Abstract
Objective
Free fatty acid receptor 1 (FFAR1) is highly expressed in enteroendocrine cells of the small intestine and pancreatic beta cells, where FFAR1 agonists function as GLP-1 and insulin secretagogues, respectively. Most efficacious are so-called second-generation synthetic agonists such as AM5262, which, in contrast to endogenous long-chain fatty acids are able to signal through both IP3/Ca2+ and cAMP pathways. Whereas IP3 signaling is to be expected for the mainly Gq-coupled FFAR1, the mechanism behind FFAR1-induced cAMP accumulation remains unclear, although originally proposed to be Gs mediated.
Methods and results
When stimulated with AM5262, we observe that FFAR1 can activate the majority of the Gα proteins, except - surprisingly - members of the Gs family. AM5262-induced FFAR1-mediated transcriptional activation through cAMP response element (CREB) was blocked by the specific Gq inhibitor, YM253890. Furthermore, in Gq-deficient cells no CREB signal was observed unless Gq or G11 was reintroduced by transfection. By qPCR we determined that adenylate cyclase 2 (Adcy2) was highly expressed and enriched relative to the nine other Adcys in pro-glucagon expressing enteroendocrine cells. Co-transfection with ADCY2 increased the FFAR1-induced cAMP response 4-5-fold in WT HEK293 cells, an effect fully inhibited by YM253890. Moreover, co-transfection with ADCY2 had no effect in Gq-deficient cells without reintroduction of either Gq or G11. Importantly, although both AM5262/FFAR1 and isoproterenol/β2 adrenergic receptor (β2AR) induced cAMP production was lost in Gs-deficient cells, only the β2AR response was rescued by Gs transfection, whereas co-transfection with ADCY2 was required to rescue the FFAR1 cAMP response. In situ hybridization demonstrated a high degree of co-expression of ADCY2 and FFAR1 in enteroendocrine cells throughout the intestine. Finally, in the enteroendocrine STC-1 and GLUTag cell lines AM5262-induced cAMP accumulation and GLP-1 secretion were both blocked by YM253890.
Conclusions
Our results show that Gq signaling is responsible not only for the IP3/Ca2+ but also the cAMP response, which together are required for the highly efficacious hormone secretion induced by second-generation FFAR1 agonists - and that ADCY2 presumably mediates the Gq-driven cAMP response.
Keywords: FFAR1, GPR40, Adenylate cyclase 2, GLP-1, ADCY2, Gq
Highlights
-
•
FFAR1 can activate all types of Gα proteins - except members of the Gs family.
-
•
FFAR1 stimulation of cAMP is mediated through Gq and adenylate cyclase 2.
-
•
Adenylate cyclase 2 is highly expressed and enriched in Ffar1 expressing enteroendocrine cells.
-
•
FFAR1-induced GLP-1 secretion is entirely blocked by the Gq-inhibitor YM254890.
1. Introduction
Free fatty acid receptor 1 (FFAR1) (also known as GPR40) is a Gq-coupled long-chain fatty acid (LCFA) receptor primarily expressed in beta cells of the endocrine pancreas and enteroendocrine cells (EECs) of the small intestine [[1], [2], [3], [4]]. FFAR1 senses LCFAs released from dietary triglycerides and acts in synergy with the Gs-coupled sensor of 2-monoacylglycerol, GPR119, to strongly stimulate glucagon-like peptide 1 (GLP-1) release. In the islets, FFAR1 senses both LCFAs released from chylomicrons and the arachidonic acid metabolite 20-HETE released from β-cells and in an autocrine manner enhances glucose-dependent insulin secretion [5,6]. The first synthetic FFAR1 agonists for the treatment of diabetes were developed more than a decade ago. Initially, Takeda pioneered the field with the prototype first-generation FFAR1 agonists, TAK875 (or fasiglifam) [7], which reached clinical Phase III, showing meaningful improvements in glucose tolerance in diabetic patients [8]. Unfortunately, off-target liver toxicity terminated the program [[8], [9], [10], [11], [12], [13]]. However, as FFAR1 was now a clinically proven anti-diabetic target, even more efficacious, second-generation FFAR1 agonists were developed, with the Amgen compound, AM5262 as the prototype [14]. The key feature of second-generation agonists is their ability to induce significantly greater incretin hormone secretion both in vitro and in vivo [15].
FFAR1 is a family A GPCR that was generally known to couple to Gq and stimulate hormone secretion through classical inositol triphosphate (IP3) accumulation, Ca2+ release, and further downstream signaling [1,2,16]. Unexpectedly, we discovered that second-generation agonists such as AM5262, in contrast to first-generation compounds and endogenous LCFAs, not only induced IP3/Ca2+ responses, but also increased cAMP production [15]. Combined cAMP and IP3 accumulation achieved by either activating FFAR1 with a first-generation agonist (only Gq) and simultaneously activating the Gs-coupled GPR119 or TGR5 receptors or when activating FFAR1 with a second-generation agonist was shown to generate a greater incretin hormone secretion [15,17]. Given that in the context of GPCR signaling cAMP generation is classically associated with activation of Gs [18], the original interpretation was that these second-generation compounds could bias FFAR1 into signaling through both Gq and Gs [15]. Curiously, however, a recent study investigating FFAR1's direct coupling to G proteins by measuring BRET between the heterotrimeric G protein subunits Gα and Gβγ revealed no significant activation of Gs for either first or second-generation FFAR1 agonists [19].
In the present study, we expand the characterization of the second-generation FFAR1 agonist using a variety of tools, including pharmacological inhibitors, proximity-based BRET assays, and genetically engineered cells lacking the expression of specific G proteins, to define the G protein-coupling profile of FFAR1. We observe that the strong cAMP production induced by second-generation FFAR1 agonists does not depend on Gs, but rather is a Gq-mediated effect driven by activation of the atypical adenylate cyclase 2 (ADCY2), which we find is particularly highly expressed and enriched in FFAR1 expressing enteroendocrine cells in the gut. Importantly, specific inhibition of Gq signaling completely abolishes AM5262-induced GLP-1 secretion in STC-1 and GLUTag cells.
2. Materials & methods
2.1. Compounds
All compounds were dissolved in DMSO. TAK875 and AM5262 were synthesized as previously described [14,18]. YM254890 was purchased from Wako; Carbachol (cat# 212385) and Isoproterenol (cat# I6504) from Sigma.
2.2. Plasmids
The receptor construct of human FFAR1 was inserted into the pCMV-tag2B vector whereas CAMYEL [20], human β2AR, human M1R, and Gα, Gβ and Gγ proteins were all expressed via the pcDNA3.1 (+) vector.
2.3. Cell culture, plating, and transfection
WT HEK293T cells were maintained in Dulbecco's Modified Eagle's Medium 1966 with GlutaMAX™ supplemented with 10% fetal bovine serum (Sigma–Aldrich), 1% l-glutamine and 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C with 5% CO2. HEK293A Parent (wildtype - unmodified) [21], -GsKO (ΔGNAS/GNAL) [22] and -GqKO (ΔGNAQ/GNA11) [21,23] cells were maintained in Dulbecco's Modified Eagle's Medium 1885 with GlutaMAX™ supplemented with 10% heat-inactivated fetal bovine serum and 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C with 5% CO2.
All HEK293 (HEK293T, HEK293A Parent, -GsKO and -GqKO) cells were plated in white poly-D-lysine-coated 96-well plates (35.000/well). The following day plates were transiently transfected with 0.3 μl/well Lipofectamine-2000 (ThermoFisher), with a maximal total DNA of 80 ng/well according to the manufacturer's protocol in Opti-MEM (Gibco) and supplemented with fresh medium after 5 h, or DMEM with low serum (0.5%) for use in the reporter assay.
GLUTag (kindly provided by Frank Reimann and Fiona Gribble) and STC-1 (ATCC: CRL-3254) cells were maintained in Dulbecco's Modified Eagle's Medium 1885 with GlutaMAX™ supplemented with 10% heat-inactivated fetal bovine serum and 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C with 5% CO2.
2.4. BRET based G protein activation assay and cAMP assay
The degree of G protein activation was monitored using bioluminescence resonance energy transfer (BRET) (Figure 1A). This method relies on dissociation of the G protein, which leads to increase in BRET intensity upon interaction of the masGRKct-Rluc8 (membrane anchored) and the Venus-γ2 constructs. Likewise, the intracellular cAMP was monitored using BRET based on a construct consisting of cAMP binding protein (Exchange protein activated by cAMP (Epac)) flanked by a BRET pair; Renilla luciferase (Rluc) and yellow fluorescent protein (YFP). This construct is called CAMYEL (cAMP sensor using YFP-Epac-Rluc) and monitors cAMP levels through a conformational change upon cAMP binding to Epac, resulting in a loss of BRET intensity (Figure 3C). HEK293A [21,22] cells in 96-well plates were transfected with hFFAR1 or hM1R (20 ng and 5 ng, respectively) with 1 ng Gα protein, 0.166 ng β1, 0.333 ng masGRKct-Rluc8, 0.166 ng Venus-γ2 and 20 ng empty vector for the G protein assay. For cAMP determination, the cells were transfected with 20 ng receptor, 50 ng CAMYEL and 1 ng G protein if listed. The following day cells were washed twice with 100 μl/well HBSS (Gibco, Life Technologies) and pre-incubated for 30 min at 37 °C with 85 μl HBSS. Coelenterazine h (ThermoFisher) was added at a 5 μM final concentration and agonists were added 5 min later. Plates were read, after 5 min incubation for the G protein assay and after 10 min in the cAMP assay, on a CLARIOstar Plus plate reader. The BRET signal was calculated as the ratio of the emission intensity at 535 nm (citrine/YFP) to the emission intensity at 475 nm (Renilla luciferase). Determinations were made in triplicates. cAMP determination in STC-1 were done by LTX lipofectamine transfection of 50 ng 22F cAMP plasmid (GloSensor™ Promega). The following day cells were washed with 100 μl/well HBSS (Gibco, Life Technologies) and added 99 μl/well of HBSS with 2% v/v Glosensor™ and 1 mM IBMX. AM5262 +/− either 1 μM YM254890, 5 μM Edelfosine or 5 μM Chelerythrine chloride were added and plates were measured for 1 min intervals for 1 h of total luminesence. Determinations were made in triplicates.
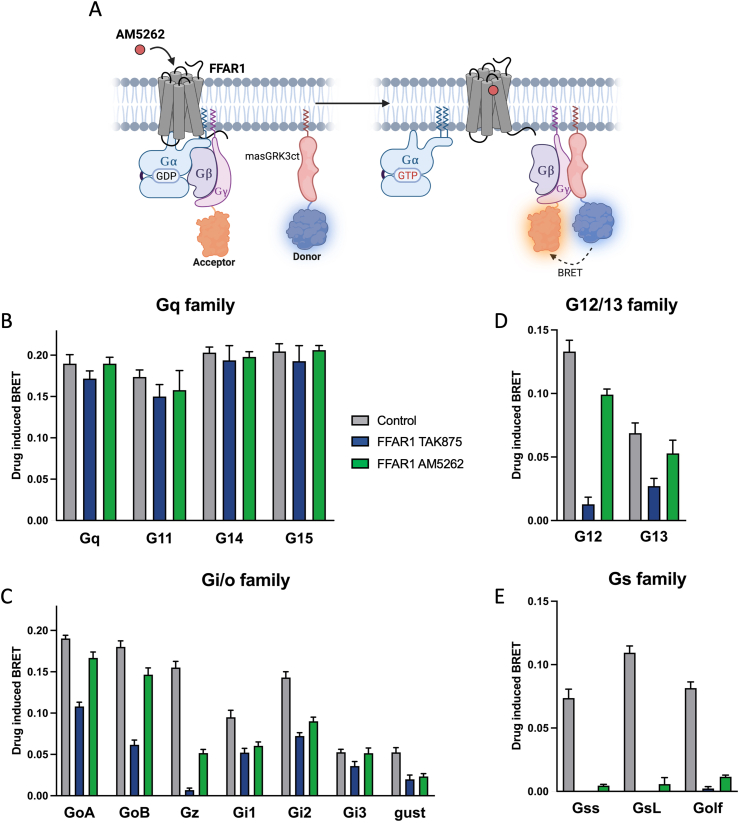
Figure 1.
FFAR1 activation of individual Gα subunits measured with a BRET-based βγ release assay.PanelA - Schematic overview of the BRET βγ release assay. The BRET donor Rluc8 is fused to the C-terminal fragment of GPCR kinase 3 anchored to the membrane by a N-terminal myristoylated peptide (masGRK3ct-Rluc8) and the acceptor mVenus is fused to γ2, which is released from the Gα subunit during receptor activation to interact with the tagged GRK3ct and thereby increase the BRET signal. Co-transfection of FFAR1 and individual Gα proteins of choice in HEK293 cells enables testing G protein activation via specific Gα′s. PanelB-E - Ligand-induced BRET response testing all 16 Gα proteins with FFAR1 induced by 1 μM TAK875 (blue columns) or AM5262 (green columns) and a positive control receptor for each G protein family (grey columns); PanelB - Gq family with muscarinic M1R activated by 1 μM carbachol as control; PanelC - Gi/o family with dopamine 2 receptor (D2R) activated by 10 μM dopamine as control; PanelD - G12/13 family with the endothelin A receptor activated by 100 nM endothelin as control; PanelE - Gs family with the β2AR activated by 1 μM isoproterenol as control. Bars and error represent mean ± SEM for three independent experiments performed in triplicate.
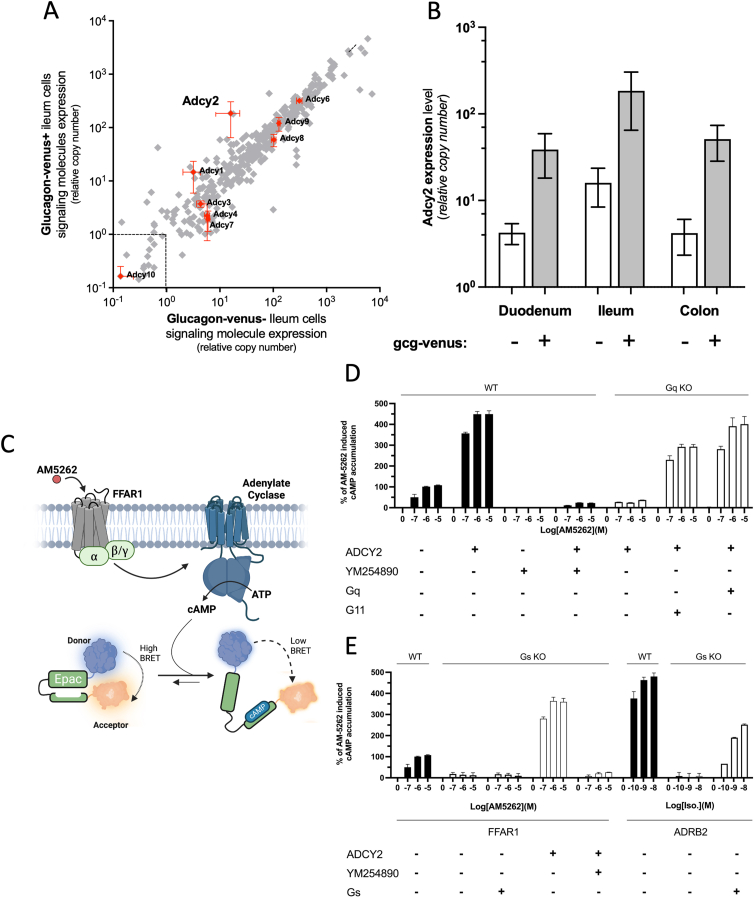
Figure 3.
Identification of ADCY2 as potential mediator of the FFAR1-induced Gq-dependent cAMP accumulation. Panel A– Expression of the ten adenylate cyclases highlighted in orange symbols in FACS purified GLU-Venus positive enteroendocrine GLP-1 cells (y-axis) versus expression in neighboring GLU-Venus negative mucosal cells (x-axis) as determined using a customized qPCR array of GPCR signal transduction genes (grey symbols) in cells isolated from the ileum of proglucagon GLU-Venus reporter mice. Dotted lines indicate detection limit corresponding to CT 35. Panel B – Expression of Adcy2 in FACS-purified GLU-Venus positive cells (grey columns) and negative mucosal cells (open columns) from the duodenum, ileum, and colon of the GLU-Venus reporter mice. Data are from 3 individual experiments, shown with mean ± SEM. Full scattergrams for duodenum and colon are shown in Figure S4. Panel C - Schematic overview of the BRET-based CAMYEL assay used for measuring cAMP accumulation within HEK293 cells (Panels D and E). Panels D and E - FFAR1-induced cAMP accumulation in response to AM5262 10−7 to 10−5 M as % of Emax observed in WT cells without ADCY2 co-transfection in WT HEK293 cells (black columns) and Gαq deficient HEK293 cells (Panel D) and in WT (black columns) and Gαs deficient HEK293 cells (Panel E) (white columns) +/− co-transfection with ADCY2 and +/− Gq inhibitor (YM254890), and +/− reintroduction of Gα proteins as indicated below the panels. To the right in Panel E are as control shown cAMP accumulations in response to isoproterenol in WT and Gs-deficient HEK293 cells transfected with the β2AR without and with reintroduction of Gs as indicated. Bars and error represent the mean ± SEM for 3 three independent experiments performed in triplicate.
2.5. CREB luciferase reporter assay
A cAMP response element-binding protein (CREB) reporter vector (Promega) was used to monitor the activity of cAMP/PKA signalling. Luciferase expression is controlled by the cAMP response element (CRE) promoter, which is activated by the CREB protein downstream of accumulation of cAMP and activation of PKA signalling (Figure 2A). HEK293A cells were transfected with hFFAR1, hM1R, hβ2AR or empty vector control (20 ng, 5 ng, 5 ng, 20 ng, respectively) and 30 ng CREB reporter vector (Promega) and supplemented in low serum (0.5%) medium overnight (ON) after transfection (Figure 2A). For rescue experiments in G protein depleted cells, co-transfection with individual G protein (1 ng and 0.1 ng for G12/13) subunits was performed on the subsequent day. Ligands +/− Gi inhibitor (ON PTX treatment 100 ng/ml) or Gq inhibitors (1 μM YM254890 or 5 μM edelfosine added 30 min prior to ligand) were added and cells incubated for 5 h at 37 °C, 5% CO2. After incubation, plates were washed with 200 μl/well DPBS (Gibco, Life Technologies) and 50 μl DPBS was added along with 50 μl Steadyliteplus (PerkinElmer). Plates were shielded from light and incubated for 10 min while gently shaking. Luciferase activity was measured on an EnVision (PerkinElmer). Determinations were made in triplicates.
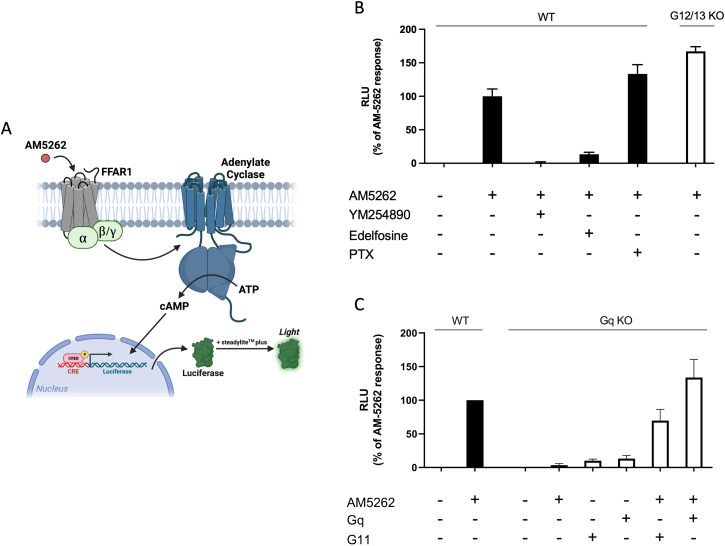
Figure 2.
Identification of Gα subunit driving FFAR1-induced cAMP accumulation as measured by CREB transcriptional activation.PanelA - Schematic overview of the cAMP Response Element (CRE) luciferase reporter assay where cells are co-transfected with CREB reporter and FFAR1 expression plasmids. Luciferase expression is controlled by the CRE promoter, which is activated by the CRE Binding (CREB) protein downstream of cAMP and an intracellular increase in cAMP therefore leads to increased luminescence. PanelB - CREB response in wildtype HEK293A (black bars) and CRISPR gene-edited G12/13 deficient HEK293A cells (white bars) transfected with FFAR1 in response to 1 μM AM5262 in the presence of specific inhibitors of Gq (YM254890), Phospholipase C (Edelfosine) and Gi/o (PTX); PanelC - CREB response in the gene-edited Gq deficient HEK293A cell line (white columns) transfected with FFAR1 in the absence or presence of 1 μM AM5262 and reintroduction of Gq and G11 by transfection as indicated. The response in WT HEK293A cells (black columns) for the indicated conditions are shown for comparison. Bars and error represent the mean ± SEM for 3 three independent experiments performed in triplicate.
2.6. GLP-1 secretion
STC-1 and GLUTag cells were seeded in 24 well plates that had been precoated with poly-D-lysine for 15 min at RT followed by PBS washing. The following day cells were stimulated with secretion buffer (138 mM NaCl, 4.5 mM KCl, 4.2 mM NaHCO3, 1.2 mM NaH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, and 10 mM HEPES supplemented with 0.1% (wt/vol) fatty acid-free BSA) containing 10 μM AM5262 alone or in combination with 1 μM YM254890 and left to incubate for 2 h at 37 °C. The supernatants were collected and stored at −80 °C. GLP-1 was measured using the V-PLEX ® MSD MULTI-ARRAY Assay System GLP-1 Total KIT (cat# K1503PD-1) according to the manufacturer's protocol. Measurements were determined in duplicates.
2.7. qPCR array
Total RNA was prepared using NucleoSpin RNA, Mini kit for RNA purification (Macherey–Nagel) followed by RT-PCR using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Real-time quantitative PCR (qPCR) was performed using SYBR green Real-Time PCR System (Sigma). Amplifications were carried out in 12 μl reaction solutions containing 6 μl 2× SYBR green, 0.12 μl 10 pmol/μl forward and reverse primer. PCR conditions were one cycle of 2 min at 95 °C followed by 45 amplification cycles with quantification mode activation. The PCR reaction ended with a melting cycle and cooling of the samples. CT values were calculated using formula 2–ΔΔCt to calculate the relative fold gene expression of samples compared to a control sample. Housekeeping reference genes were Actb, Gapdh, Hprt1, Tbp and Ywas. A threshold of Ct = 35 was used for undetectable targets.
2.8. Fluorescence-activated cell sorting (FACS) and quantitative PCR (qPCR)
The mice used in this study were maintained according to European and Danish guidelines for the care and safe use of experimental animals. Pancreas and the three divisions of the intestine; ileum, duodenum and, colon were collected from GLU-Venus transgenic male mice to obtain single-cell suspension through washing, mincing and collagenase treatment as previously described [24]. Cells were FACS sorted into venus-positive and negative cells based on fluorescence at 530 nm and 580 nm directly into lysis buffer (Ambion), and mRNA was purified using the “RNAqueous-Micro” micro-scale RNA isolation kit (Ambion, Catalogue #1931). mRNA was DNAse treated and converted into cDNA using Superscript III (Invitrogen). A custom-designed 384 well qPCR from Lonza (Copenhagen, DK) for signaling molecules analysis containing primers for 376 genes involved in the cell signaling machinery together with 8 housekeeping genes as controls was performed. Primer library can be found in Appendix 1. To gain intergenic comparable copy numbers, a genomic DNA sample was used as a calibrator as described previously [25].
2.9. Tissue preparation, RNAscope in situ hybridization and immunohistochemistry
Small segments of duodenum, ileum, colon, and pancreas were collected from 3 male C57BL/6 mice, fixed for 24 h in 4% formaldehyde, dehydrated and embedded in paraffin blocks. 5 μm sections were used for RNAscope labelling and subsequent immunohistochemistry (IHC). For detection of Ffar1, Adcy2 and Gcg commercially available RNAscope Multiplex Fluorescent Assay V2 (Advanced Cell Diagnostics) and probes against Ffar1 (Ref.N 464311), Adcy2 (Ref. N 462031-C3), and Gcg (Ref. N 400601-C2) (Advanced Cell Diagnostics), were used according to the manufacturer's protocols. To visualize additional EECs, sections after RNAscope were blocked with 5% donkey serum and incubated with antibodies against PYY (1:3000; Acris), CCK (1:8000, donated by Poulsen SS), and 5HT (1:3200, Abcam) overnight. After washing sections were incubated with species-specific secondary antibodies conjugated to Alexa Flour 690 and mounted with ProLong™ Gold Antifade Mountant with DAPI (Invitrogen). Slides were imaged using Zeizz Axio Observer microscope equipped with Axiocam 702 camera.
3. Results
3.1. G protein signaling profile of FFAR1
To identify G protein interaction partners for FFAR1 upon ligand stimulation, we used a BRET-based method where all 16 Gα subunits were tested individually in unmodified form. In the assay the BRET signal occurred between the donor Rluc8 fused to the C-terminal fragment of GPCR kinase3 anchored to the membrane and the acceptor mVenus fused to Gγ2, which is released during receptor-mediated G protein activation (Figure 1A) [26].
Using both first- (TAK875) and second-generation (AM5262) FFAR1 agonists at 1 μM, a robust BRET response was observed for both FFAR1 agonists with all four members of the Gq family, i.e. Gq, G11, G14, and G15 (Figure 1B). The efficacy was comparable to the response observed with the positive control prototype Gq coupled muscarinic 1 receptor (M1R) stimulated with a full agonist (Figure 1B grey columns). AM5262 also induced a strong BRET response with all the Gi/o family members except Gz, albeit variable in efficacy dependent upon the individual Gα subunit. Importantly, the response was in all cases of similar magnitude as observed with the positive control prototype Gi/o-coupled dopamine 2 receptor (D2R) stimulated with a full agonist (Figure 1C). That was also the case for activation of G12 and G13 where the BRET response for AM5262 and FFAR1 was similar to that of the endothelin 1 receptor (ET1R) (Figure 1D). In contrast, the response to the first-generation FFAR1 agonist TAK875 was lower or much lower for the Gi/o and G12/13 family members dependent upon the individual Gα subunits (Figure 1C,D).
For the Gs family, FFAR1 did not exhibit any BRET response when stimulated with TAK875, consistent with previous studies where cAMP accumulation was not observed with this ligand [15]. However, stimulation of FFAR1 with AM5262, which previously have been shown to elicit a strong cAMP response [15], surprisingly only resulted in a negligible Gs-mediated BRET response, as compared to the response observed with the prototype Gs-coupled β2AR (Figure 1E).
Thus, we find that FFAR1, when stimulated with AM5262, can activate the full range of Gα proteins with the notable exception of the Gs family, which had been inferred to be responsible for the robust cAMP accumulation previously reported with this class of FFAR1 agonists [15].
3.2. The cAMP response of FFAR1 is dependent on Gq activation
In order to determine which Gα subunit that drives the cAMP response induced by AM5262 through FFAR1, we initially used the cAMP Response Element Binding (CREB) luciferase transcriptional reporter assay as a readout [20]. Wildtype and gene-edited HEK293A cells, deficient in various Gα subunits [21,22], were transfected with either FFAR1 or as a control, β2AR in combination with the CREB reporter plasmid and different pharmacological pathway inhibitors.
AM5262 stimulation of FFAR1 induced a robust CREB response (Figure 2B), which was eliminated by the Gq inhibitor, YM254890, and strongly inhibited by the PLC inhibitor edelfosine (Emax 13.5% ± 1.7% of control). The response was increased by the Gi/o inhibitor PTX (Emax 133 ± 8%) consistent with loss of the inhibitory effect of endogenous Gi on cAMP levels. In HEK293 cells lacking G12 and G13 we observed a similar, even larger CREB response to AM5262 (Emax 167 ± 4%) indicating that G12/13 are dispensable for AM5262-induced cAMP response (Figure 2B). Thus, although FFAR1 can activate Gi/o and G12/13 (Figure 1), the AM5262 induced CREB response is dependent solely on activation of the Gq, PLC pathway. To further substantiate this notion, we used a Gq deficient HEK293A cell line which still express all other Gα subunits including Gs [22] and, importantly, has an intact CREB response to the activation of the Gs-coupled β2AR demonstrating that the classical Gs pathway is intact in these cells (Figure S1). Nevertheless, in these cells the CREB response to AM5262-stimulated FFAR1 was totally eliminated. Importantly, the response was rescued by co-transfection with either Gq or G11 (Emax 134 ± 27% for Gq and 69.8 ± 16.8% for G11) (Figure 2C).
Together the data strongly indicates that the cAMP response induced by FFAR1 upon AM5262 stimulation is mediated not through Gs but rather through the Gq, PLC pathway.
3.3. Adcy2 is highly expressed and enriched as compared to the other Adcys in enteroendocrine cells
Since the FFAR1-mediated cAMP accumulation appeared to be Gq, and not Gs, driven, we reasoned that it might be mediated through one of the non-classical adenylate cyclases that can be activated through Gq signaling. To explore this hypothesis in cells with endogenous FFAR1 expression and function, we determined the expression of all the different isoforms of adenylate cyclase by qPCR in FACS-purified enteroendocrine cells expressing the proglucagon, GLU-Venus reporter [4,24].
Among the ten adenylate cyclases, adenylate cyclase 2 (Adcy2), stood out in being surprisingly not only the second highest expressed cyclase but also in being approximately 12-fold enriched in the pro-glucagon expressing GLP-1 cells from the ileum as compared to the neighboring GLU-Venus negative cells (Figure 3A). Adcy2 was similarly highly expressed and enriched in GLU-Venus positive enteroendocrine cells of the duodenum (9-fold), and the colon (12-fold) (Figure 3B, Figure S1).
Thus, ADCY2, which is activated by Gq signaling [27,28], is surprisingly highly expressed and enriched in enteroendocrine cells and could accordingly be involved in the FFAR1-induced cAMP response and hormone secretion.
3.4. FFAR1-mediated cAMP accumulation is dependent on ADCY2 and Gq but not Gs
Using a CAMYEL BRET assay to measure cAMP production in the cells [20] (Figure 3C), we observed that co-transfection of FFAR1 with ADCY2 in WT HEK293A cells led to a 4.5-fold increase in maximal AM5262-induced cAMP response (Emax 449 ± 7%) - a response that importantly was almost fully inhibited by the specific Gq inhibitor YM254890 (Figure 3D). In Gq/G11 deficient HEK293A cells, the large AM5262 cAMP response observed upon FFAR1/ADCY2 co-transfection was almost eliminated but was rescued by reintroducing either Gq (Emax 401 ± 37%) or G11 (293 ± 11%) (Figure 3D).
In cells deficient in Gs/Golf the FFAR1-mediated cAMP response to AM5262 was lost, which was surprising as the cells still expressed normal levels of the Gq family. The response was, however, not rescued by reintroducing Gs, showing that the lack of response was not caused by the lack of Gs (Figure 3E). Interestingly, the expression of Adcy2, which is normally already rather low in HEK293 cells, was further reduced in the Gs deficient HEK293 cells (CT values < 35) (Figure S2). Thus, when FFAR1 was co-transfected with ADCY2 in the Gs-deficient cells, the AM5262 induced cAMP response was completely restored (Emax 360 ± 17%). In addition this response was basically eliminated by the Gq blocker, YM254890 (Figure 3E) as previously observed in WT HEK293A cells (Figure 3D). This AM5262-induced, FFAR1/ADCY2-mediated cAMP response was of almost similar magnitude as the isoproterenol-induced β2AR-mediated cAMP response observed in WT HEK293A cells, a response which, as expected, was eliminated in Gs-deficient cells and – as expected - rescued by introduction of Gs, as previously reported (Figure 3E) [29]. It is also noteworthy that the introduction of ADCY2 strictly increased cAMP levels in response to AM5262 stimulation, as no constitutive activity was detected.
Thus, co-expression of ADCY2, with FFAR1 in HEK293A cells greatly enhances the cAMP response to AM5262 in an entirely Gq dependent manner.
3.5. Endogenous Ffar1 is highly co-expressed with Adcy2 in enteroendocrine cells
To understand the endogenous co-expression of Ffar1 and Adcy2, we used RNAscope in situ hybridization in the murine intestine. The RNAscope probes for Ffar1 and Adcy2 both generated strong fluorescent signal in populations of scattered enteroendocrine-like cells in the intestinal epithelium demonstrating that Adcy2 is expressed in basically all cells expressing Ffar1 as shown for the ileum in Figure 4A. However, Adcy2 was also expressed in many enteroendocrine-like cells that did not express Ffar1 (Figure 4A). Triple RNAscope staining demonstrated co-expression of Adcy2 with both Ffar1 and proglucagon (Gcg) in enteroendocrine cells in e.g. the ileum (Figure 4B). Combination of Adcy2 FISH with immunohistochemical staining for PYY or CCK showed that Adcy2 is also expressed in CCK and PYY cells (Figure 4B). Similar patterns of Adcy2 co-expression with CCK, Gcg, and PYY were observed in duodenum, ileum and colon (Figure S5). Interestingly, however, Adcy2 was not expressed in intestinal 5HT-storing enterochromaffin cells (Figure S5), which also do not express Ffar1 as GLP-1 from neighboring L-cells is the main driver of nutrient-induced secretion through the Gs-coupled GLP-1 receptor [30].
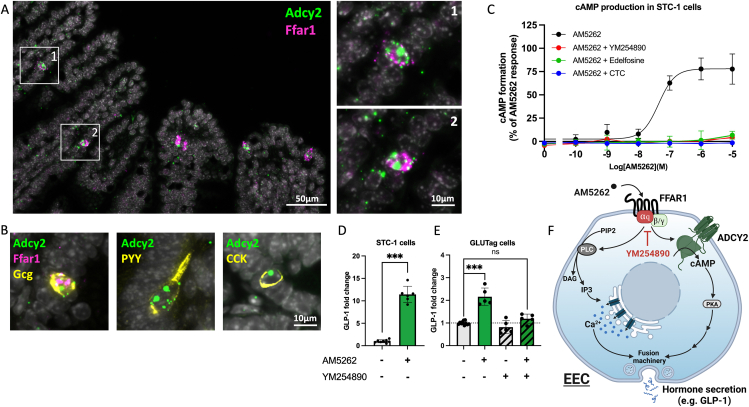
Figure 4.
Adcy2 expression and FFAR1-induced cAMP accumulation and GLP-1 secretion in enteroendocrine cells and cell lines. Panel A – Co-expression of Adcy2 with Ffar1 in enteroendocrine cells in murine ileum as determined by RNAscope in situ hybridization; A1 – zoom-in corresponding to box 1; A2 – zoom-in corresponding to box 2. Panel B – Colocalization of Adcy2 and peptide hormones in murine ileal enteroendocrine cells; left panel - multiplex RNAscope in situ hybridization of Adcy2, Ffar1, and Gcg – proglucagon/‘GLP-1’; middle panel – co-localization of Adcy2 (RNAscope) and PYY (Immunohistochemistry); right panel – colocalization of Adcy2 (RNAscope) and CCK (immunohistochemistry). Similar histological data including co-staining for 5HT for duodenum, Ileum and colon are shown in Supplementary Figure S5. Panel C – cAMP accumulation in STC-1 cells in response to AM5262 (black), AM5262 + Gq-inhibitor, YM254890 (red), PLC-inhibitor edelfosine (green) or PKC-inhibitor chelerythrine chloride, CTC (blue). Data represents mean ± SEM for 3 independent experiments performed in triplicate for AM5262 and 2 independent experiments performed in triplicates for the inhibitors. Panel D – GLP-1 secretion from STC-1 cells stimulated with 1 μM AM5262; mean ± SEM from 3 independent experiments. Panel E - GLP-1 secretion from GLUTag cells stimulated with AM5262 1 μM in the absence or presence of the specific Gq-inhibitor YM254890; mean ± SEM from 3 independent experiments. Statistical significance was calculated using two-tailed Mann–Whitney t test: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Panel F – Simplified schematic overview in enteroendocrine cell (EEC) of the proposed dual signal-transduction pathway activation by FFAR1 in response to second generation agonists (here AM5262) where Gq activates both the PLC/IP3/Ca2+ pathway and the ADCY2/cAMP/PKA pathways acting in synergy to stimulate enteroendocrine hormone secretion (GLP-1) and that both signaling pathways are blocked by the Gq inhibitor YM254890.
In the pancreatic islets Ffar1 was as expected strongly expressed in the majority of centrally located presumably β-cells, which in contrast to the Ffar1 expressing enteroendocrine cells did not show any sign of Adcy2 expression (Figure S6). The Gcg expressing pancreatic α-cells did not express Adcy2, which however was clearly detected in a few unidentified cells at the perifery of the islets (Figure S6).
Thus, Adcy2 appears to be strongly expressed in Ffar1 expressing, gut hormone producing intestinal enteroendocrine cells.
3.6. AM5262-induced cAMP and GLP-1 secretion in enteroendocrine cells is Gq mediated
All of the functional studies described above were performed in various forms of transfected HEK-293 cells. To evaluate to what degree the effect of AM5262 on gut hormone secretion is dependent upon Gq in enteroendocrine cells, we turned to two different immortalized enteroendocrine cell lines, STC-1 and GLUTag cells [30, 31]. As shown in Figure 4D, 1 μM of AM5262 induced a 11.4 ± 0.7-fold stimulation of GLP-1 secretion in the STC-1 cells, which as demonstrated by qPCR endogenously express the relevant signaling proteins: Ffar1, Gnaq, and – importantly - Adcy2 as well as proglucagon (Figure S3). AM5262 in a dose-dependent manner increased intracellular cAMP in the STC-1 cells with an EC50 of 44.4 nM, a response that importantly was blocked by the Gq inhibitor YM254890 as well as the PLC inhibitor edelfosine and the PKC-inhibitor chelerythrine chloride (Figure 4C). In the prototype enteroendocrine GLUTag cell line, which has been used extensively for studies of gut hormone secretion [32], AM5262 also stimulated GLP-1 secretion, i.e. 2.2 ± 0.2-fold, a response that was totally blocked by the specific Gq inhibitor, YM254890 (Figure 4E).
It is concluded that Gq is responsible for the full enteroendocrine secretagogue effect of second-generation FFAR1 agonists by activating not only the classical PLC/IP3/Ca2+ signaling pathway but also a non-classical ADCY2/cAMP signaling conceivably acting in synergy, independently of Gs (Figure 4F).
4. Discussion
The aim of the present study was to clarify the mechanism of the robust cAMP response observed upon activation of FFAR1 by second-generation synthetic agonists - here AM5262 – which is responsible for their highly efficacious GLP-1 secretagogue activity. We find that FFAR1 can activate not only the Gq family of G proteins but also members of the Gi and G12/13 families, but surprisingly not the Gs family. Importantly, the FFAR1-induced cAMP accumulation is likely mediated through Gq activation of the non-classical ADCY2, which was found to be particularly highly expressed and enriched in FFAR1 and gut hormone expressing enteroendocrine cells.
4.1. FFAR1 activates ADCY2 through Gq independently of Gs
cAMP accumulation is traditionally linked to Gs stimulation of the classical adenylate cyclases (ADCYs). In several cases the mere presence of Gαs subunits is specifically required for the ADCY function as they in the absence of Gs cannot be activated even by direct modulators such as forskolin [33]. Nevertheless, already in the 1990's it was shown that Gi/o and Gq, and later also G12/13, could stimulate cAMP accumulation through activation of the non-classical adenylate cyclase ADCY2 [28]. This was proposed to be mediated by βγ release or via PKC. In the original study the ADCY2-mediated cAMP accumulation stimulated by the Gq-coupled M1R was in fact dependent on co-transfection with a constitutively active Gs [28]. More recently it has, however, been shown that carbachol-induced M1R activation can lead to cAMP accumulation without co-activation by Gs and that this was mediated through activation of AKAP79, PKC and ADCY2 [27]. In the present study Gs was completely dispensable for the FFAR1-induced accumulation of cAMP as shown with the BRET-based Epac sensor in the Gs deficient HEK293 cells; importantly, only in the presence of ADCY2. This was not the case for the classical Gs-coupled β2AR, where Gs was essential for receptor-induced cAMP accumulation. Further studies are required to identify the precise molecular mechanism through which FFAR1 is activating ADCY2 in a strictly Gq dependent manner.
4.2. Adcy2 is highly expressed and enriched in enteroendocrine cells
ADCY2 is mainly known to be expressed in the brain and in skeletal muscle but is in fact also found in endocrine tissues such as the adrenal and parathyroid glands (proteinatlas.org). In the present study, we find by qPCR analysis of FACS-purified cells and by RNAscope in situ hybridization that Adcy2 in the intestine is highly expressed selectively in gut hormone producing enteroendocrine cells. This opens for the possibility that not only FFAR1, but also other Gq- or perhaps even Gi/o coupled receptors e.g. the Gi-coupled short chain fatty acid receptor, FFAR3/GPR41 known to stimulate hormone secretion could possibly do so in a similar manner through activation of ADCY2 in enteroendocrine cells.
Our original observation of robust stimulation of cAMP accumulation by FFAR1 was confirmed by Ho and coworkers using the same WT HEK293 cells, and similar cAMP assays [34]. However, using CHO-K1 cells Rives and coworkers were unable to demonstrate significant Gs coupling of FFAR1 and only observed a minor cAMP accumulation in response to FFAR1 activation [19]. In view of our present observation that ADCY2 most likely is responsible for the FFAR1-induced cAMP production these different results are conceivably caused by differences in ADCY2 expression in the two different cell types employed. Thus, in our WT HEK293 cells ADCY2 is expressed at a relatively low but sufficient level to obtain a FFAR1-mediated cAMP response (Figure S2). On the contrary, the gene-edited Gs-deficient HEK293 cells barely have detectable ADCY2 expression, and we do not observe any FFAR1-mediated cAMP response. However, by introducing ADCY2, but not Gs, in these cells we could restore a robust cAMP response to FFAR1 stimulation. Most importantly, Adcy2 is highly expressed in the endogenous enteroendocrine cells and cell lines where Ffar1 is normally expressed as demonstrated here by both qPCR in FACS purified cells and by RNAscope in situ hybridization.
Interestingly Adcy2 is not expressed in the pancreatic β-cells which is the other main cells type of the body with high Ffar1 expression. This means that the important function of FFAR1 in glucose-induced insulin secretion via autocrine sensing of 20-HETE probably is mediated only though a classical Gq/IP3/Ca2+ pathway as previously described [5,6].
4.3. First vs. second generation or partial versus full FFAR1 agonists
Second-generation FFAR1 agonists are more efficacious in respect of stimulating GLP-1 secretion both ex vivo and in vivo than first-generation compounds [15]. Originally, we assumed that this was a qualitative difference in second-generation agonist being able to activate not only Gq - like first-generation compounds - but also Gs and thereby provide an additional cAMP response to act in synergy with the IP3/Ca2+ response. However, we now know that the cAMP response is also mediated through Gq, meaning that the difference in efficacy likely is a ‘simple’ quantitative difference similar to partial versus full agonism. In fact, the second-generation agonists are also more efficacious in respect to stimulating IP3 accumulation than first-generation agonists and at least for some of the first-generation agonists a very small cAMP response may be detected [15]. However, it cannot be excluded that other G proteins or accessory proteins could be involved. Although the agonists in the BRET-based assay of the present study appear to be rather equal in respect to activating Gq family members, the prototype first-generation agonist, TAK875 was clearly less efficacious in activating Gi/o and in particular G12/13 family members (Figure 1). Although Gi/o and G12/13 were not directly involved in the cAMP response to AM5262 (Figure 2), future studies may reveal more complex interactions between the G proteins that perhaps can explain the inability of TAK875 to stimulate cAMP - beyond just being a partial agonist.
Although being chemically relatively similar, the two FFAR1 agonist classes display clear positive allosteric cooperativity as they in radioligand binding assays compete for binding within each class while they enhance binding of members of the ‘other’ class [15]. Notably X-ray structures have subsequently shown that the two classes of agonists bind to two distinct sites on FFAR1 [35,36]. First-generation agonists bind to the so-called outer-leaflet, lipid-exposed binding site whereas second-generation agonists bind to the also lipid-exposed ‘inner leaflet’ binding site of FFAR1 [37]. However, how agonist binding to the inner leaflet site promotes higher Gq activation efficacy and robust ADCY2-mediated cAMP accumulation remains to be determined. Interestingly, the endogenous long chain fatty acid agonists, which likely can bind to both sites, only activates PLC/IP3/Ca2+ and not cAMP [15,37].
Author contributions (CRediT roles)
MT, MHP, JJ, TWS: Conceptualization. MT, MHP: Project administration. MT, MHP, JEP: Methodology, Formal analysis. MT, MHP, JEP, TA, MSE: Investigation. TWS, MHP: Funding acquisition and Resources. MT, JEP, TWS, WA: Visualization. TWS, MT: Supervision. MHP, MT: Writing - original draft. MT, MHP, TWS, JEP, JJ, approved for publication by all authors: Writing - review & editing.
Acknowledgement
We thank Frank Reimann and Fiona Gribble for providing the GLU-Venus mouse. We also thank Asuka Inoue for providing the CRISPR edited HEK293 G protein KO cells and Pernille Baumann Toft for helping with GLP-1 secretion studies in GLUTag cells. The project was supported by Challenge Grant NNF140C0013655 (T.W.S.) and Grant NNF17OC0024830 (M.H.P.), both from the Novo Nordisk Foundation. The Novo Nordisk Foundation Center for Basic Metabolic Research (www.metabol.ku.dk) is supported by an unconditional grant, NNF10CC1016515 from the Novo Nordisk Foundation to University of Copenhagen. Lastly, we thank Sofie Liljewall for generation of illustrations made with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101757.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003 Mar 13;422(6928):173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 2.Briscoe C.P., Tadayyon M., Andrews J.L., Benson W.G., Chambers J.K., Eilert M.M., et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003 Mar 28;278(13):11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 3.Edfalk S., Steneberg P., Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008 Sep;57(9):2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabol. 2008 Dec;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trauelsen M., Lückmann M., Frimurer T.M., Schwartz T.W. The HETE is on FFAR1 and pancreatic islet cells. Cell Metabol. 2018 Feb 6;27(2):273–275. doi: 10.1016/j.cmet.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Tunaru S., Bonnavion R., Brandenburger I., Preussner J., Thomas D., Scholich K., et al. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Commun. 2018 Jan 12;9(1):177. doi: 10.1038/s41467-017-02539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito R., Tsujihata Y., Matsuda-Nagasumi K., Mori I., Negoro N., Takeuchi K. TAK-875, a GPR40/FFAR1 agonist, in combination with metformin prevents progression of diabetes and β-cell dysfunction in Zucker diabetic fatty rats. Br J Pharmacol. 2013 Oct;170(3):568–580. doi: 10.1111/bph.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancini A.D., Poitout V. GPR40 agonists for the treatment of type 2 diabetes: life after “TAKing” a hit. Diabetes Obes Metabol. 2015 Jul;17(7):622–629. doi: 10.1111/dom.12442. [DOI] [PubMed] [Google Scholar]
- 9.Burant C.F., Viswanathan P., Marcinak J., Cao C., Vakilynejad M., Xie B., et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012 Apr 14;379(9824):1403–1411. doi: 10.1016/S0140-6736(11)61879-5. [DOI] [PubMed] [Google Scholar]
- 10.Desai D., Mehta D., Mathias P., Menon G., Schubart U.K. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabetes Care. 2018 Aug;41(8):1631–1638. doi: 10.2337/dc17-1379. [DOI] [PubMed] [Google Scholar]
- 11.Marcinak J.F., Munsaka M.S., Watkins P.B., Ohira T., Smith N. Liver safety of fasiglifam (TAK-875) in patients with type 2 diabetes: review of the global clinical trial experience. Drug Saf. 2018;41(6):625–640. doi: 10.1007/s40264-018-0642-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang L., Prasad B., Salphati L., Chu X., Gupta A., Hop C.E.C.A., et al. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos. 2015 Mar;43(3):367–374. doi: 10.1124/dmd.114.061580. [DOI] [PubMed] [Google Scholar]
- 13.Otieno M.A., Snoeys J., Lam W., Ghosh A., Player M.R., Pocai A., et al. Fasiglifam (TAK-875): mechanistic investigation and retrospective identification of hazards for drug induced liver injury. Toxicol Sci. 2018 Jun 1;163(2):374–384. doi: 10.1093/toxsci/kfx040. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Liu J.J., Dransfield P.J., Zhu L., Wang Z., Du X., et al. Discovery and optimization of potent GPR40 full agonists containing tricyclic spirocycles. ACS Med Chem Lett. 2013 Jun 13;4(6):551–555. doi: 10.1021/ml300427u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauge M., Vestmar M.A., Husted A.S., Ekberg J.P., Wright M.J., Di Salvo J., et al. GPR40 (FFAR1) - combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metabol. 2015 Jan;4(1):3–14. doi: 10.1016/j.molmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotarsky K., Nilsson N.E., Flodgren E., Owman C., Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003 Feb 7;301(2):406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- 17.Hauge M., Ekberg J.P., Engelstoft M.S., Timshel P., Madsen A.N., Schwartz T.W. Gq and Gs signaling acting in synergy to control GLP-1 secretion. Mol Cell Endocrinol. 2017 Jul 5;449:64–73. doi: 10.1016/j.mce.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002 Sep;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 19.Rives M.-L., Rady B., Swanson N., Zhao S., Qi J., Arnoult E., et al. GPR40-Mediated Gα12 activation by allosteric full agonists highly efficacious at potentiating glucose-stimulated insulin secretion in human islets. Mol Pharmacol. 2018 Jun;93(6):581–591. doi: 10.1124/mol.117.111369. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L.I., Collins J., Davis R., Lin K.-M., DeCamp D., Roach T., et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem. 2007 Apr 6;282(14):10576–10584. doi: 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrage R., Schmitz A.-L., Gaffal E., Annala S., Kehraus S., Wenzel D., et al. The experimental power of FR900359 to study Gq-regulated biological processes. Nat Commun. 2015 Dec 14;6 doi: 10.1038/ncomms10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stallaert W., van der Westhuizen E.T., Schönegge A.-M., Plouffe B., Hogue M., Lukashova V., et al. Purinergic receptor transactivation by the β2-adrenergic receptor increases intracellular Ca2+ in nonexcitable cells. Mol Pharmacol. 2017 May;91(5):533–544. doi: 10.1124/mol.116.106419. [DOI] [PubMed] [Google Scholar]
- 23.Free R.B., Chun L.S., Moritz A.E., Miller B.N., Doyle T.B., Conroy J.L., et al. Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol Pharmacol. 2014 Jul;86(1):96–105. doi: 10.1124/mol.113.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker H.E., Habib A.M., Rogers G.J., Gribble F.M., Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009 Feb;52(2):289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelstoft M.S., Park W.-M., Sakata I., Kristensen L.V., Husted A.S., Osborne-Lawrence S., et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metabol. 2013 Sep 4;2(4):376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuho I., Martemyanov K.A., Lambert N.A. Monitoring G protein activation in cells with BRET. Methods Mol Biol. 2015;1335:107–113. doi: 10.1007/978-1-4939-2914-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J.X., Cooper D.M.F. AKAP79, PKC, PKA and PDE4 participate in a Gq-linked muscarinic receptor and adenylate cyclase 2 cAMP signalling complex. Biochem J. 2013 Oct 1;455(1):47–56. doi: 10.1042/BJ20130359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustig K.D., Conklin B.R., Herzmark P., Taussig R., Bourne H.R. Type II adenylylcyclase integrates coincident signals from Gs, Gi, and Gq. J Biol Chem. 1993 Jul 5;268(19):13900–13905. [PubMed] [Google Scholar]
- 29.Milligan G., Inoue A. Genome editing provides new insights into receptor-controlled signalling pathways. Trends Pharmacol Sci. 2018 May;39(5):481–493. doi: 10.1016/j.tips.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Lund M.L., Egerod K.L., Engelstoft M.S., Dmytriyeva O., Theodorsson E., Patel B.A., et al. Enterochromaffin 5-HT cells - a major target for GLP-1 and gut microbial metabolites. Mol Metabol. 2018 May;11:70–83. doi: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi L., Shuai T., Da J., Ezra B., Luis S.-D. In-vitro GLP-1 release assay using STC-1 cells. Bio Protoc. 2020 Aug 20;10(16) doi: 10.21769/BioProtoc.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gribble F.M., Williams L., Simpson A.K., Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003 May;52(5):1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 33.Green D.A., Clark R.B. Direct evidence for the role of the coupling proteins in forskolin activation of adenylate cyclase. J Cyclic Nucl Res. 1982;8(5):337–346. [PubMed] [Google Scholar]
- 34.Ho J.D., Chau B., Rodgers L., Lu F., Wilbur K.L., Otto K.A., et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat Commun. 2018 Apr 25;9(1):1645. doi: 10.1038/s41467-017-01240-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava A., Yano J., Hirozane Y., Kefala G., Gruswitz F., Snell G., et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014 Sep 4;513(7516):124–127. doi: 10.1038/nature13494. [DOI] [PubMed] [Google Scholar]
- 36.Lu J., Byrne N., Wang J., Bricogne G., Brown F.K., Chobanian H.R., et al. Structural basis for the cooperative allosteric activation of the free fatty acid receptor GPR40. Nat Struct Mol Biol. 2017 Jul;24(7):570–577. doi: 10.1038/nsmb.3417. [DOI] [PubMed] [Google Scholar]
- 37.Lückmann M., Trauelsen M., Frimurer T.M., Schwartz T.W. Structural basis for GPCR signaling by small polar versus large lipid metabolites-discovery of non-metabolite ligands. Curr Opin Cell Biol. 2020 Apr;63:38–48. doi: 10.1016/j.ceb.2019.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.