Abstract
BACKGROUND:
Among patients with schizophrenia, nonadherence to oral atypical antipsychotics (OAAs) leads to increased risk of relapses, which entails substantial economic burden.
OBJECTIVE:
To evaluate the impact on health care costs and relapse rates of switching patients with schizophrenia from OAAs to once-monthly paliperidone palmitate (PP1M), with subsequent transitions to once-every-3-months (PP3M) and once-every-6-months paliperidone palmitate (PP6M).
METHODS:
A 36-month Markov model was developed from a Medicaid payer’s perspective. Two non–mutually exclusive subpopulations of adults with schizophrenia who were nonadherent to OAAs were considered: (1) recently relapsed and (2) young adults (aged 18-35). Patients were assumed nonadherent to OAAs until switching treatments, which was permissible multiple times during the 36-month period. Patients switching to PP1M could subsequently transition to PP3M and PP6M. Relapse rates were assumed consistent across treatments based on patients’ adherence. Model inputs were literature based. PP6M transition rates were assumed similar to PP3M. Cost savings were reported at the plan level and per patient switched.
RESULTS:
In a hypothetical health plan of 1 million Medicaid beneficiaries, an estimated 10,053 adults with schizophrenia were nonadherent to OAAs, among whom 7,454 were recently relapsed and 4,002 were young adults. Switching 5% of recently relapsed adults (N = 373) from OAAs to PP1M prior to subsequent relapse resulted in 541 relapses avoided and plan-level savings of $8.2M after 3 years. Incorporating transitions to PP3M/PP6M increased net savings to $9.1M and 631 relapses were avoided. Among young adults, switching 5% (N = 200) from OAAs to PP1M saved $1.8M at the plan level with 178 relapses avoided after 3 years. Including transitions to PP3M/PP6M, 3-year plan-level savings were $2.0M with 223 relapses avoided. Per recently relapsed patient switched to PP1M, and subsequently to PP3M/PP6M, cumulative 3-year cost savings were $22,100 and $24,300, respectively. Among young adults, corresponding 3-year cost savings per patient were $8,900 and $9,800.
CONCLUSIONS:
Switching nonadherent patients from OAAs to PP1M results in substantial cost savings and reduces relapse rates. Incorporating transitions to PP3M/PP6M leads to incremental cost savings and additional relapses avoided.
Plain language summary
Nonadherence to oral antipsychotics among patients with schizophrenia can increase their risk of hospitalization. Long-acting antipsychotics, with a lower dosing frequency than traditional antipsychotics, have been found to improve adherence to treatment, which reduces the risk of hospitalization. This projection exercise found that switching patients from oral to certain long-acting antipsychotics would reduce the number of hospitalizations, resulting in health care cost savings. Switching patients earlier in their disease course would result in additional cost savings.
Implications for managed care pharmacy
Among recently relapsed patients with schizophrenia who are nonadherent to oral atypical antipsychotics, switching to once-monthly/once-every-3-months/once-every-6-months paliperidone palmitate resulted in fewer relapses compared with remaining on oral atypical antipsychotics, leading to substantial plan-level cost savings with incremental benefits when incorporating transitions to once-every-6-months paliperidone palmitate. Notably, cost savings were observed when patients were switched prior to experiencing a subsequent relapse, and findings were similar among nonadherent young adults who are at a higher risk of schizophrenia relapse.
Schizophrenia is a severe and persistent mental disorder that typically emerges in young adulthood and is characterized by periods of delusions, hallucinations, and catatonic behavior, among other debilitating symptoms, which remit and recur over time.1 Accordingly, schizophrenia is associated with a substantial economic burden, with one estimate of $281.6B in societal costs in the United States in 2020.2 Symptomatic relapse is particularly costly because of increased hospitalizations and use of other health care resources. One recent study demonstrated that health care costs among recently relapsed patients with schizophrenia can total more than $34,000 per patient per year (2018 USD).3 With patients experiencing an average of 9 relapses over a mean 5.5-year observation period, management of frequent relapses undeniably adds to the considerable clinical and economic burden of schizophrenia.4 As such, a primary goal of schizophrenia treatment is relapse prevention.5
Daily oral atypical antipsychotics (OAAs) are the mainstay frontline treatment for schizophrenia,6 and although effective, they are associated with high levels of nonadherence, particularly among young adults (ie, aged 18-35 years), complicating disease management and leading to an increased risk of relapse and hospitalization and higher health care costs.7-14 Long-acting injectables (LAIs), such as once-monthly paliperidone palmitate (PP1M),15 are recommended by schizophrenia treatment guidelines for patients who are either nonadherent or refractory to initial treatment5,6 and have been shown to be effective at preventing relapse and hospitalization in patients with schizophrenia, thus reducing the ensuing costs.8-10,12,13,16,17 For patients who have been adequately treated with PP1M for 4 or more months, a longer-acting formulation, once-every-3-months paliperidone palmitate (PP3M), has been approved for use,18 and preliminary analyses have shown incremental benefits for those transitioning from PP1M to PP3M.8,19,20 The newest formulation, once-every-6-months paliperidone palmitate (PP6M), recently received approval for patients who have been stabilized on 1 of the 2 highest doses of PP1M15 or PP3M,18 is the first LAI with a twice-yearly dosing regimen for the treatment of schizophrenia.21
To date, little is known about the effect on the relapse rate and economic burden of switching nonadherent patients with schizophrenia from OAAs to PP1M and transitioning those eligible to PP3M and PP6M, particularly from the perspective of Medicaid. Medicaid is the largest payer for mental health care services in the United States with a higher prevalence of schizophrenia compared with national estimates (2.16%-4.01% vs 0.25%-0.64%).1,22,23 A cost model was developed to evaluate the long-term (ie, 3-year) impact on relapse rates and health care costs of switching nonadherent patients with schizophrenia from OAAs to PP1M, incorporating subsequent transitions to PP3M and PP6M, with a focus on nonadherent, recently relapsed adults and nonadherent young adults (ie, aged 18-35 years) insured through Medicaid. Given limited real-world evidence on PP3M and the novel PP6M formulation, this study aims to provide valuable economic and clinical projections for the benefit of patients, physicians, and payers.
Methods
MODEL STRUCTURE AND DESIGN
A Markov model with 3-month cycles over a 36-month time frame (Figure 1) was adapted from previously published analyses that used a 36-month decision tree model to assess the economic impact of transitioning nonadherent recently relapsed patients from OAAs to PP1M.16 The current model builds upon this prior work by additionally estimating the incremental relapses avoided and cost savings from a Medicaid payer’s perspective when considering subsequent transitions to PP3M and PP6M.
FIGURE 1.
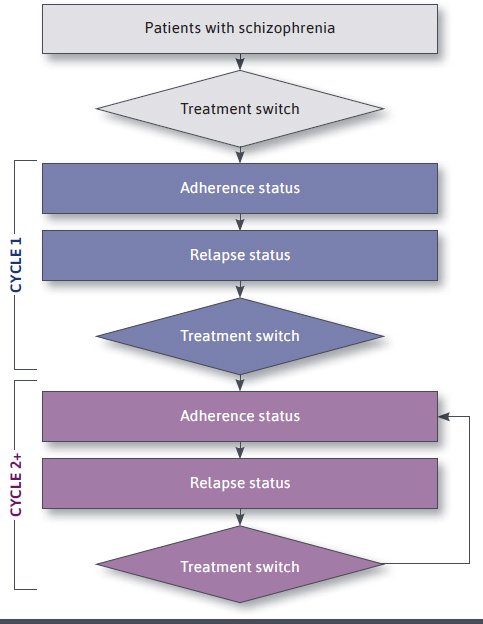
Model Structure
Furthermore, the following 2 non–mutually exclusive subpopulations of adults with schizophrenia who were nonadherent to OAAs were considered: (1) patients who had experienced a recent relapse (ie, in the previous 6 months) and (2) young adults (ie, aged 18-35 years) regardless of recent relapse history. LAIs are recommended by schizophrenia treatment guidelines for patients with poor or uncertain levels of adherence to antipsychotic (AP) treatment, which may lead to relapse.5,6 Additionally, young adults represent a particularly vulnerable subgroup of patients with schizophrenia given their increased propensity for poor adherence, higher risk for relapse, and incurred health care costs,7,12 underscoring the importance of early intervention in this group.
A 3-month cycle length was chosen, as prior work has demonstrated that among patients with schizophrenia receiving APs, the average time between relapse episodes was 12.5 weeks.4 At every 3-month cycle over the 36-month time frame, patients may or may not be adherent to treatment (whereby adherence rates differed according to treatment) and may or may not experience a relapse. At the end of the cycle, patients could continue receiving the same treatment or switch treatments.
Among both subpopulations, outcomes were reported when the proportion of patients switched from OAAs to PP1M was 5%, 10%, and 50% to reflect a wide spectrum of health plan cost containment strategies. Specifically, the average LAI utilization rate reported among Medicaid beneficiaries with schizophrenia is approximately 10%24; 5% was selected for consistency with prior work16,17 and 50% was selected to elucidate the impact of a more optimistic scenario. Additionally, the impact of switching from OAAs to PP1M was investigated such that patients who experienced a recent relapse could switch prior to or following their first subsequent relapse, and young adults could switch prior to or following their first relapse (or subsequent relapse if they have prior history of relapse), to reflect real-world clinical decision making. Based on these objectives, the following 4 scenarios were explored: switching 5% (Scenario 1), 10% (Scenario 2), and 50% (Scenario 3) of patients from OAAs to PP1M before they experienced any (subsequent) relapse; and switching 50% of patients from OAAs to PP1M immediately after their first (subsequent) relapse (Scenario 4).
The model evaluated the number of relapses avoided and health care costs, including treatment- and relapse-related costs, among patients who switched to PP1M, patients who had switched to PP1M with subsequent transitions to PP3M and PP6M, and those who remained on OAAs at 1, 2, and 3 years. Cost savings were reported at the plan level and per patient switched.
MODEL ASSUMPTIONS
The 36-month Markov model incorporated the following assumptions: patients who remained treated with OAAs continued to be nonadherent until they switched treatment and the risk of relapse among OAA, PP1M, PP3M, and PP6M was equal at equal adherence (a conservative assumption due to the limited literature on relapse risk among patients receiving these treatments with similar rates of adherence). Patients could switch treatment multiple times; however, patients transitioning from PP1M to PP3M or PP6M could only do so after two 3-month cycles of treatment with PP1M; transitions to PP3M and PP6M only occurred when patients were stable (ie, did not relapse) in their latest 3-month cycle, and transitions only occurred at corresponding dose strengths, with transitions to PP6M only allowed if patients were receiving one of the 2 highest doses of PP1M (156 mg or 234 mg)15 or PP3M (546 mg or 819 mg).18 To mimic possible real-world treatment patterns, as of the third cycle or later, patients treated with PP3M were permitted to switch back to treatment with an OAA or PP1M, and as of the fourth cycle or later, patients treated with PP6M were permitted to switch back to treatment with an OAA, PP1M, or PP3M; patients who had switched to PP1M were still counted as part of the PP1M treatment arm, irrespective of treatment switches. Patients who did not experience a relapse and remained on OAAs may have switched to another OAA. In Scenario 4, 50% of patients experiencing a relapse were switched to PP1M immediately following the event; the remaining 50% of patients continued to be treated with OAAs and were not eligible to switch to PP1M.
MODEL INPUTS
Population Inputs. Population inputs used to identify the 2 subpopulations are summarized in Supplementary Table 1 (767.2KB, pdf) , available in online article. Published literature of the Medicaid schizophrenia population from the past 5 years was used to identify the prevalence of schizophrenia and the proportion of patients currently treated with OAAs who were nonadherent, recently relapsed, and young adults irrespective of prior relapse.12,13,23,25,26
Clinical and Cost Inputs. Clinical and cost model inputs are summarized in Supplementary Tables 2 and 3 (767.2KB, pdf) ; rates of adherence (ie, the proportion of patients with a proportion of days covered [PDC] ≥80%), relapse, and switching as well as relapse costs were based on published literature.12,13,23,27,28 For switching rates, 14% of patients were estimated to be eligible for transition from PP1M to PP3M, 13.6% from PP3M to PP6M, and 13.2% for a direct transition from the 2 highest does of PP1M to PP6M. These estimates were based on real-world evidence showing the proportion of patients adequately treated with PP1M for 4 months who transition to PP3M per label at corresponding dose strengths,18 the proportion transitioning from PP1M to PP3M, and the proportion on the 2 highest doses of either PP1M or PP3M20; transition rates for PP6M were assumed similar to PP3M. Treatment costs were based on unit costs for corresponding doses, obtained from RED BOOK Online,29 and adjusted for adherence status; across treatments, treatment costs were assumed 90% among adherent patients and 38.7% among nonadherent patients based on a weighted average of the PDC from prior research.25 A 70% treatment discount, considering both statutory and inflation discounts, was used for PP1M and PP3M based on literature30; for PP6M, a statutory 23.1% minimum discount was applied,31 and the inflation discount was conservatively assumed to be 10% less than that of PP1M and PP3M, as PP6M is a newer product.
The inputs remained constant across the 36-month model duration and for each scenario.
ONE-WAY SENSITIVITY ANALYSIS
A one-way sensitivity analysis (OSA) was conducted to assess the impact of varying model inputs on per-patient costs. Probabilities of relapse obtained from published literature were used to create upper and lower bounds.16,17 The OSA applied ±30% to rates of adherence, rates of transitioning to PP3M and PP6M, and relapse costs, because the probabilities of adherence used in the model were conservative and consistent with lower bounds identified in the literature. OAA treatment costs were varied by ±30%, and PP1M, PP3M, and PP6M treatment cost discounts were varied by ±20%.
Results
TARGET POPULATIONS
In a hypothetical health plan of 1 million Medicaid beneficiaries, a total of 7,454 (0.75%) adult patients with schizophrenia were nonadherent to OAAs and had experienced a recent relapse and 4,002 (0.40%) young adults (ie, aged 18-35 years) with schizophrenia were nonadherent to OAAs, regardless of recent relapse (Supplementary Figure 1 (767.2KB, pdf) ).
NONADHERENT, RECENTLY RELAPSED ADULTS
Scenario 1: Switching 5% of Patients to PP1M Before Subsequent Relapse. By switching 5% of nonadherent, recently relapsed adults (N = 373) from OAAs to PP1M prior to subsequent relapse, a total of 225, 177, and 139 relapses were avoided at years 1, 2, and 3, respectively (Figure 2), which yielded total cumulative net cost savings of $3.3M, $2.8M, and $2.2M, respectively, at the plan level (Figure 3).
FIGURE 2.
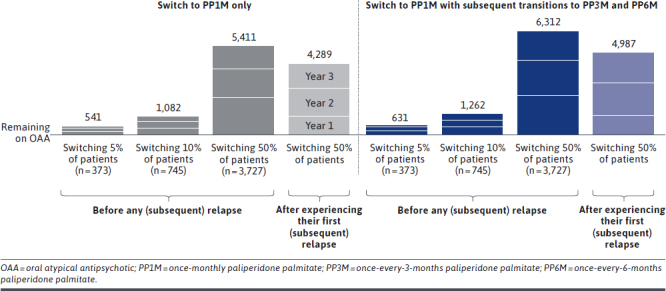
Total Number of Avoided Relapses Associated With Switching Nonadherent, Recently Relapsed Adults From OAA to PP1M, With Subsequent Transitions to PP3M and PP6M, Compared With Remaining on Treatment With OAA
FIGURE 3.
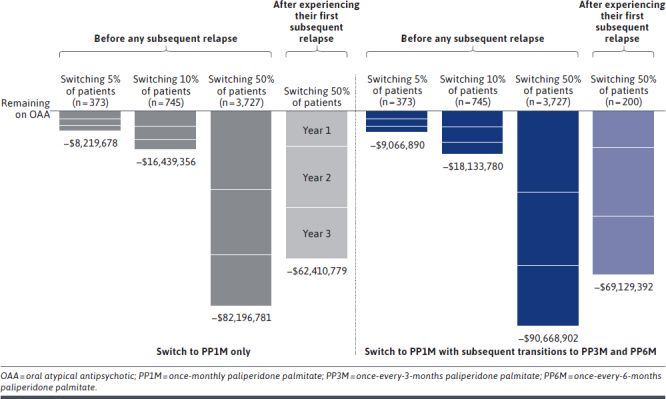
Total Cumulative Plan-Level Net Cost Savings Associated With Switching Nonadherent, Recently Relapsed Adults From OAA to PP1M, With Subsequent Transitions to PP3M and PP6M, Compared With Remaining on Treatment With OAA
Scenario 2: Switching 10% of Patients to PP1M Before Subsequent Relapse. By doubling the proportion of nonadherent, recently relapsed adults switched from OAA to PP1M prior to a subsequent relapse (N = 745), the total number of relapses avoided at 1, 2, and 3 years was 450, 354, and 278, respectively (Figure 2), which yielded total cumulative net cost savings at the plan level of $6.6M, $5.5M, and $4.3M, respectively (Figure 3).
Scenario 3: Switching 50% of Patients to PP1M Before Subsequent Relapse. When 50% of nonadherent, recently relapsed patients with schizophrenia (N = 3,727) were switched from OAA to PP1M before subsequent relapse, the total number of relapses avoided was 2,252, 1,769, and 1,390 at 1, 2, and 3 years, respectively (Figure 2), and the total cumulative net cost savings at the plan level were $33.1M, $27.5M, and $21.5M, respectively (Figure 3).
Scenario 4: Switching 50% of Patients to PP1M After the First Relapse. When waiting until after the first subsequent relapse to switch 50% of the eligible nonadherent, recently relapsed patients with schizophrenia (N = 3,727) from OAA to PP1M, the total number of relapses avoided decreased to 1,126, 1,741, and 1,422 at 1, 2, and 3 years, respectively (Figure 2), and the total cumulative net cost savings at the plan level were $15.2M, $25.9M, and $21.3M, respectively (Figure 3).
Incorporating Subsequent Transitions From PP1M to PP3M and PP6M. Across all scenarios, allowing for subsequent transitions for eligible patients from PP1M to PP3M and PP6M increased the total number of relapses avoided to 631, 1,262, and 6,312 when 5%, 10%, and 50% of patients were switched to PP1M prior to a subsequent relapse, respectively (Figure 2). When 50% of patients were switched to PP1M after a subsequent relapse, the total number of relapses avoided increased to 4,987. The incremental relapses avoided associated with incorporating transitions to PP3M and PP6M resulted in cumulative plan-level savings of $9.1M when switching 5% of nonadherent, recently relapsed patients, $18.1M when switching 10%, and $90.7M when switching 50% to PP1M prior to a subsequent relapse (Figure 3). Cumulative plan-level savings were $69.1M when switching 50% of patients to PP1M after a subsequent relapse, incorporating transitions to PP3M and PP6M. Compared with the costs associated with relapse, treatment costs accounted for 5% of the total plan-level costs (Supplementary Tables 4 and 5 (767.2KB, pdf) ).
NONADHERENT YOUNG ADULTS
Scenario 1: Switching 5% of Patients to PP1M Before Any (Subsequent) Relapse. By switching 5% of the nonadherent young adults (N = 200) from OAAs to PP1M prior to their first relapse, or prior to their subsequent relapse if they experienced a recent relapse (preceding entry into the model), the total number of relapses avoided was 70, 59, and 49 at years 1, 2, and 3, respectively (Figure 4), resulting in total cumulative net cost savings of $646,000, $623,000, and $514,000, respectively, at the plan level (Figure 5).
FIGURE 4.
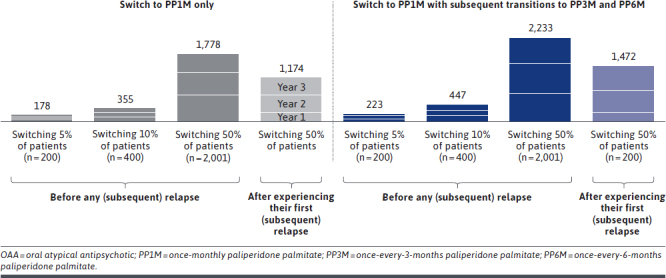
Total Number of Avoided Relapses Associated With Switching Nonadherent Young Adults From OAA to PP1M, With Subsequent Transitions to PP3M and PP6M, Compared With Remaining on Treatment With OAA
FIGURE 5.
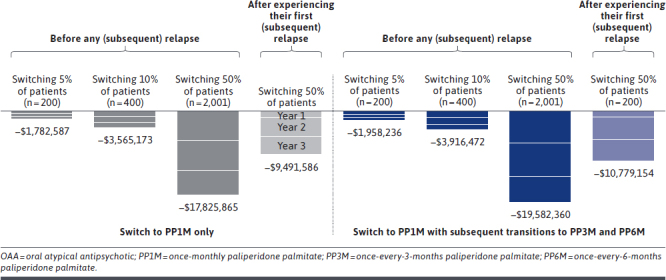
Total Cumulative Plan-Level Net Cost Savings Associated With Switching Nonadherent Young Adults From OAA to PP1M, With Subsequent Transitions to PP3M and PP6M, Compared With Remaining on Treatment With OAA
Scenario 2: Switching 10% of Patients to PP1M Before Any (Subsequent) Relapse. By switching 10% of the nonadherent young adults (N = 400) from OAAs to PP1M prior to their first relapse, or prior to their subsequent relapse if they experienced a recent relapse, the total number of relapses avoided was 140, 117, and 98 at years 1, 2, and 3, respectively (Figure 4), resulting in total cumulative net cost savings of $1.3M, $1.2M, and $1.0M, respectively, at the plan level (Figure 5).
Scenario 3: Switching 50% of Patients to PP1M Before Any (Subsequent) Relapse. By switching 50% of the nonadherent young adults (N = 2,001) from OAAs to PP1M prior to their first relapse, or prior to their subsequent relapse if they experienced a recent relapse, the total number of relapses avoided was 704, 586, and 488 at years 1, 2, and 3, respectively (Figure 4), resulting in total cumulative net cost savings of $6.5M, $6.2M, and $5.1M, respectively, at the plan level (Figure 5).
Scenario 4: Switching 50% of Patients to PP1M After the First (Subsequent) Relapse. By switching 50% of the nonadherent young adults (N = 2,001) from OAAs to PP1M after their first relapse, or after their first subsequent relapse if they experienced a recent relapse, the total number of relapses avoided decreased to 231, 478, and 465 at years 1, 2, and 3, respectively (Figure 4), resulting in total cumulative net cost savings of $1.3M, $4.0M, and $4.2M, respectively, at the plan level (Figure 5).
Incorporating Subsequent Transitions From PP1M to PP3M and PP6M. Across all scenarios, allowing nonadherent young adult patients who were eligible to transition from PP1M to PP3M and PP6M increased the total number of relapses avoided to 223, 447, and 2,233 when 5%, 10%, and 50% of patients were switched to PP1M prior to a subsequent relapse, respectively (Figure 4). Switching 50% of patients to PP1M after a (subsequent) relapse resulted in 1,472 cumulative relapses avoided after 3 years. Accordingly, plan-level savings over the 3 years increased to $2.0M when switching 5% of nonadherent young adult patients, $3.9M when switching 10%, and $19.6M when switching 50% to PP1M prior to a (subsequent) relapse (Figure 5). Cumulative plan-level savings increased to $10.8M when 50% of nonadherent young adult patients were switched to PP1M after a (subsequent) relapse (Supplementary Tables 6 and 7 (767.2KB, pdf) ).
COST SAVINGS PER PATIENT SWITCHED AND PER MEMBER PER MONTH
Switching 5%, 10%, or 50% of nonadherent, recently relapsed adult patients with schizophrenia from OAAs to PP1M prior to a subsequent relapse resulted in cost savings per patient switched of $8,884 at year 1, $7,391 at year 2, and $5,780 at year 3 (Supplementary Figure 2 (767.2KB, pdf) ), or $0.28, $0.23, and $0.18 per member per month (PMPM; based on a plan of 1 million members), respectively. Allowing for subsequent transitions to PP3M and PP6M yielded higher cost savings per patient switched of $9,220, $8,385, and $6,723, (Supplementary Figure 2 (767.2KB, pdf) ), or $0.29, $0.26, and $0.21 PMPM, respectively.
Switching 5%, 10%, or 50% of nonadherent young adult patients with schizophrenia from OAAs to PP1M prior to (subsequent) relapse resulted in cost savings per patient switched of $3,227 at year 1, $3,112 at year 2, and $2,570 at year 3 (Supplementary Figure 3 (767.2KB, pdf) ), or $0.05, $0.05, and $0.04 PMPM, respectively. Allowing for subsequent transitions to PP3M and PP6M yielded higher cost savings per patient switched of $3,405, $3,522, and $2,860 (Supplementary Figure 3 (767.2KB, pdf) ), or $0.06, $0.06, and $0.05 PMPM, respectively.
ONE-WAY SENSITIVITY ANALYSIS
After varying selected model inputs in the OSA, Supplementary Figures 4-9 (767.2KB, pdf) show the input parameters that influenced the net cost per patient switched from OAAs to PP1M, allowing subsequent transitions to PP3M and PP6M, if 5%, 10%, or 50% of patients were switched before any subsequent relapse. Based on the OSA, net cost savings were consistently observed across all parameters for both the nonadherent recently relapsed and nonadherent young adult subpopulations across all 3 years, with the exception of the treatment cost discount for PP1M in year 1 among nonadherent young adults. In year 1, net cost savings per patient switched ranged from −$16,276 to −$4,790 for nonadherent, recently relapsed adult patients and −$8,221 to $241 for nonadherent young adult patients with schizophrenia. The parameters with the largest influence on the net cost per patient switched to PP1M were the relapse ratio (the difference in the relapse rate by AP adherent/nonadherent status) and cost, whereas transitions rates to PP6M were the least influential; however, the influence of each parameter varied over each year and subpopulation.
Discussion
Over a 3-year period, this Markov model showed that switching nonadherent, recently relapsed patients with schizophrenia from OAAs to PP1M resulted in relapses avoided and cost savings at the plan level compared with remaining on OAA treatment. Additionally, allowing those eligible to subsequently transition to PP3M and PP6M led to incremental cost savings and relapses avoided. Notably, plan-level cost savings were higher when patients were switched prior to experiencing any (subsequent) relapse, providing economic justification for switching patients to LAIs earlier in their disease course. The cost of relapse was high in general, representing the majority of total plan-level costs. As such, treatment costs were more than fully offset by the substantial reductions in relapse costs when patients were switched to LAIs. Plan-level cost savings were similarly observed among nonadherent young adult patients. The risk of relapse in this subpopulation is high following treatment discontinuation32; thus, interventions that aim to lower this risk are of clinical value. Incorporating subsequent transitions to longer dosing intervals (ie, PP3M and PP6M) resulted in additional relapses avoided and further cost savings.
The economic value of switching patients from OAA to PP1M projected by this model corroborates the results of prior retrospective claims analyses showing cost savings attributable to fewer hospitalizations.10,12 The current study extends the findings of a previous long-term model that analyzed the plan-level savings from a Medicaid perspective associated with switching nonadherent, recently relapsed patients from OAA to PP1M.16 To provide a more comprehensive assessment that includes the newest formulations of the drug, the current 3-year model incorporated subsequent transitions from PP1M to PP3M and PP6M. Moreover, an additional subpopulation of young adults was evaluated given that young adults with schizophrenia represent a particularly nonadherent, vulnerable population, for whom avoiding relapse through early intervention has been found to be associated with better neurocognitive outcomes.33 Longer-acting formulations were consistently associated with incremental benefits such that over a 3-year period, allowing 50% of recently relapsed patients to transition from OAA to PP1M only, and incorporating subsequent transitions to PP3M and PP6M, respectively, resulted in cumulative plan-level cost savings of $82.2M vs $90.7M compared with remaining on OAA, driven by 5,411 vs 6,312 relapses avoided. In the young adult subpopulation, the corresponding cost savings were $17.8M and $19.6M for the transition from OAAs to PP1M and upon incorporating subsequent transitions to PP3M and PP6M, respectively, driven by 1,778 and 2,233 relapses avoided. Accordingly, these estimated cost savings provide a foundation for payers to consider strategies for increasing the utilization rates of these treatments to potentially reduce the occurrence of relapses and decrease associated costs among patients with schizophrenia. Cost projections can thus be a valuable tool for health care plan administrators to proactively forecast their budgets to optimize the allocation of funds across multiple prevalent and/or expensive conditions. However, real-world data are warranted to determine if these findings translate to clinical practice.
Among patients with schizophrenia, clinical disease management can be challenging if patients are nonadherent, because poor adherence to OAAs is a major risk factor for inadequate symptom control and relapse,11,34 resulting in substantial health care utilization and costs.7-14 The current findings are in line with recent evidence between retrospective and post hoc analyses showing that patients who transition to treatment with PP3M were more likely to be adherent to treatment and experience fewer relapses and hospitalizations than those who remain on PP1M.8,20 With PP6M having recently received regulatory approval for adult patients with schizophrenia,21 and until real-world evidence becomes available for the drug, this study provides the first projection of favorable cost outcomes associated with the twice-yearly dosing regimen.
Compared with switching 50% of patients from OAAs to PP1M (allowing subsequent transitions to PP3M and PP6M) after a subsequent relapse, when 50% of nonadherent recently relapsed adults were switched before a subsequent relapse, more than 1,300 relapses were avoided and an additional $21.2M in cost savings were observed at the plan level, consistent with the projection of prior models.16,17 Importantly, the results were consistent among nonadherent young adults, demonstrating that in the long term, cost savings were the highest when patients were switched from OAAs to PP1M before they experienced their next relapse and when subsequent transitions to PP3M and PP6M were incorporated. Together, the evidence suggests that the upfront use of PP1M for treating patients with schizophrenia early in the course of disease, prior to experiencing a relapse, is cost saving and clinically impactful. These findings support schizophrenia treatment guideline recommendations to initiate LAIs as first-line treatment in patients at high risk of nonadherence.5,6 Although not studied as a distinct population, recently relapsed young adults may represent a particularly vulnerable population warranting further research.
LIMITATIONS
This study was subject to certain limitations. Model inputs and assumptions were based on available data in published literature and may be subject to uncertainty or limited generalizability. Real-world analyses to confirm the findings are required to draw definitive conclusions. As PP6M is a newer product, the treatment discount was based on assumptions, and real-world rebates may vary widely; nonetheless, sensitivity analysis demonstrated consistent net cost savings when this input was varied. Additionally, the model included 2 subpopulations nonadherent to OAAs with Medicaid coverage. These subpopulations were not mutually exclusive based on recent relapse history. Finally, results of the model should be interpreted with caution as they may not be generalizable to the overall population with schizophrenia or those with other types of insurance coverage.
Conclusions
In a hypothetical health plan of 1 million Medicaid beneficiaries, switching nonadherent, recently relapsed patients with schizophrenia from OAAs to PP1M before a subsequent relapse can yield substantial cost savings and relapses avoided. When these patients were subsequently transitioned to PP3M and/or to PP6M, further incremental cost savings and relapses avoided were observed. Switching before relative to after a subsequent relapse was more beneficial from an economic and clinical perspective, yielding additional cost savings and relapses avoided. Plan-level cost savings were greater when a higher proportion of patients were switched from OAAs to PP1M. These results were also observed in nonadherent young adults regardless of recent relapse history. This subpopulation has a greater risk for relapse; hence, providing early and effective AP treatment for younger patients may lead to a significant reduction in overall health care costs associated with managing schizophrenia.
ACKNOWLEDGMENTS
Medical writing support was provided by Loraine Georgy, PhD, and Christine Tam, MSc, employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC., which funded the development and conduct of this study and manuscript.
REFERENCES
- 1.National Institute of Mental Health. Schizophrenia. Accessed September 2, 2021. https://www.nimh.nih.gov/health/topics/schizophrenia
- 2.Schizophrenia & Psychosis Action Alliance. Societal costs of schizophrenia & related disorders. 2021. [Google Scholar]
- 3.Patel C, Emond B, Morrison L, et al. Risk of subsequent relapses and corresponding healthcare costs among recently-relapsed Medicaid patients with schizophrenia: A real-world retrospective cohort study. Curr Med Res Opin. 2021;37(4):665-74. doi:10.1080/03007995.2021.1882977 [DOI] [PubMed] [Google Scholar]
- 4.Lafeuille MH, Gravel J, Lefebvre P, et al. Patterns of relapse and associated cost burden in schizophrenia patients receiving atypical antipsychotics. J Med Econ. 2013;16(11):1290-9. doi:10.3111/13696998.2 013.841705 [DOI] [PubMed] [Google Scholar]
- 5.The American Pyschiatric Association. Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Assocation; 2020. [Google Scholar]
- 6.The University of South Florida; Florida Medicaid Drug Therapy Management Program. 2019-2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. 2020. [Google Scholar]
- 7.Huang A, Amos TB, Joshi K, et al. Understanding healthcare burden and treatment patterns among young adults with schizophrenia. J Med Econ. 2018;21(10):1026-35. doi:10.1080/13696998.2018.1500370 [DOI] [PubMed] [Google Scholar]
- 8.Mathews M, Gopal S, Singh A, et al. Comparison of relapse prevention with 3 different paliperidone formulations in patients with schizophrenia continuing versus discontinuing active antipsychotic treatment: A post-hoc analysis of 3 similarly designed randomized studies. Neuropsychiatr Dis Treat. 2020;16:1533-42. doi:10.2147/NDT.S221242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel C, Emond B, Lafeuille MH, et al. Real-world analysis of switching patients with schizophrenia from oral risperidone or oral paliperidone to once-monthly paliperidone palmitate. Drugs Real World Outcomes. 2020;7(1):19-29. doi:10.1007/s40801-019-00172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilon D, Muser E, Lefebvre P, et al. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry. 2017;17(1):207. doi:10.1186/s12888-017-1358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higashi K, Medic G, Littlewood KJ, Diez T, Granstrom O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manjelievskaia J, Amos TB, El Khoury AC, et al. A comparison of treatment patterns, healthcare resource utilization, and costs among young adult Medicaid beneficiaries with schizophrenia treated with paliperidone palmitate or oral atypical antipsychotics in the US. J Med Econ. 2018;21(12):1221-9. doi:10.1080/1 3696998.2018.1527608 [DOI] [PubMed] [Google Scholar]
- 13.Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable anti-psychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-68. doi:10.18553/jmcp.2015.21.9.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-9. doi:10.1176/appi.ajp.161.4.692 [DOI] [PubMed] [Google Scholar]
- 15.U.S Food and Drug Administration. INVEGA SUSTENNA® - Highlights of prescribing information. Accessed September 2, 2020. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+SUSTENNA-pi.pdf
- 16.El Khoury AC, Pilon D, Morrison L, et al. Projecting the long-term economic impact of once-monthly paliperidone palmitate versus oral atypical antipsychotics in Medicaid patients with schizophrenia. J Manag Care Spec Pharm. 2020;26(2):176-85. doi:10.18553/jmcp.2020.26.2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Khoury AC, Pilon D, Morrison L, et al. The prospective economic impact of once monthly paliperidone palmitate versus oral atypical antipsychotics in Medicaid patients with schizophrenia. Curr Med Res Opin. 2019;35(3):395-405. doi:10.1080/03007995.2018.1558195 [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. INVEGA TRINZA® - Highlights of prescribing information. Accessed September 2, 2020. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+TRINZA-pi.pdf
- 19.Morrison L, Lin D, Pilon D, et al. Economic analysis of switching patients with schizophrenia from oral atypical antipsychotics to once-monthly paliperidone palmitate and to once-every-three-months paliperidone palmitate. [Virtual Poster] presented at: ISPOR 2021 Virtual Conference; May 17-20, 2021. [Google Scholar]
- 20.Lin D, Pilon D, Zhdanava M, et al. Medication adherence, healthcare resource utilization, and costs among Medicaid beneficiaries with schizophrenia treated with once-monthly paliperidone palmitate or once-every-three-months paliperidone palmitate. Curr Med Res Opin. 2021:37(4):675-83. doi:10.1080/03007995.2021.1882412 [DOI] [PubMed] [Google Scholar]
- 21.U.S Food and Drug Administration. INVEGA HAFYERA™ - Highlights of prescribing information. Accessed January 10, 2022. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+HAFYERA-pi.pdf
- 22.Medicaid.gov. Behavioral Health Services. Accessed September 1, 2021. https://www.medicaid.gov/medicaid/benefits/behavioral-health-services/index.html
- 23.Pilon D, Patel C, Lafeuille MH, et al. Prevalence, incidence and economic burden of schizophrenia among Medicaid beneficiaries. Curr Med Res Opin. 2021;37(10):1811-9. doi:10.1080/03007995.2 021.1954894 [DOI] [PubMed] [Google Scholar]
- 24.Patel C, Pilon D, Gupta D, et al. National and regional description of healthcare measures among adult Medicaid beneficiaries with schizophrenia within the United States. J Med Econ. 2022;25(1):792-807. doi:10.1080/13696998.2022.2084234 [DOI] [PubMed] [Google Scholar]
- 25.Pilon D, Tandon N, Lafeuille M-H, et al. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39(10):1972-85.e2. doi:10.1016/j. clinthera.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 26.Lafeuille MH, Laliberte-Auger F, Lefebvre P, et al. Impact of atypical long-acting injectable versus oral anti-psychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry. 2013;13:221. doi:10.1186/1471-244X-13-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453-60. doi:10.4088/jcp.v67n0317 [DOI] [PubMed] [Google Scholar]
- 28.Young-Xu Y, Duh MS, Muser E. et al. Impact of paliperidone palmitate versus oral atypical antipsychotics on health care resource use and costs in Veterans with schizophrenia. J Clin Psychiatry. 2016;77(10):e1332-41. doi:10.4088/JCP.16m10745 [DOI] [PubMed] [Google Scholar]
- 29.IBM Watson Health THA. IBM Micromedix RED BOOK Online. Accessed January 10, 2022. https://www.ibm.com/products/micromedex-red-book/details
- 30.Kelly C. Price inflation rebates and the spectre of 100% discounts In Medicare Part D. Accessed December 6, 2021.https://pink.pharmaintelligence.informa.com/PS141533/Price-Inflation-Rebates-And-The-Spectre-Of-100-Discounts-In-Medicare-Part-D
- 31.Centers for Medicare & Medicaid Services. Medicaid Drug Rebate Program (MDRP). Accessed December 8, 2021. https://www.medicaid.gov/medicaid/prescription-drugs/medicaid-drug-rebate-program/index.html
- 32.Leclerc E, Noto C, Bressan RA, et al. Determinants of adherence to treatment in first-episode psychosis: A comprehensive review. Braz J Psychiatry. 2015;37(2):168-76. doi:10.1590/1516-4446-2014-1539 [DOI] [PubMed] [Google Scholar]
- 33.Rund BR, Barder HE, Evensen J, et al. Neurocognition and duration of psychosis: A 10-year follow-up of first-episode patients. Schizophr Bull. 2016;42(1):87-95. doi: 10.1093/schbul/sbv08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llorca PM. Partial compliance in schizophrenia and the impact on patient outcomes. Psychiatry Res. 2008;161(2):235-47. doi:10.1016/j.psychres.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 35.Pilon D, Joshi K, Tandon N, et al. Treatment patterns in Medicaid patients with schizophrenia initiated on a first-or second-generation long-acting injectable versus oral antipsychotic. Patient Prefer Adherence. 2017;11:619-29. doi :10.2147/PPA.S127623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healthcare Cost and Utilization Project (HCUP). Weighted national estimates from HCUP National (Nationwide) Inpatient Sample (NIS). Accessed January 10, 2022. https://hcupnet.ahrq.gov
- 37.Healthcare Cost and Utilization Project (HCUP). Statistical brief #189: hospital readmissions involving psychiatric disorders, 2012. Accessed January 10, 2022. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb189-Hospital-Readmissions-Psychiatric-Disorders-2012.jsp


