Summary
Pregnant women in resource-limited settings are highly susceptible to anemia and iron deficiency, but the etiology of postpartum anemia remains poorly defined. To inform the optimal timing for anemia interventions, changes in iron deficiency-attributable anemia through pregnancy and postpartum need to be understood. In 699 pregnant Papua New Guinean women attending their first antenatal care appointment and following up at birth and 6 and 12 months postpartum, we undertake logistic mixed-effects modeling to determine the effect of iron deficiency on anemia and population attributable fractions, calculated from odds ratios, to quantify the contribution of iron deficiency to anemia. Anemia is highly prevalent during pregnancy and 12 months postpartum, with iron deficiency increasing the odds of anemia during pregnancy and, to a lesser extent, postpartum. Iron deficiency accounts for ≥72% of anemia during pregnancy and 20%–37% postpartum. Early iron supplementation during and between pregnancies could break the cycle of chronic anemia in women of reproductive age.
Keywords: anemia, iron deficiency, pregnancy, postpartum, malaria, Papua New Guinea
Graphical abstract
Highlights
-
•
Pregnant women in Papua New Guinea are at high risk of anemia and iron deficiency
-
•
High anemia prevalence from the first antenatal care visit to 12 months postpartum
-
•
Iron deficiency is the main cause of anemia in pregnancy but less so postpartum
Davidson et al. report that anemia is highly prevalent in pregnant Papua New Guinean women and during the first 12 months postpartum. Iron deficiency is the main contributor to anemia in pregnancy but less so postpartum. Iron supplementation early during and between pregnancies could alleviate anemia in women of reproductive age.
Introduction
Anemia in pregnancy is a major global public health problem, particularly in resource-limited regions, where every second pregnant woman is estimated to be anemic.1,2 Anemia in pregnancy contributes significantly to maternal morbidity and mortality and increases the risk of adverse neonatal outcomes.3,4,5,6 Consequently, reducing anemia by 50% in women of reproductive age is the second goal of the World Health Organization’s (WHO) “Global Nutrition Targets for 2025.”7
Approximately half of all anemia cases in pregnancy worldwide are attributed to iron deficiency.2 Pregnant women have an increased susceptibility to iron deficiency due to the high iron requirements of pregnancy.8,9 Whether women remain susceptible in the postpartum period, and for how long, is unknown. In high-income settings, the postpartum period is typically considered a time of low iron deficiency risk, as the iron stores of healthy women who take iron supplements typically return to prepregnancy levels within weeks of birth.10,11 However, it remains unclear how hemoglobin and iron levels change from pregnancy through to the postpartum period in settings with a high burden of infections and under-nutrition.12 Continued anemia postpartum consigns women to poor health and increases the likelihood of entering subsequent pregnancies already anemic.13,14 Understanding hemoglobin and iron level changes in pregnancy and into the postpartum period will inform when anemia interventions will be most effective.
The WHO’s principal recommendation for the prevention of maternal anemia is universal oral iron and folate supplementation throughout pregnancy.15 This is a widely implemented recommendation that is supported by a large Cochrane Review of iron supplementation trials in pregnancy.16 Postpartum, the WHO recommends iron supplementation for the first 6–12 weeks in settings where anemia is a moderate or severe public health problem (population prevalence ≥ 20%).12 Despite this, iron supplementation is not considered part of routine postpartum care.12 To further understand the benefit of extending iron supplementation into the postpartum period, there is an urgent need for longer-term information on the prevalence of anemia and iron deficiency in the first year postpartum.
Furthermore, anemia has a complex etiology. In addition to iron deficiency, there are other important causes including micronutrient deficiencies (vitamins A and B12), genetic conditions (e.g., thalassemia), and infectious diseases.17,18,19 In malaria endemic settings, Plasmodium spp. (species) infection is a major determinant of anemia in pregnancy,19 with key prevention strategies including intermittent preventative treatment in pregnancy and use of insecticide-treated bed nets.20 Thus, in settings with more than one cause of anemia, iron supplementation may not be enough to reduce the burden of anemia. Determining the relative contributions of these risk factors to anemia, during pregnancy and postpartum periods, is important for the planning and implementation of anemia prevention strategies.
To better understand anemia in pregnancy and postpartum and to inform anemia prevention strategies, we determined the prevalence of anemia and iron deficiency during pregnancy and the first 12 months postpartum in a prospective cohort of women in Papua New Guinea and quantified the time-varying effect of iron deficiency on hemoglobin and anemia in pregnancy and postpartum.
Results
Cohort characteristics
In a cohort of 699 pregnant women recruited at first antenatal visit (Table 1), the median age was 26 years (interquartile range [IQR]: 22–30), the median gestation age was 30 weeks ( IQR: 28–32 weeks), and 75% (522/699) of women were multigravida. Genetic polymorphisms were common: 94% (637/680) had a low or intermediate complement receptor 1 (CR1) expression genotype, 16% (109/673) had α+-thalassemia, and 5% (36/681) had Southeast Asian ovalocytosis (SAO). Bed net use was moderate (63%, 440/698), and Plasmodium spp. infection detected by PCR was 12% (73/601); 42.5% (31/73) of infections were P. falciparum, 48% (35/73) were Plasmodium vivax, and 9.5% (7/73) were mixed infections (Table 1). Mean hemoglobin level at enrollment was 96.3 g/L (standard deviation [SD]: 14.5), with 82% (483/587) anemic (hemoglobin < 110 g/L). Iron deficiency (ferritin < 15 μg/L) was also highly prevalent in 81% (448/552). Enrollment sociodemographic, clinical, and diagnostic measures were similar for women who returned at birth (n = 638), 6 months postpartum (n = 552), and 12 months postpartum (n = 365) and in those who were lost to follow-up by 12 months postpartum (n = 334) (Table S1). Non-participation at each stage was due to loss-to-follow-up, relocation out of the province, or withdrawal (Figure S1).
Table 1.
Cohort characteristics at enrollment
| Sociodemographic details | n/N (%)a | |
|---|---|---|
| Enrollment clinic | Vunapope | 184/699 (26.3) |
| Nonga | 83/699 (11.9) | |
| Keravat | 125/699 (17.9) | |
| Napapar | 158/699 (22.6) | |
| Paparatava | 149/699 (21.3) | |
| Age (years) | median (IQR), range | 26 (22–30), 16–49 |
| Gravidity | primigravida | 177/699 (25.3) |
| multigravida | 522/699 (74.7) | |
| Highest level of education | primary or less | 325/698 (46.6) |
| Employment status | not employed | 531/699 (76.0) |
| Smoking status | never smoked | 427/697 (61.3) |
| current/past smoker | 270/697 (38.7) | |
| Bed net use |
owns bed net | 527/698 (75.5) |
| net used last night |
440/698 (63.0) |
|
| Clinical measures | ||
| Gestational age (weeks)b | median (IQR), range | 30 (28–32), 26–40 |
| MUAC (cm) | mean (SD), range | 26 (3), 13.5–43.7 |
| MUAC > 23 cm | 592/692 (84.7) | |
| MUAC ≤ 23 cm | 101/692 (14.4) | |
| Body mass index (kg/m2) | median (IQR), range | 25.3 (3.2), 17·9–34·3 |
| Feverc | yes | 91/687 (13.2) |
| Diagnostic measures | ||
| Hemoglobin level (g/L) | mean (SD), range | 96.3 (14.5), 41–145 |
| Anemia status | anemicd | 483/587 (82.3) |
| Ferritin level (μg/L) | median (IQR), range | 9.2 (5.3, 18.6), 0·6–292·4 |
| Iron statuse | iron deficient | 448/552 (81.2) |
| iron replete | 104/552 (18.8) | |
|
Plasmodium spp. infection (PCR) |
negative | 528/601 (87.9) |
| positive | 73/601 (12.2) | |
| P. falciparum | 31/601 (5.2) | |
| P. vivax | 35/601 (5.8) | |
| mixed |
7/601 (1.2) |
|
| Genetic polymorphisms | ||
| α+-thalassemia | wild type | 564/673 (83.8) |
| heterozygous | 87/673 (12.9) | |
| homozygous | 22/673 (3.3) | |
| CR1 deficiencyf | H/H | 43/680 (6.3) |
| H/L | 252/680 (37.1) | |
| L/L | 385/680 (56.6) | |
| SAO | normal | 645/681 (94.7) |
| SAO | 36/681 (5.3) | |
IQR, interquartile range; MUAC, mid-upper arm circumference; spp, species; PCR, polymerase chain reaction; CR1, complement receptor 1; H/H: high CR1 expression; H/L: intermediate CR1 expression; L/L: low CR1 expression; SAO, Southeast Asian ovalocytosis.
Or otherwise stated.
Gestational age was estimated from fundal height measurements using a previously published formula in women with fundal height measurements ≥24 cm.21
Self-reported history of fever during the pregnancy prior to their first antenatal care appointment.
Anemia defined as hemoglobin <110 g/L.
Iron deficient: ferritin <15 μg/L; iron replete: ferritin ≥15 μg/L and C-reactive protein (CRP) ≤10 mg/L, determined by enzyme-linked immunosorbent assays.
Allele abbreviations correspond to CR1 red blood cell surface expression levels: H allele, high expression; L allele, low.
At birth, women were asked how often they took antenatal iron folic acid supplements during their pregnancy; 65% (348/534) responded “most days”; 16% (87/534) responded “twice per week”; 7% (37/534) responded “a few times per month”; 9% (46/534) responded “only a few times during their entire pregnancy”; and 3% (16/534) “stopped taking it.” Most of these women (93%, 316/343) started taking iron supplements at their first antenatal care appointment. At 6 and 12 months postpartum, 1.4% (8/550) and 1.7% (6/360) of women reported that they were taking iron folate supplements. Women were also asked about any other medication used in pregnancy; 54% (342/635) recalled taking antimalarials at least once. No women reported taking multiple micronutrient supplementation during pregnancy or postpartum.
Hemoglobin and ferritin dynamics, and burden of anemia and iron deficiency in pregnancy and postpartum
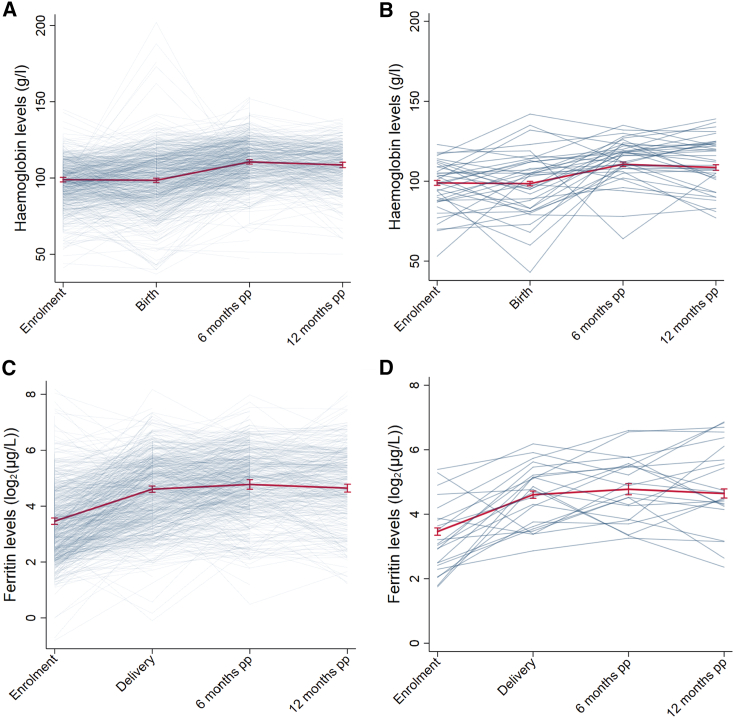
At the population level, mean hemoglobin concentration remained stable during pregnancy (adjusted mean difference of −0.44 g/L; 95% confidence interval [CI]: −2.37, 1.49; p = 0.65 at birth compared with enrollment; Figure 2; Tables S2 and S3) and then increased in the postpartum period (adjusted mean difference of 11.63 g/L; 95% CI: 9.62, 13.65; p < 0.001 and 9.63 g/L; 95% CI: 7.38, 11.87; p < 0.001 at 6 and 12 months postpartum, respectively, compared with birth). At the individual level, hemoglobin concentration showed substantial variation over time (within-woman SD = 13.02 g/L); compared with at birth, hemoglobin levels were lower at 6 months postpartum in 25% of women, higher in 74%, and did not change in 1%. The population mean ferritin concentration in pregnancy was dynamic, with a 2.3-fold increase in the geometric mean ferritin from enrollment to birth (95% CI: 2.11, 2.50; p < 0.001) and a further increase by 6 months postpartum (1.22-fold increase in adjusted geometric mean compared with birth; 95% CI: 1.12, 1.34; p < 0.001) before stabilizing (0.93-fold increase in geometric mean at 12 months compared with 6 months; 95% CI: 0.84, 1.03; p = 0.14).
Figure 2.
Hemoglobin (Hb) and ferritin dynamics over time (enrollment, birth, and 6 and 12 months postpartum [pp])
(A and B) Observed individual trajectories of Hb levels (g/L) (A) for the entire cohort and (B) for a randomly selected subset of women who have levels available at all evaluation times.
(C and D) Observed individual trajectories of ferritin levels (log2(μg/L)) (C) for the entire cohort and (D) for a randomly selected subset of women who have levels available at all evaluation times. Superimposed on the plots are the estimated mean Hb and ferritin levels over the study period (set at mean levels or prevalence of enrollment maternal factors: age, MUAC, gravidity, smoking status, clinic location, history of fever, Plasmodium spp. infection, and genetic polymorphisms).
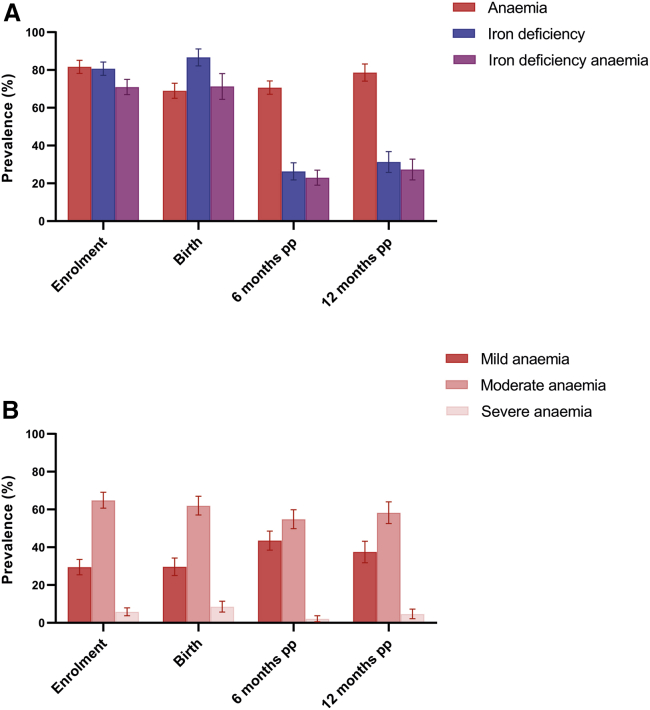
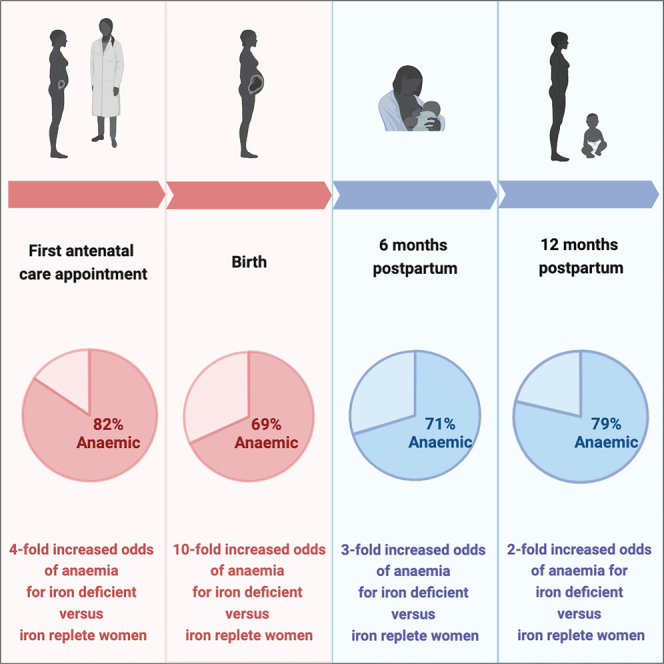
Anemia (hemoglobin < 110 g/L) was highly prevalent throughout pregnancy, with 82% (483/587) anemic at enrollment and 69% (372/537) at birth (Figure 3A). The majority of this anemia was moderate-to- severe (hemoglobin < 100 g/L) at enrollment and birth (∼70%) (Figure 3B). Postpartum, anemia (hemoglobin < 120 g/L) prevalence remained high, with >70% of women anemic at 6 (375/531) and 12 months postpartum (281/356). The proportion of moderate-to-severe anemia (hemoglobin < 110 g/L) was lower postpartum, at 57% (212/375) and 63% (176/281) for 6 and 12 months postpartum, respectively. Iron deficiency (ferritin < 15 μg/L) was also highly prevalent in pregnancy (enrollment: 81%, 448/552; birth: 87%, 171/196), but prevalence declined to 26% (104/398) by 6 months postpartum and 31% (80/257) by 12 months postpartum. Similar declines were observed for iron-deficiency anemia, decreasing from 71% (325/455) at enrollment down to 27% (67/247) by 12 months postpartum.
Figure 3.
Prevalence (95% CI) of anemia, iron deficiency, and iron-deficiency anemia at enrollment, birth, and 6 and 12 months postpartum (pp)
(A) In pregnancy (enrollment, birth), anemia is defined as Hb <110 g/L; iron deficiency is defined as ferritin <15 μg/L; iron-deficiency anemia is defined as ferritin <15 μg/L and Hb <110 g/L. In postpartum (6 months pp, 12 months pp), anemia is defined as Hb <120 g/L; iron deficiency is defined as ferritin <15 μg/L; iron-deficiency anemia is defined as ferritin <15 μg/L and Hb <120 g/l.
(B) In pregnancy (enrollment, birth), anemia is defined as mild: Hb < 110 g/L and ≥100 g/L; moderate: Hb < 100 and ≥70 g/L; severe: Hb < 70 g/L. In postpartum (6 months pp, 12 months pp), anemia is defined as mild: Hb < 120 g/L and ≥110 g/L; moderate: Hb < 110 and ≥80 g/L; severe: Hb < 80 g/L.
Associations between iron deficiency, hemoglobin levels, and anemia
To quantify the association between iron stores and the outcomes, hemoglobin levels, and anemia, univariable and multivariable mixed-effects modeling was performed (Table 2). Models included ferritin and hemoglobin measurements from enrollment, birth, and 6 and 12 month postpartum evaluation times; thus, the effect measures represent the averages across all evaluation times. It was assumed that a concurrent measurement of ferritin/iron deficiency affects hemoglobin/anemia (see causal diagram in Figure 1). In multivariable analysis, iron deficiency was associated with a lower mean hemoglobin level over the entire study period (−8.07 g/L; 95% CI: −10.12, −6.01; p < 0.001). This corresponded to a 4.60-fold increased odds (95% CI: 2.79, 7.58; p < 0.001) of anemia in those who were iron deficient compared with iron-replete women over the entire study period. When the anemia outcome measure of moderate-to-severe anemia was used, the odds were still increased for iron-deficient individuals during pregnancy and postpartum (Table S4). Other variables associated with lower mean hemoglobin levels (and anemia) over pregnancy and postpartum included being multigravida (−2.63 g/L; 95% CI: −5.26, −0.01; p = 0.05; compared with primigravida), Plasmodium spp. infection at enrollment (−4.12 g/L; 95% CI: −7.30, −0.93; p = 0.01; compared with uninfected), and having α+-thalassemia (−5.78 g/L; 95% CI: −8.48, −3.08; p < 0.001; compared with wild types) (Table 2).
Table 2.
Associations between iron stores and enrollment confounders, and hemoglobin levels and anemia over the entire study period
| Variable | Hemoglobin levels (g/L) |
Anemiaa |
|||
|---|---|---|---|---|---|
| Unadjusted mean difference (95% CI); p value | Adjusted mean difference (95% CI); p value | Unadjusted odds ratio (95% CI); p value | Adjusted odds ratio (95% CI); p value | ||
| Iron stores | |||||
| Ferritin (log2(μg/l))b | – | 1.99 (1.41, 2.58); <0.001 | – | 0.65 (0.57, 0.73); <0.001 | – |
| Iron deficiencyc |
replete | Ref. | Ref. | Ref. | Ref. |
| deficient |
−8.29 (−10.20, −6.38); <0.001 |
−8.07 (−10.12, −6.01); <0.001 |
4.26 (2.70, 6.71); <0.001 |
4.60 (2.79, 7.58); <0.001 |
|
| At enrollment | |||||
| Age (years) | – | −0.001 (−0.17, 0.16); 0.99 | 0.03 (−0.18, 0.23); 0.79 | 1.00 (0.97, 1.03); 0.93 | 1.01 (0.96, 1.05); 0.74 |
| Gravidity | primigravida | Ref. | Ref. | Ref. | Ref. |
| multigravida | −2.65 (−4.76, −0.54); 0.·01 | −2.63 (−5.26, −0.01); 0.05 | 1.34 (0.94, 1.92); 0.11 | 1.18 (0.67, 2.08); 0.56 | |
| Smoking status | never | Ref. | Ref. | Ref. | Ref. |
| current/past | −0.91 (−2.78, 0.95); 0.34 | −1.24 (−3.28, 0.80); 0.23 | 1.23 (0.89, 1.69); 0.21 | 1.45 (0.93, 2.27); 0.11 | |
| MUAC (cm) | 0.63 (0.32, 0.93); <0.001 | 0.51 (0.13, 0.90); 0.01 | 0.90 (0.86, 0.95); <0.001 | 0.89 (0.82, 0.96); 0.01 | |
| >23 cm | Ref. | – | Ref. | – | |
| ≤23 cm | −3.59 (−6.23, −0.95); 0.01 | – | 1.61 (1.00, 2.61); 0.05 | – | |
| Feverd | no | Ref. | Ref. | Ref. | Ref. |
| yes | −0.95 (−3.68, 1.77); 0.49 | −0.10 (−2.97, 2.78); 0.95 | 1.03 (0.64, 1.64); 0.91 | 0.84 (0.46, 1.56); 0.59 | |
| Plasmodium spp. (PCR) | negative | Ref. | Ref. | Ref. | Ref. |
| positive | −6.48 (−9.41, −3.55); <0.001 | −4.12 (−7.30, −0.93); 0.01 | 2.55 (1.44, 4.49); <0.001 | 1.99 (0.92, 4.26); 0.08 | |
| α+-Thalassemia | wild type | Ref. | Ref. | Ref. | Ref. |
| Het/Hom | −5.74 (−8.17, −3.32); <0.001 | −5.78 (−8.48, −3.08); <0.001 | 2.86 (1.78, 4.62); <0.001 | 3.15 (1.58, 6.28); 0.001 | |
| CR1 deficiency | H/H | Ref. | Ref. | Ref. | Ref. |
| H/L | 2.20 (−1.71, 6.12); 0.27 | 2.96 (−1.19, 7.11); 0.16 | 0.99 (0.51, 1.93); 0.97 | 0.68 (0.26, 1.75); 0.42 | |
| L/L | 2.08 (−1.73, 5.89); 0.29 | 2.72 (−1.34, 6.79); 0.19 | 1.01 (0.53, 1.95); 0.97 | 0.69 (0.27, 1.75); 0.43 | |
| SAO | normal | Ref. | Ref. | Ref. | Ref. |
| SAO | −3.22 (−7.34, 0.91); 0.13 | −2.50 (−6.64, 1.64); 0.24 | 1.16 (0.56, 2.39); 0.70 | 0.90 (0.37, 2.17); 0.81 | |
Estimated mean hemoglobin differences and odds ratios for anemia were derived from linear and logistic mixed-effects models, respectively, with a random effect for the individual-specific intercept. Adjusted models included enrollment variables listed in the table and time (enrollment, birth, and 6 and 12 months postpartum). CI, confidence interval; Het, heterozygous; Hom, homozygous; CR1, complement receptor 1; H/H, high CR1 expression; H/L, intermediate CR1 expression; L/L, low CR1 expression; SAO, Southeast Asian ovalocytosis.
Anemia defined as hemoglobin <110 g/L in pregnancy and hemoglobin <120 g/L in the postpartum period.
Ferritin transformed to log base-2 due to positively skewed distribution, thus the coefficient/odds ratio represents the change associated with a 2-fold increase in ferritin.
Iron deficient: ferritin <15 μg/L in pregnancy and postpartum; iron replete: ferritin ≥15 μg/L and CRP ≤10 mg/L in pregnancy and ferritin ≥15 μg/L and CRP ≤5 mg/L postpartum.
Self-reported history of fever during the pregnancy prior to their first antenatal care appointment.
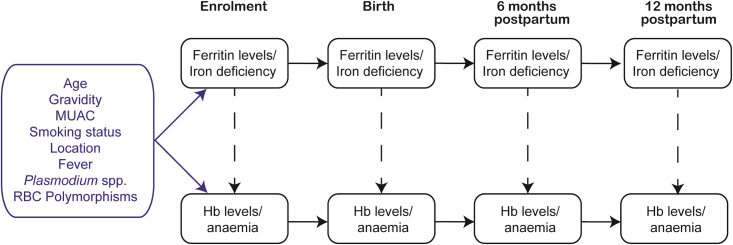
Figure 1.
Causal diagram depicting the relationships between ferritin levels/iron deficiency and hemoglobin (Hb) levels/anemia at enrollment, birth, 6 months postpartum, and 12 months postpartum
Potential confounders, presented as a single node in the blue box, include age, gravidity, mid-upper arm circumference (MUAC), location of enrollment clinic, smoking status, history of fever, Plasmodium spp. infection, and red blood cell (RBC) polymorphisms. Vertical dashed arrows depict associations between concurrent iron stores and Hb levels/anemia—the key associations of interest. Adherence to iron supplements was not included as it is not a common cause confounder and was only recorded at a single time point (birth).
Associations between anemia risk factors gravidity, Plasmodium spp. infection, α+-thalassemia, and ferritin levels/iron deficiency were assessed though multivariable mixed-effects modeling (Table S5). In multivariable analysis, multigravida women had increased odds of iron deficiency, while Plasmodium spp. infection at enrollment was associated with decreased odds of iron deficiency. α+-Thalassemia showed no significant associations with iron status, and there were no significant interactions between these risk factors and iron deficiency (likelihood ratio test p values ranged from 0.14 to 0.76) (Tables S6 and S7).
Differences in anemia etiology between pregnancy and postpartum periods
To investigate how anemia etiology differed between pregnancy and postpartum periods, an interaction term was included between evaluation time and iron deficiency in the regression models. Iron deficiency was associated with substantially increased odds of all anemia during pregnancy (enrollment adjusted odds ratio [aOR] = 4.18; 95% CI: 2.22, 7.90; p < 0.001; birth aOR 10.21; 95% CI: 3.42, 30.48; p < 0.001) (Table 3). A similar trend was observed for the association between iron deficiency and moderate-to-severe anemia during pregnancy (Table S8). At 6 months postpartum, the odds of anemia increased 3.28-fold (95% CI: 1.68, 6.43; p = 0.001) for those who were iron deficient versus iron replete (Table 3). By 12 months postpartum, the odds of anemia were only 2-fold increased for women who were iron deficient versus iron replete (aOR = 1.81; 95% CI: 0.75, 4.38; p = 0.19) (Table 3). The odds of moderate-to-severe anemia was increased for iron-deficient individuals at both 6 (aOR = 4.06; 95% CI: 2.34, 7.03; p = 0.001) and 12 months postpartum (aOR = 2.36; 95% CI: 1.22, 4.57; p = 0.01) (Table S8).
Table 3.
Time-varying contributions of iron deficiency to anemia at enrollment, birth, and 6 and 12 months postpartum
| Evaluation time | Anemia adjusted ORa (95% CI); p value | Population attributable fraction (95% CI)b | |
|---|---|---|---|
| Iron deficiencyc | enrollment | 4.18 (2.22, 7.90); <0.001 | 72% (49, 85) |
| birth | 10.21 (3.42, 30.48); <0.001 | 89% (66, 96) | |
| 6 months postpartum | 3.28 (1.68, 6.43); 0.001 | 37% (13, 62) | |
| 12 months postpartum | 1.81 (0.75, 4.38); 0.19 | 20% (0, 56) |
Anemia defined as hemoglobin <110 g/L in pregnancy and hemoglobin <120 g/L postpartum. Population attributable fractions for other important anemia risk factors identified in Table 2, such as Plasmodium spp. infection and α+-thalassemia, were not able to be determined due to low numbers of observations in the exposed, non-anemic groups.
Adjusted ORs for anemia were derived from multivariable logistic mixed-effects models with a random effect for the individual-specific intercept. Models included an interaction term included between iron deficiency and time (enrollment, birth, and 6 and 12 months postpartum) and adjusted for age, mid-upper arm circumference, gravidity, smoking status, residence, fever, and genetic polymorphisms.
Population attributable fractions for anemia were calculated using the formula: [prevalence × (OR-1)]/[prevalence (OR-1) + 1] and 95% confidence limits were calculated using confidence limits for ORs.
Women classified as iron deficient: ferritin <15 μg/L; replete: ferritin ≥15 μg/L and CRP ≤10 mg/L in pregnancy and ferritin ≥15 μg/L and CRP ≤5 mg/L postpartum.
To further quantify the contribution of iron deficiency to anemia at enrollment, birth, and 6 and 12 month postpartum evaluation times specifically, population attributable fractions were calculated from aORs (Table 3). Iron deficiency accounted for 72% (95% CI: 49, 85) and 89% (95% CI: 66, 96) of anemia cases at enrollment and birth. Similarly, iron deficiency accounted for 62% (95% CI: 37, 78) and 84% (95% CI: 50, 95) of moderate-to-severe anemia cases at enrollment and birth (Table S8). In contrast, iron deficiency accounted for 37% (95% CI: 13, 62) and 20% (95% CI; 0, 56) of anemia cases at 6 and 12 months postpartum (Table 3). Iron deficiency accounted for 44% (95% CI: 22, 65) and 30% (95% CI: 5, 57) of moderate-to-severe anemia cases at 6 and 12 months postpartum respectively (Table S8).
To investigate how long lasting the effect of iron stores on hemoglobin levels is, iron stores were included in the mixed-effects models as lagged effects—iron stores from the preceding evaluation time (depicted in Figure S2). No discernible associations were found, indicating that concurrent iron stores are the more important determinant of hemoglobin levels and anemia (Tables S9 and S10).
Discussion
Pregnant women are at risk of anemia and iron deficiency due to physiological changes and increased iron demands that occur in pregnancy. However, the burden of anemia in the postpartum period and the contribution of iron deficiency to postpartum anemia are largely unknown. In the current study of Papua New Guinean women, hemoglobin levels remained low throughout the study, with anemia prevalent in ≥69% from enrollment through to 12 months postpartum. Iron deficiency was associated with the greatest increase in odds of anemia throughout pregnancy and up to 12 months postpartum, demonstrating that it is a key risk factor for anemia, alongside Plasmodium spp. infection and α+-thalassemia. Notably, the relative contribution of iron deficiency to anemia changed over time; iron deficiency contributed to 72% of anemia at first antenatal visit but only 20% of anemia by 12 months postpartum. Current anemia prevention strategies delivered during antenatal care in this setting where women present later in pregnancy are not sufficient to address the high burden of anemia during pregnancy and postpartum periods. Anemia prevention strategies delivered earlier in pregnancy, postpartum, or in women of reproductive age targeting anemia etiologies relevant for reproductive stage may also be warranted to reduce the burden of anemia.
Global estimates suggest that approximately 50% of all anemia in pregnancy is attributable to iron deficiency1; in the present study, 72%–89% of anemia in pregnancy was attributed to iron deficiency. This suggests that a large proportion of anemia in pregnancy in this setting would be amenable to iron supplementation. Daily prenatal iron and folic acid supplementation was recommended to the women in this study as per Papua New Guinea National guidelines.22 In self-reported data on iron supplement use collected after birth, 65% (343/534) of women reported taking iron supplements “most days” during their pregnancy. However, almost all (93%, 316/343) of these women only started taking iron supplements at their first antenatal care appointment (enrollment), which typically occurred late on in pregnancy (median 30 [IQR 28–32] gestational weeks) where women would be likely at, or close to, the nadir of iron status in pregnancy. Iron supplementation may have contributed to the observed increase in ferritin concentration between enrollment and birth. Despite this increase, the proportion of women classified as anemic or iron deficient did not change between enrollment and birth, potentially because not enough time or doses between first antenatal care appointment were realized to achieve clinically meaningful benefits. Given that the majority of women were already iron deficient at their first antenatal care appointment and presented to this appointment during the second or third trimester of pregnancy, the WHO’s primary recommendation of universal supplementation during pregnancy may not be sufficient to prevent maternal anemia in settings where first antenatal care appointments tend to occur later in pregnancy.
Another opportunity to prevent anemia exists postpartum, between pregnancies, when women are attending regular medical appointments for infant checkups and immunizations. Iron supplementation is not routinely provided as part of postnatal care in Papua New Guinea. In line with this, only ∼1.5% of women in this study reported that they were taking iron folate supplements at 6 and 12 months postpartum. Iron supplementation could be extended into the postpartum period for women with moderate-to-severe anemia, through existing health systems, to improve women’s hemoglobin and iron reserves before their next pregnancy. This intervention strategy could reduce the risk of anemia in subsequent pregnancies, as well as address postpartum anemia caused by iron deficiency. There is a paucity of literature on the prevalence and etiology of postpartum anemia in resource-limited settings worldwide. The high burden of postpartum anemia (>70%) observed highlights that the postpartum period should not be considered a time of low anemia risk in this setting. However, the proportion of anemia attributable to iron deficiency was lower postpartum than in pregnancy, ∼20% by 12 months postpartum. Aside from iron deficiency, Plasmodium spp. infection was a key risk factor for anemia in this cohort. Thus, the effective implementation of malaria prophylaxis should be encouraged, as per standard of care. Bed net use in this cohort was moderate (63% reported using a net the night prior to enrollment) but could be strengthened further as an anemia prevention strategy, along with regular rapid diagnostic testing for malaria. While Plasmodium spp. infection (and also α+-thalassemia) were significant risk factors for anemia, their prevalence was relatively low and could not account for all the remaining attributable risk. Other potential anemia etiologies that were not assessed here, such as infection with intestinal helminths and other micronutrient deficiencies (e.g., vitamins A and B12), may also be important.23,24 Quantifying these intervenable risk factors will inform a potential suite of interventions that address infections and multiple nutritional needs and further reduce the significant burden of anemia in pregnancy and postpartum.
The consistently low population mean hemoglobin levels throughout the study period suggest that women of reproductive age in this setting experience a cycle of chronic anemia, with hemoglobin levels never being fully restored between pregnancies. In order to substantially reduce anemia in settings where it is highly prevalent (≥20%), the WHO recently proposed that iron supplementation be provided to all menstruating women7 rather than just during pregnancy and postpartum periods. This strategy has not been widely implemented but has proved successful in regions of Vietnam and India, which saw anemia reductions of 20% and 24%, respectively, in women after 12 months of iron supplementation.25,26 Another option is to target adolescent girls through schools. This approach was adopted in Ghana, where a cohort of adolescent girls received weekly iron-folic supplementation, resulting in a 26% reduction in anemia after 9 months of implementation.27 These programs provided intermittent iron supplementation, which has similar efficacy to the daily regime but with fewer side effects.16 Similar interventions in Papua New Guinea may be valuable but need to consider challenges in health service access, constraints in infrastructure, and low community outreach frequency, especially in remote and rural areas. However, given the public health problem anemia poses in this setting, it is a high priority for improving the health and well-being of women. This is particularly relevant in light of the WHO Global Nutrition target to halve anemia in women of reproductive age by 50% in 20257; a goal that is unlikely to be achieved in the near term in settings like Papua New Guinea that experience a high burden of maternal anemia without a push for further action or new intervention strategies.
As an alternative to oral iron supplementation, iron can be given intravenously. Modern intravenous iron formulations have been deemed safe and effective in preventing anemia in both pregnancy and postpartum periods in recent randomized controlled trials (reviewed in Qassim et al.,28 Lewkowitz et al.,29 and Sultan et al.30). The key advantage of this strategy is that a single infusion of intravenous iron is required, so there are no compliance concerns. However, significant health system capacity building and strengthening would need to take place to successfully deliver intravenous iron to all moderately to severely anemic pregnant and postpartum women attending health care facilities in Papua New Guinea.
Limitations of the study
In East New Britain, Papua New Guinea, ∼90% of women attend antenatal care, and our study participants were representative of pregnant women attending five antenatal clinics that provide >75% of antenatal services in the province. A limitation of our study was that it did not capture women not attending antenatal care, typically women living in hard-to-reach areas with presumed poorer health, which may lead us to underestimate the true population prevalence of anemia and iron deficiency. Loss to follow-up (48% by 12 months postpartum) may also introduce bias. However, in our analyses, we used mixed-effects modeling with maximum likelihood estimation, which uses all participant outcome data regardless of completeness across time points. This estimation method provides unbiased effect estimates in the presence of attrition assuming a “missing-at-random” missing data mechanism. Furthermore, given that enrollment characteristics were similar at follow-up time points, attrition should not significantly impact study estimates or study conclusions. It should also be noted that the population attributable fractions were calculated using ORs, and given that anemia was a common outcome in our study population, this will result in an overestimation of the population attributable fractions. In terms of external validity, the relative contribution of iron deficiency, genetic polymorphisms, malaria, and other anemia risk factors will vary with the local conditions. In accordance with this, prevention and control strategies for maternal anemia will need to be tailored to the setting-specific anemia etiology. Despite these limitations, some generalizations from this study can be made. The fact that 69% of women in this study were still anemic at birth in this cohort, coupled with the stagnant global prevalence of anemia in pregnancy over the last two decades, suggests that the WHO primary recommendation of universal antenatal iron folic acid supplementation is either not being implemented successfully, or early enough, or is not effective enough to prevent maternal anemia in resource-limited settings. Based on the high prevalence of anemia observed during the first 12 months postpartum in this study, we can postulate that populations experiencing a similarly high burden of anemia during pregnancy will continue to have high anemia prevalence in the postpartum period without intervention. Given that every second pregnant woman is estimated to be anemic in resource-limited settings,1 anemia in the postpartum period is likely to be a widespread yet neglected condition globally.
Conclusion
Maternal anemia was highly prevalent in pregnancy and postpartum in our population, with iron deficiency a major risk factor in pregnancy, but less so in the postpartum period. Iron supplementation provided both early during pregnancy and between pregnancies, in conjunction with malaria prevention strategies, could break the cycle of chronic anemia in women of reproductive age. Research is needed to determine the effectiveness and feasibility of providing iron supplementation earlier in pregnancy, as part of routine postpartum care, and the optimal frequency and duration of supplementation required to improve the health of women in this region, as well as other settings where maternal anemia is highly prevalent.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human serum samples | Healthy Mothers Healthy Babies cohort study in this paper | N/A |
| Human whole blood samples | Healthy Mothers Healthy Babies cohort study in this paper | N/A |
| Critical commercial assays | ||
| Ferritin ELISA kit | Immunology Consultants Laboratory Immunoperoxidase Assay | Cat#E−80F |
| CRP ELISA kit | Elisakit, | Cat#EK-0040 |
| QIAamp ® DNA and Blood Minikit | Qiagen | Cat#51104 |
| Oligonucleotides | ||
| α2/3.7 Forward CCCCTCGCCAAGTCCACCC | Geneworks | N/A |
| α2 Reverse AGACCAGGAAGGGCCGGTG | Geneworks | N/A |
| 3.7 Reverse AAAGCACTCTAGGGTCCAGCG | Geneworks | N/A |
| 4.2 Forward GGTTTACCCATGTGGTGCCTC | Geneworks | N/A |
| 4.2 Reverse CCCGTTGGATCTTCTCATTTCCC | Geneworks | N/A |
| CR1 Exon 22 Forward TTCACATTGGATAGCCCAGAGC | Geneworks | N/A |
| CR1 Exon 22 Reverse CCCTTGTAAGGCAAGTCTGG | Geneworks | N/A |
| SAO Forward GGGCCCAGATGACCCTCTGC | Geneworks | N/A |
| SAO Reverse GCCGAAGGGGTGATGGCGGGTG | Geneworks | N/A |
| P. falciparum forward TATTGCTTTTGAGAGGTTTTGTTACTTTG | Bioneer Pacific | N/A |
| P. falciparum reverse ACCTCTGACATCTGAATACGAATGC | Bioneer Pacific | N/A |
| P. falciparum probe ACGGGTAGTCATGATTGAGTT | Bioneer Pacific | N/A |
| P. vivax forward GCTTTGTAATTGGAATGATGGGAAT | Bioneer Pacific | N/A |
| P. vivax reverse ATGCGCACAAAGTCGATACGAAG | Bioneer Pacific | N/A |
| P. vivax probe AGCAACGCTTCTAGCTTA | Bioneer Pacific | N/A |
| Software and algorithms | ||
| STATA Version 15 | StataCorp, College Station, TX, USA | N/A |
Resource availability
Lead contact
For further information and requests for resources should be directed to and will be fulfilled by the lead contact, Freya Fowkes (freya.fowkes@burnet.edu.au).
Materials availability
This study did not generate new unique reagents.
Experimental model and Study participant details
Study setting
Papua New Guinea is a resource-limited country in the Western Pacific with a population of approximately 9 million people. The majority (87%) of Papua New Guineas population reside in rural and remote areas,31 where rugged terrain, poor roads, and limited infrastructure restrict access to health services. A chronic shortage of health providers is another major health system constraint,32 with an estimated nine health providers per 10,000 people nationwide.33 At an estimated 594 per 100,000 live births, in 2013 Papua New Guinea has one of the highest maternal mortality ratios in the world.34
This study took place in the rural province of East New Britain province, in the Islands Region of Papua New Guinea. Five hospitals/health centers were selected as sentinel sites: Nonga Hospital, Saint Mary’s Hospital (Vunapope), Keravat rural Hospital, Paparatava Health Center and Naparpar Health Center. These encompass the two largest urban hospitals and three busy health facilities in rural areas of East New Britain, and account for over 75% of antenatal care services in the province according to the Provincial Health Office.
The National Guideline for Obstetrics and Gynecology in Papua New Guinea recommends women book antenatal care after missing 2–3 menstrual periods.35 However, a national health survey review found fewer than half of all pregnant women attended one antenatal care visit.33 As part of routine antenatal care in Papua New Guinea, daily iron folate supplementation is provided to all women throughout pregnancy. Intermittent preventive treatment of malaria is also routinely delivered in antenatal care visits. Deworming is recommended,35 but there are no reliable statistics on the implementation of this intervention.36 There are no national guidelines on postnatal care after hospital discharge. For those diagnosed as anemic in pregnancy, iron folic acid supplementation is recommended for 1–3 months postpartum,35 however, postnatal care is not recorded consistently and there is limited data on this intervention and routine care more broadly.
Subject details
This prospective cohort study of 699 pregnant women took place between March 2015 and December 2018. Pregnant women of any gravidity were randomly selected to participate through a dice roll, while attending one of the afore-mentioned five local clinics/hospitals as previously described.37,38 Inclusion criteria required that participants be 1) at least 16 years old; 2) attending their first antenatal care appointment for the current pregnancy; 3) reside within the catchment area of the healthcare facility; 4) intending to live in East New Britain for the subsequent 12 months; and 5) be willing to participate. The original study was powered on the primary outcome of birthweight and a sample size of 700 was calculated to detect a clinically meaningful difference of at least 120g in birthweight with a power of 90% and a significance level of 5%, and where the standard deviation of birthweight was 475g for the exposures malaria, sexually transmitted infections, tuberculosis, anemia and iron deficiency (assuming prevalence of approximately 30%). The analysis of anemia and iron deficiency during pregnancy and postpartum presented here represents secondary outcomes. Follow-up visits took place at birth, 1-, 6- and 12-month postpartum, at the health facility where possible, or at their residence.
At enrollment, following informed consent, women completed interviews, covering demographic details, obstetric history, and accounts of illness and medication use in the current pregnancy. Physical examinations were performed, collecting anthropometric and fundal height measurements. Gestational age was calculated from enrollment fundal height measurements (excluding <24cm and twins) using a previously published formula.21 Venous blood samples were collected, then again at birth, 6- and 12-month postpartum. At each timepoint, hemoglobin concentration was determined using a Hemocue haemoglobinometer (Hemocue, Ängelholm, Sweden). Plasmodium spp. infection was determined by ACCESSBIO CareStart Malaria HRP2/pLDH (Pf/PAN) Combo and women with a positive diagnosis were supported to receive appropriate treatment (Artemether lumefantrine). Intermittent preventive treatment (sulfadoxine-pyremethamine) and iron folate supplementation was provided to all women during antenatal visits by the antenatal clinic, as per national guidelines. Data on uptake and adherence of iron folate supplements during pregnancy was collected retrospectively at the birth. At 6- and 12-month postpartum women were asked to report use of any medication, but adherence data was not collected. All women had the same survey data and samples collected, and the same laboratory tests performed.
Ethics approval was obtained from Alfred Health, Australia (348/14), and the Medical Research Advisory Council, National Department of Health, Papua New Guinea (14/27).
Method details
Ferritin and CRP ELISAs for iron deficiency diagnosis
Ferritin (Immunology Consultants Laboratory Immunoperoxidase Assay) and C-reactive protein (CRP) (Elisakit) was determined by enzyme-linked immunosorbent assay after the cohort study was completed in serum samples at enrollment, birth, 6- and 12-month postpartum. ELISAs for Ferritin and CRP were run in 96-well plates, following the corresponding kit protocols. To obtain absolute quantification of Ferritin and CRP concentration, standard curves were established using an 8-fold dilution of standards with known concentrations, which were provided in the kits and run in duplicate. Negative controls, or 'blanks', containing ELISA reagents but no standard or serum sample, were also included in all ELISA plates. Furthermore, positive controls were incorporated in all ELISA plates, which consisted of at least two serum samples collected from Melbourne controls.
Detection of P. Falciparum and P. Vivax infection
Quantitative polymerase chain reaction (qPCR) was utilized to detect peripheral P. falciparum and P. vivax parasitaemia, following a previously published protocol.39 The 18S ribosomal RNA gene was amplified with specific primers and probes for either P. falciparum or P. vivax. Standard curves were generated for absolute quantification of P. falciparum and P. vivax, using a 7-fold serial dilution of the control plasmids, ranging from 78,125 copies/μL to 5 copies/μL, in quadruplicate. To ensure reproducibility, 12% of all samples were run as repeats. Negative controls were included in all PCR reactions, which contained PCR master mix but no DNA. Assays were performed on a QuantStudio 7 Flex Real-Time PCR System (ThermoScientific) in 384-well plate format. P. falciparum and P. vivax qPCR reagent and cycling conditions specified in Additional File 1, Table S11.
Genotyping of red blood cell polymorphisms
DNA was extracted from red blood cell samples, previously separated from venous blood plasma, using the QIAamp DNA and Blood Minikit (Qiagen) manufacturer spin protocol. RBC polymorphisms α+-thalassemia, CR1 deficiency and SAO were typed using polymerase chain reactions (PCRs). The two most common α+-thalassemia deletions in Melanesian populations, 3.7kb and 4.2kb deletions, were typed by multiplex PCR as previously described,40 with modifications. SAO (27bp deletion in the band 3 gene) and CR1 polymorphisms (exon 22, A/G substitution at 3650) were typed using established methods.41,42 All PCR reagent volumes, cycling and gel conditions are specified in Additional File 1, Table S12.
Quantification and statistical analysis
Data were analyzed using STATA Version 15 (StataCorp, College Station, TX, USA). To determine the association between iron status (ferritin concentration, iron deficient/replete) and the outcomes hemoglobin levels or anemia, univariable and multivariable linear or logistic mixed-effects modeling were performed with time included as a categorical variable, and a random effect for the individual-specific intercepts was included to account for between-women variability at enrollment. To determine the association between iron status at preceding evaluation times and hemoglobin levels/anemia, ferritin and iron deficiency were also included as lagged effects (Figure S2). Anemia was defined as hemoglobin <110 g/L in pregnancy and hemoglobin <120 g/L in postpartum, according to WHO guidelines.43 Iron deficiency was defined as ferritin <15 μg/L; iron replete was defined as ferritin ≥15 μg/L and CRP ≤5 mg/L or ≤10 mg/L (in pregnancy and postpartum respectively, to take into account raised ferritin due to inflammation), as per WHO recommendation.8 Multivariable models were adjusted for enrollment confounders determined a priori: maternal age, gravidity, mid-upper arm circumference, location of enrollment clinic, smoking, self-reported history of fever in pregnancy, PCR detected Plasmodium spp. infection, α+-thalassemia, CR1 deficiency, and SAO - informed by directed acyclic graphs (Figure 1). Effect modification by time was investigated by adding an interaction term between time and iron status. Population attributable fractions were calculated using the odds ratios from these mixed-effects multivariable models and the formula [prevalence x (OR-1)]/[prevalence (OR-1) + 1]. To determine associations between anemia risk factors: gravidity, Plasmodium spp. infection, α+-thalassemia, and ferritin levels/iron deficiency, univariable and multivariable mixed-effects modellings were performed. Adherence to iron supplements was not adjusted for in the above models as it was not assumed to be a common cause confounder and was only recorded at a single time-point mid follow-up.
Acknowledgments
First and foremost, we thank the women for their participation. We thank the Healthy Mothers Healthy Babies staff in Kokopo, Papua New Guinea, for performing study administration, interviews, sample collection, and data entry: Dr. Stenard Hiasihri, Essie Koniel, Thalia Wat, Noelyne Taraba, Chris Sohenaloe, Dorish Palagat, Zoe Saulep, Elizabeth Walep, Irene Daniels, Gabriella Kalimet-Tade, Noreen Tamtilik, Ellen Kavang, Wilson Kondo, Allan Tirang, Michael Palauva, Ioni Pidian, Teddy Wanahau, Eremas Amos, Bettie Matonge, Elice Adimain, Thelma Punion, and Lucy Palom. Thank you to the invaluable project support from Burnet Institute Melbourne, especially Stanley Luchters, Rodney Stewart, Kellie Woiwod, James Lawson, Lisa Davidson, Vivian Newton, Lisa Vitasovich, Paul Agius for statistics advice, Long Nguyen for database support, and Clarissa Moreira for providing the study flow chart included in the supplemental information. For ongoing leadership and technical guidance to the Healthy Mothers Healthy Babies program, we also thank Prof. Michael Toole and Prof. Caroline Homer. Our special thanks to the National Department of Health, the East New Britain Provincial Administration, the Provincial Health Authority, and Catholic Health Services and participating health facilities (Nonga General Hospital, St Mary’s Vunapope, Kerevat Rural Hospital, Napapar Health Center, Paparatava Health Center) for enthusiastically facilitating our research team to work alongside them. Specific thanks to Mr. Nicholas Larme, Mr. Levi Mano, Dr. Ako Yap, Mr. Moses Bogandri, Mr. Benedict Mode, Dr. Pinip Wapi, Dr. Felix Diaku, Dr. Tanmay Bagade, Dr. Delly Babona, Sr. Placidia Nohan, Sr. Theonila Wat, and Sr. Rebecca Penaia, who have provided invaluable support and advice throughout the planning and implementation of this work in East New Britain.
This work was funded by the Burnet Institute with philanthropic support provided by numerous private and business donors in Australia and PNG, including the Bank South Pacific PNG Community Grant, the Gras Foundation, the Fidelity Foundation, the June Canavan Foundation, the Naylor Steward Ancillary Fund, and the Chrysalis Foundation. E.M.D. was supported by an Australian Postgraduate Award. Several authors receive funding from the National Health and Medical Research Council of Australia: Career Development Fellowship and Investigator Grants to F.J.I.F. and L.J.R., Senior Research Fellowship and Investigator Grant to J.G.B. and J.A.S., Program Grant to J.G.B. and B.S.C., and Postgraduate Research Scholarship to C.J.M. M.J.L.S. received a Basser Research Entry Scholarship from the Royal Australasian College of Physicians Foundation (2018 and 2020). The Burnet Institute is supported by the NHMRC Independent Research Institutes Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Support scheme.
Authors contributions
J.G.B., C.J.M., M.J.L.S., F.J.I.F., P.B., and B.S.C. provided major contributions to the Healthy Mothers Heathy Babies study design, with input from A.E., P.M.S., W.P., L.J.R., and E.K. M.J.L.S., E.P., P.M., H.S., P.H., W.P., D.K., K.T., R.S., R.F., and B.K. undertook field work and data collection. E.M.D. and D.H.O. performed and interpreted iron deficiency assays. E.M.D., J.A.S., and F.J.I.F. conceived and designed the statistical plan. E.M.D. and F.J.I.F. drafted the manuscript, and all authors approved the final report.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: July 5, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101097.
Supplemental information
Data and code availability
-
•
The data analyzed during the current study are not publicly available due to study participants’ privacy, but de-identified data reported in this paper will be shared by the lead contact to applicants who provide a sound proposal to the Medical Research Advisory Council, National Department of Health, Papua New Guinea.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.World Health Organization . World Health Organization; 2015. The Global Prevalence of Anaemia in 2011. [Google Scholar]
- 2.Stevens G.A., Finucane M.M., De-Regil L.M., Paciorek C.J., Flaxman S.R., Branca F., Peña-Rosas J.P., Bhutta Z.A., Ezzati M., Nutrition Impact Model Study Group Anaemia Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Global Health. 2013;1:16–25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozuki N., Lee A.C., Katz J., Child Health Epidemiology Reference Group Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J. Nutr. 2012;142:358–362. doi: 10.3945/jn.111.149237. [DOI] [PubMed] [Google Scholar]
- 4.Balarajan Y., Ramakrishnan U., Ozaltin E., Shankar A.H., Subramanian S.V. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 5.Brabin B.J., Hakimi M., Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J. Nutr. 2001;131:604S–614S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 6.Young M.F., Oaks B.M., Tandon S., Martorell R., Dewey K.G., Wendt A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019;1450:47–68. doi: 10.1111/nyas.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . World Health Organization; 2014. Global Nutrition Targets 2025: Anaemia Policy Brief. WHO/NMH/NHD/14.4) [Google Scholar]
- 8.World Health Organization . World Health Organization; 2004. Assessing the Iron Status of Populations. [Google Scholar]
- 9.Bothwell T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000;72 doi: 10.1093/ajcn/72.1.257S. 257S-64S. [DOI] [PubMed] [Google Scholar]
- 10.Milman N., Agger A.O., Nielsen O.J., Iron supplementation during pregnancy Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan. Med. Bull. 1991;38:471–476. [PubMed] [Google Scholar]
- 11.Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann. Hematol. 2011;90:1247–1253. doi: 10.1007/s00277-011-1279-z. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . World Health Organization; 2016. Guideline: Iron Supplementation in Postpartum Women. [PubMed] [Google Scholar]
- 13.Bodnar L.M., Cogswell M.E., Scanlon K.S. Low income postpartum women are at risk of iron deficiency. J. Nutr. 2002;132:2298–2302. doi: 10.1093/jn/132.8.2298. [DOI] [PubMed] [Google Scholar]
- 14.Scholl T.O., Reilly T. Anemia, iron and pregnancy outcome. J. Nutr. 2000;130(Suppl) doi: 10.1093/jn/130.2.443S. 443S-7S. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; 2012. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. [PubMed] [Google Scholar]
- 16.Peña-Rosas J.P., De-Regil L.M., Garcia-Casal M.N., Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015;2015:CD004736. doi: 10.1002/14651858.CD004736.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gernand A.D., Schulze K.J., Stewart C.P., West K.P., Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat. Rev. Endocrinol. 2016;12:274–289. doi: 10.1038/nrendo.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning L., Laman M., Rosanas-Urgell A., Michon P., Aipit S., Bona C., Siba P., Mueller I., Davis T.M.E. Severe anemia in Papua New Guinean children from a malaria-endemic area: a case-control etiologic study. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kai O.K., Roberts D.J. The pathophysiology of malarial anaemia: where have all the red cells gone? BMC Med. 2008;6:24. doi: 10.1186/1741-7015-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . World Health Organization; 2017. Implementing Malaria in Pregnancy Programs in the Context of World Health Organization Recommendations on Antenatal Care for a Positive Pregnancy Experience. [Google Scholar]
- 21.Nosten F., McGready R., Simpson J.A., Thwai K.L., Balkan S., Cho T., Hkirijaroen L., Looareesuwan S., White N.J. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 22.National Department of Health Papua New Guinea . Government of Papua New Guinea; 2010. National Health Plan 2011-2020, Volume 1 Policies and Strategies. [Google Scholar]
- 23.Brooker S., Hotez P.J., Bundy D.A.P. Hookworm-Related anaemia among pregnant women: a systematic review. PLoS Neglected Trop. Dis. 2008;2:e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwangi M.N., Prentice A.M., Verhoef H. Safety and benefits of antenatal oral iron supplementation in low-income countries: a review. Br. J. Haematol. 2017;177:884–895. doi: 10.1111/bjh.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey G.J., Montresor A., Cavalli-Sforza L.T., Thu H., Phu L.B., Tinh T.T., Tien N.T., Phuc T.Q., Biggs B.A. Elimination of iron deficiency anemia and soil transmitted helminth infection: evidence from a fifty-four month iron-folic acid and de-worming program. PLoS Neglected Trop. Dis. 2013;7:e2146. doi: 10.1371/journal.pntd.0002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguayo V.M., Paintal K., Singh G. The Adolescent Girls' Anaemia Control Programme: a decade of programming experience to break the inter-generational cycle of malnutrition in India. Publ. Health Nutr. 2013;16:1667–1676. doi: 10.1017/S1368980012005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghana Health Service, Ghana Education Service, UNICEF-Ghana, Emory University of Global Health Institute, Centres for Disease Control and Prevention (CDC) UNICEF-Ghana Cantonments; 2019. Impact Evaluation of a School-Based Integrated Adolescent Nutrition and Health Programme with Iron and Folic-Acid Supplementation Intervention Among Adolescent Girls in Ghana. [Google Scholar]
- 28.Qassim A., Grivell R.M., Henry A., Kidson-Gerber G., Shand A., Grzeskowiak L.E. Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta-analysis. Med. J. Aust. 2019;211:367–373. doi: 10.5694/mja2.50308. [DOI] [PubMed] [Google Scholar]
- 29.Lewkowitz A.K., Gupta A., Simon L., Sabol B.A., Stoll C., Cooke E., Rampersad R.A., Tuuli M.G. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. J. Perinatol. 2019;39:519–532. doi: 10.1038/s41372-019-0320-2. [DOI] [PubMed] [Google Scholar]
- 30.Sultan P., Bampoe S., Shah R., Guo N., Estes J., Stave C., Goodnough L.T., Halpern S., Butwick A.J. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019;221:19–29.e3. doi: 10.1016/j.ajog.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The World Bank . 2022. World Bank Open Data. Riural Population (% of Total Population)https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS 2018. [Google Scholar]
- 32.Wilson A.N., Melepia P., Suruka R., Hezeri P., Kabiu D., Babona D., Wapi P., Spotswood N., Bohren M.A., Vogel J.P., et al. Quality newborn care in East New Britain, Papua New Guinea: measuring early newborn care practices and identifying opportunities for improvement. BMC Pregnancy Childbirth. 2022;22:462. doi: 10.1186/s12884-022-04735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Department of Health Papua New Guinea . National Department of Health; 2021. Annual Review, Assessment of Sector Performance 2016-2020 National Report. Posrt Moresby: Papua New Guinea. [Google Scholar]
- 34.Kassebaum N.J., Bertozzi-Villa A., Coggeshall M.S., Shackelford K.A., Steiner C., Heuton K.R., Gonzalez-Medina D., Barber R., Huynh C., Dicker D., et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mola G. Manual of standard management in obstetrics and Gynaecology for doctors, HEOs and nurses in Papua New Guinea. Port Moresby. 2016 Seventh Edition. [Google Scholar]
- 36.UNICEF . 2016. Papua New Guinea National Nutrition Policy 2016-2026. [Google Scholar]
- 37.Scoullar M.J.L., Boeuf P., Peach E., Fidelis R., Tokmun K., Melepia P., Elijah A., Bradshaw C.S., Fehler G., Siba P.M., et al. Mycoplasma genitalium and other reproductive tract infections in pregnant women, Papua New Guinea, 2015-2017. Emerg. Infect. Dis. 2021;27:894–904. doi: 10.3201/eid2703.201783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peach E., Morgan C., Scoullar M.J.L., Fowkes F.J.I., Kennedy E., Melepia P., Homiehombo P., Au L., Luchters S., Umbers A.J., et al. Risk factors and knowledge associated with high unintended pregnancy rates and low family planning use among pregnant women in Papua New Guinea. Sci. Rep. 2021;11:1222. doi: 10.1038/s41598-020-79103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosanas-Urgell A., Mueller D., Betuela I., Barnadas C., Iga J., Zimmerman P.A., del Portillo H.A., Siba P., Mueller I., Felger I. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar. J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong S.S., Boehm C.D., Higgs D.R., Cutting G.R. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood. 2000;95:360–362. [PubMed] [Google Scholar]
- 41.Jarolim P., Palek J., Amato D., Hassan K., Sapak P., Nurse G.T., Rubin H.L., Zhai S., Sahr K.E., Liu S.C. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc. Natl. Acad. Sci. USA. 1991;88:11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang L., Rundles J.R., Hamilton D.R., Wilson J.G. Quantitative alleles of CR1: coding sequence analysis and comparison of haplotypes in two ethnic groups. J. Immunol. 1999;163:4939–4945. [PubMed] [Google Scholar]
- 43.World Health Organization . World Health Organization; 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The data analyzed during the current study are not publicly available due to study participants’ privacy, but de-identified data reported in this paper will be shared by the lead contact to applicants who provide a sound proposal to the Medical Research Advisory Council, National Department of Health, Papua New Guinea.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.