Abstract
The detection of nutrients in the gut influences ongoing and future feeding behavior as well as the development of food preferences. In addition to nutrient sensing in the intestine, the hepatic portal vein plays a considerable role in detecting ingested nutrients and conveying this information to brain nuclei involved in metabolism, learning, and reward. Here, we review mechanisms underlying hepatic portal vein sensing of nutrients, particularly glucose, and how this is relayed to the brain to influence feeding behavior and reward. We additionally highlight several gaps where future research can provide new insights into the effects of portal nutrients on neural activity in the brain and feeding behavior.
Keywords: Hepatic Portal Vein, Food Intake, Reward, Nutrient Sensing
Summary.
We review mechanisms underlying hepatic portal vein sensing of nutrients, particularly glucose, and its influence on feeding behavior. We additionally highlight gaps where future research can refine our knowledge of how portal nutrients modulate brain activity and food intake.
Food intake is governed by a complex set of biological mechanisms involving the body and brain.1, 2, 3 In particular, nutrient sensing in the gastrointestinal tract is fundamental to regulating energy homeostasis and feeding behavior.2 The body contains multiple organs with a wide variety of transporters and sensors that detect several nutrients, including but not limited to carbohydrates, proteins, and fats. The detection of glucose, the main source of energy for all cells in the body, is of particular metabolic importance.
Circulating blood glucose concentrations are maintained within a tight range, and major fluctuations can cause serious disturbances such as blurred vision, fatigue, and in extreme cases, loss of consciousness or death.4 To maintain glucose homeostasis, the body has specialized systems for glucose sensing.5, 6, 7 To deal with excess glucose, the body prepares for incoming nutrients with metabolic responses and mechanisms to curb additional food intake. Conversely, when energy is low, the body initiates counterregulatory processes to conserve and produce glucose. To this end, levels of glucose (and other nutrients) are sensed in the gastrointestinal tract and hepatic portal area and trigger the initiation of appropriate metabolic, digestive, or counterregulatory processes. In turn, the signals generated by peripheral nutrient detection are communicated to the brain to modulate current and future food intake.
After absorption from the gastrointestinal tract, nutrients enter the circulatory system through mesenteric capillary beds that flow into the hepatic portal vein (HPV). The HPV is exposed to the largest range of concentrations of glucose in the body.8, 9, 10 Therefore, the HPV may be the most important area in the body to sense glucose levels and trigger appropriate behavioral and physiological changes.
Here, we focus on glucose sensing within the HPV and how it influences food intake. After briefly summarizing the anatomy of the HPV, we review literature on the effects of HPV glucose sensing on food intake. We additionally discuss putative gut-brain mechanisms that relay glucose-related information to the brain to influence feeding behavior and reward processing. Last, we discuss current gaps in knowledge and future research directions that will lead to a better mechanistic understanding of both portal glucose sensing and the portal control of feeding behavior. We suggest that HPV glucose sensing, compared with intestinal glucose sensing, is often overlooked and should be revisited to gain a more complete understanding of the gut-brain control of feeding behavior.
Anatomy of the HPV
A portal system describes a venous system where blood passes through 2 capillary beds before returning to the heart. Accordingly, the hepatic portal system comprises a series of veins that carries blood from the capillary beds of the gastrointestinal tract and spleen to the capillary beds of the liver before being returned to the heart.11,12 The majority of ingested nutrients that are absorbed through the gastrointestinal tract pass through the HPV before being processed and filtered in the liver.11,12
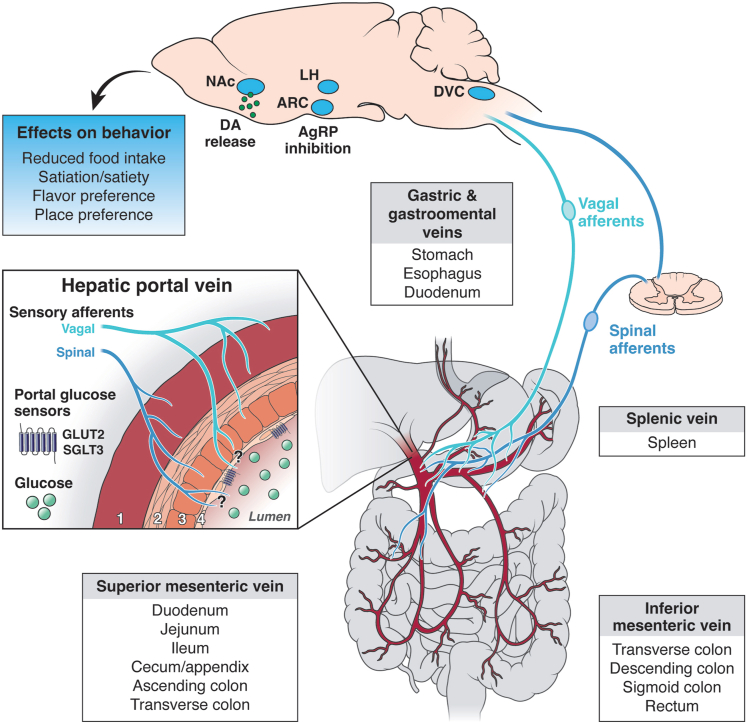
The HPV receives input from 3 major veins that carry blood from distinct parts of the gastrointestinal tract (Figure 1).12 Most proximal to the liver are the gastric and gastro-omental veins, which carry blood from the distal esophagus, stomach, and duodenum to the portal vein. Just ventral to this junction is the splenic vein, which carries blood from the spleen. The inferior mesenteric vein (which carries blood from the transverse, descending, and sigmoid colon, as well as the rectum) joins with the splenic vein just before the HPV junction. Last, the superior mesenteric vein carries blood from the duodenum, jejunum, ileum, cecum, and ascending and transverse colon. All of these veins converge to the HPV before it enters the liver.12
Figure 1.
HPV nutrient sensing and feeding behavior. Schematic depicting putative connectivity between the HPV, sensory afferents, and key feeding centers in the brain. The portal vein begins at the confluence of the superior mesenteric and splenic veins, carrying blood from the intestines and spleen. Vagal and spinal afferents that innervate the entire hepatic portal area relay this information to brain, likely to the dorsal vagal complex (DVC). Glucose infused in the portal vein modulates activity in this brainstem region in addition to feeding centers within the hypothalamus (such as the arcuate hypothalamic nucleus [ARC] and LH) and results in increased dopamine (DA) release in the striatum, including in the nucleus accumbens (NAc). Glucose detected in the portal vein ultimately decreases feeding behavior. The HPV box depicts the anatomy of the vein, which consists of 4 layers (from outer to inner): (1) tunica adventitia, a layer of connective tissues with collagen and elastic fibrils; (2 and 3) tunica media, which comprises a longitudinal smooth muscle layer (2) and a layer of circular smooth muscle (3); and (4) tunica interna, a thin layer of endothelial cells that line the lumen of the HPV. Sensory afferents innervate the outer 3 layers (1–3), and it remains unclear how far they project toward the lumen, and where glucose sensors (SGLT3 and GLUT2) are expressed. Hormonal and metabolic pathways (not depicted here) also contribute to energy balance control and likely interact with portal glucose sensing to influence feeding behavior.
Light and electron microscopy have uncovered the structural organization of the HPV, which is composed of 3 distinct layers (Figure 1, insert).13,14 The external layer (tunica adventitia) is a layer of connective tissue containing collagen and elastic fibrils. The middle layer (tunica media) comprises 2 distinct smooth muscle layers. The outer layer is composed of smooth muscle, which is aligned longitudinally and spans the proximal to distal parts of the vein. Below is a layer of circular smooth muscle which is aligned circumferentially about the vein. The inner layer (tunica interna) comprises a thin layer of endothelial cells that line the lumen of the HPV.
Sensory afferents, which convey HPV signals to the central nervous system, innervate the HPV throughout these layers (Figure 1, insert). The nature of this innervation and its influence in regulating food intake are described throughout this review. It is worth noting that the majority of experiments discussed in this review catheterize and infuse glucose in the HPV slightly above or below the junction of the superior mesenteric and portal veins.10 Thus, any feeding effects with HPV infusions can be attributed to nutrient sensing that occurs within or downstream of this region of the HPV, though it does not exclude the possibility that nutrient sensing occurs within smaller input veins as well.
Effects of HPV Glucose Sensing on Feeding Behavior
Russek15 was the first to show that infusions of glucose into the HPV in the dog cause termination of an ongoing meal. Subsequent studies have replicated this effect showing that portal glucose infusions decrease food intake across species, including rodents, rabbits, chickens, and dogs.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 This effect on feeding is localized to the portal vein and not the to jugular15,25,27,29 or caval20 veins, suggesting that the anorectic effects are triggered by local sensing within the HPV or liver, rather than sensing elsewhere in circulation or in the brain. While overwhelming evidence supports a role for HPV glucose sensing for inhibiting food intake, it is worth noting that there are other studies showing no effect, or even increases, in feeding following HPV glucose infusions.17,23,34, 35, 36, 37, 38, 39, 40, 41, 42
There are several experimental factors that may have led to contradictory results, including the energy state of the animal, infusion parameters, and food used in intake tests. First, in contrast to experiments where animals were fed ad libitum, experiments in which animals were food restricted overnight prior to portal infusion generally did not observe reductions in food intake.17,23,34, 35, 36,39,41,43 Long-term (eg, overnight) food restriction impacts mechanisms underlying motivation and metabolism, such as those underlying glycogen storage, that likely interact with portal nutrient sensing, perhaps explaining these results. In contrast, acute food restriction (3–4 hours before food intake tests) does not influence the intake suppression elicited by portal glucose infusions.32,33
Second, the parameters of the portal infusion (eg, rate and concentration) may have consequences on the experimental outcome. In the rat, glucose is absorbed from the gut at a constant rate of 3 mg/min per 100 g body weight for up to 2 hours after a meal.44 Additionally, portal vein blood flows at ∼10 mL/min and portal glucose levels are between 0.8–2.0 g/L after a meal.25 The experimental parameters that produce the most reliable portal feeding effects use infusion rates of approximately 0.1 mL/min (or less) and infuse over several hours before the feeding tests begin.25,27, 28, 29,32,33 These are likely the most consistent parameters, as they mimic physiological rates of glucose absorption. The timing of HPV infusion in relation to the feeding test is a large source of variability in the literature that we will return to when we discuss satiety/satiation subsequently.
Third, the quality of the test diet may impact the ability to observe food intake reductions following portal glucose infusion. Several studies failed to report decreases in food intake if the subjects’ maintenance diet was used as the test diet, and many (but not all) of those that reported decreases used a novel test diet. Therefore, it has been suggested that the use of novel foods and contexts may improve the ability to observe food intake changes following portal glucose infusions.25 Consistent with this, most failures to obtain effects of portal glucose infusions on food intake use within-subject designs, which often confound sensory and metabolic cues that can be used to learn about the nutrient value of the test food.
While these experimental conditions can influence whether HPV glucose infusions inhibit food intake, other considerations do not seem to affect experimental outcome. For example, testing in the light vs dark phase does not have an impact, nor does the consistency of the food (ie, solid vs liquid food).25,27,29
Does Portal Glucose Impact Satiety or Satiation?
Food intake can be reduced in several ways, and an examination of meal microstructure can provide insights into the behavioral function of HPV nutrient sensing. Two main processes exist to decrease feeding; those of satiation and satiety.45,46 Satiation is the perception of fullness during a meal that eventually terminates ongoing eating. On the other hand, satiety refers to feelings of fullness between meals which prevent future eating from occurring. Whether portal glucose sensing influences satiation or satiety is debated. Baird et al29 systematically tested temporal parameters for hepatic portal glucose–mediated decreases in feeding and found that portal glucose does not terminate an ongoing meal, but rather decreases the sizes of subsequent meals. This result was interpreted as an effect on satiety, as portal glucose infusion had longer-term, rather than immediate, effects on feeding behavior.
In contrast, other studies support the argument that portal nutrient sensing decreases the size of ongoing meals, implying an effect on satiation.31,47 It is worth noting that these studies involved remotely triggered portal infusions in rats consuming an ongoing meal, whereas Baird et al29 measured feeding responses with an intraoral glucose infusion. Although the experimental paradigm in Langhans et al31 was arguably more naturalistic, the results of HPV glucose infusions on meal size were inconsistent, as only 1 dose of glucose (1 mmol), but not lower or higher doses, elicited feeding effects.
Other studies supporting a role for HPV glucose sensing on satiation, or at least on short-term feeding behavior, examined lick microstructure.16,24,30 These studies gave short (1–5 minutes) portal glucose infusions to rats prior to lick spout access. After these brief portal infusions, licking behavior was initially decreased compared with saline infusion controls, but this was a transient effect, persisting for about 10 minutes.16,24 Baird et al30 gave a 2-hour portal glucose infusion prior to and during 90-minute feeding tests in which rats could lick for glucose. Similar to the aforementioned studies, licking was decreased relative to control animals in the first 10 minutes of access, but there were no differences between groups after 15 minutes.
Therefore, while most studies support the idea that HPV glucose sensing reduces feeding, the satiation or satiety mechanisms through which it does so are not fully understood. With recent technology enabling easy continuous and automated meal pattern monitoring, future studies could revisit these questions to systematically manipulate infusion parameters and examine effects on meal patterns throughout the entire light-dark cycle rather than for just a few meals.
Glucose Sensors in the HPV for Food Intake Control
Here, we describe putative glucose sensing mechanisms in the HPV for food intake control. Given the role of glucose transporter 2 (GLUT2) in pancreatic glucose sensing,48 GLUT2 was investigated as a potential HPV glucose sensor. GLUT2 knockout mice display disrupted glycemic responses to HPV infusions of high concentrations of glucose, and this is not rescued by GLUT2 re-expression in the liver.49 Interestingly, however, despite the necessity of GLUT2 for the hypoglycemic responses to portal glucose, GLUT2 is not responsible for the food intake–suppressive effects of portal glucose.33 Rather, sodium-dependent glucose transporter 3 (SGLT3) is a low-affinity glucose-activated ion channel that senses glucose50 in many tissues including the HPV51 and is likely involved in the anorectic effect of portal glucose. Unlike other SGLTs (eg, SGLT1), SGLT3 lacks the ability to transport glucose and is therefore considered a glucose sensor rather than a transporter.50,52 There are several lines of pharmacological evidence suggesting that SGLT3 is an HPV sensor that contributes to food intake control.33 First, α-methylglucopyranoside, a glucose analog and substrate for all SGLTs but not GLUTs, mimicked the effects of portal glucose on food intake. Second, phlorizin, a competitive inhibitor of SGLTs, blocked the effects of portal glucose on food intake. Third, portal infusion of 3-O-methyl-D-glucopyranose, which is transported by SGLT1 but does not bind SGLT3, had no effect on food intake. Together, these experiments implicate SGLT3 as an HPV glucose sensor for food intake control.33 Gaining direct in vivo evidence for HPV glucose sensing by SGLT3 should be an important area for future investigation. It also remains possible that other transporters or sensors are involved, though there is currently no literature suggesting a role for others in the HPV-mediated control of food intake.
Are HPV Infusions of Glucose Rewarding?
Food preference is controlled in part by the perceived nutrient value of the food.53 Energy-dense foods send a positive message to the brain, reinforcing the intake of the particular food. Does HPV glucose sensing contribute to this “food reward,” and if so, does it therefore promote learning about nutrient content? Is it involved in the processes that integrate sensory signals, such as flavor and taste, with nutrient value to further reinforce eating of calorically dense or glucose-rich foods?
Tordoff and Friedman25 were the first to demonstrate that portal glucose infusions are not aversive, but in fact are able to condition flavor-nutrient preferences (ie, flavor preferences that result from pairing nonnutritive flavor cues with postingestive nutrients). In this study, rats were given standard laboratory chow that was flavored with either nonnutritive chocolate or chicken flavors. During conditioning sessions, one flavor of chow was presented during glucose infusions and the other flavor of chow was presented during saline infusions into the portal vein. When later given a choice between the 2 flavored chows, rats selected the flavor that was paired with HPV glucose infusion. Importantly, the rats had no previous experience with the flavored chow; hence, new learning about the postingestive consequences was more likely to occur.
In contrast, Ackroff et al54 did not find that HPV infusions of glucose supported flavor nutrient learning. Here, HPV infusions of glucose paired with a noncaloric flavor did not yield a preference. One methodological difference between these 2 studies is that Tordoff and Friedman25 used flavor cues that contained calories, whereas Ackroff et al54 used flavor cues without calories. This suggests that both pre- and postabsorptive mechanisms are necessary for portal glucose to support flavor-nutrient learning.
While these studies have opposing conclusions regarding a role for portal glucose sensing in flavor-nutrient learning, other accumulating evidence suggests that portal glucose is reinforcing and activates neural mechanisms that promote learning. Portal glucose infusions stimulate dopamine release in the nucleus accumbens shell,55,56 which is involved in tracking reinforcer value, representing reward-guided motivation, and associating contextual elements with rewarding stimuli.57, 58, 59, 60 The fact that glucose detected in the HPV increases dopamine in the nucleus accumbens shell argues that it is reinforcing and may help the animal link the nutrient value of the glucose with sensory stimuli such as taste, flavor, and context. Consistently, glucose (but not mannitol) infusions in the HPV condition a place preference in rats,55 demonstrating the ability of HPV glucose to support learning about contextual cues, in addition to flavor/taste cues. While portal glucose is likely rewarding, its power to reinforce behavior has yet to be fully characterized. Future studies could examine the role of portal glucose in supporting reward-guided learning, such as operant responding for portal glucose infusion or serving as an unconditioned stimulus in Pavlovian conditioning.
Taken together, these studies demonstrate that glucose has the ability to reduce food intake, condition nutrient preferences, and influence dopamine signaling, depending on experimental conditions. However, when considering the role of HPV glucose sensing in the development of food preferences, and food intake more generally, it is important to consider that the HPV is just one part of a complex system for the control of feeding behavior.61 Therefore, despite the aforementioned examples of how HPV glucose sensing influences ingestive behaviors, there are many other metabolic signals originating in the gut (eg, gastric distension, nutrient sensing in small intestine, release of hormonal signals [such as leptin, ghrelin, cholecystokinin (CCK), etc.]) that communicate with the brain and contribute to feeding behavior.2,3,9,62
HPV-to-Brain Pathways for Food Intake Control
How are signals from HPV glucose sensors transmitted to the brain to exert effects on food intake? The HPV is innervated by both vagal63,64 and spinal65 afferents. Accordingly, it is thought that glucose sensors in the HPV likely signal via the vagus nerve66,67 or through spinal afferents68 to the brain and ultimately manifest physiological or behavioral effects.
In particular, afferent nerve innervation of the HPV is necessary for the anorectic feeding effect of portal glucose infusions. Chemical or mechanical ablation of afferent nerve fibers in the HPV abolishes the reductions in food intake by portal glucose.32,33 However, common hepatic branch vagotomy does not abolish the anorectic effect of portal glucose.33 Interestingly, celiac-superior mesenteric ganglionectomy, which ablates spinal afferent neurons innervating the gut and HPV, attenuates the satiating effect of intestinal nutrients.69 It is possible that part of this effect reflects downstream HPV nutrient sensing, rather than direct intestinal nutrient sensing. Therefore, the food intake inhibition by HPV glucose may be mediated by spinal afferent signaling, though to our knowledge, there is no published evidence that directly tests this.
In line with these behavioral findings, the transmission of HPV glucose signals to the brain appears to be dependent on spinal, but not vagal, afferents.70 Spinal afferents, whose cell bodies lie in the dorsal root ganglion (DRG), synapse in the dorsal horn of the spinal cord. In turn, second-order spinal neurons send projections to the brain, including to feeding-relevant regions such as the dorsal vagal complex (DVC) and parabrachial nucleus (PBN).65,71,72 Each of these areas projects to hypothalamic feeding centers including, but not limited to, the arcuate hypothalamic nucleus, lateral hypothalamus (LH), and ventromedial hypothalamus, among others.73 Spinal transection at T5 or splanchnic nerve ablation prevents neuronal firing in the LH in response to HPV glucose infusion.70 In contrast, signals traveling through the vagus nerve synapse only with brainstem neurons in the DVC.63,66,74 Vagotomy does not abolish, but rather increases, LH firing, suggesting that the vagus nerve is not required for, but may play a modulatory role in, communicating HPV glucose signals to the brain.70 A recent study applied a transsynaptic virus to the HPV and revealed labeling within the DVC, most notably within the dorsal motor nucleus of the vagus (DMX) with only sparse labeling in the nucleus of the solitary tract (NTS).75 These data suggest that the DVC is a primary target of HPV-innervating spinal and vagal afferents.
Recently, Goldstein et al76 examined the effects of portal glucose signaling on activity in hypothalamic, hunger-sensitive agouti-related protein (AgRP)–expressing neurons in the arcuate hypothalamic nucleus, whose activity is decreased upon consumption of food. They found that direct HPV infusion of glucose reduces AgRP neuron activity, and that this neural response requires spinal but not vagal signaling.76 Additionally, slow onset hypoglycemia is relayed via spinal (and not vagal) afferents, and these signals are communicated to feeding centers such as the DVC and VMH.68,77 These studies use celiac-superior mesenteric ganglionectomy as a strategy to ablate spinal afferent neurons; however, this manipulation also affects sympathetic and potentially vagal efferent activity.78 While these studies highlight a likely role for HPV-spinal afferent-brain signaling on neural activity, in the future, more specific gain- and loss-of-function manipulations of spinal afferent neurons will clarify the role of spinal gut-brain signaling on feeding behavior.
In addition to the aforementioned experimental caveats, the HPV glucose infusions in experiments examining neural activity were much shorter in duration and higher in glucose concentrations than what has been used to demonstrate the HPV feeding effects. Thus, while these studies provide useful information on the gut-brain pathways and central circuits through which portal glucose may influence feeding behavior, interpretations are limited until future studies can control for the parameters of glucose infusion across behavioral and physiological readouts. With in vivo imaging techniques becoming more standard in the field of gut-brain signaling,79 it will be important to use these platforms to determine how HPV glucose impacts activity in feeding circuits beyond AgRP neurons. Further, combining these studies with manipulations of vagal or spinal afferent neurons will enable a more complete understanding of the gut-brain connectivity that connects HPV sensing to food intake control.
Toward a Vein-to-Brain Pathway for HPV Glucose Sensing
While the anorectic effect induced by portal glucose has been attributed to portal SGLT3, the mechanistic details of this signaling—from portal glucose sensing to signaling within the brain—remain elusive. SGLT3 (and other potential glucose sensors) may be present on cells within the HPV or on sensory afferents innervating the portal vein. Perhaps due to antibody issues, visualization of SGLT3 via immunohistochemistry has not been successful,33 and the cell types in which this glucose sensor is expressed in the HPV are currently unknown.
SLGT3 may be localized to cells that make contact with the HPV lumen, or they may be on the terminal endings of sensory afferents innervating various layers of the portal vein wall. The nature of vagal and spinal innervation across the defined layers of the HPV remains a current topic of investigation. There is ample evidence demonstrating that sensory fibers course across and possibly terminate within the tunica adventitia layer of the HPV. Tracing studies using DiI injected in to the nodose ganglia find fine varicose endings terminating in the tunica adventitia.63,65 Similarly, CGRP (a marker for sensory afferents) is found in the tunica adventitia,65 and also in the smooth and longitudinal muscle layers, and sparsely in the tunica interna.80 Garcia-Luna et al75 examined the depth of sensory innervation of the HPV by systematically performing injections of a transsynaptic anterograde tracer on the surface, within the muscular wall, and in the lumen of the HPV. Injection of virus into the muscular layers resulted in the most robust and consistent labeling of neurons in the brain compared with surface injections, whereas injections into the lumen did not yield any expression (though this could be explained by blood flow preventing the virus from binding to epithelial cells). Therefore, afferents terminate in several anatomical levels of the HPV, likely most densely in the tunica adventitia and media. It is still unknown how far sensory afferents protrude toward the lumen of the HPV.
Despite increasing sets of single-cell sequencing data for vagal and spinal afferent neurons, it remains unclear whether sensory afferents within the HPV directly sense glucose. Neurons in the DRG and nodose ganglion express only very low levels of SGLTs or GLUT2.81,82 Therefore, instead of directly sensing glucose, it is also possible that vagal and spinal afferents in the HPV communicate with cells that line the lumen of the HPV.33,83 Indeed, this could be similar to the reported synapses between vagal afferents and intestinal cells for glucose sensing.84, 85, 86 The HPV cell types in which SGLT3 is expressed are unknown. In fact, more generally, to our knowledge the HPV cell types and the RNAs and proteins that they express have not been systematically profiled. Applying sequencing approaches to profile HPV cell types would fill a major gap in our knowledge and likely help inform mechanisms for nutrient sensing. Furthermore, while there are several brain regions that receive information about portal glucose sensing, the necessity of these regions (and the cell types within) for controlling feeding behavior remain to be tested.
HPV Sensing of Other Nutrients on Feeding Behavior
While it is well documented that the HPV sodium modulates salt (NaCl) intake,87, 88, 89 sensing mechanisms for caloric nutrients besides glucose have received less attention for their role in ingestive behavior. However, it is likely that the HPV plays a role in sensing protein, fat, and some metabolic hormones. We briefly discuss this literature here.
Amino Acids/Proteins
Infusion of amino acids into the portal vein strongly suppresses ongoing food intake.20 More recent studies suggest that the HPV senses protein to indirectly inhibit feeding behavior. Indeed, a series of studies by Mithieux et al32 revealed that the digestion of protein begins the process of intestinal gluconeogenesis, which is subsequently sensed in the HPV and reduces feeding behavior.90,91 Here, the intestinal digestion of proteins releases oligopeptides in the HPV, which bind mu opioid receptors lining the portal vein wall.90 Mu opioid receptors in the HPV are necessary for protein-induced gluconeogenesis.90 This endogenously produced glucose is sensed by the HPV8,92,93 to decrease food intake32,90,94 and neural activation in hypothalamic brain nuclei.32 We also note that dietary fiber and gastric bypass surgery are known triggers of intestinal gluconeogenesis.91,95,96 The food intake-suppressive and other beneficial metabolic effects of intestinal gluconeogenesis are nicely summarized in a recent review.91
Fat
Ingested fat is broken down in the intestine into fatty acids, some of which are absorbed into portal circulation to decrease food intake.97 Portal infusions of both short- and long-chain fatty acids substantially decrease the size of the first meal and subsequent 24-hour food intake in rats.98,99 Using similar paradigms to those described previously, infusions of fatty acids decrease food intake of the subsequent meal by about 50% (compared with about 30% for glucose). There is differential potency of certain fatty acids to decrease food intake, which may reflect differential vagal afferent detection of distinct fatty acids.100 It is worth noting that a large proportion long-chain fatty acids are absorbed via the lymphatic system,101 and it remains unclear whether a meaningful amount enters portal circulation under physiological conditions.
Little is known about the effects of portal lipid sensing on brain activity. Goldstein et al76 infused intralipid (an emulsion of phospholipids and triglycerides that provides essential fatty acids) into the HPV. They found that, in contrast to glucose infusion, intralipids had no effect on activity of hypothalamic AgRP neurons. However, the effects of HPV fatty acid infusion on AgRP neuron activity, as well as activity in other feeding centers in the brain, remains unknown. Furthermore, additional work is needed to determine the role of vagal and spinal afferents in the HPV sensing of fatty acids and how this information is relayed to central feeding regions to influence behavior.
Finally, we argue for caution in interpreting the results from studies that infused fat into the HPV, as this has not yet been demonstrated to condition food preferences. It is possible that such decreases in food intake may be a result of nonspecific malaise pathways rather than of specific feeding mechanisms.102, 103, 104
Gut Peptides
In addition to protein and fat, the HPV can sense gut peptides that influence feeding behavior. Glucagon-like peptide-1 (GLP-1) is produced by enteroendocrine L cells and is secreted into the portal vein after a meal. The receptor for GLP-1 is expressed in the portal vein105,106 and GLP-1 infusion into the portal vein stimulates vagal afferent nerve activity.83,107 Furthermore, infusion of GLP-1 into the portal vein decreases the size of an ongoing but not future meal in rats, independent of the vagus nerve.47 In addition to GLP-1, several other gut peptides have been studied, though less extensively. HPV infusions of CCK,108 glucagon,41 and a combination of insulin and glucose (but not insulin alone)31 have been shown to decrease subsequent feeding behavior. Last, there is also evidence that other metabolites produced from the metabolism of nutrients in the intestine (eg, pyruvate, L-lactate, and glycerol, to name a few) are sensed via the vagus nerve in the hepatic portal area to decrease satiety and food intake.109,110
The GLP-1 receptor is expressed at levels at least 3 times higher than SGLTs in nodose ganglion and DRG neurons81,82 and has been visualized on nerve terminals within the HPV.83 Vagal afferents that terminate in the HPV are also activated by serotonin or CCK.111 Therefore, we speculate that GLP-1 receptor signaling on sensory afferents, and perhaps other endocrine mechanisms similar to those within the well-studied intestinal-vagal-brain axis, also exists for HPV nutrient sensing. These mechanisms, as well as their potential relevance to food intake control, will be revealed through future studies.
Conclusions
Though there is extensive evidence that glucose (and other nutrients) act via the HPV to exert widespread effects on feeding and metabolism, our knowledge of the HPV sensors and gut-brain mechanisms that mediate these effects is in its infancy. As a great deal of this work was performed years if not decades ago, it will be important to revisit these studies with modern techniques to better understand (1) the intricacies of the HPV glucose effects on feeding, (2) the sensors and locations of said sensors that mediate these effects, (3) the neural connections that transmit these signals to the brain, and (4) the in vivo activity dynamics in feeding circuits that are triggered by HPV glucose to impact subsequent food intake.
Several major conceptual questions also remain regarding the effects of HPV glucose on feeding behavior. First, how much of the nutrient sensing occurs in the HPV vs the liver? Studies that denervate the HPV have suggested that direct HPV glucose sensing is physiologically relevant to food intake control32,33 but have not ruled out a role for the liver in nutrient sensing. Manipulating glycogen or adenosine triphosphate levels within the liver influences food intake,112, 113, 114, 115, 116 and therefore it has been speculated that there are sensors for these signaling molecules within the liver. Tissue-specific knockouts, selective afferent manipulations, and in vivo activity monitoring of sensory neurons will be helpful to precisely determine the contributions of sensing in the HPV as opposed to downstream metabolic effects from the liver.
Relatedly, how much does HPV nutrient sensing account for the postingestive signaling effects on feeding behavior? In other words, what are the relative contributions of food and nutrient sensing within the stomach, small intestine, HPV, liver, etc., on food intake? Furthermore, how can we separate the effects of HPV sensing from other metabolic and hormonal signals that are released upon food ingestion? These questions are difficult to answer, as one cannot perform a simple “loss-of-function” study with the HPV. Again, advances in tissue-specific gene knockout strategies, more specific afferent ablation techniques, and genetic models to manipulate and record activity in specific cell types will help us get closer to this answer.
Finally, because it appears that HPV-detected glucose can condition flavor preferences, what is the interplay between HPV glucose sensing and food sensory cues? Where do these 2 streams of information converge in the brain to influence learning about associations between sensory and nutritive food components? There have been major strides in understanding flavor-nutrient preferences,117,118 with a focus on enteroendocrine cells119 and the vagus nerve.120,121 However, considerably less is known about how nutrient sensing by the HPV fits into this puzzle. Further research in these areas will add to what we know about the development of food preferences, satiation, and satiety, and potentially inform treatments for obesity and metabolic disease.122
Acknowledgments
CRediT Authorship Contributions
Sam Z. Bacharach: Conceptualization, Writing - original draft, Writing - Review and editing, Visualization.
Michael G. Tordoff: Writing - Review & editing.
Amber L. Alhadeff: Writing - Review & editing, Visualization.
Footnotes
Conflicts of Interest The authors disclose no conflicts.
References
- 1.Andermann M.L., Lowell B.B. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95:757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemmensen C., Müller T.D., Woods S.C., et al. Gut-brain cross-talk in metabolic control. Cell. 2017;168:758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shechter A., Schwartz G.J. Gut-brain nutrient sensing in food reward. Appetite. 2018;122:32–35. doi: 10.1016/j.appet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasserman D.H. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296:E11–E21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley S., Moheet A., Seaquist E.R. Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr Rev. 2019;40:768–788. doi: 10.1210/er.2018-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verberne A.J.M., Sabetghadam A., Korim W.S. Neural pathways that control the glucose counterregulatory response. Front Neurosci. 2014;8:38. doi: 10.3389/fnins.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachsmuth H.R., Weninger S.N., Duca F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. 2022;54:377–392. doi: 10.1038/s12276-021-00677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croset M., et al. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 9.Soty M., Gautier-Stein A., Rajas F., et al. Gut-brain glucose signaling in energy homeostasis. Cell Metab. 2017;25:1231–1242. doi: 10.1016/j.cmet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Strubbe J.H., Bruggink J.E., Steffens A.B. Hepatic portal vein cannulation for infusion and blood sampling in freely moving rats. Physiol Behav. 1988;65:885–887. doi: 10.1016/s0031-9384(98)00248-0. [DOI] [PubMed] [Google Scholar]
- 11.Pellerito J., Polak J., Anatomy, editors. normal doppler signatures of abdominal vessels. In: Introduction to Vascular Ultrasonography. 7th ed. Elsevier; New York, NY: 2019. pp. 439–449. [Google Scholar]
- 12.Shah V., Kamath P. In: Feldman M., Friedman L.S., Brandt L.J., editors. Vol. 92. Elsevier; New York, NY: 2019. Portal hypertension and variceal bleeding; pp. 1443–1470. (Sleisenger and Fordtran’s Gastrointestinal and Liver Disease). [Google Scholar]
- 13.Ts’ao C.H., Glagov S., Kelsey B.F. Special structural features of the rat portal vein. Anat Rec (Hoboken) 1970;166:529–539. doi: 10.1002/ar.1091660310. [DOI] [PubMed] [Google Scholar]
- 14.Dong H.-M., Ichimura K., Sakai T. Structural organization of hepatic portal vein in rat with special reference to musculature, intimal folds, and endothelial cell alignment. Anat Rec (Hoboken) 2010;293:1887–1895. doi: 10.1002/ar.21246. [DOI] [PubMed] [Google Scholar]
- 15.Russek M. Demonstration of the influence of an hepatic glucosensitive mechanism on food-intake. Physiol Behav. 1970;5:1207–1209. doi: 10.1016/0031-9384(70)90218-0. [DOI] [PubMed] [Google Scholar]
- 16.Campbell C.S., Davis J.D. Licking rate of rats is reduced by intraduodenal and intraportal glucose infusion. Physiol Behav. 1974;12:357–365. doi: 10.1016/0031-9384(74)90110-3. [DOI] [PubMed] [Google Scholar]
- 17.Novin D., Sanderson J.D., Vanderweele D.A. The effect of isotonic glucose on eating as a function of feeding condition and infusion site. Physiol Behav. 1974;13:3–7. doi: 10.1016/0031-9384(74)90298-4. [DOI] [PubMed] [Google Scholar]
- 18.Booth D.A., Jarman S.P. Inhibition of food intake in the rat following complete absorption of glucose delivered into the stomach, intestine or liver. J Physiol. 1976;259:501–522. doi: 10.1113/jphysiol.1976.sp011479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanderWeele D.A., Skoog D.R., Novin D. Glycogen levels and peripheral mechanisms of glucose-induced suppression of feeding. Am J Physiol. 1976;231:1655–1659. doi: 10.1152/ajplegacy.1976.231.6.1655. [DOI] [PubMed] [Google Scholar]
- 20.Yin T.H., Tsai W.H., Barone F.C., et al. Effects of continuous intramesenteric infusion of glucose and amino acids on food intake in rats. Physiol Behav. 1979;22:1207–1210. doi: 10.1016/0031-9384(79)90278-6. [DOI] [PubMed] [Google Scholar]
- 21.Russek M., Lora-Vilchis M.C., Islas-Chaires M. Food intake inhibition elicited by intraportal glucose and adrenaline in dogs on a 22 hour-fasting/2 hour feeding schedule. Physiol Behav. 1980;24:157–161. doi: 10.1016/0031-9384(80)90028-1. [DOI] [PubMed] [Google Scholar]
- 22.Shurlock T.G., Forbes J.M. Evidence for hepatic glucostatic regulation of food intake in the domestic chicken and its interaction with gastro-intestinal control. Br Poult Sci. 1981;22:333–346. doi: 10.1080/00071688108447893. [DOI] [PubMed] [Google Scholar]
- 23.Lacy M.P., Van Krey H.P., Skewes P.A., et al. Effect of intrahepatic glucose infusions on feeding in heavy and light breed chicks. Poult Sci. 1985;64:751–756. doi: 10.3382/ps.0640751. [DOI] [PubMed] [Google Scholar]
- 24.Novin D., Robinson K., Culbreth L.A., et al. Is there a role for the liver in the control of food intake? Am J Clin Nutr. 1985;42:1050–1062. doi: 10.1093/ajcn/42.5.1050. [DOI] [PubMed] [Google Scholar]
- 25.Tordoff M.G., Friedman M.I. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 26.Rusby A.A., Anil M.H., Chatterjee P., et al. Effects of intraportal infusion of glucose and lysine on food intake in intact and hepatic-vagotomized chickens. Appetite. 1987;9:65–72. doi: 10.1016/0195-6663(87)90054-7. [DOI] [PubMed] [Google Scholar]
- 27.Tordoff M.G., Friedman M.I. Hepatic control of feeding: effect of glucose, fructose, and mannitol infusion. Am J Physiol. 1988;254:R969–R976. doi: 10.1152/ajpregu.1988.254.6.R969. [DOI] [PubMed] [Google Scholar]
- 28.Tordoff M.G., Tluczek J.P., Friedman M.I. Effect of hepatic portal glucose concentration on food intake and metabolism. Am J Physiol. 1989;257:R1474–R1480. doi: 10.1152/ajpregu.1989.257.6.R1474. [DOI] [PubMed] [Google Scholar]
- 29.Baird J.P., Grill H.J., Kaplan J.M. Intake suppression after hepatic portal glucose infusion: all-or-none effect and its temporal threshold. Am J Physiol. 1997;272:R1454–R1460. doi: 10.1152/ajpregu.1997.272.5.R1454. [DOI] [PubMed] [Google Scholar]
- 30.Baird J.P., Grill H.J., Kaplan J.M. Effect of hepatic glucose infusion on glucose intake and licking microstructure in deprived and nondeprived rats. Am J Physiol. 1999;277:R1136–R1143. doi: 10.1152/ajpregu.1999.277.4.R1136. [DOI] [PubMed] [Google Scholar]
- 31.Langhans W., Grossmann F., Geary N. Intrameal hepatic-portal infusion of glucose reduces spontaneous meal size in rats. Physiol Behav. 2001;73:499–507. doi: 10.1016/s0031-9384(01)00479-6. [DOI] [PubMed] [Google Scholar]
- 32.Mithieux G., Misery P., Magnan C., et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2:321–329. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Delaere F., Duchampt A., Mounien L., et al. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab. 2012;2:47–53. doi: 10.1016/j.molmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baile C.A., Zinn W., Mayer J. Feeding behavior of monkeys: glucose utilization rate and site of glucose entry. Physiol Behav. 1971;6:537–541. doi: 10.1016/0031-9384(71)90201-0. [DOI] [PubMed] [Google Scholar]
- 35.Yin T.H., Tsai C.T. Effects of glucose on feeding in relation to routes of entry in rats. J Comp Physiol Psychol. 1973;85:258–264. doi: 10.1037/h0035012. [DOI] [PubMed] [Google Scholar]
- 36.Stephens D.B., Baldwin B.A. The lack of effect of intrajugular or intraportal injections of glucose or amino-acids on food intake in pigs. Physiol Behav. 1974;12:923–929. doi: 10.1016/0031-9384(74)90139-5. [DOI] [PubMed] [Google Scholar]
- 37.Vanderweele D.A., Novin D., Rezek M., et al. Duodenal or hepatic-portal glucose perfusion: evidence for duodenally-based satiety. Physiol Behav. 1974;12:467–473. doi: 10.1016/0031-9384(74)90124-3. [DOI] [PubMed] [Google Scholar]
- 38.Rezek M., Havlicek V., Novin D. Satiety and hunger induced by small and large duodenal loads of isotonic glucose. Am J Physiol. 1975;229:545–548. doi: 10.1152/ajplegacy.1975.229.3.545. [DOI] [PubMed] [Google Scholar]
- 39.Bellinger L.L., Trietley G.J., Bernardis L.L. Failure of portal glucose and adrenaline infusions or liver denervation to affect food intake in dogs. Physiol Behav. 1976;16:299–304. doi: 10.1016/0031-9384(76)90136-0. [DOI] [PubMed] [Google Scholar]
- 40.Rezek M., Novin D. Hepatic-portal nutrient infusion: effect on feeding in intact and vagotomized rabbits. Am J Physiol. 1977;232:E119–E130. doi: 10.1152/ajpendo.1977.232.2.E119. [DOI] [PubMed] [Google Scholar]
- 41.Strubbe J.H., Steffens A.B. Blood glucose levels in portal and peripheral circulation and their relation to food intake in the rat. Physiol Behav. 1977;19:303–307. doi: 10.1016/0031-9384(77)90342-0. [DOI] [PubMed] [Google Scholar]
- 42.Rezek M., Havlicek V., Friesen H. Hepatic-portal glucose and insulin levels: relationship to glucose-induced satiety and hunger. J Nutr. 1979;109:1665–1672. doi: 10.1093/jn/109.10.1665. [DOI] [PubMed] [Google Scholar]
- 43.Bellinger L.L., Williams F.E. The effect of portal and jugular infused glucose, mannitol and saline on food intake in dogs. Physiol Behav. 1989;46:693–698. doi: 10.1016/0031-9384(89)90353-3. [DOI] [PubMed] [Google Scholar]
- 44.Niewoehner C.B., Gilboe D.P., Nuttall F.Q. Metabolic effects of oral glucose in the liver of fasted rats. Am J Physiol. 1984;246:E89–E94. doi: 10.1152/ajpendo.1984.246.1.E89. [DOI] [PubMed] [Google Scholar]
- 45.Bellisle F., Drewnowski A., Anderson G.H., et al. Sweetness, satiation, and satiety. J Nutr. 2012;142:1149S–1154S. doi: 10.3945/jn.111.149583. [DOI] [PubMed] [Google Scholar]
- 46.Blundell J.E., Lawton C.L., Cotton J.R., et al. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr. 1996;16:285–319. doi: 10.1146/annurev.nu.16.070196.001441. [DOI] [PubMed] [Google Scholar]
- 47.Rüttimann E.B., Arnold M., Hillebrand J.J., et al. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guillam M.T., Hümmler E., Schaerer E., et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 49.Burcelin R., Dolci W., Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–1648. doi: 10.2337/diabetes.49.10.1643. [DOI] [PubMed] [Google Scholar]
- 50.Díez-Sampedro A., Hirayama B.A., Osswald C., et al. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A. 2003;100:11753–11758. doi: 10.1073/pnas.1733027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong C.T., Yet S.F., Lever J.E. Cloning and expression of a mammalian Na+/amino acid cotransporter with sequence similarity to Na+/glucose cotransporters. J Biol Chem. 1993;268:1509–1512. [PubMed] [Google Scholar]
- 52.Barcelona S., Menegaz D., Díez-Sampedro A. Mouse SGLT3a generates proton-activated currents but does not transport sugar. Am J Physiol Cell Physiol. 2012;302:C1073–C1082. doi: 10.1152/ajpcell.00436.2011. [DOI] [PubMed] [Google Scholar]
- 53.Tordoff M. In: Chemical Senses Volume 4: Appetite and Nutrition. Friedman M.I., Tordoff M.G., Kare M.R., editors. Marcel Dekker; New York, NY: 1991. Metabolic basis of learned food preferences; pp. 239–260. [Google Scholar]
- 54.Ackroff K., Yiin Y.-M., Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliveira-Maia A.J., Roberts C.D., Walker Q.D., et al. Intravascular food reward. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L., Han W., Lin C., et al. Sugar metabolism regulates flavor preferences and portal glucose sensing. Front Integr Neurosci. 2018;12:57. doi: 10.3389/fnint.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bossert J.M., Poles G.C., Wihbey K.A., et al. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beyene M., Carelli R.M., Wightman R.M. Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience. 2010;169:1682–1688. doi: 10.1016/j.neuroscience.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saddoris M.P., Cacciapaglia F., Wightman R.M., et al. Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J Neurosci. 2015;35:11572–11582. doi: 10.1523/JNEUROSCI.2344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sackett D.A., Moschak T.M., Carelli R.M. Nucleus accumbens shell dopamine mediates outcome value, but not predicted value, in a magnitude decision-making task. Eur J Neurosci. 2020;51:1526–1538. doi: 10.1111/ejn.14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sclafani A., Ackroff K. Commentary: sugar metabolism regulates flavor preferences and portal glucose sensing. Front Integr Neurosci. 2019;13:4. doi: 10.3389/fnint.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zanchi D., Depoorter A., Egloff L., et al. The impact of gut hormones on the neural circuit of appetite and satiety: a systematic review. Neurosci Biobehav Rev. 2017;80:457–475. doi: 10.1016/j.neubiorev.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Berthoud H.R., Kressel M., Neuhuber W.L. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol. 1992;186:431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- 64.Berthoud H.-R., Neuhuber W.L. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann N Y Acad Sci. 2019;1454:42–55. doi: 10.1111/nyas.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berthoud H.-R. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:827–835. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 66.Adachi A., Shimizu N., Oomura Y., et al. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- 67.Niijima A. Blood glucose levels modulate efferent activity in the vagal supply to the rat liver. J Physiol. 1985;364:105–112. doi: 10.1113/jphysiol.1985.sp015733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bohland M., Matveyenko A.V., Saberi M., et al. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes. 2014;63:2866–2875. doi: 10.2337/db13-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sclafani A., Ackroff K., Schwartz G.J. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav. 2003;78:285–294. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- 70.Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. Am J Physiol. 1973;225:1089–1095. doi: 10.1152/ajplegacy.1973.225.5.1089. [DOI] [PubMed] [Google Scholar]
- 71.King G.W. Topology of ascending brainstem projections to nucleus parabrachialis in the cat. J Comp Neurol. 1980;191:615–638. doi: 10.1002/cne.901910408. [DOI] [PubMed] [Google Scholar]
- 72.Menétrey D., Basbaum A.I. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 73.Saper C.B., Loewy A.D. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 74.Adachi A. Electrophysiological study of hepatic vagal projection to the medulla. Neurosci Lett. 1981;24:19–23. doi: 10.1016/0304-3940(81)90352-9. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Luna C., Sanchez-Watts G., Arnold M., et al. The medullary targets of neurally conveyed sensory information from the rat hepatic portal and superior mesenteric veins. eNeuro. 2021;8 doi: 10.1523/ENEURO.0419-20.2021. ENEURO.0419-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldstein N., McKnight A.D., Carty J.R.E., et al. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021;33:676–687.e5. doi: 10.1016/j.cmet.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujita S., Donovan C.M. Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes. 2005;54:3258–3264. doi: 10.2337/diabetes.54.11.3258. [DOI] [PubMed] [Google Scholar]
- 78.Berthoud H.R., Powley T.L. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 79.Alhadeff A.L. Monitoring in vivo neural activity to understand gut–brain signaling. Endocrinology. 2021;162:bqab029. doi: 10.1210/endocr/bqab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carrier N., Connat J.L. CGRP innervation and receptors during aging of male and female hepatic rat portal veins. Neurobiol Aging. 1996;17:53–60. doi: 10.1016/0197-4580(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 81.Kupari J., Häring M., Agirre E., et al. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019;27:2508–2523.e4. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Usoskin D., Furlan A., Islam S., et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 83.Vahl T.P., Tauchi M., Durler T.S., et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 84.Bohórquez D.V., Chandra R., Samsa L.A., et al. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42:3–13. doi: 10.1007/s10735-010-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bohórquez D.V., Samsa L.A., Roholt A., et al. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bohórquez D.V., Shahid R.A., Erdmann A., et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blake W.D., Lin K.K. Hepatic portal vein infusion of glucose and sodium solutions on the control of saline drinking in the rat. J Physiol. 1978;274:129–139. doi: 10.1113/jphysiol.1978.sp012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tordoff M.G., Schulkin J., Friedman M.I. Hepatic contribution to satiation of salt appetite in rats. Am J Physiol. 1986;251:R1095–R1102. doi: 10.1152/ajpregu.1986.251.6.R1095. [DOI] [PubMed] [Google Scholar]
- 89.Tordoff M.G., Schulkin J., Friedman M.I. Further evidence for hepatic control of salt intake in rats. Am J Physiol. 1987;253:R444–R449. doi: 10.1152/ajpregu.1987.253.3.R444. [DOI] [PubMed] [Google Scholar]
- 90.Duraffourd C., De Vadder F., Goncalves D., et al. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell. 2012;150:377–388. doi: 10.1016/j.cell.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 91.Gautier-Stein A., Mithieux G. Intestinal gluconeogenesis: metabolic benefits make sense in the light of evolution. Nat Rev Gastroenterol Hepatol. 2023;20:183–194. doi: 10.1038/s41575-022-00707-6. [DOI] [PubMed] [Google Scholar]
- 92.Mithieux G. A novel function of intestinal gluconeogenesis: central signaling in glucose and energy homeostasis. Nutrition. 2009;25:881–884. doi: 10.1016/j.nut.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 93.Mithieux G., Bady I., Gautier A., et al. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab. 2004;286:E370–E375. doi: 10.1152/ajpendo.00299.2003. [DOI] [PubMed] [Google Scholar]
- 94.Penhoat A., Mutel E., Amigo-Correig M., et al. Protein-induced satiety is abolished in the absence of intestinal gluconeogenesis. Physiol Behav. 2011;105:89–93. doi: 10.1016/j.physbeh.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 95.De Vadder F., Kovatcheva-Datchary P., Zitoun C., et al. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 96.Troy S., Soty M., Ribeiro L., et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Leonhardt M., Langhans W. Fatty acid oxidation and control of food intake. Physiol Behav. 2004;83:645–651. doi: 10.1016/j.physbeh.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 98.Jambor de Sousa U.L., Arnold M., Langhans W., et al. Caprylic acid infusion acts in the liver to decrease food intake in rats. Physiol Behav. 2006;87:388–395. doi: 10.1016/j.physbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Jambor de Sousa U.L., Benthem L., Arsenijevic D., et al. Hepatic-portal oleic acid inhibits feeding more potently than hepatic-portal caprylic acid in rats. Physiol Behav. 2006;89:329–334. doi: 10.1016/j.physbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 100.Mélone J. Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst. 1986;17:231–241. doi: 10.1016/0165-1838(86)90060-3. [DOI] [PubMed] [Google Scholar]
- 101.Ramírez M., Amate L., Gil A. Absorption and distribution of dietary fatty acids from different sources. Early Hum Dev. 2001;65:S95–S101. doi: 10.1016/s0378-3782(01)00211-0. [DOI] [PubMed] [Google Scholar]
- 102.Pelchat M.L., Grill H.J., Rozin P., et al. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol. 1983;97:140–153. [PubMed] [Google Scholar]
- 103.Halford J.C., Blundell J.E. Pharmacology of appetite suppression. Prog Drug Res. 2000;54:25–58. doi: 10.1007/978-3-0348-8391-7_2. [DOI] [PubMed] [Google Scholar]
- 104.Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutr Neurosci. 2022;5:159–188. doi: 10.1080/10284150290013059. [DOI] [PubMed] [Google Scholar]
- 105.Aulinger B.A., Perabo M., Seeley R.J., et al. Rapid hepatic metabolism blunts the endocrine action of portally infused GLP-1 in male rats. Am J Physiol Endocrinol Metab. 2020;318:E189–E197. doi: 10.1152/ajpendo.00298.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andersen D.B., Grunddal K.V., Pedersen J., et al. Using a reporter mouse to map known and novel sites of GLP-1 receptor expression in peripheral tissues of male mice. Endocrinology. 2021;162:bqaa246. doi: 10.1210/endocr/bqaa246. [DOI] [PubMed] [Google Scholar]
- 107.Nakabayashi H., Nishizawa M., Nakagawa A., et al. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 108.Qian M., Wu G.S., Adem A., et al. CCK-8 can inhibit ingestive behavior by acting on the liver. Neuroreport. 1999;10:359–362. doi: 10.1097/00001756-199902050-00027. [DOI] [PubMed] [Google Scholar]
- 109.Langhans W., Egli G., Scharrer E. Selective hepatic vagotomy eliminates the hypophagic effect of different metabolites. J Auton Nerv Syst. 1985;13:255–262. doi: 10.1016/0165-1838(85)90014-1. [DOI] [PubMed] [Google Scholar]
- 110.Langhans W., Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst. 1987;18:13–18. doi: 10.1016/0165-1838(87)90129-9. [DOI] [PubMed] [Google Scholar]
- 111.Horn C.C., Friedman M.I. Separation of hepatic and gastrointestinal signals from the common ‘hepatic’ branch of the vagus. Am J Physiol Regul Integr Comp Physiol. 2004;287:R120–R126. doi: 10.1152/ajpregu.00673.2003. [DOI] [PubMed] [Google Scholar]
- 112.Friedman M. Obesity and the hepatic control of feeding behavior. Drug News Perspect. 2007;20:573–578. doi: 10.1358/dnp.2007.20.9.1162243. [DOI] [PubMed] [Google Scholar]
- 113.Friedman M., Tordoff M. In: Liver Innervation and the Control of Hepatic Function. Shimazu T., editor. John Libbey & Company Ltd; London, United Kingdom: 1996. Hepatic metabolic signal for control of food intake: stimulus generation, signal transduction and neural transmission; pp. 373–380. [Google Scholar]
- 114.Rawson N.E., Friedman M.I. Phosphate loading prevents the decrease in ATP and increase in food intake produced by 2,5-anhydro-D-mannitol. Am J Physiol. 1994;266:R1792–R1796. doi: 10.1152/ajpregu.1994.266.6.R1792. [DOI] [PubMed] [Google Scholar]
- 115.López-Soldado I., Zafra D., Duran J., et al. Liver glycogen reduces food intake and attenuates obesity in a high-fat diet-fed mouse model. Diabetes. 2015;64:796–807. doi: 10.2337/db14-0728. [DOI] [PubMed] [Google Scholar]
- 116.López-Soldado I., Guinovart J.J., Duran J. Hepatic overexpression of protein targeting to glycogen attenuates obesity and improves hyperglycemia in db/db mice. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.969924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berthoud H.-R., Morrison C.D., Ackroff K., et al. Learning of food preferences: mechanisms and implications for obesity & metabolic diseases. Int J Obes (Lond) 2021;45:2156–2168. doi: 10.1038/s41366-021-00894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sclafani A., Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buchanan K.L., Rupprecht L.E., Kaelberer M.M., et al. The preference for sugar over sweetener depends on a gut sensor cell. Nat Neurosci. 2022;25:191–200. doi: 10.1038/s41593-021-00982-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li M., Tan H.-E., Lu Z., et al. Gut-brain circuits for fat preference. Nature. 2022;610:722–730. doi: 10.1038/s41586-022-05266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan H.-E., Sisti A.C., Jin H., et al. The gut-brain axis mediates sugar preference. Nature. 2020;580:511–516. doi: 10.1038/s41586-020-2199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cotero V., Graf J., Miwa H., et al. Stimulation of the hepatoportal nerve plexus with focused ultrasound restores glucose homoeostasis in diabetic mice, rats and swine. Nat. Biomed. Eng. 2022;6:683–705. doi: 10.1038/s41551-022-00870-w. [DOI] [PMC free article] [PubMed] [Google Scholar]