Abstract
Objectives:
Dementia caregivers (CGs) are at heightened risk for developing problems with anxiety and depression. Much attention has been directed toward developing and deploying interventions designed to protect CG health but few have been supported by rigorous empirical evidence. Technology-based interventions that are effective, scalable, and do not add greatly to CG burden are of particular interest.
Methods:
We conducted a nine-month randomized controlled trial in 63 homes evaluating People Power Caregiver (PPCg), a system of sensors in the home connected to cloud-based software that alerts CGs about worrisome deviations from normal patterns (e.g., falls, wandering).
Results:
CGs in the active condition had significantly less anxiety than those in the control condition at the six-month assessment. Greater anxiety reduction in the active condition at the six-month assessment was associated with greater interaction with PPCg via SMS text messages. There were no differences in anxiety at the three-month or nine-month assessments or in depression at any assessment.
Conclusions:
PPCg shows promise for reducing anxiety associated with caring for a PWD.
Clinical implications:
Technology-based interventions can help reduce CG anxiety, a major adverse consequence of caregiving that may be difficult to treat due to other demands on caregiver time and energy.
Keywords: Dementia, Caregivers, Interventions, Technology, Anxiety
Introduction
Alzheimer’s disease (AD) and related dementias can produce profound deficits in cognitive, emotional, language, and motor functioning. Because these diseases are progressive, people with dementia (PWDs) become increasingly impaired and dependent on formal (paid) or informal (unpaid) caregivers (CGs) to provide functional, psychological, and economic support. With the worldwide “graying” of the population and the increasing prevalence of dementia with age (e.g., by 2050, an estimated 18.5% of individuals in the US between the ages of 75–84 and 36.6% of those 85 years or older will have AD; Hebert, Weuve, Scherr, & Evans, 2013), dementia caregiving is becoming increasingly common. In the U.S., 11 million family members and friends are estimated to provide approximately 27 hours of care per week for PWDs (Alzheimer’s Association, 2022).
Given the scope of dementia caregiving, the extensive demands it makes on CG time and energy, and the heightened risk that CGs face for significant health declines (see below), a great deal of attention is being directed toward developing and deploying interventions designed to protect CG health. Against this backdrop, technology-based interventions that are effective, scalable, and do not add greatly to CG burden are of particular interest.
Impact on CG mental health
Many studies report that CGs suffer “collateral damage” as PWDs experience dementia’s progressive, unrelenting decline. Not surprisingly, the associated worry, uncertainty, stress, burden, loneliness, and exposure to the PWD’s suffering (Monin & Schulz, 2009; Richardson, Lee, Berg-Weger, & Grossberg, 2013; Schulz, Beach, Czaja, Martire, & Monin, 2020) can have adverse effects on CGs’ mental health.
CGs manifest up to four-fold increases in rates of depression, three-fold increases in seeking treatment for anxiety, greater use of psychotropic medications, and greater suicidal ideation compared to non-caregiving adults of similar age (Collins & Kishita, 2020; Cuijpers, 2005; Dura, Stukenberg, & Kiecolt-Glaser, 1991; Kaddour & Kishita, 2020; Kolanowski, Fick, Waller, & Shea, 2004; O’Dwyer, Moyle, Zimmer-Gembeck, & De Leo, 2013; Victor, et al., 2021). These elevations in psychiatric disorders are even more striking given that their prevalence normally stabilizes or decreases for people over the age of 65 (Blazer, 1994).
These group-level comparisons between CGs and non-CGs are important for establishing aggregate risk; however, they can obscure important individual differences. Early studies of individual differences in CG health focused on the impact of “external” and demographic factors (e.g., greater health risk for low income and spousal CGs; Schulz, Visintainer, & Williamson, 1990). Subsequent research (Ornstein & Gaugler, 2012; Schulz, O’Brien, Bookwala, & Fleissner, 1995) emphasized the influence of PWDs’ behavioral and psychological symptoms (e.g., agitation and apathy). We have found poor CG health to be strongly associated with deficits in PWD emotional functioning (e.g., Brown, et al., 2018; Brown, et al., 2020; Chen, et al., 2017; Hua, et al., 2019; Otero & Levenson, 2017).
Interventions to protect CG and PWD health
CG Interventions: Current status.
As public awareness of the vulnerability of dementia caregivers has increased, a large number of caregiver interventions have been developed, many of which have shown promise in initial efficacy trials (Gitlin & Hodgson, 2015). One challenge facing many of these interventions has been the high rate of attrition (Bernstein, Grill, & Gillen, 2021; Schulz, Martire, & Klinger, 2005). As caregiving demands heighten over time, CGs can find it increasingly difficult to continue participating in interventions and associated research programs that make significant demands on their limited time and energy.
Comprehensive reviews have concluded that many interventions that fared well in initial efficacy trials neither translated into successful real-world effectiveness trials nor were disseminated into large-scale community use (Gitlin & Hodgson, 2015; Gitlin, Marx, Stanley, & Hodgson, 2015; Schulz, Martire, & Klinger, 2005). An even more sobering note was sounded in a recent comprehensive review of caregiver interventions (Butler, et al., 2020) that included almost 600 unique studies. The authors concluded that among these myriad studies and interventions, only the multi-component REACH II (Belle, et al., 2006; Luchsinger, et al., 2018) and collaborative care (Possin, et al., 2017; Possin, et al., 2019) interventions were supported by a least “low-strength” empirical evidence. Of the other interventions, they concluded: “For all other interventions and outcomes, we found the uncertainty of the evidence was too high to draw conclusions.” (page ES-2; Butler, et al., 2020).
The promise of technology-based interventions.
The preceding review has highlighted numerous converging factors that underscore the pressing need for new CG interventions that are less demanding of CG time and energy, more scalable to larger populations, more personalizable, more flexible over time, and better supported by rigorous empirical evidence. Accordingly, there has been increasing interest in using modern digital technologies such as remote sensors, smartphones, wearables, and associated apps to support caregivers and PWDs (AARP, 2016; Bui, Park, & Giap, 2022; Daly Lynn, et al., 2019; Heins, et al., 2021; Pappada, Chattat, Chirico, Valente, & Ottoboni, 2021). Technology-based interventions can provide CGs with much needed 24/7 backup for many of the more taxing caregiving activities, especially those that require high levels of vigilance and attention (e.g., protecting PWDs from harm). Not surprisingly, many of the first wave technology-based solutions were home-safety devices repurposed for use with dementia CGs. For example, Personal Emergency Response Systems--the devices popularized in the “help me I’ve fallen” ads--generated US sales of $2.5 billion in 2020 (Global Industry Analysts, 2022). Unfortunately, most of these devices are “set and forget” (e.g., not capable of learning and adapting to the different behavioral patterns and changing needs of CGs and PWDs) and require the wearer to initiate requests for help (which may not be possible due to incapacity and/or impaired judgment) rather than enabling the technology to detect dangerous situations and request needed help.
Technology-based interventions seem well-suited for addressing the worry and need for vigilance (Mahoney, et al., 2003; Schulz & Sherwood, 2008) that many CGs experience and that likely contribute to their heightened vulnerability to anxiety disorders (Joling, et al., 2010; Joling, et al., 2015). If these interventions were only able to reduce CG anxiety, they would still make an enormously important contribution. However, technology in the modern age can also increase human connection (e.g., Facebook, dating apps, shared-interest groups). In addition, new technologies have targeted “affective computing”, including producing “socially-sensitive” robotic companions (e.g., Breazeal, Ostrowski, Singh, & Park, 2019; Ostrowski, DiPaola, Partridge, Park, & Breazeal, 2019).
Despite the great promise of harnessing technology to help caregivers and the increasing number of technology-based products on the market, there has been a paucity of rigorous research evaluating their ability to improve the health and well-being of CGs and PWDs. Among the few published studies, most are small-N pilot studies or “proof-of-concept” demonstrations (e.g., Austrom, et al., 2015; Guerra, Rodrigues, Demain, Figueiredo, & Sousa, 2013). In the previously cited comprehensive review of caregiver interventions (Butler, et al., 2020), only three studies were identified that evaluated in-home assistive technology. All three were classified as “pilot studies” and none met the minimal research standards for inclusion in the article’s main analyses. Similarly, for robot-assisted interventions, only one of seven studies identified met the research standards for inclusion in the main analysis. This study (Moyle, et al., 2017), which evaluated a robotic baby harp seal, did not report CG outcomes (effects on PWD engagement and agitation were mixed).
Methods
Participants
63 PWDs and their primary caregivers were recruited through: (a) the Memory and Aging Center at the University of California, San Francisco (N = 11); and (b) a professional recruitment firm (Recruitment Partners) that specializes in populating clinical trials for Alzheimer’s disease and related dementias (N = 52). PWDs from the Memory and Aging Center were being seen as outpatients and had expressed interest in participating in research projects. Participants from Recruitment Partners were recruited from dementia care and caregiver support groups located in West Coast communities and were typically under the care of local neurologists and physicians.
We originally planned to recruit 80 PWDs and their CGs. However, the onset of COVID-19 restrictions made it impossible to conduct in-home installations of the assistive technology (see below) for new participants. Thus, we ended recruitment of new participants earlier than planned, but continued the study for those who already had their systems installed.
Apparatus
People Power Caregiver (PPCg) was developed with support from a Small Business Innovation Research grant from the National Institute on Aging. It consists of a set of artificial intelligence cloud services, an iPhone mobile app, a gateway device, wireless battery-operated sensors, an iPad mini, and a Google Home voice controller. The sensors, which have adhesive strips that enable easy attachment to surfaces in the home, consisted of: (a) five motion sensors, (b) three entry sensors, (c) two temperature sensors, (d) one water leak sensor, and (e) six motion sensing night lights. The gateway device communicated with the sensors via the ZigBee wireless protocol and uploaded sensor information to the cloud where machine learning-based artificial intelligence software learned the regular patterns of activity in each home and adjusted to changes in these patterns that occurred over time. Once patterns were established, the software detected short-term deviations that might signal worrisome events (e.g., door openings at unusual times, levels of motion in locations and at times that differed from normal patterns) and provided alerts (via SMS text messages and notifications pushed to the app) to the primary CG and, when appropriate, to designated friends and family members.
The second production version of PPCg, which was evaluated in this research, primarily focused on alerting CGs to potentially dangerous situations and providing them with a resource that could reduce the need for constant vigilance, decrease worry, and make CG forays away from home feel safer. It also provided several features intended to maintain social connections and reduce CG isolation: (a) an iPad-based “digital picture frame” enabled friends and families to share photos and videos; and (b) CGs were encouraged to set up a “trusted circle” of friends and families who could provide backups for receiving alerts and assistance when needed.
Although PPCg has been in a process of continual improvement and feature development since its inception, the system used by participants in the present study was “frozen” throughout the course of the study (other than minor bug fixes that were applied to all systems). This ensured that all homes, regardless of when they started participating, had the same system.
Measures
Although a full questionnaire battery including demographics and other health and well-being measures was administered, for this study we focused on anxiety and depression, which were the two CG mental health problems primarily targeted by PPCg.
Anxiety.
Caregivers completed the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988), a 21-item self-report scale of how bothered they were by different anxiety symptoms in the previous month (e.g., “nervous”; 0 = “Not at all,” 3 = “Severely – it bothered me a lot”). Scores ranged from 0 to 63, with higher scores indicating greater anxiety. This measure has shown reasonable levels of reliability and validity (Beck, Epstein, Brown, & Steer, 1988; Fydrich, Dowdall, & Chambless, 1992).
Depression.
Caregivers completed the Center for Epidemiological Studies Depression scale (CES-D; Radloff, 1977), a 20-item self-report scale of how frequently they experienced different depression symptoms in the previous week (e.g., “I felt sad”; 0 = “Rarely [Less than 1 day],” 3 = “Most or all of the time [5–7 days]”). Scores ranged from 0 to 60, with higher scores indicating greater depression. This measure has been validated for measuring depression in older adults (Beekman, et al., 1997; Haringsma, Engels, Beekman, & Spinhoven, 2004).
Procedures
CGs for PWDs living in California and other West Coast cities were recruited via local advertisement, clinic referrals, and community CG networks. An initial screening was conducted to determine whether the following eligibility criteria were met: (a) the CG was the primary unpaid (“informal”) spousal or other adult familial CG living with a PWD; (b) the PWD had received a diagnosis of dementia from a medical professional (people diagnosed with mild cognitive impairment were not included); (c) the home had Wi-Fi and internet service; and (d) the CG had an iPhone and associated cellular service. CGs who met these criteria were asked to complete the baseline questionnaire assessment online and an appointment was scheduled for our technicians to come to their home, install PPCg, and provide the CG with instructions as to its use. After the system was installed and verified to be working properly, the CG was randomly assigned to either: (a) fully activated condition (N = 34; all sensors and all associated alerts were activated); or (b) control condition (N = 29; only the water leak and moisture sensor and associated alerts were activated). The study lasted for a total of nine months with the questionnaire package administered again at three months, six months, and nine months. The four administrations of the questionnaires were essentially identical with the exception that some demographic questions were not repeated and user satisfaction items were added to the six-month and nine-month assessments.
During the course of the study, there were no additional planned interactions with participants beyond the communications to and from the PPCg system and the questionnaires. However, participants were offered technical support via People Power staff if needed. At the end of the nine-month study, participants in the control condition were offered the opportunity to have their PPCg systems fully activated for an additional nine-month period without charge and to continue completing the questionnaire packages at 12, 15, and 18 months.
Participants in both conditions were paid $175 for their participation in the nine-month study (prorated if all questionnaires were not completed). Participants in the control condition who agreed to have their systems fully activated and continue completing the questionnaires were paid an additional $75 (prorated if all questionnaires were not completed).
Results
Of the 66 participants randomized to the two experimental conditions, 63 (95%) completed all four questionnaires. All subsequent analyses only used data from these 63 participants, thus, ensuring that the same participants were included at all time points and that no missing data needed to be estimated.
Preliminary analyses
Analysis of CG demographics revealed that the sample was relatively old (mean age = 63.7, standard deviation = 11.6), female (78%), well-educated (33% completed a four-year college; 37% had advanced degrees), financially secure (32% had annual household income of $100,000 or more), and non-Hispanic White or European American (76%). CGs were primarily spouses or partners of PWDs (67%) or their adult children (27%).
Reliabilities for both the anxiety and depression baseline measures were high (Cronbach’s alpha: anxiety = .88; depression = .90).
Demographics and baseline measures for the active and control conditions were compared using χ² tests for categorical variables and independent sample t-tests for continuous variables. These revealed no differences for CG demographic variables of age, education, household income, ethnicity, or relationship to the PWD. A difference was found for sex, with the proportion of male caregivers greater for the active condition than the control condition (active = 32% male; control = 10% male; χ² = 4.39, p = .036). Examination of the baseline assessments for the two outcome variables revealed no differences in depression. However, the difference in anxiety approached significance (active M = 8.12, control M = 11.8; t [61] = 1.90, p = .062). Key comparisons between the active and control conditions are presented in Table 1.
Table 1.
Demographic and baseline assessments: Fully activated versus control conditions
| Demographics | Fully activated | Control | Statistical comparison |
|---|---|---|---|
|
| |||
| Gender (% male) | 32.4% | 10.3% | χ2(1) = 4.39, p=.036 |
| Age: Mean (SD) | 62.85 (12.71) | 64.62 (10.29) | t(61) = .60, p = .551 |
| Anxiety: Mean (SD) | 8.12 (7.21) | 11.83 (8.28) | t(61) = 1.90, p = .062 |
| Depression: Mean (SD) | 18.53 (11.22) | 19.76 (10.67) | t(61) = .443, p = .659 |
Main analyses
Because preliminary analyses comparing the active and control conditions revealed significant differences in CG sex and differences in baseline anxiety that approached significance, we initially conducted an overall 2 X 2 X 3 (Outcome measure X Condition X Assessment) repeated-measures MANCOVA using CG sex and baseline levels of the outcome measures as covariates. This MANCOVA revealed a significant interaction of Outcome measure X Condition, F(1,59) = 5.47, p = .023. We then computed separate 2 X 3 (Condition X Assessment) MANCOVAs for the anxiety and depression measures (with gender and baseline level of the outcome measure as covariates).
For anxiety, the Condition effect approached significance, F(1,59) = 3.45, p = .068, partial eta2 = .055, with anxiety levels lower in the active condition than in the control condition (active: M = 8.56, SE = .73; control: M = 10.61, SE = .80). In addition, although the linear effect for the Condition X Assessment interaction was not significant, F(1,59) = .02, p = .882, partial eta2 = .000, the quadratic effect for this interaction approached significance, F(1,59) = 3.50, p = .066, partial eta2 = .056. Decomposing this interaction further, univariate GLM’s revealed that at the six-month assessment, anxiety levels (adjusted for the covariates) were significantly lower in the treatment condition compared to the control condition, F(1,59) = 6.50, p = .013, partial eta2 = .099 (active: M = 7.30, SE =.99; control: M = 11.10, SE = 1.07). Anxiety levels did not different between the active and control conditions at either the three-month, F(1,59) = .61, p = .44, or 9-month assessments, F(1,59) = 1.00, p = .32. These findings are depicted in Figure 1.
1.
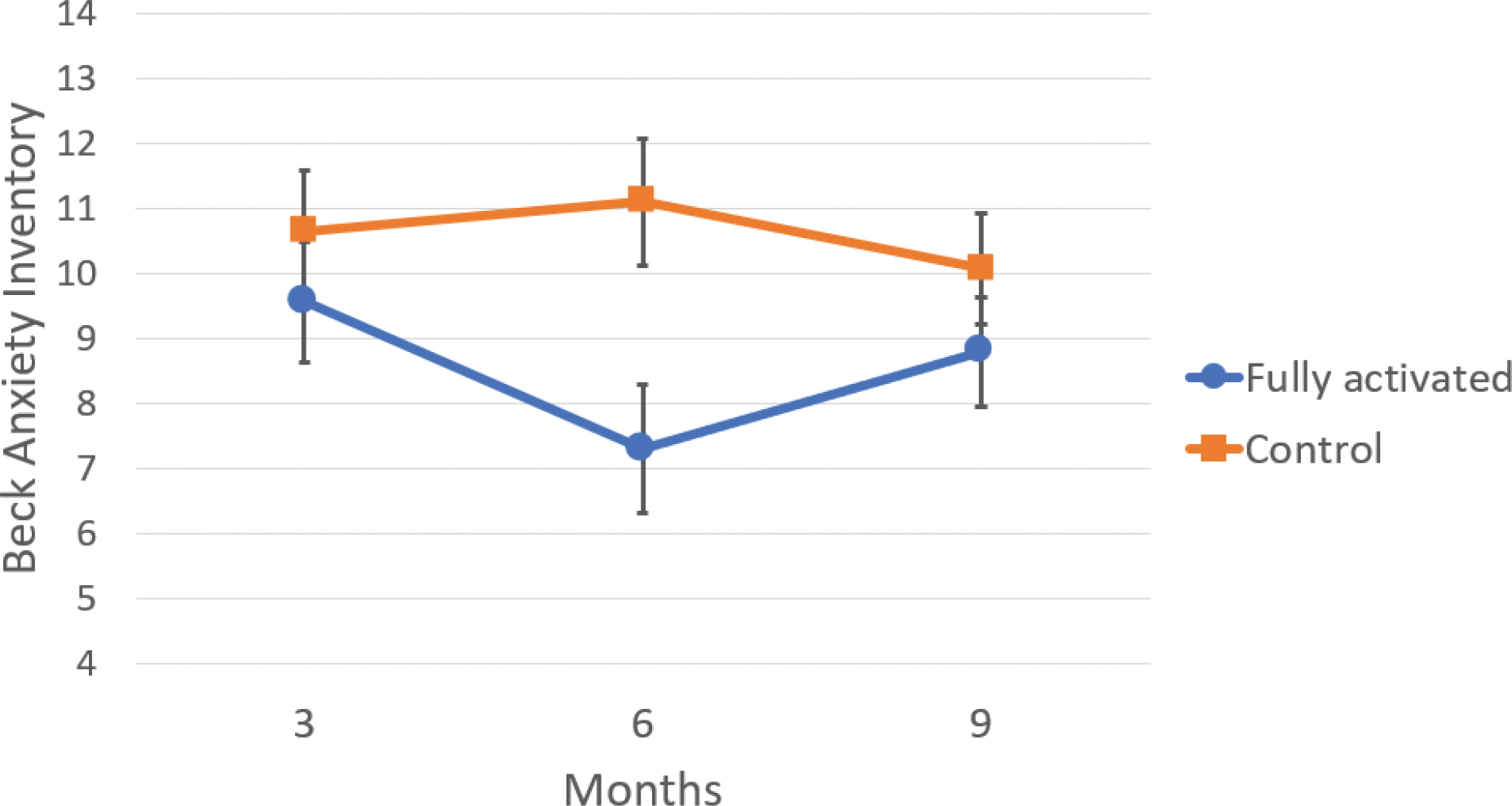
Beck Anxiety Inventory CG mean scores and standard errors at three, six, and nine month assessments for fully activated and control conditions (scores are adjusted for CG sex and baseline anxiety).
For depression, the Condition effect was not significant, F(1,59) = .03, p = .86. In addition, both the linear and quadratic effect for the Condition X Assessment interaction were not significant, linear: F(1,59) = 1.26, p = .27; quadratic: F(1,59) = .03, p = .86. Because none of these effects were significant or approached significance, we did not decompose them further.
To summarize, analyses revealed evidence that the fully activated version of PPCg had a beneficial effect in reducing CG anxiety at the six-month assessment compared to the control condition. These benefits were not found at the three-month or nine-month assessments and did not extend to CG depression.
Exploratory analyses
Because our analyses found lower anxiety at the six-month assessment in the fully activated condition compared to the control condition, we conducted exploratory analyses to determine if greater anxiety reduction was associated with more interactions between the user and the PPCg system. Using only participants in the fully activated condition, we computed an anxiety change score (six-month score minus three-month score). We then correlated this change score with the number of SMS text messages (including push notifications sent through the app) sent by PPCg to the CG (e.g., alerts, warnings) and the number of SMS text message replies sent by the CG to PPCg (e.g., clarifying whether help was needed) between the time of the three-month and six-month assessments. These analyses revealed that greater reduction in CG anxiety was associated with more text messages sent from PPCg to the CG, r(31)=−.36, p =.039. A similar relationship between greater reduction in CG anxiety and more text messages sent from the CG to PPCg approached significance, r(31) = −.30, p = .086.
Discussion
In this article we report what to our knowledge is the first rigorous assessment of a technology-based solution designed to reduce some of the adverse health effects experienced by CGs of PWDs. Using a randomized controlled trial with a nine-month longitudinal assessment, we evaluated a fully activated version of PPCg that learned patterns of activity in each home and provided warnings when worrisome deviations occurred from those patterns and that provided some tools for sustaining social contact between CGs and their friends and families. Although many studies of clinical interventions utilize a “treatment as usual” or “waiting list” control condition, we set a higher bar (i.e., participants in our control condition received the same PPCg hardware at the start of the study and the same professional installation and instruction as in the active condition). This kind of “active control” condition helps isolate the specific effects of the intervention from some of the more non-specific ones (e.g., amount of interaction with the research team). Moreover, the version of PPCg provided in the control condition was not merely inert hardware. Rather, it provided warnings regarding water leaks and moisture conditions that many of our participants reported were quite valuable.
Given the steep competition afforded by this control condition and the fact that our recruitment had to end early due to COVID restrictions on in-person installations (thus, leaving the study underpowered compared to the original plan), we consider it quite encouraging that we still found evidence of a benefit for CGs in the fully activated condition compared with the control condition. Moreover, exploratory analyses revealed that greater interactions with PPCg in the fully activated condition were associated with greater reductions of anxiety, thus, suggesting that CGs who received a larger “dose” of PPCg did better.
Importantly, we had very little attrition (i.e., 5%) in our 9-month study. This contrasts with the higher rates of attrition often reported in CG research (Bernstein, Grill, & Gillen, 2021; Schulz, Martire, & Klinger, 2005). Our lack of attrition may reflect the lower demands placed on CGs by a technology-based intervention like PPCg compared to more typical interventions based on information/education, health-promoting activities (meditation, exercise), or support groups, all of which require the CG to find the time and energy to engage in these activities (which may become increasingly difficult as the PWDs’ disease progresses). Given the higher attrition rates and lack of rigorous empirical support for the effectiveness of most CG interventions (both conventional and technology-based; Butler, et al., 2020), we consider our findings to be sufficiently encouraging to continue pursuing these kinds of technology-based solutions in the future.
Having sounded this optimistic note, it is important to acknowledge the less encouraging aspects of our findings as well. First, the benefit for CGs was only found for reducing anxiety; we found no indication that this version of PPCg helped reduce CG depression. Given that both anxiety and depression are known to be significant health problems encountered by many CGs (e.g., Collins & Kishita, 2020; Joling, et al., 2010; Kaddour & Kishita, 2020), it will be important to bolster the social connection features and add other features (e.g., wellness advice, encouragement) in future versions of PPCg to address more directly the likely pathways leading to depression. Second, the reduction of CG anxiety did not appear until the time of the six-month assessment and was no longer found at the time of the final nine-month assessment. Thus, an important goal for future versions of PPCg will be to start delivering benefits sooner and sustain them longer. Finally, we note that several of our findings only approached statistical significance at the adopted .05 level. Future studies will benefit from larger sample sizes that are more adequately powered to detect small-medium effect sizes.
PPCg: Why it works
Most of the empirical evidence we presented pertains to whether PPCg helps CGs and not to why it is helpful. As for the latter, we speculate that PPCg provides the kind of behind-the-scenes, never fatiguing, always alert, and not too demanding round-the-clock assistance that CGs need. And this need only becomes greater as the PWD’s disease progresses and the CG’s resources dwindle due to increasing care responsibilities, fatigue, and being in a state of hyper-vigilance (Mahoney, et al., 2003; Schulz & Sherwood, 2008). Computer-based systems excel at repetitive tasks and, with a few exceptions (e.g., periodic maintenance needs) the quality of their performance does not decline over time. For a dementia CG, just knowing that they have some backup entity that’s “on duty” when they are distracted, exhausted, angry, depressed, or need to leave the PWD alone in the home can provide some respite from the worries and stress that are an inevitable part of caring for a person who has a progressive, debilitating, and ultimately fatal disease. Thus, technology like PPCg, which takes on some of the demands CGs face, can partially interrupt the pathway of events that foster CG anxiety.
A comment obtained from PPCg user (an adult daughter caring for her father) is illustrative:
“I was a brand new, sleep-deprived caregiver when [PPCg] came along, running up and downstairs every time my father made a noise. [PPCg] gave me back my peace of mind. I could glance at the app, check his whereabouts, and close my eyes again reassured that he was safe. As his only child, I was taking care of him by myself and [PPCg] became one of the first offers of true support in helping me protect both his safety and his desire to feel independent again.”
For CGs to be able to rely on technology like PPCg and be comfortable delegating some of their responsibilities, technology-based systems must prove themselves to be reasonably accurate, with acceptably low levels of false positives (providing warnings when nothing is amiss) and false negatives (failing to provide warnings when something is amiss). Making accurate determinations that users can rely on in real-world high-stakes situations represents an enormous challenge for artificial intelligence and machine learning based technologies. In the case of dementia caregiving, these technologies must deal with additional complexities including: (a) different needs of CGs for PWDs with various types of dementia and their associated disabilities, and (b) adapting to changing patterns of demands and needs that occur as the PWD’s disease progresses.
Limitations
Although our research has a number of strengths (e.g., advanced hardware and software system that is personalized and adaptive, intervention makes limited demands on CGs after system installation, randomized controlled trial design with an active control condition), there are also limitations. Our West Coast sample was largely non-Hispanic White or European American and was relatively high on education, income, and proficiency with technology. Thus, additional research (currently ongoing) will be needed to determine whether PPCg would be embraced by and its benefits generalize to other populations. We also recognize that many events in the lives of the CGs and PWDs in our study could have influenced CG anxiety and depression (e.g., changes in social support, COVID confinement). Our research design depended on randomization to equalize these influences across experimental conditions. However, there are clearly advantages to measuring and evaluating these factors directly. Finally, we did not collect the “ground-truth” data necessary to determine the influence of system accuracy on benefits received by CGs. We plan to add the ability to obtain these kinds of information to future versions of PPCg (e.g., regular messaging with CGs to determine if PPCg’s warnings of worrisome events were accurate and if worrisome events occurred that PPCg missed).
Technological solutions for dementia CGs: Looking ahead
Many issues will need to be addressed if technology-based solutions for dementia CGs are to be developed, improved, and deployed successfully in the future. The architecture of in-home systems like PPCg can easily be expanded with additional sensors to cover more rooms, doors, and windows, which can be important when dealing with larger homes and special situations (e.g., multiple-resident care facilities). Advances in cloud computing and data processing and storage now make it possible for companies to scale their services to work with information from essentially unlimited numbers of homes and other settings. Over time, sensor technology will continue to improve (e.g., new radar-based and Wi-Fi motion sensing devices for detecting falls), battery life will lengthen, and monitoring and alerting software will become more sensitive and accurate. Future systems will feature improved ways of summarizing information from sensors and wearables that are maximally helpful for family members and healthcare providers. Although systems like PPCg are much less expensive than paid in-home or institutional care and economies of scale are likely to reduce prices further, they are still expensive. Innovative solutions (e.g., private insurance and Medicare reimbursements, subsidies, rental plans) will be needed to make these systems affordable for lower-income families.
Another important aspect of the future of technology-based solutions for dementia CGs will be the integration of wearable devices (e.g., Chen, et al., 2022). Systems like PPCg, with sensors mounted on home surfaces, will always be challenged by uncertainties as to which person is the source of detected changes in motion, door openings, etc. Moreover, these devices create a monitoring perimeter that is essentially limited to the area within the home. Integrating wearable devices (watches, pendants, bracelets, etc.) can provide valuable personalized information to help resolve uncertainties about the source of worrisome events (e.g., is it really the PWD who opened the front door at midnight?) and can continue to provide information when the wearer has left the home (e.g., GPS-based location data can help deal with wandering in PWDs; Cullen, et al., 2022). At present, many wearable devices operate with their own propriety systems for accessing and processing information. In the future it will be critical to develop common protocols so that these devices can share information more readily with home-based systems like PPCg to improve the accuracy of alerts and warnings and to extend the perimeter of protection beyond the confines of the home.
Finally, we note the importance of offering technology-based solutions to rural and ethnic minority populations who are often underserved by existing dementia care services and supportive interventions for CGs (e.g., Bernstein, et al., 2019; Gallagher-Thompson, 2006). Technology-based solutions can be readily adapted to support different languages and could be deployed in rural areas that are distant from major dementia care centers. Of course, significant challenges will need to be addressed if these kinds of devices are to be successfully introduced and deployed in diverse communities in culturally-sensitive ways.
Clinical Implications.
There is a pressing need for new interventions to protect the health of dementia CGs that are less demanding of their time and energy, more scalable to larger populations, more personalizable, more flexible over time, and better supported by rigorous empirical evidence.
In a randomized controlled trial, a new technology-based intervention (People Power Caregiver) that uses in-home sensors, machine learning, and artificial intelligence software to warn dementia CGs of worrisome situations (e.g., PWD falls and wandering, home fires and flooding) was found to reduce CG anxiety.
Acknowledgments
Preparation of this manuscript was supported by National Institute on Aging grants awarded to Robert W. Levenson (R01 AG041762, P01 AG019724, SB1 AG059458) and Gene Wang (SB1 AG059458). We thank our caregivers and people living with dementia for their participation.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [RWL], upon reasonable request.
References cited
- AARP (2016). Caregivers and technology: What they want and need. Washington, D.C.: AARP. [Google Scholar]
- Alzheimer’s Association (2022). 2022 Alzheimer’s disease facts and figures. Alzheimers Dement, 18, 700–789. [DOI] [PubMed] [Google Scholar]
- Austrom MG, Geros KN, Hemmerlein K, McGuire SM, Gao S, Brown SA, Callahan CM, & Clark DO (2015). Use of a multiparty web based videoconference support group for family caregivers: Innovative practice. Dementia (London), 14, 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56, 893–897. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, & Van Tilburg W (1997). Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med, 27, 231–235. [DOI] [PubMed] [Google Scholar]
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, Gitlin LN, Klinger J, Koepke KM, Lee CC, Martindale-Adams J, Nichols L, Schulz R, Stahl S, Stevens A, Winter L, Zhang S, & Resources for Enhancing Alzheimer’s Caregiver Health, I.I.I. (2006). Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med, 145, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A, Harrison KL, Dulaney S, Merrilees J, Bowhay A, Heunis J, Choi J, Feuer JE, Clark AM, Chiong W, Lee K, Braley TL, Bonasera SJ, Ritchie CS, Dohan D, Miller BL, & Possin KL (2019). The Role of Care Navigators Working with People with Dementia and Their Caregivers. J Alzheimers Dis, 71, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein OM, Grill JD, & Gillen DL (2021). Recruitment and retention of participant and study partner dyads in two multinational Alzheimer’s disease registration trials. Alzheimer’s Research & Therapy, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer D (1994). Geriatric psychiatry. Hales, Robert E.; Yudofsky, Stuart C.; Talbott, John A. (1994). The American Psychiatric Press textbook of psychiatry (2nd ed.). (pp. 1405–1421). Washington, DC, US: American Psychiatric Association. xxiii, 1694 pp. Washington, DC: American Psychiatric Association. [Google Scholar]
- Breazeal CL, Ostrowski AK, Singh N, & Park HW (2019). Designing Social Robots for Older Adults. The Bridge (Washington), 49, 22. [Google Scholar]
- Brown CL, Lwi SJ, Goodkind MS, Rankin KP, Merrilees J, Miller BL, & Levenson RW (2018). Empathic accuracy deficits in patients with neurodegenerative disease: Association with caregiver depression. American Journal of Geriatric Psychiatry, 26, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Wells JL, Hua AY, Chen KH, Merrilees J, Miller BL, & Levenson RW (2020). Emotion Recognition and Reactivity in Persons With Neurodegenerative Disease Are Differentially Associated With Caregiver Health. Gerontologist, 60, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui LK, Park M, & Giap TT (2022). eHealth interventions for the informal caregivers of people with dementia: A systematic review of systematic reviews. Geriatr Nurs, 48, 203–213. [DOI] [PubMed] [Google Scholar]
- Butler M, Gaugler JE, Talley KMC, Abdi HI, Desai PJ, Duval S, Forte ML, Nelson VA, Ng W, Ouellette JM, Ratner E, Saha J, Shippee T, Wagner BL, Wilt TJ, & Yeshi L (2020). Care interventions for people living with dementia and their caregivers. Comparative effectiveness review Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Chen KH, Casey JJ, Connelly DE, Merrilees J, Yang CM, Miller BL, & Levenson RW (2022). Lower activity linkage between caregivers and persons with neurodegenerative diseases is associated with greater caregiver anxiety. Psychophysiology, 59, e14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Wells JL, Otero MC, Lwi SJ, Haase CM, & Levenson RW (2017). Greater experience of negative non-target emotions by patients with neurodegenerative diseases is related to lower emotional well-being in caregivers. Dementia and Geriatric Cognitive Disorders, 44, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RN, & Kishita N (2020). Prevalence of depression and burden among informal care-givers of people with dementia: a meta-analysis. Ageing & Society, 40, 2355–2392. [Google Scholar]
- Cuijpers P (2005). Depressive disorders in caregivers of dementia patients: A systematic review. Aging & Mental Health, 9, 325–330. [DOI] [PubMed] [Google Scholar]
- Cullen A, Md Khadimul Anam M, Smith MD, Lithander FE, Mícheál Ó B, & Henderson EJ (2022). Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors, 22, 3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly Lynn J, Rondon-Sulbaran J, Quinn E, Ryan A, McCormack B, & Martin S (2019). A systematic review of electronic assistive technology within supporting living environments for people with dementia. Dementia (London), 18, 2371–2435. [DOI] [PubMed] [Google Scholar]
- Dura JR, Stukenberg KW, & Kiecolt-Glaser JK (1991). Anxiety and depressive disorders in adult children caring for demented parents. Psychol Aging, 6, 467–473. [DOI] [PubMed] [Google Scholar]
- Fydrich T, Dowdall D, & Chambless DL (1992). Reliability and validity of the Beck Anxiety Inventory. Journal of Anxiety Disorders, 6, 55–61. [Google Scholar]
- Gallagher-Thompson D (2006). The family as the unit of assessment and treatment in work with ethnically diverse older adults with dementia. Ethnicity and the dementias (2nd ed.) (pp. (2006), p 2119–2508). [Google Scholar]
- Gitlin LN, & Hodgson N (2015). Caregivers as therapeutic agents in dementia care: The context of caregiving and the evidence base for interventions. Family caregiving in the new normal (pp. (2015), p 2305–2693). [Google Scholar]
- Gitlin LNP, Marx KPMPH, Stanley IHBA, & Hodgson NPRNF (2015). Translating evidence-based dementia caregiving interventions into practice: State-of-the-science and next steps. The Gerontologist, 55, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Industry Analysts (2022). Personal emergency response systems global market.
- Guerra SR, Rodrigues SP, Demain S, Figueiredo DM, & Sousa LX (2013). Evaluating proFamilies-dementia: adopting photovoice to capture clinical significance. Dementia (London), 12, 569–587. [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman AT, & Spinhoven P (2004). The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry, 19, 558–563. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins P, Boots LMM, Koh WQ, Neven A, Verhey FRJ, & de Vugt ME (2021). The Effects of Technological Interventions on Social Participation of Community-Dwelling Older Adults with and without Dementia: A Systematic Review. J Clin Med, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua AY, Wells JL, Haase CM, Chen KH, Rosen HJ, Miller BL, & Levenson RW (2019). Evaluating Patient Brain and Behavior Pathways to Caregiver Health in Neurodegenerative Diseases. Dementia and Geriatric Cognitive Disorders, 47, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling KJ, van Hout HPJ, Schellevis FG, van der Horst HE, Scheltens P, Knol DL, & van Marwijk HWJ (2010). Incidence of depression and anxiety in the spouses of patients with dementia: A naturalistic cohort study of recorded morbidity with a 6-year follow-up. The American Journal of Geriatric Psychiatry, 18, 146–153. [DOI] [PubMed] [Google Scholar]
- Joling KJ, van Marwijk HWJ, Veldhuijzen AE, van der Horst HE, Scheltens P, Smit F, & van Hout HPJ (2015). The two-year incidence of depression and anxiety disorders in spousal caregivers of persons with dementia: Who is at the greatest risk? The American Journal of Geriatric Psychiatry, 23, 293–303. [DOI] [PubMed] [Google Scholar]
- Kaddour L, & Kishita N (2020). Anxiety in informal dementia carers: A meta-analysis of prevalence. Journal of Geriatric Psychiatry and Neurology, 33, 161–172. [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Fick D, Waller JL, & Shea D (2004). Spouses of persons with dementia: Their healthcare problems, utilization, and costs. Research in Nursing & Health, 27, 296–306. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Burgio L, Mittelman M, Dunner I, Levine JA, Hoyos C, Tipiani D, Henriquez Y, Kong J, Silver S, Ramirez M, & Teresi JA (2018). Comparative Effectiveness of 2 Interventions for Hispanic Caregivers of Persons with Dementia. J Am Geriatr Soc, 66, 1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DF, Jones RN, Coon DW, Mendelsohn AB, Gitlin LN, & Ory M (2003). The Caregiver Vigilance Scale: Application and validation in the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) project. American Journal of Alzheimer’s Disease and Other Dementias, 18, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin JK, & Schulz R (2009). Interpersonal effects of suffering in older adult caregiving relationships. Psychol Aging, 24, 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W, Jones CJ, Murfield JE, Thalib L, Beattie ERA, Shum DKH, O’Dwyer ST, Mervin MC, & Draper BM (2017). Use of a Robotic Seal as a Therapeutic Tool to Improve Dementia Symptoms: A Cluster-Randomized Controlled Trial. J Am Med Dir Assoc, 18, 766–773. [DOI] [PubMed] [Google Scholar]
- O’Dwyer ST, Moyle W, Zimmer-Gembeck M, & De Leo D (2013). Suicidal ideation in family carers of people with dementia: a pilot study. Int J Geriatr Psychiatry, 28, 1182–1188. [DOI] [PubMed] [Google Scholar]
- Ornstein K, & Gaugler JE (2012). The problem with “problem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient-caregiver dyad. Int Psychogeriatr, 24, 1536–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski AK, DiPaola D, Partridge E, Park HW, & Breazeal C (2019). Older Adults Living With Social Robots: Promoting Social Connectedness in Long-Term Communities. IEEE Robotics & Automation Magazine, 26, 59–70. [Google Scholar]
- Otero MC, & Levenson RW (2017). Lower visual avoidance in dementia patients is associated with greater psychological distress in caregivers. Dementia and Geriatric Cognitive Disorders, 43, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappada A, Chattat R, Chirico I, Valente M, & Ottoboni G (2021). Assistive Technologies in Dementia Care: An Updated Analysis of the Literature. Front Psychol, 12, 644587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Merrilees J, Bonasera SJ, Bernstein A, Chiong W, Lee K, Wilson L, Hooper SM, Dulaney S, Braley T, Laohavanich S, Feuer JE, Clark AM, Schaffer MW, Schenk AK, Heunis J, Ong P, Cook KM, Bowhay AD, Gearhart R, Chodos A, Naasan G, Bindman AB, Dohan D, Ritchie C, & Miller BL (2017). Development of an adaptive, personalized, and scalable dementia care program: Early findings from the Care Ecosystem. PLoS Med, 14, e1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Merrilees JJ, Dulaney S, Bonasera SJ, Chiong W, Lee K, Hooper SM, Allen IE, Braley T, Bernstein A, Rosa TD, Harrison K, Begert-Hellings H, Kornak J, Kahn JG, Naasan G, Lanata S, Clark AM, Chodos A, Gearhart R, Ritchie C, & Miller BL (2019). Effect of Collaborative Dementia Care via Telephone and Internet on Quality of Life, Caregiver Well-being, and Health Care Use: The Care Ecosystem Randomized Clinical Trial. JAMA Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Richardson TJ, Lee SJ, Berg-Weger M, & Grossberg GT (2013). Caregiver health: health of caregivers of Alzheimer’s and other dementia patients. Curr Psychiatry Rep, 15, 367. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Czaja SJ, Martire LM, & Monin JK (2020). Family caregiving for older adults. Annual Review of Psychology, 71, 635–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Martire LM, & Klinger JN (2005). Evidence-based caregiver interventions in geriatric psychiatry. Psychiatric Clinics of North America. Special Issue: Evidence-Based Geriatric Psychiatry, 28, 1007–1038. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, & Fleissner K (1995). Psychiatric and Physical Morbidity Effects of Dementia Caregiving Prevalence, Correlates, and Causes. The Gerontological Society of America, 35, 771–791. [DOI] [PubMed] [Google Scholar]
- Schulz R, & Sherwood PR (2008). Physical and Mental Health Effects of Family Caregiving. Journal of Social Work Education, 44, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Visintainer P, & Williamson GM (1990). Psychiatric and physical morbidity effects of caregiving. J Gerontol, 45, P181–191. [DOI] [PubMed] [Google Scholar]
- Victor CR, Rippon I, Quinn C, Nelis SM, Martyr A, Hart N, Lamont R, & Clare L (2021). The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging & Mental Health, 25, 1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [RWL], upon reasonable request.


