Abstract
Cyst infection is a frequent and serious complication of autosomal dominant polycystic kidney disease (ADPKD). Hematogenous spread via bacterial translocation in the intestine is considered to be the main cause, so intestinal flora may be involved. However, the exact role of the intestinal flora in cyst infection in ADPKD is unknown. We report a 66-year-old woman and a 56-year-old man with ADPKD who had severe hepatic cyst infection. We analyzed the microbiome of infected cyst content, feces, and saliva in these two patients. The microbiome of patient 1 showed various bacteria in an infected cyst, whereas that of patient 2 showed only one bacterium. In both patients, the composition of the microbiome of the cyst content was quite different from those of feces and saliva, and the main bacteria in the infected cyst content represented a small proportion of those in feces and saliva. Lactobacilli were not almost detected in the infected cyst content though some lactobacilli are endemic in the gastrointestinal tract and the saliva. The association between bacteria in cysts and those in feces or saliva remains uncertain, and further research on this topic is needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13730-022-00767-2.
Keywords: ADPKD, Cyst infection, Infected cyst, Polycystic kidney disease
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited disease [1], and cyst infection is a frequent and serious complication. Studies estimated that 30–50% of patients with ADPKD experience some form of kidney infection during their lifetime [2, 3], although cyst infection leading to hospitalization is much less frequent, occurring in approximately 9% of patients [4]. These infections sometimes become resistant to treatment and can be fatal, even when appropriate antibiotics are administered [4–6]. However, there remains a considerable lack of knowledge concerning cyst infection in ADPKD. The most common causative bacteria were reported to be enterobacteria [4, 5, 7]. Previously, we reported that cyst infection via urinary tract infections or cholangitis is rare and that the main cause of cyst infection is considered to be hematogenous spread via bacterial translocation in the intestine. Therefore, the intestinal flora is considered important for cyst infection, and it was found to play important roles in many kinds of diseases [8]; however, its role in cyst infection in ADPKD is unknown.
In this article, we present the analysis of the microbiome of infected cysts, feces, and saliva in two patients with ADPKD. As far as we know, this is the first report on the microbiome in patients with cyst infection in ADPKD.
Case presentation
Case 1
This patient was a 66-year-old Japanese woman with ADPKD who had been on dialysis for 14 years. She had undergone renal transcatheter arterial embolization (TAE) 7 years previously and had complete anuria. She developed left flank pain and fever and received an oral antimicrobial (cefcapene pivoxil hydrochloride hydrate, 200 mg/day). Her symptoms improved temporarily, but then they recurred, and 7 days later, she was referred to our hospital. This was her first episode of cyst infection. At admission, her body temperature was 38.0 °C and she had mild left flank pain. Her serum C-reactive protein level was 14.1 mg/dL, and her white blood cell count, 6200/µL. Her blood culture test was negative.
Imaging examinations
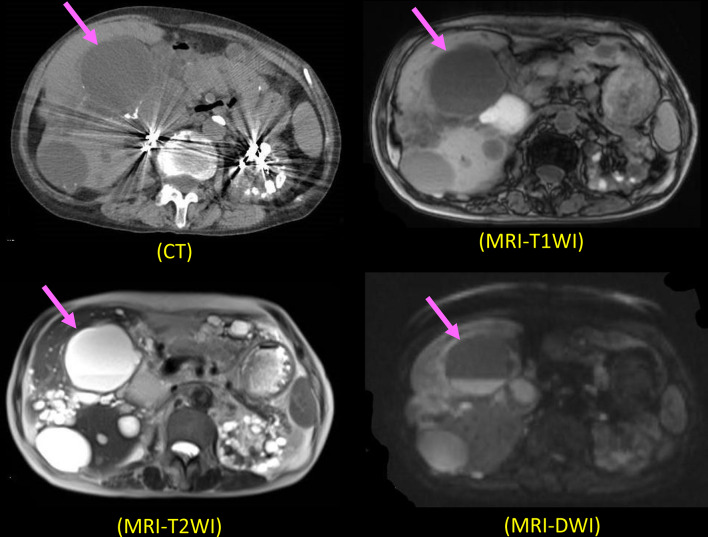
Abdominal computed tomography (CT) was performed, as reported previously [9, 10]. Magnetic resonance tomography (MRI) was also performed to obtain transverse and sagittal T1-weighted images (T1WI), T2-weighted images (T2WI), and diffusion-weighted images (DWI), as reported previously [9, 10]. We diagnosed an infected hepatic cyst according to our diagnostic criteria [9, 10] (Supplementary File 1). The cyst showed intracystic fluid–fluid level and high intensity on the MRI DWI (Fig. 1).
Fig. 1.
CT and MRI findings (T1WI, T2WI, and DWI) in Case 1. The infected hepatic cyst shows iso-density with normal cyst on CT and iso-intensity with normal cysts on T1WI and T2WI. It showed intracystic fluid–fluid level and high intensity on DWI
Hospital treatment
We administered an intravenous antimicrobial (cefmetazole sodium, 1 g/day). However, because of the continued fever and large size of the infected renal cyst (diameter: 6 cm), we decided to drain the cyst. The cyst content was dark, but the cyst content culture test result was negative. We also obtained samples of feces and saliva and compared her microbiome with that of the cyst content. These samples were both taken on the day when cyst drainage was performed. After cyst drainage, her symptoms improved gradually, and she was discharged from the hospital.
Microbial characterization
We identified 106 genera and 11 phyla of bacteria and archaea in the microbiomes of the cyst content, feces, and saliva of the patient (Additional File 2). The top 10 most abundant genera and phyla in the infected cyst content are shown in Fig. 2. In the cyst content, the prevalence of Clostridiaceae;g was the highest (15%), followed by Blautia (13%) and Enterobacterales (11.4%). However, the proportions of the 10 most abundant genera and phyla in the cyst content were not as high in feces and saliva, although Streptococcus represented 25% of the genera in saliva (Fig. 2). The V3-V4 regions of 16S ribosomal RNA gene sequence of T1 have been deposited in GenBank/DDBJ/EMBL under accession number SAMD00291257.
Fig. 2.
Top 10 most abundant genera in infected cyst content of Case 1 is presented compared with other categories
Case 2
This patient was a 56-year-old Japanese man with ADPKD who had been on dialysis for 15 years. He had undergone renal TAE 6 years previously and had complete anuria. He was transferred to our hospital from other hospital, where he had been hospitalized for 2 months because of a refractory hepatic cyst infection. At that hospital, he had received an oral antimicrobial (moxifloxacin hydrochloride, 400 mg/day) and an intravenous one (panipenem/betamipron, 0.5 g/day) for a total of 2 months. At admission to our hospital, his body temperature was 37.0 °C and he had a dull pain throughout the abdomen. His serum C-reactive protein level was 17.1 mg/dL, and his white blood cell count, 8,900/µL. He had a history of one cyst infection 15 months previously.
Imaging examinations
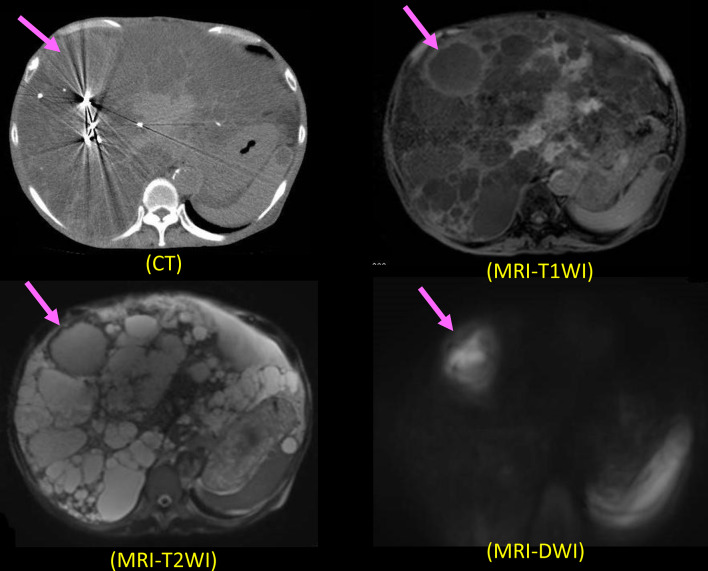
Abdominal CT and MRI were performed, as reported previously [9, 10]. We diagnosed an infected hepatic cyst according to our diagnostic criteria [9, 10] (Additional File 1). MRI (DWI) showed high intensity in the cyst, and MRI (T1-weighted image and T2-weighted image) and CT showed cyst wall thickening (Fig. 3).
Fig. 3.
CT and MRI findings (T1WI, T2WI, and DWI) in Case 2. The infected hepatic cyst shows slightly higher density than normal cyst on CT. The infected renal cyst shows a higher intensity on DWI and T1WI than normal cysts, while it has a lower intensity on T2WI. Cyst wall thickening is seen on all images of MRI
Hospital treatment
At our hospital, we initiated intravenous antimicrobial administration (cefmetazole sodium, 1 g/day), but the patient’s symptoms worsened, so we switched to intravenous administration of meropenem, 0.5 g/day. However, the symptoms still did not improve. Therefore, we drained the cyst on day 16 after transfer to our hospital. The cyst content was dark, and the cyst content culture test identified Escherichia coli. As in Case 1, we also obtained samples of feces and saliva for analysis. These samples were both taken on the day when cyst drainage was performed. After cyst drainage, his symptoms improved gradually, and he was discharged from the hospital.
Microbial characterization
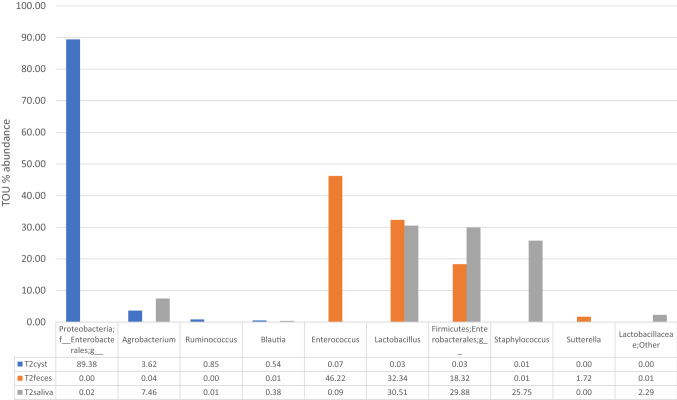
We identified 106 genera and 11 phyla of bacteria and archaea in the microbiomes of the cyst content, feces, and saliva of the patient (Additional File 3). The top 10 most abundant genera and phyla in the infected cyst content are shown in Fig. 4. In the cyst content, the prevalence of Proteobacteria f was the highest (89.4%), followed by Agrobacterium (3.6%) and Ruminococcus (0.9%). In the feces, the most common of these 10 most abundant genus or phyla were Enterococcus (46.2%), followed by Lactobacillus (32.3%), and Firmicutes (18.3%); in the saliva, the percentage of Lactobacillus was 30.5%; of Firmicutes, 29.9%; and of Staphylococcus was 25.8% (Fig. 4). The V3-V4 regions of 16S ribosomal RNA gene sequence of T2 have been deposited in GenBank/DDBJ/EMBL under accession number SAMD00291258.
Fig. 4.
Top 10 most abundant genera in infected cyst of Case 2 is presented compared to other categories
Discussion and conclusion
We present two different cases of patients with ADPKD and hepatic cyst infection. The microbiome of the infected cyst in Case 1 revealed various bacteria, even though this was the patient’s first episode of a cyst infection. In contrast, the microbiome of the infected cyst in Case 2 revealed a single bacterium, even though the infection had been present for two months.
The finding in Case 1 suggests that cyst infection can be caused by multiple bacteria simultaneously, which is consistent with our previous study on the causative bacteria found in cultures of the cyst content and blood from patients with cyst infection [5]. In that study, we reported that, in some patients, multiple bacteria were cultured in blood or cyst content, and in about half of the patients with positive results for both blood and cyst content culture tests the causative bacteria were not the same in the blood and cyst content cultures. Our current study showed that a much greater variety of bacteria can be present simultaneously in the same infected cyst than we found in our previous study. Taken together, our findings suggest that bacteria cultured in blood or cyst content represent only some of the bacteria causing the infection. In fact, blood culture test may be false negative, for example, it was reported that the sensitivity of blood culture test is approximately 70% for critically ill patients and even lower for fastidious microorganisms [11, 12]. If physicians encounter patients with a cyst infection that is resistant to antimicrobial therapy, it may be important to consider that bacteria cultured in blood or cyst content may represent only some of the causative bacteria.
In contrast, our findings in Case 2 suggest that a cyst infection can also be caused by a single type of bacterium. The cyst culture test revealed E coli, which was susceptible to cefmetazole and meropenem. However, antimicrobial therapy with cefmetazole or meropenem was ineffective in this patient. This antimicrobial resistance might have been due to poor penetration of these antibiotics into the cyst because both cefmetazole and meropenem are water-soluble antibiotics. This suggestion is supported by our previous finding that penetration of meropenem into cysts is poor [13].
Interestingly, in both cases the composition of the cyst content microbiome was quite different from that of feces or saliva. In addition, the proportion of the main genera and phyla in the cyst content was much lower than that in feces and saliva. These results might be inconsistent with our hypothesis that the bacteria that caused the cyst infection were major intestinal bacteria. However, our finding that the causative bacteria were not highly prevalent in feces and saliva indicates that bacterial mass may not be important for bacterial translocation. We suspect that it is related to the ability of each bacterial species to invade and translocate across the intestinal mucosal barrier. Indeed, the most commonly translocated bacteria are oxygen-tolerant pathobionts including Enterococcus, Enterobacteriaceae (e.g., E. coli and Klebsiella spp.), and viridans streptococci [14]. Previous studies showed that the microbiome was affected by previous exposure to antibiotics [14]. Specifically, cephalosporines and quinolones decreased the abundance of E. coli, cephalosporins and carbapenems increased the abundance of Enterococcus spp., and carbapenems and quinolones strongly decreased the abundance of anaerobic bacteria [15]. Thus, the intestinal microbiome after administration of antibiotics might differ from the intestinal microbiome before administration of antibiotics, which is a limitation of this study.
As we expected, lactobacilli were not almost detected in the infected cyst content though some lactobacilli are endemic in the gastrointestinal tract and the saliva. This result supported our hypothesis that intestinal probiotic bacteria are not causative bacteria of cyst infection [16]. Specific strains of lactobacilli may influence the host immune system by promoting production of the antibody immunoglobulin A (IgA) and probiotics have been shown to be effective for colitis [17, 18]. It is also frequently taken as a starter in fermented milk as part of the daily diet. Intestinal probiotic bacteria may have a role to play in preventing cyst infection and further studies are needed to clearly elucidate their role in preventing cyst infection.
This is the first report on the microbiome in patients with cyst infection in ADPKD, however this is a report of just two cases. The association between bacteria in cysts and those in feces and saliva remains uncertain, and further research on this topic is needed.
In conclusion, we present two cases of cyst infection in ADPKD in which the microbiome of the cyst content was quite different. Cyst infection in ADPKD can be caused by a single bacterium and by multiple bacteria. The composition of the microbiome of cyst content can be quite different from that of feces and saliva. Lactobacilli were not almost detected in the infected cyst content. More research is needed on this topic.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 Additional File 2 of Microbiome of infected cysts, feces and saliva in patients with autosomal dominant polycystic kidney disease: case reports. The analysis of the microbiome of infected cysts, feces, and saliva in the patients of Case 1. (XLSX 14 KB)
Supplementary file3 Additional File 3 of Microbiome of infected cysts, feces and saliva in patients with autosomal dominant polycystic kidney disease: case reports. The analysis of the microbiome of infected cysts, feces, and saliva in the patients of Case 2. (XLSX 14 KB)
Acknowledgements
This manuscript was checked for language content by a native English-speaking medical editor at Yamada Translation Bureau, Inc. (Tokyo, Japan).
Funding
This work was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS KAKENHI Grant number JP18K08227 and JP19K17758). This study was also supported in part by a Grant-in-Aid for Progressive Renal Disease Research from the Ministry of Health, Labour and Welfare of Japan and by Okinaka Memorial Institute for Medical Research, Toranomon Hospital.
Data availability
Our data is available by contacting the author.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest (COI).
Ethical statement
The study was approved by the Institutional review board of Toranomon Hospital.
Patient consent
Written consent to participate in this study and publish this information was obtained from the patient described in the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tatsuya Suwabe, Email: suwabetat@gmail.com.
Junichi Hoshino, Email: jhoshinoind@gmail.com.
References
- 1.Torres VE. Polycystic kidney disease autosomal-dominant and recessive forms. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 2.Gardner KD, Jr, Evan AP. Cystic kidneys: an enigma evolves. Am J Kidney Dis. 1984 doi: 10.1016/s0272-6386(84)80002-5. [DOI] [PubMed] [Google Scholar]
- 3.Schwab SJ, Bander SJ, Klahr S. Renal infection in autosomal dominant polycystic kidney disease. Am J Med. 1987 doi: 10.1016/0002-9343(87)90005-2. [DOI] [PubMed] [Google Scholar]
- 4.Sallee M, Rafat C, Zahar JR, Paulmier B, Grunfeld JP, Knebelmann B, et al. Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.01870309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suwabe T, Araoka H, Ubara Y, Kikuchi K, Hazue R, Mise K, et al. Cyst infection in autosomal dominant polycystic kidney disease: causative microorganisms and susceptibility to lipid-soluble antibiotics. Eur J Clin Microbiol Infect Dis. 2015 doi: 10.1007/s10096-015-2361-6. [DOI] [PubMed] [Google Scholar]
- 6.Suwabe T, Ubara Y, Higa Y, Nakanishi S, Sogawa Y, Nomura K, et al. Infected hepatic and renal cysts: differential impact on outcome in autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2009 doi: 10.1159/000214211. [DOI] [PubMed] [Google Scholar]
- 7.Jouret F, Lhommel R, Beguin C, Devuyst O, Pirson Y, Hassoun Z, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.06900810. [DOI] [PubMed] [Google Scholar]
- 8.Sebastian Domingo JJ, Sanchez SC. From the intestinal flora to the microbiome. Rev Esp Enferm Dig. 2018 doi: 10.17235/reed.2017.4947/2017. [DOI] [PubMed] [Google Scholar]
- 9.Suwabe T, Ubara Y, Sumida K, Hayami N, Hiramatsu R, Yamanouchi M, et al. Clinical features of cyst infection and hemorrhage in ADPKD: new diagnostic criteria. Clin Exp Nephrol. 2012 doi: 10.1007/s10157-012-0650-2. [DOI] [PubMed] [Google Scholar]
- 10.Suwabe T, Ubara Y, Ueno T, Hayami N, Hoshino J, Imafuku A, et al. Intracystic magnetic resonance imaging in patients with autosomal dominant polycystic kidney disease: features of severe cyst infection in a case-control study. BMC Nephrol. 2016 doi: 10.1186/s12882-016-0381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beutz M, Sherman G, Mayfield J, Fraser VJ, Kollef MH. Clinical utility of blood cultures drawn from central vein catheters and peripheral venipuncture in critically ill medical patients. Chest. 2003 doi: 10.1378/chest.123.3.854. [DOI] [PubMed] [Google Scholar]
- 12.Nieman AE, Savelkoul PHM, Beishuizen A, Henrich B, Lamik B, MacKenzie CR, et al. A prospective multicenter evaluation of direct molecular detection of blood stream infection from a clinical perspective. BMC Infect Dis. 2016 doi: 10.1186/s12879-016-1646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamanoue S, Suwabe T, Ubara Y, Kikuchi K, Hazue R, Mise K, et al. Cyst infection in autosomal dominant polycystic kidney disease: penetration of meropenem into infected cysts. BMC Nephrol. 2018 doi: 10.1186/s12882-018-1067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017 doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota—a systematic review. J Infect. 2019 doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Suwabe T. Cyst infection in autosomal dominant polycystic kidney disease: our experience at Toranomon Hospital and future issues. Clin Exp Nephrol. 2020 doi: 10.1007/s10157-020-01928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boirivant M, Amendola A, Butera A. Intestinal microflora and immunoregulation. Mucosal Immunol. 2008 doi: 10.1038/mi.2008.52. [DOI] [PubMed] [Google Scholar]
- 18.Kotani Y, Kunisawa J, Suzuki Y, Sato I, Saito T, Toba M, et al. Role of Lactobacillus pentosus Strain b240 and the Toll-like receptor 2 axis in Peyer's patch dendritic cell-mediated immunoglobulin A enhancement. PLoS One. 2014 doi: 10.1371/journal.pone.0091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file2 Additional File 2 of Microbiome of infected cysts, feces and saliva in patients with autosomal dominant polycystic kidney disease: case reports. The analysis of the microbiome of infected cysts, feces, and saliva in the patients of Case 1. (XLSX 14 KB)
Supplementary file3 Additional File 3 of Microbiome of infected cysts, feces and saliva in patients with autosomal dominant polycystic kidney disease: case reports. The analysis of the microbiome of infected cysts, feces, and saliva in the patients of Case 2. (XLSX 14 KB)
Data Availability Statement
Our data is available by contacting the author.