Abstract
BACKGROUND:
Medication therapy management (MTM) and comprehensive medication management (CMM) have been practiced by clinical pharmacists as a predominantly manual activity with interventions documented in a record-keeping system. Program evaluations, largely based on estimations of projected savings and utilization reductions, have not accurately predicted actual claims and utilization changes, leading many to doubt the efficacy of medication management.
OBJECTIVE:
To assess the impact on actual medical claims of a novel artificial intelligence (AI) platform that identifies members and provides decision support to clinicians in performing telephonic interventions similar to MTM and CMM with high-risk Medicaid members.
METHODS:
This retrospective observational study used mixed-effects regression models that flexibly account for general trends in cost, as measured by actual claims, to identify the amount of savings and associated impact. To study the economics, total cost of care (TCoC), defined as all medication costs plus all noncapitated medical costs, was evaluated. Utilization was evaluated through the number of emergency department (ED) visits, hospital admissions, bed days, and readmissions. The study included 2,150 predominantly middle-aged (aged 40-64 years) Medicaid members with an average of 10 medications for chronic conditions among an average of 25 total medications. The analysis considered cost and utilization data from August 2017 through April 2019. Interventions occurred between January 2018 and February 2019.
RESULTS:
Statistically significant correlations were found between receiving interventions and decreased costs and utilization. The economic study found a 19.3% reduction in the TCoC (P < 0.001) that, applied to a preintervention monthly cost of $2,872, yielded a savings of $554 per member per month (PMPM). Medication costs showed a 17.4% reduction (P < 0.001), which, when applied to preintervention cost of $1,110, yielded a savings of $192 PMPM. The utilization study found a 15.1% reduction in ED visits (P = 0.002), a 9.4% reduction in hospital admissions (P = 0.008), and a 10.2% reduction in bed days (P = 0.01). Return on investment is 12.4:1 based on TCoC savings and program costs.
CONCLUSIONS:
This study evaluated the CMM-Wrap program, which used an advanced AI platform integrated with health plan data, clinical pharmacists trained in disease management, telephonic patient engagement, and closed-loop provider coordination. The results correlate cost and utilization savings with the program. The TCoC savings of $554 PMPM translates to approximately $1.2M a month and more than $14M annually for the 2,150 members in the study. We believe Medicaid and Medicare payment of AI enhanced telephonic CMM services would substantially decrease government health care expenditures, whereas improving health program expansion to Medicaid members with similar risks could save the Health Plan $109M annually. For instance, we estimate that California’s Medicaid (Medi-Cal) program could save more than $1B annually by applying the program’s observed impact to a similar high-risk cohort (about 1.6%) of Medi-Cal members. Additionally, benefits will accrue to nonmanaged health plans based on the savings themselves.
What is already known about this subject
A comprehensive medication management (CMM) longitudinal approach combining medication with disease management is more effective than an episodic or medication only approach.
Cost avoidance models that estimate savings are not an accurate means of establishing value for medication therapy management or CMM. Analysis needs to be based on actual claims.
Providing intervention documentation strictly to the patient is not an effective means to achieve adoption of pharmacist recommendations.
What this study adds
CMM, extended with advanced artificial intelligence (AI), substantially reduces the total cost of care and utilization as measured by claims.
Clinical decision support, including AI, longitudinal lab data, information visualization, and action plan simulation, enable more efficient, effective, and investigative interventions.
Empowering pharmacists with AI costs less than zero as a strong return on investment exceeding 12.4:1 was observed.
Inland Empire Health Plan (health plan) is a large, managed Medicaid health plan serving 1.3 million members in Southern California under California’s Medicaid (Medi-Cal) program. Surveyor Health’s artificial intelligence platform (AI platform) unifies population health with telemedicine to identify and prioritize members at risk and provide AI decision support for interventions with robust data collection and reporting and proprietary MedRiskScores (risk scores). Preveon Health (clinical team) is a disease therapy management provider that performs telephonic interventions by teams of medical assistants and clinical pharmacists trained in disease management.
In November 2017, the health plan began the CMM-Wrap program for its Medicaid population as a pilot intended to “wrap around” and expand on typical medication management programs, such as medication therapy management (MTM), but with additional comprehensive medication management (CMM) features and enabled with AI (for a comparison of these 3 programs, see Supplementary Table 1 (1.3MB, pdf) , available in online article). A retrospective observational study was conducted to evaluate the impact of advanced medication management interventions on actual cost and utilization data for 2,150 Medicaid members who were in the program.
The program’s design was guided by the principles of traditional MTM and CMM, for example, it incorporated CMM’s focus on the whole person, member lab history, and longitudinal management of member chronic diseases, not just medications. Unlike most CMM programs where pharmacists are physically embedded in a clinic, it operated as a remote telephonic service; no face-to-face interventions were employed, thus physical assessment of members was not possible. For care coordination, clinicians closed the loop with providers for adoption of recommendations through faxed reports and phone calls when needed but without benefit of collaborative practice agreements.
Although MTM is typically reimbursed for the Medicare population, this is generally not the case for the Medicaid population, and studies evaluating the impact of MTM and CMM services on the Medicaid population are lacking.
Actual utilization and cost data for members who received interventions were evaluated with 6 hypotheses: The CMM-Wrap program is associated with decreases in total cost of care (TCoC) (1) and medication costs (2) and is associated with decreased occurrences of hospital admissions (3), hospital readmissions (4), bed days (5), and emergency department (ED) visits (6).
Methods
STUDY DESIGN
This observational study examined 2,150 of the health plan’s Medicaid members who received their first telephonic intervention between January 2018 and February 2019 (study period window). The analysis considered their cost and utilization data from August 2017 through April 2019 (observation window). An advanced regression model was used because members started receiving treatment at different times during the study period window and a simple pre/post model offered a less robust analytical approach. The advanced model effectively allowed the regression to measure differences between members of the same cohort who have and have not yet received treatment across multiple months. Members had a mean (SD) of 11.7 (2.2) months of preintervention data and a mean of 8.8 (2.38) months of postintervention data.
Multiple linear regression analyses were used to estimate the correlation between receiving interventions and their impact on health care costs and utilization. The linear regressions compared the health plan’s costs and utilization for each member before and after their first intervention as well as compared members who had and had not yet received an intervention. Indicator variables accounted for other potentially confounding concurrent programs.
PARTICIPANTS
Enrollment requirements were based on a chronic medication threshold, defined as a minimum number of medications being taken by a member for chronic conditions as identified by the health plan. At the start of the study period window, the threshold was 10. On August 1, 2018, the threshold was decreased to 8 because all members who met the threshold of 10 had already been contacted. There were no medication cost threshold requirements; however, a higher number of medications for chronic conditions was associated with higher than average medication costs. Members had multiple comorbidities and an average of 25 medications, 10 of which on average were for chronic conditions, including asthma, chronic obstructive pulmonary disease, depression, diabetes, and hypertension. This represented a high pill count burden with concomitantly high medication risks as well as a substantial opportunity to optimize care. Although the number of medications treating chronic conditions was used to select members, all of their medications and conditions were evaluated in a holistic review. Additional baseline characteristics are shown in Table 1. Members were enrolled an average of at least 18.3 months before their first intervention based on enrollment data from January 2017 onward.
TABLE 1.
Baseline Characteristicsa
| Cohort | Study cohort (n = 2,150) | Plan population during study (N = 1,200,000) |
|---|---|---|
| Baseline medications | ||
| Mean medication count | 25.3 | 2.0 |
| Mean count of medications for chronic conditions | 10.3 | 0.5 |
| Baseline demographics, n (%) | ||
| Male | 778 (36.2) | 549,600 (45.8) |
| English speaking | 1,844 (85.8) | 926,400 (77.2) |
| Spanish speaking | 305 (14.2) | 273,600 (22.8) |
| Aged 0-12 years | 0 (0) | 361,200 (30.1) |
| Aged 13-18 years | 1 (0) | 170,400 (14.2) |
| Aged 19-39 years | 78 (3.6) | 351,600 (29.3) |
| Aged 40-64 years | 1,827 (85.0) | 243,600 (20.3) |
| Aged 65-79 years | 213 (9.9) | 56,400 (4.7) |
| Aged 80 and older years | 31 (1.4) | 16,800 (1.4) |
aData such as race, education, employment, homelessness, and homeownership were not available.
STUDY DATA
The 3 teams worked together for 1 year to meticulously review and verify the cost and utilization data and complete the regression analysis.
TCoC is defined as all medication costs, plus all noncapitated medical costs including inpatient, ED, physician, outpatient, laboratory and radiology, community and home care, transportation, long-term care (LTC), and hospice. The health plan’s actuarial office provided claims data for all noncapitated medical cost categories. The health plan identified other concurrent programs that might confound the results; indicator variables were included for each. The health plan provided all medical cost, utilization, and program enrollment data in summary format, aggregated by member and reporting month. Medication fill costs came from the health plan’s pharmacy benefits manager (PBM). First DataBank’s MedKnowledge was employed as evidence upon which the AI and analytics operated.
ARTIFICIAL INTELLIGENCE AND USE OF THE AI PLATFORM
The AI platform is a cloud application that regularly validates and integrates enrollment, demographics, conditions, and laboratory data from the health plan with medication fill data from the PBM. This application created as complete a profile as possible, optimized for an intervention and built from the health plan’s data, without requiring access to an electronic health record (EHR). The AI platform provided decision support in identifying the medications most likely to be active and the indications for each medication. This provided an optimized starting point for medication reconciliation and discussions with the member.
Risk scores helped visualize medication and regimen level risks for the entire regimen both before and after the intervention, simulating the impact of prospective changes of therapy. Drug-disease contraindications of individual medications were highlighted. Pairs of medications were assessed for drug-drug interactions and duplicate therapy. Additive effects occur when multiple medications pose the same side effect risks (e.g., nausea) and are used together in a regimen, increasing the likelihood that the risk will be realized.
The AI platform uniquely applied Bayesian probabilistic inference to indicate the risk each medication and the regimen as a whole have for each possible adverse effect. More broadly, these risks were combined to create a vector of elements, statistically compared and contrasted with other vectors to understand each individual and the population by the absolute and relative risks within.
CLINICAL WORKFLOW
The clinical team included 3 clinical pharmacists and 3 medical assistants. Each comprehensive intervention took between 15 and 45 minutes (depending on case complexity) and averaged about 30 minutes to complete. This was half the time the same clinical team required for comprehensive medication reviews without the AI platform, enabling the small team to double their productivity while increasing their focus on condition management. On average each member received 3.5 interventions within 14 months, ranging from 1 to 9 interventions.
Member Selection.
Every month the AI platform identified eligible members and placed them in its intervention pool in a random order for selection by the clinical team.
Clinical Decision Support for Interventions.
The clinical team used the AI platform’s clinical application to understand the member’s history, view health status, perform medication reconciliation, identify medication risks, compose a prospective action plan, view a simulation of the action plan’s impact, weigh its trade-offs, and revise it as needed with guidance from the visual simulation of changes considered, then configure reporting documents for automatic generation. A clinician’s description of using the clinical application during a typical intervention is shown in “An Example Intervention (1.3MB, pdf) ” in the Supplementary Materials (available in online article).
Multiple Interventions.
Members were automatically eligible for additional interventions if they still met the targeting criteria 3 months after each intervention unless more frequent interventions were warranted and scheduled by the clinical team.
As seen in Figure 1, the application was highly visual, showing individual risks for each medication as well as the regimen risk from all medications before and after the intervention. This avoided interventions that resolve one problem only to invisibly create new problems.
FIGURE 1.
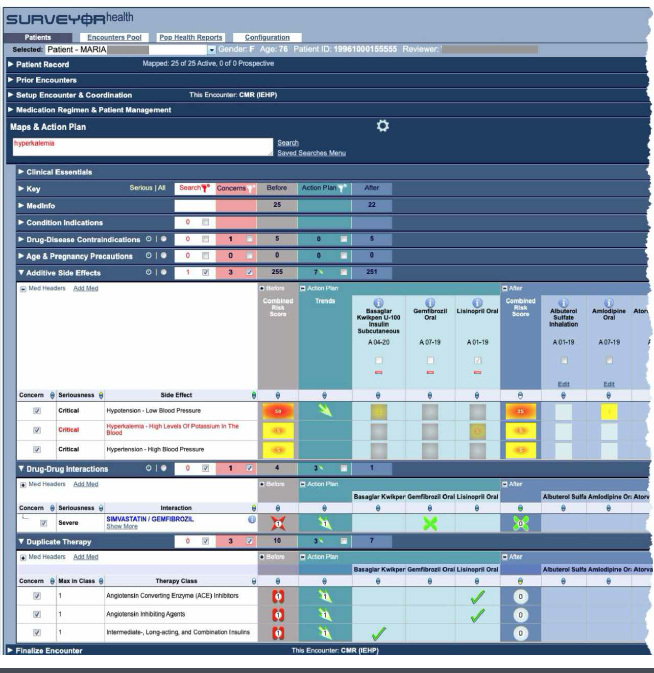
The Surveyor Health Clinical Application
Communications and Care Coordination.
Communication materials were automatically generated to ensure consistency and save time. A customized patient care plan and an updated medication list were mailed to the member, while a consultation summary with recommendations was faxed to their primary care provider (PCP). If the member could not be reached or declined the consultation, a report was sent only to their PCP. When severe risks were identified, the clinical team phoned the PCP or specialist.
CLINICAL ACTION PLAN RECOMMENDATIONS
There were 7,485 interventions with 46,090 recommended actions during the study. Each intervention averaged 3.4 medication-related actions and 2.8 general actions for a total of 6.2. The primary changes needed were to discontinue medications, many of which were duplicative. Medication-related actions (percentage of interventions) were as follows: 15,579 discontinuations (84.6%), 2,477 replacements (27.8%), 3,267 order refills (31.6%), 1,116 “add medication” (13.1%), 2,857 dosage increase/decrease/titrate/hold (32.3%), and 32 “correct improper administration” (0.4%). General actions included the following: 6,289 “add or update labs” (79.8%), 7,786 “patient education provided” (38.5%), 2,792 “provider needs to provide patient education” (31.6%), 2,390 “provider needs to perform medication reconciliation” (25.1%), and 1,505 custom recommendations (17.6%).
VARIABLES
Monthly outcome variables were as follows: (1) TCoC; (2) medication costs; (3) ED visits; (4) hospital admissions; (5) bed days; and (6) readmissions. The models regress multiple independent variables: receipt of an intervention, enrollment in various other programs, the days since the program started, and the square of the days since it started.
BIAS
The outcome variables are not subjective measurements, but rather objective measurements, and are not subject to response shift bias.1 Associations discussed and estimated may be confounded by several factors—measured or unmeasured—as this was not a randomized clinical trial. To mitigate these concerns, a flexible modeling of time was included to address the possibility that changes in cost could have occurred in absence of the intervention, as well as the possibility that the associations could be explained by a member’s simultaneous enrollment in other programs.
STUDY SIZE
After applying the filters, 2,150 members and 43,993 months of available cost and utilization data were analyzed (Figure 2).
FIGURE 2.
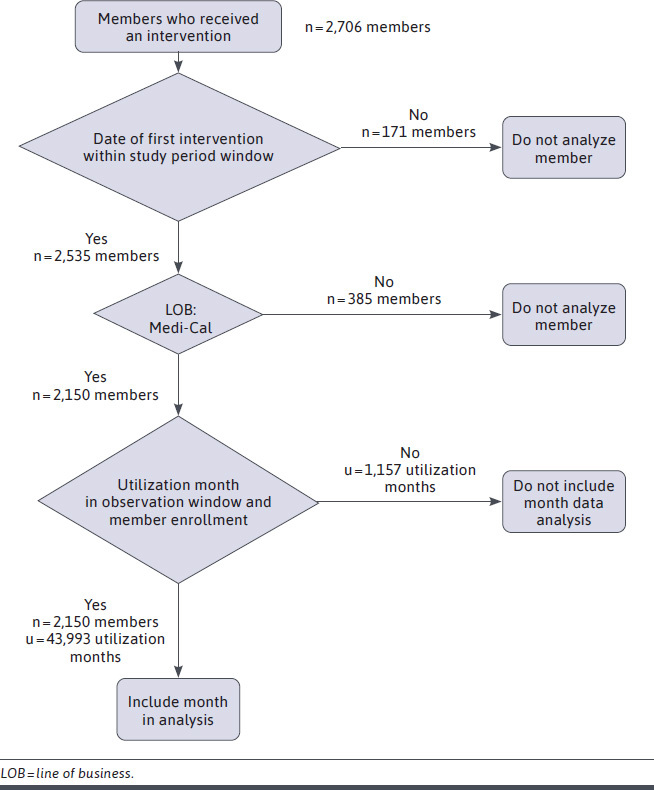
STROBE Flowchart of Member and Monthly Data Inclusion in Analysis
A member was considered for inclusion if their first intervention was finalized within the study period window. The cohort was further reduced to members enrolled in California’s Medicaid (Medi-Cal) at the time of their first intervention. Monthly cost and utilization data were included in the analysis for only those members who fell within the observation window. Cost and utilization data were only included for months when a member was enrolled.
QUANTITATIVE VARIABLES
All cost data is represented in US dollars as positive numbers or zero. All utilization data are positive integers or zero. Program indicator variables are either zero (member not enrolled) or 1 (enrolled). These data are sourced from the health plan and its PBM. Days since the start of the observation window is a positive integer representing roughly the number of days since the beginning of the observation window; it is included to account for any time-variant effect outside of the program.
STATISTICAL METHODS
The analysis consisted of 6 linear mixed-effects regression models.2 A mixed model was used to account for each member having multiple months of data. Python and its implementation of mixed linear regression models in statsmodels.formula.api.mixedlm,3 with a random intercept on RECORD_ID, was used. This implementation is in line with the LME4 implementation in R (R Foundation) and other mature statistical packages.4
Medication costs and TCoC were log transformed to account for the right skew of the data. Most monthly costs were zero or near zero but ranged much higher, some surpassing $100,000. As part of the transform, $1.00 was added to every cost before the transformation to avoid performing the log of zero. The distribution of residuals for TCoC in logarithmic space is shown in Supplementary Figure 4 (1.3MB, pdf) (available in online article).
Likewise, utilization data were also log transformed, but because those numbers were not much larger than 10 on average (and never larger than 30), 0.0001 was added before performing the transformation.
The basic regression model is defined as:
INDEPENDENT_VAR = intercept + ß1 INTERVENTION_RECEIVED + β2 DAYS_SINCE_START + β3 DAYS_SINCE_START2 + β4..16 PROGRAM_INDICATORS + ε
where β1 (beta_1) is the estimated effect of receiving an intervention.
The regression controlled for time and various types of programs that some members were enrolled in, such as behavioral health, community-based adult services, health homes, in-home support services, LTC facilities, low-income subsidy, pain management, palliative care, and Medicare Parts A and B. Each program has a unique indicator variable in the regression models; this group of variables is denoted as β4..16 PROGRAM_INDICATORS.
The following are regressed on the above model with random intercept on RECORD_ID:
log(MED_COST), log(TOTAL_COST), log(ADMITS), log(BED_DAYS), log(READMITS), and log(ER_VISITS)
The P values for beta_1 (the estimated treatment effect) were adjusted to reduce the occurrence of type I errors when performing multiple hypothesis tests by applying a Holm-Bonferroni error correction to the 2 cost-based hypotheses and separately to the 4 utilization-based hypotheses.
Results
Statistically significant correlations were found between receiving an intervention and decreased costs and utilization. Savings, based on claims, are reported as per member per month (PMPM).
REGRESSION ANALYSIS DETAILS
Complete regression results are displayed in Table 2. They show that 5 of the 6 cost and utilization hypotheses indicate statistically significant reductions. There was a statistically significant 19.3% (P < 0.001) decrease in TCoC PMPM. When applied to the mean preintervention monthly TCoC of $2,872 PMPM, this yields a savings of $554 PMPM (Supplementary Figure 1 (1.3MB, pdf) , available in online article). This translates to more than $14M saved annually for the study cohort. When looking at medication costs alone, there is a statistically significant 17.3% (P < 0.001) decrease which when applied to the mean preintervention medication cost of $1,110 PMPM yields a medications savings of $192 PMPM (Supplementary Figure 2 (1.3MB, pdf) , available in online article).
TABLE 2.
Regression Analysis Details
| Outcome variablea | Costs | Utilization | ||||
|---|---|---|---|---|---|---|
| Total cost of care | Medication costs | ED visits | Hospital admissions | Hospital bed days | Hospital readmissions | |
| Estimated treatment effect | ||||||
| Intervention received | −0.214 | −0.19 | −0.164 | −0.099 | −0.107 | −0.027 |
| Change, % | −19.3 | −17.3 | −15.1 | −9.4 | −10.2 | −2.7 |
| P valueb | <0.001 | < 0.001 | 0.002 | 0.008 | 0.01 | 0.149 |
| SE | 0.023 | 0.024 | 0.052 | 0.037 | 0.042 | 0.019 |
| Covariant, n (P value) | ||||||
| Intercept | 6.56 (0.001) | 5.748 (0.001) | −8.18 (0.001) | −8.82 (0.001) | −8.77 (0.001) | −9.159 (0.001) |
| Daysc | 0 (0.001) | 0 (0.001) | 0 (0.998) | 0 (0.197) | 0 (0.209) | 0 (0.038) |
| Days squaredd | 0 (0.001) | 0 (0.001) | 0 (0.492) | 0 (0.877) | 0 (0.877) | 0 (0.455) |
| Indicator variables for participation in another plan program, n (P value) | ||||||
| BH | 0.206 (0.001) | 0.041 (0.344) | 0.11 (0.172) | 0.10 (0.070) | 0.12 (0.049) | 0.075 (0.008) |
| CA | 0.122 (0.225) | −0.169 (0.110) | −0.21 (0.259) | −0.06 (0.652) | −0.10 (0.520) | −0.239 (0.001) |
| HHP | 0.122 (0.014) | 0.140 (0.007) | 0.22 (0.048) | −0.01 (0.908) | −0.01 (0.880) | 0.013 (0.755) |
| INS | 0.321 (0.001) | 0.275 (0.001) | 0.22 (0.001) | 0.20 (0.001) | 0.22 (0.001) | 0.055 (0.005) |
| Landmarke | 0.282 (0.001) | 0.329 (0.001) | 0.29 (0.001) | 0.23 (0.001) | 0.27 (0.001) | 0.072 (0.002) |
| LI | 0.397 (0.002) | 0.078 (0.556) | 0.18 (0.531) | 0.27 (0.184) | 0.30 (0.188) | 0.028 (0.787) |
| LTC resident | 1.112 (0.001) | 0.213 (0.022) | 0.71 (0.001) | 1.50 (0.001) | 1.80 (0.001) | 0.921 (0.001) |
| LTC services | 0.203 (0.218) | 0.321 (0.062) | −0.55 (0.107) | −1.39 (0.001) | −1.61 (0.001) | −0.793 (0.001) |
| Med AB | −2.277 (0.001) | −3.180 (0.001) | −0.58 (0.001) | −0.31 (0.005) | −0.35 (0.005) | −0.097 (0.089) |
| MyPathf | 0.024 (0.757) | 0.069 (0.384) | −0.63 0.001) | 0.11 (0.336) | 0.10 (0.458) | 0.229 (0.001) |
| PAIN | 1.121 (0.001) | 0.306 (0.055) | 0.17 (0.625) | −0.03 (0.891) | −0.04 (0.885) | −0.111 (0.379) |
| ToC | 0.530 (0.001) | 0.154 (0.244) | −0.42 (0.152) | −0.31 (0.135) | −0.32 (0.166) | −0.053 (0.618) |
aAll outcome variables have been log transformed.
bTreatment P values adjusted with Holm-Bonferroni. Costs were adjusted as a family of 2, and utilizations were adjusted as a family of 4.
cNumber of days since start of observation window.
dThe square of the days variable.
eIn-home care and education.
fHome-based care for advanced disease patients.
BH = behavioral health integration & complex care initiative; CA = community-based adult services; ED = emergency department; HHP = health homes program; INS = in-home support services; LI = Medicare low-income subsidy; LTC = long-term care; Med AB = Medicare Part A or B; PAIN = pain management center of excellence; SE = standard error; ToC = transitions of care management.
Utilization claims also showed statistically significant results including a 15.1% decrease in ED visits (P = 0.002), 9.4% reduction in hospital admissions (P = 0.008), and 10.2% reduction in bed days (P = 0.01). There was no statistically significant correlation in the number of readmissions observed.
SUMMARY OF SIGNIFICANT REDUCTIONS
As seen in Figure 3, reductions occurred across the board. Risks were reduced, paralleling a similar reduction in number of ED visits, hospitalizations and bed days. Even greater reductions in the costs were observed.
FIGURE 3.
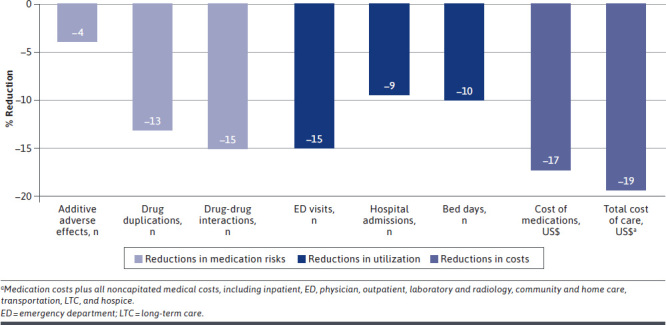
Summary Chart of Significant Reductions by Percentage
EXPLORATIONS FOUND ADDITIONAL STATISTICALLY SIGNIFICANT CORRELATIONS
In further explorations, medication risk scores were regressed and showed a decreased risk of adverse drug events correlated with receiving interventions. Risk scores showed statistically significant (P < 0.001) reductions of 15.2% serious drug-drug interactions, 13.1% duplications of therapy, and 3.9% additive adverse events. Although we cannot assume causation, this shift is consistent with the reductions in ED visits (15.1%) and hospitalizations (9.4%) as seen in Figure 3.
After the study period ended, the health plan provided all noncapitated medical costs with categorizations and details on costs on which we had not anticipated access. They were explored with regressions, and we are reporting on the statistically significant results which were found in inpatient, ED, physician, outpatient, laboratory and radiology, community and home care, transportation, LTC, and hospice categories (Supplementary Figure 3 (1.3MB, pdf) , available in online article).
As applied to their pretreatment means, some of the decreases in costs were 9.1% inpatient ($66.47; P = 0.008), 24.2% specialist ($85.85; P < 0.001), 10.0% ED ($5.93; P < 0.001), 7.1% primary care provider ($1.07; P = 0.003), 13.0% lab/radiology ($5.43; P < 0.001), and 6.9% community home care ($4.41; P < 0.001). Only 1 category showed increased costs: 2.3% hospice ($0.11; P < 0.001), but these costs were insubstantial.
The health plan changed the categorization of some costs during the study, which could impact some of these categorizations but not the overall totals or the primary hypotheses.
EVALUATING POTENTIAL CONFOUNDERS
Participation in the health plan’s other programs were considered as possible confounders. The regression found that the confounding programs, which provided services such as home care, decreased the TCoC savings estimate for the CMM-Wrap program by 1.0%. Without accounting for confounders, the regression reported a 20.3% reduction in TCoC costs, whereas when accounting for the confounders (covariants in Table 2) the percentage reduction was 19.3%. Note that this does not indicate anything about the efficacy of those programs on their own, only on their impact on the members of this study during the study window (Supplementary Table 2 (1.3MB, pdf) , available in online article).
Discussion
RETURN ON INVESTMENT
For return on investment (ROI) calculations, we use the sum of AI platform cost ($24 PMPM) and clinical team cost ($17 PMPM) as the investment amount of $41 PMPM for an average of 3.5 interventions per member. The regression estimated effect of $554 savings for TCoC was used. An ROI of 12.4:1 was observed based on TCoC, which before intervention was about 38.6% medication costs and 61.4% nonmedication costs.
GENERALIZABILITY
These results are potentially generalizable to Medicaid members who are aged 40 years or older with 8 or more medications for chronic conditions. Our study did not limit members by the occurrence of specific chronic or acute diseases. Comparative analysis with the health plan’s Medicare population reveals similar risks, indicating potential applicability to Medicare.
COMPARISON WITH RELATED RESEARCH
Review of the literature did not reveal any other study that shares the CMM-Wrap program’s distinctive competence or its results. In 2015, a meta-analysis across 44 MTM studies concluded that “medication therapy management interventions may reduce the frequency of some medication-related problems…but the evidence is insufficient with respect to improvement in health outcomes.”6 More recent studies have shown results and key lessons; comparisons follow.
Savings and ROI need to be based on claims, not estimates. Studies that attempt to establish ROI frequently use cost avoidance estimates yet assigning values to recommendations frequently overrepresents savings. In 1 instance, estimated savings predicted an ROI of up to 1.8 times, yet the actual ROI turned out to be −3.3 times when calculated from claims with the average cost increase of $1,244.7 An ROI claim of 12 times made in 20088 was changed to a 1.29 times estimate after a 10-year retrospective analysis by the same MTM system. Yet the 12 times figure was included in an often-cited literature review in 2014.9 A pharmacist-delivered CMM-like program reviewed utilization across 312 patients and matched comparators providing 5,705 intervention actions.10 No significant changes in admissions were observed, but ED visits increased for the comparators only. Based on estimated hospital and ED costs, the program reports a benefit:cost ratio of between 2.2:1 and 2.6:1. A 1-year retrospective study of a 2016 Texas CMM program,11 where pharmacists reviewed EHR records of 3,280 patients and worked with independent physicians to assess estimates, resulted in $361.28 per member per year of estimated savings. The CMM-Wrap program demonstrates savings, based on actual claims, of $6,648 per member per year and an ROI exceeding 12 times.
Preidentification of medication-related problems is essential. When reviews were guided by preidentification of medication-related problems (MRP), significant changes in the number of admissions and ED visits were seen.12 Only reviews with preidentification of MRPs, primarily adherence, resulted in statistically significant findings. The AI powering the CMM-Wrap program always preidentifies and visualizes medication-related risks and shows how each is mitigated by the action plan, providing advanced information and guidance to the pharmacist who then applies their own clinical judgment to develop the plan.
Multiple interventions. Fifty percent of deprescribed medications are prescribed again within a year,13 quantifying the understanding that sustaining therapeutic changes requires ongoing management. The CMM-Wrap program utilized up to 9 interventions within 14 months when needed to achieve and sustain changes.
Closing the loop with providers. MTM requires that patients be sent standardized documents but does not require notification to the provider. None of the studies discussed above described any follow-through of interventions recommended. As is typical with CMM, the CMM-Wrap program’s clinical team always sent reports to providers and called them when needed.
LIMITATIONS
A retrospective observational study always limits conclusions on causality. The health plan’s administrative costs were not included in our calculations; however, the costs of the clinicians and the platform, including costs of administering eligibility and enrollment, were. Remaining administrative costs were considered to be insignificant by the health plan and therefore were not included.
Clinical outcomes were also not analyzed in relation to the member’s diseases or medication issues identified by the clinical team. Although statistically significant reductions in ED visits, admissions, and bed days were seen, reasons for visits or specific disease associations were not analyzed. Plan members with higher TCoC tended to have interventions conducted on them sooner than those with lower TCoC; this was not by design but may have been due to the reduction in chronic medication threshold over the study period window.
Conclusions
It has become of paramount importance to provide the most effective health care at the lowest cost while ensuring availability of and accessibility to care. The objectives of this study were to assess the impact of the CMM-Wrap program on actual medical claims using a novel AI platform in selecting high-risk Medicaid members and then supporting, aiding, and guiding pharmacist interventions. This study demonstrates that pharmacists and medical assistants, trained in disease management and working hand in hand with advanced AI to provide telephonic CMM medication management services, reduces the cost of care and decreases ED and hospital utilization, which may be considered to be indicators of improved health.
This study establishes that CMM interventions delivered telephonically reduced utilization and costs without face-to-face care and provide a cost-effective means to manage chronic disease and associated medications at scale. These results are particularly relevant in the context of the COVID-19 pandemic, during which telehealth delivered care continues to grow and evolve, whereas in person primary doctor visits have become challenging to deliver. In this environment it becomes even more important to have “multiple eyes” (team-based care including the pharmacist) on higher risk members (eg, with uncontrolled chronic disease managed primarily through medications) so that care can be coordinated and optimized, preventing critical health care issues from occurring.
These results are based on analysis of actual medical claims for a substantial cohort over a meaningful period of time. Few other studies of medication management programs meet all of these criteria (claims, scale, length). Thus, this study provides meaningful evidence, further supporting adoption of similar programs by payers and health care organizations.
An analysis of the health plan’s membership produced an additional observation worth reflecting on: middle-aged Medicaid recipients have medication-risk challenges and potential outcomes similar to Medicare’s seniors when their medication risks and interventions are evaluated. About 1.6% of each population, totaling 2 million nationally, would benefit from similar AI-powered telephonic CMM interventions, potentially leading to substantial savings as members’ medications are optimized and these members avoid ED and hospital utilization. Extrapolating the potential cost reductions points to billions of dollars that can be saved.
With an observed ROI of 12.4:1, this program demonstrates significant economic value to the government payer net of costs. Provider-payer support for CMM performed by pharmacists is strongly warranted by the results of this study. Medicaid and Medicare payment for these comprehensive medication management services would ensure they are delivered more frequently, widely, and consistently. We believe Medicaid and Medicare payment of AI enhanced telephonic CMM services would substantially decrease government health care expenditures while improving health. Additionally, benefits will accrue to nonmanaged health plans based on the savings themselves.
ACKNOWLEDGMENTS
The authors acknowledge and thank others who participated in this study. The Inland Empire Health Plan data teams in the actuarial services and health care informatics departments provided the cost and utilization data. The Preveon Clinical Team performed the actual interventions and their informatics team provided additional data. In the preparation of this manuscript, third-party reviewers contributed valuable opinions and suggestions.
REFERENCES
- 1.Howard GS. Response-shift bias: a problem in evaluating interventions with pre/post self-reports. Eval Rev. 1980;4(1):93-106. [Google Scholar]
- 2.Lindstrom M, Bates D. Newton-Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J Am Stat Assoc. 1988;83(404):1014-22. doi:10.2307/2290128 [Google Scholar]
- 3.Statsmodels.org. Statsmodels v0.12.2: statsmodels.formula.api.mixedlm API. Updated February 2, 2021. Accessed May 7, 2021. https://www.statsmodels.org/stable/generated/statsmodels.formula.api.mixedlm.html
- 4.Statsmodels.org. Linear mixed effects models: comparing R imer to Statsmodels MixedLM. March 25, 2021. Accessed May 7, 2021. https://www.statsmodels.org/devel/examples/notebooks/generated/mixed_lm_example.html#Comparing-R-lmer-to-statsmodels-MixedLM
- 5.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-28. doi.org/10.2105/ajph.86.5.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings a systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76-87. doi:10.1001/jamainternmed.2014.5841 [DOI] [PubMed] [Google Scholar]
- 7.Barner JC, Roberson K, Richards KM, Makhinova T, Nduaguba SO. Clinical and economic outcomes of the Texas Medicaid Medication Therapy Management (MTM) Pilot: asthma and COPD. October 18, 2016. Accessed November 6, 2020. https://hhs.texas.gov/sites/default/files/documents/laws-regulations/reports-presentations/2016/sb1-medicaid-mtm-pilot-oct-2016.pdf
- 8.Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc. 2008;48:203-11. [DOI] [PubMed] [Google Scholar]
- 9.Ai AL, Carretta H, Beitsch, et al. Medication therapy management programs: promises and pitfalls. J Manag Care Pharm. 2014;20(12):1162-82. doi:10.18553/jmcp.2014.20.12.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moczygemba LR, Alshehri AM, Harlow LD 3rd, et al. Comprehensive health management pharmacist-delivered model: impact on healthcare utilization and costs. Am J Manag Care. 2019;25(11):554-60. [PubMed] [Google Scholar]
- 11.Chung TH, Hernandez RJ, Libaud-Moal A, et al. The evaluation of comprehensive medication management for chronic diseases in primary care clinics, a Texas delivery system reform incentive payment program. BMC Health Serv Res. 2020;20(1):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferries, E, Dye JT, Hall B, Ndehi L, Schwab P, Vaccaro J. Comparison of medication therapy management services and their effects on health care utilization and medication adherence . J Manag Care Spec Pharm. 2019:25(6);688-95. doi:10.18553/jmcp.2019.25.6.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez AI, Abner EL, Jicha GA, et al. One-year evaluation of a targeted medication therapy management intervention for older adults. J Manag Care Spec Pharm. 2020:26(5);520-28. doi:10.18553/jmcp.2020.26.4.520 [DOI] [PMC free article] [PubMed] [Google Scholar]


