spectrometry and DNAJB9 staining are powerful tools in the diagnosis of FGN, though these were not conclusive in our case.
Few reports have described DNAJB9-negative cases with fibrillar polytypic Ig deposition. As in our case, Andeen et al. reported six suspected FGN cases with negative DNAJB9 staining [1]. In these cases, fibrillar deposits with diameters of approximately 20 nm were commonly observed. However, after re-examination, three cases with polytypic Ig deposits remained descriptive diagnoses, two of which had monotypic Ig deposits and were reclassified as probable immunotactoid glomerulopathy (ITG). One case was classified as amyloid light-chain (γ)-type amyloidosis with crescentic GN. These revised diagnoses are inapplicable to our case due to light-chain restriction, organized deposits with hollow cores, and absent DFS-positive staining. Of the three remaining cases, two showed mild mesangial expansion with mesangial Ig deposition, while the other case showed focally crescentic GN with large immune deposits, similar to our case. Focally crescentic GN was present in the 64-year-old woman, who presented with creatinine of 2.5 mg/dL, hematuria, proteinuria, suspected autoimmune disease (ANA positive, Sjögren syndrome-related antigen (SS)-A positive, and SS-B positive), progressing to ESKD 25 months after kidney biopsy. Immunofluorescence staining showed that IgG, IgM, C3, and C1q were positive in the glomerular, focal arterial, and tubular basement membranes. Electron microscopy revealed fibrils with diameters ranging from 22 to 27 nm and tubuloreticular inclusions. Based on these findings, the case was similar to lupus-like immune complex-mediated GN, which differs from our case.
However, some cases of GN with monotypic DNAJB9-negative fibrillar deposits have been reported. Nasr et al. reported a patient with monoclonal gammopathy (IgG with λ light chain) who developed DNAJB9 negative FGN leading to ESKD [7]. LC–MS/MS revealed that the glomerular deposits contained a high number of spectra for the γ1 heavy chain, and no detected spectra for DNAJB9, κ, or λ light chains. Using immunochemical and molecular methods, the glomerular deposition of Congo red-negative was determined to be an immunoglobulin heavy chain. Similar to this case, Kudose et al. classified 29 cases with fibrillar IgG deposits and apparent light-chain restriction on standard immunofluorescence on frozen tissue by staining for Congo red, DNAJB9, and IgG subtypes [9]. Patients were reclassified into four groups: DNAJB9-positive FGN (n = 14), monotypic DNAJB9-positive FGN (n = 7), GN with polytypic DNAJB9-negative fibrillar IgG deposits (n = 2), and GN with monotypic DNAJB9-negative fibrillar IgG deposits (n = 6). Interestingly, among the cases, only the monotypic DNAJB9-positive group showed an almost membranous proliferative or mesangial pattern with no subepithelial deposits. However, the monotypic DNAJB9-negative group showed a membranous pattern with subepithelial deposits. These findings indicate that the pathogenesis may differ between DNAJB9 positive and negative cases. These negative cases exhibited many features reminiscent of ITG, including focally parallel fibril alignment and strong association with chronic lymphocytic leukemia. In our case, the subepithelial deposits were similar, but the deposits were accompanied by polytypic Ig and hump-like morphology, different from previous cases.
IF findings in FGN include ill-defined deposits that stain most intensely for IgG, typically accompanied by C3, k, and λ, and occasionally also associated with staining for C1q, IgM, and/or IgA. According to a cohort from the Mayo Clinic [10], all 66 cases were positive for IgG with a mean intensity of 2.5+ in glomeruli by immunofluorescence. A total of 47% cases were positive for IgM (mean intensity of positive cases, 1.0+) and 28% cases positive for IgA (mean intensity of positive cases, 1.0+). Glomerular staining for IgM and IgA was weaker than that for IgG in all cases except one, in which IgA staining was stronger than that for IgG, resembling our case [10]. Thus, because IgA-dominant deposition is very rare in FGN, it is necessary to exclude other GNs with IgA-dominant deposits, such as IgA-dominant infection-related GN. However, in our case, there were no systemic symptoms derived from vasculitis or prodromal streptococcal infection, suggestive of IgA vasculitis or infection-related IgA-dominant GN. Nasr et al. also reported cases of coexisting DNAJB9 associated FGN and IgA nephropathy (IgAN). FGN with concurrent IgAN (FGN-IgAN) is also very rare and was present in 1.8% of 847 FGN cases (11). They revealed that the clinical presentation and demographics of FGN-IgAN were closer to those of FGN than IgAN. Histology showed that all cases exhibited smudgy glomerular staining for IgG and DNAJB9, with fibrillary deposits and granular mesangial staining for IgA. Our case differs from FGN-IgAN in that IgA is stained segmentally along the capillaries with IgG deposition.
In conclusion, we report a case of IgA-dominant GN with DNAJB9-negative fibrillar polytypic immunoglobulin deposits in the subepithelium. To the best of our knowledge, this is the first report of this unique case. This case shows the morphology of the organized structures was consistent with FGN, despite IgA-dominant hump-like deposits and negative DNAJB9 staining. Thus, the need to evaluate DNAJB9-negative cases with organized deposits with accurate electron microscopic diagnosis, and subsequent deposit analysis using LMD and LC–MS/MS to reveal the disease entity is emphasized.
Acknowledgements
We are grateful to Yuriko Sawa, Norihiko Suzuki, Ryoko Sakamoto, and Naoko Asano for their excellent technical assistance. We would like to thank Editage (www.editage.com) for the English language editing.
Author contributions
RM and KM were responsible for the manuscript, interpretation of data, and writing of this paper. The patient was attended by SF. SS, KN, KT, and SM participated in composing the manuscript and providing advice for the concept. All the authors were involved in drafting, reviewing, and approving the final manuscript.
Funding
The authors declare no relevant financial support.
Data availability
The data generated and/or analyzed during the current case report are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional and National Research Committee at which the studies were conducted with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent for the publication of this report was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andeen NK, et al. Fibrillary glomerulonephritis clinicopathologic features and atypical casese from a multi-institutional cohort. Clin J Am Soc Nephrol. 2019;14:1741–1750. doi: 10.2215/CJN.03870319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenstock JL, et al. Fibrillary and immunotactoid glomerulonephritis: disintc entities with different clinical and pathologic features. Kidney Int. 2003;63:1450–1461. doi: 10.1046/j.1523-1755.2003.00853.x. [DOI] [PubMed] [Google Scholar]
- 3.Javaugue V, et al. Long-term kidney disease outcomes in fibrillary glomerulonephritis: a case series of 27 patients. Am J Kidney Dis. 2013;62:679–690. doi: 10.1053/j.ajkd.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Nasr SH, et al. DNAJB9 is a specific immunohistochemical marker for fibrillary glomerulonephritis. Kidney Int Rep. 2018;3:56–64. doi: 10.1016/j.ekir.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasari S, et al. DnaJ heat shock protein family B member 9 is a novel biomarker for fibrillary GN. J Am Soc Nephrol. 2018;29:51–56. doi: 10.1681/ASN.2017030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andeen NK, et al. DnaJ homolog subfamily B member 9 is a putative autoantigen in fibrillary GN. J Am Soc Nephrol. 2018;29:231–239. doi: 10.1681/ASN.2017050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasr SH, et al. Heavy chain fibrillary glomerulonephritis: a case report. Am J Kidney Dis. 2019;74:276–280. doi: 10.1053/j.ajkd.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Alexander MP, et al. Congophilic fibrillary glomerulonephritis: a case series. Am J Kidney Dis. 2018;72:325–336. doi: 10.1053/j.ajkd.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Kudose S, et al. Diagnostic approach to glomerulonephritis with fibrillar IgG deposits and light chain restriction. Kidney Int Rep. 2021;6:936–945. doi: 10.1016/j.ekir.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr SH, et al. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol. 2011;6:775–784. doi: 10.2215/CJN.08300910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Said SM, et al. Characteristics of patients with coexisting DNAJB9-associated fibrillary glomerulonephritis and IgA nephropathy. Clin Kidney J. 2021;14:1681–1690. doi: 10.1093/ckj/sfaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and/or analyzed during the current case report are available from the corresponding author on reasonable request.