Abstract
BACKGROUND:
Severe hypoglycemia is a significant barrier to optimizing insulin therapy in both type 1 and type 2 diabetes and places a burden on the US health care system because of the high costs of hypoglycemia-related health care utilization.
OBJECTIVE:
To compare the frequency of sensor-detected severe hypoglycemic events (SHEs) among a population of continuous glucose monitoring (CGM) users on insulin therapy after initiation of the InPen smart insulin pen (SIP) system and to estimate the potential hypoglycemia-related medical cost savings across a population of SIP users.
METHODS:
SIP users of all ages with type 1 or type 2 diabetes were required to have at least 90 days of SIP use with a connected CGM device. The last 14 days of sensor glucose (SG) data within the 30-day period prior to the start of SIP use (“pre-SIP”) and the last 14 days of SG data, along with the requirement of at least 1 bolus entry per day within the 61- to 90-day period after SIP start (“post-SIP”), were analyzed. Sensor-detected SHEs (defined as ≥10 minutes of consecutive SG readings at <54 mg/dL) were determined. Once factored, the expected medical intervention rates and associated costs were calculated. Intervention rates and costs were obtained from the literature.
RESULTS:
There were 1,681 SIP + CGM users from March 1, 2018, to April 30, 2021. The mean number of sensor-detected SHEs per week declined from 0.67 in the pre-SIP period to 0.58 in the post-SIP period (P = 0.008), which represented a 13% reduction. Assuming a range of 5%-25% of all sensor-detected SHEs resulted in a clinical event, the estimated cost reduction associated with reduced SHEs was $12-$59 and $110-$551 per SIP user per month and per year, respectively. For those aged at least 65 years, there were 166 SIP+CGM users and the reduction in the mean number of sensor-detected SHEs per week between the pre-SIP and post-SIP periods was 31%.
CONCLUSIONS:
Use of the SIP system with a connected CGM is associated with reduced sensor-detected severe hypoglycemia, which may result in significant cost savings.
Plain language summary
People with diabetes sometimes experience low levels of blood sugar, called hypoglycemia, that may require care from a health care provider or facility. We compared the number of hypoglycemia events among people before and after the start of treatment with a smart insulin pen. We found that people experienced less hypoglycemia after using the smart insulin pen, then estimated the cost savings that may result from a reduction in health care use.
Implications for managed care pharmacy
We find a reduction in sensor-detected severe hypoglycemia after the start of smart insulin pen therapy in a population of people with diabetes using continuous glucose monitoring. We extend this finding by estimating the potential cost savings from reduced hypoglycemia-related health care utilization that may be associated with smart insulin pen use. The potential economic impact of smart insulin pen use may be useful for formulary placement and decision-making among payers and decision makers.
Fewer than 20% of youths aged younger than 18 years with type 1 diabetes (T1D) in the United States achieve the recommended treatment goal of glycated hemoglobin less than 7.5%, and only 21% of adults with T1D achieve the recommended glycated hemoglobin of less than 7.0%.1 Hypoglycemia is a common complication among people with diabetes, especially those using insulin, and places a burden on both the patient and the health care system. Although there is variability in how and when individuals with T1D show symptoms of hypoglycemia, the occurrence of the time below target glucose range (TBR) of less than 70 mg/dL (level 1 hypoglycemia)2 and less than 54 mg/dL (level 2 hypoglycemia)2 are associated with an increased risk of severe hypoglycemia (SH).3 Although international consensus has not assigned a glucose concentration to level 3 (severe) hypoglycemia,4 the occurrence of and failure to recognize level 2 hypoglycemia (<54 mg/dL; ie, serious and clinically significant) increases the risk of level 3 (severe) hypoglycemia. The challenge of current American Diabetes Association clinical targets is to minimize TBR while maintaining or improving the time in range (TIR).5
Adults with T1D have about 2 episodes of mild (self-treated) hypoglycemia per week, whereas about 30% of adults with T1D experience severe (requiring help) hypoglycemia at least once per year with several factors, such as disease duration, increasing its incidence.6 The T1D Exchange Registry also shows that 6%-10% of adults, depending on the age group, experience severe hypoglycemic events (SHEs; needing assistance or resulting in seizure or loss of consciousness) every 3 months.1 In addition, numerous studies have shown the elevated risk for hypoglycemia in the older adult population.7-10 Adults with type 2 diabetes (T2D) using insulin experience a lower rate of mild events and SHEs than those living with T1D, but event frequency rises progressively with the increasing duration of insulin therapy.6
SH places a high burden on the US health care system because of the high costs of hypoglycemia-related health care utilization in the form of hospitalizations and emergency department (ED) visits. Among US adults aged 18 years and older, 8.25 million hospital discharges were reported with diabetes as any listed diagnosis and 60,000 involved hypoglycemia (2.2 per 1,000 adults with diabetes) in 2018.11 Similarly, among the same population and time period, 17 million ED visits were reported with diabetes as any listed diagnosis and 242,000 involved hypoglycemia (9.6 per 1,000 adults with diabetes).11 One earlier study estimated the average cost for inpatient hypoglycemia admissions and ED visits related to hypoglycemia to be $17,564 and $1,387, respectively12; therefore, any reduction in health care utilization due to SHEs may have a significant economic benefit on the US health care system.
THE ROLE OF SMART INSULIN PENS IN MULTIPLE DAILY INSULIN THERAPY
The InPen smart insulin pen (SIP) system (Medtronic) is the first US Food and Drug Administration-cleared SIP.13 The system can be paired with a continuous glucose monitor (CGM) and includes a smartphone-based diabetes management application that contains a bolus calculator that factors in multiple metrics (insulin-on-board, insulin sensitivity, insulin-carbohydrate ratio, and ambient glucose level) and can address challenges in optimizing therapy.14 SIPs support users on multiple daily injection (MDI) therapy with algorithms determining optimal bolus insulin doses while accounting for active insulin and sensor glucose (SG) values to help avoid dangerous hypoglycemia.15,16
SIP features allow opportunities to safely deliver more frequent rapid-acting insulin doses because the dose calculator takes insulin-on-board into account when calculating the dose, thereby decreasing the risk of insulin stacking that can result from a previous dose of insulin. In a retrospective study of 1,721 SIP users (both youths and adults with T1D or T2D), the TIR improved as the number of daily bolus injections increased, without increasing the TBR.17 These findings suggest that SIPs can be used to calculate and deliver more frequent correction doses of insulin, when needed, to safely improve the TIR without an increased risk of hypoglycemia. Another analysis of data from SIP users (N = 122), who were above the recommended daily TBR goal of less than 4%5 prior to SIP use, showed that the TBR was reduced from an average of 8.0% to 6.3% after starting SIP therapy. This resulted in 25 fewer minutes per day in the hypoglycemic range. These findings suggest that by using SIPs, individuals at high risk for SH may significantly reduce their TBR without compromising their overall glycemic control.18
In this retrospective real-world data analysis, we sought to evaluate the impact of the SIP paired with CGM (SIP + CGM) on SH (<54mg/dL) by comparing the frequency of sensor-detected SHEs among a population of CGM users on insulin therapy before and after the initiation of the SIP system. As an added metric to measure or predict meaningful glycemic events, low blood glucose index (LBGI) values based on CGM readings were also used to quantify the risk of hypoglycemia from preperiod to postperiod for each SIP user. Finally, we applied findings from the literature to estimate the potential hypoglycemia-related medical cost savings across a population of SIP users.
Methods
STUDY OBJECTIVE AND POPULATION
The objective of the data analysis was to assess the change in sensor-detected SHEs among people with T1D or T2D after adding treatment via SIPs to the existing therapy regimen. The existing therapy regimen included MDIs and CGM for a minimum of 30 days before the start of SIP therapy. Users with less than an average of 1 bolus dose per day after the start of SIP therapy were excluded from the study, as this implies the possibility that SIP was not the only rapid-acting therapy regimen being used.
STUDY MEASURES
The study index date was the SIP therapy start date, defined by the first day the user administered a bolus insulin dose with the SIP. The preperiod measure included the 30 days before the index date, and the postperiod measure included post-index days 61-90. Days 0-60 of treatment with SIP were excluded and considered as the period of getting acquainted and established with the SIP system and therapy regimen, during which time there is often known variability in behaviors and outcomes.
The insulin dose data, including doses manually logged by the user and automatically logged from the SIP device and the glucose data originating from the CGM device, were deidentified and collected from the SIP app. The CGM transmits values every 5 minutes (real time), resulting in up to 288 SG values per day. Based on the existing literature, 14 days of CGM data are sufficient for estimating glucose outcome measures.19,20 Therefore, the latest 14-day period in each study period (pre-SIP and post-SIP use) was used to derive the hypoglycemic metric. Each SIP user included in the analysis met the SG point requirement of 4,032 SGs (288 SGs × 14 days) in both periods.
Sensor-detected SHEs were identified with a combination of SG points and duration (as shown in Figure 1). First, SH SG points were identified by values of less than 54 mg/dL. Next, consecutive SH SG points having a duration of more than 600 seconds (10 minutes) qualified as SHEs. Finally, consecutive SHEs that were less than 30 minutes apart were merged into a single event. Based on this definition, the total count of events per person per 14-day measurement period was calculated to obtain the mean number of SHEs (MNSHEs) in each study period. We also assessed MNSHEs among SIP users aged 65 years and older separately because of the known elevated risk of hypoglycemia among this subgroup.7-10
FIGURE 1.
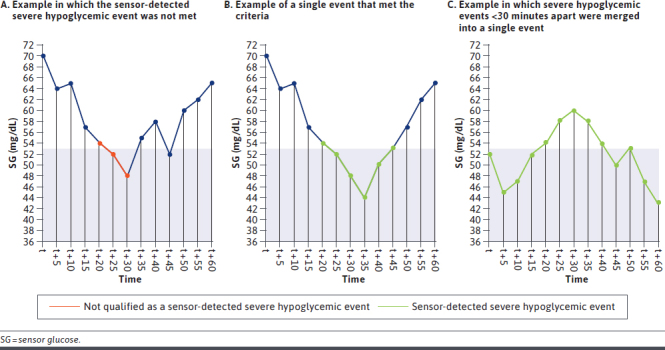
Sensor-Detected Severe Hypoglycemic Events, as Determined by the SG Level of <54 mg/dL for ≥10 Consecutive Minutes
SIP users' preperiod MNSHEs per week were paired with postperiod MNSHEs per week and tested for statistical differences with the Wilcoxon signed-rank test. The LBGI value was derived from a logarithmic transformation of self-monitoring of blood glucose readings to make the transformed data symmetric around 0 and then grouped into 3 SH risk categories: low (LBGI < 2.5), moderate (LBGI between 2.5 and 5), and high risk (LBGI > 5).21 Given that the sampling frequency of self-monitoring of blood glucose and CGM systems are different, the computation of LBGI values from CGM readings is biased and a correction is needed.22 Hence, a linear transformation of LBGI values was used to adapt the characteristics of the CGM system, and SIP users’ preperiod LBGI values were paired with postperiod LBGI values and tested for statistical differences with the paired t-test. All data analysis was conducted in Python software (Python Software Foundation) using the scipy.stats library.
ASSUMPTIONS AND ESTIMATES
To estimate the potential hypoglycemia-related medical cost savings across a population of SIP users, a cost calculation was performed by applying the values of key parameters obtained from the literature to the results from the pre-SIP and post-SIP data analysis. Key parameters in the cost calculation included potential health care utilization rates resulting from SHEs and the associated costs of those events. The parameter inputs were obtained from studies that included individuals with T1D or T2D. Table 1 summarizes the expected medical intervention rates and associated costs for SH-related hospitalization, ED visits, and ambulance transportation that formed the basis of this cost calculation. All costs obtained from the literature were adjusted to represent 2021 US dollars using the medical care component of the Consumer Price Index.29
TABLE 1.
Estimates for Cost Calculation
| Parameter | Estimate | Population | Reference |
|---|---|---|---|
| Severe hypoglycemic events requiring hospitalization, % | 6.7 | T1D and T2D | Heller 201623 |
| Severe hypoglycemic events requiring emergency department visit, % | 14.5 | T1D and T2D | Heller 201623 Foos 201524 |
| Severe hypoglycemic events requiring ambulance transportation, % | 29.3 | T1D and T2D | Heller 201623 |
| Hospitalization cost for a severe hypoglycemia event, $ | 16,160a | Adults with T2D or discharged with a diagnosis of hypoglycemia | Curkendall 201125 Goyal 201726 Pawaskar 201827 Quilliam 201112 |
| Emergency department visit cost for a severe hypoglycemia event, $ | 2,001a | Adults with T2D or EMS activations in which dispatch recorded a complaint of a “diabetic problem” | Kaufmann 201928 Pawaskar 201827 Quilliam 201112 |
| Ambulance cost for a severe hypoglycemia event, $ | 821a | EMS activations in which dispatch recorded a complaint of a “diabetic problem” | Kaufmann 201928 |
a All costs were standardized to 2021 using the medical care component of the Consumer Price Index.
EMS = Emergency Medical Services; T1D = type 1 diabetes; T2D = type 2 diabetes.
To estimate cost savings, the literature-based event rates and costs were applied to the change in sensor-detected SHEs estimated in the data analysis. Three key assumptions were applied to the cost-savings calculation to obtain reasonable estimates of the economic impact of SIPs. First, to estimate a population-based savings from reduced SHEs, we considered the probability of having any sensor-detected SHEs during the measurement period. To avoid overestimating potential savings, we applied a weighted average that represented the observed event rate in the pre-SIP period to the cost calculation. Second, we conducted an analysis based on 5%-25% of sensor-detected SHEs resulting in a clinical event requiring intervention. This range of probabilities acknowledges that not all sensor-detected events will result in a clinical event but also accounts for uncertainty, as the link between sensor-derived events and clinical events resulting in intervention has not been established.30 Finally, annual cost savings also included a factor to account for the therapy attrition rate based on the rate observed in the SIP user population.
The annualized savings per user was calculated by applying the estimated cost of an SHE to the estimated change in sensor-detected events in the post-SIP period, with adjustments for retention and the probability of having any event as described above. The cost-savings equation is described further in the Supplementary Materials (139.1KB, pdf) (available in online article).
Results
After applying sensor data inclusion criteria, there were 1,681 SIP + CGM users from March 2018 to April 2021 included in the analysis (Table 2). Overall, there were 2,250 sensor-detected SHEs in the pre-SIP period and 1,956 events in the post-SIP period; each study period represented a 2-week measurement window as described above. The descriptive statistics and distribution of sensor-detected SHEs during the 2-week measurement window are shown in Supplementary Tables 1 and 2 (139.1KB, pdf) . The mean number of sensor-detected SHEs per week declined by 13%, from 0.67 in the pre-SIP period to 0.58 in the post-SIP period (P = 0.008), as shown in Table 3. For the subset of users aged 65 years and older, there were 166 SIP + CGM users and the mean number of sensor-detected SHEs per week declined by 31%, from 0.36 in the pre-SIP period to 0.25 in the post-SIP period (P = 0.033).
TABLE 2.
Demographics and Characteristics of Study Population
| Adolescents (age <18 years) | Adults (age 18-64 years) | Elderly (age ≥65 years) | NAa | |
|---|---|---|---|---|
| (N = 397) | (N = 957) | (N = 166) | (N = 161) | |
| Age, mean ± SD, years | 12 ± 4 | 41 ± 13 | 72 ± 6 | |
| Sex (M/F/NAa) | 178/187/32 | 380/520/57 | 69/81/16 | |
| Diabetes type (T1D/T2D/NAa) | 377/2/18 | 821/79/57 | 96/58/12 | |
| Weight, mean ± SD, kg | 47.0 ± 21.2 | 80.2 ± 21.2 | 81.5 ± 22.9 | |
| Duration of diabetes, mean ± SD, years | 5 ± 5 | 20 ± 14 | 30 ± 6 |
a Users who did not self-report demographic data within the InPen app.
F = female; M = male; NA = not applicable; T1D = type 1 diabetes; T2D = type 2 diabetes.
TABLE 3.
Mean Number of Sensor-Detected Severe Hypoglycemic Events
| Metrics | Pre-SIP (N = 1,681) | Post-SIP (N = 1,681) | P value |
|---|---|---|---|
| Users with sensor-detected severe hypoglycemic events, n (%) | 661 (39.3) | 626 (37.2) | |
| Number of sensor-detected severe hypoglycemic events per week | 1,125 | 978 | |
| Sensor-detected severe hypoglycemic events per week, mean ± SD | 0.67 ± 1.50 | 0.58 ± 1.27 | 0.008 |
SIP = smart insulin pen.
As for the LBGI metric, the change in each SH risk category from the preperiod to postperiod is summarized in Supplementary Table 3 (139.1KB, pdf) . Notably, about 50% (38 of 78) of those in the moderate-risk category improved to the low-risk category and the other 50% did not change. The average LBGI value for those in the moderate-risk category decreased from 3.15 in the preperiod to 2.55 in the postperiod (P < 0.001).
Based on the results from the data analysis and published literature in Table 1, we estimated the potential cost savings associated with a reduction in sensor-detected SHEs from a US payer perspective. By applying the intervention rates and costs for SH-related hospitalization (6.7%, $16,160), ED visits (14.5%, $2,001), and ambulance transportation (29.3%, $821), the total estimated cost reduction per month associated with reduced sensor-detected SHEs for this population of 1,681 SIP + CGM users is $19,234-$98,915 (Supplementary Table 4 (139.1KB, pdf) ) when assuming that 5%-25% of sensor-detected SHEs result in a clinical event.
To obtain the monthly cost-savings estimate that accounted for the probability of having any event, we applied a weighted average that represented the proportion of users with an event in the pre-SIP period (39.3%) to the cost calculation. Annual cost savings also included a factor to account for the therapy attrition rate. Results from the cost calculation yielded savings of $12-$59 per SIP user per month, or $110-$551 per SIP user per year.
Results from the sensitivity analysis are described in Table 4. Annual savings per user ranged from $110 in the scenario in which only 5% of sensor-detected SHEs result in a clinical event to $551 in the scenario in which 25% of sensor-detected SHEs result in a clinical event.
TABLE 4.
Cost Savings Assuming Sensor-Detected Events Result in Clinically Defined Severe Hypoglycemic Events
| Proportion, % | Savings per user per month, $ | Annual savings per user, $ |
|---|---|---|
| 25 | 59 | 551 |
| 20 | 48 | 441 |
| 15 | 36 | 330 |
| 10 | 24 | 220 |
| 5 | 12 | 110 |
Discussion
Fewer than one-third of people with diabetes on insulin therapy achieve glycemic targets,31 which can have negative short- and long-term consequences for health outcomes. In particular, SH is a significant barrier to optimizing insulin therapy in both T1D and T2D and places a high-cost burden on the health care system because of hypoglycemia-related health care utilization. The advent of SIP offers the potential to provide dosing support to MDI users; given that most people in the United States who are on intensive insulin therapy to manage their diabetes use injections,32 there is a potential for a substantial impact on improving glycemic control in a large number of people with diabetes. Initial data analyses suggest that SIP use is associated with more frequent correction doses and improved TIR without compromising the TBR.17,33 In fact, SIP users at high risk for hypoglycemia prior to SIP initiation experienced a reduction of the TBR, resulting in 25 fewer minutes per day in the hypoglycemic range.18 Therefore, using SIP to calculate and deliver more frequent correction doses to safely improve the TIR without increasing the risk of hypoglycemia has the potential for meaningful impacts in intensive insulin injection therapy and may result in a reduction in health care spending cost.
In this retrospective real-world analysis evaluating the impact of a SIP + CGM, we extend what is known about the benefit of SIP therapy and quantify important potential economic benefits. Among the SIP users included in the study, we found that the frequency of sensor-detected SHEs decreased by 13% after initiation of the SIP system. This finding is in line with the earlier analysis from Smith et al that demonstrated an 11% reduction in the TBR (2.54% vs 2.27%; P < 0.001) among MDI + CGM users after initiating SIP therapy.18 In another study comparing the benefits of realtime vs flash CGM, there was no significant reduction in the percentage of time in clinically relevant hypoglycemia (<54 mg/dL) for the cohort that used real-time CGM in both study periods.34 To that end, our study provides the first real-world assessment of the potential incremental economic benefit of SIP in a population of people who added SIP therapy to an existing CGM regimen.
Our analysis includes projected cost savings across a range of probabilities (5%-25%) that sensor-detected SHEs result in clinical SHEs requiring health care utilization. We find that even in a scenario of low event rates, SIP users may experience a reduction in SHE-related health care utilization and cost. At a population level, in which nearly 30% of adults with T1D experience 2 SHEs per year, these findings have meaningful economic implications for patients and payers. Notably, the reduction in sensor-detected SHEs per week for SIP users aged 65 years and older (N = 166) was 31% (15 vs 10 in the preperiod and postperiod, respectively) compared with the overall reduction of 13% (281 vs 245 in the preperiod and postperiod, respectively) when assuming that 25% of sensor-detected SHEs result in a clinical event, suggesting that the use of the SIP may provide unique therapeutic benefits and cost savings in older individuals. Changes in health care utilization and potential savings related to additional or longer-term benefits of SIP therapy should be considered in the future, along with indirect costs (eg, productivity and quality of life) and the cost of therapy, to allow a more complete economic assessment of SIP therapy.
The strength of this study is that it is the first study of the impact of SIP use on sensor-detected SHEs in a real-world population of SIP users. This retrospective study includes a wide age range of SIP users and people with either T1D or T2D. By combining this real-world analysis of sensor-detected SHEs with previously published SHE rates and costs, we provide important information on the potential economic impact of SIP use that may be useful for decision-making among patients, physicians, and payers.
LIMITATIONS
There are several limitations to our study. First, the population included in this study does not include a direct comparator arm, so we cannot be certain that the reduction in sensor-detected SHEs is a result of the introduction of SIP therapy. However, all people included in the study had a preperiod with no SIP use to control for any within-user variation that may contribute to the change in the preperiod to postperiod event rates. Second, the user data captured by the SIP and CGM do not include complete clinical, diagnostic, or demographic detail to allow for more robust risk adjustment or assessment of therapy impact among subgroups. Third, the literature-based cost estimates are based on a range of populations (Commercial, Medicare, various age groups, and diabetes type) and may not accurately represent the real costs and utilization of our study population. Finally, the actual rate of clinical events that occur after sensor-detected events is not known. However, based on both modeling and clinical studies, the cost reduction may be closer to that in the lower half of the sensitivity analysis cases in Table 4. Although our study presents a wide range of potential cost savings, future work bridging the gap between sensor events and clinical events will further inform this work. Additionally, future studies should consider the indirect cost impacts of SIP therapy on productivity and health-related quality of life, as well as the potential for longer-term savings associated with therapy, to assess the full benefit of SIP therapy.
Conclusions
Use of the SIP system is associated with reduced sensor-detected SHEs, which may result in significant cost savings, assuming sensor-detected SHEs result in clinical-defined SHEs.
REFERENCES
- 1.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA 1c for type 1 diabetes: A consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40(12):1622-30. doi:10.2337/dc17-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C. The association of biochemical hypoglycemia with the subsequent risk of a severe hypoglycemic event: Analysis of the DCCT data set. Diabetes Technol Ther. 2019;21(1):1-5. doi:10.1089/dia.2018.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: A joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155-7. doi:10.2337/dc16-2215 [DOI] [PubMed] [Google Scholar]
- 5.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-603. doi:10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frier BM. Hypoglycaemia in diabetes mellitus: Epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711-22. doi:10.1038/nrendo.2014.170 [DOI] [PubMed] [Google Scholar]
- 7.DuBose SN, Weinstock RS, Beck RW, et al. Hypoglycemia in older adults with type 1 diabetes. Diabetes Technol Ther. 2016;18(12):765-71. doi:10.1089/dia.2016.0268 [DOI] [PubMed] [Google Scholar]
- 8.Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513-7. doi:10.2337/dc09-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorda CB, Ozzello A, Gentile S, et al. ; HYPOS-1 Study Group of AMD. Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol. 2015;52(5):845-53. doi:10.1007/s00592-015-0713-4 [DOI] [PubMed] [Google Scholar]
- 10.Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people - a less well recognized risk factor for frailty. Aging Dis. 2015;6(2):156-67. doi:10.14336/AD.2014.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Accessed on February 3, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html [Google Scholar]
- 12.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care. 2011;17(10):673-80. [PubMed] [Google Scholar]
- 13.Kiang T. Department of Health and Human Services Food and Drug Administration 510(k) summary letter correspondence for Companion Medical InPen System, indications for use approval form. Department of Health & Human Services. July 26, 2016. Accessed on March 5, 2021. https://www.accessdata.fda.gov/cdrh_docs/pdf16/k160629.pdf [Google Scholar]
- 14.Harbison R, Hecht M, MacLeod J. Building a data-driven multiple daily insulin therapy model using smart insulin pens. J Diabetes Sci Technol. 2022;16(3): 610-6. doi:10.1177/1932296820951225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klonoff DC, Kerr D. Smart pens will improve insulin therapy. J Diabetes Sci Technol. 2018;12(3):551-3. doi:10.1177/1932296818759845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kompala T, Neinstein AB. Smart insulin pens: Advancing digital transformation and a connected diabetes care ecosystem. J Diabetes Sci Technol. 2022;16(3):596-604. doi:10.1177/1932296820984490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith M, Thanasekaran S, Gaetano A, MacLeod J. Increased number of daily boluses positively impacts glycemia in injection therapy with smart insulin pens. Presented at: ADCES 2020 Poster Presentation. [Google Scholar]
- 18.Smith M, Thanasekaran S, Im G, Gaetano A, Lewis J, MacLeod J. Smart insulin pens improve timbe below range in multiple daily insulin therapy. Presented at: AMCP 2020 Poster Presentation. doi:10.18553/jmcp.2020.26.4-a.s1 [Google Scholar]
- 19.Xing D, Kollman C, Beck RW, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther. 2011;13(3):351-8. doi:10.1089/dia.2010.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314-6. doi:10.1089/dia.2017.0455 [DOI] [PubMed] [Google Scholar]
- 21.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870-5. doi:10.2337/diacare.21.11.1870 [DOI] [PubMed] [Google Scholar]
- 22.Fabris C, Patek SD, Breton MD. Are risk indices derived from CGM interchangeable with SMBG-based indices? J Diabetes Sci Technol. 2016;10(1):50-9. doi:10.1177/1932296815599177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heller SR, Frier BM, Herslv ML, Gundgaard J, Gough SCL. Severe hypoglycaemia in adults with insulin-treated diabetes: impact on healthcare resources. Diabet Med. 2016;33(4):471-7. doi:10.1111/dme.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foos V, Varol N, Curtis BH, et al. Economic impact of severe and nonsevere hypoglycemia in patients with Type 1 and Type 2 diabetes in the United States. J Med Econ. 2015;18(6):420-32. doi:10.3111/13696998.2015.1006730 [DOI] [PubMed] [Google Scholar]
- 25.Curkendall SM, Zhang B, Oh KS, Williams SA, Pollack MF, Graham J. Incidence and cost of hypoglycemia among patients with type 2 diabetes in the United States: Analysis of a health insurance database. J Clin Outcomes Manag. 2011;18(10):455-62. [Google Scholar]
- 26.Goyal RK, Sura SD, Mehta HB. Direct medical costs of hypoglycemia hospitalizations in the United States. Value Health. 2017;20(9):PA498. doi:10.1016/j.jval.2017.08.562 [Google Scholar]
- 27.Pawaskar M, Iglay K, Witt EA, Engel SS, Rajpathak S. Impact of the severity of hypoglycemia on health - related quality of life, productivity, resource use, and costs among US patients with type 2 diabetes. J Diabetes Complications. 2018;32(5):451-7. doi:10.1016/j.jdiacomp.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann MA, Nelson DR, Kaushik P, Mann NC, Mitchell B. Hypoglycemia emergencies: Factors associated with prehospital care, transportation status, emergency department disposition, and cost. Prehosp Emerg Care. 2019;23(4):453-64. doi:10.1080/10903127.2018.1528322 [DOI] [PubMed] [Google Scholar]
- 29.US Bureau of Labor Statistics. Consumer Price Index (CPI) databases. Accessed on April 6, 2021. https://www.bls.gov/cpi/data.htm [Google Scholar]
- 30.Divilly P, Martine-Edith G, Mahmoudi Z, et al. Rates of sensor detected hypoglycaemia and patient reported hypoglycaemia; preliminary data from the Hypo-Metrics Trial. Presented at: ATTD 2022 Oral Presentations Session 3. doi:10.1089/dia.2022.2525.abstracts [Google Scholar]
- 31.Selvin E, Parrinello CM, Daya N, Bergenstal RM. Trends in insulin use and diabetes control in the U.S.: 1988-1994 and 1999-2012. Diabetes Care. 2016;39(3):e33-5. doi:10.2337/dc15-2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Draznin B, Aroda VR, Bakris G, et al. 7. Diabetes technology: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S97-112. doi:10.2337/dc22-S007 [DOI] [PubMed] [Google Scholar]
- 33.Smith M, Lewis J, Thanasekaran S, Gaetano A, Im G, MacLeod J. Smart insulin pens allow correction doses as needed without compromising time below range. Presented at: Diabetes Technology Society 2020 Poster Presentation. doi:10.1177/1932296821996093 [Google Scholar]
- 34.Reddy M, Jugnee N, Anantharaja S, Oliver N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: The extension phase of the I HART CGM study. Diabetes Technol Ther. 2018;20(11):751-7. doi:10.1089/dia.2018.0252 [DOI] [PMC free article] [PubMed] [Google Scholar]


