Summary
Determining the prognostic association of different immune cell types in the tumor microenvironment is critical for understanding cancer biology and developing new therapeutic strategies. However, this is challenging in certain cancer types, where the abundance of different immune subsets is highly correlated. In this study, we develop a computational method named TimiGP to overcome this challenge. Based on bulk gene expression and survival data, TimiGP infers cell-cell interactions that reveal the association between immune cell relative abundance and prognosis. As demonstrated in metastatic melanoma, TimiGP prioritizes immune cells critical in prognosis based on the identified cell-cell interactions. Highly consistent results are obtained by TimiGP when applied to seven independent melanoma datasets and when different cell-type marker sets are used as inputs. Additionally, TimiGP can leverage single-cell RNA sequencing data to delineate the tumor immune microenvironment at high resolutions across a wide range of cancer types.
Keywords: tumor immune microenvironment, transcriptome-based method, cell-cell interaction network, prognostic associations of immune cells, prognostic model
Graphical abstract

Highlights
-
•
TimiGP illustrates cell-cell interactions and prognostic associations of immune cells
-
•
A robust method to dissect the tumor immune microenvironment from bulk expressions
-
•
Adaptive to high-resolution analysis utilizing single-cell RNA-seq
-
•
Applicable to various sequencing platforms and distinct cancer types
Inspired by the dynamic balance of immune system, Li et al. develop a computational method, TimiGP, to uncover relationships between cellular context and clinical results, It holds promise for making biological discoveries and predicting disease progression, which may enhance our understanding of various cancer types and improve personalized medical approaches.
Introduction
Cancers are composed of not only tumor cells but also various non-cancerous cells that constitute a complex tumor microenvironment. Immune cells are one of the essential non-malignant tumor components that interact with each other to regulate cancer evolution.1,2 These cells, including cytotoxic cells such as CD8+ T cells3 and natural killer (NK) cells,4 as well as immunosuppressive cells such as regulatory T cells (Tregs)5 and myeloid-derived suppressor cells (MDSCs),6 form a tangled tumor immune microenvironment (TIME). Recently, accumulating evidence has suggested that the TIME drastically impacts the clinical outcomes of patients with cancer.7,8,9,10 However, due to immune cell crosstalk and co-infiltration, analyses of their impact on clinical outcomes have often led to conclusions contradictory to each other or opposite to their established anti- or pro-tumor roles.9 Therefore, a comprehensive understanding of how the TIME influences the biological and clinical behaviors of cancers is needed to facilitate the development of novel therapeutic strategies.
To investigate the TIME, traditional immunophenotyping approaches, such as immunohistochemistry (IHC) and flow cytometry, are widely used in clinical practice. However, these methods are only able to analyze a small number of markers; therefore, only a few cell types can be assessed simultaneously. Single-cell RNA sequencing (scRNA-seq) has emerged as a powerful technique that enables transcriptomic profiling and cell subtyping at a higher resolution.11 However, currently, it is impractical in clinical practice due to its high cost and requirement of high sample quality, not to mention that some cell types may be lost and the expression profiles may be distorted during tumor dissociation.12 By contrast, RNA-seq is amendable for low-quality samples and can portray the transcriptome profile from bulk tissues, including various cell types. RNA-seq-based assays have been widely utilized for molecular profiling in clinical practice.13,14 Therefore, bulk transcriptomic profiling retains its advantage as an important approach to studying the TIME due to its mature technologies, tissue availability, and low cost.
Over the last two decades, multiple marker expression- or deconvolution-based approaches (e.g., xCell,15 CIBERSORTx16) have been proposed to dissect cellular heterogeneity in the TIME.15,16,17,18,19,20,21,22,23,24,25 However, most of these methods are designed to estimate immune cell infiltration levels rather than immune interactions. Though a few methods enable the inference of cell-cell communication from bulk sequencing data (e.g., CCCExplorer,26 ICELLNET27), these require knowledge of ligand-receptor interactions and hence limit their application only to known intercellular signaling communications.28 Considering our rudimentary knowledge of the TIME, computational methods that comprehensively dissect immune cell-cell interaction networks and their association with clinical outcomes remain an unmet need.
To fill this void, we developed TimiGP (tumor immune microenvironment illustration based on gene pairs), a computational method to investigate the TIME by inferring cell-cell interactions and the prognostic value of immune cells. Our approach combines survival statistics with bulk transcriptomic profiles to construct an immune cell-cell interaction network in which the edge (e.g., X → Y) indicates that a high X/Y ratio is associated with favorable prognosis. By analyzing the topology of the network, our method enables the identification of immune cell types critical in prognosis and anti-tumor immunity. In this study, we applied TimiGP to metastatic melanoma and demonstrated how TimiGP resolves the prognostic bias (i.e., the estimation of the clinical value of immune cells contradictory to their biological function) caused by immune co-infiltration. Referring to the cell-cell interaction network, we also built a prognostic model to denote the clinical utility of TimiGP, which exhibited high interpretability and accuracy across multiple independent datasets. To extend the application of TimiGP, we integrated scRNA-seq results and utilized the method to analyze different scales of cell-cell interactions, such as in the entire tumor microenvironment or within T cell subpopulations. Finally, we applied TimiGP to 23 solid tumor types and revealed the heterogeneity of prognostic association of immune cells in pan-cancer. This work will improve the understanding of the TIME with implications for novel target identification and prognostic model construction, eventually facilitating personalized therapies.
Results
Gene pair analysis disentangles expression correlations caused by immune cell co-infiltration
In the tumor microenvironment, different immune cells are often closely regulated and hence co-infiltrate.29 To demonstrate this, we applied eight transcriptome-based cell-type quantification methods15,16,17,18,19,20,21,22,23,24,25 to metastatic melanoma, which is known to be a highly immune-infiltrated cancer type (immune “hot”) (Table S1).30,31 As expected, estimated cell abundances showed strong positive correlations with each other (Figure S1A). Although such co-occurrence patterns enable the identification of multicellular communities (e.g., EcoTyper21), they may also cause “prognostic bias” when the clinical significance of immune cells is inappropriately estimated. We examined the association between prognosis and inferred immune cell infiltration (Table S2A). As shown in Figure 1A, 44 cell types were significantly associated with prognosis (p < 0.05), with the majority (93.2%) associated with a favorable prognosis (hazard ratio [HR] < 1), including many cell types reported to play pro-tumor roles (e.g., plasmacytoid dendritic cells32). This systematic tendency of association between high infiltration of almost all immune cells with superior survival is an instance of prognostic bias. Similarly, at the gene expression level, most prognostic immune marker genes (IMGs) (90.5%), including many negative immune regulators (e.g., PD-L1, LAG3, TIGIT, IDO1),33,34 were associated with improved survival (Figure 1B; Table S2B), likely also due to a high level of immune co-infiltration as evidenced by the predominant positive correlation between their IMGs (Figures 1C and S2B). Taken together, these results highlight the need for new methods to reduce the potential influence of immune co-infiltration in order to reveal the true relations and roles of immune cells.
Figure 1.
Gene pairing was a potential solution of prognostic bias caused by immune cell co-infiltration
(A) Volcano plot showing that the majority of immune cells are positively associated with prognosis. The infiltration was estimated by 8 transcriptome-based cell-type methods shown as different shapes. The percentage of cell types associated with favorable (red) or unfavorable (blue) prognosis is highlighted in the bar graphs.
(B) Volcano plot showing that the majority of immune marker genes (IMG) are positively associated with prognosis. The text labels well-known immune stimulators/cytotoxic markers (red) and inhibitors/checkpoints (blue). The percentage of IMGs associated with favorable (red) or unfavorable (blue) prognosis is stressed in the bar graphs.
(C) Heatmap of Pearson correlation coefficients (PCC) of prognostic IMGs’ expression.
(D) Example of PD-L1 and CXCL10 showing that the high correlation between the two genes’ expression leads to prognostic bias, and their gene pair can solve the bias. Top panel: Kaplan-Meier (KM) curve showing the overall survival of patients with high (red) or low (blue) expression of CXCL10 (left) or PD-L1(right) using the median as a cutoff; bottom panel: scatterplot showing the Pearson correlation between the two genes (left) and KM curve showing the survival impact of CXCL10-to-PD-L1 ratio (right). The p value shown in the KM plot is calculated by the log rank test by comparing the survival of two groups.
(E) Representative schema showing how the correlated infiltration affects the prognostic evaluation of cells and indicating that the relative estimation (e.g., relative abundance) is a better measurement than absolute infiltration. All analyses have been performed with the metastatic melanoma dataset in The Cancer Genome Atlas (TCGA_SKCM06).
See also Figures S1 and S2 and Tables S1 and S2.
Immune-related gene pairs have been reported as predictive biomarkers in previous studies.35,36 To mitigate the above-mentioned bias, we propose focusing on the pairwise relation between gene expressions to investigate the prognostic effect (Figures 1D and S2). For example, in Figure 1D, both CXCL10 and PD-L1 were associated with longer survival, although they have opposing functions—promoting (CXCL10) versus suppressing (PD-L1) anti-tumor immunity, respectively. The positive prognostic value of PD-L1 is likely caused by its positive correlation with genes promoting anti-tumor immunity (e.g., CXCL10, which increases T cell infiltration and interferon γ [IFN-γ] production and causes PD-L1 expression37). Interestingly, a high PD-L1-to-CXCL10 ratio was associated with significantly shorter survival, suggesting that the relative expression level of immune genes may better reflect the biological/clinical behaviors of cancers. Inspired by such gene pairs, we considered estimating the relative abundance at the cellular level to reduce the influence of co-infiltration and therefore inferring the prognostic value of immune cells more accurately. As shown in Figure 1E, although the absolute infiltrations of immune effectors and suppressors are positively correlated, their relative abundance enables us to capture subtle differences between them and reveal the prognostic associations of these cells in line with their biological functions. As an extension of this idea, we developed a computational method called TimiGP.
The TimiGP framework
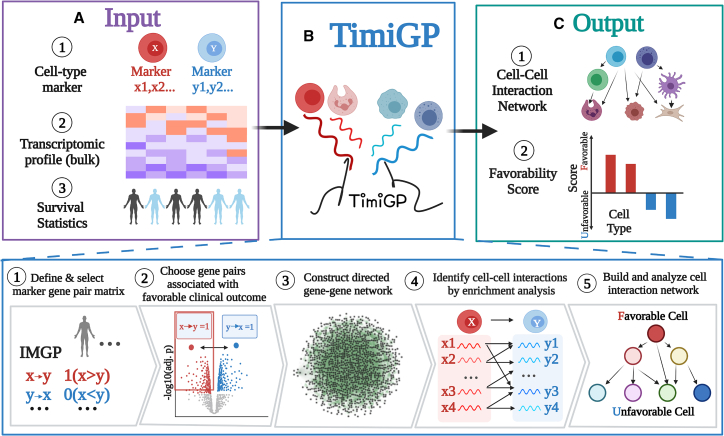
TimiGP is designed to study the TIME by inferring cell-cell interaction networks and prognostic associations of infiltrating immune cells from bulk tissue transcriptomes (Figure 2). To achieve this, the method requires three inputs: immune cell-type-specific markers, expression profiles, and patient survival statistics. The statistical framework of TimiGP consists of five major steps as follows (Figure 2; STAR Methods).
Figure 2.
TimiGP framework
Schematic depicting the TimiGP framework. TimiGP required three inputs: (1) immune cell-type markers, (2) transcriptomic profiles, and (3) survival statistics, including event (e.g., death, recurrence) and time to event of the same cohort. TimiGP infers cell-cell interaction networks and prioritizes critical immune cells affecting prognosis (favorability score) through five steps: (1) defining and selecting marker gene pair matrix, (2) choosing gene pairs associated with favorable prognosis, (3) constructing the directed gene-gene network, (4) identifying cell-cell interactions by enrichment analysis, and (5) building and analyzing cell-cell interaction network.
First, with bulk tissue transcriptomes, TimiGP selects the expression of immune cell-type marker genes and compares the differential expression between IMG pairs (IMGPs) in each sample. The resultant score (marker pair score [MPS]) of each pairwise comparison is set to 1 if the expression of gene x is higher than y; otherwise, it is 0. Second, TimiGP uses univariate cox regression to evaluate associations between IMGPs and prognosis, choosing prognostic IMGPs whose MPSs of 1 are significantly associated with a favorable prognosis (HR < 1; false discovery rate [FDR] < 0.05).
Third, by connecting selected IMGPs, TimiGP constructs a directed gene-gene network. Fourth, TimiGP identifies cell-cell interactions by examining the enrichment of their cross-cell marker gene pairs in the prognostic gene-gene network. For example, an interaction X → Y is defined when significantly more prognostic marker pairs from cell X to cell Y are observed than expected by chance (FDR < 0.05), indicating that a high X/Y ratio in the TIME is associated with a favorable prognosis.
Finally, by connecting all the cell-cell interactions, TimiGP builds a network at the cell level in which the edge represents the cell-cell interaction, for example, pointing from X to Y. Through topological analysis, the cell-cell network can be organized in a hierarchical manner: cells in the top layer have a higher number of outdegree and are more likely to be associated with favorable prognosis (favorable cell types), while cells in the bottom layer have a higher level of indegree and tend to be associated with an inferior prognosis (unfavorable cell types). Based on the in- and outdegrees of the cells, TimiGP calculates a favorability score for each cell, which prioritizes their prognostic association and anti-tumor potential in the TIME.
TimiGP illustrates clinically relevant TIME in metastatic melanoma
To demonstrate the capabilities of TimiGP, we applied it to metastatic melanoma cohorts, where, as mentioned, immune co-infiltration can cause prognostic bias. We started with IMGs of 22 cell types reported by Bindea et al.,38 of which the performance has been extensively validated.39,40,41,42,43,44 In addition, we kept markers of two special types: the cytotoxic cells denoted by common cytotoxic markers of, for example, anti-tumor CD8 T cells and NK cells, and the tumor cells, as evidence of immune evasion. Both unique cell types were set as positive and negative controls, respectively.
Among the 462 possible cell pairs, TimiGP identified 54 cell-cell interactions, including cytotoxic cells → mast cells, cytotoxic cells → neutrophils, cytotoxic cells → tumor cells, and cytotoxic cells → immature dendritic cells (iDCs) as the top ranked (Figure 3A; Table S3). These results suggest that cytotoxic cells are associated with a favorable prognosis, as expected, due to their anti-tumor functions. Similarly, the interactions that linked type 1 helper T cells (Th1s) to several other cell types were also prioritized to be associated with superior survival. It is notable that the interaction cytotoxic cells → tumor cells was identified as one of the most significant interactions, indicating the effectiveness of TimiGP.
Figure 3.
TimiGP infers immune cell-cell interaction network and prioritizes critical cell type affecting prognosis
(A) Dot plot of the top 10 cell-cell interactions ranked by FDR. All these interactions were also evaluated by permutation tests with resultant FDR less than 3%.
(B) Chord diagram of all cell-cell interactions. The arrow (X → Y) represents cell-cell interactions from favorable cell X (color of the outer ring and arrow) to unfavorable cell Y (color of the inner ring), indicating that a high X/Y ratio is associated with a favorable prognosis. The wider the arrow, the smaller the FDR.
(C) A hierarchical cell-cell interaction network based on degrees. The node represents the cell type, and its color shows the difference between outdegree and indegree. The edge represents cell-cell interaction, and its transparency shows the FDR.
(D) Bar plot of the favorability score to evaluate each cell type’s favorable (orange) or unfavorable (blue) role in anti-tumor immunity and prognosis.
All analyses have been performed with the TCGA_SKCM06 datasets using modified Bindea2013 markers. Abbreviation of cell types: T, T cell; Th, helper T cell; Th1, type 1 Th; Th2, type 2 Th; Tfh, follicular Th; Tcm, central memory T cell; Tem, effector memory T cell; cytotoxic, cytotoxic cell (common cytotoxic markers of anti-tumor CD8 T cells, Tγδ, and NK cells); NK, natural killer cell; DC, dendritic cell; iDC, immature DC; aDC, activated DC; Mast, mast cell; Tumor, tumor cell.
See also Table S3.
By connecting the 54 cell-cell interactions, TimiGP constructed a directed interaction network (Figure 3B). Importantly, the network exhibits interesting topology features: some nodes (cell types) have large outdegrees (leaving edges), while others have large indegrees (entering edges). As such, the interaction network can be reorganized into a hierarchical network (Figure 3C). The top layer (indegree = 0) is composed of activated dendritic cells (aDCs), Th1s, and CD56dim NK cells, together with the positive control (cytotoxic cells), which tended to be associated with favorable prognosis compared with other cell types. In contrast, the bottom layer (outdegree = 0) contains prognostically unfavorable cell types including mast cells and iDCs, as well as the negative control (tumor cells). The majority of cells have both leaving and entering edges (indegree > 0 and outdegree > 0) and thus were positioned in the middle layer of the hierarchical network.
To more precisely characterize the prognostic association of cells in the interaction network, especially those in the middle layer, TimiGP calculates a “favorability score” for each cell type by counting degrees. Since most immune cells have been reported to have dual roles associated with prognosis, the “favorability score” is a combination of both sides: (1) “favorable scores” indicate positive prognostic association and potential anti-tumor function, and (2) “unfavorable scores” indicate negative prognostic association and possible pro-tumor function. This combined “favorability score” prioritizes critical immune cell types that may affect the prognosis of patients with cancer, indicating their anti- or pro-tumor roles. As shown in Figure 3D, the result is consistent with network analysis and previous studies,3,45,46,47,48 with cytotoxic cells (positive control) demonstrating the highest favorable score and the tumor cells (negative control) showing the highest unfavorable score. Altogether, these results demonstrate the ability of TimiGP to effectively infer cell-cell interactions and the overall prognostic effects of different cell types.
TimiGP demonstrates robust performance when applied to different datasets or with different cell-type markers
To evaluate the robustness of TimiGP, we further applied it to seven additional metastatic melanoma datasets comprising both microarray and RNA-seq data49,50,51,52,53,54,55,56 (Table S1). As shown in Figure 4A, the cell-cell interaction networks exhibited significant similarity across different datasets in metastatic melanoma (p < 0.0002). TimiGP was able to identify the control interaction (cytotoxic cells → tumor cells) in all datasets (Figure 4B). As shown, the cytotoxic cell was classified as a favorable cell type, and the tumor was prioritized as an unfavorable cell type (Figures 4C and 4D). The consistent cell-cell interaction network and the correct identification of controls in all eight datasets demonstrate the robustness of TimiGP in identifying immune cell-cell interactions and their prognostic associations.
Figure 4.
TimiGP robustly infers immune cell-cell interactions and their favorability score on prognosis
(A–D) Application of TimiGP to eight metastatic melanoma datasets using modified Bindea2013 markers. Beyond TCGA_SKCM06 (dark gray), another 7 independent melanoma datasets were analyzed, including three metastatic melanoma datasets (red), two melanoma datasets for metastatic lymph nodes (blue), and two immunotherapy cohorts (anti-CTLA-4, purple; anti-PD-1, orange).
(A) The similarity of cell-cell interaction networks across eight metastatic melanoma datasets. For each pairwise comparison, p < 0.0002.
(B) The dot plot showing the enrichment results of cytotoxic-to-tumor interactions across different datasets.
(C and D) Bar graph of favorable scores of cytotoxic cells (C) and unfavorable scores of tumor cells (D) across different datasets.
(E and F) Application of TimiGP to TCGA_SKCM06 using 3 different cell-type marker annotations.
(E) Venn diagram showing the numbers and overlap of IMGs.
(F) Bar plot of the favorable score (top) and unfavorable score (bottom) of similar cell types identified.
The text label shows the rank of the cell favorability in each annotation set. The non-immune cell type, tumor cell, has been removed in Bindea2013 annotation before TimiGP analysis. Abbreviation of cell types: T, T cell; Th, helper T cell; Th1, type 1 Th; Th2, type 2 Th; Th17, type 17 Th; Tcm, central memory T cell; Tem, effector memory T cell; aCD4 T, activated CD4 T cell; aCD8 T, activated CD8 T cell; Cytotoxic, cytotoxic cell (common cytotoxic markers of anti-tumor CD8 T cells, Tγδ, and NK cells)38; NK, natural killer cell; DC, dendritic cell; iDC, immature DC; pDC, plasmacytoid DC.
Since TimiGP relies on cell-type markers, we next examined whether it remains robust using different cell-type annotations (Figure 4E). Compared with the cell-type markers used previously, signatures generated by Charoentong et al.57 include more mutually exclusive cell-subtype markers, while modified cell-type markers based on Xu et al.58 have only 47 genes used to represent 17 cell types. Though the cell classifications were distinct, TimiGP still identified CD8 T cells → neutrophils (Figure S3A) and Th1 → neutrophils (Figure S3B) from three analyses, which indicates the negative prognostic value of neutrophil-to-lymphocyte ratios, in line with recent studies.59,60,61,62,63 Furthermore, consistently estimated in three analyses, activated T cells and NK cells contributed to the favorable prognosis, whereas neutrophils were relatively unfavorable (Figure 4F).
Moreover, we also used LM22 cell-type markers defined in a CIBERSORT study22 for TimiGP to analyze the same cohort. Compared with CIBERSORT22 and CIBERSORTx16 (Figure 1A), TimiGP was able to identify the association of immune cells with clinical outcomes more consistent with their biological functions2,9,46 (Figures S3C and S3D). Collectively, TimiGP demonstrated stable performance when using different cell-type annotations to infer immune cell-cell interactions and functions associated with prognosis.
TimiGP facilitates the development of IMGP-based prognostic models with immunological insights
Immune-related gene pairs have been reported as emerging biomarkers to predict clinical outcomes.35,36,64 Traditional methods select signatures from prognostic marker pairs, while TimiGP yields the enriched IMGPs that not only provide clinical insights but also reflect the cell-cell interactions (Figure 5A), which is supposed to facilitate the model construction. Therefore, we next investigated the potential of TimiGP in the development of gene expression-based prognostic models.
Figure 5.
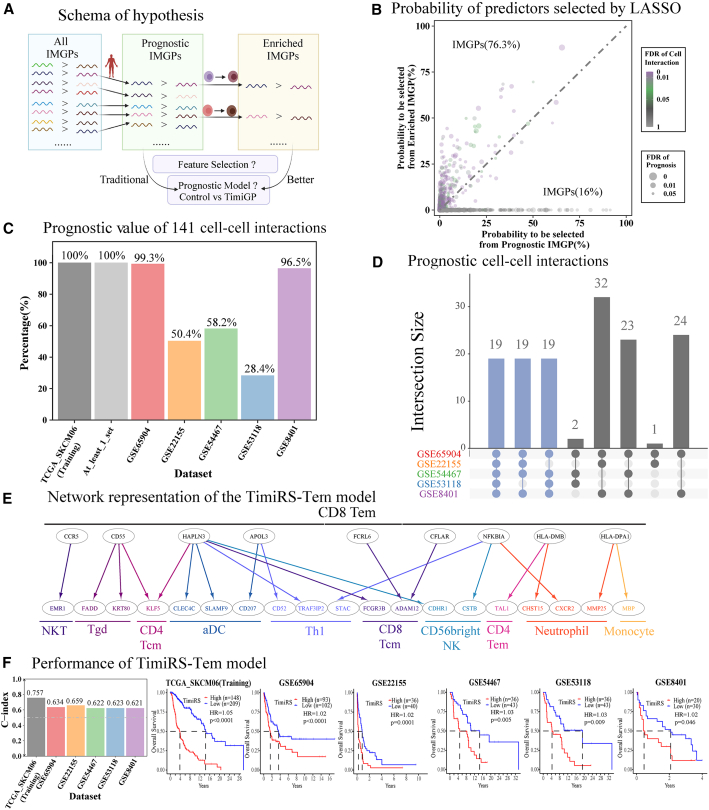
TimiGP assists with feature selection and IMGP-based prognostic model development
(A) Schema showing the rationale that TimiGP benefits feature selection with immunological insights and the hypothesis that it facilitates the development of prognostic models.
(B) Scatterplot of the probability of IMGPs selected from prognostic (x axis) and/or enriched candidates (y axis) by LASSO. The dot size shows the FDR calculated by survival analysis, and the color shows the FDR of potential cell-cell interactions annotated by this IMGP according to enrichment analysis. The text label shows the percentage of both selected IMGPs in this area of 544 candidates belonging to prognostic and enriched IMGPs simultaneously.
(C) Percentage of cell-cell interaction-based TimiRS models that demonstrated statistical significance (p < 0.05) in both training dataset (TCGA_SKCM06) and five independent validation datasets. “At_least_1_set” is the percentage of these models validated by at least one validation set.
(D) Upset plot showing the numbers and overlap of cell-cell interaction-based TimiRS models validated by five independent validation sets.
(E) A network representation of 21 pairwise features in the TimiRS-Tem model. Nodes represent IMGs. The color of the node label represents different immune cells. Edges describe pairwise relations. The edge from node x to node y denotes that a high x-to-y expression ratio is associated with better prognosis in patients whose color is the same as the color of the cell types at the lower layer.
(F) Bar plot of C index (left) and KM curves (right) showing the performance of the TimiRS-Tem model.
Abbreviation of cell types in Charoentong2017 annotation: Tem, effector memory T cell; Th1, type 1 helper T cell; Tgd, gamma delta T cell (Tγδ); Tcm; central memory T cells; aDC, activated dendritic cell; NK, natural killer cell; NKT, natural killer T cell.
See also Figure S4 and Table S4. Probability of IMGPs selected from enriched or prognostic candidates by LASSO, related to Figure 5, Table S5. Prognostic value of cell-cell interactions, related to Figure 5, Table S6. Model information about TimiRS-Tem and TimiRS-control model, related to Figure 5.
We compared the probability of an IMGP being selected from enriched pairs versus prognostic pairs using LASSO Cox regression65 (STAR Methods). This time, we still used the TCGA_SKCM06 cohort but with the cell-type markers summarized by Charoentong et al.,57 which includes mutually exclusive with more immune cell subtypes. As a result, there were 544 candidates taken from both of the input pools (Figure 5B; Table S4). Among these gene pairs, 76% IMGPs were more likely to be selected from enriched pairs than prognostic pairs to construct the model, suggesting that TimiGP benefits feature selection by filtering the correlated but noisy features.
In addition, the majority of candidate IMGPs denote high-confidence cell-cell interactions between immune cells (Figure 5B; Table S4). This suggests that these cell-cell interactions are potential signatures for prognostic models. To examine this, we designed TimiRS, the risk score calculated by the percentage of IMGP signatures with an MPS of 0, and inspected the prognostic value of each cell-cell interaction in one training set and five independent validation sets49,50,51,52,53 (STAR Methods). The analysis revealed all cell-cell interactions significantly associated with prognosis in at least one validation set (Figure 5C; Table S5), and 19 cell-cell interactions were able to stratify survival across all five independent datasets (Figure 5D). These results suggest that TimiGP uncovers cell-cell interactions of immune cells whose representative IMGPs show potential for both biological research and clinical applications.
Considering that single cell-cell interactions are insufficient to portray the TIME, we further integrated several interactions and developed an upgraded prognostic model. We noticed that the top prognostic interactions were related to effector memory CD8 T cells (CD8 Tems)-to-other cells, which supports the favorable role of CD8 Tems in anti-tumor immunity (Figure S4A). This is consistent with the characteristics of CD8 Tems, which undergo clonal expansion and exert anti-tumor function in the context of persistent antigen exposure.66,67 Therefore, we selected the top 21 IMGPs ranked by the LASSO selection probability to represent CD8 Tems → other cells interactions and constructed the TimiRS-Tem model (Figure 5E; Table S6; STAR Methods). As a control, 21 IMGP signatures were selected from prognostic pairs in the same way (Table S6). Compared with the control model (Figures S4B and S4C), TimiRS-Tem achieved a higher ability of patient stratification and concordance index (0.621–0.659) in five independent validation sets49,50,51,52,53 (Figure 5F). Its performance was consistently better than the control model when evaluated by the time-dependent receiver operating characteristic (ROC) curve, specifically the AUC (area under the curve) (Figures S4D and S4E). Taken together, these results suggest that TimiGP may facilitate the development of prognostic models by considering biological motivation and immunological dependency.
TimiGP elucidates the tumor microenvironment at different scales
Over the past decade, scRNA-seq has generated a large amount of cell-type annotations of different tissues at a granular level.68 scRNA-seq-derived cell-type signatures have obvious advantages over bulk RNA-seq for their high specificity and resolution.69 Therefore, we next sought to evaluate whether TimiGP was capable of dissecting the cell-cell interactions and functions at different scales by leveraging scRNA-seq-derived markers (Figure 6A).
Figure 6.
TimiGP can adjust the study resolution by utilizing cell-type markers from scRNA-seq
(A) A hierarchical schema showing tumor and microenvironment components. TimiGP was applied to metastatic melanoma (TCGA_SKCM06) to zoom out (green) and zoom in (blue) the tumor microenvironment.
(B and C) Chord diagram of cell-cell interaction network (B) and the favorability score of selected cell types (C) to illustrate the entire tumor microenvironment using the markers summarized by scRNA-seq analysis on metastatic melanoma.
(D and E) Chord diagram of cell-cell interaction network (D) and the favorability score of selected cell types (E) to illustrate the zoom-in T cell subpopulations using the markers summarized by scRNA-seq of pan-cancer T cell analysis. The color of cell types shows the top favorable (red) or unfavorable (blue) cell types, heterogeneous Texs (pink) and Tregs (green).
Abbreviation of cell types: CAF, cancer-associated fibroblast; Endo, endothelial cells; B, B cell; T, T cell; Tn, naive T cell; Th, helper T cell; Th1, type Th; Th17, type 17 Th; Tfh, follicular Th; Treg, regulatory T cell; Tex, exhausted T cell; Tc17, type 17 T cell; Tm, memory T cell; Tem, effector memory T cell; Trm, tissue-resident memory T cell; Temra, terminally differentiated effector memory or effector T cell.
We first applied TimiGP to dissect the entire tumor microenvironment of metastatic melanoma. In addition to immune cells, stromal cells are another critical component that shape the microenvironment.70 In 2016, Tirosh et al. applied scRNA-seq to 19 patients with metastatic melanoma and profiled tumor cells, immune cells, endothelial cells (Endos), and cancer-associated fibroblasts (CAFs).71 Using cell-type markers identified in that study, we profiled the cell-cell interactions between cancer cells and non-cancerous cells in the tumor microenvironment (Figure 6B). Compared with immune cells, cancer cells and stromal cells were associated with poor prognosis, consistent with the original scRNA-seq study71 (Figure 6C). This indicates that TimiGP can be utilized to study cell-cell interactions beyond cancer and immune cells.
Next, we tested the capability of TimiGP for high-resolution analysis. Using the T cell-subtype markers identified from scRNA-seq analysis,72 we repeated TimiGP in the same metastatic melanoma cohorts. Consequently, GZMK+ CD8 and CD4 Tems contributed to a favorable prognosis, consistent with the analysis described above (Figures 6D and 6E). In addition, IFNG+ follicular helper cells (Tfhs)/Th1s and ISG+ T cells, identified as potential tumor-reactive types in the original scRNA-seq analysis,72 were also identified as anti-tumor cell types more strongly associated with a favorable prognosis than other cells. Our results also highlighted the heterogeneity of exhausted T cells (Texs) and Tregs from the perspective of prognostic association. For example, the scRNA-seq study revealed that the CD8+ TCF7+ Tex population had lower expansion and proliferation indices and a higher expression of exhausted and inhibitory markers compared with CD8+ GZMK7+ Texs.72,73 TimiGP demonstrated the heterogeneity of CD8+ Tex subtypes based on cell-cell interactions and clinical functions, which further supported that the CD8+ GZMK7+ Tex was less favorable in prognosis than CD8+ TCF7+ Texs. In summary, TimiGP is capable of delineating the cell-cell interaction network within subpopulations and identifying their potential functions at a more granular level using scRNA-seq.
Taken together, these results suggest that TimiGP is a versatile method for analyzing both detailed cell subpopulations and broader tumor microenvironments. By leveraging high-resolution cell-subtyping markers derived from scRNA-seq, TimiGP makes it possible to depict the TIME with high resolution using low-resolution bulk sequencing data.
TimiGP shows high potential in pan-cancer analysis
Finally, we applied TimiGP to 23 solid cancer types to showcase the generalization of TimiGP in studying the TIME (Table S7). As before, we set cytotoxic cells as the positive control and the tumor cells as the negative control. Although cell-cell interactions vary across those cancer types, the impact of cytotoxic cells → other cells or other cells → tumor cells interactions tends to remain consistent (Figures S5A and S5B). Furthermore, the prognostic association of some immune cells demonstrated similar patterns across most cancer types (Figure 7). For example, anti-tumor immune cells, such as B cells and cytotoxic cells, were more favorable regarding prognosis than tumor cells and pro-tumor immune cells, such as neutrophils and Th2s across many cancer types.
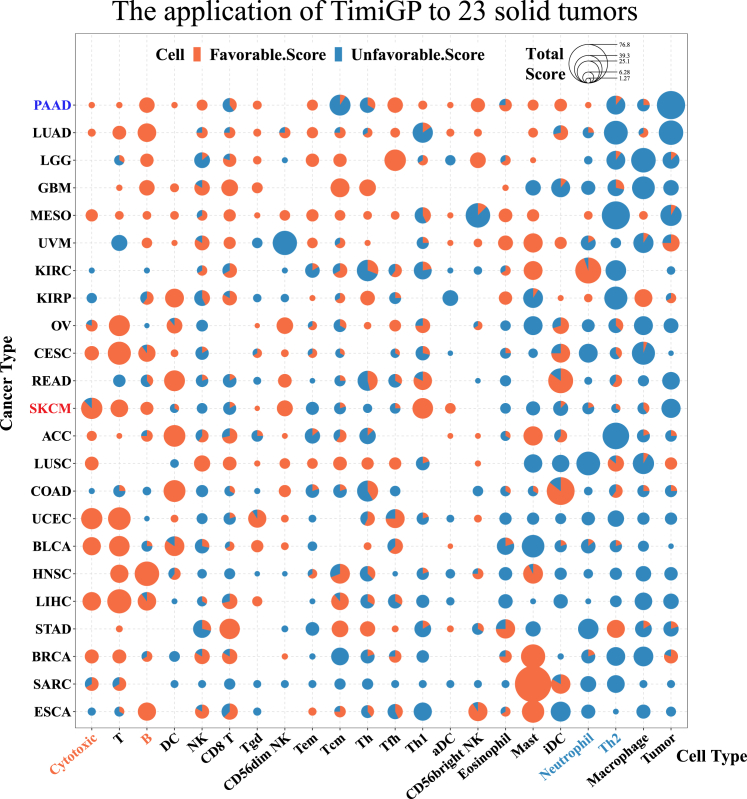
Figure 7.
TimiGP reveals the prognostic association of immune cells in 23 solid tumors
Scatterpie chart showing TimiGP favorability score of cell types (x axis) defined by Bindea et al. across 23 TCGA cancer types (y axis). Each dot is a tiny pie chart showing the percentage of favorable (orange) or unfavorable (blue) scores. The area of the dot represents the sum of both scores. Abbreviation of cell types: B, B cell; T, T cell; Th, helper T cell; Th1, type 1 Th; Th2, type 2 Th; Tfh, follicular Th; Tcm, central memory T cell; Tem, effector memory T cell; Tgd, gamma delta T cell (Tγδ); Cytotoxic, cytotoxic cell (common cytotoxic features of anti-tumor CD8 T cells, Tγδ, and NK cells); NK, natural killer cell; DC, dendritic cell; iDC, immature DC; aDC, activated DC; Mast, mast cell; Tumor, tumor cell. Abbreviation of cancer types: ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower-grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; UCEC, uterine corpus endometrial carcinoma; UVM, uveal melanoma.
Moreover, TimiGP also revealed the heterogeneity of prognostic association of immune cells between distinct cancer types, which was consistent with the previous studies2,7,9 (Figure 7). Interestingly, pancreatic cancer, a typically immune “cold” tumor with low immune infiltration,74,75 could pass our filter on immune marker expressions and be amenable for TimiGP analysis (Figures S5C and S5D). As expected, immune cold pancreatic cancer exhibited distinct cell-cell interaction networks compared with immune hot melanoma. Of note, although the interaction of cytotoxic cells → tumor cells, the preset controls, was identified in pancreatic cancer, cytotoxic cells themselves were not prioritized as a predominant favorable cell type for anti-tumor immunity and prognosis. Instead, our analysis indicated that B cells, reported as salient features of pancreatic cancer,76,77,78 might play a more important role in anti-cancer immunity in this cancer type, highlighting the variability in immune responses against different cancer types. Altogether, these results demonstrate that TimiGP is a promising method to analyze TIME interactions and the heterogeneity of prognostic association of immune cells across different cancers.
Discussion
In this study, we develop TimiGP as a new method for decoding cell-cell interactions and the prognostic associations of immune cells from gene expression data. Instead of using individual immune cell infiltration levels inferred from previous deconvolution methods,15,16,17,18,19,20,21,22,23,24,25 TimiGP starts with pairwise relations at the expression level and estimates prognostic association of immune cells through network analysis, which avoids the prognostic bias probably caused by immune cell co-infiltration. It should also be noted that the cell-cell interactions identified by TimiGP are statistical interactions, indicating that the relative abundance of the two “interacted” cells are associated with good prognosis, referring to the dynamic balance of the immune system1,79 (Figure S6A). These interaction are different from the physical ligand-receptor-based interactions described in Armingol et al.28 Despite this, we found that interactions derived by TimiGP could also be evidenced by the traditional methods. For example, scSeqComm80 identified cell-cell interactions between macrophages and Endos, between macrophages and CAFs, and between T cells and macrophages from the scRNA-seq data.71 TimiGP also identified similar interactions from the bulk RNA-seq using the cell-type markers selected from the same scRNA-seq study71 (Figure 6B). Although the definition of interaction and the data type between the two methods were different, this indicates that TimiGP interactions may also be a clue of some ligand-receptor communications, which are not limited within the existing ligand-receptor knowledge.
TimiGP also paves the path to define comprehensive cell atlases (Figure S6B). Since it compares the paired gene expression in individual tumor samples, it does not require complicated normalization, making it applicable for integrative analysis with the transcriptomic profiles obtained from different specimens (e.g., fresh, frozen, and formalin-fixed paraffin-embedded [FFPE]) and sequencing platforms (e.g., RNA-seq, microarray). Furthermore, TimiGP is capable of the comprehensive assessment of the tumor microenvironment at different scales. It only depends on reliable cell-type markers and does not require complex training on gene signature expression references. This allows it to directly utilize cell-type markers obtained from scRNA-seq analysis and hence be flexible to achieve a higher resolution of cell subpopulations or a broader scale of the tumor microenvironment, such as the entire microenvironment, the TIME, and T cell subtypes as shown in our studies.
Compared with predictors based on individual gene expressions, gene pair-based prognostic models have better normalizing robustness, predicting accuracy, and translational potential.81,82,83,84 While recent studies have revealed the advantage of immune-related gene pairs in developing predictive signatures,35,64 it remains statistically and computationally challenging to identify the best combination of gene pair signatures due to the quadratically combinatorial complexity. As we have demonstrated, TimiGP can be used to build effective prognostic models by focusing on marker gene pairs associated with cell-cell interactions. Since TimiGP provides interpretable IMGP signatures by evaluating immune contexture, the corresponding prognostic model, TimiRS, can be validated across multiple independent datasets without complex model and training/testing/tuning processes. This suggests the necessity of understanding the TIME to develop prognostic models instead of just relying on computational modeling. In this study, we developed a simple but effective prognostic model, TimiRS-Tem, based on CD8 Tems to showcase how TimiGP efficiently facilitates the development and endows high performance and interpretability to the prognostic model. Such TimiGP-based models are conducive to summarizing why and how predictors work in a certain cohort for further investigation, which will overcome another limitation of machine learning methods and expedite personalized therapy in cancer treatment. Altogether, TimiGP will offer substantial potential in clinical practice following further optimization.
The cellular context may have a profound impact on cell-cell interactions.28 Although our main focus was melanoma in this study, using immune cell-type marker gene sets that are not cancer-type specific, TimiGP is able to examine and compare the immune landscape across different cancer types. Indeed, our results indicate that many immune cells, such as B cells and Th2s, exhibit similar patterns regarding prognostic associations across different cancer types (Figure 7). On the other hand, we also observed notable heterogeneity in the prognostic association of immune cells. For instance, the TimiGP analysis revealed a positive association of mast cells with kidney renal clear cell carcinoma (KIRC) and head and neck squamous cell carcinoma (HNSC) but a negative association with bladder urothelial carcinoma (BLCA) and stomach adenocarcinoma (STAD). These results are consistent with a prior study that summarized the prognostic value of immune cells in over 70,000 patients from nearly 3,000 studies.9 In addition, we identified distinct cell-cell interaction networks between immune hot melanoma and immune cold pancreatic cancer. These results highlight the important impact of cellular context on cell-cell interactions.
In summary, TimiGP represents a broadly applicable framework to dissect the cell-cell interactions and prognostic associations of infiltrating cells. This strategy can be used to investigate the clinically relevant tumor microenvironment at different scales and enables high-resolution analysis from low-resolution bulk profiles with the help of scRNA-seq markers. The framework could be extended to other diseases for which expression profiles and clinical statistics are available. It systematically determines the associations between cellular context and clinical outcomes through network analysis. Informed by such insights, TimiGP could therefore have utility for biological discovery and prediction. Given the method’s versatility (Figure S6B), we anticipate that TimiGP will be a useful asset for the practice of personalized medicine.
Limitations of the study
Despite the great promise we have shown, this study has three important limitations. First, to effectively identify prognostic gene pairs, TimiGP requires a large gene expression dataset with matched survival information. The power of the survival analysis is determined by the sample size, the number of events, and the quality of the survival information. For data with relatively poor-quality survival, we suggest using relaxed statistical thresholds to identify prognostic gene pairs or, alternatively, choosing the top-ranked gene pairs. Second, since the cell-cell interactions are identified based on enrichment analysis, the sensitivity to identify a specific interaction is affected by the number of corresponding marker genes. Cell types with fewer marker genes are less likely to be identified as having significant interactions. Fortunately, the wide application of scRNA-seq analysis in cancer immunology has generated high-quality marker gene sets for most immune cell types. Finally, no wet-lab experiments were performed to validate the important findings. Although rigorous computational analyses and statistical tests were employed to strengthen the findings, the lack of experimental validation raises some degree of uncertainty about the reliability of the inference of cell-cell interaction. Future research should consider exploring appropriate strategies for experimental validation while accounting for the complexities and uncertainties associated with the system under investigation. Experimental validation of TimpGP results can be achieved by two approaches: (1) the generation of cellular composition data (e.g., from scRNA-seq or immune mass cytometry analysis) from a cohort of decent size with high-quality survival information and (2) the use of the functional assay to test the effect of immune cell ratios on cancer development and progression.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| TimiGP | This study | https://github.com/CSkylarL/TimiGP |
| Cytoscape (v3.9.0) | Shannon et al. (2003)85 | https://cytoscape.org/ |
| R (v4.1.0) | R CRAN | https://cran.r-project.org/ |
| dplyr R package (v1.0.7) | Wickham et al. (2021)86 | https://cran.r-project.org/web/packages/dplyr/index.html |
| reshape R package (v0.8.8) | Wickham (2007)87 | https://cran.r-project.org/web/packages/reshape/index.html |
| tidyr R package (v1.1.3) | Wickham88 | https://cran.r-project.org/web/packages/tidyr/index.html |
| stringr R package (v1.4.0) | Wickham (2019)89 | https://cran.r-project.org/web/packages/stringr/index.html |
| data.table R package (v1.14.0) | Dowle et al. (2019)90 | https://cran.r-project.org/web/packages/data.table/index.html |
| roxygen2 R package (v7.1.1) | Wickham et al.91 | https://cran.r-project.org/web/packages/roxygen2/index.html |
| devtools R package (v2.4.2) | Wickham et al. (2021)92 | https://cran.r-project.org/web/packages/devtools/index.html |
| doParallel R package (v1.0.16) | Analytics and Weston (2014)93 | https://cran.r-project.org/web/packages/doParallel/index.html |
| foreach R package (v1.5.1) | Wallig and Weston (2020)94 | https://cran.r-project.org/web/packages/foreach/index.html |
| survival R package (v3.2-11) | Therneau and Grambsch (2000)95 | https://cran.r-project.org/web/packages/survival/index.html |
| survivalROC R package (v1.0.3) | Heagerty et al. (2013)96 | https://cran.r-project.org/web/packages/survivalROC/index.html |
| glmnet R package (v4.1-2) | Friedman et al. (2010)97; Simon et al. (2011)65 | https://cran.r-project.org/web/packages/glmnet/index.html |
| scales R package (v1.1.1) | Wickham and Seidel (2020)98 | https://cran.r-project.org/web/packages/scales/index.html |
| ggplot2 R package (v3.3.5) | Wickham (2016)99 | https://cran.r-project.org/web/packages/ggplot2/index.html |
| survminer R package (v0.4.9) | Kassambara et al. (2021)100 | https://cran.r-project.org/web/packages/survminer/index.html |
| scatterpie R package (v0.1.6) | Guangchuang (2021)101 | https://cran.r-project.org/web/packages/scatterpie/index.html |
| VennDiagram R package (v1.6.20) | Chen (2018)102 | https://cran.r-project.org/web/packages/VennDiagram/index.html |
| gridExtra R package (v2.3) | Auguie and Antonov (2017)103 | https://cran.r-project.org/web/packages/gridExtra/index.html |
| ggrepel R package (v0.9.1) | Slowikowski et al. (2021)104 | https://cran.r-project.org/web/packages/ggrepel/index.html |
| ComplexHeatmap R package (v2.10.0) | Gu et al. (2016)105 | https://www.bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html |
| RColorBrewer R package (v1.1-2) | Neuwirth and Neuwirth (2014)106 | https://cran.r-project.org/web/packages/RColorBrewer/index.html |
| circlize R package (v0.4.13) | Gu et al. (2014)107 | https://cran.r-project.org/web/packages/circlize/index.html |
| immunedeconv | Sturm et al. (2019)25 | https://github.com/omnideconv/immunedeconv |
| MCP-counter | Becht et al. (2016)17 | https://github.com/ebecht/MCPcounter |
| TIMER | Li et al. (2016)18; Li et al. (2017)19; Li et al. (2020)20 | http://timer.cistrome.org/ |
| EcoTyper | Luca et al. (2021)21 | https://ecotyper.stanford.edu/carcinoma/ |
| CIBERSORTx | Newman et al. (2019)16 | https://cibersortx.stanford.edu/ |
| CIBERSORT | Newman et al. (2015)22 | http://cibersort.stanford.edu/ |
| EPIC | Racle et al. (2017)24 | https://github.com/GfellerLab/EPIC |
| xCELL | Aran et al. (2017)15 | https://github.com/dviraran/xCell |
| quanTIseq | Finotello et al. (2019)23 | https://github.com/icbi-lab/quanTIseq |
| Deposited data | ||
| Bulk RNA-seq of The Cancer Genome Atlas | Weinstein et al. (2013)108 | https://gdac.broadinstitute.org/ |
| Microarray of Metastatic melanoma validation data | Cirenajwis et al. (2015)49; Jönsson et al. (2010)51; Jayawardana et al. (2015)50; Mann et al. (2013)52; Xu et al. (2008)53 | GEO: GSE65904; GSE22155; GSE54467; GSE53118; GSE8401 |
| Bulk RNA-seq of metastatic melanoma validation data | Van Allen et al. (2015)54; Liu et al. (2019)55; Lee et al. (2021)56 |
Original data: phs000452.v2.p1; phs000452.v3.p1 Preprocessed data: https://zenodo.org/record/4540874 |
| Other | ||
| Cell-Type Markers: Bindea2013 | Bindea et al. (2013)38 | https://doi.org/10.1016/j.immuni.2013.10.003 |
| Cell-Type Markers: Charoentong2017 | Charoentong et al. (2017)57 | https://doi.org/10.1016/j.celrep.2016.12.019 |
| Cell-Type Marker: Xu2018 | Xu et al. (2018)58 | https://doi.org/10.1158/0008-5472.can-18-0689 |
| Cell-Type Marker: Newman2015(LM22) | Newman et al. (2015)22 | https://doi.org/10.1038/nmeth.3337 |
| Cell-Type Marker: Tirosh2016(metastatic melanoma scRNA-seq) | Tirosh et al. (2016)71 | https://doi.org/10.1126/science.aad0501 |
| Cell-Type Marker: Zheng2021(pan-cancer T cell scRNA-seq) | Zheng et al. (2021)72 | https://doi.org/10.1126/science.abe6474 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Chao Cheng (chao.cheng@bcm.edu).
Materials availability
This study did not generate new unique reagents.
Method details
Data collection and preprocessing
The Cancer Genome Atlas (TCGA)108 RNA-seq dataset for 23 solid tumors (ACC, BRCA, COAD, GBM, KIRC, LGG, LUAD, MESO, PAAD, SARC, STAD, UVM, BLCA, CESC, ESCA, HNSC, KIRP, LIHC, LUSC, OV, READ, SKCM, UCEC) was downloaded from Firehose (https://gdac.broadinstitute.org/). This dataset consisted of RSEM-normalized gene expression data for 20,501 genes. Of the SKCM samples, 368 are metastatic tumors (TCGA_SKCM06),109 among which, 357 samples have survival statistics for TimiGP analysis and prognostic model training. In addition, five independent microarray datasets, including GSE6590449 (N = 195), GSE2215551 (N = 76), GSE5446750 (N = 79), GSE5311852 (N = 79), GSE840153 (N = 50), were downloaded from Gene Expression Omnibus (GEO) at the probe set level and then transferred to the gene level. For genes with multiple probe sets, we selected the probe sets with the highest average hybridization intensity to represent the corresponding genes. Only metastatic melanoma samples were included to evaluate the robustness of the TimiGP method and to validate prognostic models. Besides, two preprocessed RNA-seq datasets of metastatic melanoma patients who received anti-CTLA-4 (phs000452.v2.p1, N = 37) or anti-PD-1 therapy (phs000452.v3.p1, N = 121) were acquired via ZENODO repository (https://zenodo.org/record/4540874)56 and used for additional evaluation of TimiGP method. The clinical characteristics of the patients from these metastatic melanoma datasets were summarized in Table S1, and the survival statistics of TCGA pan-cancer were shown in Table S7.
Immune infiltration estimation and prognostic value evaluation
The immune infiltration estimated by MCP-counter, TIMER, CIBERSORT, EPIC, xCELL, quanTIseq was downloaded from TIMER2.0 Website15,17,18,19,20,22,23,24,25 (http://timer.cistrome.org/infiltration_estimation_for_tcga.csv.gz). The results of TCGA_SKCM06 cohorts were selected for this study. Of note, “Absolute Score” was used as the result of CIBERSORT to ensure the result is comparable across samples.
As for CIBERSORTx and EcoTyper, the input was the TPM normalized expression of TCGA_SKCM06 cohorts. According to the instructions with CIBERSORTx, we chose the LM22 signature matrix, disabled batch correction, absolute run mode, and 100 permutations. As for the cell states estimation, we ran EcoTyper with default parameters. Those inferred cell/state infiltrations were used for survival analysis, in which the HR and p-value were calculated using the Cox regression model that fitted infiltration of each cell type as a continuous variable. (Figure 1A, Table S1A). The co-infiltration between these cells were evaluated by Pearson correlation and hierarchical clustering (Figure S1).
IMG’s prognostic value evaluation
The immune marker genes (IMGs) were collected from several studies38,57,58 and supplemented by well-known immune-related regulatory genes. Their prognostic values were analyzed by the univariate Cox regression that fitted expression of each gene as a continuous variable (Figure 2B, Table S1B). The FDR is calculated by adjusting the p values from the Cox regression using the Benjamini-Hochberg method. The prognostic IMGs (FDR <0.05) were further evaluated by Pearson correlation and hierarchical clustering to demonstrate the co-expression patterns (Figure 1C). The co-expression pattern of all collected IMGs and the cell-type markers used in MCP-counter, TIMER, CIBERSORT(x), EPIC, xCELL, quanTIseq are analyzed in the same way, respectively (Figure S1B).
TimiGP framework
Conceptualized from the dynamic balance of immune systems (Figure S6A), TimiGP is designed to infer cell-cell interactions and prognostic association of immune cells based on transcriptomic profiles of bulk tissues. Therefore, the method’s inputs are immune cell-type markers, gene expression profiles, and patient survival statistics. TimiGP performs the following steps, graphically depicted in Figure 2.
Step 1: Define and select marker gene pair matrix
Under the hypothesis that the expression ratios between IMGs capture relative abundances of immune cell infiltration and depict critical immune interactions, TimiGP defines marker pair score (MPS) by comparing the expression between IMGPs. After logrithm transformation and gene-wise median normalization, it defines a binary variable for a pair of selected genes using the following equation:
| (Equation 1) |
where and represent the expression of genes x and y in sample S.
By applying this function to all possible pairs of marker genes in each sample, TimiGP generates an MPS matrix. The non-informative IMGPs with constant values (0 or 1) in more than 90% of samples are filtered out.
In addition to the default binary representation described above, TimiGP provides an option that uses the expression difference between IMGPs as a continuous variable:
| (Equation 2) |
where and represent the log-transformed expression of genes x and y in sample S. The non-informative IMGPs with a value less than 0.1 are filtered out.
The default binary mode represents a more conservative analysis and results in a list of high-confident cell-cell interactions, which is used in this study. Compared to it, the continuous option is more sensitive to identifying prognostic gene pairs and cell-cell interactions.
Step 2: choose gene pairs associated with favorable prognosis
Based on MPS, TimiGP utilizes univariate Cox regression to select prognostic marker pairs. Each gene pair can be represented as x > y or x < y, with opposite prognostic associations (HR > 1 vs. HR < 1) in their MPS. To remove the duplicates, we only consider the x > y positively associated with prognosis (HR < 1). Therefore, the IMGPs with “HR < 1, FDR <0.05” are selected as prognostic IMGP associated with a favorable prognosis. The FDR is calculated by adjusting the p values from the Cox regression using the Benjamini-Hochberg method.
Step 3: Construct the directed gene-gene network
Based on the prognostic importance of IMGPs, TimiGP makes pairwise connections between every favorable and unfavorable gene (nodes) and constructs a directed gene-gene network. The directionality of the edges reflects the relationship between the expression levels of the genes and their impact on patient prognosis. For example, the edge from x to y (x→y) indicates that higher relative expression of gene x versus gene y is associated with improved survival.
Step 4: Identify cell-cell interactions by enrichment analysis
To transform the marker gene-gene network to the cell level, TimiGP performs enrichment analysis to identify cell-cell interactions that are overrepresented in the network compared to what would be expected by chance. The resultant cell-cell interaction provides insights into the prognostic effect of relative abundance between cells in the TIME. An immunological assumption behind this step is that the balance between pro- and anti-tumor cells may determine effective or suppressive TIME and subsequently impact prognosis (Figure S6A).
In detail, first, for a directed cell pair X →Y, TimiGP enumerates all possible marker gene pairs to form a set of cross-cell marker gene pairs. Assuming cell X and Y have LX and LY markers respectively, the set was defined as:
| (Equation 3) |
Next, TimiGP performs the over-representation analysis to statistically examine whether the marker gene pairs for a specific cell pair X→Y is more prevalent in the gene-gene network than expected by chance. Specifically, it calculates the enrichment ratio and p value based on hypergeometric distribution110:
| (Equation 4) |
| (Equation 5) |
Where N is the total number of all annotated IMGPs (including all cell types) including both directions, such as gene Xi→Yj and Yj→Xi, M is the number of IMGPs for a specific cell-cell interaction such as X→Y, n is the total number of prognostic IMGPs, and k is the number of prognostic IMGPs for X→Y as presented the gene-gene network.
If the IMGPs of a cell pair S(X→Y) are significantly enriched in the prognostic gene-gene network (FDR <0.05), X→Y is identified as a significant cell-cell interaction, suggesting that the relative abundance between X and Y is associated with prognosis: the higher X-to-Y ratio, the longer patient survival. In the interaction, Cell X tends to be an anti-tumor cell associated with a favorable prognosis, while cell Y is likely to be a pro-tumor cell type associated with an inferior outcome.
In addition, TimiGP has included a permutation-based procedure to control FDR. To this end, the marker gene sets for all cell types were shuffled while preserving their original sizes, and based on them the p values for all cell pairs were recalculated. After N permutations, the FDR of the kth most significant interaction (with a p value of ) based on the original markers was calculated as follows:
| (Equation 6) |
Step 5: Build and analyze the cell-cell interaction network
By connecting all significant interactions, TimiGP constructs a network consisting of directed cell-cell interactions associated with favorable prognosis. The network provides a complete portrait of the TIME. In the network, each node represents a specific cell type and an edge indicates that the high relative abundance between the two cell types in TIME is associated with favorable prognosis. Based on the in- and out-degrees of nodes, the directed network can be reorganized into a hierarchical structure with three layers: the top layer includes all cell types without entering edges (in-degree = 0), the bottom layer includes all cell types without leaving edges (out-degree = 0), and the middle layer includes all the other cell types with both leaving and entering edges (in-degree>0 and out-degree>0). Furthermore, TimiGP calculated a favorability score, which was composed of two values (a favorable score and an unfavorable score) as follows:
| (Equation 7) |
In the equation, Out(X) and In(X) represent the number of out-degree and in-degree for node cell X, respectively. The favorability score evaluates the relative role of cell X among all k cell types in the network. A higher favorable score indicates that the corresponding cell type is generally associated with good prognosis and mainly functions as anti-tumor cells in the TIME. In contrast, a higher unfavorable score indicates a negative prognostic association and pro-tumor roles.
Flexibility of TimiGP framework
TimiGP is a flexible framework that can adapt to different scenarios and allow for a broader range of applications (Figure S6B). Starting from gene pairs in the individual sample, TimiGP could be utilized for different sequencing platforms (Figure 4A). Taking advantage of different biomarker references derived from bulk and single-cell RNA-seq, TimiGP can be applied to investigate the tumor microenvironment at different scales (Figures 4E and 4F and 6). All sets of cell-type markers used in this study were integrated into the TimiGP R package. With data in different cancers or diseases, the TimiGP framework is applicable to pan-cancer analysis (Figure 7).
Evaluate the robustness of TimiGP
To evaluate the robustness, we applied TimiGP to seven additional metastatic melanoma datasets49,50,51,52,53,54,55 (Table S1). Due to the variation in sample sizes and survival data qualify, we applied a different threshold to identify prognostic marker gene pairs (Step 2): FDR <0.05 for GSE65904 and GSE8401; p-value <0.01 for GSE22155; p-value <0.05 for GSE54467, GSE53118, phs000452.v2.p1, phs000452.v3.p1. For each dataset, we constructed an cell-cell interaction using the TimiGP algorithm. The consistency between these networks (e.g., between A and B) was examined by calculating their Tversky index:
| (Equation 8) |
In the equation, | is the number of shared interactions (e.g., ) between network A and B, and the is the number of interactions in the smaller network (i.e., the largest number of interactions that can be shared by A and B). The index takes a value within [0,1] with 0 and 1 indicating the worst and the best consistency, respectively.
The statistical significance of the similarity is evaluated based on the hypergeometric distribution:
| (Equation 9) |
Where N is the total number of potential cell-cell interactions annotated by given cell-type markers.
Feature selection for prognostic model
Feature selection is a critical step in biomarker expression-based models. Since we aimed to demonstrate the application of TimiGP in this area rather than develop a clinical prognostic model, we referred to the current gene pair-based model35 and utilized regularized model with the L1 penalty (least absolute shrinkage and selection operator, LASSO) to select features(glmnet R package v4.1-2).65,97 The penalty parameter was estimated by 10-fold cross-validation and measured by Harrel C-index. We chose “lambda.1se” as the value of λ. To evaluate the robustness of the selected IRGPs, feature selection was repeated 1000 times in 80% randomized TCGA_SKCM06 cohorts. Since the cell-type markers summarized by Charoentong, P. et al.57 have the largest number of mutually exclusive genes in this study, we chose their expression profiles as the input for further analysis(Figure 5). To prove TimiGP can benefit feature selection, we compared the probability of the same IMGP selected from prognostic and enriched pairs by LASSO. Notably, prognostic pairs were obtained from the second step of TimiGP as control, which was used by current methods, and enriched pairs were from the fourth step, representing the cell-cell interactions (Figure 2). The probability that marker pair x→y is selected as a predictor will be calculated below (Figure 5B):
| (Equation 10) |
To examine if TimiGP can facilitate the prognostic model development, we next selected the top 21 IMGPs following the selection probability for prognostic models. The number of IMGPs was determined by 10-fold cross-validation according to C-index in the TCGA_SKCM06 training set. The signature for the control model was picked from prognostic pairs, and the signature for the Tem model was selected from enriched pairs that represent Effector memory CD8 T cell (CD8 Tem)-to-other cells interactions with strong confidence (FDR <0.0001).
TimiRS model construction and validation
Since the gene pair represents the intuitive notion that its high relative abundance is associated with a good prognosis, the risk score (TimiRS), on the contrary, is calculated by the percentage of predictors whose MPS are 0.
| (Equation 11) |
We considered TCGA_SKCM06 as a training set because the signature was selected based on the cohorts and validated the models in five independent datasets (GSE65904,49 GSE22155,51 GSE54467,50 GSE53118,52 GSE840153). Among 141 cell-cell interactions identified by TimiGP, each interaction has a list of supportive enriched pairs. They were applied as individual models to calculate TimiRS in training and five validation sets. The prognostic model of these signatures was evaluated by Cox regression which fits TimiRS as a continuous variable (p-Value <0.05, Figures 4C and 4D).
As for TimiRS-control and TimiRS-Tem models, we used the signatures selected from prognostic pairs (control) or enriched pairs related to CD8 Tem →other cells interactions (Tem) to calculate TimiRS in training and five independent datasets. The performance of each model was evaluated by the C-index, the ability of patient stratification (Kaplan-Meier estimate using the median as a cutoff), and time-dependent receiver operating characteristic (ROC).
Pan-cancer TimiGP analysis
Considering the quality of survival statistics, we selected 23 solid tumors (ACC, BRCA, COAD, GBM, KIRC, LGG, LUAD, MESO, PAAD, SARC, STAD, UVM, BLCA, CESC, ESCA, HNSC, KIRP, LIHC, LUSC, OV, READ, SKCM, UCEC) from TCGA for pan-cancer analysis108 (Table S7). All samples with the survival information were used to perform TimiGP analysis per cancer type. To be consistent across 23 tumor types, we selected the top 4352 prognostic IMGPs following p-value in step 2 for each cancer type. The number was determined by the cancer type with the least number of prognostic IMGPs (p-value <0.05). We visualized the TimiGP results by scatterpie R package (v0.1.6) (Figure 7). We also selected the top 10 cell-cell interactions related to Cytotoxic cell → Other cells or Other cells → Tumor cells interactions ranked by the number of cancer types from which the interactions were identified.
Quantification and statistical analysis
The major analysis and package development were based on R (v4.1.0) and R libraries (dplyr (v1.0.7),86 reshape (v0.8.8),87 tidyr (v1.1.3),88 stringr (v1.4.0),89 data.table (v1.14.0),90 roxygen2 (v7.1.1),91 devtools (v2.4.2),92 doParallel (v1.0.16),93 foreach (v1.5.1),94 scales (v1.1.1),98 ggplot2 (v3.3.5),99 scatterpie (v0.1.6),101 VennDiagram (v1.6.20),102 gridExtra (v2.3),103 ggrepel (v0.9.1),104 ComplexHeatmap (v2.10.0),105 RColorBrewer (v1.1-2),106 circlize (v0.4.13)107). The hierarchical network in Figure 3C was visualized by Cytoscape (v3.9.0)85 and structured by yFile with default parameters. If not mentioned in the specific method section, the cox regression and C-index were performed using survival R package (v3.2-11).95 Time-dependent ROC at 3 years and the corresponding AUC (Area under the ROC Curve) were calculated by survivalROC R package (v1.0.3).96 The Kaplan-Meier estimate was performed through survminer R package (v0.4.9).100
Additional resources
The TimiGP R package and updates are available via GitHub: https://github.com/CSkylarL/TimiGP. And all codes and intermediate results in this study are deposited in https://github.com/CSkylarL/MSofTimiGP.
Acknowledgments
This work was supported by National Cancer Institute of the National Institute of Health Research Project grants (R01CA234629–01 to J.Z. and R01CA269764 to C.C.); an AACR-Johnson & Johnson Lung Cancer Innovation Science grant (18-90-52-ZHAN to J.Z.); the MD Anderson Physician Scientist Program (J.Z.); the MD Anderson Lung Cancer Moon Shot Program (J.Z.); and the Cancer Prevention Research Institute of Texas (CPRIT) (RR180061 to C.C.). C.C. is a CPRIT Scholar in Cancer Research. The authors would like to acknowledge the support of the High Performance Computing for research facility at the University of Texas MD Anderson Cancer Center for providing computational resources that have contributed to the research results reported in this paper. The graphical abstract and Figures 1E, 2, 4A, and S6 were created with BioRender.com.
Author contributions
Conceptualization, C.L. and C.C.; methodology, C.L. and C.C.; software: C.L.; validation: C.L.; formal analysis, C.L.; investigation, C.L., B.Z., and E.S.; resources, C.C. and J.Z.; data curation, C.C. and C.L.; writing – original draft, C.L.; writing – review & editing, J.Z., C.C., A.R., L.W., M.J.T., B.Z., and E.S.; visualization, C.L.; supervision, J.Z. and C.C.; funding acquisition, J.Z. and C.C.
Declaration of interests
J.Z. reports grants from Merck, grants and personal fees from Johnson and Johnson and Novartis, and personal fees from Bristol Myers Squibb, AstraZeneca, GenePlus, Innovent, and Hengrui outside the submitted work.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: July 18, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101121.
Contributor Information
Jianjun Zhang, Email: jzhang20@mdanderson.org.
Chao Cheng, Email: chao.cheng@bcm.edu.
Supplemental information
This table includes (A) Prognostic Value of Immune cell infiltration estimated by existing methods; (B) Prognostic Value of Immune marker genes collected for this study.
Data and code availability
All bulk RNA-seq and microarray data and cell type markers are publicly available. Accession numbers for these datasets are listed in the key resources table. The original code for TimiGP is publicly available as of the publication date for non-profit academic use. The TimiGP package and updates are available via GitHub: https://github.com/CSkylarL/TimiGP. All codes in this study are deposited in GitHub: https://github.com/CSkylarL/MSofTimiGP. Any additional information required in this paper is available from the lead contact upon request.
References
- 1.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Galon J., Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Raskov H., Orhan A., Christensen J.P., Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 5.Togashi Y., Shitara K., Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 6.Veglia F., Sanseviero E., Gabrilovich D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021;21:485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridman W.H., Zitvogel L., Sautès-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 8.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni D., Angell H.K., Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer. 2020;20:662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 10.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 11.Wagner A., Regev A., Yosef N. Revealing the vectors of cellular identity with single-cell genomics. Nat. Biotechnol. 2016;34:1145–1160. doi: 10.1038/nbt.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lähnemann D., Köster J., Szczurek E., McCarthy D.J., Hicks S.C., Robinson M.D., Vallejos C.A., Campbell K.R., Beerenwinkel N., Mahfouz A. Eleven grand challenges in single-cell data science. Genome Biol. 2020;21:1–35. doi: 10.1186/s13059-020-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marco-Puche G., Lois S., Benítez J., Trivino J.C. RNA-Seq perspectives to improve clinical diagnosis. Front. Genet. 2019;10:1152. doi: 10.3389/fgene.2019.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuksin M., Morel D., Aglave M., Danlos F.-X., Marabelle A., Zinovyev A., Gautheret D., Verlingue L. Applications of single-cell and bulk RNA sequencing in onco-immunology. Eur. J. Cancer. 2021;149:193–210. doi: 10.1016/j.ejca.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Aran D., Hu Z., Butte A.J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18:220–314. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman A.M., Steen C.B., Liu C.L., Gentles A.J., Chaudhuri A.A., Scherer F., Khodadoust M.S., Esfahani M.S., Luca B.A., Steiner D., et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019;37:773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becht E., Giraldo N.A., Lacroix L., Buttard B., Elarouci N., Petitprez F., Selves J., Laurent-Puig P., Sautès-Fridman C., Fridman W.H., de Reyniès A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218–220. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B., Severson E., Pignon J.-C., Zhao H., Li T., Novak J., Jiang P., Shen H., Aster J.C., Rodig S., et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174–216. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T., Fan J., Wang B., Traugh N., Chen Q., Liu J.S., Li B., Liu X.S. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T., Fu J., Zeng Z., Cohen D., Li J., Chen Q., Li B., Liu X.S. TIMER2. 0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luca B.A., Steen C.B., Matusiak M., Azizi A., Varma S., Zhu C., Przybyl J., Espín-Pérez A., Diehn M., Alizadeh A.A., et al. Atlas of clinically distinct cell states and ecosystems across human solid tumors. Cell. 2021;184:5482–5496.e28. doi: 10.1016/j.cell.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finotello F., Mayer C., Plattner C., Laschober G., Rieder D., Hackl H., Krogsdam A., Loncova Z., Posch W., Wilflingseder D., et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019;11 doi: 10.1186/s13073-019-0638-6. 34-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racle J., de Jonge K., Baumgaertner P., Speiser D.E., Gfeller D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife. 2017;6 doi: 10.7554/eLife.26476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturm G., Finotello F., Petitprez F., Zhang J.D., Baumbach J., Fridman W.H., List M., Aneichyk T. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics. 2019;35:i436–i445. doi: 10.1093/bioinformatics/btz363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi H., Sheng J., Gao D., Li F., Durrans A., Ryu S., Lee S.B., Narula N., Rafii S., Elemento O., et al. Transcriptome analysis of individual stromal cell populations identifies stroma-tumor crosstalk in mouse lung cancer model. Cell Rep. 2015;10:1187–1201. doi: 10.1016/j.celrep.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Noël F., Massenet-Regad L., Carmi-Levy I., Cappuccio A., Grandclaudon M., Trichot C., Kieffer Y., Mechta-Grigoriou F., Soumelis V. Dissection of intercellular communication using the transcriptome-based framework ICELLNET. Nat. Commun. 2021;12:1–16. doi: 10.1038/s41467-021-21244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armingol E., Officer A., Harismendy O., Lewis N.E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet. 2021;22:71–88. doi: 10.1038/s41576-020-00292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varn F.S., Wang Y., Mullins D.W., Fiering S., Cheng C. Systematic Pan-Cancer Analysis Reveals Immune Cell Interactions in the Tumor MicroenvironmentPan-Cancer Analysis of Immune Cell Interactions. Cancer Res. 2017;77:1271–1282. doi: 10.1158/0008-5472.CAN-16-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluger H.M., Zito C.R., Barr M.L., Baine M.K., Chiang V.L.S., Sznol M., Rimm D.L., Chen L., Jilaveanu L.B. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin. Cancer Res. 2015;21:3052–3060. doi: 10.1158/1078-0432.CCR-14-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G., Zhu X., Liu C. Characterization of Immune Infiltration and Construction of a Prediction Model for Overall Survival in Melanoma Patients. Front. Oncol. 2021;11:639059. doi: 10.3389/fonc.2021.639059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou B., Lawrence T., Liang Y. The role of plasmacytoid dendritic cells in cancers. Front. Immunol. 2021;12:4414. doi: 10.3389/fimmu.2021.749190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhai L., Ladomersky E., Lenzen A., Nguyen B., Patel R., Lauing K.L., Wu M., Wainwright D.A. IDO1 in cancer: a Gemini of immune checkpoints. Cell. Mol. Immunol. 2018;15:447–457. doi: 10.1038/cmi.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18:155–214. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B., Cui Y., Diehn M., Li R. Development and validation of an individualized immune prognostic signature in early-stage nonsquamous non–small cell lung cancer. JAMA Oncol. 2017;3:1529–1537. doi: 10.1001/jamaoncol.2017.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auslander N., Zhang G., Lee J.S., Frederick D.T., Miao B., Moll T., Tian T., Wei Z., Madan S., Sullivan R.J., et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat. Med. 2018;24:1545–1549. doi: 10.1038/s41591-018-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgovanovic D., Song M., Wang L., Zhang Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020;8 doi: 10.1186/s40364-020-00228-x. 49-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bindea G., Mlecnik B., Tosolini M., Kirilovsky A., Waldner M., Obenauf A.C., Angell H., Fredriksen T., Lafontaine L., Berger A., et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Bagaev A., Kotlov N., Nomie K., Svekolkin V., Gafurov A., Isaeva O., Osokin N., Kozlov I., Frenkel F., Gancharova O., et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39:845–865.e7. doi: 10.1016/j.ccell.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y., Sun A., Zhao Y., Ying W., Sun H., Yang X., Xing B., Sun W., Ren L., Hu B., et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257–261. doi: 10.1038/s41586-019-0987-8. [DOI] [PubMed] [Google Scholar]
- 41.Tamborero D., Rubio-Perez C., Muiños F., Sabarinathan R., Piulats J.M., Muntasell A., Dienstmann R., Lopez-Bigas N., Gonzalez-Perez A. A pan-cancer landscape of interactions between solid tumors and infiltrating immune cell PopulationsTumor features associated to immunophenotypes. Clin. Cancer Res. 2018;24:3717–3728. doi: 10.1158/1078-0432.CCR-17-3509. [DOI] [PubMed] [Google Scholar]
- 42.Ghorani E., Reading J.L., Henry J.Y., Massy M.R.d., Rosenthal R., Turati V., Joshi K., Furness A.J.S., Ben Aissa A., Saini S.K., et al. The T cell differentiation landscape is shaped by tumour mutations in lung cancer. Nat. Can. (Que.) 2020;1:546–561. doi: 10.1038/s43018-020-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z., Li M., Jiang Z., Wang X. A comprehensive immunologic portrait of triple-negative breast cancer. Transl. Oncol. 2018;11:311–329. doi: 10.1016/j.tranon.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabrita R., Lauss M., Sanna A., Donia M., Skaarup Larsen M., Mitra S., Johansson I., Phung B., Harbst K., Vallon-Christersson J., et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 45.Dudek A.M., Martin S., Garg A.D., Agostinis P. Immature, semi-mature, and fully mature dendritic cells: toward a DC-cancer cells interface that augments anticancer immunity. Front. Immunol. 2013;4:438. doi: 10.3389/fimmu.2013.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy K., Weaver C. Garland science; 2016. Janeway's Immunobiology. [Google Scholar]
- 47.Komi D.E.A., Redegeld F.A. Role of mast cells in shaping the tumor microenvironment. Clin. Rev. Allergy Immunol. 2020;58:313–325. doi: 10.1007/s12016-019-08753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cózar B., Greppi M., Carpentier S., Narni-Mancinelli E., Chiossone L., Vivier E. Tumor-Infiltrating Natural Killer CellsTumor-infiltrating Natural Killer Cells. Cancer Discov. 2021;11:34–44. doi: 10.1158/2159-8290.CD-20-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cirenajwis H., Ekedahl H., Lauss M., Harbst K., Carneiro A., Enoksson J., Rosengren F., Werner-Hartman L., Törngren T., Kvist A., et al. Molecular stratification of metastatic melanoma using gene expression profiling: Prediction of survival outcome and benefit from molecular targeted therapy. Oncotarget. 2015;6:12297–12309. doi: 10.18632/oncotarget.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayawardana K., Schramm S.J., Haydu L., Thompson J.F., Scolyer R.A., Mann G.J., Müller S., Yang J.Y.H. Determination of prognosis in metastatic melanoma through integration of clinico-pathologic, mutation, mRNA, microRNA, and protein information. Int. J. Cancer. 2015;136:863–874. doi: 10.1002/ijc.29047. [DOI] [PubMed] [Google Scholar]
- 51.Jönsson G., Busch C., Knappskog S., Geisler J., Miletic H., Ringnér M., Lillehaug J.R., Borg Å., Lønning P.E. Gene expression profiling–based identification of molecular subtypes in stage IV melanomas with different clinical outcome. Clin. Cancer Res. 2010;16:3356–3367. doi: 10.1158/1078-0432.CCR-09-2509. [DOI] [PubMed] [Google Scholar]
- 52.Mann G.J., Pupo G.M., Campain A.E., Carter C.D., Schramm S.-J., Pianova S., Gerega S.K., De Silva C., Lai K., Wilmott J.S., et al. BRAF mutation, NRAS mutation, and the absence of an immune-related expressed gene profile predict poor outcome in patients with stage III melanoma. J. Invest. Dermatol. 2013;133:509–517. doi: 10.1038/jid.2012.283. [DOI] [PubMed] [Google Scholar]
- 53.Xu L., Shen S.S., Hoshida Y., Subramanian A., Ross K., Brunet J.-P., Wagner S.N., Ramaswamy S., Mesirov J.P., Hynes R.O. Gene expression changes in an animal melanoma model correlate with aggressiveness of human melanoma metastases. Mol. Cancer Res. 2008;6:760–769. doi: 10.1158/1541-7786.MCR-07-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Allen E.M., Miao D., Schilling B., Shukla S.A., Blank C., Zimmer L., Sucker A., Hillen U., Foppen M.H.G., Goldinger S.M., et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu D., Schilling B., Liu D., Sucker A., Livingstone E., Jerby-Arnon L., Zimmer L., Gutzmer R., Satzger I., Loquai C., et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med. 2019;25:1916–1927. doi: 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J.S., Nair N.U., Dinstag G., Chapman L., Chung Y., Wang K., Sinha S., Cha H., Kim D., Schperberg A.V., et al. Synthetic lethality-mediated precision oncology via the tumor transcriptome. Cell. 2021;184:2487–2502.e13. doi: 10.1016/j.cell.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charoentong P., Finotello F., Angelova M., Mayer C., Efremova M., Rieder D., Hackl H., Trajanoski Z. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Xu L., Deng C., Pang B., Zhang X., Liu W., Liao G., Yuan H., Cheng P., Li F., Long Z., et al. TIP: A Web Server for Resolving Tumor Immunophenotype ProfilingTIP: Tracking Tumor Immunophenotype. Cancer Res. 2018;78:6575–6580. doi: 10.1158/0008-5472.CAN-18-0689. [DOI] [PubMed] [Google Scholar]
- 59.O'Dwyer R.T., Dennehy C., Sui J.S.Y., Kelly C.M., Calvert P., McCaffrey J. American Society of Clinical Oncology; 2019. Neutrophil to Lymphocyte Ratio (NLR): A Prognostic Marker in Melanoma Patients Receiving Immunotherapy. [Google Scholar]
- 60.Cohen J.T., Miner T.J., Vezeridis M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020;7:MMT47. doi: 10.2217/mmt-2020-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartlett E.K., Flynn J.R., Panageas K.S., Ferraro R.A., Sta. Cruz J.M., Postow M.A., Coit D.G., Ariyan C.E. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer. 2020;126:76–85. doi: 10.1002/cncr.32506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capone M., Giannarelli D., Mallardo D., Madonna G., Festino L., Grimaldi A.M., Vanella V., Simeone E., Paone M., Palmieri G., et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer. 2018;6:74–77. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma J., Kuzman J., Ray A., Lawson B.O., Khong B., Xuan S., Hahn A.W., Khong H.T. Neutrophil-to-lymphocyte Ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci. Rep. 2018;8:4044–4046. doi: 10.1038/s41598-018-22425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang R.-z., Mao M., Zheng J., Liang H.-q., Liu F.-l., Zhou G.-y., Huang Y.-q., Zeng F.-y., Li X. Development of an immune-related gene pairs index for the prognosis analysis of metastatic melanoma. Sci. Rep. 2021;11:1253–1312. doi: 10.1038/s41598-020-80858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon N., Friedman J., Hastie T., Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J. Stat. Software. 2011;39:1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P., Côté A.L., de Vries V.C., Usherwood E.J., Turk M.J. Induction of postsurgical tumor immunity and T-cell memory by a poorly immunogenic tumor. Cancer Res. 2007;67:6468–6476. doi: 10.1158/0008-5472.CAN-07-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wistuba-Hamprecht K., Martens A., Heubach F., Romano E., Geukes Foppen M., Yuan J., Postow M., Wong P., Mallardo D., Schilling B., et al. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients. Eur. J. Cancer. 2017;73:61–70. doi: 10.1016/j.ejca.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svensson V., Vento-Tormo R., Teichmann S.A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 2018;13:599–604. doi: 10.1038/nprot.2017.149. [DOI] [PubMed] [Google Scholar]
- 69.Papalexi E., Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 2018;18:35–45. doi: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- 70.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 71.Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., Treacy D., Trombetta J.J., Rotem A., Rodman C., Lian C., Murphy G., et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng L., Qin S., Si W., Wang A., Xing B., Gao R., Ren X., Wang L., Wu X., Zhang J., et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. 2021;374:abe6474. doi: 10.1126/science.abe6474. [DOI] [PubMed] [Google Scholar]
- 73.Miller B.C., Sen D.R., Al Abosy R., Bi K., Virkud Y.V., LaFleur M.W., Yates K.B., Lako A., Felt K., Naik G.S., et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., Neoptolemos J.P. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.22. 16022-22. [DOI] [PubMed] [Google Scholar]
- 75.Hilmi M., Bartholin L., Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J. Gastroenterol. 2018;24:2137–2151. doi: 10.3748/wjg.v24.i20.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spear S., Candido J.B., McDermott J.R., Ghirelli C., Maniati E., Beers S.A., Balkwill F.R., Kocher H.M., Capasso M. Discrepancies in the tumor microenvironment of spontaneous and orthotopic murine models of pancreatic cancer uncover a new immunostimulatory phenotype for B cells. Front. Immunol. 2019;10:542. doi: 10.3389/fimmu.2019.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castino G.F., Cortese N., Capretti G., Serio S., Di Caro G., Mineri R., Magrini E., Grizzi F., Cappello P., Novelli F., et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tewari N., Zaitoun A.M., Arora A., Madhusudan S., Ilyas M., Lobo D.N. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer. 2013;13:436–510. doi: 10.1186/1471-2407-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cicchese J.M., Evans S., Hult C., Joslyn L.R., Wessler T., Millar J.A., Marino S., Cilfone N.A., Mattila J.T., Linderman J.J., Kirschner D.E. Dynamic balance of pro-and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018;285:147–167. doi: 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baruzzo G., Cesaro G., Di Camillo B. Identify, quantify and characterize cellular communication from single-cell RNA sequencing data with scSeqComm. Bioinformatics. 2022;38:1920–1929. doi: 10.1093/bioinformatics/btac036. [DOI] [PubMed] [Google Scholar]
- 81.Donald G., Christian d.A. Classifying gene expression profiles from pairwise mRNA comparisons. Stat. Appl. Genet. Mol. Biol. 2004;3:1–22. doi: 10.2202/1544-6115.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leek J.T. The tspair package for finding top scoring pair classifiers in. Bioinformatics. 2009;25:1203–1204. doi: 10.1093/bioinformatics/btp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patil P., Bachant-Winner P.-O., Haibe-Kains B., Leek J.T. Test set bias affects reproducibility of gene signatures. Bioinformatics. 2015;31:2318–2323. doi: 10.1093/bioinformatics/btv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen R., Luo L., Jiang H. Identification of gene pairs through penalized regression subject to constraints. BMC Bioinf. 2017;18:466–511. doi: 10.1186/s12859-017-1872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wickham H., Romain F., Lionel H. 2021. Kirill Müller (2020) Dplyr: A Grammar of Data Manipulation. R package version 0.8. 4. [Google Scholar]
- 87.Wickham H. Reshaping data with the reshape package. J. Stat. Software. 2007;21:1–20. [Google Scholar]
- 88.Wickham H.T. 2021. Tidy Messy Data: R Package Version 1.1. 3.https://CRAN. R-project. org/package= tidyr URL. [Google Scholar]
- 89.Wickham H. Stringr: Simple, consistent wrappers for common string operations. R package version. 2019;1:86–182. [Google Scholar]
- 90.Dowle M., Srinivasan A., Gorecki J., Chirico M., Stetsenko P., Short T., Lianoglou S., Antonyan E., Bonsch M., Parsonage H. 2019. Package ‘data. Table’. Extension of ‘data. Frame. [Google Scholar]
- 91.Wickham H., Danenberg P., Csárdi G., Eugster M. 2020. roxygen2: In-Line Documentation for R. R package version 7. [Google Scholar]
- 92.Wickham H., Hester J., Chang W., Hester M.J. 2021. Package ‘devtools’. [Google Scholar]
- 93.Analytics R., Weston S. doParallel: Foreach parallel adaptor for the parallel package. R package version. 2014;1:2014. [Google Scholar]
- 94.Wallig M., Weston S. 2020. Foreach: Provides Foreach Looping Construct. [Google Scholar]
- 95.Therneau T.M., Grambsch P.M. Modeling survival data: extending the Cox model. Springer; 2000. The cox model; pp. 39–77. [Google Scholar]
- 96.Heagerty P.J., Saha-Chaudhuri P., Saha-Chaudhuri M.P. GitHub; 2013. Package ‘survivalROC’. [Google Scholar]
- 97.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 98.Wickham H., Seidel D. 2020. scales: Scale Functions for Visualization. R package version 1.1. 1. [Google Scholar]
- 99.Wickham H. Vol. 10. Springer-Verlag New York; 2016. Package ‘ggplot2’: Elegant Graphics for Data Analysis. 978-970. [Google Scholar]
- 100.Kassambara A., Kosinski M., Biecek P., Fabian S. Vol. 2021. 2021. Survminer: Drawing Survival Curves Using Ggplot2.https://CRAN. R-project. org/package= survminer. R package version 0.4 9 URL. [Google Scholar]
- 101.Guangchuang Y. Vol. 7. 2021. Scatterpie: Scatter Pie Plot. R Package. version 0.1. [Google Scholar]
- 102.Chen H. 2018. VennDiagram: Generate High-Resolution Venn and Euler Plots.https://CRAN. R-project. org/package= VennDiagram R package version 1.6. 20. [Google Scholar]
- 103.Auguie B., Antonov A. 2017. gridExtra: Miscellaneous Functions for "Grid" Graphics. R Package Version 2.3. Computer Software]https://CRAN. R-project. org/package= gridExtra [Google Scholar]
- 104.Slowikowski K., Schep A., Hughes S., Dang K., Lukauskas S., Irisson J., Kamvar Z., Ryan T., Christophe D., Hiroaki Y. Automatically position non-overlapping text labels with ‘ggplot2’. R Package Version 0. 2021;9:1. [Google Scholar]
- 105.Gu Z., Eils R., Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 106.Neuwirth E., Neuwirth M.E. ColorBrewer Palettes; 2014. Package ‘RColorBrewer’. [Google Scholar]
- 107.Gu Z., Gu L., Eils R., Schlesner M., Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 108.Cancer Genome Atlas Research Network. Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R.M., Ozenberger B.A., Ellrott K., Shmulevich I., Sander C., Stuart J.M. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akbani R., Akdemir K., Aksoy B., Albert M., Ally A., Amin S., Arachchi H., Arora A., Auman J., Ayala B., et al. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boyle E.I., Weng S., Gollub J., Jin H., Botstein D., Cherry J.M., Sherlock G. GO:: TermFinder—open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table includes (A) Prognostic Value of Immune cell infiltration estimated by existing methods; (B) Prognostic Value of Immune marker genes collected for this study.
Data Availability Statement
All bulk RNA-seq and microarray data and cell type markers are publicly available. Accession numbers for these datasets are listed in the key resources table. The original code for TimiGP is publicly available as of the publication date for non-profit academic use. The TimiGP package and updates are available via GitHub: https://github.com/CSkylarL/TimiGP. All codes in this study are deposited in GitHub: https://github.com/CSkylarL/MSofTimiGP. Any additional information required in this paper is available from the lead contact upon request.