Abstract
Objective
Medium chain fatty acids (MCFAs), which are fatty acids with chain lengths of 8–12 carbon atoms, have been shown to reduce food intake in rodents and humans, but the underlying mechanisms are unknown. Unlike most other fatty acids, MCFAs are absorbed from the intestine into the portal vein and enter first the liver. We thus hypothesized that MCFAs trigger the release of hepatic factors that reduce appetite.
Methods
The liver transcriptome in mice that were orally administered MCFAs as C8:0 triacylglycerol (TG) was analyzed. Circulating growth/differentiation factor 15 (GDF15), tissue Gdf15 mRNA and food intake were investigated after acute oral gavage of MCFAs as C8:0 or C10:0 TG in mice. Effects of acute and subchronic administration of MCFAs as C8:0 TG on food intake and body weight were determined in mice lacking either the receptor for GDF15, GDNF Family Receptor Alpha Like (GFRAL), or GDF15.
Results
Hepatic and small intestinal expression of Gdf15 and circulating GDF15 increased after ingestion of MCFAs, while intake of typical dietary long-chain fatty acids (LCFAs) had no effect. Plasma GDF15 levels also increased in the portal vein with MCFA intake, indicating that in addition to the liver, the small intestine contributes to the rise in circulating GDF15. Acute oral provision of MCFAs decreased food intake over 24 h compared with a LCFA-containing bolus, and this anorectic effect required the GDF15 receptor, GFRAL. Moreover, subchronic oral administration of MCFAs reduced body weight over 7 days, an effect that was blunted in mice lacking either GDF15 or GFRAL.
Conclusions
We have identified ingestion of MCFAs as a novel nutritional approach that increases circulating GDF15 in mice and have revealed that the GDF15-GFRAL axis is required for the full anorectic effect of MCFAs.
Keywords: Medium-chain fatty acids, Growth/differentiation factor 15, Satiety, Lipid metabolism, Food intake, Hepatokine
Graphical abstract
Highlights
-
•
Intake of medium-chain fatty acids (MCFAs) induces Gdf15 in liver and small intestine.
-
•
MCFA intake increases plasma GDF15 levels in systemic circulation and portal vein.
-
•
The GDF15-GFRAL axis is required for the full anorectic effect of MCFAs.
1. Introduction
The majority of fat in the diet and in the human body comprise triacylglycerols (TGs) containing long-chain fatty acids (LCFAs) with a chain length of more than 13 carbon atoms. In contrast, medium-chain fatty acids (MCFAs), defined by a chain length of 8–12 carbon atoms, are far less abundant. Coconut and palm kernel oils are rich food sources of MCFAs. Due to their shorter carbon tail, MCFAs have a higher ionization at physiological pH, and hence are more soluble in aqueous biological fluids than LCFAs [1]. These properties affect their digestion, absorption, and cellular metabolism. In the 1950s, it was first described that MCFAs are absorbed into the portal vein after digestion, which is in contrast to lymphatic uptake of LCFAs from the small intestine [2]. This observation initially rendered MCFAs a clinical nutrition strategy in patients with fat malabsorption [3]. Subsequent work in rodents somewhat serendipitously noted that diets rich in TG with MCFAs (MCTs) resulted in lower body weight and reduced fat deposition compared with diets with LCFA-containing TGs (LCTs) [4,5]. Since then, it has been shown that MCT-rich diets improve insulin-stimulated glucose metabolism [6,7] and satiety [8] in humans. Regarding its effects on satiety, it has been shown that rats eat less of a MCT-containing high-fat diet (HFD) compared with rats consuming an iso-caloric LCT-HFD [9]. Humans also eat ∼10% less of a HFD over 14 days, when the MCT content of the diet is 40E% compared with 20E% or 31E% [10]. Following acute intake of 10–30g of MCT oil or coconut oil, which is naturally rich in MCFAs, healthy young men and women reported an increased feeling of satiety compared with ingestion of LCT [11,12]. Moreover, energy intake was reduced by 14–40% in lean as well as individuals with obesity during a subsequent ad libitum meal or consecutive meals after consumption of 11–39g of MCT oil compared with ingestion of similar amount of LCTs [11,[13], [14], [15], [16], [17], [18], [19]]. A recent meta-analysis thus supports the notion that MCTs decrease energy intake [8], however, the underlying mechanisms are unknown.
Recently, it was proposed that MCFAs act on hypothalamic proopiomelanocortin (POMC) neurons to signal a state of satiety [20]. This conflicts with the physiology of how dietary MCFAs are absorbed from the gut and processed in the body. Non-esterified MCFAs are absorbed into the portal vein and enter the liver where they are predominantly metabolized, resulting in elevated circulating ketone bodies [21,22]. In agreement, the presence of MCFAs in the systemic circulation is low to undetectable in humans [23,24]. Conversely, in patients with a portacaval shunt, which connects the portal vein to vena cava thereby bypassing the liver, circulating MCFA levels after infusion of MCFAs in the upper small bowel were increased ∼10-fold more compared with control individuals without such a shunt [25]. This highlights that dietary MCFAs are predominantly taken up and metabolized during the first-pass in the liver. Moreover, an important role for the liver in the satiety-inducing effects of MCFAs is supported by an elegant study in rats, in which identical amounts of MCFAs were infused either into the portal vein or into the vena cava of fasted rats [26]. While portal vein infusion reduced food intake during the first meal by 40%, MCFA infusion into the vena cava had no effect [26]. Collectively, these data led to the present hypothesis that metabolism of MCFAs in the liver generates specific signals that inhibit appetite. To test this hypothesis, we performed transcriptome analysis of livers from mice gavaged with an oil rich in MCTs and identified the emerging putative satiety factor, growth/differentiation factor 15 (GDF15) [27,28], as a potential mediator of the observed appetite-lowering effect.
2. Materials and methods
2.1. Animals
The mouse studies were conducted at University of Copenhagen, Denmark and at German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany, respectively. C57BL/6j male mice were obtained from commercial breeders (Janvier, FR). The GFRAL knockout (KO) and WT littermates were generated as previously reported [29,30]. All experiments were performed at 22 °C with a 12:12 h light–dark cycle. Mice had ad libitum access to water and chow diet (Altromin 1324, Brogaarden, Denmark), or when indicated a high-fat diet (HFD) (D12492, Research Diets, US) or a high-fat, high sucrose (HFHS) diet (D12331, Research Diets, US). These experiments were approved by the Danish Animal Experimentation Inspectorate and complied with the European convention for protection of vertebrate animals used for scientific purposes. The GDF15 KO mice and WT littermates were generated as described previously [31]. After weaning, mice were housed in groups of two GDF15 KO or WT mice per cage, respectively. Mice were housed at 22 °C with a 12:12 h light–dark cycle and ad libitum access to water and chow diet (ssniff V1534-300, ssniff Spezialdiäten GmbH, Soest, Germany) or HFHS diet (E15772-34, ssniff Spezialdiäten GmbH, Soest, Germany), respectively. These experiments were approved by the LAVG Brandenburg, Germany, and complied with the European convention for protection of vertebrate animals used for scientific purposes.
2.2. Protocols
2.2.1. Acute oral gavage study for whole genome transcriptome analysis
Single-housed, chow-fed, two-hours fasted male C57Bl/6j mice (12 weeks of age) were randomized by body weight (BW) into either the control group or for MCT administration (Figure 1A–D). Mice were either orally gavaged with water or C8:0 MCT oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark) (10 μl/g BW). After 60 min, the mice were euthanized and their liver was harvested, snap-frozen in liquid nitrogen and stored at −80 °C until later whole genome transcriptome analysis.
Figure 1.
Oral administration of medium-chain triacylglycerol increases circulating GDF15. a-d, Whole-genome sequencing of livers collected one hour after oral administration with a C8:0 medium-chain triacylglycerol (MCT) oil or water (a) with a volcano plot (b) depicting detected transcripts over the threshold of 1 FPKM comparing MCT oil with water, a heatmap (c) of the 10 most up- or downregulated genes, and genes encoding for secreted factors (d). e,f, plasma growth/differentiation factor 15 (GDF15) and blood β-hydroxybutyrate concentrations at indicated time points in chow-fed male C57Bl/6j mice that were orally gavaged at 0 h with 200 μl of C8:0 MCT oil or a long-chain triacylglycerol (LCT) oil (corn oil) (n = 13–14). g-j, plasma GDF15 (n = 12), blood β-hydroxybutyrate concentrations, cumulative food intake (n = 12–13), and 24 h delta body weight at indicated time points in high-fat, high-sucrose (HFHS)-fed diet-induced obese (DIO) male C57Bl/6j mice that were orally gavaged at 0 h with 250 μl of C8:0 MCT oil or LCT oil (corn oil). k, Gdf15 mRNA abundance in indicated tissues at indicated time points (in minutes) in chow-fed male C57Bl/6j mice that were orally gavaged at 0 min with C8:0 MCT oil. Data were analyzed by a two-way RM ANOVA (e–i) with Šidák post hoc tests, when ANOVA revealed significant interactions; an unpaired, two-tailed student's t-test (j); and with one-way ANOVA (k,l) within each tissue (k) or between groups (l) with Šidák post hoc tests, when ANOVA revealed a significant difference among groups (i, j). ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001 different from LCT oil (or from 0 min (k)). ##p < 0.01 different from water.
2.2.2. Acute oral gavage study for GDF15 time course in chow-fed mice
Single-housed, chow-fed, four-hours fasted male C57Bl/6j mice (12–16 weeks of age) were orally gavaged with 250 μl of 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden) and corn oil (LCT oil; Coop, Denmark) (Figure 1E–F). Blood was sampled from the tail-vein before and one, two, four, and 24 h after administration, and β-hydroxybutyrate was measured using a handheld ketone meter with test strips (FreeStyle Precision β-ketone; Abbott Laboratories, CH, IL, US) on whole blood. The mice were allowed access to chow food after four hours.
2.2.3. Acute oral gavage study for GDF15 time course in diet-induced obese (DIO) mice
Single-housed, four-hours fasted male HFHS-fed DIO C57Bl/6j mice (25–30 weeks of age) were orally gavaged with 250 μl of 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden) or LCT oil (corn oil; Coop, Denmark) (Figure 1G–H). Blood was sampled from the tail-vein before and one and four hours after administration, and β-hydroxybutyrate was measured using a handheld ketone meter with test strips (FreeStyle Precision β-ketone; Abbott Laboratories, CH, IL, US) on whole blood samples before and one hour after administration.
2.2.4. Acute oral gavage food intake study in DIO mice
In a subsequent study, four-hours fasted single-housed male HFHS-fed DIO C57Bl/6j mice (30–35 weeks of age) were orally gavaged with 250 μl of 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden) or corn oil (LCT oil; Coop, Denmark) one hour prior to the dark phase (Figure 1I–J). Body weight was measured prior to and 24 h after oral administration of the oils. Throughout the experiment, mice were allowed ad libitum access to HFD that was weighted before and four, six, 16, and 24 h after oil administration.
2.2.5. Acute oral gavage study for Gdf15 tissue mRNA levels
Single-housed, two-hours fasted chow-fed male C57Bl/6j mice (12 weeks of age) were randomized by BW into different time point groups for tissue collection (0, 60, 120 min) (Figure 1K). All mice, except those euthanized at time point 0, were orally administered with C8:0 MCT oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark) (10 μl/g BW). Mice were decapitated and liver, kidney, m. gastrocnemius (SkM), duodenum, jejunum, ileum, colon, inguinal white adipose tissue (iWAT), and interscapular brown adipose tissue (BAT) were harvested, snap-frozen in liquid nitrogen and stored at −80 °C until Gdf15 mRNA analyses.
2.2.6. Acute oral gavage study for GDF15 measurement in portal vein
Double-housed, two-hours fasted chow-fed male C57Bl/6j mice (12 weeks of age) were randomized into three groups and orally gavaged with C8:0 MCT oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark), LCT in the form of olive oil (#8001-25-0, Sigma, Denmark) or water (all 5 μl/g BW) (Figure 1L). One hour later, mice were transferred to a closed chamber with isoflurane pumped into the chamber for sedation. The mice were kept sedated through a tube connected to 3% isoflurane. When reflexes were absent, an incision to the abdominal cavity was performed. The portal vein was localized and stabilized by a tweezer. A 25g needle was inserted gently into the portal vein, and blood was collected.
2.2.7. Acute oral gavage study with multiple control oils for circulating GDF15, ketone bodies, glucose and insulin levels in DIO mice
Two-hours fasted single-housed male HFHS-fed DIO C57Bl/6j mice (30–35 weeks of age) were orally gavaged with 5 μl/g BW of C8:0 MCT oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark), C10:0 MCT oil (glyceryl tridecanoate, #621-71-6, Sigma, Denmark), corn oil (#8001-30-7, Sigma, Denmark), lard oil (#8016-28-2, Sigma, Denmark) or C18:1 LCT oil (glyceryl trioleate, #122-32-7, Sigma, Denmark) (Figure 2A, B & Fig. S1 A, B). Blood was sampled from the tail-vein before and one hour after administration, and glucose (Contour XT; Bayer, Germany) and β-hydroxybutyrate (FreeStyle Precision β-ketone; Abbott Laboratories, CH, IL, US) were measured using handheld meters with test strips before and one hour after administration.
Figure 2.
Oral administration of both C8:0 and C:10 medium-chain triacylglycerol oil increases circulating GDF15 and lowers food intake in obese mice. a,b, plasma growth/differentiation factor 15 (GDF15) (a) and blood β-hydroxybutyrate (b) levels determined in tail-vein blood sampled before (0 h) and one hour after gavage with 5 μl/g body weight of indicated oils. c, cumulative food intake over 24 h of a high-fat, high-sucrose (HFHS) diet after administration of 5 μl/g body weight of indicated oils (n = 9–11). Data were analyzed by a two-way RM ANOVA with Šidák post hoc tests, when ANOVA revealed significant interactions. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 difference between 0 h and 1 h; #p < 0.05, ##p < 0.01 difference between C10:0 medium-chain triacylglycerol (MCT) oil and corn oil at indicated time point; §p < 0.05, §§p < 0.01 difference between C8:0 MCT oil and corn oil at indicated time point.
2.2.8. Acute oral gavage study with multiple control oils for food intake in DIO mice
Single-housed male HFHS-fed DIO C57Bl/6j mice (30–35 weeks of age) were orally gavaged with 5 μl/g BW of C8:0 MCT oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark), C10:0 MCT oil (glyceryl tridecanoate, #621-71-6, Sigma, Denmark), corn oil (#8001-30-7, Sigma, Denmark), lard oil (#8016-28-2, Sigma, Denmark) or C18:1 LCT oil (glyceryl trioleate, #122-32-7, Sigma, Denmark) one hour prior to the dark phase (Figure 2C). Throughout the experiment, mice had ad libitum access to HFHS diet that was weighed before and 7, 17, and 24 h after oil administration.
2.2.9. Acute oral gavage study with multiple control oils for plasma free fatty acid, triacylglycerol, total ghrelin and glucagon like peptide 1 (GLP-1) measurements in DIO mice
Fed single-housed male HFHS-fed DIO C57Bl/6j mice (32–37 weeks of age) were orally gavaged with 5 μl/g BW of C8:0 MCT oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark), C10:0 MCT oil (glyceryl tridecanoate, #621-71-6, Sigma, Denmark), corn oil (#8001-30-7, Sigma, Denmark), and C18:1 LCT oil (glyceryl trioleate, #122-32-7, Sigma, Denmark) and trunk blood was sampled one hour after administration (Fig. S1C–F).
2.2.10. Acute oral gavage food intake studies in GFRAL KO
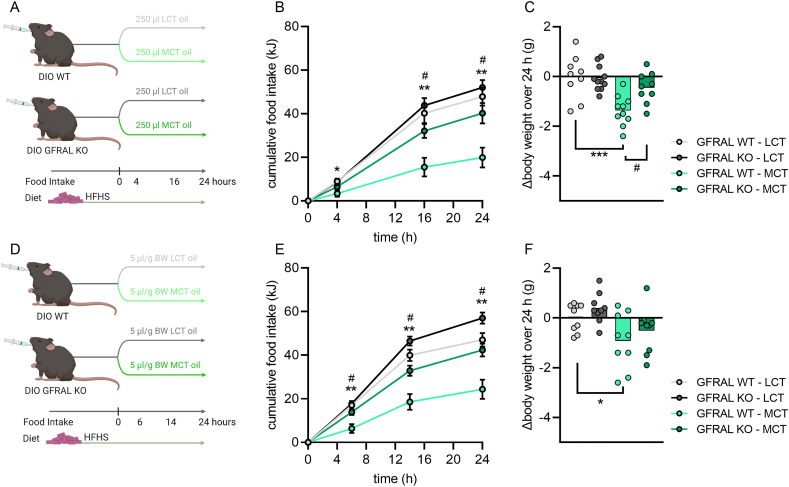
Single-housed four-hours fasted male and female HFD-fed DIO WT and GFRAL KO mice (30–50 weeks of age) were orally gavaged with 250 μl or 5 μl of oil per gram BW, respectively, of 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden) or LCT oil (corn oil; Coop, Denmark) one hour prior to the dark phase (Figure 3A–F). Body weight was measured prior to and 24 h after oral administration of the oils. Throughout the experiment, mice were allowed ad libitum access to HFHS diet that was weighed prior to and four, 16, and 24 h after oil administration.
Figure 3.
Lowering of food intake by acute intake of medium-chain triacylglycerol is dependent on GFRAL. a, d schematic of the oral administration of C8:0 medium-chain triacylglycerol (MCT) and long-chain triacylglycerol (LCT; corn oil) oils in high-fat, high-sucrose (HFHS) diet-induced obese (DIO) male wild-type (WT), and GDNF Family Receptor Alpha Like (GFRAL) knock-out (KO) mice. b,e, cumulative food intake over 24 h of a HFHS diet and c,f, 24-hours delta body weight after acute administration of 250 μl oil (b,c; n = 7–11) or 5 μl oil/g body weight (e,f; n = 9). Data were analyzed by two-way RM ANOVA (b,e) with Šidák post hoc tests when ANOVA revealed significant interactions; by two-way ANOVA (c,f) with Šidák post hoc tests when ANOVA revealed significant interactions. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 different from LCT oil within WT. #p < 0.05 different from GFRAL KO within MCT.
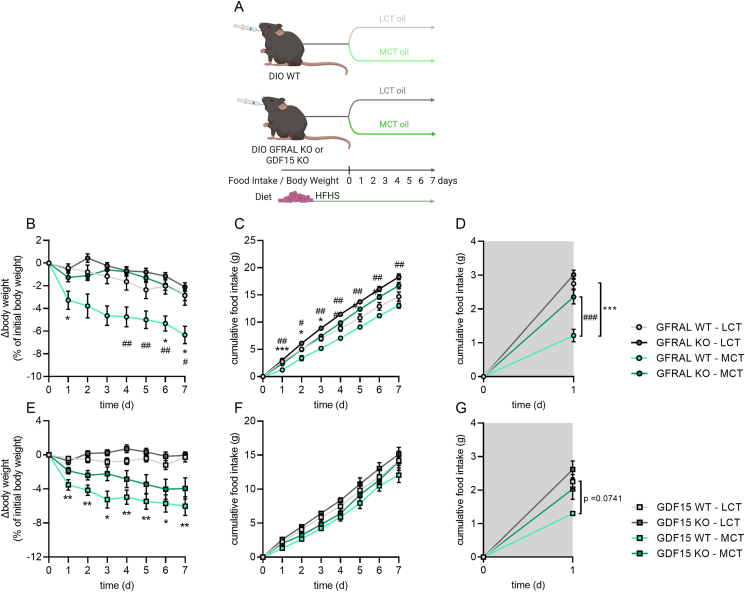
Subchronic oral gavage studies in GFRAL KO and GDF15 KO (Figure 4A–G): Single-housed male HFD-fed DIO GFRAL KO and double-housed HFHS-fed GDF15 KO male mice and their corresponding DIO WT littermates (20–50 weeks of age) were daily orally gavaged with 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden) or LCT oil (corn oil; Coop, Denmark) at a volume of 5 μl per gram body weight one hour prior to the dark phase for seven consecutive days. Throughout the experiment, mice were allowed ad libitum access to HFHS diet. In the GDF15 KO study, blood was sampled before and one hour after the last oral gavage administration of 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden) or LCT oil (corn oil; Coop, Denmark) oil on day seven (Fig. S2). On day seven, one hour after administration, GDF15 KO and WT littermates were decapitated and liver harvested, snap-frozen in liquid nitrogen, and stored at −80 °C until further analyses (Fig. S2).
Figure 4.
The anorectic effects of medium-chain triacylglycerols during subchronic treatment are in part mediated by the GDF15-GFRAL axis. a, schematic of subchronic daily oral administration of C8:0 medium-chain triacylglycerol (MCT) or long-chain triacylglycerol (LCT; corn oil) oils in high-fat, high-sucrose (HFHS) diet-induced obese (DIO) male wild-type (WT), GDNF Family Receptor Alpha Like (GFRAL) knock-out (KO) and growth/differentiation factor 15 (GDF15) KO mice. b-g, body weight change (b,e) and cumulative food intake of a HFHS diet during 7 days of daily oral gavage administration of MCT and LCT oils (c,f) as well as the accumulated food intake specifically during the first 24 h of the subchronic intervention (d,g). For the study in GFRAL KO and WT control mice (b–d) n = 7–9. For the study in GDF15 KO and WT control mice n = 7–9 for body weight (e); n = 2–4 for subchronic food intake (f) because most mice were double housed and some mice shredded food; n = 4–5 for food intake during the first day (g) because most mice were double-housed. Data were analyzed by two-way RM ANOVA with Šidák post hoc tests, when ANOVA revealed significant interactions. ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001 different from LCT oil within WT. #p < 0.05, ##p < 0.01, ###p < 0.001 different from GFRAL KO within MCT.
2.3. RNAseq
The RNA sequencing was carried out by BGI Genomics laboratory in Hong Kong via nanoball sequencing technology. Raw 150bp paired reads have been adapter trimmed and quality controlled. The filtered sequences resulted in ∼ 9 GB reads per sample. Subsequently, an additional quality control was assessed on the 9 GB filtered reads by using FASTQC V0.11.9 and aligned to the mouse reference GRCm38.102 using STAR (V2.7.4). The gene abundance was calculated by Stringtie (V2.1.3b) and expressed by fragment per kilobase of exon per million (FPKM). Differentially gene expression (DEG) was calculated via R (V4.1.2) utilizing the limma package (V3.50.1). Genes which showed a p-value <0.05 (adjusted for multiple testing via benjamini-hochberg) and an absolute log2foldchange >1 have been assigned as DEG. The heatmap of the 10 most up/down-regulated genes was based on their z-scores. Screening for secreted genes was done via VerSeDa (http://genomics.cicbiogune.es/VerSeDa) @07.12.2021 via full search “All methods positive and curated (extracellular)”.
2.4. PCR
RNA from tissues was isolated by Trizol-Chloroform (peqGOLD TriFastTM, 30-2010P, VWR or QIAGEN RNeasy® Lipid Tissue Mini Kit) extraction in homogenized tissues and precipitation with Isopropanol. Genomic DNA was removed (DNAse I, EN0521, Fisher Scientific; RiboLock RNAse Inhibitor, EO0382, Fisher Scientific) and 1 μg RNA was transcribed into cDNA using LunaScript RT Super Mix (E3010L, New England Biolabs). Afterwards gene expression was quantified by qPCR using SYBR Green (Luna Universal qPCR Master Mix, M3003E, New England Biolabs) on a VIA7 Real Time PCR System (4453534, Thermo Fischer Scientific). Primer efficiency was determined with an experiment-specific standard curve and target gene expression was normalized to Rpl13a or Hprt. (Gdf15: FW:CCGAGAGGACTCGAACTCAG RW:ACCCCAATCTCACCTCTGGA. Rpl13a: FW:GGAGGGGCAGGTTCTGGTAT RW:TGTTGATGCCTTCACAGCGT. Hprt: FW: CTCATGGACTGATTATGGACAGGAC RW:GCAGGTCAGCAAAGAACTTATAGCC. Atf4: FW: GGAATGGCCGGCTATGG RW:TCCCGGAAAAGGCATCCT. Chop (Ddit3): FW:CTGGAAGCCTGGTATGAGGAT RW:CAGGGTCAAGAGTAGTGAAGGT).
2.5. Plasma analyses
Blood samples were transferred to EDTA-coated tubes and were kept on ice until centrifugation. For the determination of glucagon-like peptide 1 (GLP-1) levels, the dipeptidyl peptidase 4 inhibitor valine pyrrolidide (0.01 mmol/l) was added. Plasma was separated from the blood after centrifugation (5 min; 4 °C at 13,000 rpm). Samples were stored at −80 °C until plasma analyses. Plasma GDF15 concentration was measured by Quantikine ELISA Mouse/Rat GDF15 immunoassay (Catalog #MGD150, R&D Systems, Denmark) following the manufacturer's instruction. Plasma insulin concentration was determined using an ELISA kit (80-INSRTU-E10; ALPCO Diagnostics, Salem, NH, USA) following the manufacturer's instruction. Plasma fatty acid (FA) (Wako Chemicals BmbH, Germany) and plasma triacylglycerol (TG) (triacylglycerol GPO-PAP kit, Roche Diagnostics, Mannheim) were measured colorimetrically on an autoanalyzer (Pentra C400 analyzer, Horiba, Japan). Plasma total GLP-1 was measured with a U-PLEX kit (K1525UK-1; Meso Scale Discovery (MSO), Maryland, US) and plasma total ghrelin was measured with an ELISA kit (#EZRGRT-91K; Sigma, Denmark) following the manufacturers' instructions.
2.6. Statistics
All data are expressed as means ± SEM in the xy-plots, and with means ± SEM and also individual data points in the bar graphs. The statistical analyses performed are described in each figure legend. For the two-way repeated measures ANOVA in Figure 3, Figure 4 and Fig. S3, genotype and oil were combined into one factor (e.g., GFRAL WT – LCT) and interaction with time was investigated. Statistical significance was defined as p < 0.05. Statistical analyses were performed in GraphPad PRISM 8 (GraphPad, CA, US).
3. Results
To explore specific hepatic signals potentially involved in the hypophagic effects of MCT, we investigated the effect of dietary intake of MCFAs on liver gene expression, by orally administering an oil containing only C8:0 MCTs to mice and collecting livers one hour later to perform whole genome transcriptome analysis (Figure 1A). MCT oil containing C8:0 FAs was chosen, because it is known to be the most ketogenic MCFA [21] indicating that it might have the most pronounced effect on liver metabolism. This revealed that MCTs significantly impacted hepatic gene expression, with 724 transcripts showing differential expression compared with control mice that received saline (Figure 1B; adj. p < 0.05). Among the regulated transcripts was the putative satiety factor Gdf15 [27,28], which was among the top 10 most up-regulated genes (Figure 1C), being increased 13-fold; and among the eight MCFA-regulated genes that encode proteins predicted to be secreted (Figure 1D). To further investigate this finding of increased Gdf15 mRNA and to control for macronutrient class and the calories administered, we treated mice with MCT oil or corn oil as a LCT oil and measured plasma GDF15 levels over 24 h. MCT oil, but not LCT oil, increased circulating GDF15 by sevenfold, reaching peak levels of 280 pg/ml after one hour and returning to baseline within 24 h (Figure 1E). Then, we examined the effect of MCT oil on circulating GDF15 in HFHS-fed diet-induced obese (DIO) mice, a mouse model of human obesity [32]. In DIO mice, MCT oil increased plasma GDF15 levels sevenfold, peaking at ∼2000 pg/ml after one hour, returning to near baseline levels after four hours (Figure 1G). This rise in GDF15 coincided with a 40% reduction in food intake over 24 h and led to a greater loss of body weight during that time compared with animals receiving LCT oil (Figure 1I, J). MCT oil administration resulted in a pronounced and immediate increase in the ketone body, β-hydroxybutyrate, in both chow-fed (from 0.5 to 1.3 mM) and in DIO mice (from 0.5 to 3 mM) (Figure 1F, H). We next sought to understand whether the increase in Gdf15 mRNA is specific to the liver. We gavaged mice with MCT oil and sacrificed them after one or two hours, and compared these results with mice sacrificed in the 2-hours fasted state. Treatment with MCT oil increased hepatic Gdf15 mRNA levels by ∼40-fold within one hour (Figure 1K). MCT also increased Gdf15 mRNA abundance in duodenum, jejunum, ileum, and kidney, albeit to a considerable lesser extent than in liver (Figure 1K). Gdf15 mRNA abundance remained unchanged in colon, white and brown adipose tissue, and skeletal muscle following intake of MCT oil (Figure 1K). Given the increase in Gdf15 mRNA in several intestinal regions, we assessed GDF15 levels in blood from the portal vein, which carries blood from the intestine to the liver. Portal vein plasma GDF15 was sevenfold higher following gavage with MCT oil compared with LCT oil or water (Figure 1L). Combined with the gene expression data, this implies that the small intestine contributes to the increased GDF15 levels seen in the portal vein and, together with the liver, likely also to the systemic circulation after MCFA administration. In sum, our findings are consistent with previous reports showing that MCFAs can acutely reduce food intake [11,[13], [14], [15], [16], [17], [18], [19]], and we identify a novel association between MCFA-induced hypophagia and increased circulating GDF15. Furthermore, we find increased Gdf15 expression in intestine, kidney, and liver after MCT intake, with the largest effect occurring in the liver.
To determine if other MCFAs have similar effects on circulating GDF15 and food intake, we administered C8:0 or C10:0 MCT oils through oral gavage to DIO mice. We compared the outcomes with those of corn oil, lard oil (which has a higher saturated fat content), and C18:1 TG oil, which, like the MCT oils, is pure, consisting solely of C18:1-TG. We observed that both C8:0 and C10:0 MCT oils significantly increased circulating GDF15 to similar extent (∼seven- to eightfold) one hour after oral administration (Figure 2A). In contrast, no effect was observed with corn, lard, and C18:1 LCT oils (Figure 2A). Both C8:0 and C10:0 MCT oils increased circulating β-hydroxybutyrate, with C8:0 MCT oil eliciting a larger increase (Figure 2B), confirming previous findings that C8:0 MCFAs are more ketogenic than C10:0 MCFAs [21]. Blood glucose levels were lower one hour after administration in mice gavaged with C10:0 MCT oil and tended to be lower (p = 0.12) with gavage of C8:0 MCT oil (Fig. S1A). These changes in blood glucose were accompanied by an increase in plasma insulin levels with administration of C8:0 MCT and C10:0 MCT oils (Fig. S1B). In contrast, blood glucose and insulin levels were not altered with administration of any of the control LCT oils (Fig. S1 A, B). After gavage with C8:0 MCT and C10:0 MCT oils, plasma FA levels were not different from LCT control oils, while circulating TG levels were lower compared to corn oil and C18:1 LCT oil (Figs. S1C and D). These data support the concept that LCTs are absorbed from the gut through the lymphatic system and enter the systemic circulation as TG-rich chylomicrons; whereas MCFAs are absorbed via the portal vein and are predominantly metabolized by the liver, largely limiting their entry into the systemic circulation. Notably, C10:0 MCT oil reduced food intake over a 24-hour period to a similar extent as C8:0 MCT oil administration relative to all three LCT control oils (Figure 2C). Neither total plasma ghrelin nor GLP-1 levels were affected by oral MCFA administration (Figs. S1E and F). This collective evidence strongly supports the notion that dietary MCFAs – and not just C8:0 alone - increase circulating GDF15 and decrease food intake.
To understand whether GDF15 mediates the acute anorectic effects of dietary MCFAs, we used DIO wild-type (WT) mice and DIO mice lacking the GDF15 receptor, GDNF Family Receptor Alpha Like receptor (GFRAL KO). GFRAL is exclusively expressed in the hindbrain and has been shown to mediate the anorectic effects of GDF15 [27]. We performed two experiments (Figure 3A, D). First, we gavaged WT and GFRAL KO mice with the same absolute amount of either MCT or LCT oil (Figure 3A). Second, because DIO GFRAL KO mice are generally slightly heavier than their WT counterparts [33], we treated a separate cohort of mice with the same relative amount of MCT oil (5 μl of oil per gram body weight; Figure 3D). In both experiments, the reduction in food intake associated with MCT oil relative to treatment with LCT oil was blunted in GFRAL KO mice (Figure 3B, E). A similar trend could be observed for the change in body weight over 24 h, with MCT resulting in greater loss of body weight compared with LCT, an effect that was somewhat dampened in GFRAL KO (Figure 3C, F). Overall, these data indicate that the full anorectic effects of dietary MCFAs require the GDF15-GFRAL axis.
Next, we aimed to understand whether subchronic treatment with dietary MCFAs can reduce body weight and what role the GDF15-GFRAL pathway plays in these effects. We gavaged DIO GFRAL KO, DIO GDF15 KO and corresponding DIO WT mice daily with either MCT or LCT oil for seven days (Figure 4A). Overall, MCT administration moderately reduced body weight over seven days, however, this effect was mostly driven by a pronounced decrease in body weight during the first day (Figure 4B, E). Food intake followed a similar trend, with a MCT-induced reduction in food intake during the first 24 h that was blunted in both GFRAL and GDF15 KO mice compared with the respective WT mice (Figure 4 C–G). During the remaining six days, food intake was mostly similar between MCT and LCT groups (Figure 4C, F). On the last day of the subchronic treatment, MCT oil administration still increased hepatic Gdf15 mRNA levels by sevenfold (Fig. S2A) and circulating GDF15 by sevenfold to ∼3400 pg/ml one hour after gavage (Fig. S2B). The increase in hepatic Gdf15 mRNA abundance following the gavage after subchronic treatment with MCT occurred without changes in mRNA levels of Atf4 and Chop (Ddit3) (Fig. S2A), which belong to the integrated stress response pathway that has been shown to induce Gdf15 expression [34]. Similarly, the increase in Gdf15 mRNA following a single gavage with MCT (Figure 1A–D) was not accompanied by increases in mRNA of Atf4, and Chop (Ddit3) (see supplemental spreadsheets). Collectively, these data demonstrate that dietary MCFAs moderately reduce body weight over seven days. This outcome is likely driven by the hypophagia that occurs during the first 24 h, and the GDF15-GFRAL axis is partially required for this effect.
We next wanted to understand whether this pronounced and consistent effect of MCFAs to decrease food intake especially within the first 24 h of administration in male mice extends to female mice. Hence, we used female DIO WT and DIO GFRAL KO mice and observed a MCT-induced reduction in cumulative 24 h food intake relative to LCT oil administration in DIO WT mice (Fig. S3A and B). As in male mice, these effects were blunted in female mice, when GFRAL was lacking (Fig. S3A and B). Thus, the GDF15-GFRAL axis partially regulates the acute anorectic effects of dietary MCFAs both in male and female mice.
4. Discussion
Herein, we applied liver whole genome transcriptome analysis to screen for signals involved in the satiety effect of MCFAs. This identified GDF15 as a hepatic factor induced acutely by intake of MCFAs. Of great interest, we could demonstrate that the anorectic effects of dietary MCFAs are in part mediated by the GDF15-GFRAL axis. While the induction of Gdf15 mRNA levels is particularly high in the liver and likely drive most of the increase in systemic GDF15 levels, we also provide evidence strongly suggesting a sizable contribution of small intestine-derived GDF15 to the increase in circulating GDF15 in response to dietary MCFA.
GDF15 has gained attention as a potential treatment target for obesity. GDF15 reduces food intake by activating the GFRAL receptor, which is located in the area postrema and the nucleus tractus solitarius of the hindbrain [27]. GDF15-induced suppression of food intake has been observed in mice, rats, and nonhuman primates [28]. Unlike other hormones that regulate energy balance, such as leptin, GDF15 levels in the blood do not vary substantially in response to fasting or overeating [34]. Instead, it is widely acknowledged that GDF15 is a stress signal and thus often found at higher levels in individuals with chronic conditions such as cancer and mitochondrial myopathy [35]. There are indications that elevated GDF15 correlates with cancer-associated cachexia, an effect that is reversed by an antibody-mediated inhibition of GDF15-GFRAL activity, at least in mice [36]. The exact role of GDF15 in stress conditions is not yet clear, but it may exert protective effects. For example, blocking GDF15 in mice with experimental sepsis, a condition associated with high GDF15 levels, increases mortality [37]. We have also found that GDF15 levels increase in response to metabolic stress, such as physical exercise [30,38]. It is key to consider physiology versus pathophysiology when evaluating the biological consequences of elevated GDF15 levels [35,39]. Although GDF15 levels are higher after exercise, they return to baseline levels within 8–24 h, and people that are physically fit appear to have lower plasma GDF15 levels [40]. In contrast, in disease states like cancer, circulating GDF15 remains chronically elevated, likely triggering different biological outcomes. Some drugs and chemicals can also increase endogenous GDF15 levels, which can lead to weight loss in rodents by activating GFRAL signaling in the hindbrain [41]. However, the specific role of elevated GDF15 in the anorectic effects of these substances may be difficult to determine due to the pleiotropic toxic nature of many potent GDF15 secretagogues.
Intake of MCFAs is among the first known nutritional interventions that can affect circulating GDF15 levels. Previously, plasma GDF15 concentration increased ∼2.5-fold in mice following four hours of feeding with a lysine-deficient diet compared with chow diet [34], pointing to a potential role of GDF15 as a regulator of amino acid homeostasis. One human study found increased GDF15 levels after glucose ingestion [42], which is, however, not found in most other studies [34,43]. It is not yet clear whether a high flux of MCFAs, which are not typically found in large amounts in the body or diet, into intestinal and hepatic cells represents a form of distress or eustress. However, we did not detect increases in mRNA of genes associated with the integrated stress response pathway.
Given that the anorectic effects of dietary MCFAs was only in part mediated by the GDF15-GFRAL axis, other hypophagic and satiety-inducing mechanisms could be involved. We herein appear to rule out an involvement of ghrelin and GLP-1, since the total circulating levels of these two hormones were unaffected by MCFAs. However, since we assessed their total levels, it remains possible that the proportion of acylated ghrelin and/or active GLP-1 were altered, thereby modulating food intake. Moreover, other gut-derived hormones could also induce satiety and thus play a role in the observed hypophagic effect [44]. Another possibility is that the MCFA-induced increase in circulating ketone bodies, which have been suggested to induce satiety [45,46], partially contributes to the reduction in food intake observed in response to MCT administration. Alternatively, satiety could be mediated by one or more of the other secreted factors identified induced by MCT administration (Figure 1D). For example, ANGPTL8, which has been linked to the regulation of food intake through a CNS receptor action [47], increased with MCT administration. Another intriguing secreted factor, insulin-like growth factor binding protein-1 (IGFB1), was induced by MCT oil and has previously been found to increase insulin sensitivity [48], raising the possibility that IGFB1 contributes to the described insulin-sensitizing effects of MCT-rich diets [6,7]. Notably, we observed an acute glucose-lowering effect of MCTs accompanied by increased insulin levels. Unraveling the mechanism behind MCFA-induced insulin secretion is a subject of future interest, which may involve direct effects on pancreatic cells [49,50] or indirect effects via gut-derived incretins in the portal vein.
The mechanism behind the MCFA-induced increase in Gdf15 mRNA is not yet understood. Intake of MCFAs did not increase mRNA levels of Atf4 and Chop, suggesting that the integrated stress response pathway is not involved in the induction of GDF15, as was also observed in other physiological stimuli such as exercise [51]. Interestingly, it has been shown that activation of the cellular stress sensor, AMPK, can increase hepatic GDF15 levels [52].
In summary, we have identified a nutritional approach that acutely increases circulating GDF15 levels and reduces food intake and body weight in mice. The GDF15-GFRAL axis is required for the full anorectic effects of dietary MCFAs that occur shortly after treatment.
Funding information
This study was supported by the Novo Nordisk Foundation (grant# NNF20OC0063744), Arla Food for Health, the Danish Dairy Research Foundation. PhD scholarship of J.M.K. was supported by a research grant from the Danish Cardiovascular Academy, which is funded by the Novo Nordisk Foundation, grant # NNF20SA0067242, and the Danish Heart Foundation. S.H., A-M.L. and A.M.F. were funded by the Danish Diabetes Academy, funded by the Novo Nordisk Foundation (grant# NNF17SA0031406). A.M.F. was also funded directly by the Novo Nordisk Foundation (grant# NNF22OC0074110). M.J. and A.S. were supported by the German Ministry of Education and Research (BMBF: DZD grant 82DZD03D03) and the Brandenburg State. M.K. was supported by the Deutsche Forschungsgemeinschaft (DFG; KL 3285/5-1), the German Center for Diabetes Research (DZD; 82DZD03D03and 82DZD03D1Y), the Novo Nordisk Foundation (NNF; NNF19OC0055192) and the Deutsche Diabetes Gesellschaft (DDG). C.C. was supported by a research grant from Independent Research Fund Denmark (0134-00254B). The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark, and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.cbmr.ku.dk) (Grant number NNF18CC0034900).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Bente Kiens reports financial support was provided by Novo Nordisk Foundation. Maximilian Kleinert reports financial support was provided by Arla Food For Health. Bente Kiens reports financial support was provided by Danish Dairy Research Foundation. Josephine Maria Kanta reports financial support was provided by Danish Cardiovascular Academy. Anne-Marie Lundsgaard reports financial support was provided by Danish Diabetes Academy. Stephanie Holm reports financial support was provided by Danish Diabetes Academy. Andreas Machel Fritzen reports financial support was provided by Danish Diabetes Academy. Andreas Machel Fritzen reports financial support was provided by Novo Nordisk Foundation. Markus Jahnert reports financial support was provided by German Ministry of Education and Research. Annette Schurmann reports financial support was provided by German Ministry of Education and Research. Markus Jahnert reports financial support was provided by The Brandenburg State. Anette Schurmann reports financial support was provided by The Brandenburg State. Maximilian Kleinert reports financial support was provided by Deutsche Forschungsgemeinschaft. Maximilian Kleinert reports financial support was provided by German Center for Diabetes Research. Maximilian Kleinert reports financial support was provided by Novo Nordisk Foundation. C.C. is co-founder of Ousia Pharma ApS, a biotech company developing therapeutics for obesity. C.C. is also on the editorial board of Molecular Metabolism.
Acknowledgements
We thank Irene Bech Nielsen, Betina Bolmgren, Charlotte Sashi Aier Svendsen, and Annemette Overgaard Brethvad for skilled technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101760.
Contributor Information
Andreas M. Fritzen, Email: amfritzen@sund.ku.dk.
Maximilian Kleinert, Email: maximilian.kleinert@dife.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure S1.
Effect of oral administration of C8:0 and C10:0 medium-chain triacylglycerol oils on circulating glucose, insulin, fatty acid, triacylglycerol, ghrelin and glucagon-like peptide 1 (GLP-1) levels. a-e, blood glucose (a) and plasma insulin (b) determined in blood from the tail-vein sampled before (0 h) and one hour after gavage with 5 μl of indicated oils per gram body weight. Plasma fatty acid (c), plasma triacylglycerol (d), plasma total ghrelin (e), and plasma total GLP-1 (f) levels determined in trunk blood collected one hour after gavage with 5 μl of indicated oils per gram body weight (n = 8). Data were analyzed by a two-way RM ANOVA (a-b) with Šidák post hoc tests, when ANOVA revealed significant interactions, or with one-way ANOVA (c-e) with Dunnett post hoc test, respectively, when ANOVA revealed significant interactions. ∗p < 0.01, ∗∗p < 0.01, ∗∗∗p < 0.001 between indicated.
Figure S2.
Hepatic expression of Gdf15 and circulating levels of GDF15 are still acutely induced after seven days of daily oral gavage administration of medium-chain triacylglycerols. a, hepatic Gdf15, Atf4, and Chop mRNA expressions one hour after acute administration of C8:0 medium-chain triacylglycerol (MCT) and long-chain triacylglycerol (LCT; corn oil) oils performed after seven days of daily oral gavage administration of the same oils in HFHS DIO male mice. b, circulating plasma growth/differentiation factor 15 (GDF15) levels before and 1 h after acute administration of MCT and LCT oils performed after 7 days of daily oral gavage administration of the same oils in HFHS DIO male mice. n = 8–9. Data were analyzed by an unpaired, two-tailed student's t-test within each gene (a) and two-way RM ANOVA (b) with Šidák post hoc tests, when ANOVA revealed significant interactions. ∗p < 0.05 different from LCT oil, ∗∗∗p < 0.001 different from LCT oil within 1h time point.
Figure S3.
The acute anorectic effects of medium-chain triacylglycerols are in part mediated by the GDF15-GFRAL axis in female mice. a,b, accumulated food intake of a HFHS diet over 24 h (a) and 24-hour body weight change (b) after acute administration of 5 μl of C8:0 medium-chain triacylglycerol (MCT) and long-chain triacylglycerol (LCT; corn oil) oils per gram body weight in WT and GFRAL KO HFHS-DIO female mice (n = 9–12). Data were analyzed by a two-way ANOVA (a) and two-way RM ANOVA (b) with Šidák post hoc tests, when ANOVA revealed significant interactions. ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001 different from LCT oil (within genotype in a,b). #p < 0.05, ##p < 0.01, different from GFRAL KO within MCT.
Data availability
Data will be made available on request.
References
- 1.Bach A.C., Babayan V.K. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36(5):950–962. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- 2.Bloom B., Chaikoff I.L., Reinhardt Intestinal lymph as pathway for transport of absorbed fatty acids of different chain lengths. Am J Physiol. 1951;166(2):451–455. doi: 10.1152/ajplegacy.1951.166.2.451. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes J., van de Kamer J.H., Weijers H.A. Differences in absorption of the various fatty acids studied in children with steatorrhea. J Clin Investig. 1962;41(3):488–494. doi: 10.1172/JCI104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crozier G., Bois-Joyeux B., Chanez M., Girard J., Peret J. Metabolic effects induced by long-term feeding of medium-chain triglycerides in the rat. Metab Clin Exp. 1987;36(8):807–814. doi: 10.1016/0026-0495(87)90122-3. [DOI] [PubMed] [Google Scholar]
- 5.Lavau M.M., Hashim S.A. Effect of medium chain triglyceride on lipogenesis and body fat in the rat. J Nutr. 1978;108(4):613–620. doi: 10.1093/jn/108.4.613. [DOI] [PubMed] [Google Scholar]
- 6.Eckel R.H., Hanson A.S., Chen A.Y., Berman J.N., Yost T.J., Brass E.P. Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes. 1992;41(5):641–647. [PubMed] [Google Scholar]
- 7.Lundsgaard A.-M., Fritzen A.M., Sjøberg K.A., Kleinert M., Richter E.A., Kiens B. Small amounts of dietary medium-chain fatty acids protect against insulin resistance during caloric excess in humans. Diabetes. 2021;70(1):91–98. doi: 10.2337/db20-0582. [DOI] [PubMed] [Google Scholar]
- 8.Maher T., Clegg M.E. A systematic review and meta-analysis of medium-chain triglycerides effects on acute satiety and food intake. Crit Rev Food Sci Nutr. 2021;61(4):636–648. doi: 10.1080/10408398.2020.1742654. [DOI] [PubMed] [Google Scholar]
- 9.Bray G.A., Lee M., Bray T.L. Weight gain of rats fed medium-chain triglycerides is less than rats fed long-chain triglycerides. Int J Obes. 1980;4(1):27–32. [PubMed] [Google Scholar]
- 10.Stubbs R.J., Harbron C.G. Covert manipulation of the ratio of medium- to long-chain triglycerides in isoenergetically dense diets: effect on food intake in ad libitum feeding men. Int J Obes Relat Metab Disord : J Int Associat Study Obes. 1996;20(5):435–444. [PubMed] [Google Scholar]
- 11.Kinsella R., Maher T., Clegg M.E. Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav. 2017;179:422–426. doi: 10.1016/j.physbeh.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Norgren J., Sindi S., Sandebring-Matton A., Kåreholt I., Daniilidou M., Akenine U., et al. Ketosis after intake of coconut oil and caprylic acid-with and without glucose: a cross-over study in healthy older adults. Front Nutr. 2020;7:40. doi: 10.3389/fnut.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolls B.J., Gnizak N., Summerfelt A., Laster L.J. Food intake in dieters and nondieters after a liquid meal containing medium-chain triglycerides. Am J Clin Nutr. 1988;48(1):66–71. doi: 10.1093/ajcn/48.1.66. [DOI] [PubMed] [Google Scholar]
- 14.Van Wymelbeke V., Himaya A., Louis-Sylvestre J., Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr. 1998;68(2):226–234. doi: 10.1093/ajcn/68.2.226. [DOI] [PubMed] [Google Scholar]
- 15.Coleman H., Quinn P., Clegg M.E. Medium-chain triglycerides and conjugated linoleic acids in beverage form increase satiety and reduce food intake in humans. Nutr Res (New York, N.Y.) 2016;36(6):526–533. doi: 10.1016/j.nutres.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Maher T., Sampson A., Goslawska M., Pangua-Irigaray C., Shafat A., Clegg M.E. Food intake and satiety response after medium-chain triglycerides ingested as solid or liquid. Nutrients. 2019;11(7) doi: 10.3390/nu11071638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St-Onge M.-P., Mayrsohn B., O'Keeffe M., Kissileff H.R., Choudhury A.R., Laferrère B. Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur J Clin Nutr. 2014;68(10):1134–1140. doi: 10.1038/ejcn.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Wymelbeke V., Louis-Sylvestre J., Fantino M. Substrate oxidation and control of food intake in men after a fat-substitute meal compared with meals supplemented with an isoenergetic load of carbohydrate, long-chain triacylglycerols, or medium-chain triacylglycerols. Am J Clin Nutr. 2001;74(5):620–630. doi: 10.1093/ajcn/74.5.620. [DOI] [PubMed] [Google Scholar]
- 19.Maher T., Deleuse M., Thondre S., Shafat A., Clegg M.E. A comparison of the satiating properties of medium-chain triglycerides and conjugated linoleic acid in participants with healthy weight and overweight or obesity. Eur J Nutr. 2021;60(1):203–215. doi: 10.1007/s00394-020-02235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes V.R., Michael N.J., van den Top M., Zhao F.-Y., Brown R.D., De Souza D., et al. A Neural basis for Octanoic acid regulation of energy balance. Mol Metabol. 2020;34:54–71. doi: 10.1016/j.molmet.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St-Pierre V., Vandenberghe C., Lowry C.-M., Fortier M., Castellano C.-A., Wagner R., et al. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front Nutr. 2019;6:46. doi: 10.3389/fnut.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57(6):943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias V.C., Fung E., Snyder F.F., Carter R.J., Parsons H.G. Effects of medium-chain triglyceride feeding on energy balance in adult humans. Metab Clin Exp. 1990;39(9):887–891. doi: 10.1016/0026-0495(90)90295-n. [DOI] [PubMed] [Google Scholar]
- 24.Vistisen B., Nybo L., Xu X., Høy C.-E., Kiens B. Minor amounts of plasma medium-chain fatty acids and no improved time trial performance after consuming lipids. J Appl Physiol (Bethesda, Md. : 1985) 2003;95(6):2434–2443. doi: 10.1152/japplphysiol.00118.2003. [DOI] [PubMed] [Google Scholar]
- 25.Linscheer W.G., Patterson J.F., Moore E.W., Clermont R.J., Robins S.J., Chalmers T.C. Medium and long chain fat absorption in patients with cirrhosis. J Clin Investig. 1966;45(8):1317–1325. doi: 10.1172/JCI105438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jambor de Sousa U.L., Arnold M., Langhans W., Geary N., Leonhardt M. Caprylic acid infusion acts in the liver to decrease food intake in rats. Physiol Behav. 2006;87(2):388–395. doi: 10.1016/j.physbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang D., Day E.A., Townsend L.K., Djordjevic D., Jørgensen S.B., Steinberg G.R. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17(10):592–607. doi: 10.1038/s41574-021-00529-7. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y., Walker K., Min X., Hale C., Tran T., Komorowski R., et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci Transl Med. 2017;9(412) doi: 10.1126/scitranslmed.aan8732. [DOI] [PubMed] [Google Scholar]
- 29.Frikke-Schmidt H., Hultman K., Galaske J.W., Jørgensen S.B., Myers M.G., Seeley R.J. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol Metabol. 2019;21:13–21. doi: 10.1016/j.molmet.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein A.B., Nicolaisen T.S., Ørtenblad N., Gejl K.D., Jensen R., Fritzen A.M., et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat Commun. 2021;12(1):1041. doi: 10.1038/s41467-021-21309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao E.C., Koniaris L.G., Zimmers-Koniaris T., Sebald S.M., Huynh T.V., Lee S.J. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20(10):3742–3751. doi: 10.1128/MCB.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinert M., Clemmensen C., Hofmann S.M., Moore M.C., Renner S., Woods S.C., et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14(3):140–162. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 33.Klein A.B., Nicolaisen T.S., Johann K., Fritzen A.M., Mathiesen C.V., Gil C., et al. The GDF15-GFRAL pathway is dispensable for the effects of metformin on energy balance. Cell Rep. 2022;40(8):111258. doi: 10.1016/j.celrep.2022.111258. [DOI] [PubMed] [Google Scholar]
- 34.Patel S., Alvarez-Guaita A., Melvin A., Rimmington D., Dattilo A., Miedzybrodzka E.L., et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metabol. 2019;29(3):707–718.e8. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breit S.N., Brown D.A., Tsai V.W.-W. The GDF15-GFRAL pathway in Health and metabolic disease: friend or foe? Annu Rev Physiol. 2021;83:127–151. doi: 10.1146/annurev-physiol-022020-045449. [DOI] [PubMed] [Google Scholar]
- 36.Suriben R., Chen M., Higbee J., Oeffinger J., Ventura R., Li B., et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat Med. 2020;26(8):1264–1270. doi: 10.1038/s41591-020-0945-x. [DOI] [PubMed] [Google Scholar]
- 37.Luan H.H., Wang A., Hilliard B.K., Carvalho F., Rosen C.E., Ahasic A.M., et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178(5):1231–1244.e11. doi: 10.1016/j.cell.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinert M., Clemmensen C., Sjøberg K.A., Carl C.S., Jeppesen J.F., Wojtaszewski J.F.P., et al. Exercise increases circulating GDF15 in humans. Mol Metabol. 2018;9:187–191. doi: 10.1016/j.molmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johann K., Kleinert M., Klaus S. The role of GDF15 as a myomitokine. Cells. 2021;10(11) doi: 10.3390/cells10112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conte M., Martucci M., Mosconi G., Chiariello A., Cappuccilli M., Totti V., et al. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol. 2020;11:915. doi: 10.3389/fimmu.2020.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu J.F., Zhu M.Q., Xie B.C., Shi X.C., Liu H., Zhang R.X., et al. Camptothecin effectively treats obesity in mice through GDF15 induction. PLoS Biol. 2022;20(2) doi: 10.1371/journal.pbio.3001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schernthaner-Reiter M.H., Kasses D., Tugendsam C., Riedl M., Peric S., Prager G., et al. Growth differentiation factor 15 increases following oral glucose ingestion: effect of meal composition and obesity. Eur J Endocrinol. 2016;175(6):623–631. doi: 10.1530/EJE-16-0550. [DOI] [PubMed] [Google Scholar]
- 43.Martinussen C., Svane M.S., Bojsen-Møller K.N., Jensen C.Z., Kristiansen V.B., Bookout A.L., et al. Plasma GDF15 levels are similar between subjects after bariatric surgery and matched controls and are unaffected by meals. Am J Physiol Endocrinol Metab. 2021;321(4):E443–E452. doi: 10.1152/ajpendo.00190.2021. [DOI] [PubMed] [Google Scholar]
- 44.St-Onge M.-P., Jones P.J.H. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr. 2002;132(3):329–332. doi: 10.1093/jn/132.3.329. [DOI] [PubMed] [Google Scholar]
- 45.Stubbs B.J., Cox P.J., Evans R.D., Cyranka M., Clarke K., de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring, Md.) 2018;26(2):269–273. doi: 10.1002/oby.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deemer S.E., Plaisance E.P., Martins C. Impact of ketosis on appetite regulation-a review. Nutri Res (New York, N.Y.) 2020;77:1–11. doi: 10.1016/j.nutres.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Chen S., Feng M., Zhang S., Dong Z., Wang Y., Zhang W., et al. Angptl8 mediates food-driven resetting of hepatic circadian clock in mice. Nat Commun. 2019;10(1):3518. doi: 10.1038/s41467-019-11513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajwani A., Ezzat V., Smith J., Yuldasheva N.Y., Duncan E.R., Gage M., et al. Increasing circulating IGFBP1 levels improves insulin sensitivity, promotes nitric oxide production, lowers blood pressure, and protects against atherosclerosis. Diabetes. 2012;61(4):915–924. doi: 10.2337/db11-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang T., Chen P., Stanley C.A., Hoshi T., Li C. Mechanisms of octanoic acid potentiation of insulin secretion in isolated islets. Islets. 2019;11(4):77–88. doi: 10.1080/19382014.2019.1566683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanbar S.S., Martin J.M. Stimulation by octanoate of insulin release from isolated rat pancreas. Metab Clin Exp. 1967;16(5):482–484. doi: 10.1016/0026-0495(67)90140-0. [DOI] [PubMed] [Google Scholar]
- 51.Townsend L.K., Medak K., Weber A.J., Dibe H., Shamshoum H., Wright D.C. CHOP is dispensable for exercise-induced increases in GDF15. J Appl Physiol (Bethesda, Md. : 1985) 2022;132(2):413–422. doi: 10.1152/japplphysiol.00698.2021. [DOI] [PubMed] [Google Scholar]
- 52.Townsend L.K., Weber A.J., Day E.A., Shamshoum H., Shaw S.J., Perry C.G.R., et al. AMPK mediates energetic stress-induced liver GDF15. FASEB J : Off Publ Fed Am Soc Exp Biol. 2021;35(1) doi: 10.1096/fj.202000954R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.