Abstract
BACKGROUND:
A key therapeutic goal of metastatic renal cell carcinoma (mRCC) treatment is delayed disease progression. The degree to which early therapeutic success affects downstream outcomes is not well established.
OBJECTIVE:
To assess the clinical and economic impact of early vs delayed disease progression in patients with mRCC treated with first-line (1L) tyrosine kinase inhibitors (TKIs) followed by second-line (2L) therapy in the US Veterans Health Administration (VHA) database.
METHODS:
Adult patients newly diagnosed with mRCC who were treated with a TKI as 1L therapy and who progressed to 2L therapy from October 1, 2013, through March 31, 2018, were identified from the US VHA database. Patients were stratified by median time from initiation of 1L therapy to initiation of 2L therapy into early (median time or sooner)and delayed (longer than the median) progression cohorts. Clinical outcomes (time to 2L therapy discontinuation, time to third-line [3L] treatment initiation, and overall survival) were assessed descriptively, and health care resource utilization and costs were compared between patients in the early and those in the delayed progression cohorts. Survival analyses (Kaplan-Meier curves) were used to estimate descriptively the median time to discontinuation, time to next line of treatment, and time to death for each cohort. Multivariate analysis was performed to adjust for the influence of differences in cohort characteristics, and Cox proportional hazards models were used to descriptively assess the impact of predictive factors on clinical outcomes.
RESULTS:
289 patients were included in the analysis: 145 in the early progression cohort and 144 in the delayed progression cohort. Baseline characteristics were similar between the early and delayed progression cohorts. Median time from 1L therapy initiation to 2L therapy discontinuation was 7.9 months in the early progression cohort and 18.0 months in the delayed progression cohort, whereas time from 1L therapy initiation to 3L therapy initiation was 9.4 and 21.8 months, respectively; overall survival was 19.7 and 36.4 months, respectively. Descriptive analysis revealed generally lower risks for 2L therapy discontinuation (HR = 0.40, 95% CI = 0.31-0.52), 3L therapy initiation (HR = 0.42, 95% CI = 0.32-0.55), and death (HR = 0.46, 95% CI = 0.33-0.64) for those with delayed progression. After adjustment for possible confounding factors, comparative analysis during the follow-up period showed that delayed progression was associated with a shorter median all-cause hospital length of stay (0.4 days vs 0.8 days for early progression; P = 0.0004), fewer pharmacy visits (3.57 vs 4.08 visits; P = 0.0266), and lower total health care costs ($10,342 vs $13,388; P = 0.0347) per patient per month.
CONCLUSIONS:
In patients with mRCC, early progression after 1L therapy initiation is associated with generally worse clinical outcomes and statistically significantly greater health care resource utilization and costs than delayed progression. This finding highlights the importance of initiating therapy with an optimal 1L treatment regimen that has been proven to delay disease progression.
What is already known about this subject
The treatment landscape for metastatic renal cell carcinoma (mRCC) is changing with the introduction of new, effective first-line (1L) therapies.
Early disease progression after initiation of 1L therapy is associated with worse survival prognosis in patients with mRCC.
What this study adds
The time from initiation of 1L therapy to initiation of second-line therapy for mRCC is an important real-world prognostic indicator because delayed progression is associated with generally superior overall survival, as well as significantly lower health care resource utilization and costs, compared with early progression (as estimated through claims analysis).
These data reinforce the importance of initiating mRCC treatment with 1L medications that have been shown to prolong overall survival and time to disease progression.
Global estimates of renal cell carcinoma (RCC) incidence range from 0.8 to 18 per 100,000 people; the highest incidence rates are found in North America and Northern Europe.1 In the United States, an estimated 73,750 new cases of kidney and renal pelvis cancer were expected to be diagnosed in 2020, and an estimated 14,830 deaths were expected to be attributable to these cancers.2 Although the age-adjusted incidence of kidney and renal pelvis cancer has been stable among the US population during the past 10 years,2 the incidence of RCC has successively increased by birth cohort, starting with individuals born in 1955.3 As early-stage disease is often asymptomatic, an estimated 25%-33% of cases have progressed to metastatic RCC (mRCC) by the time of diagnosis.4,5 Moreover, 20%-40% of cases initially diagnosed as localized disease will progress to mRCC.5 The difference in prognosis is profound among local, regional, and metastatic kidney and renal pelvis cancer, with 5-year survival rates of 92.6%, 70.4%, and 13.0%, respectively.2
Although the main goal of systemic pharmacologic treatment in patients with mRCC is complete response, in practice, clinicians must sometimes aim to prevent or delay progression and extend survival.6 Historically, tyrosine kinase inhibitors (TKIs) such as sunitinib and pazopanib have been the standard first-line (1L) therapy for patients with mRCC. Despite the expansion of treatment options for patients with mRCC during the past decade—which now include mechanistic target of rapamycin inhibitors and immune checkpoint inhibitors—and data supporting combination therapy regimens, monotherapy with TKIs continues to prevail in clinical practice.7-11
Furthermore, the therapeutic landscape for mRCC has changed as a result of the increased availability of viable second-line (2L) therapies, which have been linked to improved overall survival.12,13 Yet the clinical and economic ramifications of increased use of 2L therapy have not been extensively studied in the real-world setting. The extent to which characteristics such as duration of 1L therapy have prognostic significance for 2L therapy is also unknown. As 1L and 2L therapeutic options grow more diverse, datadriven insights are needed for clinicians to select optimal sequences of regimens for real-world populations. To that end, we conducted a retrospective, claims-based analysis to evaluate the impact of duration of progression from initiation of 1L therapy to initiation of 2L therapy on clinical outcomes, health care resource utilization (HCRU), and costs among patients with mRCC.
Methods
DATA SOURCE
The IMPACT RCC (Impact of Early Progression Versus Delayed Progression Among Patients With Metastatic Renal Cell Carcinoma Treated With Tyrosine Kinase Inhibitors) trial was a retrospective, observational, claims-based study that used data from the US Veterans Health Administration (VHA) Medical Inpatient, Outpatient, and Decision Support System datasets. The VHA is the largest integrated health care system in the United States, providing inpatient, outpatient, and long-term care to more than 9 million enrollees.14
Relevant diagnoses were identified by using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes found in medical (inpatient and outpatient) claims. Systemic therapies for mRCC were identified by using Healthcare Common Procedure Coding System codes from outpatient records and National Drug Code numbers from pharmacy records.
PATIENT SELECTION AND IDENTIFICATION
The study population consisted of veterans with 1 or more diagnoses of RCC (ICD-9-CM codes 189.0, 189.1; ICD-10-CM codes C64, C65) during the study period and 1 or more diagnoses of metastasis (ICD-9-CM codes 196, 197, 198, 199; ICD-10-CM codes C77, C78, C79, C80, C45.9) on or after the date of the initial RCC diagnosis during the patient identification window of October 1, 2013, through March 31, 2018. The date of the first diagnosis of metastasis during this period was defined as the mRCC diagnosis date.
To be included in the analysis population, patients were required to have 1 or more claims for any RCC systemic therapy during the patient identification window. The date of the first medication claim was considered the index date and marked the start of 1L therapy. The 6-month period preceding the index date was defined as the baseline period. The treatments constituting 1L therapy included all systemic therapies recorded within 14 days of the index date. Patients were required to be aged 18 years or older on the index date and to have been enrolled continuously for medical and pharmacy benefits through the VHA from at least 6 months before the mRCC diagnosis date until at least 6 months after the index date.
Patients were excluded from the analysis population if they were pregnant at any time during the study period; died within 14 days of the index date; or had evidence of receiving any mRCC systemic therapy during the 6 months preceding the index date, any cancer diagnoses (other than RCC) during the 6 months before the mRCC diagnosis date, or any TKI-TKI or TKI-mechanistic target of rapamycin combinations as their 1L treatment regimen.
To limit variability arising from differences in 1L treatment regimens, we included in the analysis only patients receiving a TKI—the drug class most commonly used as 1L therapy for mRCC in clinical practice.7-11 Eligible patients had evidence of advancement to 2L therapy, which was defined as the addition of a new systemic therapy, a switch to or substitution with a new systemic therapy, or reinitiation of the index systemic therapy after a gap of more than 90 days from the end of prior therapy (whichever occurred first).11,15 These patients were required to have been continuously enrolled for medical and pharmacy benefits through the VHA until at least 6 months after the 2L initiation date, unless the patient died more than 14 days after the index date (1L therapy initiation). Patients were followed from the index date until they died or the follow-up period ended (March 31, 2018), whichever occurred first.
This retrospective database analysis did not involve the collection, use, or transmittal of personally identifiable information. As such, this study did not require institutional review board approval; it is considered exempt according to 45CFR46.101(b)(4): Existing Data and Specimens - No Identifiers (Common Rule). The dataset and the security of the offices where the data are housed meet HIPAA requirements.
STUDY MEASURES
Patient Characteristics.
Demographic characteristics including race, sex, and age on the index date were obtained for all patients. Clinical characteristics assessed during the baseline period included the Quan-Charlson Comorbidity Index (CCI), National Cancer Institute Comorbidity Index score, select individual comorbidities, time from mRCC diagnosis date to index date (≤ 1 year or > 1 year), and allcause HCRU and costs.
Outcomes.
Clinical outcomes were descriptively evaluated during the follow-up period and included time to 2L therapy discontinuation (time from the index date to 2L therapy discontinuation or death, whichever occurred first), time to 3L therapy initiation (time from the index date to 3L therapy initiation or death, whichever occurred first), and overall survival (time from the index date to death due to any cause). Initiation of the next line of treatment was considered a proxy for estimating disease progression,9 as clinically confirmed progression cannot be captured by using claims data. Patients’ clinical outcomes were evaluated until their death or the end of the study, and patients without an event of interest during the evaluation period were censored.
The economic outcomes in this study were comparatively evaluated and included all-cause and mRCC-related HCRU and direct health care costs for inpatient, outpatient, and pharmacy visits from the index date to the end of follow-up (ie, patient death or the end of the study, whichever occurred first). Inpatient HCRU (including length of stay [LOS]) and costs included care received in all acute care (eg, hospital and emergency department stays > 24 hours) and extended care (eg, nursing home) settings. Outpatient HCRU and costs included care received in doctors’ offices, emergency departments (stays shorter than 24 hours), laboratories, and other outpatient settings. Pharmacy HCRU and costs included all prescriptions from inpatient and outpatient settings. In addition to these costs, medical costs were computed as the sum of inpatient and outpatient costs, and total health care costs were computed as the sum of medical and pharmacy costs. HCRU and costs were considered related to mRCC if they occurred on a day when a diagnosis code for RCC was recorded. Pharmacy visits and costs were considered related to mRCC if they included a prescription for mRCC-related systemic therapy.
STATISTICAL ANALYSIS
Patients were stratified into 1 of 2 cohorts on the basis of the Kaplan-Meier curve-derived median time from initiation of 1L therapy to initiation of 2L therapy: early progression (the median time or sooner) and delayed progression (longer than the median time). All study variables were summarized descriptively for both cohorts by using means, SDs, and interquartile ranges for continuous variables, and numbers and percentages for categorical variables. For HCRU and cost outcomes only, the Student’s t-test was used to evaluate the statistical significance of differences in continuous measures, and the chi-square test, for differences in categorical measures, between the early and delayed progression cohorts. Survival analyses (Kaplan-Meier curves) were used to descriptively estimate the median times to discontinuation, next treatment, and death for each cohort. HCRU and costs were estimated per patient per month (PPPM) by dividing each patient’s total number of visits and total costs by the length of follow-up, and then calculating the group mean by using these values; the means were then compared. The costs were adjusted to 2018 US dollars by using the medical care component of the US Department of Labor’s Consumer Price Index.16
To adjust for the influence of differences in cohort characteristics (demographic characteristics [age, sex, and race], baseline clinical factors [Quan-CCI, time from mRCC diagnosis date to index date], and economic parameters [numbers of inpatient, outpatient, and pharmacy visits]) on clinical and economic outcomes, multivariate analysis was performed. Cox proportional hazards models were used to descriptively assess the impact of the aforementioned predictive factors on clinical outcomes. Data are reported as hazard ratios (HRs) and 95% CIs. Generalized linear models with negative binomial and gamma distributions were used to generate adjusted estimates for HCRU and costs, respectively.
For all statistical comparisons, P < 0.05 was considered significant. Analyses were performed using SAS statistical software for Windows, version 9.4 (SAS Institute).
Results
STUDY POPULATION
The analysis population comprised 289 patients newly diagnosed with mRCC who had received a TKI as 1L treatment and progressed to 2L treatment (Figure 1). The median time to 2L treatment initiation was 6.0 months. Patients with a time to 2L treatment initiation shorter than or equal to the median value were assigned to the early progression cohort (n = 145), and patients with a time to 2L treatment initiation longer than the median were assigned to the delayed progression cohort (n = 144).
FIGURE 1.
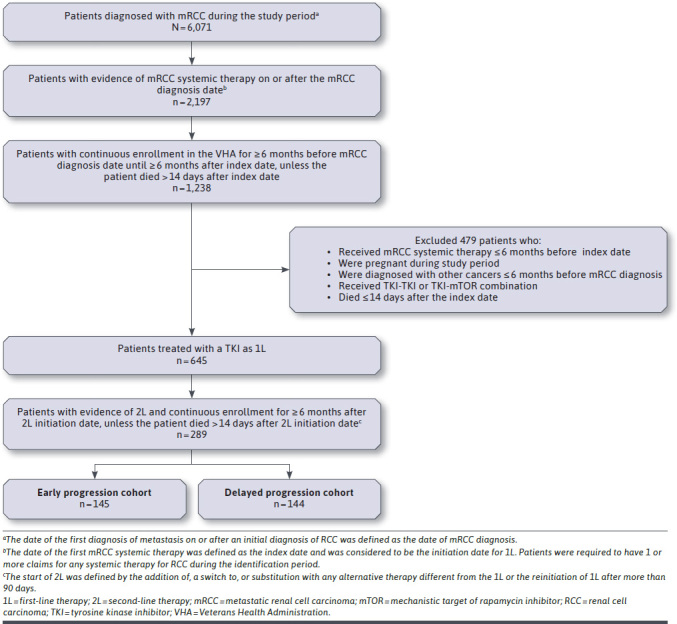
Patient Selection Criteria
For the overall study population, the mean age at the index date was 67.4 years, and 70.6% of patients were aged 65 years or older. Most patients were White (79.9%), and 97.9% of patients were male. The comorbidity burden was high, as indicated by an average Quan-CCI score of 9.02. No significant differences in baseline demographics or clinical characteristics were found between the early and delayed progression cohorts (Table 1). During the 6-month period before the initiation of 1L treatment, HCRU and costs PPPM were numerically higher, but not statistically significantly so, in the early progression cohort than in the delayed progression cohort.
TABLE 1.
Baseline Characteristics of the Study Population (N = 289)
| Parameters | Early progression cohort (n = 145) | Delayed progression cohort (n = 144) | ||
|---|---|---|---|---|
| Categorical variablesa | ||||
| Age, years | ||||
| < 65 | 39 (26.90) | 46 (31.94) | ||
| ≥ 65 | 106 (73.10) | 98 (68.06) | ||
| Sex | ||||
| Male | 143 (98.62) | 140 (97.22) | ||
| Female | 2 (1.38) | 4 (2.78) | ||
| Race | ||||
| White | 119 (82.07) | 112 (77.78) | ||
| Non-White | 26 (17.93) | 32 (22.22) | ||
| Comorbidities | ||||
| Any malignancy | 140 (96.55) | 140 (97.22) | ||
| COPD | 24 (16.55) | 27 (18.75) | ||
| Diabetes without chronic complications | 54 (37.24) | 53 (36.81) | ||
| Metastatic solid tumor | 126 (86.90) | 130 (90.28) | ||
| Renal disease | 40 (27.59) | 47 (32.64) | ||
| Continuous variablesb | ||||
| Age, years | 68.04 (7.93) | 68.00 (64.00-71.00) | 66.73 (8.19) | 67.00 (63.00-72.00) |
| Comorbidity | ||||
| Quan-CCI score | 8.88 (2.77) | 9.00 (8.00-11.00) | 9.17 (2.80) | 9.00 (8.00-11.00) |
| NCI score | 1.91 (1.94) | 1.6 (0.00-2.94) | 1.92 (1.75) | 1.6 (0.00-2.94) |
| Time from mRCC diagnosis to index date,c days | 100.10 (172.96) | 33.00 (9.00-111.00) | 103.24 (163.36) | 46.00 (13.00-106.50) |
| Length of stay, days | 0.61 (1.22) | 0.00 (0.00-0.67) | 0.40 (0.94) | 0.00 (0.00-0.42) |
| Mean no. of visitsd | ||||
| Inpatient | 0.21 (0.39) | 0.00 (0.00-0.33) | 0.16 (0.32) | 0.00 (0.00-0.17) |
| Outpatient | 3.10 (2.22) | 2.67 (1.67-3.83) | 2.70 (1.92) | 2.33 (1.33-3.67) |
| Pharmacy | 2.58 (2.08) | 2.00 (1.00-3.50) | 2.56 (1.94) | 2.33 (1.00-3.58) |
| Mean all-cause costs,d $ | ||||
| Inpatient stay | 2,677 (5,191) | 0 (0-3,486) | 1,925 (4,196) | 0 (0-1,891) |
| Outpatient stay | 2,196 (2,127) | 1,682 (984-2,644) | 1,866 (1,745) | 1,453 (649-2,564) |
| Pharmacy | 338 (1,276) | 85 (22-274) | 210 (425) | 72 (25-188) |
| Medical | 4,874 (5,858) | 2,677 (1,336-6,065) | 3,791 (4,948) | 1,945 (649-5,470) |
| Total | 5,212 (6,371) | 2,755 (1,443-6,321) | 4,000 (5,197) | 2,013 (771-5,676) |
aData are n (%).
bData are mean (SD) and median (interquartile range).
cThe index date refers to the date on which first-line therapy was started.
dPer patient per month during the 6 months before the index date.
CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; mRCC=metastatic renal cell carcinoma; NCI = National Cancer Institute.
CLINICAL OUTCOMES
Clinical outcomes are presented descriptively. Kaplan-Meier analyses revealed generally more favorable clinical outcomes in patients in the delayed progression cohort. Median overall survival was 19.7 months for the early progression cohort and 36.4 months for the delayed progression cohort (HR = 0.46, 95% CI = 0.33-0.64; Figure 2). Median time to discontinuation of 2L therapy was 7.9 months in the early progression cohort and 18.0 months in the delayed progression cohort (HR = 0.40, 95% CI = 0.31-0.52), and median time to initiation of 3L therapy was 9.4 and 21.8 months, respectively(HR = 0.42, 95% CI = 0.32-0.55; Supplementary Figure 1 (451.6KB, pdf) , available in online article).
FIGURE 2.
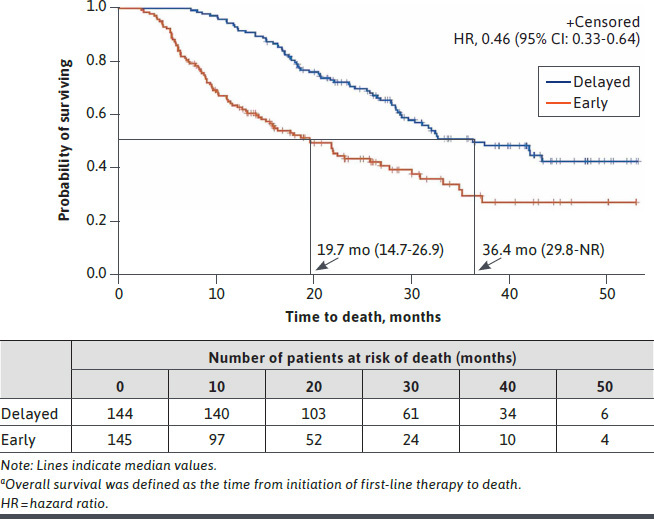
Kaplan-Meier Curves for Overall Survivala by Progression Status Cohort
Clinical outcomes were evaluated descriptively by cohort and baseline variables in order to provide information on positive and negative prognostic factors. Risk of discontinuing 2L treatment was 60% lower among patients in the delayed progression cohort than in the early progression cohort (HR = 0.40, 95% CI = 0.31-0.52; Supplementary Figure 2A (451.6KB, pdf) , available in online article). The delayed progression cohort was 58% less likely to initiate 3L treatment during the study period (HR = 0.42, 95% CI = 0.320.55; Supplementary Figure 2B (451.6KB, pdf) , available in online article). For both 2L treatment discontinuation and 3L treatment initiation, no other notable discrepancies in risk factors were identified. Patients in the delayed progression cohort had a 54% lower risk of death during the study period than those in the early progression cohort (HR = 0.46, 95% CI = 0.33-0.64; Supplementary Figure 2C (451.6KB, pdf) , available in online article). A duration of 1 year or less from mRCC diagnosis to initiation of 1L treatment coincided with a lower risk of death (HR = 0.52, 95% CI = 0.30-0.90), whereas more inpatient visits during the 6 months before the initiation of 1L treatment coincided with a higher risk of death (HR = 2.29, 95% CI = 1.34-3.93).
HEALTH CARE RESOURCE UTILIZATION
HCRU outcomes were compared statistically between cohorts. Significant differences in unadjusted HCRU and costs were identified between the early progression and delayed progression cohorts. On a PPPM basis, patients in the delayed progression cohort had significantly shorter allcause (0.5 days) and mRCC-related (0.3 days) hospital LOS than the early progression cohort (all-cause LOS = 0.9 days, P = 0.0212; mRCC-related LOS = 0.7 days, P = 0.0171), and fewer all-cause and mRCC-related inpatient and outpatient visits and fewer mRCC-related pharmacy visits (Supplementary Figure 3 (451.6KB, pdf) , available in online article). The delayed progression cohort incurred lower all-cause and mRCC-related inpatient, outpatient, medical, and total costs (all PPPM) than the early progression cohort (Supplementary Figure 4 (451.6KB, pdf) , available in online article). Pharmacy costs related to mRCC were similar between the 2 cohorts.
After adjustment for possible confounding variables, all-cause (0.4 vs 0.8 days; P = 0.0004) and mRCC-related (0.2 vs 0.7 days; P< 0.0001) hospital LOS (PPPM) remained significantly shorter in the delayed progression cohort than in the early progression cohort. In addition, the delayed progression cohort had significantly fewer all-cause and mRCC-related pharmacy visits PPPM, and fewer mRCC-related outpatient visits PPPM, than the early progression cohort (Figure 3 and Supplementary Figure 5 (451.6KB, pdf) , available in online article). Results of the adjusted health care cost analysis were consistent with those of the unadjusted analysis (Figure 4 and Supplementary Figure 6 (451.6KB, pdf) , available in online article).
FIGURE 3.
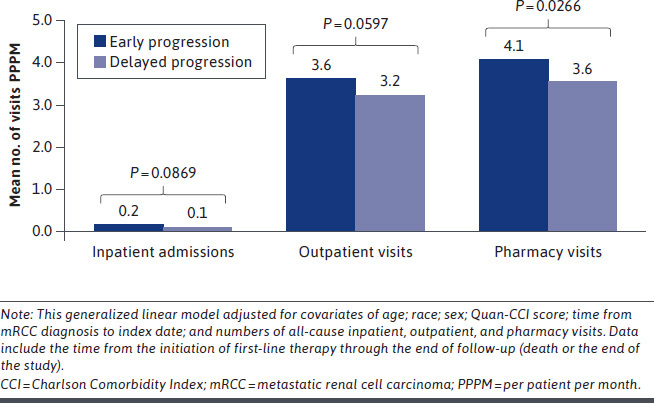
Adjusted Mean All-Cause Health Care Resource Utilization PPPM
FIGURE 4.
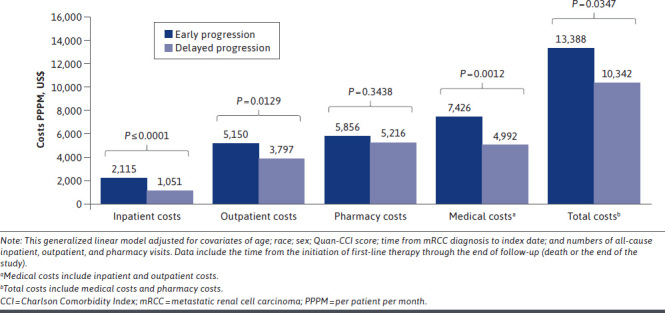
Adjusted Mean All-Cause Health Care Costs PPPM
Discussion
Data from this retrospective, claimsbased cohort analysis demonstrate that there are directional differences in clinical outcomes, including progression to further lines of therapy and overall survival, and statistically significant differences in HCRU and costs among patients with mRCC who received TKI monotherapy as 1L treatment and progressed to 2L therapy earlier rather than later. Although inherent and unavoidable bias precluded statistical comparison of clinical outcomes (see the Limitations section), descriptive analysis revealed that a longer interval between initiation of 1L therapy and initiation of 2L therapy corresponded with generally more favorable clinical outcomes, and comparative analysis revealed significantly fewer all-cause and disease-specific inpatient and outpatient visits and lower associated costs. Notably, baseline demographics and patient characteristics were generally similar between the early and delayed progression cohorts; the only baseline factor that corresponded with clinical outcomes was a lower risk of death among patients with 1 year or less between mRCC diagnosis and systemic therapy.
Because it uses time to next line of treatment as a proxy for disease progression in real-world clinical practice,9 our study adds to accumulating evidence demonstrating a connection between time to progression after initiation of 1L therapy with a TKI and clinical and economic outcomes in patients with mRCC. Using data from a retrospective chart review, Harada et al8 determined that patients with mRCC who discontinued 1L TKI therapy within 6 months (n = 103) had a worse prognosis for overall survival than did patients who continued 1L TKI treatment for 6 months or more (n = 89; 4-year survival rate, 36.6% vs 66.8%). In their retrospective, registry-based study of 1,209 patients receiving targeted therapies for mRCC, Lakomy et al17 found that, among patients who received everolimus as 2L therapy, associations existed between 1L progression-free survival for 9 months or more and both longer 2L overall survival and longer 2L progression-free survival. In cohorts stratified by time to progression after failure of 1L TKI therapy, Ishihara et al18 evaluated the influence of early and delayed progression on clinical outcomes among Japanese patients with mRCC receiving targeted 2L therapy from January 2007 to March 2016. In their study population (N = 60), patients with a time to progression longer than the median 8.8 months had significantly longer overall survival from the start of 2L therapy (9.6 months) than did patients whose time to progression was shorter than the median (28.0 months; P = 0.0036). The delayed progression group also experienced longer progression-free survival from the start of 2L therapy (10.2 months, vs 5.0 months in the early progression group; P = 0.0002). Chen et al19 expanded on the observed correlation between duration of therapy and clinical outcomes, demonstrating that remaining on 1L or 2L treatment for 3 months or more was associated with longer median overall survival in patients with mRCC.
Our study builds on these findings, concurrently analyzing the influence of time to progression on clinical outcomes, HCRU, and health care costs during the entirety of systemic therapy for mRCC, from initiation of 1L therapy through the end of the follow-up interval. These data highlight the importance of initiating mRCC treatment with 1L therapies, including newer immuno-oncology (IO)-based combination regimens, that have been shown to prolong time to disease progression, to improve patient prognosis, and to potentially reduce the associated clinical and economic burden.
To reduce variability introduced by different 1L therapies, in this study we included only patients who had received 1L monotherapy with a TKI. Moreover, because of data availability, the study period ended in March 2018—just before US Food and Drug Administration approvals of new IO and TKI combination therapies. Thus, our findings should be interpreted in the context of a rapidly changing treatment landscape. Although TKIs remain a mainstay of mRCC treatment, the introduction of new pharmacologic systemic therapies has greatly enhanced the variety of treatment options and combinations. A paradigm shift toward combination immunotherapy in mRCC management, which includes IO combination options, is reflected in current treatment guidelines for advanced kidney cancer. Although TKI monotherapies are, as of 2020, still among the treatment options cited by the National Comprehensive Cancer Network guidelines for stage IV kidney cancer with clear cell histology (version 1.2021),20 IO combinations are among the preferred regimens, particularly for patients categorized as having a poor or intermediate prognosis based on International Metastatic Renal Cell Carcinoma Database Consortium criteria.6 The 2019 consensus statement on immunotherapy for the treatment of advanced RCC, developed by the Society for Immunotherapy of Cancer, advocates 1L combination therapy for many scenarios among patients who are candidates for immunotherapy.21 With this wealth of treatment options comes a challenge for clinicians and patients regarding choosing the therapy that has the greatest likelihood of clinical benefit.22 Given the associations that we and other researchers have established between time to progression and downstream clinical outcomes, early use of treatments known to delay disease progression may be a factor to consider as part of the treatment decision-making process.
The ability to gauge individual prognosis on the basis of patient-related and clinical factors is less often applied to patients with mRCC who are initiating 2L or subsequent lines of therapy than to those beginning de novo treatment.23 The analyses reported above point to the importance of time to progression (as generally estimated in this study by the initiation of new treatment) as a prognostic variable during 1L therapy; notably, these estimations generally align with progression-free survival in clinical trials of novel IO-TKI combination therapies that included TKI monotherapies as comparators.24,25
Moreover, time to progression has been included in a new prognostic model for overall survival. Along with tumor burden, Derosa et al26 identified time from 1L to 2L treatment as a new independent prognostic factor for overall survival in patients previously treated for mRCC. Our results should be interpreted with caution given the chronology of 1L combination therapy approval and uptake. Nonetheless, they warrant future research that similarly evaluates time to progression among patients receiving IO-TKI combinations in the real-world setting and that clinically validates progression estimates.
A notable caveat to the HCRU and cost analyses in this study is that the data were analyzed on a PPPM basis. Using this metric, we found that HCRU and costs were lower in the delayed progression cohort than the early progression cohort, though in some cases they were comparable. We anticipate, however, that the extended overall survival in the delayed progression cohort would increase absolute HCRU and costs. Lower monthly HCRU but higher absolute total HCRU has been reported for patients with mRCC receiving targeted therapies,27 as have higher treatment costs for therapies associated with longer survival.28 In addition, in this study we assessed HCRU and costs during the entire evaluation period, including treatment gaps, to comprehensively capture the impact of early progression in real-world clinical practice. Future investigation may focus on economic outcomes directly associated with time receiving treatment.
LIMITATIONS
Several general limitations apply to the interpretation of our study findings. As with all claims analyses, interpretation of results is limited to identification of associations rather than inference of causality. Moreover, the available data are subject to potential coding errors and a lack of corroborating evidence that a diagnostic code listed on a health claim reflects actual presence of a disease. In addition, certain clinical and disease-specific information that might have prognostic significance may not be captured within claims data. For example, neither Eastern Cooperative Oncology Group Performance Status nor the scores based on risk criteria from the International Metastatic RCC Database Consortium or Memorial Sloan Kettering Cancer Center could be captured in our dataset, which precluded patient stratification by prognostic risk status.
There are also limitations specific to the study design. Chief among these are inherent differences in time to events between cohorts, which are due to necessary survival through 2L treatment initiation in the delayed cohort (immortal time). These differences precluded statistical comparison of clinical outcomes because of the potential for bias, although time-sensitive (PPPM) analysis of economic outcomes controlled for this confounder. The dataset was limited to years for which data were available; thus the results should be interpreted in the context of the rapidly changing treatment landscape for mRCC. The predominantly male study population also limits generalizability to the broader population of patients with mRCC. Nonetheless, the data regarding time to estimated progression as a prognostic indicator in different study populations support the validity of our findings beyond the VHA population. Further, data availability and attrition parameters limited our sample size to fewer than 300 patients. In addition, algorithms were used to define lines of therapy on the basis of administrative claims, which may not reflect the definition of lines of therapy used in clinical practice.
Finally, progression was estimated by using initiation of the next line of therapy as a proxy. Progressionfree survival could not, however, be directly estimated through clinically confirmed progression, and a regimen may have been switched for reasons other than disease progression (clinical confirmation of the reason for 2L treatment initiation was not available in the data). The results warrant future research to confirm these trends through clinically validated observation of progression.
Conclusions
This analysis of real-world data demonstrates tangible differences in outcomes between patients with mRCC who progressed from 1L TKI monotherapy to 2L therapy earlier and those who progressed later. Descriptive analysis suggests that patients with a shorter interval from 1L initiation to 2L initiation also have generally shorter overall survival, and comparative analysis reveals significantly greater HCRU and health care costs (PPPM) among these patients. These findings underscore the need to optimize 1L therapy through use of treatments that have been proven to improve overall survival and delay disease progression, which may include newer treatment strategies such as immunotherapy combinations. As the use of 1L combination therapies becomes more widespread in the initial management of mRCC, future studies are warranted to evaluate their real-world impact on disease progression, clinical outcomes, and economic burden.
DATA AVAILABILITY
The dataset supporting the conclusions in this article is available from the US Veterans Health Administration. However, restrictions apply to the availability of these data, which were used under license for this study and are not publicly available.
ACKNOWLEDGMENTS
Shivani Pandya provided study design and analysis support for this manuscript, and Crystal Murcia, PhD, STATinMED Research, provided medical writing support-all funded by the study sponsors.
REFERENCES
- 1.Medina-Rico M, Ramos HL, Lobo M, Romo J, Prada JG. Epidemiology of renal cancer in developing countries: review of the literature. Can Urol Assoc J. 2018;12(3):E154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer stat facts: kidney and renal pelvis cancer. Surveillance, Epidemiology, and End Results Program. 2020. Accessed April 28, 2020. https://seer.cancer.gov/statfacts/html/kidrp.html
- 3.Zheng T, Zhu C, Bassig BA, et al. The long-term rapid increase in incidence of adenocarcinoma of the kidney in the USA, especially among younger ages. Int J Epidemiol. 2019;48(6):1886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205. [DOI] [PubMed] [Google Scholar]
- 5.Pal SK, Ghate SR, Li N, et al. Real-world survival outcomes and prognostic factors among patients receiving first targeted therapy for advanced renal cell carcinoma: a SEER-Medicare database analysis. Clin Genitourin Cancer. 2017;15(4):e573–82. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Jonasch E, Michaelson MD, et al. NCCN guidelines insights: kidney cancer, version 2.2020. J Natl Compr Canc Netw. 2019;17(11):1278–85. [DOI] [PubMed] [Google Scholar]
- 7.Maroun R, Mitrofan L, Benjamin L, et al. Real life patterns of care and progression free survival in metastatic renal cell carcinoma patients: retrospective analysis of cross-sectional data. BMC Cancer. 2018;18(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada K, Nozawa M, Uemura M, et al. Treatment patterns and outcomes in patients with unresectable or metastatic renal cell carcinoma in Japan. Int J Urol. 2019;26(2):202–10. [DOI] [PubMed] [Google Scholar]
- 9.Maroun R, Fleury L, Nachbaur G, Maunoury F, Vanhille JL, Durand-Zaleski I. Real-world costs and outcomes in metastatic renal cell carcinoma patients treated with targeted therapies: a cohort study from the French health insurance database. Curr Med Res Opin. 2017;33(10):1755–62. [DOI] [PubMed] [Google Scholar]
- 10.Schmidinger M, Pichler R, Loidl W, et al. Real-world evidence data on metastatic renal-cell carcinoma treatment in Austria: the RELACS study. Clin Genitourin Cancer. 2019;17(5):e957–67. [DOI] [PubMed] [Google Scholar]
- 11.Pal S, Gong J, Mhatre SK, et al. Realworld treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer. 2019;19(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratta R, Verzoni E, Di Maio M, et al. Exposure to multiple lines of treatment and survival of patients with metastatic renal cell carcinoma: a realworld analysis. Clin Genitourin Cancer. 2018;16(4):e735–42. [DOI] [PubMed] [Google Scholar]
- 13.Wagstaff J, Jones R, Hawkins R, et al. Treatment patterns and clinical outcomes in patients with renal cell carcinoma in the UK: insights from the RECCORD registry. Ann Oncol. 2016;27(1):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagalman E. The number of veterans that use VA health care services: a fact sheet. June 3, 2014. Accessed April 5, 2018. https://fas.org/sgp/crs/misc/R43579.pdf
- 15.Thompson M. Deriving line of therapy: an algorithmic approach in a realworld oncology database. Paper RW06. Presented at EU2019: The Clinical Data Science Conference, November 10-13, 2019. Accessed December 21, 2020. https://www.lexjansen.com/phuse/2019/rw/RW06.pdf
- 16.Bureau of Labor Statistics. Consumer Price Index - December 2018. News release. January 11, 2019. Accessed June 16, 2020. https://www.bls.gov/news.release/archives/cpi_01112019.pdf
- 17.Lakomy R, Poprach A, Bortlicek Z, et al. Utilization and efficacy of second-line targeted therapy in metastatic renal cell carcinoma: data from a national registry. BMC Cancer. 2017;17(1):880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara H, Kondo T, Yoshida K, et al. Time to progression after first-line tyrosine kinase inhibitor predicts survival in patients with metastatic renal cell carcinoma receiving second-line molecular-targeted therapy. Urol Oncol. 2017;35(9):542.e1–e9. [DOI] [PubMed] [Google Scholar]
- 19.Chen VJ, Hernandez-Meza G, Agrawal P, et al. Time on therapy for at least three months correlates with overall survival in metastatic renal cell carcinoma. Cancers (Basel). 2019;11(7):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCCN Clinical Practice Guidelines in Oncology. Kidney cancer. V1.2021. National Comprehensive Cancer Network. Accessed September 23, 2020. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf [DOI] [PubMed]
- 21.Rini BI, Battle D, Figlin RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer. 2019;7(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed SH, Elbaghdady N, Alorabi M. Application of the American Society of Clinical Oncology frameworks to compare tyrosine kinase inhibitors used in first line treatment of metastatic renal cell carcinoma: had we solved the mystery? Expert Rev Anticancer Ther. 2017;17(11):1061–70. [DOI] [PubMed] [Google Scholar]
- 23.Derosa L, Bayar MA, Albiges L, Le Teuff G, Escudier B. A new prognostic model for survival in second line for metastatic renal cell carcinoma: development and external validation. Angiogenesis. 2019;22(3):383–95. [DOI] [PubMed] [Google Scholar]
- 24.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27. [DOI] [PubMed] [Google Scholar]
- 25.Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derosa L, Bayar MA, Albiges L, Le Teuff G, Escudier B. A new prognostic model for survival in second line for metastatic renal cell carcinoma: development and external validation. Angiogenesis. 2019;22(3):383–95. [DOI] [PubMed] [Google Scholar]
- 27.Simard H, Sabbagh R, Ouellet S, Richard P, Jeldres C. The impact of targeted therapy on healthcare resource use in patients with metastatic renal cell carcinoma: the University of Sherbrooke experience. Can Urol Assoc J. 2018;12(9):E373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazha S, Tanguay S, Kapoor A, et al. Use of targeted therapy in patients with metastatic renal cell carcinoma: clinical and economic impact in a Canadian real-life setting. Curr Oncol. 2018;25(6):e576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions in this article is available from the US Veterans Health Administration. However, restrictions apply to the availability of these data, which were used under license for this study and are not publicly available.


