Abstract
Ganoderma lucidum is a valuable medical macrofungus with a myriad of diverse secondary metabolites, in which triterpenoids are the major constituents. This paper introduced the germplasm resources of genus Ganoderma from textual research, its distribution and identification at the molecular level. Also we overviewed G. lucidum in the components, the biological activities and biosynthetic pathways of ganoderic acid, aiming to provide scientific evidence for the development and utilization of G. lucidum germplasm resources and the biosynthesis of ganoderic acid.
Keywords: chemical constituents, Ganoderma lucidum (Leyss. ex Fr.) Karst., ganoderic acid, germplasm resources, pharmacological activities, secondary metabolism regulation
1. General situation of genus Ganoderma germplasm resources
Ganoderma lucidum (Leyss. ex Fr.) Karst., also known as “Lingzhi”, “Reishi” and “Yeongji”, mainly grows on rotten roots in tropical and subtropical regions. It has two parts, kidney-shaped semicircular or nearly circular cap and stipe (Chen & Li, 2004). As a medicinal mushroom, G. lucidum has anti-HIV and anti-tumor activities, and can also treat coronary heart disease, diabetes, hypertension and other diseases (Lin, 2019, Xie et al., 2021). With the increase of human diseases and the enhancement of health care awareness, G. lucidum has attracted more and more attention.
1.1. Textual research of genus Ganoderma
G. lucidum has a history of more than 6800 years in China, and its culture can be traced back to the Hemudu Period (5000 B.C.) (Yuan et al., 2018). In the historical data of the pre-Qin period, there are many textual researches on the medicinal effects of G. lucidum. As the “fairy medicine” sought by the first Emperor of Qin (221 B.C.), G. lucidum has a high historical status since ancient times (Zhang, 2022). Compendium of Materia Medica records that the medicinal effect of G. lucidum is suitable for people of any constitution. Its medicinal properties are warm-natured. It can treat mental diseases, nourish the spleen and stomach, increase wisdom, enhance memory, and is beneficial to longevity. Shennong's Classic of Materia Medica divides G. lucidum into five categories according to the colours: Qing Zhi, Chi Zhi, Huang Zhi, Bai Zhi, and Hei Zhi. Qing, Chi, Huang, Bai and Hei mean light green, red, yellow, white and black in colour in Chinese, respectively. In addition, there is a type of Zi Zhi, where Zi means purple in colour (Lin, 2019). In 2000, Chinese Pharmacopoeia was published for the first time to confirm the medicinal value of genus Ganoderma. In 2001, the Ministry of Health issued the List of Fungal Species that Can Be Used in Health Food that recognized the medicinal value of three Ganoderma species: G. lucidum, G. sinensis J.D. Zhao, L.W. Hsu & X.Q. Zhang, and G. tsugae Murr. (Shi et al., 2012) in China.
1.2. Distribution of genus Ganoderma
Ganoderma was established in 1881 (Karsten, 1881). Donk put forward the concept of Ganoderma subfamily in 1933, and promoted the subfamily to family in 1948 (Chen & Li, 2004). At present, according to the statistics of Index Fungorum (http://www.indexfungorum.org/names/Names.asp), 489 records of genus Ganoderma can be found (as of October 2022). The genus Ganoderma is represented by more than 260 species, which are distributed in northern and southern Europe, Central Africa, South America, East Asia and other regions (Cai, He, & An, 2016). China has abundant germplasm resources with wide distribution. There are over 100 species of Ganoderma, distributed in the southwestern, northwestern, and northeastern regions (Wu, Dai & Lin, 2004).
Among the three mentioned species of genus Ganoderma with medicinal value, G. lucidum mainly distributed in East Asia. G. lucidum is mainly distributed in Hubei, Guangxi, Guangdong, Jilin and Jiangxi provinces in China. G. sinensis grows mainly in China, Korea and Japan in the world. In China, it grows mainly in Guangxi, Guizhou, Hainan, Taiwan and Yunnan Provinces. G. tsugae are mainly distributed in Northwest and Northeast of China and the United States. (Wu, Dai, & Lin, 2004). Table 1 showed the distribution of G. lucidum, G. sinensis and G. tsugae in China.
Table 1.
Distribution of G. lucidum, G. Sinensis and G. tsugae in various provinces of China.
| Provinces | Number of species |
|---|---|
| Beijing | 3 |
| Hebei | 6 |
| Tianjin | 1 |
| Heilongjiang | 4 |
| Jilin | 8 |
| Liaoning | 1 |
| Inner Mongolia Autonomous Region | 3 |
| Gansu | 1 |
| Shanxi | 2 |
| Shandong | 3 |
| Henan | 3 |
| Shaanxi | 1 |
| Jiangsu | 6 |
| Zhejiang | 8 |
| Fujian | 20 |
| Taiwan | 6 |
| Jiangxi | 9 |
| Anhui | 13 |
| Hubei | 4 |
| Hunan | 8 |
| Guangdong | 16 |
| Hong Kong Special Administrative Region | 2 |
| Hainan | 78 |
| Guangxi Zhuang Autonomous Region | 33 |
| Sichuane | 26 |
| Tibet Autonomous Region | 5 |
| Guizhou | 49 |
| Yunnan | 41 |
Ganoderma germplasm resources are abundant and have broad industrial prospects. At present, G. lucidum is the most species available in the market, so we will mainly focus on G. lucidum for the subsequent discussion. It is urgent to breed high-quality G. lucidum varieties to meet market demand. G. lucidum are mainly classified into two kinds: wild G. lucidum and cultivated G. lucidum. In order to promote the development of G. lucidum industry, it is necessary to collect its germplasm resources, conduct metabolome, transcriptome and proteome sequencing, select and breed high quality G. lucidum varieties. By comparing cultivars such as Xianzhi No. 1, Xianzhi No. 2 and Xianzhi No. 3, Xu et al. (2021) found that the genus Ganoderma spore and sporocarp of Xianzhi No. 3 had the highest content of polysaccharides, triterpenes and other bioactive substances. Wang and others collected more than 50 wild varieties of G. lucidum, adopted systematic breeding method, and finally selected the short growth cycle, good fecundity, stability and adaptability of ‘Yu Ze’ G. lucidum (Xia, Yang, Li & Xia, 2018). The breeding of each exceptional G. lucidum cultivar holds great potential for widespread adoption.
1.3. Identification of genus Ganoderma germplasm at molecular level
The application and development of molecular biology provided a new method for the identification of genus Ganoderma germplasm resources. Based on the whole genome sequencing results of G. lucidum, DNA sequence and cluster analysis can eliminate the interference caused by external environmental factors and human factors, and improve the accuracy of Ganoderma species identification at the molecular level. As early as 2012, Chen’s team has completed the genomic framework map of G. lucidum to reveal the molecular mechanism of active ingredient biosynthesis in Chinese medicine, which established a solid foundation for accelerating the selection and breeding of high quality and high yielding G. lucidum varieties (Chen et al., 2012). Recently, the following genomes of the genus Ganoderma have been published in National Center for Biotechnology Information (NCBI): G. lucidum, G. multipileum, G. sinense, G. leucocontextum, G. tsugae, G. meredithae, G. boninense, Ganoderma sp. (https://www.ncbi.nlm.nih.gov/genome/?term=ganoderma). The publication of these genomes has laid a solid foundation for the identification of germplasm resources within the Ganoderma genus.
Random amplified polymorphic DNA (RAPD) (Huang, Xu, & Zhou., 2013), internal transcribed spacer (ITS) (Schoch et al., 2012), simple sequence repeat (SSR) (He et al., 2022), inter-simple sequence repeat (ISSR) (He et al., 2022), sequence-related amplified polymorphism (SRAP) (Sun et al., 2006), sequence characterized amplified regions (SCAR) (Kwon, Lee, & Park, 2019) and other technologies have been widely used in the identification of genus Ganoderma. Zhang used SSR molecular markers to construct molecular IDs for the genus Ganoderma, and used the Genetics Statistics software and ID Analysis software to analyze the specificity index and molecular IDs, resulting in the successful clustering of 11 Ganoderma strains into four categories. Researchers found that 15 specific ISSR primers can be used for intraspecific identification and polymorphism analysis of G. lucidum (He et al., 2022). Sun et al. (2006) was the first to apply SRAP technology to the systematics of genus Ganoderma strains in 2006 and successfully classified these 31 Ganoderma strains into five groups. RAPD technology was used to classify eight species of genus Ganoderma into two groups (Wang, Su, & Lv, 2011). G. lucidum was distinguished from Asia and Europe by ITS sequence analysis (Liao et al., 2015). Using 60 primer pairs, 24 SSR loci with polymorphic bands were screened based on the results of cluster analysis to obtain the affinities among the strains, which is useful for analyzing the genetic diversity of strains (Xu et al., 2020). Therefore, the development of molecular biology provided new methods for Ganoderma classification. Researchers can more clearly delineate genus Ganoderma by sequencing whole genome and building a phylogenetic tree.
2. Research progress of main chemical constituents in G. lucidum
G. lucidum, as a model organism of fungi, has a wide range of chemical composition, including polysaccharides, triterpenoids, proteins, amino acids, sterols, alkaloids, trace elements and other components (Zhang et al., 2021). Polysaccharides and triterpenoids are the main active components of G. lucidum (Zhang, Zhou, & Song, 2018). The triterpenoids have complex structures. According to the different functional groups and side chain structures connected to triterpenoids, they are divided into ganoderic acids, ganoderiol, ganoderal and ganolactone.
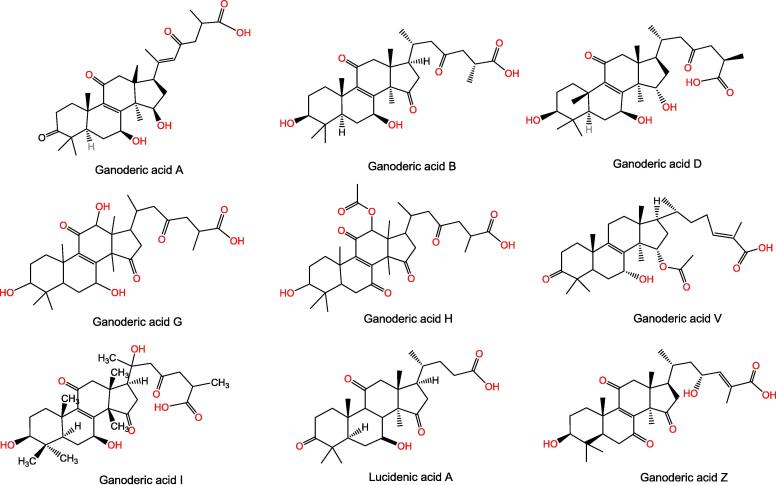
Ganoderic acids are the main triterpenoids compounds, and their physiological activity is mainly determined by different side chain groups. The content of ganoderic acids (ganoderic acid A and ganoderic acid B) is one of the main bases for evaluating the quality of G. lucidum. The higher the content of ganoderic acids is, the better the quality of G. lucidum (Yan et al., 2019) is. In 1982, for the first time scientists isolated the two kinds of ganoderic acids (ganoderic acid A and ganoderic acid B) from the genus Ganoderma, ganoderic acid A and ganoderic acid B (Kubota, Asaka, Miura, & Mori, 1982). At present, there are more than 380 triterpenoids isolated from genus Ganoderma, among which there are about 171 ganoderic acids (Baby, Johnson, & Govindan, 2015). Chemical structures of ganoderic acids were in Fig. 1. The research shows that the composition and content of ganoderic acid vary in, different species, different parts, different growth periods and different producing areas. Yan et al. (2019) compared the contents of ganoderic acid from different origins and found that the total contents of ganoderic acid A and ganoderic acid B were the highest in G. lucidum from Anhui Province of China. The triterpene content of wild G. lucidum is far lower than that of cultivated G. lucidum, and the total triterpene content is only one tenth of that of cultivated G. lucidum. Among the 12 kinds of ganoderic acids, ganoderic acid A accounts for the highest proportion (Jia et al., 2017). Ganoderic acid is extracted from the cap and stipe of G. lucidum (Zhang et al., 2020). The content of ganoderic acid in the cap is higher than in the stipe, and the main ganoderic acid in the cap is ganoderic acid T, Mk, Me and S. Ganoderic acid in the stipe mainly includes ganoderic acid A, B and C. In addition, at different growth stages of G. lucidum, the content of ganoderic acid A and D was higher at the maturity stage, while the content of ganoderic acid B, C2 and G was higher at the bud stage (Ren et al., 2020).
Fig. 1.
Chemical structures of ganoderic acids.
We focus mainly on G. lucidum, G. tsugae, and G. sinensis, the three species with medicinal value to explore the distribution pattern and characteristics of the content of ganoderic acids in genus Ganoderma. G. sinensis does not contain ganoderic acids A, B, C2, G, E or other ganoderic acid triterpenoids, while G. lucidum is rich in ganoderic acid triterpenoids (Ding, Huang, Qiu, Liang, & Wang, 2009). G. tsugae contains ganoderic acid A, B, C and I, ganodermanontriol, ganoderiol A, ganodermatrio, lucidone A, lucidenic acid C and LM1 (Liu, 2009). Therefore, the triterpenoids in G. lucidum and G. tsugae are closer to each other, while G. sinensis contains fewer triterpenoids, but mainly polysaccharides and sphingolipids. In addition, the content of triterpene acids in the fruiting body is higher than that in the spore powder; The broken-wall spore powder had higher triterpene acids content than the unbroken-wall spore powder; Compared with G. lucidum strains not Spaceship-Carried, the triterpene acids content of G. lucidum strains Spaceship-Carried increased.
3. Pharmacological activities of ganoderic acids
The secondary metabolism process of plants is the result of the plant adaptation to the ecological environment changes in long-term evolution, and it plays an important role in dealing with the relationship between plants and the ecological environment. Ganoderic acid, the main secondary metabolite of G. lucidum, has a complex chemical structure and extensive pharmacological activities (Chen, 2020), with anti-toxic and anti-bacterial effects, it prevents cardiovascular diseases, protects the liver and prevents epilepsy (Xing, Liu, He, & Chen, 2017).
Cancer is one of the diseases with the highest mortality. At present, chemotherapy is the main treatment scheme for cancer, and chemotherapy-related fatigue (CRF) is one of the main side effects of chemotherapy on patients, which seriously affects the recovery of patients’ physical functions. Relieving chemotherapy-related fatigue is also one of the main medical problems at present. The latest experimental research showed that ganoderic acid can improve mitochondrial activity, promote ATP production, increase sugar content, reduce lactic acid content, and improve the function of muscle fatigue, thus ganoderic acid provides the basis for clinical treatment of CRF (Abulizi et al., 2021). Furthermore, ganoderic acid can specifically bind to tubulin, which provides a direction for microtubulin-targeted anticancer drug design (Kohno et al., 2017). Sorafenib (SRF) has been recognized as the treatment of advanced liver cancer as early as 2007, but its nonspecific intake will cause serious side effects (Zhu, Zheng, Wang, & Chen, 2017). Ganoderic acid can assist SRF in the synergistic treatment of liver cancer, and constructing nano lipidic carriers (NLCs) loaded with SRF and ganoderic acid can effectively restore liver parameters, non-liver parameters and inflammatory indicators, which are close to normal levels (Wang et al., 2021). The normal levels of TNF-α in macrophage can inhibit tumorigenesis and viral replication but its overexpression is the main cause of asthma (Landskron, Marjorie, Thuwajit, Thuwajit, & Hermoso, 2014). Both aqueous extract of three medicinal herbs (G. lucidum, Sophora flavescens Ait and Glycyrrhiza uralensis Fischer) and ganoderic acid in G. lucidum can inhibit the production of TNF-α. Ganoderic acid C1 has the strongest inhibitory effect on TNF-α, which is dose-dependent and non-cytotoxic. TNF-α is not completely inhibited, but the level of TNF-α is regulated by signal pathway (Liu et al., 2015). Treatment of cervical cancer cells (Hela cells) with different concentrations of ganoderic acid T, and then irradiated by gamma rays, can induce apoptosis and necrosis of HeLa cells, and with the increase of ganoderic acid T concentration, the necrosis rate also increases (Shao et al., 2021).
Ganoderic acid not only has irreplaceable effect on cancer treatment, but also has high curative effect on the treatment of other diseases; such as multiple sclerosis (MS) (Castillo-Trivino, Braithwaite, Bacchetti, & Waubant, 2013), major depression (MDD) (Bao et al., 2021), cardiovascular disease (Ren, 2019), renal fibrosis and other diseases (Geng et al., 2020). Ganoderic acid A enters the central nervous system through the blood–brain barrier and activates the bile acid receptor FXR to promote the formation of regenerative myelin in the central nervous system for repair and regeneration (Jia et al., 2021). It also regulates the neuroimmune system to achieve antidepressant effect (Bao et al., 2021). Ganoderic acid A can improve renal fibrosis and relieve renal dysfunction by inhibiting the over-activation of TGF-β/Smad signal, the expression of fibronectin and the deposition of extracellular matrix (ECM) in kidney (Geng et al., 2020). Ganoderic acid A activates PI3K/AKT and mTOR by up-regulating miR-153, thereby improves the symptoms of cerebral hypoxia, provides a scheme for the treatment of cardiovascular and cerebrovascular diseases (Li et al., 2020). It also inhibits the release of histamine from cells and promotes the digestion of various organs of the digestive system (Lei, 2019). Ganoderic acid A can inhibit the expression of pro-inflammatory factors and promote the expression of anti-inflammatory factors, which in turn inhibit inflammatory activity. β-Galactosidase is a senescence-related marker. Ganoderic acid D can delay aging in human amniotic mesenchymal stem cell (HAMSC) by activating the PERK/NRF2 signaling pathway, drastically limiting the production of β-galactosidase in a dose-dependent manner, having no cytotoxic side effect, preventing cell cycle arrest, and improving telomerase activity (Xu et al., 2020). The researchers also found that ganoderic acid T induces P53 protein expression and promotes apoptosis (Tang, Wu, Wang, Sun, & Ouyang, 2015). In addition, ganoderal can delay Alzheimer’s disease through DNA methylation (Lai et al., 2019).
4. Biosynthetic pathways of ganoderic acid
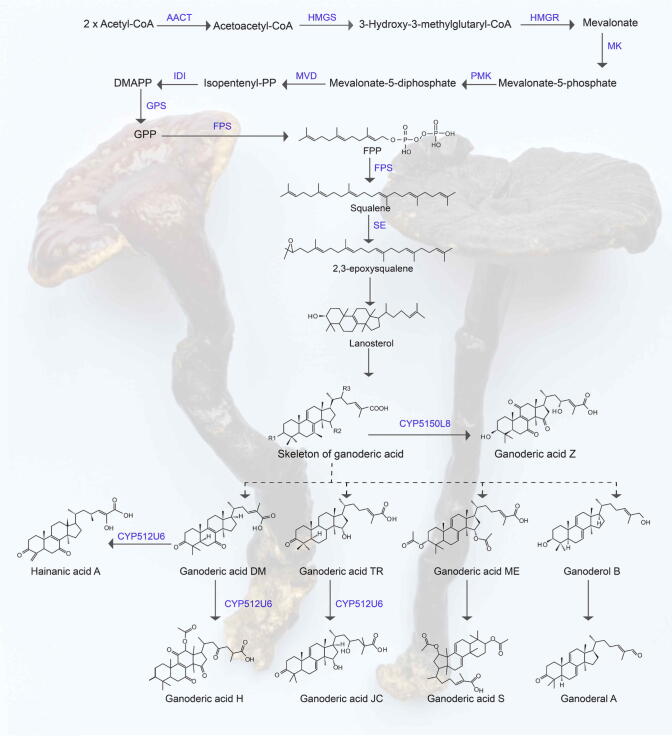
Ganoderic acid which is structurally similar to highly oxidized lanosterol-derived compounds of triterpenoids containing a carboxyl structure (Zhao, Xu, & Zhong, 2011), and is an important secondary metabolite of G. lucidum. It was found that the synthetic pathway of ganoderic acid follows the mevalonate pathway (Shiao, 2013). The biosynthesis of ganoderic acid has three stages: the generation of mevalonate precursors, the synthesis of the lanosterol skeleton, and the biosynthesis of ganoderic acid. However, the specific synthetic pathway from lanosterol to ganoderic acid is very complex, and in recent decades, there has been no specific pathway, that represents a difficult problem in the research field. It is speculated that there may be a series of redox reactions of lanosterol. The synthetic pathway of lanosterol has been studied in detail, but the biosynthetic process from lanosterol to ganoderic acid has not been studied in detail so far. The ganoderic acid biosynthesis pathway was shown in Fig. 2.
Fig. 2.
Biosynthetic pathway of ganoderic acid (Shiao, 2013; Ren et al., 2013).
Lanosterol synthase (LS), farnesyl pyrophosphate synthase (FPS), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) and squalene synthase (SQS) are the key enzymes in the ganoderic acid synthesis pathway (Shi, Ren, Mu, & Zhao, 2010). The synthesis of lanosterol from 2,3-epoxysqualene is catalyzed by LS, and the expression of lanosterol synthase gene and the content of ganoderic acid are positively correlated (Luo, Li, & Xu, 2022). LaeA is a global regulatory gene, and the production of ganoderic acid is positively correlated with the transcription level of LaeA gene (Luo et al., 2022). HMGR is the rate-limiting enzyme which is responsible for catalyzing the reduction of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) to mevalonate (Zhao, Zhong, Liang, Wang, & Jong, 2004). The synthesis of farnesyl pyrophosphate (FPP) into squalene (SQ) is at a metabolic branch point, this chemical reaction is catalyzed by SQS. Therefore, LS, FPS, HMGR and SQS play a decisive role in the synthesis of ganoderic acid.
Many regulatory factors influence the biosynthesis of ganoderic acid. The newly sequenced genome of G. lucidum in Changbai Mountain, Jilin Province, China, 360 genes are identified to be involved in triterpenoid biosynthesis, 300 genes belong to cytochrome P450 (CYP450), and 18 genes are the main genes of triterpenoid biosynthesis pathway, which is the genome with the largest number of triterpenoid biosynthesis in G. lucidum genome report at present. It is speculated that the increase of triterpenoid genes is one of the main reasons for the increase of triterpenoid content (Tian et al., 2021). It was found that when HMGR, FPS and SQS transcripts reached their highest levels, so did ganoderic acid B, C2 and G (Ren et al., 2020). Studies have shown that multiple action factors regulate the levels of ganoderic acid by regulating the levels of reactive oxygen species (ROS). For example, Cu2+ stress can regulate three key enzyme genes in the ganoderic acid biosynthesis pathway by regulating ROS levels. Under Cu2+ stress, ROS were produced excessively, and the expression of key enzymes (SQS, oxidosqualene cyclase, HMGR) genes is up-regulated, which in turn regulating ganoderic acid biosynthesis and mycelial growth (Gao et al., 2019). Increased ROS content accelerates methyl jasmonate (MeJA)-induced ganoderic acid biosynthesis (Jiang et al., 2019). AreA is a GATA-type transcription factor that inhibits ganoderic acid biosynthesis through the induction of intracellular NO production (Zhu, Sun, Shi, & Song, 2019). Under heat stress (HS) conditions, ROS content raised, regulatory heat shock protein (HSP) expression enriched, and eventually ganoderic acid content increased (Zhang et al., 2016). Nitrogen oxides also play an irreplaceable role in the regulation of ganoderic acid biosynthesis. Nitric oxide (NO) can reduce the content of mitROS by inhibiting the activity of aconitase, which in turn affects the biosynthesis of ganoderic acid. APSES family transcription factors are unique to fungi (Doedt et al., 2004). GlSwi6, an APSES family transcription factor, was identified from G. lucidum and found to be involved in G. lucidum substratum development and regulation of ganoderic acid biosynthesis (Zhang et al., 2018). Calcium signal plays a key role in calcium-regulated phosphatase-mediated regulation of ganoderic C acid biosynthesis, and Ca2+ can enhance ganoderic acid biosynthesis (Han & Zhong, 2020).
Previous studies have shown that many CYP450 genes are involved in Ganoderma triterpene synthesis. CYP512U6 is an important gene responsible for Ganoderma triterpene synthesis, and the CYP512U6 gene was highly related to the content of eight Ganoderma triterpene components. Therefore, the researchers speculated that the contribution of the CYP512U6 gene may not have been limited to the synthesis of hainanic acid H, ganoderic acid Jc, and ganoderic acid ZXYL (Jiang et al., 2022). CYP5150L8 is also a key gene that regulates lanosterol into ganoderic acid biosynthesis. In vitro enzyme experiments showed that CYP5150L8 catalyzes the three-step oxidation of lanosterol at c-26 to synthesize 3-hydroxy-lanosta-8,24-dien-26-oic acid (HLDOA): First the methyl group is oxidized to hydroxyl group, followed by the conversion of hydroxyl group to formyl group, and finally the conversion of formyl group to carboxyl group (Lu et al., 2020). Glutamine synthase (GS) is a central nitrogen metabolizing enzyme that plays an important role in the nitrogen regulatory network and secondary metabolism of fungi. GS activity is inhibited under nitrate condition, which adversely affects the biosynthesis of ganoderic acid (Zhu et al., 2021). Histone acetylation plays an important role in regulating the growth and secondary metabolic synthesis of fungi. It was found that histone acetylation can regulate the growth and development of G. lucidum through its global regulatory factors, and then can affect the biosynthesis of polysaccharides (Wang et al., 2020). By cloning PacC gene in response to environmental changes, it was found that PacC plaied an important role in regulating the transcription level of key enzyme genes, intermediate metabolites and ganoderic acid content in the ganoderic acid biosynthesis pathway (Wu et al., 2016). The analysis of three ceramide synthases from G. lucidum (lag1, lag2 and lag3) revealed that RNA interference inhibition of lag1 reduced the synthesis of ganoderic acid in the mutant, while dual inhibition of lag2/lag3 increased the synthesis of ganoderic acid in the mutant (Tao et al., 2021).
5. Prospects
In recent years, G. lucidum has been a hot spot for researchers. The genus Ganoderma and its relatives, its species and their morphologically diversity are delineated at the molecular level, that provides conditions for improving Ganoderma breeding and germplasm resources. Ganoderic acids are some of the main substances in G. lucidum that exerts pharmacological activity. Different ganoderic acids can coordinate with each other and play a role together. Due to the influence of a variety of exogenous and endogenous substances on ganoderic acids biosynthesis, the biosynthesis pathway from lanosterol to ganoderic acid is unclear. How to improve the content of ganoderic acid in G. lucidum has been an important research direction so far. In order to investigate the key compounds that play a regulatory function in its secondary metabolism, mine critical genes, and enhance ganoderic acid production, we analyzed the different kinds of ganoderic acids, their pharmacological activity and biosynthetic pathways.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (No. 2021C02074 and 2021C02073), Zhejiang Provincial Natural Science Foundation of China (No. LR21H280002) and Zhejiang Key Agricultural Enterprise Institute (No. 2017Y20001).
Contributor Information
Zongqi Yang, Email: yangzongqi@zstu.edu.cn.
Dongfeng Yang, Email: ydf807@sina.com.
References
- Abulizi A., Hu L., Ma A., Shao F., Zhu H., Lin S.…Yang B. Ganoderic acid alleviates chemotherapy-induced fatigue in mice bearing colon tumor. Acta Pharmacologica Sinica. 2021;42(10):1703–1713. doi: 10.1038/s41401-021-00669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby S., Johnson A.J., Govindan B. Secondary metabolites from Ganoderma. Phytochemistry. 2015;114:66–101. doi: 10.1016/j.phytochem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Bao H., Li H., Jia Y., Xiao Y., Luo S., Zhang D.…Du J. Ganoderic acid a exerted antidepressant-like action through FXR modulated NLRP3 inflammasome and synaptic activity. Biochemical Pharmacology. 2021;188 doi: 10.1016/j.bcp.2021.114561. [DOI] [PubMed] [Google Scholar]
- Cai X., He W., An F. Research progress on ganoderma lucidum germplasm resources. Modern Agricultural Science and Technology. 2016;6:99–100. [Google Scholar]
- Castillo-Trivino T., Braithwaite D., Bacchetti P., Waubant E. Rituximab in relapsing and progressive forms of multiple sclerosis: A systematic review. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0066308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Li K. Resources, taxonomy, ecological distribution, exploitation and utilization of ganodermataceae from china. Acta Agriculturae Universitis Jiangxiensis. 2004;26(1):89–95. [Google Scholar]
- Chen S., Xu J., Liu C., Zhu Y., Nelson D.R., Zhou S., Li C., Wang L., Guo X., Sun Y., Luo H.M., Li Y., Song G.Y., Bernard H., Anthony L., Qian J., Li J.Q., Luo X., Shi L.C., He L., Xiang L., Xu X.L., Niu Y.Y., Li Q.S., Han M.V., Yan H.X., Zhang J., Chen H.M., Lv A.P., Wang Z., Liu m.z., David C.S., Sun C. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nature Communications. 2012;3(1):913. doi: 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. The pharmacological effects of triterpenoids from Ganoderma lucidum and the regulation of its biosynthesis. Advances in Biological Chemistry. 2020;10(2):55–65. [Google Scholar]
- Ding P., Huang H.B., Qiu J.Y., Liang Y.J., Wang H.L. Study on the differences of Ganoderma lucidum and Ganoderma sinense by HPTLC and HPLC fingerprint chromatogram. West China Journal of Pharmaceutical Sciences. 2009;24(4):404–406. [Google Scholar]
- Doedt T., Krishnamurthy S.P., Bockmühl D., Tebarth B., Stempel C., Russell C.L.…Ernst J.F. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Molecular Biology of the Cell. 2004;15(7):3167–3180. doi: 10.1091/10.1091/mbc.E03-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Shi L., Zhang T., Ren A., Jiang A., Yu H., Zhao M. Cross talk between calcium and reactive oxygen species regulates hyphal branching and ganoderic acid biosynthesis in Ganoderma lucidum under copper stress. Applied and Environmental Microbiology. 2019;84(13):e00438–e00518. doi: 10.1128/AEM.00438-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Ma A., He J., Wang L., Jia Y., Shao G.…Yang B. Ganoderic acid hinders renal brosis via suppressing the TGF- β/Smad and MAPK signaling pathways. Acta Pharmacologica Sinica. 2020;41(5):670–677. doi: 10.1038/s41401-019-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Zhong J. Role of calcineurin-responsive transcription factor CRZ1 in ganoderic acid biosynthesis by Ganoderma lucidum. Process Biochemistry. 2020;95:166–173. [Google Scholar]
- He L., Yao K., Xu X., Li F., Luo X. Intraspecific identification of lingzhi medicinal mushroom, ganoderma lingzhi (agaricomycetes), using ISSR markers. International Journal of Medicinal Mushrooms. 2022;24(4):43–52. doi: 10.1615/IntJMedMushrooms.2022043141. [DOI] [PubMed] [Google Scholar]
- Huang H., Xu G., Zhou S. Identification of genetic relationship among several chinese strains of Ganoderma with RAPD and rDNA molecular markers. Journal of Jianghan University (Natural Science Edition) 2013;41(2):70–74. [Google Scholar]
- Jia H.Y., Wang Y.T., Zhang Z.H., Feng N., Liu Y.F., Zhou S.…Tang Q.J. Determination of triterpenoids in Ganodema lucidum from different areas and species by HPLC. Microbiology China. 2017;67(18):5147–5158. [Google Scholar]
- Jia Y., Zhang D., Li H., Luo S., Xiao Y., Han L., Zhou F., Wang C., Feng L., Wang G., Wu P., Xiao C.J., Yu H.J., Du J., Bao H.k. Activation of FXR by ganoderic acid A promotes remyelination in multiple sclerosis via anti-inflammation and regeneration mechanism. Biochemical Pharmacology. 2021;185 doi: 10.1016/j.bcp.2021.114422. [DOI] [PubMed] [Google Scholar]
- Jiang A.L., Liu Y.N., Liu R., Ren A., Ma H.Y., Shu L.B., Shi L., Zhu J., Zhao M.W. Integrated proteomics and metabolomics analysis provides insights into ganoderic acid biosynthesis in response to methyl jasmonate in Ganoderma lucidum. International Journal of Molecular Sciences. 2019;20(24):6116. doi: 10.3390/ijms20246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Li Z., Dai Y., Liu Z., Han X., Li Y.…Fu Y. Massive genome investigations reveal insights of prevalent introgression for environmental adaptation and triterpene biosynthesis in Ganoderma. Molecular Ecology Resources. 2022;00:1–18. doi: 10.1111/1755-0998.13718. [DOI] [PubMed] [Google Scholar]
- Karsten P.A. Enumeratio Boletinarum et Polyporarum Fennicarum systemate novo dispositorum. Revue Mycologique. 1881;3(9):16–19. [Google Scholar]
- Kohno T., Hai-Bang T., Zhu Q.C., Amen Y., Sakamoto S., Tanaka H., Morimoto S., Shimizu K. Tubulin polymerization-stimulating activity of Ganoderma triterpenoids. Journal of Natural Medicines. 2017;71(2):457–462. doi: 10.1007/s11418-017-1072-y. [DOI] [PubMed] [Google Scholar]
- Kubota T., Asaka Y., Miura I., Mori H. Structures of ganoderic acid A and B, two new lanostane type bitter triterpenes from Ganoderma lucidum (FR.) KARST. Helvetica Chimica Acta. 1982;65(2):611–619. [Google Scholar]
- Kwon O.C., Lee C.S., Park Y.J. SNP and SCAR markers for specific discrimination of antler-shaped Ganoderma lucidum. Microorganisms. 2019;7(1):12. doi: 10.3390/microorganisms7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai G., Guo Y., Chen D., Tang X., Shuai O., Yong T.…Wu Q. Alcohol extracts from ganoderma lucidum delay the progress of alzheimer's disease by regulating DNA methylation in rodents. Frontiers in Pharmacology. 2019;10:272. doi: 10.3389/fphar.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G., Marjorie D., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. Journal of Immunol Research. 2014;20(14):149–185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei R. Protective effect of ganoderic acid against the streptozotocin induced diabetes, inflammation, hyperlipidemia and microbiota imbalance in diabetic rats. Saudi Journal of Biological Sciences. 2019;26(8):1961–1972. doi: 10.1016/j.sjbs.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lou B., Zhang Y., Zhang C. Ganoderic Acid A exerts the cytoprotection against hypoxia-triggered impairment in PC12 cells via elevating microRNA-153. Phytotherapy Research. 2020;34(3):640–648. doi: 10.1002/ptr.6556. [DOI] [PubMed] [Google Scholar]
- Liao B., Chen X., Han J., Dan Y., Wang L., Jiao W.…Chen S. Identification of commercial ganoderma (lingzhi) species by ITS2 sequences. Chinese Medicine. 2015;10(1):22. doi: 10.1186/s13020-015-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. A combined study of Chinese and western medicine to interpret the discussion of Ganoderma lucidum in agriculture god’s canon of materia medica. Chinese Journal of Pharmacology and Toxicology. 2019;33(09):645–646. [Google Scholar]
- Liu C., Yang N., Song Y., Wang L., Zi J., Zhang S.W., Dunkin D., Busse P., Weir D., Tversky J., Rachel L.M., Joseph G., Zhan X.J., Li X.M. Ganoderic acid C1 isolated from the anti-asthma formula, ASHMI™ suppresses TNF-α production by mouse macrophages and peripheral blood mononuclear cells from asthma patients. International Immunopharmacology. 2015;27(2):224–231. doi: 10.1016/j.intimp.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Xie C., Wang Y., Liu Y., Han J., Shi L.…Zhao M. Function of ceramide synthases on growth, ganoderic acid biosynthesis and sphingolipid homeostasis in Ganoderma lucidum. Phytochemistry. 2020;172 doi: 10.1016/j.phytochem.2020.112283. [DOI] [PubMed] [Google Scholar]
- Luo Q., Li N., Xu J.W. A methyltransferase LaeA regulates ganoderic acid biosynthesis in Ganoderma lingzhi. Frontiers in Microbiology. 2022;13 doi: 10.3389/fmicb.2022.1025983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren A., Ouyang X., Shi L., Jiang A.L., Mu D.S., Li M.J., Han Q., Zhao M.W. Molecular characterization and expression analysis of GlHMGS, a gene encoding hydroxymethylglutaryl-CoA synthase from Ganoderma lucidum (Ling-zhi) in ganoderic acid biosynthesis pathway. World Journal of Microbiology and Biotechnology. 2013;29:523–531. doi: 10.1007/s11274-012-1206-z. [DOI] [PubMed] [Google Scholar]
- Ren X., Wang J., Huang L., Cheng K., Yang H. Comparative studies on bioactive compounds, ganoderic acid biosynthesis and antioxidant activity of pileus and stipes of Ganoderma lucidum fruiting body at different growth stages. International Journal of Medicinal Mushrooms. 2020;22(2):133–144. doi: 10.1615/IntJMedMushrooms.2020033683. [DOI] [PubMed] [Google Scholar]
- Ren L. Protective effect of ganoderic acid against the streptozotocin induced diabetes, inflammation, hyperlipidemia and microbiota imbalance in diabetic rats. Journal of Biological Sciences. 2019;26(8):1961–1972. doi: 10.1016/j.sjbs.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding C, Fungal Barcoding Consortium Author L Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C.S., Feng N., Zhou S., Zheng X.X., Wang P., Zhang J.S., Huang Q. Ganoderic acid t improves the radiosensitivity of heLa cells via converting apoptosis to necroptosis. Toxicology Research. 2021;10(3):531–541. doi: 10.1093/toxres/tfab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Ren A., Mu D., Zhao M. Current progress in the study on biosynthesis and regulation of ganoderic acids. Applied Microbiology & Biotechnology. 2010;88(6):1243–1251. doi: 10.1007/s00253-010-2871-1. [DOI] [PubMed] [Google Scholar]
- Shi F., Ding Z., Chen S., Tong X. Research progress on resource and identification of Ganoderma. World Science and Technology (Modernization of Traditional Chinese Medicine and Materia Medica) 2012;1181:107–134. [Google Scholar]
- Shiao M.S. Triterpenoid natural products in the fungus Ganoderma lucidum. Journal of the Chinese Chemical Society. 2013;39(6):669–674. [Google Scholar]
- Sun S.J., Gao W., Lin S.Q., Zhu J., Xie B.G., Lin Z.B. Analysis of genetic diversity in Ganoderma population with a novel molecular marker SRAP. Applied Microbiology and Biotechnology. 2006;72(3):537–543. doi: 10.1007/s00253-005-0299-9. [DOI] [PubMed] [Google Scholar]
- Tang W., Wu Y., Wang Y.Q., Sun P.L., Ouyang J.J. Inhibition of ganoderic acid mediated by P53 protein on cancer cells proliferation. Science and Technology of Food Industry. 2015;36(11):193–196. [Google Scholar]
- Tao Y., Han X., Ren A., Li J., Song H., Xie B., Zhao M. Heat stress promotes the conversion of putrescine to spermidine and plays an important role in regulating ganoderic acid biosynthesis in Ganoderma lucidum. Applied Microbiology and Biotechnology. 2021;105(12):1–13. doi: 10.1007/s00253-021-11373-0. [DOI] [PubMed] [Google Scholar]
- Tian Y.Z., Wang Z.F., Liu Y.D., Zhang G.Z., Li G. The whole-genome sequencing and analysis of a Ganoderma lucidum strain provide insights into the genetic basis of its high triterpene content. Genomics. 2021;113(1):840–849. doi: 10.1016/j.ygeno.2020.10.015. [DOI] [PubMed] [Google Scholar]
- Wang L., Su H., Lv S. Molecular markers and phylogenetic relationships of eight Ganoderma lucidum strains. Journal of Xinxiang University. 2011;28(3):236–239. [Google Scholar]
- Wang Q., Xu M., Zhao L., Wang F., Li Y., Shi G., Ding Z. Transcriptome dynamics and metabolite analysis revealed the candidate genes and regulatory mechanism of ganoderic acid biosynthesis during liquid superficial-static culture of Ganoderma lucidum. Microbial Biotechnology. 2020;14(2):600–613. doi: 10.1111/1751-7915.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Sun L., Wen M.Y., Tan Y.C., Almalki W.H., Katouah H., Kazmi I., Afzal O., Altamimi A.S.A., Al-Abbasi F.A., Sarwar B., Mahfoozur R. Nano lipidic carriers for codelivery of sorafenib and ganoderic acid for enhanced synergistic antitumor efficacy against hepatocellular carcinoma. Saudi Pharmaceutical Journal. 2021;29(8):843–856. doi: 10.1016/j.jsps.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Dai Y., Lin Y. Stuny on the ganodermataceae of China I. Guizhou Science. 2004;22(2):27–33. [Google Scholar]
- Wu X., Dai Y., Lin Y. Stuny on the ganodermataceae of China III. Guizhou Science. 2004;22(4):36–40. [Google Scholar]
- Wu F., Zhang G., Ren A., Dang Z., Shi L., Jiang A., Zhao M. The ph-responsive transcription factor pacC regulates mycelial growth, fruiting body development, and ganoderic acid biosynthesis in Ganoderma lucidum. Mycologia. 2016;108(6):1104–1113. doi: 10.3852/16-079. [DOI] [PubMed] [Google Scholar]
- Xia Q., Yang Z., Li M., Xia D. Selective breeding research on new cultivar “Yuze Lingzhi” of Ganoderma lucidum. Lishizhen Medicine and Materia Medica Research. 2018;29(4):987–989. [Google Scholar]
- Xie Y.K., Zhang J., Yu X., Yan C.Y. Advances in studies on polysaccharides from Ganoderma and their biological activities. Chinese Traditional and Herbal Drugs. 2021;52(17):5414–5429. [Google Scholar]
- Xing K., Liu Y., He Z., Chen S. Progress in ganoderic acid research. Acta Edulis Fungi. 2017;24(3):96–103. [Google Scholar]
- Xu Y., Yuan H., Luo Y., Zhao Y., Xiao J. Ganoderic acid D protects human amniotic mesenchymal stem cells against oxidative stress-induced senescence through the PERK/NRF2 signaling pathway. Oxidative Medicine and Cellular Longevity. 2020;46(3):18–22. doi: 10.1155/2020/8291413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang Y., Huang Q., Wang X., Li Z., Li M., Fu Y. Breeding of a new Ganoderma lucidum variety‘Xianzhi 3’. Acta Edulis Fungi. 2021;28(3):39–46. [Google Scholar]
- Yan Z., Hao L., Zhang L., Kang C., Ma T., Cui Y., Zheng Z. Comparison of main chemical constituents in Ganoderma lucidum fruiting bodies collected from three producing regions. Food Science. 2019;40(06):248–254. [Google Scholar]
- Yuan Y., Wang Y., Sun G., Wang Y., Cao L., Shen Y., Yuan B., Han D., Huang L. Archaeological evidence suggests earlier use of Ganoderma in neolithic china. Science Bulletin. 2018;63(13):1180–1188. [Google Scholar]
- Zhang X., Ren A., Li M.J., Cao P.F., Chen T.X., Zhang G., Shi L., Jiang A.L., Zhao M.W. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis, and hyphal branching of Ganoderma lucidum via Cytosolic Ca2. Applied and Environmental Microbiology. 2016;82(14):4112–4125. doi: 10.1128/AEM.01036-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Ren A., Shi L., Zhu J., Jiang A., Shi D., Zhao M. Functional analysis of an APSES transcription factor (glswi6) involved in fungal growth, fruiting body development and ganoderic-acid biosynthesis in Ganoderma lucidum. Microbiological Research. 2018;207:280–288. doi: 10.1016/j.micres.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhou T., Song X. Research progress on active ingredients and pharmacological action of Ganoderma lucidum. Journal of Anhui Agricultural Sciences. 2018;46(3):18–22. [Google Scholar]
- Zhang X., Zhang W., Chen X., Lan J., Yin C. Comparative study of active constituents of fruiting bodies and spores from two Ganoderma lucidum strains. Journal of Chinese Medicinal Materials. 2020;43(02):363–367. [Google Scholar]
- Zhang T., Guo J., Ma Q., Kong F., Zhou M., Xie Q.…Zhao Y. Study on chemical constituents from fruiting bodies of Ganoderma calidophilum. China Journal of Chinese Materia Medica. 2021;46(7):1783–1789. doi: 10.19540/j.cnki.cjcmm.20201223.602. [DOI] [PubMed] [Google Scholar]
- Zhang X. The study about early formation of Chinese ganoderma culture. Regional Culture Study. 2022;28(1):49–59. [Google Scholar]
- Zhao M.W., Zhong J.Y., Liang W.Q., Wang N., Jong S.C. Analysis of squalene synthase expression during the development of Ganoderma lucidum. Journal of Microbiology and Biotechnology. 2004;14(1):116–120. [Google Scholar]
- Zhao W., Xu J., Zhong J. Enhanced production of ganoderic acids in static liquid culture of Ganoderma lucidum under nitrogen-limiting conditions. Bioresource Technology. 2011;102(17):8185–8190. doi: 10.1016/j.biortech.2011.06.043. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zheng B., Wang H., Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacologica Sinica. 2017;38(5):614–622. doi: 10.1038/aps.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Sun Z., Shi D., Song S. Dual functions of area, a GATA transcription factor, on influencing ganoderic acid biosynthesis in Ganoderma lucidum. Environmental Microbiology. 2019;21(11):4166–4179. doi: 10.1111/1462-2920.14769. [DOI] [PubMed] [Google Scholar]
- Zhu J., Song S.Q., Sun Z.H., Lian L.D., Shi L., Ren A., Zhao M.W. Regulation of glutamine synthetase activity by transcriptional and posttranslational modifications negatively influences ganoderic acid biosynthesis in Ganoderma lucidum. Environmental Microbiology. 2021;23(2):1286–1297. doi: 10.1111/1462-2920.15400. [DOI] [PubMed] [Google Scholar]