Abstract
Seven Tesla magnetic resonance spectroscopy (7T MRS) offers a precise measurement of metabolic levels in the human brain via a non-invasive approach. Studying longitudinal changes in brain metabolites could help evaluate the characteristics of disease over time. This approach may also shed light on how the age of study participants and duration of illness may influence these metabolites. This study used 7T MRS to investigate longitudinal patterns of brain metabolites in young adulthood in both healthy controls and patients. A four-year longitudinal cohort with 38 patients with first episode psychosis (onset within 2 years) and 48 healthy controls was used to examine 10 brain metabolites in 5 brain regions associated with the pathophysiology of psychosis in a comprehensive manner. Both patients and controls were found to have significant longitudinal reductions in glutamate in the anterior cingulate cortex (ACC). Only patients were found to have a significant decrease over time in γ-aminobutyric acid, N-acetyl aspartate, myo-inositol, total choline, and total creatine in the ACC. Together we highlight the ACC with dynamic changes in several metabolites in early-stage psychosis, in contrast to the other 4 brain regions that also are known to play roles in psychosis. Meanwhile, glutathione was uniquely found to have a near zero annual percentage change in both patients and controls in all 5 brain regions during a four-year follow-up in young adulthood. Given that a reduction of the glutathione in the ACC has been reported as a feature of treatment-refractory psychosis, this observation further supports the potential of glutathione as a biomarker for this subset of patients with psychosis.
Introduction
Proton magnetic resonance spectroscopy (MRS) allows for the non-invasive quantification of multiple compounds that are related to metabolism, neurotransmission, and other processes (called brain metabolites in this manuscript) in the human brain [1]. This methodology has been used extensively over the last two decades to study such brain metabolites in neuropsychiatric disorders in vivo [2, 3]. Compared to scans performed at lower field strengths, 7 Tesla (7T) MRS provides better spectral resolution with an increase in the number of resolved spectral features and improved separation of spectral lines of individual metabolites, such as glutamine and glutamate [4–6]. As a result, 7T can provide better precision measurements in brain metabolites, particularly those with smaller signals in the spectrum, such as glutathione, γ-aminobutyric acid (GABA), glutamine, and N-acetylaspartyl glutamate (NAAG) [4–6]. Nevertheless, to date, there have only been a handful of 7T MRS studies for patients with psychotic disorders [7–16].
Results for brain metabolites and their changes in patients with psychosis may not be perfectly consistent: for example, when patients with schizophrenia were compared with healthy controls, both increases and decreases in GABA and glutamate levels have been reported [13, 16–25]. Several factors may cause these inconsistent findings such as the heterogeneity of the disease and possible differences in MRS techniques. Some important factors could also be the age of the study participants, their duration of illness, and medication. There is evidence that some brain metabolites measured by 3T MRS change over time, which is either due to disease progression or in response to, or as a consequence of, therapeutic interventions [26, 20, 27–36]. Recent meta-analysis studies of cross-sectional 3T MRS data have observed alterations in glutamatergic metabolites in patients with schizophrenia and bipolar disorder, and associations between glutamatergic metabolites and illness severity/states [18, 37, 38]. The longitudinal approach is just starting in 7T MRS and psychiatric research, except for one pilot study with a small number of controls (n=10) and FEP patients (n=21) on glutamate for one brain region, the dorsal anterior cingulate cortex (dorsal ACC) [15]. The lack of a longitudinal landscape for multiple brain metabolites in different brain regions measured by 7T MRS is currently a major knowledge gap.
Here we hypothesize that longitudinal assessment of glutamate in 7T MRS, which separates the signal of glutamate from other metabolites more precisely, may provide complementary information to recent meta-analysis publications on 3T MRS data [18, 37, 38]. We further hypothesize that a systematic assessment of 10 brain metabolites (not limited to glutamate but also other key neurotransmitters/brain metabolites) in 5 brain regions that are functionally interconnected but distinct in the pathophysiology of early-stage psychosis [39–42] will provide a comprehensive longitudinal landscape in molecular changes associated with early-stage psychosis. Psychosis is central to schizophrenia (SZ) but is also frequently seen in severe mental illness, such as bipolar disorder and major depressive disorder [43–45]. Thus, many recent publications have studied patients with psychosis in different DSM diagnoses to look for their shared pathological signatures by using MRS and other brain imaging methods [structural magnetic resonance imaging (MRI), functional MRI, and positron emission tomography (PET)] [11, 26, 46–50]. Accordingly, we also looked for the shared pathological signatures among FEP patients in a cross-disease manner.
Accordingly, this paper reports the findings of a longitudinal study of both FEP patients and healthy controls for multiple brain metabolites in 5 brain regions assessed by 7T MRS. This longitudinal study expands our previously published cross-sectional study of 7T MRS with 81 FEP patients and 91 healthy controls that reported a number of metabolic abnormalities in patients: lower levels of GABA, glutamate, glutathione, and N-acetylaspartate (NAA) in the ACC; lower levels of NAA in the thalamus and orbitofrontal region; and a reduction in glutathione in the thalamus in FEP patients compared with healthy controls [16]. The present longitudinal cohort has 38 FEP patients and 48 healthy controls who returned for at least one follow-up evaluation over 4 years.
Study participants and Methods
Participants
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board and all study participants provided written informed consent. FEP patients within 24 months of the first psychotic manifestation at their baseline visit were recruited from local in-patient and out-patient clinics in the state of Maryland. As stated in our previous publication [16], the inclusion and exclusion criteria for all study participants were: 1) between 15 and 35 years old; 2) no history of traumatic brain injury, cancer, abnormal bleeding, viral infection (such as HIV and hepatitis; note that all the MRS scans were conducted before the COVID pandemic), neurologic disorder, or mental retardation; 3) no drug or alcohol abuse in the past three years; and 4) no illicit drug (except cannabis) use in the past two months. FEP patients were assessed by psychiatrists using the Structured Clinical Interview for DSM-IV [51] and medical records. Healthy controls were defined as having no personal or immediate family history of psychosis and not currently taking psychotropic medication. Thirty-eight patients and 48 controls came back for yearly study visits, including 7T MRS scans, for up to 4 years. The average duration between the first and last visit was 651 days (standard deviation: 345 days). Patients were medicated and diagnosed with schizophrenia (n = 16), schizoaffective disorder (n = 4), schizophreniform disorder (n = 1), bipolar disorder with psychosis (n = 10), major depressive disorder with psychotic features (n = 4), and not otherwise specified psychotic disorder (n=3) at the baseline visit. To test whether diagnosis affects main results, patients were classified into non-affective groups (patients with schizophrenia, schizoaffective disorder, and schizophreniform disorder) and affective groups (patients with bipolar disorder with psychosis and major depressive disorder with psychotic features), following the classification frequently used by other groups [52–56]. The medication records were collected during follow-ups.
The Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms (SAPS) were used to evaluate the presence and severity of negative and positive symptoms in patients, respectively. Total scores, the sum of the global ratings, for SANS and SAPS were calculated and used in this study.
MR protocol
The same hardware, software, and acquisition procedures were used in the entire study. Specifically, all participants were scanned using a 7T scanner (Philips ‘Achieva’, Best, Netherlands) equipped with a 32-channel receive head coil using a protocol previously described in detail [16]. T1-weighted anatomical images were acquired for spectroscopic voxel placement and tissue segmentation using a sagittal 3-dimensional magnetization-prepared rapid acquisition with gradient echo sequence (‘MPRAGE’, field of view, 220×220×180 mm3; isotropic resolution, 0.8 mm; echo time, 1.9 milliseconds; repetition time, 4.4 milliseconds; flip angle, 7°; SENSE factor, 4; scan time, approximately 4 minutes). Spectra were recorded using the STEAM sequence (TR/TE/TM = 3000/14/33 ms, 128 excitations) with VAPOR water suppression from the thalamus (20×30×15 mm3), left orbitofrontal region (OFR; 20×20×20 mm3), ACC (30×20×20 mm3), left dorsolateral prefrontal cortex (DLPFC; 25×20×20 mm3) and left centrum semiovale (40×20×15 mm3) (Figure 1A). A non-water suppressed acquisition was also collected with 2 excitations. Figure 1B shows representative spectra from the ACC of one study participant at two time points, and the results of the spectral fitting routine. Prior to the acquisition, field homogeneity was optimized up to the 2nd order using the FASTMAP technique [57], and RF pulses calibrated using a localized power optimization scheme [58]. Scan time was 6 min 30 seconds per voxel location.
Figure 1. Magnetic Resonance Spectroscopy (MRS) Voxel Localizations and Representative Spectra.
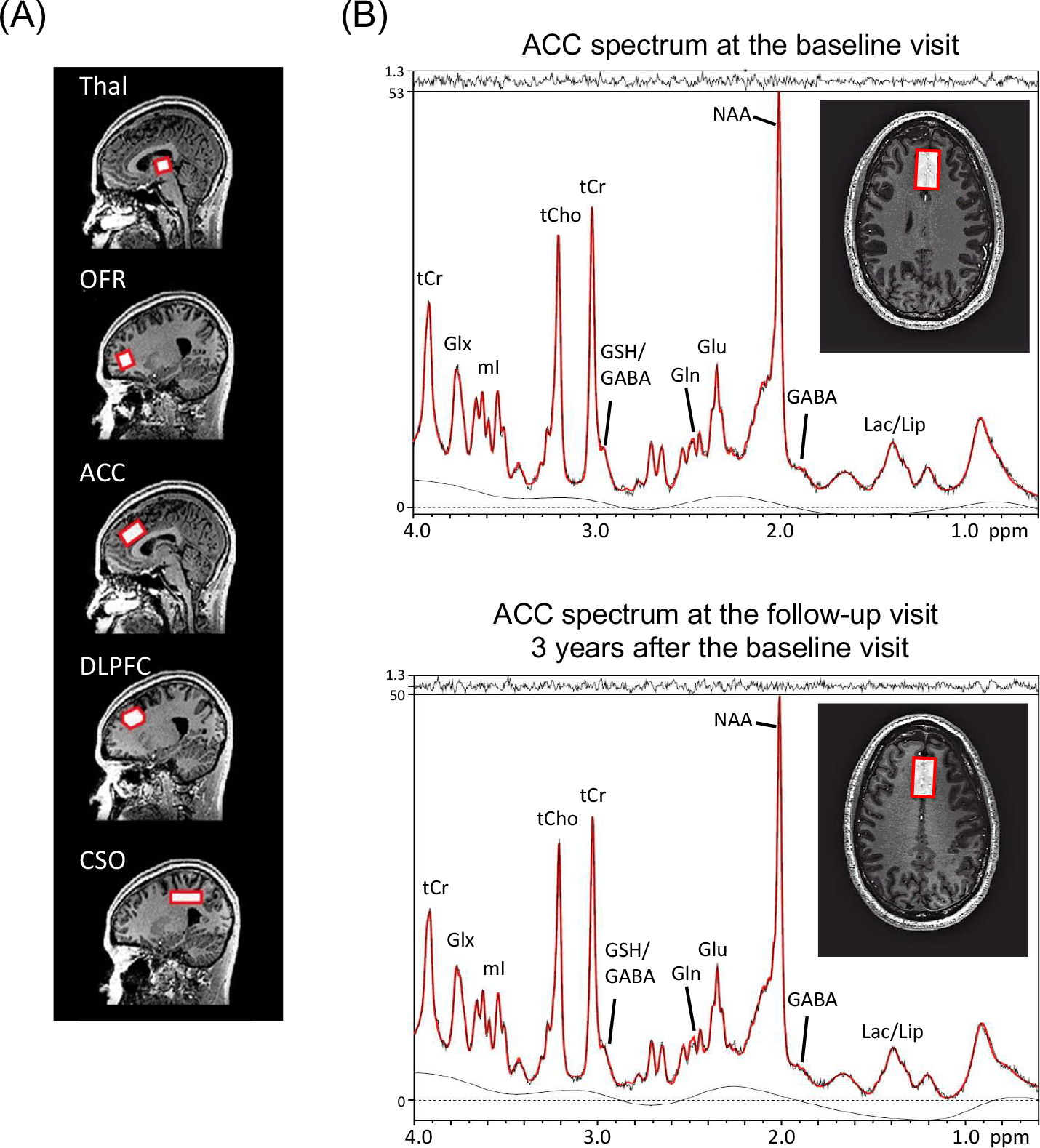
(A) Sagittal T1-weighted images, showing the locations of the five brain regions (red boxes) used for MRS in this study.
(B) Anterior cingulate cortex (ACC) spectra recorded at the baseline visit and the follow-up visit 3 years after the baseline visit in one study participant, showing the voxel location overlaid on an axial T1-weighted image at both time points with results of the LCModel analysis; the LCModel output (red line) is superimposed on the original data (black line). The residual (fit - data) is shown at the top. Abbreviations: GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Glx, glutamate plus glutamine; GSH, glutathione; Lac, lactate; Lip, lipid; NAA, N-acetylaspartate; NAAG, N-acetylaspartyl glutamate; mI, myo-inositol; tCho, phosphocholine plus glycerophosphocholine; tCr, creatine plus phosphocreatine; Thal, thalamus; OFR, orbital frontal cortex; DLPFC, dorsolateral prefrontal cortex; and CSO, centrum semiovale.
MR data processing
All spectra were analyzed with the LCModel software package [59] using a simulated basis set [60]. Spectra were fitted between 0.2 and 4.0 ppm after eddy-current correction. Prior to fitting, eddy current correction was performed using the non-suppressed water signal. Metabolite levels were estimated relative to the unsuppressed water signal. As described in our previous study [16], metabolite levels were corrected for cerebrospinal fluid (CSF) content using [X]corrected = [X]/(1 − fCSF), where [X] denotes the metabolite level output by the LCModel, and fCSF denotes the fraction of cerebrospinal fluid within the voxel. No relaxation time corrections were performed.
Metabolite levels were included in further statistical analyses only when their Cramer-Rao Lower Bounds (CRLBs) were below 20%, except for NAAG and lactate where 30% was used owing to their relatively low concentrations, following the cutoff recommended by the LCModel manual. Other measures of spectral quality, namely, the signal to noise ratio (based on the height of the NAA peak and the standard deviation of the LCModel fit residual) and full width at half maximum were also estimated using the LCModel. MRS voxel composition was determined by segmenting the anatomical T1-weighted images into gray matter, white matter, and cerebrospinal fluid using the SPM12 toolbox [61].
Twenty brain metabolites were included in the LCModel basis set as described previously [16]. After quality control as described above, the following metabolite concentration estimates were considered reliable enough for further statistical analysis: GABA, glutamate, glutamine, glutathione, lactate, myo-inositol, NAA, NAAG, total choline (phosphocholine plus glycerophosphocholine), and total creatine (creatine plus phosphocreatine). Metabolites that did not meet the defined quality control criteria (described above) were excluded from the analysis. The sample size for each metabolite in each brain region is listed in Table S1.
Attrition bias
Some study participants dropped out during the longitudinal follow-ups. To investigate possible attrition bias (i.e. whether study participants who returned for follow-up visits differed in any way from those of the original cross-sectional cohort), which could potentially affect the analysis results, we compared the level of all the measured brain metabolites in 5 brain regions obtained during the baseline visit for all study participants in our previous cross-sectional full cohort (baseline levels) [16] with those for the current longitudinal cohort. A linear regression with metabolite levels as the dependent variable, return status (returned for follow-up visits: yes or no) as the independent variable, and age, gender, race, and smoking status as covariates was performed in the control only group, while a linear regression with age, gender, race, smoking status, chlorpromazine equivalent dose, and duration of illness as covariates was performed in the patient only group. Additionally, we checked whether there was an attrition bias in the patient group from the viewpoint of symptomatic scales via linear regressions with SANS/SAPS total scores as the dependent variables, return status as the independent variable, and age, gender, race, smoking status, chlorpromazine equivalent dose, and duration of illness as covariates.
Annual percentage change (APC)
APC is an established method to quantify trends in a longitudinal change of biological measurements [62–65]. A major advantage of APC is that it allows comparisons across various cohorts, irrespective of differences in follow-up duration, aging, as well as cohort effects. In a sample set with a small age variation, such as the present case, APC provides an intuitive linear measure to study the effect of time. In addition, considering that 70% of study participants in the present study cohort had longitudinal data from two time points, APC rather than linear regression is more suitable for this study. The definition of APC is as follows:
L is the level of metabolite, D is the date of the visit, i is the ith visit, Di+1 – Di is the number of days between two visits, and n is the total number of 7T MRS visits for each study participant.
Statistical analyses
All the statistical tests conducted in this study are two-tailed tests. To compare the APC between patients and controls, we performed a Bayesian two-sample test and linear regression controlling for age, gender, race, smoking status, and gray matter %. The Benjamini-Hochberg procedure, a popular method for controlling the false discovery rate, was used for multiple comparison correction across all the measured brain metabolites and brain regions. P values corrected with the Benjamini-Hochberg procedure are presented as q values. Bayes factor was also used as a complementary indicator to q value for evaluating the significance of analysis results. Bayes factor measures the ratio of posterior probability in favor of one theory over the other. Compared with q value, Bayes factor has the advantage of directly reflecting the strength of significance and is not dependent on sample size and other extraneous factors [66]. We considered an analysis result as significant when the Bayes factor was larger than 10 and the q value was smaller than 0.05. Similarly, to test if the mean value of APC within controls or patients was different from zero, one-sample Bayesian test and one-sample T test were performed, and the same significance cutoff based on Bayes factors and q values was used to determine significance.
Next, we performed linear regressions to study the correlation between APC and demographic/clinical factors at the baseline visit, including age, smoking status, duration of illness, chlorpromazine equivalent dose [67], the usage of mood stabilizers (Yes/No), the usage of antidepressants (Yes/No), SANS total score, and SAPS total score. Since there is no fully established method to calculate equivalent doses for mood stabilizers and antidepressants, we followed the approach used in well-appreciated publications by other groups [68, 69], in which their impacts were addressed by their usage status (Yes/No).
We also evaluated correlations between APC and changes in medications during the follow-up term (between the baseline visit and the last visit), including the cumulative chlorpromazine equivalent dose, the change in the usage of mood stabilizers (Yes/No), and the change in the usage of antidepressants during the follow-up (Yes/No).
Data sharing
We can provide all the information to professional colleagues upon their requests.
Results
Longitudinal cohort
Table 1 provides the demographic information of 38 FEP patients and 48 healthy controls in the present longitudinal cohort. Most study participants were African American, reflecting the composition of the local population. Additionally, patients had a greater incidence of tobacco use than controls. Differences in these demographic factors between patients and controls were adjusted in subsequent statistical analyses.
Table 1. Demographics of study participants.
Data collected during the participants’ baseline visit is shown. T-test was performed to compare the age between first episode psychosis patients and healthy controls. Fisher exact test was used to compare gender, race, and smoking status between patients and controls.
| Characteristics | Patient | Control | Patient vs Control | ||
|---|---|---|---|---|---|
|
| |||||
| Mean | Standard deviation | Mean | Standard deviation | p-value | |
|
| |||||
| Age | 22.53 | 4.33 | 23.67 | 3.31 | 0.18 |
| Chlorpromazine equivalent dose | 274.11 | 211.67 | N/A | N/A | N/A |
| Duration of illness | 15.26 | 9.54 | N/A | N/A | N/A |
|
| |||||
| Value | Count | Value | Count | p-value | |
|
| |||||
| Race | African American | 25 | African American | 29 | 0.69 |
| Caucasian | 10 | Caucasian | 15 | ||
| Asian | 2 | Asian | 1 | ||
| Other | 1 | Other | 3 | ||
|
| |||||
| Gender | Male | 27 | Male | 26 | 0.17 |
| Female | 11 | Female | 22 | ||
|
| |||||
| Smoking status | Yes | 12 | Yes | 4 | 0.01 |
| No | 26 | No | 44 | ||
As described above, study participants in this longitudinal cohort are from the cross-sectional study in the past [16]. Some study participants in the cross-sectional study were lost to follow-up, either because of losing contact, moving out of the area, or being unwilling to return. Due to the reduced sample size, some of the differences in the baseline levels of brain metabolites between patients and controls that were observed in the previous cross-sectional cohort (full cohort) did not reach the cutoff for statistical significance after multiple comparison correction in the present longitudinal cohort. Nevertheless, between the full cohort and the present longitudinal cohort, there were no significant differences in any metabolite in any brain region in either controls (Table S2) or patients (Table S3). Furthermore, the demographic characteristics of the full cohort and those of our current longitudinal cohort were similar. In addition, we did not observe significant differences in the SANS/SAPS total scores in patients between the full cohort and the present longitudinal cohort (SANS total score: p-value = 0.33; SAPS total score: p-value = 0.20).
To study longitudinal changes in brain metabolites in the present longitudinal cohort, we employed the APC [62–65] as a quantitative indicator (see Method section). No significant longitudinal changes in voxel tissue fractions were observed (Table S4). We assessed the longitudinal changes in 10 brain metabolites in 5 brain regions. We did not observe any significant differences in APCs of any brain metabolite in any brain region between patients with non-affective and those with affective psychosis (Table S5). Based on this publication, we conducted the main analysis in a cross-disease manner, that is, analyzed the FEP patients as one patient group instead of analyzing each diagnosis separately. We observed several patterns as described below.
Longitudinal reductions in ACC glutamate in both patients and controls.
We observed a negative mean value of the APC for ACC glutamate in both patients and controls, indicating that ACC glutamate levels decreased over time (Figure 2A, Figure S1A, Table 2). In controls, the mean value of the APC of ACC glutamate was −2.51%, whereas in patients the mean value of the APC was −5.03%. One-sample t-test and Bayesian test supported the significance of reduction over time in ACC glutamate in both patients and controls (Bayes factor > 10 and q-value < 0.05) (Figure 2A, Figure S1A, Table 2). These data indicate that longitudinal glutamate changes exist even in young adulthood, possibly as a normal aging process. On average, patients had a faster decline in ACC glutamate compared to controls, though it did not reach statistical significance after multiple comparison correction (p-value = 0.01, q-value = 0.09, Bayes factor = 5.65). Note that, our previous study found that ACC glutamate was significantly lower in patients compared to controls at baseline [16]. The longitudinal reduction in glutamate was only observed in the ACC, not in other brain regions.
Figure 2. Boxplots of the Annual Percentage Change (APC) in brain metabolites in the Anterior Cingulate Cortex (ACC).
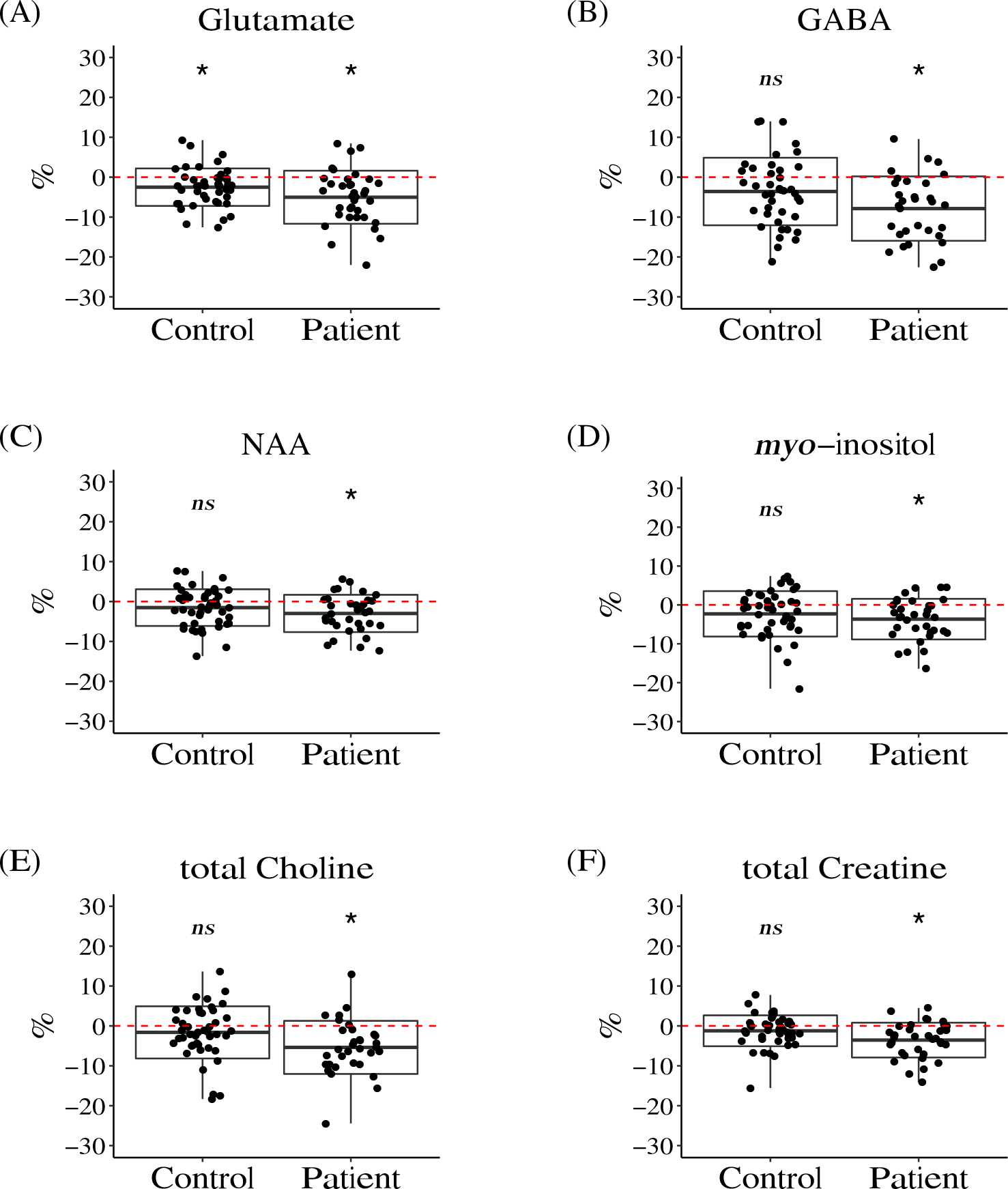
Significant longitudinal reductions were observed in glutamate (A) in both patients and controls, while for γ-aminobutyric acid (GABA) (B), N-acetylaspartate (NAA) (C), myo-inositol (D), total choline (E), and total creatine (F), significant longitudinal reductions were observed in patients, but not in controls.
The red dashed line shows the value of zero. The box represents the standard deviation and the solid line in the middle of the box shows the mean value of the APC. The black dots represent individual study participants. Symbol * denotes significant results (i.e. APC significantly different from zero), ns denotes results that didn’t reach the threshold for significance (Bayes factor > 10 and q-value < 0.05).
Table 2. Longitudinal analysis of brain metabolites in the Anterior Cingulate Cortex (ACC).
The mean annual percentage change (APC, denoted as % in the table) was calculated to quantitatively measure the longitudinal changes. A negative value of mean APC indicates a decrease over time while a positive value indicates an increase. One-sample Bayesian test and t-test were performed to check if the mean APC in one group (healthy controls or first episode psychosis patients) was significantly different from zero. Linear regression and Bayesian two-sample test were performed to compare the APC between patients and controls. The Benjamini-Hochberg procedure was performed for multiple comparison correction. Significant results (Bayes factor > 10 and q-value < 0.05) are highlighted in bold with a gray shadow.
| region | metabolite | HC | FEP | FEP vs HC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| CRLB | % | BF | p-value | q-value | CRLB | % | BF | p-value | q-value | BF | p-value | q-value | ||
|
| ||||||||||||||
| ACC | GABA | 5.49 | −3.59 | 4.15 | 0.01 | 0.15 | 5.80 | −7.47 | 31.44 | 1.03E-03 | 0.01 | 3.50 | 0.01 | 0.10 |
| Glutamine | 5.81 | 0.88 | 0.18 | 0.65 | 0.81 | 5.57 | −1.38 | 0.22 | 0.57 | 0.77 | 0.32 | 0.39 | 0.77 | |
| Glutamate | 1.83 | −2.51 | 30.48 | 9.70E-04 | 0.02 | 1.89 | −5.03 | 372.28 | 6.44E-05 | 1.07E-03 | 5.96 | 0.01 | 0.10 | |
| Glutathione | 4.26 | −1.56 | 0.42 | 0.16 | 0.47 | 4.31 | −0.49 | 0.20 | 0.77 | 0.92 | 0.25 | 0.74 | 0.87 | |
| Lactate | 18.00 | 3.10 | 0.19 | 0.61 | 0.81 | 17.54 | −2.38 | 0.21 | 0.72 | 0.89 | 0.31 | 0.44 | 0.77 | |
| myo-inositol | 2.51 | −2.29 | 3.43 | 0.01 | 0.15 | 2.74 | −4.63 | 28.00 | 1.15E-03 | 0.01 | 2.54 | 0.01 | 0.12 | |
| NAA | 1.85 | −1.52 | 1.59 | 0.03 | 0.26 | 1.97 | −2.98 | 42.17 | 7.33E-04 | 0.01 | 0.83 | 0.08 | 0.55 | |
| NAAG | 13.39 | 2.61 | 0.18 | 0.67 | 0.81 | 14.16 | −0.40 | 0.21 | 0.94 | 0.96 | 0.27 | 0.74 | 0.87 | |
| total Choline | 2.30 | −1.62 | 0.56 | 0.11 | 0.41 | 2.29 | −5.38 | 433.75 | 5.55E-05 | 1.07E-03 | 4.46 | 0.01 | 0.10 | |
| total Creatine | 1.02 | −1.22 | 1.26 | 0.04 | 0.27 | 1.11 | −3.55 | 769.88 | 2.92E-05 | 1.07E-03 | 5.01 | 0.01 | 0.10 | |
|
| ||||||||||||||
| Centrum Semiovale | GABA | 6.65 | 0.82 | 0.22 | 0.45 | 0.81 | 7.29 | 2.44 | 0.30 | 0.33 | 0.59 | 0.25 | 0.99 | 0.99 |
| Glutamine | 12.56 | 0.64 | 0.17 | 0.76 | 0.81 | 12.03 | 8.17 | 0.39 | 0.23 | 0.52 | 0.29 | 0.54 | 0.78 | |
| Glutamate | 2.70 | −0.37 | 0.18 | 0.63 | 0.81 | 2.91 | −2.14 | 0.54 | 0.14 | 0.39 | 0.34 | 0.37 | 0.77 | |
| Glutathione | 6.13 | 0.55 | 0.17 | 0.73 | 0.81 | 6.20 | 5.47 | 0.55 | 0.14 | 0.39 | 0.82 | 0.10 | 0.56 | |
| Lactate | 24.82 | 20.62 | 1.51 | 0.03 | 0.26 | 22.50 | 17.82 | 1.01 | 0.06 | 0.22 | 0.26 | 0.70 | 0.87 | |
| myo-inositol | 2.74 | −0.73 | 0.22 | 0.41 | 0.79 | 3.03 | 1.55 | 0.22 | 0.56 | 0.77 | 0.40 | 0.27 | 0.76 | |
| NAA | 2.00 | −1.89 | 37.89 | 0.00 | 0.02 | 2.14 | −1.68 | 0.60 | 0.12 | 0.37 | 0.29 | 0.50 | 0.78 | |
| NAAG | 4.39 | −1.68 | 0.27 | 0.30 | 0.69 | 5.40 | 7.76 | 2.68 | 0.02 | 0.11 | 4.10 | 0.01 | 0.10 | |
| total Choline | 2.35 | −1.35 | 0.32 | 0.24 | 0.62 | 2.31 | 2.29 | 0.27 | 0.40 | 0.66 | 0.62 | 0.14 | 0.56 | |
| total Creatine | 1.74 | 0.21 | 0.17 | 0.71 | 0.81 | 1.80 | 0.24 | 0.20 | 0.83 | 0.92 | 0.33 | 0.40 | 0.77 | |
|
| ||||||||||||||
| DLPFC | GABA | 8.76 | −1.29 | 0.18 | 0.68 | 0.81 | 8.66 | 4.17 | 0.31 | 0.32 | 0.59 | 0.25 | 0.97 | 0.99 |
| Glutamine | 10.74 | 2.01 | 0.19 | 0.65 | 0.81 | 9.78 | 9.58 | 1.92 | 0.03 | 0.15 | 0.58 | 0.14 | 0.56 | |
| Glutamate | 2.33 | 1.08 | 0.18 | 0.61 | 0.81 | 2.22 | 2.30 | 0.36 | 0.24 | 0.52 | 0.24 | 0.87 | 0.97 | |
| Glutathione | 5.60 | 3.31 | 0.51 | 0.13 | 0.45 | 5.69 | 10.56 | 5.88 | 0.01 | 0.05 | 0.51 | 0.17 | 0.56 | |
| Lactate | 20.14 | −3.97 | 0.22 | 0.63 | 0.81 | 20.74 | 8.72 | 0.36 | 0.34 | 0.59 | 0.39 | 0.41 | 0.77 | |
| myo-inositol | 3.29 | −0.67 | 0.19 | 0.61 | 0.81 | 3.28 | 1.09 | 0.21 | 0.67 | 0.89 | 0.25 | 0.72 | 0.87 | |
| NAA | 1.91 | −2.16 | 0.89 | 0.06 | 0.34 | 1.91 | 0.56 | 0.20 | 0.71 | 0.89 | 0.48 | 0.20 | 0.59 | |
| NAAG | 13.19 | 19.85 | 0.74 | 0.09 | 0.39 | 14.14 | 13.43 | 1.47 | 0.04 | 0.19 | 0.39 | 0.35 | 0.77 | |
| total Choline | 3.57 | 3.06 | 0.47 | 0.14 | 0.45 | 3.38 | 6.99 | 1.10 | 0.06 | 0.21 | 0.36 | 0.35 | 0.77 | |
| total Creatine | 1.84 | 1.39 | 0.24 | 0.36 | 0.76 | 1.81 | 2.78 | 0.47 | 0.17 | 0.42 | 0.30 | 0.46 | 0.77 | |
|
| ||||||||||||||
| OFR | GABA | 12.61 | −2.06 | 0.20 | 0.57 | 0.81 | 13.15 | −1.20 | 0.22 | 0.82 | 0.92 | 0.31 | 0.52 | 0.78 |
| Glutamine | 13.32 | 0.88 | 0.18 | 0.78 | 0.81 | 14.25 | 11.59 | 1.61 | 0.04 | 0.19 | 0.59 | 0.17 | 0.56 | |
| Glutamate | 4.10 | 0.15 | 0.17 | 0.91 | 0.91 | 3.94 | −1.49 | 0.28 | 0.43 | 0.67 | 0.30 | 0.57 | 0.78 | |
| Glutathione | 9.38 | 5.38 | 0.76 | 0.08 | 0.39 | 9.00 | 6.71 | 1.19 | 0.05 | 0.21 | 0.26 | 0.93 | 0.99 | |
| Lactate | 25.17 | −9.28 | 0.28 | 0.41 | 0.79 | 26.00 | 15.56 | 0.63 | 0.16 | 0.41 | 0.43 | 0.32 | 0.77 | |
| myo-inositol | 5.15 | 3.26 | 0.47 | 0.14 | 0.45 | 5.19 | −0.18 | 0.21 | 0.96 | 0.96 | 0.38 | 0.34 | 0.77 | |
| NAA | 2.88 | −0.47 | 0.19 | 0.65 | 0.81 | 2.94 | −1.55 | 0.46 | 0.19 | 0.46 | 0.27 | 0.70 | 0.87 | |
| NAAG | 18.10 | 1.93 | 0.19 | 0.76 | 0.81 | 18.75 | 34.20 | 0.89 | 0.10 | 0.33 | 0.67 | 0.16 | 0.56 | |
| total Choline | 3.10 | 1.48 | 0.29 | 0.30 | 0.69 | 3.10 | −1.81 | 0.27 | 0.46 | 0.67 | 0.60 | 0.16 | 0.56 | |
| total Creatine | 2.93 | 1.52 | 0.30 | 0.29 | 0.69 | 2.84 | −1.14 | 0.27 | 0.47 | 0.67 | 0.52 | 0.19 | 0.59 | |
|
| ||||||||||||||
| Thalamus | GABA | 9.62 | −1.62 | 0.25 | 0.34 | 0.73 | 10.62 | 0.67 | 0.19 | 0.87 | 0.95 | 0.27 | 0.54 | 0.78 |
| Glutamine | 14.93 | 2.26 | 0.19 | 0.57 | 0.81 | 14.00 | −0.27 | 0.19 | 0.95 | 0.96 | 0.29 | 0.53 | 0.78 | |
| Glutamate | 4.53 | 0.82 | 0.19 | 0.56 | 0.81 | 4.62 | 0.20 | 0.18 | 0.95 | 0.96 | 0.23 | 0.93 | 0.99 | |
| Glutathione | 9.23 | 1.40 | 0.17 | 0.69 | 0.81 | 9.57 | 5.45 | 0.32 | 0.28 | 0.56 | 0.24 | 0.83 | 0.94 | |
| Lactate | 25.00 | 2.27 | 0.21 | 0.77 | 0.81 | 26.50 | 14.40 | 0.34 | 0.28 | 0.56 | 0.76 | 0.10 | 0.56 | |
| myo-inositol | 5.58 | 3.84 | 0.36 | 0.20 | 0.55 | 5.91 | 4.01 | 0.28 | 0.33 | 0.59 | 0.23 | 0.95 | 0.99 | |
| NAA | 3.07 | −0.25 | 0.17 | 0.81 | 0.83 | 3.32 | 2.21 | 0.25 | 0.41 | 0.66 | 0.29 | 0.46 | 0.77 | |
| NAAG | 18.03 | 1.50 | 0.17 | 0.77 | 0.81 | 19.07 | 4.32 | 0.26 | 0.45 | 0.67 | 0.32 | 0.43 | 0.77 | |
| total Choline | 3.61 | 6.32 | 0.68 | 0.09 | 0.39 | 3.85 | 0.82 | 0.19 | 0.79 | 0.92 | 0.27 | 0.58 | 0.78 | |
| total Creatine | 2.89 | 2.74 | 1.09 | 0.05 | 0.29 | 3.21 | 0.63 | 0.19 | 0.80 | 0.92 | 0.26 | 0.66 | 0.86 | |
Abbreviations: BF, Bayes factor; GABA, γ-aminobutyric acid; NAA, N-acetylaspartate; NAAG, N-acetylaspartyl glutamate; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; and OFR, orbital frontal region.
Longitudinal reductions in GABA, NAA, myo-inositol, total choline, and total creatine in the ACC in patients.
In addition to glutamate, we observed significant longitudinal reductions in GABA, NAA, myo-inositol, total choline, and total creatine in the ACC only in patients (Figure 2, Figure S1, Table 2). The mean value of the APC of GABA, NAA, myo-inositol, total choline, and total creatine in patients were −7.47%, −2.98%, −4.63%, −5.38%, and −3.55% respectively. The differences in the APC of these five brain metabolites in ACC between patients and controls did not reach the significance cutoff after the multiple comparison correction. Note that at baseline, our previous study found that GABA and NAA in the ACC were significantly lower in patients compared to controls, while there was no difference in myo-inositol, total choline, and total creatine between patients and controls [16]. These longitudinal reductions in GABA, NAA, myo-inositol, total choline, and total creatine in patients were only observed in the ACC, not in other brain regions.
Although we observed a significant reduction in NAA in the centrum semiovale in healthy controls, the APC was almost equivalent to the CRLB (Table 2). Thus, this observation may be artificial, caused by measurement uncertainty. We did not observe significant longitudinal changes in patients or significant differences in longitudinal changes between patients and controls for NAA in the centrum semiovale.
No significant longitudinal changes in glutathione in any brain region of both patients and controls
In the cross-sectional cohort we published for the 7T MRS study [16], the baseline levels of glutathione were significantly lower in the ACC and thalamus in patients compared with healthy controls. However, when we examined the longitudinal change in glutathione, we did not observe a significant difference in the APC of glutathione between patients and controls in any brain region (Figure 3, Figure S2, Table 2). In addition, the mean value of the APC of glutathione in any brain region in either patients or controls was not found to be significantly different from zero (Figure 3, Figure S2, Table 2). These results suggest that glutathione levels are stable in 15- to 35-year-old study participants during a 4-year follow-up. Together with the lower baseline levels of glutathione in patients observed in our previous cross-sectional study [16], it suggests that the reduction may exist prior to the onset of the disease, and continue in a stable fashion over the early course.
Figure 3. Boxplots of the Annual Percentage Change (APC) in Glutathione.
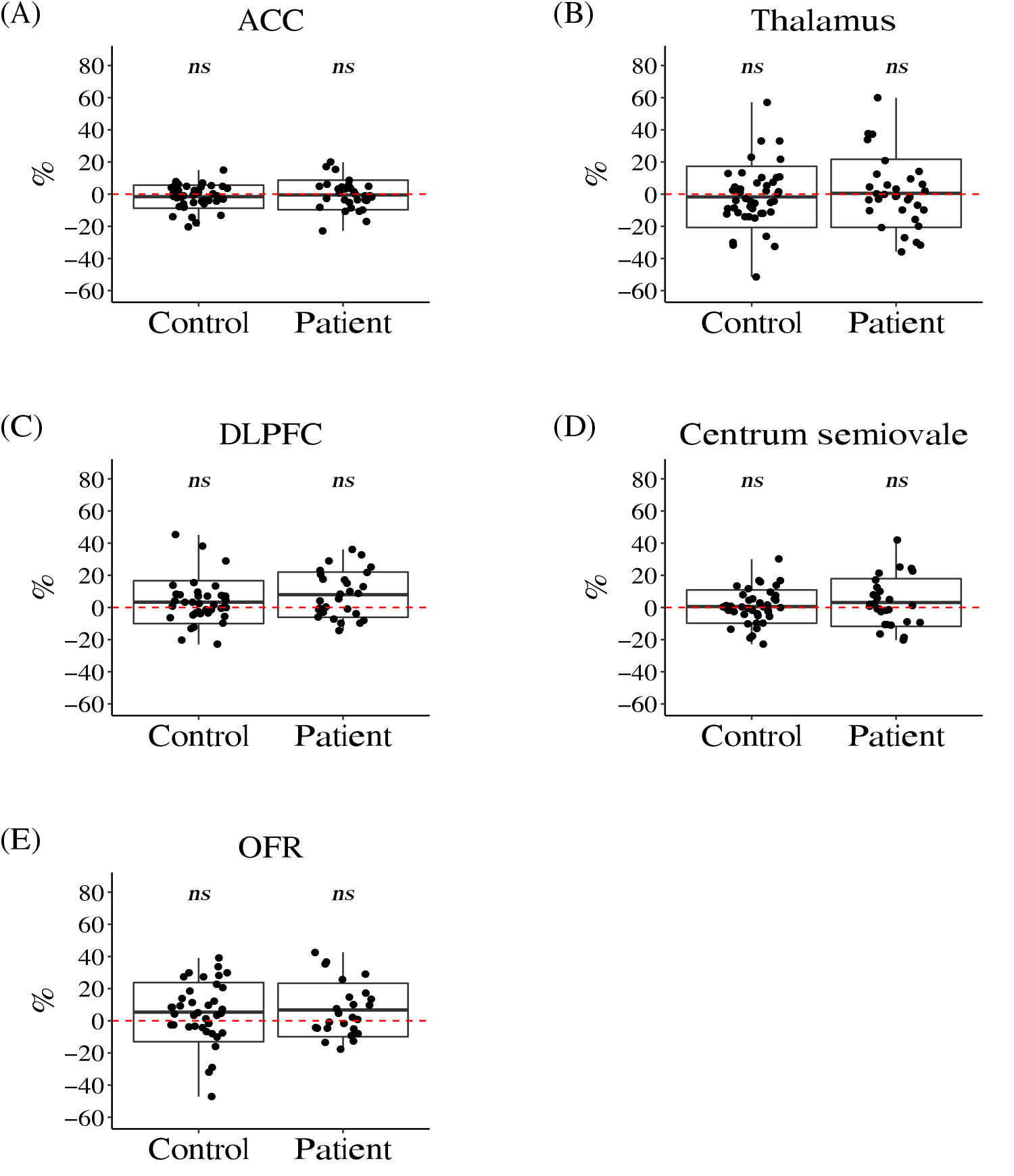
We didn’t observe significant longitudinal changes in glutathione in anterior cingulate cortex (ACC) (A), thalamus (B), dorsolateral prefrontal cortex (DLPFC) (C), centrum semiovale (D), or orbital frontal cortex (OFR) (E).
The red dashed line shows the value of zero. The box represents standard deviation and the solid line in the middle of the box shows the mean value of the APC. The black dots represent individual study participants. Symbol * denotes significant results (i.e. APC significantly different from zero), ns denotes results that didn’t reach the threshold for significance (Bayes factor > 10 and q-value < 0.05).
Additionally, we did not observe significant longitudinal changes in glutamine, lactate, or NAAG in either patients or controls in any brain region. Our previous cross-sectional study didn’t observe significant differences in these brain metabolites in any brain region between patients and controls [16]. Glutathione was unique in having a significant difference at baseline between patients and controls, but without later longitudinal changes.
Consideration of confounding factors that may affect the patient group.
In the above analyses, we considered age, gender, race, and smoking status as potential confounding factors for both patients and controls. In addition, the patient group may be influenced by intrinsic factors (disease-associated factors, such as speed and type of disease progression) and extrinsic factors (medication). To address this, we performed linear regressions for brain metabolites with significant longitudinal changes (glutamate, GABA, NAA, myo-inositol, total choline, and total creatine in the ACC) in the patient group. Since gender and race do not change over time, they were included in the linear regression as covariates. Disease severity and type of disease progression are difficult to include in the analysis, but the duration of illness, SANS total score, and SAPS total score may reflect part of their impact. Together, we evaluated the effects of age, smoking, medications, duration of illness, SANS and SAPS total scores at baseline. We also evaluated the effects of medications during the follow-up term for antipsychotics (cumulative dose) and mood stabilizers and antidepressants (status changes) (see details in the Methods section). We did not find any individual factors that exhibited a dominant or significant impact on longitudinal changes in brain metabolites (Table S6).
Discussion
In this study, we utilized 7T MRS data from FEP patients and healthy controls to study longitudinal changes in brain metabolites in a cross-disease manner within severe mental illness. We have three major findings: (i) Glutamate showed a longitudinal decline only in the ACC in both patients and controls; (ii) GABA, NAA, myo-inositol, total choline, and total creatine showed a longitudinal decline only in the ACC in patients; and (iii) glutathione levels in all the studied brain regions were relatively stable over time. Additionally, no significant longitudinal changes were observed in glutamine, lactate, or NAAG in either patients or controls in any brain regions. Our previous cross-sectional study reported no significant differences in the baseline levels of glutamine, lactate, or NAAG between patients and controls, while glutathione levels were different at baseline in the ACC and thamalus between these two groups [16]. Accordingly, glutathione was the only compound with a significant difference at baseline between patients and controls that did not show any longitudinal change.
7T MRS allows the accurate determination of multiple brain metabolites with higher precision than MRS at lower field strengths such as 3T. It is in debate whether 5 brain regions could have been studied at 3T within a tolerable scan time including measurements of GABA or glutathione, which typically require spectral-editing techniques to be reliably measured at 3T or lower field strengths. Taking such advantages of 7T, we first report a longitudinal molecular landscape in patients with early-stage psychosis in comparison with healthy controls in a comprehensive manner (10 brain metabolites in 5 brain regions).
Interestingly, the significant findings in longitudinal changes were limited to the ACC. Recent structural brain imaging has reported progressive structural changes of the ACC in schizophrenia [70–72]. The dorsal segment of the ACC (where our voxel placement is covered) forms a core part of the salience network, a large-scale neurocognitive network, together with the anterior insula and subcortical regions such as the thalamus and striatal nodes [73, 74]. This network has been proposed as one central hub involved in the core symptoms of psychotic disorders [73, 74]. Furthermore, progressive structural changes in the key nodes of this network have reportedly been related to poor prognostic outcomes in schizophrenia [75–77]. Together, the present data support the notion that the dorsal ACC may be a ‘hotbed’ for longitudinal changes of brain metabolites in early-stage psychosis.
Glutamate
The reduction of glutamate in the pathological trajectory of patients with psychosis, including those with FEP, has been reported by multiple groups [8–11, 18, 24, 26, 30, 78–80]. Antipsychotic medications were found to affect glutamate levels in patients [18, 27, 36, 81]. On the other hand, in healthy controls, cross-sectional studies observed lower glutamate levels in older study participants compared to younger ones [82, 83], and negative correlations between glutamate levels and age [18, 80]. In the present study, we observed longitudinal declines in ACC glutamate levels in both patients and controls, but with a faster rate of decline in patients. The finding of a more rapid decrease in patients in the present study suggests that these processes are accelerated compared to the normal aging process in young adulthood.
GABA, NAA, myo-inositol, total choline, and total creatine
Some studies have shown that patients with psychosis have lower levels of GABA compared to healthy controls [8, 10, 13, 21–25], although one study found higher prefrontal cortex GABA levels in unmedicated patients with schizophrenia compared to either medicated patients or controls, raising the possibility that GABA may be lowered by antipsychotic medications [84]. Another study using spectral-editing at 3T found that medial frontal GABA levels were only lower than healthy controls in older (> 35 years) medicated patients with schizophrenia, who also had a longer disease duration; the same cross-sectional study also found a greater decline in GABA with age in patients with schizophrenia compared to controls [85]. In the present study, we also showed a longitudinal reduction in GABA in medicated patients with FEP in young adulthood.
NAA is the second most abundant metabolite in the human brain [86]. It is a concentrated source of acetate and essential for myelin synthesis [87], a process that has been reported to be impaired in schizophrenia [88]. A reduction in NAA in the medial prefrontal cortex has been observed after a course of antipsychotic treatment in first-episode medication-naïve patients with schizophrenia [89]. Given that myelination occurs as the last process of cortical maturation in late adolescence and young adulthood, linking a longitudinal change in NAA with impaired myelination is one possible interpretation of the longitudinal reduction in ACC NAA. It is also possible that the reduction in NAA, in parallel to the reduction in glutamate, may underlie metabolic or microstructural changes that happen in the early stages of psychotic disorders.
Myo-inositol is utilized as a key element for intracellular signal transduction pathways [90]. It is highly enriched in astrocytes and has been used as a marker of astrocyte activity [91]. A meta-analysis reported a small, but significant, reduction in myo-inositol concentration in the medial frontal cortex in schizophrenia [92]. Our data may help to build a study framework that looks for astrocyte-neuron interactions in psychotic disorders. Choline is an essential element for membrane synthesis and cholinergic neurotransmission. Interestingly, maternal prenatal choline deficiency has been strongly linked to subsequent development of schizophrenia [93]. Total creatine (the sum of creatine and phosphocreatine, which are energy metabolites) is often used as an internal reference to quantify MRS data. Beneficial effects of exogenous creatine supplementation have been found for some neurological diseases [94]. Although one study has reported a significant reduction of total creatine in the ACC of patients with schizophrenia compared to controls [95], a meta-analysis also reported no significant abnormalities [96].
Together, GABA, NAA, myo-inositol, total choline, and total creatine have all been reported to be involved in the neuropathology of schizophrenia. In the present study, we observed significant longitudinal reductions in these metabolites in patients, which could be a reflection of disease progression or medication. Although clinical studies tend to stay descriptive, these observations may provide a useful hint to more mechanistic studies in animal models that mimic pathophysiology relevant to schizophrenia and psychosis.
Glutathione
One novel finding of the current study is that glutathione had a near zero annual change in all the brain regions studied. Glutathione is the most abundant non-enzymatic antioxidant in the central nervous system [97]. Maintaining sufficient levels of glutathione is important for protection against oxidative damage. MRS detection of glutathione levels is usually presented as an indicator of antioxidant capacity in brain tissue. Numerous groups have reported aberrant glutathione levels in the brains of patients with psychosis [9, 98–101]. Furthermore, several other studies have reported that aberrant glutathione signaling is systemic [102, 103]. Our previous 7T MRS study also observed a significantly lower glutathione level in the ACC and thalamus in patients with FEP compared to healthy controls [16]. The significant reduction of glutathione in the ACC is specifically associated with a treatment-refractory group of patients with psychosis [104]. Consistent with this observation, another study reported an association between a higher level of GSH in the ACC and a better response to antipsychotic treatment [11]. These findings suggest the presence of oxidative stress in the brain in early-stage psychosis (or even prior to the onset) [16, 105], especially in a subset of patients with poor treatment response. Here we report that glutathione levels do not show significant longitudinal changes over a 4-year timespan after a baseline measurement in patients with FEP as well as in healthy controls. The fact that glutathione levels are relatively stable over time, in contrast to the changes seen in other key brain metabolites after onset, implies its potential for diagnostic applications independent of disease stage or the effects of medications. Further studies to validate this working hypothesis are warranted, and these results may also be useful when we consider future treatments that target redox imbalance and oxidative stress. Studies with animal models have suggested that redox imbalance and oxidative stress can lead to cognitive and behavioral deficits relevant to psychotic disorders, and interventions in early stages can have a prophylactic impact [106, 107]. A constant reduction of glutathione during the disease course in patients with psychosis may help to identify a relevant subset of patients for such treatment.
Limitations and future perspectives
There are several limitations in the current study, such as a limited number of brain regions examined and a relatively narrow time window (4-year follow-up in young adulthood). While the APC estimates linear changes over time, insufficient data points were available to estimate non-linear changes. Additionally, all the patients in this study were medicated. However, at least within the cohort of the present study, we did not observe any significant correlations between APCs and factors associated with medication. Future studies with a larger sample size, a longer follow-up period, and unmedicated patients are needed to explore these effects and to expand upon the current findings. The comprehensive landscape of longitudinal changes in the brain metabolites, provided by the current study, could serve as an important foundation for future projects that investigate the relationship between the dynamic changes in brain metabolites and in clinical test scores over time.
Supplementary Material
Acknowledgments
This study is supported by The National Institute of Mental Health Grants MH-092443 (to AS), MH-094268 (to AS), MH-105660 (to AS), and MH-107730 (to AS); foundation grants from Stanley (to AS), RUSK/S-R (to AS), and a NARSAD young investigator award from the Brain and Behavior Research Foundation (to AS, KY). Study recruitment was in part funded by Mitsubishi Tanabe Pharma Corporation, Japan. LP acknowledges support from the Tanna Schulich Chair of Neuroscience and Mental Health. The authors thank Yukiko Lema for suggestions, for formatting the figures and for her role in research management, and thank Dr. Melissa A Landek-Salgado for scientific and English editions.
Footnotes
Conflict of interest
LP receives book royalties from Oxford University Press and income from the SPMM MRCPsych course. LP has received investigator-initiated educational grants from Otsuka, Janssen, and Sunovion Canada and speaker fees from Otsuka and Janssen Canada, and the Canadian Psychiatric Association. The original recruitment of study participants was partly funded by Mitsubishi Tanabe Pharma Corporation. However, this company is not involved in this specific study.
References
- 1.Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36. [DOI] [PubMed] [Google Scholar]
- 2.Poels EMP, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, et al. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. [DOI] [PubMed] [Google Scholar]
- 5.Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RAE, et al. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magn Reson Imaging. 2015;33:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tkác I, Oz G, Adriany G, Uğurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt AS, Unschuld PG, Pradhan S, Lim IAL, Churchill G, Harris AD, et al. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A (1)H MRS Study at 7 Tesla. Schizophr Res. 2016;172:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid MA, Salibi N, White DM, Gawne TJ, Denney TS, Lahti AC. 7T Proton Magnetic Resonance Spectroscopy of the Anterior Cingulate Cortex in First-Episode Schizophrenia. Schizophr Bull. 2019;45:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar J, Liddle EB, Fernandes CC, Palaniyappan L, Hall EL, Robson SE, et al. Glutathione and glutamate in schizophrenia: a 7T MRS study. Mol Psychiatry. 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakkar KN, Rösler L, Wijnen JP, Boer VO, Klomp DWJ, Cahn W, et al. 7T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biol Psychiatry. 2017;81:525–535. [DOI] [PubMed] [Google Scholar]
- 11.Dempster K, Jeon P, MacKinley M, Williamson P, Théberge J, Palaniyappan L. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol Psychiatry. 2020;25:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland LM, Pradhan S, Korenic S, Wijtenburg SA, Hong LE, Edden RA, et al. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl Psychiatry. 2016;6:e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsman A, Mandl RCW, Klomp DWJ, Bohlken MM, Boer VO, Andreychenko A, et al. GABA and glutamate in schizophrenia: a 7 T 1H-MRS study. Neuroimage Clin. 2014;6:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godlewska BR, Minichino A, Emir U, Angelescu I, Lennox B, Micunovic M, et al. Brain glutamate concentration in men with early psychosis: a magnetic resonance spectroscopy case-control study at 7 T. Transl Psychiatry. 2021;11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon P, Limongi R, Ford SD, Mackinley M, Dempster K, Théberge J, et al. Progressive Changes in Glutamate Concentration in Early Stages of Schizophrenia: A Longitudinal 7-Tesla MRS Study. Schizophrenia Bulletin Open. 2021;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang AM, Pradhan S, Coughlin JM, Trivedi A, DuBois SL, Crawford JL, et al. Assessing Brain Metabolism With 7-T Proton Magnetic Resonance Spectroscopy in Patients With First-Episode Psychosis. JAMA Psychiatry. 2019;76:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustillo JR, Mayer EG, Upston J, Jones T, Garcia C, Sheriff S, et al. Increased Glutamate Plus Glutamine in the Right Middle Cingulate in Early Schizophrenia but Not in Bipolar Psychosis: A Whole Brain 1H-MRS Study. Front Psychiatry. 2021;12:660850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merritt K, McGuire PK, Egerton A, 1H-MRS in Schizophrenia Investigators, Aleman A, Block W, et al. Association of Age, Antipsychotic Medication, and Symptom Severity in Schizophrenia With Proton Magnetic Resonance Spectroscopy Brain Glutamate Level: A Mega-analysis of Individual Participant-Level Data. JAMA Psychiatry. 2021;78:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egerton A, Modinos G, Ferrera D, McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry. 2017;7:e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marenco S, Meyer C, Kuo S, van der Veen JW, Shen J, DeJong K, et al. Prefrontal GABA Levels Measured With Magnetic Resonance Spectroscopy in Patients With Psychosis and Unaffected Siblings. Am J Psychiatry. 2016;173:527–534. [DOI] [PubMed] [Google Scholar]
- 22.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, et al. Frontal Glutamate and γ-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry. 2016;73:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naïve patients with psychosis. Psychol Med. 2020;50:2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon BJ, Kumar J, Danielmeier C. Frontal neural metabolite changes in schizophrenia and their association with cognitive control: A systematic review. Neurosci Biobehav Rev. 2022;132:224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1H-MRS study (OPTiMiSE). Mol Psychiatry. 2018;23:2145–2155. [DOI] [PubMed] [Google Scholar]
- 27.de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Jung-Cook H, et al. Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry. 2018;83:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. [DOI] [PubMed] [Google Scholar]
- 31.Goff DC, Hennen J, Lyoo IK, Tsai G, Wald LL, Evins AE, et al. Modulation of brain and serum glutamatergic concentrations following a switch from conventional neuroleptics to olanzapine. Biol Psychiatry. 2002;51:493–497. [DOI] [PubMed] [Google Scholar]
- 32.Ota M, Wakabayashi C, Sato N, Hori H, Hattori K, Teraishi T, et al. Effect of l-theanine on glutamatergic function in patients with schizophrenia. Acta Neuropsychiatrica. 2015;27:291–296. [DOI] [PubMed] [Google Scholar]
- 33.Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: Relationship with treatment response . Psychiatry Research: Neuroimaging. 2009;174:121–129. [DOI] [PubMed] [Google Scholar]
- 34.Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS, et al. Effects of Davunetide on N-acetylaspartate and Choline in Dorsolateral Prefrontal Cortex in Patients with Schizophrenia. Neuropsychopharmacology. 2013;38:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadena EJ, White DM, Kraguljac NV, Reid MA, Maximo JO, Nelson EA, et al. A Longitudinal Multimodal Neuroimaging Study to Examine Relationships Between Resting State Glutamate and Task Related BOLD Response in Schizophrenia. Front Psychiatry. 2018;9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egerton A, Bhachu A, Merritt K, McQueen G, Szulc A, McGuire P. Effects of Antipsychotic Administration on Brain Glutamate in Schizophrenia: A Systematic Review of Longitudinal 1H-MRS Studies. Front Psychiatry. 2017;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry. 2016;73:665–674. [DOI] [PubMed] [Google Scholar]
- 38.Scotti-Muzzi E, Umla-Runge K, Soeiro-de-Souza MG. Anterior cingulate cortex neurometabolites in bipolar disorder are influenced by mood state and medication: A meta-analysis of 1H-MRS studies. Eur Neuropsychopharmacol. 2021;47:62–73. [DOI] [PubMed] [Google Scholar]
- 39.Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. [DOI] [PubMed] [Google Scholar]
- 40.Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren W, Lui S, Deng W, Li F, Li M, Huang X, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–1316. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann JA, Nelson B, Ratheesh A, Treen D, McGorry PD. At-risk studies and clinical antecedents of psychosis, bipolar disorder and depression: a scoping review in the context of clinical staging. Psychol Med. 2019;49:177–189. [DOI] [PubMed] [Google Scholar]
- 44.Tonna M, Ossola P, Marchesi C, Bettini E, Lasalvia A, Bonetto C, et al. Dimensional structure of first episode psychosis. Early Interv Psychiatry. 2019;13:1431–1438. [DOI] [PubMed] [Google Scholar]
- 45.Reininghaus U, Böhnke JR, Chavez-Baldini U, Gibbons R, Ivleva E, Clementz BA, et al. Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry. 2019;18:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan SY, Brady RO, Lewandowski KE, Higgins A, Öngür D, Hall M-H. Dynamic and progressive changes in thalamic functional connectivity over the first five years of psychosis. Mol Psychiatry. 2021. 25 October 2021. 10.1038/s41380-021-01319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jauhar S, Veronese M, Nour MM, Rogdaki M, Hathway P, Turkheimer FE, et al. Determinants of treatment response in first-episode psychosis: an 18F-DOPA PET study. Mol Psychiatry. 2019;24:1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Shin SH, Santangelo B, Veronese M, Kang SK, Lee JS, et al. Dopamine dysregulation in psychotic relapse after antipsychotic discontinuation: an [18F]DOPA and [11C]raclopride PET study in first-episode psychosis. Mol Psychiatry. 2020. 14 September 2020. 10.1038/s41380-020-00879-0. [DOI] [PubMed] [Google Scholar]
- 49.Kraguljac NV, Anthony T, Morgan CJ, Jindal RD, Burger MS, Lahti AC. White matter integrity, duration of untreated psychosis, and antipsychotic treatment response in medication-naïve first-episode psychosis patients. Mol Psychiatry. 2021;26:5347–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesh TA, Maddock RJ, Howell A, Wang H, Tanase C, Daniel Ragland J, et al. Extracellular free water and glutathione in first-episode psychosis-a multimodal investigation of an inflammatory model for psychosis. Mol Psychiatry. 2021;26:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kübler U Structured Clinical Interview for DSM-IV (SCID). In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine, New York, NY: Springer; 2013. p. 1919–1920. [Google Scholar]
- 52.Jäger M, Bottlender R, Strauss A, Möller H-J. On the descriptive validity of ICD-10 schizophrenia: empirical analyses in the spectrum of non-affective functional psychoses. Psychopathology. 2003;36:152–159. [DOI] [PubMed] [Google Scholar]
- 53.Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amoretti S, Cabrera B, Torrent C, Mezquida G, Lobo A, González-Pinto A, et al. Cognitive reserve as an outcome predictor: first-episode affective versus non-affective psychosis. Acta Psychiatr Scand. 2018;138:441–455. [DOI] [PubMed] [Google Scholar]
- 55.Chung J, Miller BJ. Meta-analysis of comorbid diabetes and family history of diabetes in non-affective psychosis. Schizophr Res. 2020;216:41–47. [DOI] [PubMed] [Google Scholar]
- 56.Yung NCL, Wong CSM, Chan JKN, Chen EYH, Chang WC. Excess Mortality and Life-Years Lost in People With Schizophrenia and Other Non-affective Psychoses: An 11-Year Population-Based Cohort Study. Schizophr Bull. 2021;47:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Automatic Gruetter R., localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. [DOI] [PubMed] [Google Scholar]
- 58.Versluis MJ, Kan HE, van Buchem MA, Webb AG. Improved signal to noise in proton spectroscopy of the human calf muscle at 7 T using localized B1 calibration. Magn Reson Med. 2010;63:207–211. [DOI] [PubMed] [Google Scholar]
- 59.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 60.Soher B, Semanchuk P, Todd D, Steinberg J, Young K. Vespa: Integrated applications for RF pulse design, spectral simulation and MRS data analysis. vol. 19, Proc. Intl. Soc. Mag. Reson. Med; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. [DOI] [PubMed] [Google Scholar]
- 62.Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, et al. Differential Longitudinal Changes in Cortical Thickness, Surface Area and Volume across the Adult Life Span: Regions of Accelerating and Decelerating Change. J Neurosci. 2014;34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison TM, Joie RL, Maass A, Baker SL, Swinnerton K, Fenton L, et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Annals of Neurology. 2019;85:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry. 2017;211:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krogsrud SK, Fjell AM, Tamnes CK, Grydeland H, Mork L, Due-Tønnessen P, et al. Changes in white matter microstructure in the developing brain—A longitudinal diffusion tensor imaging study of children from 4 to 11years of age. NeuroImage. 2016;124:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarosz A, Wiley J. What Are the Odds? A Practical Guide to Computing and Reporting Bayes Factors. J Probl Solving. 2014;7:2. [Google Scholar]
- 67.Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr Bull. 2016;42 Suppl 1:S90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brady RO, Cooper A, Jensen JE, Tandon N, Cohen B, Renshaw P, et al. A longitudinal pilot proton MRS investigation of the manic and euthymic states of bipolar disorder. Transl Psychiatry. 2012;2:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nery FG, Weber WA, Blom TJ, Welge J, Patino LR, Strawn JR, et al. Longitudinal proton spectroscopy study of the prefrontal cortex in youth at risk for bipolar disorder before and after their first mood episode. Bipolar Disord. 2019;21:330–341. [DOI] [PubMed] [Google Scholar]
- 70.Palaniyappan L Progressive cortical reorganisation: A framework for investigating structural changes in schizophrenia. Neurosci Biobehav Rev. 2017;79:1–13. [DOI] [PubMed] [Google Scholar]
- 71.Fusar-Poli P, Broome MR, Woolley JB, Johns LC, Tabraham P, Bramon E, et al. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. J Psychiatr Res. 2011;45:190–198. [DOI] [PubMed] [Google Scholar]
- 72.Koo M-S, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65:746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyata J Toward integrated understanding of salience in psychosis. Neurobiol Dis. 2019;131:104414. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi T, Kido M, Sasabayashi D, Nakamura M, Furuichi A, Takayanagi Y, et al. Gray Matter Changes in the Insular Cortex During the Course of the Schizophrenia Spectrum. Front Psychiatry. 2020;11:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li M, Li X, Das TK, Deng W, Li Y, Zhao L, et al. Prognostic Utility of Multivariate Morphometry in Schizophrenia. Front Psychiatry. 2019;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liloia D, Brasso C, Cauda F, Mancuso L, Nani A, Manuello J, et al. Updating and characterizing neuroanatomical markers in high-risk subjects, recently diagnosed and chronic patients with schizophrenia: A revised coordinate-based meta-analysis. Neurosci Biobehav Rev. 2021;123:83–103. [DOI] [PubMed] [Google Scholar]
- 78.Aoyama N, Théberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RWJ, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–456. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Ren H, He Y, Li Z, Ma X, Yuan L, et al. Anterior Cingulate Cortex Glutamate Levels Are Related to Response to Initial Antipsychotic Treatment in Drug-Naive First-Episode Schizophrenia Patients. Front Psychiatry. 2020;11:553269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marsman A, Mandl RCW, van den Heuvel MP, Boer VO, Wijnen JP, Klomp DWJ, et al. Glutamate changes in healthy young adulthood. European Neuropsychopharmacology. 2013;23:1484–1490. [DOI] [PubMed] [Google Scholar]
- 81.Kaminski J, Mascarell-Maricic L, Fukuda Y, Katthagen T, Heinz A, Schlagenhauf F. Glutamate in the Dorsolateral Prefrontal Cortex in Patients With Schizophrenia: A Meta-analysis of 1H-Magnetic Resonance Spectroscopy Studies. Biol Psychiatry. 2021;89:270–277. [DOI] [PubMed] [Google Scholar]
- 82.Cleeland C, Pipingas A, Scholey A, White D. Neurochemical changes in the aging brain: A systematic review. Neurosci Biobehav Rev. 2019;98:306–319. [DOI] [PubMed] [Google Scholar]
- 83.Roalf DR, Sydnor VJ, Woods M, Wolk DA, Scott JC, Reddy R, et al. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol Aging. 2020;95:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. [DOI] [PubMed] [Google Scholar]
- 85.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyake M, Kakimoto Y, Sorimachi M. A gas chromatographic method for the determination of N-acetyl-L-aspartic acid, N-acetyl-alpha-aspartylglutamic acid and beta-citryl-L-glutamic acid and their distributions in the brain and other organs of various species of animals. J Neurochem. 1981;36:804–810. [DOI] [PubMed] [Google Scholar]
- 87.Rosenberg Roger N., Pascual Juan M.. Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease. 5th ed. Elsevier; 2015. [Google Scholar]
- 88.Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Öngür D. Myelin and Axon Abnormalities in Schizophrenia Measured with Magnetic Resonance Imaging Techniques. Biol Psychiatry. 2013;74:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zong X, Hu M, Li Z, Cao H, He Y, Liao Y, et al. N-Acetylaspartate Reduction in the Medial Prefrontal Cortex Following 8 weeks of Risperidone Treatment in First-Episode Drug-Naïve Schizophrenia Patients. Sci Rep. 2015;5:9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillaspy GE. The cellular language of myo-inositol signaling. New Phytol. 2011;192:823–839. [DOI] [PubMed] [Google Scholar]
- 91.Harris JL, Choi I-Y, Brooks WM. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci. 2015;7:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das TK, Dey A, Sabesan P, Javadzadeh A, Théberge J, Radua J, et al. Putative Astroglial Dysfunction in Schizophrenia: A Meta-Analysis of 1H-MRS Studies of Medial Prefrontal Myo-Inositol. Front Psychiatry. 2018;9:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freedman R, Ross RG. Prenatal choline and the development of schizophrenia. Shanghai Arch Psychiatry. 2015;27:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–343. [DOI] [PubMed] [Google Scholar]
- 95.Ongür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, et al. Neurometabolites in schizophrenia and bipolar disorder – A systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramírez-Expósito MJ, Martínez-Martos JM. The Delicate Equilibrium between Oxidants and Antioxidants in Brain Glioma. Curr Neuropharmacol. 2019;17:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan Y, Dempster K, Jeon P, Théberge J, Khan AR, Palaniyappan L. Acute conceptual disorganization in untreated first-episode psychosis: a combined magnetic resonance spectroscopy and diffusion imaging study of the cingulum. J Psychiatry Neurosci. 2021;46:E337–E346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, et al. Antioxidant defense in schizophrenia and bipolar disorder: A meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94–102. [DOI] [PubMed] [Google Scholar]
- 100.Wood SJ, Berger GE, Wellard RM, Proffitt T-M, McConchie M, Berk M, et al. Medial temporal lobe glutathione concentration in first episode psychosis: A 1H-MRS investigation. Neurobiol Dis. 2009;33:354–357. [DOI] [PubMed] [Google Scholar]
- 101.Sydnor VJ, Roalf DR. A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: Implications for studies of psychosis risk. Schizophr Res. 2020;226:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coughlin JM, Yang K, Marsman A, Pradhan S, Wang M, Ward RE, et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol Psychiatry. 2021;26:3502–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xin L, Mekle R, Fournier M, Baumann PS, Ferrari C, Alameda L, et al. Genetic Polymorphism Associated Prefrontal Glutathione and Its Coupling With Brain Glutamate and Peripheral Redox Status in Early Psychosis. Schizophr Bull. 2016;42:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang K, Longo L, Narita Z, Cascella N, Nucifora FC, Coughlin JM, et al. A multimodal study of a first episode psychosis cohort: potential markers of antipsychotic treatment resistance. Mol Psychiatry. 2022;27:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry. 2016;21:10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cabungcal J-H, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, et al. Juvenile Antioxidant Treatment Prevents Adult Deficits in a Developmental Model of Schizophrenia. Neuron. 2014;83:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sawa A, Seidman LJ. Is Prophylactic Psychiatry around the Corner? Combating Adolescent Oxidative Stress for Adult Psychosis and Schizophrenia. Neuron. 2014;83:991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


