Visual Abstract
Keywords: fibroblast activation protein, integrin αvβ3, heterodimer, cancer-associated-fibroblasts, PET
Abstract
Radiolabeled fibroblast activation protein (FAP) inhibitors (FAPIs) and Arg-Gly-Asp (RGD) peptides have been extensively investigated for imaging of FAP- and integrin αvβ3–positive tumors. In this study, a FAPI-RGD heterodimer was radiolabeled with 68Ga and evaluated in patients with cancer. We hypothesized that the heterodimer, recognizing both FAP and integrin αvβ3, would be advantageous because of its dual-receptor–targeting property. Methods: The effective dose of 68Ga-FAPI-RGD was evaluated in 3 healthy volunteers. The clinical feasibility of 68Ga-FAPI-RGD PET/CT was evaluated in 22 patients with various types of cancer, and the results were compared with those of 18F-FDG and 68Ga-FAPI-46. Results: 68Ga-FAPI-RGD was tolerated well, with no adverse events in any of the healthy volunteers or patients. The effective dose from 68Ga-FAPI-RGD PET/CT was 1.01 × 10−2 mSv/MBq. In clinical investigations with different types of cancer, the radiotracer uptake and tumor-to-background ratio (TBR) of primary and metastatic lesions in 68Ga-FAPI-RGD PET/CT were significantly higher than those in 18F-FDG PET/CT (primary tumors: SUVmax, 18.0 vs. 9.1 [P < 0.001], and TBR, 15.2 vs. 5.5 [P < 0.001]; lymph node metastases: SUVmax, 12.1 vs. 6.1 [P < 0.001], and TBR, 13.3 vs. 4.1 [P < 0.001]), resulting in an improved lesion detection rate and tumor delineation, particularly for the diagnosis of lymph node (99% vs. 91%) and bone (100% vs. 80%) metastases. 68Ga-FAPI-RGD PET/CT also yielded a higher radiotracer uptake and TBR than 68Ga-FAPI-46 PET/CT did. Conclusion: 68Ga-FAPI-RGD exhibited improved tumor uptake and TBR compared with 18F-FDG and 68Ga-FAPI PET/CT. This study demonstrated the safety and clinical feasibility of 68Ga-FAPI-RGD PET/CT for imaging of various types of cancer.
Tumor receptor imaging is an important component of oncologic molecular imaging and plays a key role in cancer diagnosis and management. It is made possible by the intense expression of specific biomarkers in the cellular membrane. Integrin αvβ3 is a heterodimeric transmembrane glycoprotein that is highly expressed in activated endothelial cells, newly formed blood vessels, and several types of tumor cells. In contrast, it exhibits low or no expression in normal cells (1). Therefore, integrin αvβ3 is a promising target for tumor imaging and therapy (2). The tripeptide Arg-Gly-Asp (RGD) moiety exhibits a high binding affinity and specificity for integrin αvβ3. Various RGD-based cyclic peptides have been labeled with radionuclides and extensively evaluated for PET or SPECT imaging of cancers (2). However, the rapid blood clearance of RGD peptides is one of the reasons that their application is limited in radionuclide therapy (3). The need to improve the pharmacokinetics of RGD peptides has led to multimeric strategies or conjugation with albumin-binding moieties (3,4). These approaches allow the transformation of a drug into a theranostic agent for use as both an imaging agent and a therapeutic agent.
The importance of the tumor microenvironment in cancer development and clinical prognosis is now widely appreciated (5). Besides tumor cells and tumor angiogenesis, cancer-associated fibroblasts of the tumor microenvironment are the major components of solid tumors (6). Fibroblast activation protein (FAP)–α is highly expressed in cancer-associated fibroblasts in most epithelial cancers but is weakly expressed in normal tissues, making it an attractive target for cancer imaging and therapy (7). In the past few years, quinoline-based FAP inhibitor (FAPI) PET/CT has become an active field in nuclear oncology (7,8). Various studies have demonstrated that FAPI-based radiopharmaceuticals are promising radiotracers for cancer diagnosis, staging, and restaging. They may be better alternatives for cancer types that exhibit low to moderate uptake of 18F-FDG, including gastric, pancreatic, and liver cancers (9).
Recently, a heterodimeric peptide, denoted as FAPI-RGD, was synthesized from FAPI-02 and cyclo-RGD-d-Phe-Lys (c[RGDfK]) for targeting both FAP and integrin αvβ3 receptors (10). It was radiolabeled with 68Ga for preclinical evaluation and was further assessed in a pilot clinical study. The tumor uptake and retention of 68Ga-FAPI-RGD were significantly greater than those of 68Ga-FAPI-46 and 68Ga-c(RGDfK) in mouse xenografts (10). Furthermore, this pilot clinical study on 6 patients demonstrated that 68Ga-FAPI-RGD PET/CT enabled visualization of tumor lesions with favorable imaging contrast. These results encouraged us to further explore the role of 68Ga-FAPI-RGD PET/CT for cancer imaging.
The aim of this study was to evaluate, in patients with various types of cancer, the radiotracer uptake and clinical feasibility of 68Ga-FAPI-RGD PET/CT compared with those of 18F-FDG and 68Ga-FAPI-46 PET/CT.
MATERIALS AND METHODS
Chemistry and Radiochemistry
The detailed synthesis procedure for FAPI-RGD was reported recently (10). Information on the chemicals and reagents is briefly presented in the supplemental materials (available at http://jnm.snmjournals.org). 68Ga-radiolabeling of FAPI-02, FAPI-46, c(RGDfK), and FAPI-RGD variants was performed as previously described (11–13). In brief, 25 nmol of FAPI-RGD in 1 mL of sodium acetate buffer (0.25 M) were reacted with 4 mL of 68Ga-solution (1.1 GBq in 0.6 M HCl) at 100°C for 15 min. For clinical imaging, the final product was passed through a 0.22-μm Millipore filter for sterilization of each preparation of 68Ga-FAPI-RGD. Quality control was performed using ultraviolet and radio-high-performance liquid chromatography (supplemental materials). The stability of the radiolabeled compound was determined by incubating the product in phosphate-buffered saline at 37°C and analyzing it via radio-high-performance liquid chromatography after 1, 2, and 4 h of incubation.
Clinical PET/CT Imaging in Healthy Volunteers and Patients with Cancer
The prospective clinical study protocol was approved by the institutional review board of the First Affiliated Hospital of Xiamen University and was registered at ClinicalTrials.gov (NCT05543317). Written informed consent was obtained from all healthy volunteers and patients. The dose of intravenously injected 68Ga-FAPI-RGD was calculated according to the participants’ body weight (3.0–3.7 MBq/kg), which corresponds to approximately 7–8 nmol per subject. Safety data (blood pressure, heart rate, and temperature) and adverse events were recorded before and 4 h after injection of 68Ga-FAPI-RGD. The PET/CT scanning and reconstruction protocols are presented in the supplemental materials. For dosimetry evaluation, 68Ga-FAPI-RGD PET imaging was performed at 30, 60, and 180 min after tracer injection. Time–activity curve fitting and subsequent dose calculations were performed using OLINDA/EXM software, version 1.1 (14).
All patients underwent paired 68Ga-FAPI-RGD and 18F-FDG PET/CT scans. An additional 68Ga-FAPI-46 PET/CT scan was performed for comparative purposes depending on the patient’s willingness. We evaluated the in vivo distribution pattern of 68Ga-FAPI-RGD at later time points, by performing 3-h-delayed 68Ga-FAPI-RGD PET/CT scans for all patients (because of the relatively short half-life [68 min] of 68Ga). All PET images were evaluated by 2 board-certified and experienced nuclear medicine physicians. Disagreements were resolved via consensus. For quantitative analysis, the SUVmax was used to quantify radiopharmaceutical uptake by normal organs and tumor tissues. Tracer uptake in normal organs (background) was quantified by SUVmean, which was delineated with a sphere that had a diameter of 1 cm (for small organs, including thyroid, salivary gland, and pancreas) to 2 cm (for other organs, including brain, heart, liver, kidney, spleen, muscle, and bone marrow) placed inside the organ parenchyma. The tumor-to-background ratio (TBR) was calculated as tumor SUVmax/background SUVmean.
To evaluate the diagnostic performance of 68Ga-FAPI-RGD and 18F-FDG PET imaging, the results of the visually interpreted PET images were compared with the histopathologic results (via surgery or biopsy), which were used as the gold standard for the final diagnosis. For patients for whom tissue diagnosis was not applicable, clinical and radiographic follow-up data were used as the reference standard to validate the PET/CT findings. Lesions were considered malignant on the basis of any of the following follow-up criteria: typical malignant features confirmed by multimodal medical imaging, significant progression on follow-up imaging, or a significant decrease in posttreatment tumor size. The minimum follow-up period was 3 mo. Histopathologic staining of surgical and biopsy samples was performed as previously described (15).
Statistical Analysis
All statistical analyses were conducted using Prism (version 8.0; GraphPad Software Inc.) and SPSS Statistics (version 22.0; IBM Corp.). All quantitative data are expressed as the mean. The Wilcoxon matched-pairs signed-rank test was used to compare SUVs derived from 18F-FDG, 68Ga-FAPI-RGD, and 68Ga-FAPI-46 PET/CT. The McNemar test was used to compare the lesion detectability of the different PET/CT scans. Statistical significance was defined as a P value of less than 0.05.
RESULTS
Synthesis and Radiolabeling
68Ga-FAPI-RGD was radiolabeled at a specific activity of approximately 33 GBq/μmol, with over 99% radiochemical purity after purification (Supplemental Fig. 1A). High-performance liquid chromatography analysis revealed that 68Ga-FAPI-RGD had a high stability for up to 4 h, with no significant demetallation observed in phosphate-buffered saline (>99%) (Supplemental Figs. 1B–1D).
Safety and Radiation Dosimetry in Healthy Volunteers
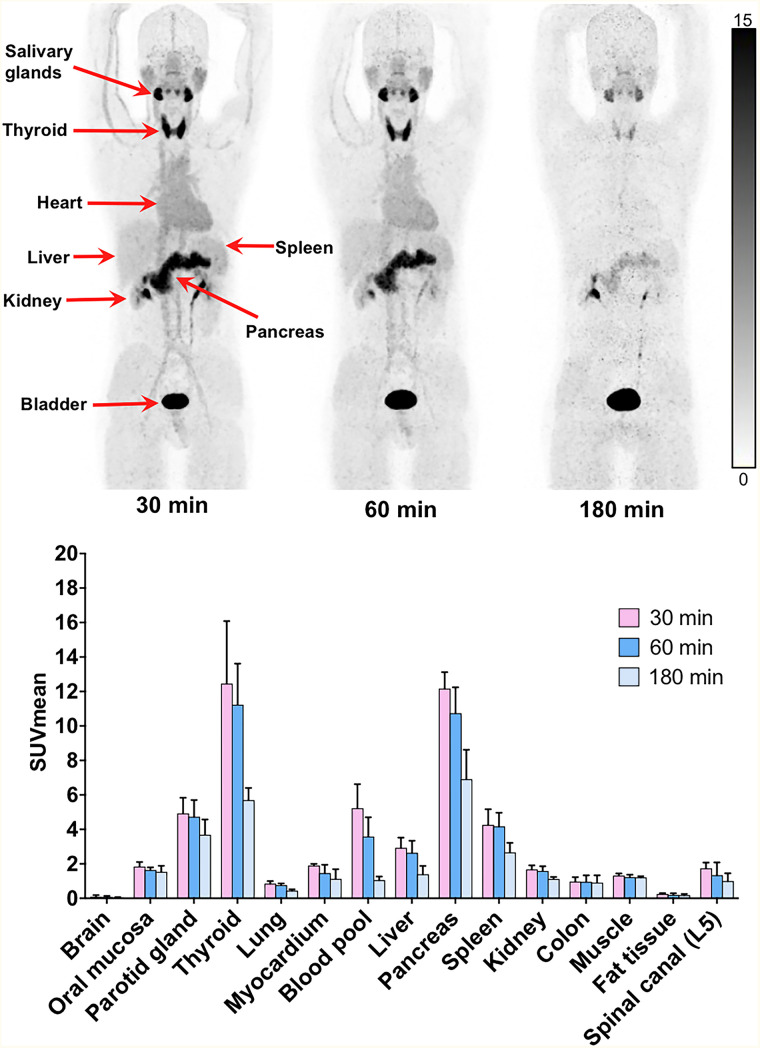
All observed vital signs (including temperature, heart rate, and blood pressure) remained normal during the injection and 4-h postinjection follow-up. 68Ga-FAPI-RGD was tolerated well, with no adverse events in any of the healthy volunteers or patients. Representative PET images and biodistribution data of healthy volunteers (n = 3) are provided in Figure 1. Tracer uptake rapidly decreased in most normal organs from 30 to 180 min, particularly in the thyroid, pancreas, and salivary glands. The 68Ga-FAPI-RGD effective dose was 1.01 × 10−2 mSv/MBq (Supplemental Table 1), which was comparable to that of 68Ga-FAPI-02 (1.80 × 10−2 mSv/MBq) (16). The organ with the highest effective dose was the thyroid (3.01 × 10−3 mSv/MBq), followed by the urinary bladder wall (1.37 × 10−3 mSv/MBq), liver (1.10 × 10−3 mSv/MBq), and lungs (1.09 × 10−3 mSv/MBq).
FIGURE 1.
68Ga-FAPI-RGD at 30, 60, and 180 min after injection in healthy volunteers, and SUVmean of normal organs at corresponding time points (n = 3).
Patients’ Characteristics
From July 1 to September 15, 2022, 22 patients with cancer (15 men; median age, 57 y; range, 34–79 y; 17 for staging and 5 for restaging) who underwent paired 68Ga-FAPI-RGD and 18F-FDG PET/CT were enrolled in this study. The median interval between the 2 scans was 4 d (range, 1–7 d). Furthermore, 7 of the 22 patients underwent additional 68Ga-FAPI-46 PET/CT for comparison. Among the 22 patients, the final diagnosis was based on the histopathologic results for 20 and diagnostic radiology results for 2. Detailed information on the enrolled patients is provided in Supplemental Table 2.
Dual-Time-Point 68Ga-FAPI-RGD PET/CT Imaging in Patients with Cancer
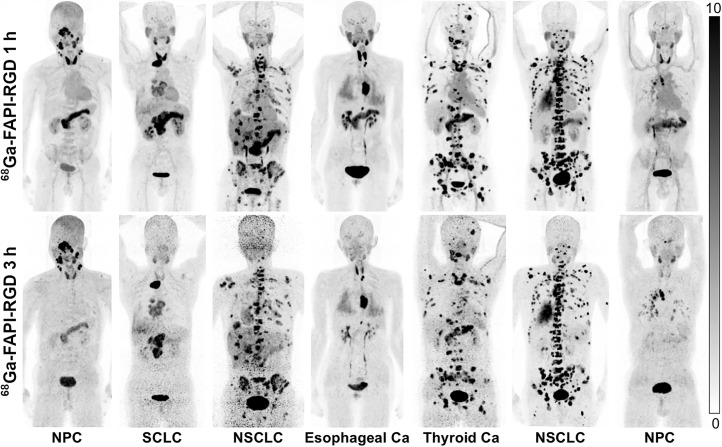
To evaluate the in vivo distribution pattern of the radiotracer and tumor uptake over time, dual-time-point 68Ga-FAPI-RGD PET/CT (1 vs. 3 h) was performed for all patients. As demonstrated in Figure 2, most tumor lesions demonstrated increased uptake over time. Specifically, SUVmax derived from the delayed scan (3 h) was significantly higher than SUVmax derived from routine scans (1 h) in the primary tumors (18.0 vs. 12.6; P < 0.001), lymph node metastases (12.1 vs. 9.3; P < 0.001), lung metastases (median, 8.0 vs. 4.6; P < 0.001), and bone metastases (16.2 vs. 13.2; P < 0.001). Interestingly, background activity decreased greatly over time. Consequently, TBR improved significantly in primary tumors with involved lymph nodes and with lung, liver, peritoneal, and bone metastases. Detailed data are presented in Supplemental Table 3.
FIGURE 2.
Maximum-intensity-projection images of 68Ga-FAPI-RGD PET/CT at 1 and 3 h after injection in different types of cancer. Ca = cancer; NPC = nasopharyngeal carcinoma; SCLC = small cell lung cancer.
Comparison of 68Ga-FAPI-RGD and 18F-FDG Uptake in Patients with Cancer
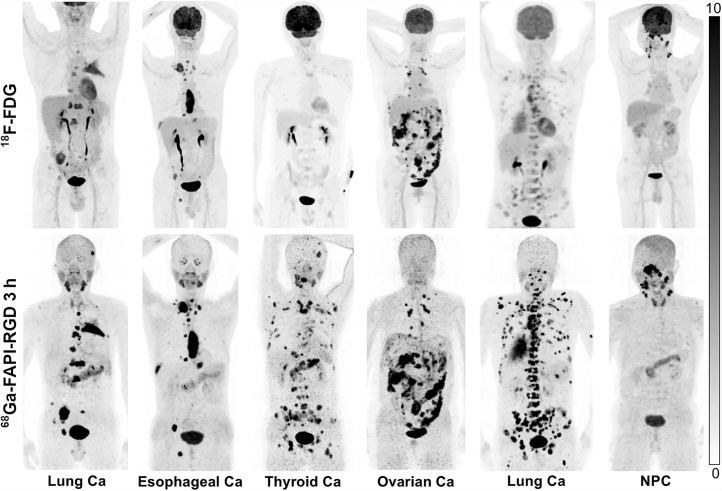
Among the 17 patients who underwent paired 68Ga-FAPI-RGD and 18F-FDG PET/CT for initial staging, 68Ga-FAPI-RGD PET/CT allowed detection of all primary tumors (19/19) with intense radiotracer uptake, whereas 18F-FDG PET/CT resulted in 3 missed tumor lesions in 1 patient with multifocal breast cancer. In all primary tumors, SUVmax was significantly higher when derived from 68Ga-FAPI-RGD PET/CT than from 18F-FDG (18.0 vs. 9.1, P < 0.001). Furthermore, the TBRs of the primary tumors from 68Ga-FAPI-RGD PET/CT were approximately 3 times greater than those from 18F-FDG PET/CT (15.2 vs. 5.5, P < 0.001). Detailed data and representative images are presented in Table 1 and Figure 3, respectively.
TABLE 1.
Comparison of SUVmax from 68Ga-FAPI-RGD and 18F-FDG PET/CT in Primary and Metastatic Tumors
| Tumor type | n | Size (cm) | 68Ga-FAPI-RGD PET/CT | 18F-FDG PET/CT | P (FAPI-RGD vs. FDG) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive tumors (n) | SUVmax | TBR | Positive tumors (n) | SUVmax | TBR | Median SUVmax | TBR | |||
| Primary | ||||||||||
| Total | 19 | 2.5 (0.6–6.5) | 19 | 18.0 (7.0–40.3) | 15.2 (7.0–57.6) | 16 | 9.1 (1.7–15.5) | 5.5 (1.7–14.3) | <0.001 | <0.001 |
| NPC | 3 | 1.9 (1.3–2.4) | 3 | 19.3 (16.5–25.8) | 13.8 (10.3–15.2) | 3 | 10.9 (7.8–11.8) | 5.5 (4.9–7.9) | 0.109 | 0.109 |
| Breast cancer* | 3 | 0.8 (0.6–0.8) | 3 | 16.2 (7.1–18.9) | 27.0 (11.8–31.5) | 0 | 2.1 (1.7–2.4) | 2.1 (1.7–2.4) | 0.109 | 0.109 |
| Esophageal cancer | 4 | 2.4 (1.8–2.7) | 4 | 30.1 (21.3–40.3) | 33.4 (23.7–57.6) | 4 | 12.1 (9.1–15.5) | 7.2 (5.4–10.2) | 0.068 | 0.068 |
| NSCLC | 3 | 2.6 (2.5–6.5) | 3 | 14.8 (12.9–18.0) | 16.1 (14.5–24.7) | 3 | 7.8 (6.8–12.9) | 10.4 (3.2–14.3) | 0.18 | 0.109 |
| SCLC | 2 | 4.2 (3.6–4.8) | 2 | 9.1 (8.9–9.2) | 7.5 (6.1–8.9) | 2 | 6.7 (5.5–7.8) | 3.4 (3.2–3.5) | NA | NA |
| Pancreatic cancer | 1 | 4.3 (NA) | 1 | 36.3 (NA) | 51.9 (NA) | 1 | 13.8 (NA) | 6.6 (NA) | NA | NA |
| Ovarian cancer | 3 | 3.8 (3.2–4.5) | 3 | 12.4 (7.0–25.6) | 12.4 (7.0–14.2) | 3 | 9.9 (8.1–12.6) | 5.7 (5.0–6.8) | 0.285 | 0.109 |
| Metastases | ||||||||||
| Lymph node mets (total) | 129 | 1.1 (0.4–3.6) | 128 | 12.1 (2.0–27.1) | 13.3 (1.8–38.7) | 117 | 6.1 (1.0–13.7) | 4.1 (1.0–14.2) | <0.001 | <0.001 |
| Lung mets | 25 | 0.9 (0.3–3.5) | 19 | 8.0 (0.9–15.7) | 15.6 (1.1–39.3) | 17 | 4.9 (0.7–7.1) | 10.0 (0.9–22.3) | <0.001 | <0.001 |
| Liver mets | 14 | 1.4 (0.6–3.9) | 14 | 11.2 (3.9–19.4) | 7.3 (3.6–16.8) | 11 | 5.3 (2.3–12.0) | 1.9 (1.0–5.0) | 0.005 | 0.001 |
| Bone mets | 80 | 1.3 (0.5–8.2) | 80 | 16.2 (5.6–49.6) | 15.6 (6.3–70.9) | 64 | 5.2 (0.9–11.8) | 4.7 (0.9–13.8) | <0.001 | <0.001 |
| Peritoneal mets | 17 | NA† | 17 | 16.0 (6.4–18.8) | 16.8 (3.6–22.9) | 17 | 10.3 (4.7–14.1) | 7.1 (2.6–10.1) | <0.001 | <0.001 |
| Other mets‡ | 13 | 0.8 (0.4–2.3) | 13 | 8.6 (4.0–10.0) | 10.0 (6.3–207.5) | 9 | 6.6 (3.4–11.9) | 3.4 (1.0–6.0) | 0.196 | 0.001 |
One patient was diagnosed with multifocal breast cancer (4 primary tumors).
Lesion size cannot be calculated because of diffuse type of peritoneal metastasis (irregular shape).
Other mets included splenic (n = 2), pleural (n = 1), adrenal (n = 1), brain (n = 4), and subcutaneous (n = 5) mets.
NPC = nasopharyngeal carcinoma; SCLC = small lung cancer; mets = metastases; NA = not applicable.
Continuous data are median and range. Quantitative data from 68Ga-FAPI-RGD and 18F-FDG PET/CT were acquired at 3 h and 1 h after injection, respectively.
FIGURE 3.
Maximum-intensity-projection images of 18F-FDG and 68Ga-FAPI-RGD PET/CT in patients with different types of cancer. Ca = cancer; NPC = nasopharyngeal carcinoma.
Among the 22 patients who underwent paired 68Ga-FAPI-RGD and 18F-FDG PET/CT, 129 lymph node metastases and 149 bone and visceral metastases were evaluated (including bone [n = 80], lung [n = 25], liver [n = 14], peritoneal [n = 17], and other [n = 13] metastases). 68Ga-FAPI-RGD PET/CT revealed a significantly higher SUVmax and TBR than did 18F-FDG PET/CT in most metastatic lesions. Interestingly, the SUVmax and TBR of lymph node metastases derived from 68Ga-FAPI-RGD PET/CT were 2 to 3 times greater than those derived from 18F-FDG PET/CT (SUVmax, 12.1 vs. 6.1 [P < 0.001]; TBR, 13.3 vs. 4.1 [P < 0.001]), resulting in a greater number of metastatic lesions detected by 68Ga-FAPI-RGD than by 18F-FDG PET/CT (99% [128/129] vs. 91% [117/129], P = 0.003). Regarding imaging of bone and visceral metastases, 68Ga-FAPI-RGD PET/CT demonstrated significantly higher radiotracer uptake and a 2- to 3-fold higher TBR than did 18F-FDG PET/CT in most tumor lesions, including the lung, liver, bone, and peritoneal metastases. Consequently, 68Ga-FAPI-RGD PET/CT yielded significantly higher lesion detection rates than 18F-FDG for the diagnosis of bone and visceral metastases (96% [143/149] vs. 79% [118/149], P < 0.001). Representative PET/CT images with FAP and integrin αvβ3 staining are presented in Figure 4.
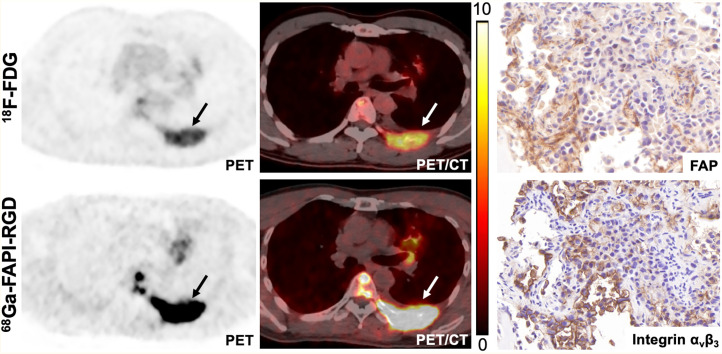
FIGURE 4.
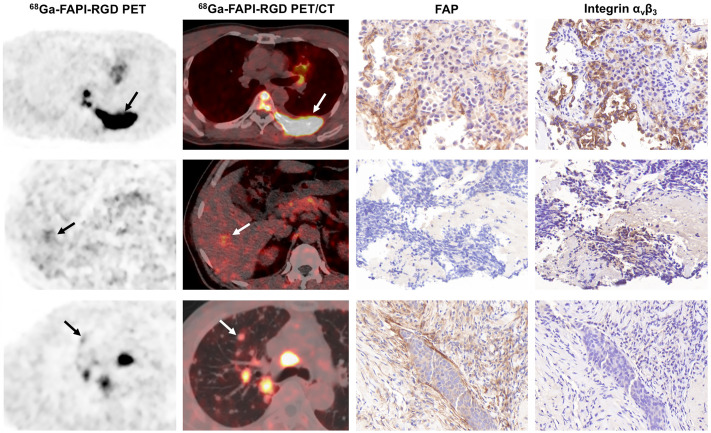
68Ga-FAPI-RGD and 18F-FDG PET/CT images in patient with metastatic lung adenocarcinoma. Immunohistochemical staining from left rib metastasis (arrow) revealed positive FAP and integrin αvβ3 expression.
Comparison of 68Ga-FAP-RGD and 68Ga-FAPI-46 Uptake in Patients with Cancer
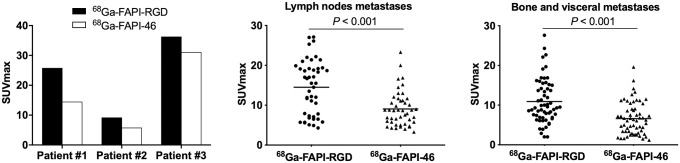
68Ga-FAPI-RGD and 68Ga-FAPI-46 PET/CT were compared among 7 patients with 5 types of cancer. Three patients were treatment-naïve, and 4 had recurrent or progressive disease. Although the SUVmax and TBR of primary tumors from 68Ga-FAPI-RGD PET/CT seemed higher than those from 68Ga-FAPI-46 (SUVmax, 25.8 vs. 14.5 [P = 0.109]; TBR, 15.2 vs. 7.6 [P = 0.109]), no statistical difference was observed (Table 2). Regarding the 102 metastatic lesions evaluated, 68Ga-FAPI-RGD PET/CT yielded a significantly higher SUVmax and TBR than did 68Ga-FAPI-46 in lymph node metastases (SUVmax, 15.5 vs. 8.7 [P < 0.001]; TBR, 13.2 vs. 8.1 [P < 0.001]), lung metastases (SUVmax, 9.5 vs. 6.4 [P = 0.004]; TBR, 18.8 vs. 12.5 [P = 0.003]), and bone metastases (SUVmax, 13.7 vs. 6.8 [P < 0.001]; TBR, 14.5 vs. 6.3 [P < 0.001]) (Fig. 5). Representative PET images are presented in Figure 6. Interestingly, in 1 patient with small cell lung cancer, 4 bone metastases and 3 liver metastases exhibited no abnormal uptake of 68Ga-FAPI-46, but uptake of 68Ga-FAPI-RGD was increased. Immunohistochemical staining of a liver metastasis demonstrated negative FAP expression but positive integrin αvβ3 expression (Fig. 7A). Notably, in another patient with nasopharyngeal carcinoma, both tracers exhibited similar uptake in several metastatic lung lesions, which demonstrated positive FAP expression and negative integrin αvβ3 expression (Fig. 7B).
TABLE 2.
Comparison of SUVmax on 68Ga-FAPI-RGD and 68Ga-FAPI-46 PET/CT Images in Primary and Metastatic Lesions
| Tumor type | n | Tumor size (cm) | Tracer | Positive lesions (n) | SUVmax | TBR | ||
|---|---|---|---|---|---|---|---|---|
| Median | P | Median | P | |||||
| Primary tumors | ||||||||
| NPC | 1 | 2.4 (NA) | 68Ga-FAPI-RGD | 25.8 | NA | 15.2 | NA | |
| 68Ga-FAPI-46 | 14.5 | 7.6 | ||||||
| SCLC | 1 | 4.8 (NA) | 68Ga-FAPI-RGD | 9.2 | NA | 6.1 | NA | |
| 68Ga-FAPI-46 | 5.8 | 3.4 | ||||||
| Pancreatic cancer | 1 | 4.3 (NA) | 68Ga-FAPI-RGD | 36.3 | NA | 51.9 | NA | |
| 68Ga-FAPI-46 | 31.1 | 38.9 | ||||||
| Total | 3 | 4.3 (2.4–4.8) | 68Ga-FAPI-RGD | 25.8 (9.2–36.3) | 0.109 | 15.2 (6.1–51.9) | 0.109 | |
| 68Ga-FAPI-46 | 14.5 (5.8–31.1) | 7.6 (3.4–38.9) | ||||||
| Metastases | ||||||||
| Lymph node mets (total) | 46 | 1.1 (0.6–2.8) | 68Ga-FAPI-RGD | 46 | 15.5 (4.3–27.1) | <0.001 | 13.2 (3.3–38.7) | <0.001 |
| 68Ga-FAPI-46 | 46 | 8.7 (3.3–23.3) | 8.1 (2.7–18.2) | |||||
| Brain mets | 4 | 0.7 (0.4–0.7) | 68Ga-FAPI-RGD | 4 | 5.8 (4.0–8.3) | 0.068 | 143.8 (80.0–207.5) | 0.068 |
| 68Ga-FAPI-46 | 4 | 3.0 (2.5–4.2) | 83.7 (65.0–85.0) | |||||
| Lung mets | 15 | 1.1 (0.5–1.4) | 68Ga-FAPI-RGD | 13 | 9.5 (2.0–15.7) | 0.004 | 18.8 (2.0–39.3) | 0.003 |
| 68Ga-FAPI-46 | 13 | 6.4 (1.6–13.3) | 12.5 (1.6–33.3) | |||||
| Liver mets | 7 | 1.1 (0.6–1.4) | 68Ga-FAPI-RGD | 7 | 6.9 (3.9–16.8) | 0.028 | 6.3 (3.6–16.8) | 0.028 |
| 68Ga-FAPI-46 | 4 | 3.4 (1.8–16.2) | 3.4 (1.8–16.2) | |||||
| Subcutaneous mets | 5 | 0.8 (0.7–0.9) | 68Ga-FAPI-RGD | 5 | 8.6 (6.6–10.0) | 0.138 | 7.2 (5.5–10.0) | 0.08 |
| 68Ga-FAPI-46 | 5 | 7.2 (6.1–9.0) | 5.1 (3.6–8.2) | |||||
| Bone mets | 29 | 1.2 (0.5–8.2) | 68Ga-FAPI-RGD | 29 | 13.7 (6.1–27.6) | <0.001 | 14.5 (6.3–27.6) | <0.001 |
| 68Ga-FAPI-46 | 25 | 6.8 (1.2–19.6) | 6.3 (1.8–21.8) | |||||
NPC = nasopharyngeal carcinoma; NA = not applicable; SCLC = small lung cancer; mets = metastases.
Data in parentheses are ranges.
FIGURE 5.
Quantitative comparison between 68Ga-FAPI-RGD and 68Ga-FAPI-46 in primary tumor (left), lymph node (middle), and bone and visceral metastases (right).
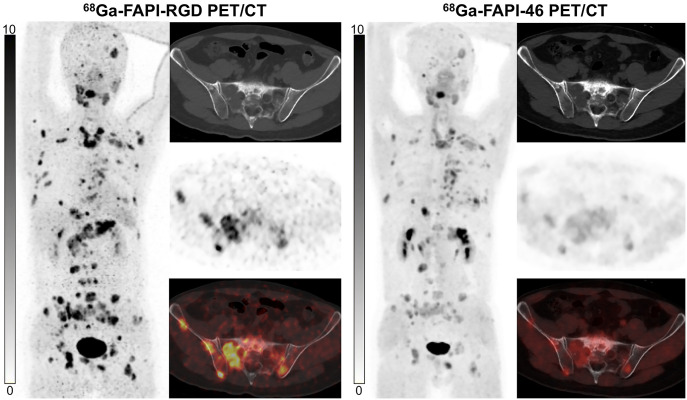
FIGURE 6.
68Ga-FAPI-RGD PET/CT shows significantly higher radiotracer uptake than 68Ga-FAPI-46 in patient with metastatic thyroid cancer.
FIGURE 7.
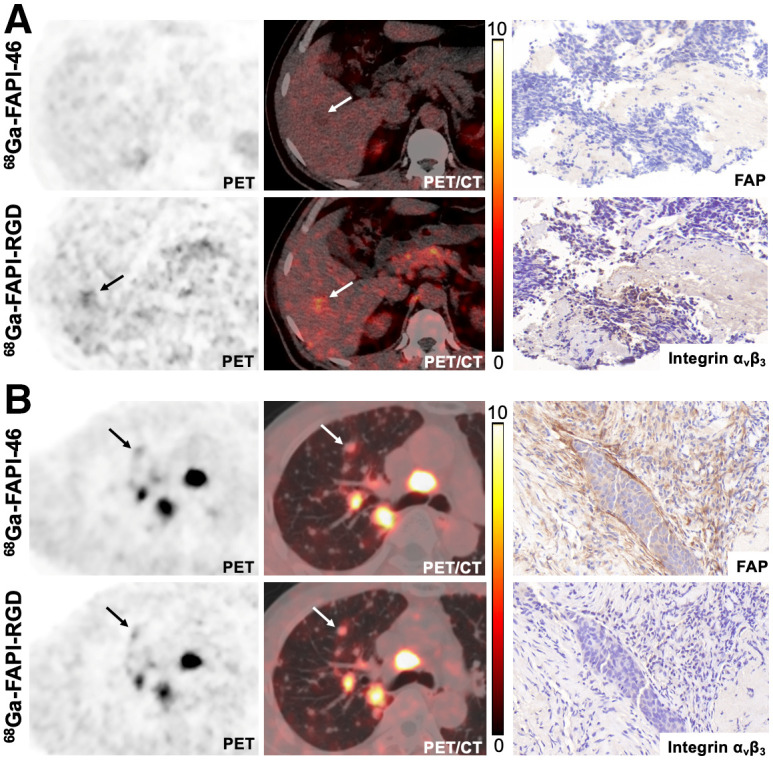
68Ga-FAPI-RGD and 68Ga-FAPI-46 PET/CT images and immunohistochemical staining in patients with metastatic small cell lung cancer (A) and nasopharyngeal carcinoma (B). Biopsy site is indicated by arrow.
DISCUSSION
As pan-cancer biomarkers, FAP with radiolabeled FAPI-based molecules (including FAPI-04/46) have yielded encouraging results for PET imaging of cancer (7,9). However, the relatively short tumor retention time may hamper the use of FAP molecules for radioligand therapy applications. We and others have applied the polyvalence effect to develop homomultimers to enhance tumor uptake and retention, and dimeric FAPI-based tracers have been synthesized and evaluated (17,18). A dual-receptor–targeting approach with a heterodimer is another strategy to improve the tumor-targeting efficacy, especially for imaging probes that recognize only 1 receptor (19). Considering that our previous data showed tumor uptake and retention of 68Ga-FAPI-RGD to be significantly greater than those of 68Ga-FAPI-46 and 68Ga-c(RGDfK) in mouse xenografts (10), we speculated that 68Ga-FAPI-RGD would be a promising radiotracer for imaging tumors expressing either FAP or integrin αvβ3. In the present study, 68Ga-FAPI-RGD, a heterodimeric PET tracer that targets both FAP and integrin αvβ3, was evaluated in 3 healthy volunteers and 22 patients with cancer. Our clinical studies demonstrated 68Ga-FAPI-RGD to be a promising PET agent that allows imaging of various types of cancer. Its dual-receptor–targeting property results in improved tumor uptake and retention, allowing imaging of tumors with either or both receptor expression patterns.
68Ga-FAPI-RGD was safe and well tolerated in all healthy volunteers and patients with cancer. The average effective whole-body dose of 68Ga-FAPI-RGD was 1.01 × 10−2 mSv/MBq, which is comparable to the effective doses of 68Ga-FAPI-02 and 68Ga-FAPI-04 (1.64 × 10−2 mSv/MBq and 1.80 × 10−2 mSv/MBq) (16). However, intense physiologic 68Ga-FAPI-RGD uptake was observed in the thyroid and pancreas, with a distribution pattern similar to that of 68Ga-FAPI dimers previously reported by us and others (17,18). Interestingly, significantly decreased 68Ga-FAPI-RGD activity was observed in normal organs (particularly in the thyroid, pancreas, and salivary glands), whereas increased uptake was observed in tumor lesions from 0.5 to 3 h after injection, resulting in optimized lesion contrast in delayed scans. Therefore, we speculate that additional delayed 68Ga-FAPI-RGD PET/CT scans may help improve the lesion detection rate and offer advantages for discrimination of tumor and nontumor lesions. However, the clinical benefits of delayed scans require further investigation in a larger patient population.
The SUVmax and TBR of primary tumors from 68Ga-FAPI-RGD PET/CT were significantly higher than those from 18F-FDG PET/CT, particularly in non–small cell lung cancer (NSCLC) and in esophageal, breast, and pancreatic cancers. The reason for this finding is that all primary tumors (19/19) were satisfactorily visualized via 68Ga-FAPI-RGD, whereas 3 breast cancer lesions were missed via 18F-FDG. 68Ga-FAPI-RGD PET/CT also demonstrated significantly greater radiotracer uptake in lymph node, bone, and visceral metastases and a significantly higher TBR than 18F-FDG PET/CT, resulting in an improved lesion detection rate, particularly for the diagnosis of lymph node (99% vs. 91%) and bone (100% vs. 80%) metastases. However, in our previous 68Ga-FAPI-RGD PET/CT study on 6 patients, the tumor uptake of 68Ga-FAPI-RGD did not differ from that of 18F-FDG (10). Possible explanations may be the limited number of patients, different cancer types investigated, and different acquisition time after injection (30–120 min vs. 60–180 min). According to the results of the present study, 68Ga-FAPI-RGD may be more suitable for imaging tumors with both FAP and integrin αvβ3 expression, particularly for NSCLC and esophageal cancer (20,21). First, the radiotracer uptake and TBR derived from 68Ga-FAPI-RGD PET/CT were higher than those from 18F-FDG in these cancer types, resulting in improved lesion detectability, particularly of liver, bone, and brain metastases. These results suggest that 68Ga-FAPI-RGD PET/CT may contribute to the diagnosis of NSCLC and esophageal cancer, especially in detecting small metastases with low-to-moderate uptake on 18F-FDG PET/CT. Second, false-positive findings in the mediastinal lymph nodes often confound interpretation of preoperative 18F-FDG PET/CT images in NSCLC and esophageal cancer. On the basis of previous publications, nonmetastatic reactive lymph nodes presenting increased 18F-FDG uptake might be correctly diagnosed either by FAP or by integrin αvβ3–targeting radiotracers (21–24). Therefore, 68Ga-FAPI-RGD PET/CT may be more suitable than 18F-FDG PET/CT for determining the preoperative lymph node status in these cancer types. Taken together, the results indicate that NSCLC and esophageal cancer may be potential indications for future clinical use of 68Ga-FAPI-RGD, and further prospective studies are warranted to confirm this possibility.
PET imaging demonstrated that the radiotracer uptake and TBR of 68Ga-FAPI-RGD were significantly higher than those of 68Ga-FAPI-46, especially in the involved lymph node, bone, and visceral metastases. Interestingly, we noted that several lesions from metastatic small cell lung cancer and thyroid cancer exhibited low uptake of 68Ga-FAPI-46 but higher uptake of 68Ga-FAPI-RGD. Histopathologic results revealed low expression of FAP but higher expression of integrin αvβ3. Therefore, 68Ga-FAPI-RGD PET/CT may be used to image tumors that are either FAP-positive or integrin αvβ3–positive, whereas 68Ga-FAPI-46 failed to visualize lesions that were FAP-negative and integrin αvβ3–positive. Moreover, both tracers showed similar uptake in lesions with FAP-positive and integrin αvβ3–negative expression, suggesting comparable FAP-targeting ability between 68Ga-FAPI-RGD and 68Ga-FAPI-46. On the basis of these findings, we speculate that 68Ga-FAPI-RGD PET/CT would be superior to 68Ga-FAPI-46 PET/CT for the diagnosis of cancer, especially when 68Ga-FAPI-46 PET/CT findings are inconclusive.
With the rather rapid washout from tumors and unsatisfactory therapeutic efficacy, 177Lu-FAPI-04/46 may not be an optimal targeting vector for radioligand therapy (25,26). Besides the improved tumor uptake of 68Ga-FAPI-RGD, the prolonged tumor retention was an unexpected finding in this study, as may be explained by the synergistic interaction between the 2 binding motifs in the heterodimer. It is possible that the binding of the first motif, even if only temporary, may first direct 68Ga-FAPI-RGD to the target surface or reduce the off-rate of 68Ga-FAPI-RGD, allowing the second binding motif to also attach to the tumor and therefore increasing the overall binding and the probability of adhering to the tumor (27). Improved tumor accumulation and prolonged tumor retention are the potential advantages of FAPI-RGD over the current FAPI variants, making it a suitable targeting vector after labeling with 177Lu/90Y/225Ac for therapeutic applications. In addition, the rapid clearance of 68Ga-FAPI-RGD in normal organs, particularly in the thyroid, salivary glands, and blood pool, may decrease the absorbed dose delivered to normal human tissues. This possibility should be extensively studied in nuclear oncology research in the future.
Our study had several limitations. First, because of the relatively short half-life of 68Ga, the in vivo distribution pattern and tumor retention of FAPI-RGD could not be fully investigated. Second, because few patients underwent paired 68Ga-FAPI-RGD/18F-FDG (n = 22) or 68Ga-FAPI-RGD/68Ga-FAPI-46 PET/CT imaging (n = 7), only a descriptive comparison was possible. Third, this study focused primarily on lesion detection rates, and the specificity of 68Ga-FAPI-RGD PET/CT requires further investigations with histopathologic confirmation. Furthermore, we were unable to compare 68Ga-FAPI-RGD PET/CT with 68Ga-RGD PET/CT in the same group of patients because of ethical considerations. 68Ga-FAPI-RGD and 68Ga-RGD PET/CT should be compared in future clinical research.
CONCLUSION
The study demonstrated the safety and clinical feasibility of 68Ga-FAPI-RGD PET/CT for imaging of various types of cancer. Further investigation of the diagnostic accuracy of 68Ga-FAPI-RGD as a PET tracer, as well as its antitumor efficacy after labeling with therapeutic radionuclides, is necessary.
DISCLOSURE
This work was funded by the National Natural Science Foundation of China (82071961), Key Scientific Research Program for Young Scholars in Fujian (2021ZQNZD 016), Fujian Natural Science Foundation for Distinguished Young Scholars (2022J01310623), Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare, Key Medical and Health Projects in Xiamen (3502Z20209002), The National University of Singapore Start-up Grant (NUHSRO/2020/133/Startup/08), NUS School of Medicine Nanomedicine Translational Research Programme (NUHSRO/2021/034/TRP/09/Nanomedicine), and National Medical Research Council (NMRC) Centre Grant Programme (CG21APR1005). Liang Zhao was partially funded by the China Scholarship Council (CSC). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Will a heterodimer that recognizes both FAP and integrin αvβ3 yield improved tumor uptake compared with 18F-FDG or 68Ga-FAPI-46?
PERTINENT FINDINGS: This clinical study revealed that the tumor uptake and TBR of 68Ga-FAPI-RGD were significantly higher than those of either 18F-FDG or 68Ga-FAPI-46 in various types of cancer.
IMPLICATIONS FOR PATIENT CARE: The dual-receptor–targeting property of 68Ga-FAPI-RGD results in improved tumor uptake compared with 18F-FDG or 68Ga-FAPI-46, enabling imaging of tumors with either or both receptor expression patterns.
REFERENCES
- 1. Pang X, He X, Qiu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen H, Niu G, Wu H, Chen X. Clinical application of radiolabeled RGD peptides for PET imaging of integrin αvβ3 . Theranostics. 2016;6:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao L, Chen H, Guo Z, et al. Targeted radionuclide therapy in patient-derived xenografts using 177Lu-EB-RGD. Mol Cancer Ther. 2020;19:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlsen EA, Loft M, Loft A, et al. Prospective phase II trial of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET/CT imaging of integrin αvβ3 for prognostication in patients with neuroendocrine neoplasms. J Nucl Med. 2023;64:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–959. [DOI] [PubMed] [Google Scholar]
- 6. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. [DOI] [PubMed] [Google Scholar]
- 7. Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loktev A, Lindner T, Mier W, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sollini M, Kirienko M, Gelardi F, Fiz F, Gozzi N, Chiti A. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:4396–4414. [DOI] [PubMed] [Google Scholar]
- 10. Zang J, Wen X, Lin R, et al. Synthesis, preclinical evaluation and radiation dosimetry of a dual targeting PET tracer [68Ga]Ga-FAPI-RGD. Theranostics. 2022;12:7180–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen X, Xu P, Shi M, et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. 2022;12:422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pang Y, Zhao L, Meng T, et al. PET imaging of fibroblast activation protein in various types of cancer using 68Ga-FAP-2286: comparison with 18F-FDG and 68Ga-FAPI-46 in a single-center, prospective study. J Nucl Med. 2023;64:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitsuyuki K, Watabe T, Naka S, et al. Evaluation of integrin αvβ3 expression in murine xenograft models: [68Ga]Ga-DOTA-C(RGDfK) PET study with immunohistochemical confirmation. Diagnostics (Basel). 2021;11:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 15. Chen H, Zhao L, Fu K, et al. Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics. 2019;9:7948–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giesel FL, Kratochwil C, Lindner T, et al. 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2019;60:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moon ES, Ballal S, Yadav MP, et al. Fibroblast activation protein (FAP) targeting homodimeric FAP inhibitor radiotheranostics: a step to improve tumor uptake and retention time. Am J Nucl Med Mol Imaging. 2021;11:476–491. [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao L, Niu B, Fang J, et al. Synthesis, preclinical evaluation, and a pilot clinical PET imaging study of 68Ga-labeled FAPI dimer. J Nucl Med. 2022;63:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boinapally S, Lisok A, Lofland G, et al. Hetero-bivalent agents targeting FAP and PSMA. Eur J Nucl Med Mol Imaging. 2022;49:4369–4381. [DOI] [PubMed] [Google Scholar]
- 20. Gao S, Wu H, Li W, et al. A pilot study imaging integrin αvβ3 with RGD PET/CT in suspected lung cancer patients. Eur J Nucl Med Mol Imaging. 2015;42:2029–2037. [DOI] [PubMed] [Google Scholar]
- 21. Dong Y, Wei Y, Chen G, et al. Relationship between clinicopathological characteristics and PET/CT uptake in esophageal squamous cell carcinoma: [18F]alfatide versus [18F]FDG. Mol Imaging Biol. 2019;21:175–182. [DOI] [PubMed] [Google Scholar]
- 22. Jin X, Liang N, Wang M, et al. Integrin imaging with 99mTc-3PRGD2 SPECT/CT shows high specificity in the diagnosis of lymph node metastasis from non-small cell lung cancer. Radiology. 2016;281:958–966. [DOI] [PubMed] [Google Scholar]
- 23. Shang Q, Zhao L, Pang Y, Meng T, Chen H. Differentiation of reactive lymph nodes and tumor metastatic lymph nodes with 68Ga-FAPI PET/CT in a patient with squamous cell lung cancer. Clin Nucl Med. 2022;47:458–461. [DOI] [PubMed] [Google Scholar]
- 24. Zhou X, Wang S, Xu X, et al. Higher accuracy of [68Ga]Ga-DOTA-FAPI-04 PET/CT comparing with 2-[18F]FDG PET/CT in clinical staging of NSCLC. Eur J Nucl Med Mol Imaging. 2022;49:2983–2993. [DOI] [PubMed] [Google Scholar]
- 25. Assadi M, Rekabpour SJ, Jafari E, et al. Feasibility and therapeutic potential of 177Lu-fibroblast activation protein inhibitor-46 for patients with relapsed or refractory cancers: a preliminary study. Clin Nucl Med. 2021;46:e523–e530. [DOI] [PubMed] [Google Scholar]
- 26. Zhao L, Chen J, Pang Y, et al. Fibroblast activation protein-based theranostics in cancer research: a state-of-the-art review. Theranostics. 2022;12:1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chittasupho C. Multivalent ligand: design principle for targeted therapeutic delivery approach. Ther Deliv. 2012;3:1171–1187. [DOI] [PubMed] [Google Scholar]