Abstract
Community-acquired pneumonia (CAP) is one of the main reasons of mortality and morbidity in elderly population, causing substantial clinical and economic impacts. However, clinically available score systems have been shown to demonstrate poor prediction of mortality for patients aged over 65. Especially, no existing clinical model can predict morbidity and mortality for CAP patients among different age stages. Here, we aimed to understand the impact of age variable on the establishment of assessment model and explored prognostic factors and new biomarkers in predicting mortality. We retrospectively analyzed elderly patients with CAP in Minhang Hospital, Fudan University. We used univariate and multiple logistic regression analyses to study the prognostic factors of mortality in each age-based subgroup. The prediction accuracy of the prognostic factors was determined by the Receiver Operating Characteristic curves and the area under the curves. Combination models were established using several logistic regressions to save the predicted probabilities. Four factors with independently prognostic significance were shared among all the groups, namely Albumin, BUN, NLR and Pulse, using univariate analysis and multiple logistic regression analysis. Then we built a model with these 4 variables (as ABNP model) to predict the in-hospital mortality in all three groups. The AUC value of the ABNP model were 0.888 (95% CI 0.854–0.917, p < 0.000), 0.912 (95% CI 0.880–0.938, p < 0.000) and 0.872 (95% CI 0.833–0.905, p < 0.000) in group 1, 2 and 3, respectively. We established a predictive model for mortality based on an age variable -specific study of elderly patients with CAP, with higher AUC value than PSI, CURB-65 and qSOFA in predicting mortality in different age groups (66–75/ 76–85/ over 85 years).
Subject terms: Health care, Risk factors
Introduction
Community-acquired pneumonia (CAP) is one of the main reasons of mortality and morbidity in elderly population, causing substantial clinical and economic impacts1,2. The elderly are inclined to pneumonia-associated death because of poor physical state, including weakened immune system, comorbidities, poor functional status, and dysphagia3–6, which makes elderly CAP even harder to cure. Thus, more attention should be paid on the introduction of accurate systems to predict the prognoses of elderly CAP patients as early as possible, and it is beneficial for early classification and further decrease the in-hospital mortality.
Clinically, there are several assessment tools widely used for predicting the hospital mortality of patients with CAP, including Confusion, Urea, Respiratory Rate, Blood Pressure, and Age ≥ 65 (CURB-65), Pneumonia Severity Index (PSI) and quick Sequential Organ Function Assessment (qSOFA)7–9. However, these score systems have been shown to demonstrate poor prediction of mortality for patients aged over 65. For example, Song et al. showed that PSI value (AUC = 0.576) was not a reliable prognostic predictor in elderly patients (aged ≥ 65 years) with CAP10. Similarly, another study reported the AUC values of CURB-65 and qSOFA were only 0.65 and 0.64, respectively, in predicting the mortality of elderly patients with a median age of 81 years (IQR 67–90)11. Consistently, Baek et al. also revealed the predictive performances of the CURB-65 and PSI were not ideal in high-aged patients (aged 80 or over with pneumonia, with AUC just being 0.61 and 0.52, respectively12. In contrast, it is reported that the AUC values of PSI and CURB-65 models in predicting mortality in young population (aged 18–64 years) were 0.87 and 0.73, respectively, significantly higher than that in elderly population (aged 65 or over)13. Therefore, the establishment of a new efficient tool to predict prognosis of elderly CAP patients is in unmet clinical need. Furthermore, in CAP patients over 65, there were still variations in the prediction of morbidity and mortality by just one available model because CAP patients of different age groups demonstrated different clinical outcomes14. However, few studies have focused on the finding of prognostic factors in predicting mortality of elderly patients with CAP accurately in different age subgroups.
In this study, we aimed to understand the impact of age on assessment model establishment and conducted an age variable-specific study of elderly patients with CAP to explore prognostic factors and new biomarkers in predicting mortality. We divided enrolled patients into three groups according to age variable: aged 66–75 years group (including 415 patients), aged 76–85 years group (394 patients enrolled), and aged over 85 years group (containing 365 patients), respectively. As a result, we found four variables with significance among all the groups of different age stage (66–75/ 76–85/ over 85 years), including Albumin, BUN, NLR and Pulse. Notably, the AUCs in predicting mortality in these three groups by the four variables were higher than PSI, CURB-65 and qSOFA.
Materials and methods
Research Objects
The study was approved by the Ethics Committee of the Minhang Hospital, Fudan University in Shanghai, China (Lot No: Medical Ethics Committee (2017) No. 42). We retrospectively enrolled patients aged over 65 years with CAP between January 1, 2018, to September 1, 2022. Informed consents were obtained from patients or their legal guardians who agreed to participate in the study. Signatures of study population were obtained, and all procedures are in accordance with the Declaration of Helsinki.
The inclusion criteria were: (1) Age > 65 years; and (2) Diagnosed with CAP based on Chinese clinical practice guideline for community-acquired pneumonia (CAP) in adults15. The exclusion criteria were: (1), Immunosuppression such as corticosteroids (> 14 days), immunosuppressed individual, eg, HIV-positive, receiving chemotherapy or radiotherapy within 90 days and transplant recipients; and (2) Patients with healthcare-associated pneumonia (HCAP).
Data collection
The following clinical data within 24 h of admission to Minhang Hospital, Fudan University were collected anonymously from electronic medical records: demographics, smoking, dysphagia, comorbidities, primary symptoms, vital signs on admission and prognosis, as well as laboratory variables (hematological data, biochemical parameters, coagulation indicators, inflammatory markers, imaging examination, etc.).
The CURB-65 (confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, systolic blood pressure < 90 mmHg or diastolic blood pressure ≤ 60 mmHg, age ≥ 65 years), qSOFA (systolic blood pressure ≤ 100 mmHg, respiratory rate ≥ 22/min, and altered cognitive state) and PSI (demographics, comorbidities, a physical examination, and laboratory and radiological findings) were measured and recorded16–18.
Statistical analysis
MedCalc software (version 20.1.0) was used for statistical analysis. Data with normal distribution were described as mean ± standard deviation and compared using Student's t-test. Data with skewed distribution were expressed as median (Inter-Quartile Range) and compared using the Mann–Whitney U nonparametric test. The classification variables were presented as percentages and compared using the Chi-squared test or Fisher's exact test. Multivariate analysis using stepwise logistic regression analysis was used to evaluate all parameters with P value < 0.05 in univariate analysis. The prediction accuracy of the prognostic factors was determined by the Receiver Operating Characteristic (ROC) curves and the area under the curves (AUC). Combination models were established using several logistic regressions to save the predicted probabilities. ROC curve analysis was performed using the saved probabilities as a new indicator. P value < 0.05 was considered statistically significant.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Minhang Hospital, Fudan University in Shanghai, and the Lot No: Medical Ethics Committee (2017) No. 42.
Ethics and informed consent statement
This study was approved by the Ethics Committee of the Minhang Hospital, Fudan University in Shanghai, and the Lot No: Medical Ethics Committee (2017) No. 42. Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Results
Clinical characteristics of patients in different age groups
After selection based on age variable and other exclusion criteria, a total of 1174 CAP patients over 65 years old (ranging from 66 to 107 years, with an average of 79 years old) were enrolled in this study (Supplementary Fig. 1). The study cohort was divided into three groups classified by age variable: group 1 (aged 66–75 years group with 415 patients, group 2 (aged 76–85 years group with 394 patients, and group 3 (aged over 85 years group containing 365 patients.
In these three age-based groups classified above, we divided each group into two populations based on clinical outcome, namely survivor group and non-survivor group. The mortality rate was 11.81% (49/415) in group 118.53% (73/394) in group 2, and 29.59% (108/365) in group 3, respectively (Fig. 1). Next, we analyzed clinically related factors and found out that different variables showed significantly different impacts between survivor group and non-survivor group across the groups classified by age intervals. Specifically, the variables in group 1 included gender, pulse, systolic pressure, leucocyte count, neutrophils count, lymphocyte count, neutrophil-to-lymphocyte ratio (NLR), c-reactive protein (CRP), procalcitonin(pct), albumin, urea nitrogen (BUN), D-dimer and cancer(p < 0.05). But in group 2, the statistically significant parameters, which were associated with the prognosis of CAP, contained age, pulse, systolic pressure, diastolic pressure, respiratory rate, leucocyte count, neutrophils count, lymphocyte count, NLR, CRP, pct, albumin, prealbumin, BUN, D-dimer and electrolyte disturbance. Regarding group 3, the variables included pulse, systolic pressure, respiratory rate, leucocyte count, lymphocyte count, NLR, CRP, pct, albumin, low-density lipoprotein, BUN, D-dimer and comorbidities (electrolyte disturbance, cancer, chronic kidney disease, congestive heart failure, coronary heart disease, hypertension) (p < 0.05) (Table 1). Collectively, our results demonstrated that factors showing prognostic values vary among different age groups of the elderly CAP patients.
Figure 1.
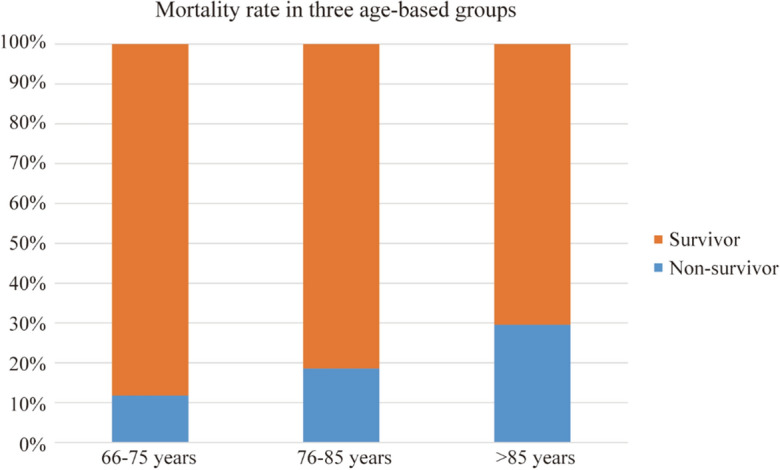
Mortality rate in three aged-based groups. The mortality rates were 11.81%, 18.53% and 29.59% in three age-based groups, respectively.
Table 1.
Basic characteristics of three age-based group patients.
| Predictive factors | Group 1 (66–75 y) (n = 415) | Group 2 (76–85 y) (n = 394) | Group 3 (> 85 y) (n = 365) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Survivor cohorts (n = 366) | Non-survivor cohorts (n = 49) | p value | Survivor cohorts (n = 321) | Non-survivor cohorts (n = 73) | p value | Survivor cohorts (n = 257) | Non-survivor cohorts (n = 108) | p value | |
| Gender (male/female) | 227/139 | 39/10 | 0.016 | 184/137 | 49/24 | 0.094 | 119/138 | 56/52 | 0.333 |
| Age (years) | 70 (68–73) | 71 (69–73) | 0.054 | 79 (77–82) | 81 (79–83) | 0.005 | 89 (86–92) | 89.5 (87–92.5) | 0.056 |
| Smoking | 90/366 | 17/49 | 0.258 | 57/321 | 13/73 | 0.993 | 26/257 | 15/108 | 0.356 |
| Pulse (/min) | 87.88 ± 17.32 | 101.76 ± 21.64 | 0.002 | 89.15 ± 13.43 | 97.12 ± 19.73 | 0.008 | 85.90 ± 16.89 | 97.47 ± 21.96 | < 0.000 |
| Systolic pressure (mmHg) | 122 (110–137) | 118 (107–133) | 0.042 | 130 (120–140) | 117 (100–140) | 0.001 | 128 (110–143) | 120 (101–140) | 0.037 |
| Diastolic pressure (mmHg) | 70 (68–80) | 75 (60–82) | 0.889 | 72 (70–80) | 69 (58–78) | 0.000 | 70 (59–79) | 70 (60–80) | 0.189 |
| Respiratory rate (/min) | 20 (19–20) | 20 (18–22) | 0.053 | 20 (19–20) | 21 (18–21) | 0.040 | 19 (18–20) | 20 (19–22) | < 0.000 |
| Dysphagia | 42/366 | 11/49 | 0.066 | 54/321 | 12/73 | 0.946 | 47/257 | 20/108 | 0.965 |
| Leucocyte count (*10^9) | 8.14 ± 4.32 | 12.51 ± 8.64 | 0.006 | 9.23 ± 3.41 | 13.97 ± 6.28 | < 0.000 | 8.79 ± 4.56 | 12.32 ± 5.38 | < 0.000 |
| Neutrophils count (*10^9) | 5.95 ± 3.95 | 10.53 ± 7.43 | 0.001 | 7.22 ± 3.41 | 12.56 ± 6.23 | < 0.000 | 9.88 ± 4.47 | 10.66 ± 5.28 | 0.395 |
| Lymphocyte count (*10^9) | 1.22 (0.84–1.67) | 0.88 (0.37–1.28) | 0.001 | 1.13 (0.82–1.52) | 0.69 (0.44–0.92) | < 0.000 | 1.16 (0.83–1.60) | 0.72 (0.51–1.20) | < 0.000 |
| Neutrophil-to-Lymphocyte Ratio (NLR) | 4.68 (2.81–8.24) | 10.69 (4.48–17.13) | < 0.000 | 5.41 (2.94–10.24) | 16.49 (11.77–25.40) | < 0.000 | 4.53 (2.61–7.86) | 13.65 (7.94–24.41) | < 0.000 |
| Platelet count (*10^9) | 223.01 ± 95.00 | 187.64 ± 93.72 | 0.018 | 221.50 ± 92.00 | 200.00 ± 95.21 | 0.148 | 202.70 ± 80.23 | 197.81 ± 98.94 | 0.778 |
| C-reactive protein CRP (ug/ml) | 61.76 ± 62.81 | 100.14 ± 80.22 | 0.022 | 62.78 ± 61.71 | 137.80 ± 102.51 | < 0.000 | 71.65 ± 62.96 | 109.56 ± 75.32 | 0.000 |
| Procalcitonin pct (ng/ml) | 0.12(0.06–0.45) | 1.35 (0.45–3.63) | < 0.000 | 0.16 (0.08–0.69) | 1.60 (0.36–7.63) | < 0.000 | 0.35 (0.10–1.55) | 0.99 (0.46–2.65) | < 0.000 |
| Albumin (g/L) | 34.49 ± 5.19 | 28.13 ± 4.89 | < 0.000 | 33.59 ± 5.38 | 27.78 ± 4.62 | < 0.000 | 32.80 ± 5.02 | 28.65 ± 4.49 | < 0.000 |
| Prealbumin (mg/L) | 130.09 ± 56.83 | 112.18 ± 49.16 | 0.235 | 128.53 ± 58.50 | 94.78 ± 45.52 | 0.003 | 110.88 ± 41.46 | 112.71 ± 59.61 | 0.852 |
| Low-density lipoprotein (mmol/L) | 2.20 ± 0.71 | 2.36 ± 1.32 | 0.520 | 2.33 ± 0.71 | 2.16 ± 0.91 | 0.348 | 2.43 ± 0.99 | 1.89 ± 0.78 | 0.002 |
| Urea nitrogen BUN (mmo/l) | 5.00 (3.80–7.10) | 9.82 (7.32–17.96) | < 0.000 | 5.70 (4.20–8.52) | 12.3 (9.68–23.33) | < 0.000 | 5.50 (3.50–8.02) | 11.80 (7.08–17.56) | < 0.000 |
| D-dimer (mg/L) | 1.23 (0.74–2.27) | 4.55 (2.32–9.57) | < 0.000 | 1.41 (0.78–2.46) | 5.26 (2.56–8.20) | < 0.000 | 1.85 (1.10–3.15) | 4.10 (1.89–8.45) | < 0.000 |
| Electrolyte disturbance | 128/366 | 11/49 | 0.201 | 60/321 | 24/73 | 0.038 | 22/257 | 35/108 | < 0.000 |
| Cancer | 30/366 | 14/49 | 0.000 | 20/321 | 5/73 | 0.855 | 8/257 | 10/108 | 0.020 |
| Chronic kidney disease | 60/366 | 7/49 | 0.747 | 19/321 | 6/73 | 0.498 | 10/257 | 15/108 | 0.001 |
| Congestive heart failure | 74/366 | 9/49 | 0.803 | 46/321 | 7/73 | 0.343 | 26/257 | 23/108 | 0.014 |
| Cerebrovascular disease | 91/366 | 14/49 | 0.669 | 80/321 | 15/73 | 0.533 | 58/257 | 30/108 | 0.410 |
| Coronary heart disease | 63/366 | 6/49 | 0.451 | 69/321 | 15/73 | 0.886 | 28/257 | 28/108 | 0.002 |
| Hypertension | 189/366 | 15/49 | 0.087 | 156/321 | 34/73 | 0.853 | 52/257 | 55/108 | < 0.000 |
| Diabetes | 89/366 | 7/49 | 0.202 | 80/321 | 18/73 | 0.971 | 40/257 | 24/108 | 0.206 |
Multiple logistic regression analysis
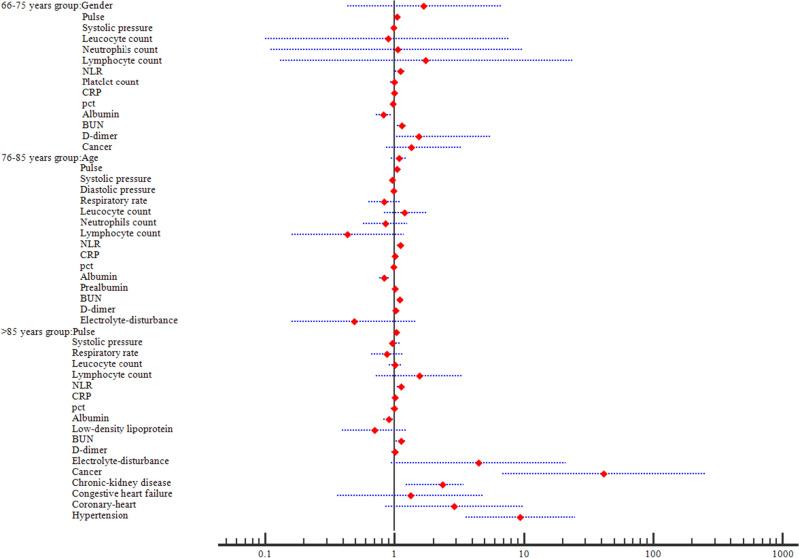
To study the prognostic factors of mortality in each age-based group, we used those significantly altered variables from univariate analysis for multiple logistic regression model analysis. In group 1, the results showed that three factors were independent risk factors, including Pulse (p = 0.041, OR 1.036, 95% CI 1.001–1.072), NLR (p = 0.026, OR = 1.112, 95% CI 1.013–1.223) and BUN (p = 0.001, OR 1.135, 95% CI 1.051–1.226) were independent risk factors, whereas Albumin (p = 0.005, OR 0.825, 95% CI 0.723–0.942) was the only independent protective factor. In group 2, five variables were demonstrated to be independently and statistically significant predictors, including Pulse (p = 0.002, OR 1.039, 95% CI 1.014–1.065), NLR (p = 0.011, OR 1.115, 95% CI 1.050–1.212), CRP (p = 0.040, OR 1.005, 95% CI 1.001–1.010), Albumin (p < 0.000, OR 0.827, 95% CI 0.759–0.901), and BUN (p = 0.000, OR 1.098, 95% CI 1.043–1.156). In group 3, eight factors were observed to independently influence the mortality, involving Pulse (p = 0.030, OR 1.027, 95% CI 1.002–1.052), NLR (p = 0.001, OR 1.125, 95% CI 1.049–1.206), Albumin (p = 0.042, OR 0.905, 95% CI 0.823–0.997), BUN (p = 0.010, OR 1.117, 95% CI 1.026–1.215), Cancer (p = 0.000, OR 41.589, 95% CI 6.802–254.273), Chronic-kidney (p = 0.032, OR 2.34, 95% CI 1.23–3.41) and Hypertension (p < 0.000, OR 9.397, 95% CI 3.539–24.954) (Table 2, Fig. 2). Moreover, the R squared value were 0.320 in Group1, 0.353 in Group 2 and 0.409 in Group 3, respectively, confirming that the data were reliable. Thus, these data demonstrated four factors with independently prognostic value were shared among all the groups, namely Albumin, BUN, NLR and Pulse.
Table 2.
Multivariate analysis for mortality in three groups.
| Variables | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Gender | 1.686 (0.427–6.657) | 0.455 | ||||
| Age | 1.077 (0.945–1.229) | 0.266 | ||||
| Smoking | ||||||
| Pulse | 1.036 (1.011–1.072) | 0.041 | 1.039 (1.014 − 1.065) | 0.002 | 1.027 (1.002–1.052) | 0.030 |
| Systolic pressure | 0.980 (0.954–1.005) | 0.117 | 0.963 (0.941 − 1.015) | 0.492 | 0.963 (0.931–1.124) | 0.056 |
| Diastolic pressure | 0.980 (0.950 − 1.011) | 0.520 | ||||
| Respiratory rate | 0.836 (0.628–1.114) | 0.221 | 0.872 (0.661–1.152) | 0.335 | ||
| Dysphagia | ||||||
| Leucocyte count | 0.885 (0.102–7.647) | 0.911 | 1.204 (0.829–1.751) | 0.330 | 1.013 (0.906–1.134) | 0.814 |
| Neutrophils count | 1.059 (0.113–9.943) | 0.960 | 0.847 (0.568–1.262) | 0.414 | ||
| Lymphocyte count | 1.744 (0.128–23.773) | 0.676 | 0.436 (0.161–1.179) | 0.102 | 1.550 (0.727–3.305) | 0.256 |
| NLR | 1.112 (1.013–1.223) | 0.026 | 1.115 (1.050–1.212) | 0.011 | 1.125 (1.049–1.206) | 0.001 |
| Platelet count | 0.998 (0.993–1.005) | 0.736 | ||||
| CRP | 0.997 (0.989–1.005) | 0.465 | 1.005 (1.001–1.010) | 0.040 | 1.005 (0.998–1.012) | 0.161 |
| pct | 0.976 (0.940–1.014) | 0.206 | 0.989(0.962–1.017) | 0.450 | 0.993 (0.949–1.039) | 0.763 |
| Albumin | 0.825 (0.723–0.942) | 0.005 | 0.827 (0.759–0.901) | < 0.000 | 0.905 (0.823–0.997) | 0.042 |
| Prealbumin | 1.005 (0.997–1.014) | 0.202 | ||||
| Low-density lipoprotein | 0.700 (0.392–1.247) | 0.226 | ||||
| BUN | 1.135 (1.051–1.226) | 0.001 | 1.098 (1.043–1.156) | 0.000 | 1.117 (1.026–1.215) | 0.010 |
| D-dimer | 1.543 (0.985–5.587) | 0.096 | 1.025 (0.973–1.080) | 0.351 | 1.007 (0.947–1.071) | 0.821 |
| Electrolyte-disturbance | 0.487 (0.161–1.472) | 0.202 | 4.465 (0.943–21.141) | 0.059 | ||
| Cancer | 1.342 (0.867–3.237) | 0.998 | 41.589 (6.802–254.273) | 0.000 | ||
| Chronic-kidney disease | 2.340 (1.230–3.410) | 0.032 | ||||
| Congestive heart failure | 1.333 (0.366–4.851) | 0.662 | ||||
| Cerebrovascular disease | ||||||
| Coronary-heart | 2.894 (0.858–9.763) | 0.087 | ||||
| Hypertension | 9.397 (3.539–24.954) | < 0.000 | ||||
| Diabetes | ||||||
Abbreviations: OR, odds ratio; CI, confidence internal; NLR, neutrophil–lymphocyte ratio; CRP, c-reactive protein; pct, procalcitonin; BUN, blood urea nitrogen.
Figure 2.
Forest plot of multivariate analysis in three age-based groups. The forest plot showed Pulse, NLR, BUN and Albumin were independent factors in group 1; five variables including Pulse, NLR, CRP, Albumin and BUN were demonstrated to be independently and statistically significant in group 2; And Pulse, NLR, Albumin, BUN, Cancer, Chronic-kidney and Hypertension were observed to independently influence the mortality in group 3.
Prediction of mortality by ROC curves
Based on above finding, we believe it is reasonable to build a model by these 4 variables (as ABNP model) to predict the in-hospital mortality across all three groups. Further analysis showed that the AUC values of the ABNP model were 0.888 (95% CI 0.854–0.917, p < 0.000), 0.912 (95% CI 0.880–0.938, p < 0.000) and 0.872 (95% CI 0.833–0.905, p < 0.000) in group 1, 2 and 3, respectively (Table 3). As a comparison, we also calculated the AUCs of CURB-65, PSI and qSOFA in all the groups (Table 3) and compared them with the value from ABNP model in predicting in-hospital mortality (Table 4, Figs. 3, 4, 5). Interestingly, the results showed that ABNP model showed superior predictive efficiency when compared to CURB-65 (AUC = 0.827, p = 0.049)/ (AUC = 0.863, p = 0.008), PSI (AUC = 0.821, p = 0.045)/ (AUC = 0.863, p = 0.040) and qSOFA (AUC = 0.766, p = 0.004)/ (AUC = 0.773, p < 0.000) in both group 1 and 2, respectively. Moreover, in group 3, even though no significant difference was observed between ABNP model and PSI (AUC = 0.860, p = 0.060), our new established model (ABNP model) still showed better performance than CURB-65 (AUC = 0.809, p = 0.009) and qSOFA (AUC = 0.728, p < 0.00). Taken together, our results supported that our model (ABNP model) owns improved predictive capacity than clinically available models (including CURB-65, PSI and qSOFA).
Table 3.
The AUC of ABNP and other assessment models in all groups.
| Group | Assessment Scores | AUC | 95%CI | p value |
|---|---|---|---|---|
| 1 | ABNP | 0.888 | 0.854–0.917 | < 0.000 |
| CURB-65 | 0.827 | 0.787–0.862 | < 0.000 | |
| PSI | 0.821 | 0.781–0.857 | < 0.000 | |
| qSOFA | 0.766 | 0.722–0.806 | < 0.000 | |
| 2 | ABNP | 0.912 | 0.880–0.938 | < 0.000 |
| CURB-65 | 0.863 | 0.825–0.895 | < 0.000 | |
| PSI | 0.863 | 0.826–0.896 | < 0.000 | |
| qSOFA | 0.773 | 0.728–0.814 | < 0.000 | |
| 3 | ABNP | 0.872 | 0.833–0.905 | < 0.000 |
| CURB-65 | 0.809 | 0.765–0.848 | < 0.000 | |
| PSI | 0.860 | 0.819–0.893 | < 0.000 | |
| qSOFA | 0.728 | 0.679–0.773 | < 0.000 |
Abbreviations: AUC, area under the curve; CI, Confidence Interval; ABNP, Albumin + BUN + NLR + Pulse model; CURB-65, confusion, urea, respiratory rate, blood pressure, and ag ≥ 65 years; PSI, Pneumonia Severity Index; qSOFA, quick Sequential Organ Function Assessment.
Table 4.
Comparison of ROC for mortality in three groups.
| Group | Comparisons | Difference between areas | 95% CI | z statistic | p value |
|---|---|---|---|---|---|
| 1 | ABNP vs. CURB-65 | 0.061 | 0.000–0.123 | 1.968 | 0.049 |
| ABNP vs.qSOFA | 0.122 | 0.039–0.205 | 2.905 | 0.004 | |
| ABNP vs.PSI | 0.067 | 0.001–0.133 | 1.991 | 0.045 | |
| 2 | ABNP vs. CURB-65 | 0.049 | 0.012–0.085 | 2.642 | 0.008 |
| ABNP vs.qSOFA | 0.139 | 0.075–0.203 | 4.254 | < 0.000 | |
| ABNP vs.PSI | 0.048 | 0.002–0.095 | 2.050 | 0.040 | |
| 3 | ABNP vs. CURB-65 | 0.062 | 0.015–0.110 | 2.607 | 0.009 |
| ABNP vs.qSOFA | 0.144 | 0.086–0.202 | 4.905 | < 0.000 | |
| ABNP vs.PSI | 0.013 | 0.036–0.062 | 0.516 | 0.060 |
Abbreviations: ROC, Receiver Operating characteristic; CI, Confidence Interval; vs, versus; ABNP, Albumin + BUN + NLR + Pulse model; CURB-65, confusion, urea, respiratory rate, blood pressure, and ag ≥ 65 years; PSI, Pneumonia Severity Index; qSOFA, quick Sequential Organ Function Assessment.
Figure 3.
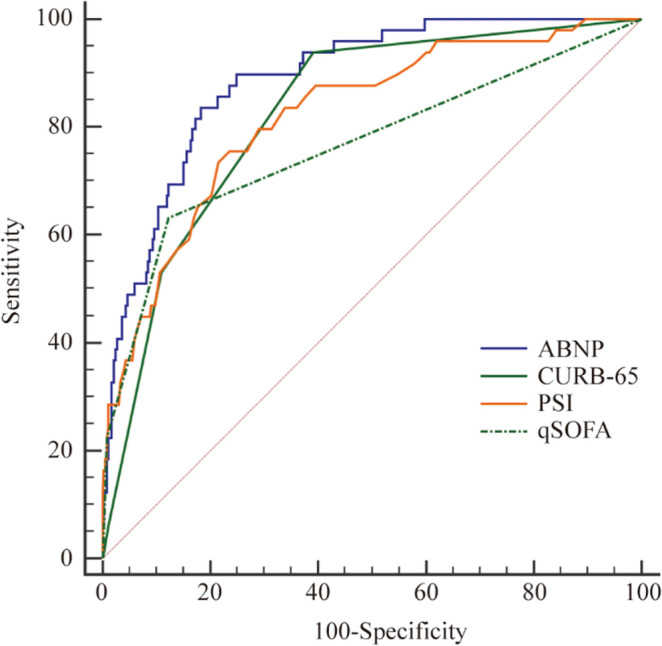
The Receiver Operating characteristic (ROC) curves of the four assessment scores for the mortality in group 1. The AUC of ABNP,CURB-65,PSI and qSOFA were 0.888, 0.827, 0.821 and 0.766, respectively.
Figure 4.
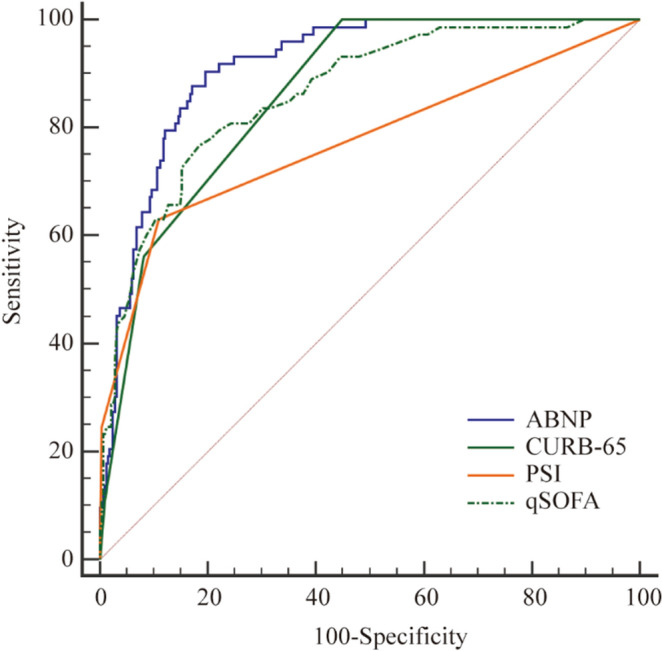
The Receiver Operating characteristic (ROC) curves of the four assessment scores for the mortality in group 2. The AUC of ABNP, CURB-65, PSI and qSOFA were 0.912, 0.863, 0.863 and 0.773, respectively.
Figure 5.
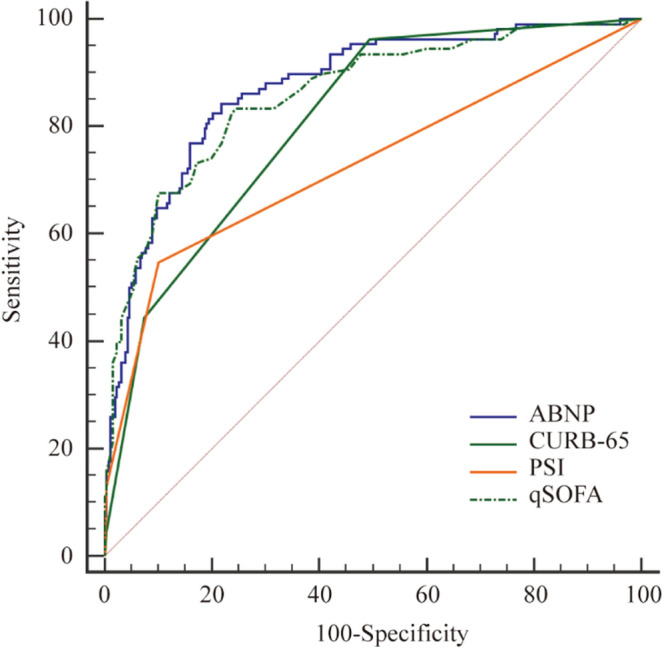
The Receiver Operating characteristic (ROC) curves of the four assessment scores for the mortality in group 3. The AUC of ABNP, CURB-65, PSI and qSOFA were 0.872, 0.809, 0.860 and 0.728, respectively.
Discussion
Currently, there are few studies reporting the prognostic factors and assessment scores in different age-based subpopulations with CAP. This study, to our best knowledge, is the first study to understand adjusted parameters for prognosis across different aged groups in elderly CAP patients. Here, we divided elderly CAP patients into three groups classified by age and established a new model (ABNP model) based on parameters derived from prognostic values shared by all the age-dependent subgroups.
Consistent with previous studies, the mortality rates were 11.81%,18.53% and 29.59% in three groups, respectively, which showed the more pronounced mortality rate with increased age19,20. The highest mortality in CAP aged over 85 years group can be explained by the death-associated comorbidities. As shown in Table 1, we observed that comorbidities showed significantly difference between survivors and non-survivors in this group, including electrolyte disturbance, cancer, chronic kidney disease, congestive heart failure, coronary heart disease and hypertension. Moreover, several factors, like cancer, chronic kidney disease and hypertension, were still independent prognostic factors after the multiple logistic analysis. However, no comorbidities were significantly association with mortality in younger cohorts. This observation is accordant with previous studies of ours and others. For instance, we previously revealed that comorbidities were independent risk factors influencing in-hospital mortality in patients over 80 years old with CAP21. As well, Ghia et al. found comorbid conditions like chronic obstructive pulmonary disease, hypertension were common risk factors for CAP in the Indian population22. On this basis, we propose that more attention should be paid to the care of comorbidities in elderly patients with CAP, especially aged over 85.
Another interesting finding is that there are four variables (albumin, BUN, NLR and pulse) independently influencing prognosis in all three age-based subgroups. These variables have been studied and used in clinic. BUN and Pulse have been applied to some assessment scores, such as CURB-65 and PSI, and demonstrated to predict the prognosis of patients with CAP23–26. Additionally, although NLR and Albumin do not belong to common assessment score systems of CAP severity such as CURB-65 and PSI, we also found out NLR and Albumin can improve the predictive ability for mortality of elderly CAP, even in age-based elderly subgroups.
NLR, short for the ratio of absolute neutrophil count to absolute lymphocyte count, has also been identified to predict adverse outcome of patients with CAP27–30. Specifically, Cataudella et al.27 found NLR predicted 30-day mortality and performed better than PSI and CURB-65 score systems. Thirty-day mortality was 30% in those with a NLR between 11.12 and 13.4%, but 50% in those with a NLR between 13.4 and 28.3. Moreover, Feng et al.31 also discovered NLR was the independent factor influencing in-hospital mortality in elderly patients with CAP and showed higher AUC value than CURB-65 (0.72 vs. 0.678, p < 0.05). Therefore, a growing number of studies emphasize the importance of NLR to improve the ability of predicting adverse outcome in CAP patients when combined with other factors. A nomogram model composed by NLR was established by Lv et al.32 to predict mortality in elderly patients with CAP, and the AUC of the model was 0.9, which was proved to be superior to CURB-65 and PSI. Collectively, NLR is a simple, easily measured, yet promising marker for predicting outcomes in patients with CAP. Its value, either alone or in conjunction with other biomarkers, need to be further investigated.
The fourth variable worth attention is the serum albumin. Typically, albumin is well-known for its important roles in immune regulation and antimicrobial33,34. Accumulating evidence show that albumin is related to the prognosis of CAP patients. In one study, Sakakibara et al.35 established a new score model including albumin to predict severe adverse events (including death) in CAP patients, which exhibits a higher AUC value (0.85) compared with the other predictive models. Furthermore, another study by Shirata et al.25 developed another albumin-based system (using cutoff as 3.0 g/dL) to predict mortality in older patients with CAP, showing a higher AUC (0.809) than that of CURB-65. In addition, albumin decreased with aging for several potential reasons such as decline in cognition, poor oral health, and dysphagia36,37. Thus, it is necessary to increase the level of albumin in elderly patients with CAP, which may improve the prognosis. Several methods can be utilized, such as direct infusion of human albumin. Also, the nasogastric feeding was another preferable option to improve the albumin in elderly patients, especially in patients with decline in cognition after stroke, dysphagia and so on38. Finally, cumulative studies also support that the nasogastric feeding tube is efficient to deliver nutrients and/or fluids to the gastrointestinal tract effectively and play a central role in the management of elderly who were malnourished or hypoalbuminemia39–42.
Notably, the new established model (ABNP model) shows superiority over clinically used tools (CURB-65, PSI and qSOFA) regarding the prediction of mortality. Notably, the AUC of ABNP model was still higher than PSI score even though there is no significant difference between them in the subgroup aged over 85 years. This could possibly be explained by the contribution of comorbidities. Multiple logistic analysis showed that comorbidities (cancer, chronic-kidney disease and hypertension) were also independent variables influencing mortality in aged over 85 years patients, besides ABNP-associated factors (albumin, BUN, NLR and pulse). Taken as a whole, we arrived a conclusion that ABNP model was an improved scoring system for prognosis prediction in elder CAP patients.
There are some limitations. Firstly, this study is a single-center study, which leads to a limited number of samples and may even cause bias in sample collection. Thus, the results of this study should be verified in multi-center, large-sample studies in the future. Secondly, our study is a retrospective observational study. This may cause several issues, including the possible poor quality of available data due to undesigned study, the possible absence of important data on potential confounding factors and differential losses to follow up on study cohort. Therefore, prospective studies, if applicable, are essential to increase the reliability. Thirdly, other clinical factors are not taken into consideration, such as antibiotic therapy and pathogen infection, and some data are missing for individual patients, such as D-dimer and prealbumin. Fourthly, some data analyzed in this study might not show authentication. For instance, odds ratio values of hypertension and cancer in our analysis were inflated. This might be due to a limited number of samples with hypertension (or cancer) in survivor or non-survivor group. This situation may be related to the special conditions in a data set and this is known as “monotone likelihood”43. Thus, we will collect more patient data and apply reconstruction of the interval estimation based on profile penalized log likelihood (PPL) to solve this concern44. Finally, we did not take functional decline or frailty into account, which could influence the prognosis of patients with CAP in elderly patients45–47. Thus, more studies with large population need to be designed in the future.
Conclusions
We established an early prediction model based on an age-group-specific study of elderly patients with CAP. The new model of the AUCs in predicting mortality in different age groups (66–75/ 76–85/ over 85 years) were higher than PSI, CURB-65 and qSOFA.
Supplementary Information
Author contributions
C.X.L. and T.P. finished the manuscript; W.X.P., Y.G., Y.M. and A.M. did the statistical analysis; W.S. and J.Y.X. summarized the data; J.H.D. and W.W. designed and supervised this study. All authors reviewed and edited the manuscript.
Funding
This research received no external funding.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: In the original version of this Article, Weixiong Peng, Yue Gao and Yang Mu were incorrectly affiliated with ‘Clinical Research Center (CRC), Medical Pathology Center (MPC), Cancer Early Detection and Treatment Center (CEDTC), Translational Medicine Research Center (TMRC), Chongqing University Three Gorges Hospital, Chongqing University, Wanzhou, Chongqing, China’. The correct affiliation is: ‘Hunan Zixing Artificial Intelligence Technology Group Co., Ltd., Hunan Province, Changsha City, China.’
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chunxin Lv and Teng Pan.
Change history
8/23/2023
A Correction to this paper has been published: 10.1038/s41598-023-40951-7
Contributor Information
Jinhai Deng, Email: jinhai.deng@kcl.ac.uk.
Wei Wei, Email: wwei0104@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-39542-3.
References
- 1.Vandana KE, Chiranjay M, Jordi R. Community-acquired bacterial pneumonia in adults: An update. Indian J. Med. Res. 2020;151:287–302. doi: 10.4103/ijmr.IJMR_1678_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Divino V, Schranz J, Early M, Shah H, Jiang M, DeKoven M. The annual economic burden among patients hospitalized for community-acquired pneumonia (CAP): A retrospective US cohort study. Curr. Med. Res. Opin. 2019;36:151–160. doi: 10.1080/03007995.2019.1675149. [DOI] [PubMed] [Google Scholar]
- 3.Yeo HJ, Byun KS, Han J, Kim JH, Lee SE, Yoon SH, et al. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: A propensity score matched analysis. Korean J. Intern. Med. 2019;34:841–849. doi: 10.3904/kjim.2018.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma R, Sandrock CE, Meehan J, Theriault N. Community-acquired bacterial pneumonia-changing epidemiology, resistance patterns, and newer antibiotics: Spotlight on delafloxacin. Clin. Drug Investig. 2020;40:947–960. doi: 10.1007/s40261-020-00953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osman M, Manosuthi W, Kaewkungwal J, Silachamroon U, Mansanguan C, Kamolratanakul S, et al. Etiology, clinical course, and outcomes of pneumonia in the elderly: A retrospective and prospective cohort study in Thailand. Am. J. Trop. Med. Hyg. 2021;104:2009–2016. doi: 10.4269/ajtmh.20-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theilacker C, Sprenger R, Leverkus F, Walker J, Häckl D, von Eiff C, et al. Population-based incidence and mortality of community-acquired pneumonia in Germany. PLoS ONE. 2021;16:e0253118. doi: 10.1371/journal.pone.0253118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ning P, Zheng Y, Luo Q, Liu X, Kang Y, Zhang Y, et al. Metabolic profiles in community-acquired pneumonia: Developing assessment tools for disease severity. Crit. Care. 2018;22:130. doi: 10.1186/s13054-018-2049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019;200:e45–67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asai N, Watanabe H, Shiota A, Kato H, Sakanashi D, Hagihara M, et al. Efficacy and accuracy of qSOFA and SOFA scores as prognostic tools for community acquired and healthcare-associated pneumonia. Int. J. Infect. Dis. 2019;84:89–96. doi: 10.1016/j.ijid.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Wang X, Lang K, Wei T, Luo J, Song Y, Yang D. Development and validation of a nomogram for predicting 28-day mortality on admission in elderly patients with severe community-acquired pneumonia. J. Inflamm. Res. 2022;15:4149–4158. doi: 10.2147/JIR.S369319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmo TA, Ferreira IB, Menezes RC, Telles GP, Otero ML, Arriaga MB, et al. Derivation and validation of a novel severity scoring system for pneumonia at intensive care unit admission. Clin. Infect. Dis. 2021;72:942–949. doi: 10.1093/cid/ciaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baek MS, Park S, Choi JH, Kim CH, Hyun IG. Mortality and prognostic prediction in very elderly patients with severe pneumonia. J. Intensive Care Med. 2020;35(12):1405–1410. doi: 10.1177/0885066619826045. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZX, Yong Y, Tan WC, Shen L, Ng HS, Fong KY. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singapore Med. J. 2018;59(4):190–198. doi: 10.11622/smedj.2017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duarte FG, Barberino MG, da Silva MS, Reis JN, Spinardi JR, de Almeida RS, et al. Incidence, aetiology and serotype coverage for pneumococcal vaccines of community-acquired pneumonia in adults: A population-based prospective active surveillance study in Brazil. BMJ Open. 2022;12(4):e059824. doi: 10.1136/bmjopen-2021-059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Huang Y, She DY, Cheng QJ, Fan H, Tian XL, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society. Chin. Med. Assoc.. Clin. Respir. J. 2018;12(4):1320–1360. doi: 10.1111/crj.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Han Y, Yu C, Guo Y, Pei P, Yang L, et al. The hospitalization burden of all-cause pneumonia in China: A population-based study, 2009–2017. Lancet Reg. Health West Pac. 2022;22:100443. doi: 10.1016/j.lanwpc.2022.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavallazzi R, Furmanek S, Arnold FW, Beavin LA, Wunderink RG, Niederman MS, et al. The burden of community-acquired pneumonia requiring admission to ICU in the United States. Chest. 2020;158(3):1008–1016. doi: 10.1016/j.chest.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv C, Shi W, Pan T, Li H, Peng W, Xu J, et al. Exploration of aging-care parameters to predict mortality of patients aged 80-years and above with community-acquired pneumonia. Clin. Interv. Aging. 2022;17:1379–1391. doi: 10.2147/CIA.S382347.PMID:36164658;PMCID:PMC9509012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghia CJ, Rambhad GS. Systematic review and meta-analysis of comorbidities and associated risk factors in Indian patients of community-acquired pneumonia. SAGE Open Med. 2022;10:20503121221095485. doi: 10.1177/20503121221095485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilg A, Moskowitz A, Konanki V, Patel PV, Chase M, Grossestreuer AV, et al. Performance of the CURB-65 score in predicting critical care interventions in patients admitted with community-acquired pneumonia. Ann. Emerg. Med. 2019;74(1):60–68. doi: 10.1016/j.annemergmed.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirata M, Ito I, Ishida T, Tachibana H, Tanabe N, Konishi S, et al. Development and validation of a new scoring system for prognostic prediction of community-acquired pneumonia in older adults. Sci. Rep. 2021;11(1):23878. doi: 10.1038/s41598-021-03440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma CM, Wang N, Su QW, Yan Y, Wang SQ, Ma CH, et al. Age, Pulse, urea, and albumin score: A tool for predicting the short-term and long-term outcomes of community-acquired pneumonia patients with diabetes. Front Endocrinol (Lausanne). 2022;13:882977. doi: 10.3389/fendo.2022.882977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cataudella E, Giraffa CM, Di Marca S, Pulvirenti A, Alaimo S, Pisano M, et al. Neutrophil-to-lymphocyte ratio: An emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J. Am. Geriatr. Soc. 2017;65(8):1796–1801. doi: 10.1111/jgs.14894. [DOI] [PubMed] [Google Scholar]
- 28.Postma D, Schuurman J, van Werkhoven C, Oosterheert JJ, van Elden L, Bonten M. Prognostic value of the neutrophil/lymphocyte ratio in patients hospitalized with community-acquired pneumonia. Contemp. Med. 2016;11(4):478–482. [Google Scholar]
- 29.Kaya Y. Relationship between neutrophil to lymphocyte ratio with presence and severity of pneumonia. J. Clin. Anal. Med. 2018;9(5):452–457. [Google Scholar]
- 30.Yang T, Wan C, Hao W, Qin J, Wen F. The prognostic and risk-stratified value of neutrophil–lymphocyte count ratio in Chinese patients with community-acquired pneumonia. Eur. J. Inflamm. 2017;15(1):22–27. doi: 10.1177/1721727X17702150. [DOI] [Google Scholar]
- 31.Feng DY, Zou XL, Zhou YQ, Wu WB, Yang HL, Zhang TT. Combined neutrophil-to-lymphocyte ratio and CURB-65 score as an accurate predictor of mortality for community-acquired pneumonia in the elderly. Int. J. Gen. Med. 2021;14:1133–1139. doi: 10.2147/IJGM.S300776.PMID:33833552;PMCID:PMC8020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv C, Li M, Shi W, Pan T, Muhith A, Peng W, et al. Exploration of prognostic factors for prediction of mortality in elderly CAP population using a nomogram model. Front Med (Lausanne). 2022;9:976148. doi: 10.3389/fmed.2022.976148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrer R, Mateu X, Maseda E, Yebenes JC, Aldecoa C, De HC, et al. Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev. Clin. Pharmacol. 2018;11:125–137. doi: 10.1080/17512433.2018.1412827. [DOI] [PubMed] [Google Scholar]
- 34.Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int. J. Mol. Sci. 2021;22:4496. doi: 10.3390/ijms22094496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakakibara T, Shindo Y, Kobayashi D, Sano M, Okumura J, Murakami Y, et al. A prediction rule for severe adverse events in all inpatients with community-acquired pneumonia: A multicenter observational study. BMC Pulm. Med. 2022;22(1):34. doi: 10.1186/s12890-022-01819-0.PMID:35022026;PMCID:PMC8753951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koponen S, Nykänen I, Savela RM, Välimäki T, Suominen AL, Schwab U. Inadequate intake of energy and nutrients is common in older family caregivers. Nutrients. 2021;13(8):2763. doi: 10.3390/nu13082763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaving G, Batstone GF, Jones RG. Age and sex variation in serum albumin concentration: An observational study. Ann. Clin. Biochem. 2016;53:106–111. doi: 10.1177/0004563215593561. [DOI] [PubMed] [Google Scholar]
- 38.Chauhan D, Varma S, Dani M, Fertleman MB, Koizia LJ. Nasogastric tube feeding in older patients: A review of current practice and challenges faced. Curr. Gerontol. Geriatr. Res. 2021;2021:6650675. doi: 10.1155/2021/6650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bischoff SC, Austin P, Boeykens K, Chourdakis M, Cuerda C, Jonkers-Schuitema C, et al. ESPEN guideline on home enteral nutrition. Clin Nutr. 2020;39(1):5–22. doi: 10.1016/j.clnu.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Gao X, Zhang Y, Zhang L, Liu S, Liu H, Zhou D, et al. Effect of home enteral nutrition on nutritional status, body composition and quality of life in patients with malnourished intestinal failure. Front Nutr. 2021;8:643907. doi: 10.3389/fnut.2021.64390738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martindale R, Patel JJ, Taylor B, Arabi YM, Warren M, McClave SA. Nutrition therapy in critically Ill patients with coronavirus disease 2019. JPEN J. Parenter Enteral. Nutr. 2020;44(7):1174–1184. doi: 10.1002/jpen.193039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu A, Xie Y, Zhong M, Wang F, Huang H, Nie L, et al. Comparison of the initiation time of enteral nutrition for critically ill patients: at admission vs. 24 to 48 h after admission. Emerg. Med. Int. 2021;2021:3047732. doi: 10.1155/2021/3047732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57(1):114–119. doi: 10.1111/j.0006-341X.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 44.Tzeng IS. To handle the inflation of odds ratios in a retrospective study with a profile penalized log-likelihood approach. J. Clin. Lab Anal. 2021;35(7):e23849. doi: 10.1002/jcla.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen T, Jia L, ChunYan J, Zhen Z, Ying S, Wei LH. Correlation of frailty status and short-term prognosis of community-acquired pneumonia in very old patients. Chin. J. Multi. Organ Dis. Elderly. 2020;19(9):646–650. [Google Scholar]
- 46.Kang Y, Fang X-Y, Wang D, Wang X-J. Activity of daily living upon admission is an independent predictor of in-hospital mortality in older patients with community-acquired pneumonia. BMC Infec. Dis. 2021;21:314. doi: 10.1186/s12879-021-06006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Hara Y, Horita N, Saigusa Y, Hirai Y, Kaneko T. Declined functional status prolonged hospital stay for community-acquired pneumonia in seniors. Clin. Interv. Aging. 2020;15:1513–1519. doi: 10.2147/CIA.S267349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].