Abstract
Metabolites from medicinal plants continue to hold significant value in the exploration and advancement of novel pharmaceuticals. In the search for plants containing compounds with anti-inflammatory effects, we observed that the ethanol (EtOH) extract obtained from the aerial components of Gouania leptostachya DC. var. tonkinensis Pit. exhibited substantial suppression of nitric oxide (NO) in vitro. In a phytochemical study on an EtOH extract of G. leptostachya, 11 compounds were purified, including one unreported compound namely gouanioside A (1). Their chemical structures were unambiguously determined through the use of various spectroscopic techniques, such as 1 and 2D NMR, IR, and HR-ESI-MS, and by producing derivatives via chemical reactions. The EtOH extract, fractions, and a new compound exerted inflammatory effects by altering NO synthesis in murine RAW264.7 macrophage cells stimulated with lipopolysaccharide. The underlying inflammatory mechanism of the new compound 1 was also explored through various in vitro experiments. The results of this study indicate the potential usefulness of new compound 1 from G. leptostachya as a treatment for inflammatory diseases.
Keywords: Gouania leptostachya, saponin, anti-inflammatory effect, gouanioside A
Introduction
The process of inflammation is intricate and linked with several factors, including bacterial infection, toxic compounds, and damaged cells [1]. Chronic inflammation is associated with approximately 40 million deaths annually, according to the World Health Organization (WHO). The inflammation process is also frequently associated with the concurrence of diseases, such as coronavirus 2019 [2], allergies [3], diabetes mellitus [4], gut diseases [3], cardiovascular disease [5], and cancer [6].
Nitric oxide (NO) plays an effector role in inflammatory disease. Cytokines such as interleukin 6 (IL-6), prostaglandin E2 (PGE2), and tumor necrosis factor alpha (TNF-α) are produced by immune effector cells [6, 7]. Thus, the inhibition of NO production and pro-inflammatory cytokines, such as PGE2, IL-6, IL-1β, and TNF-α, in addition to the manifestation of proteins such as cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS), may be useful for treating inflammatory disorders [8]. Many compounds isolated from natural products reportedly show anti-inflammatory activity [9].
Gouania leptostachya DC. var. tonkinensis Pit., belonging to the family Rhamnaceae, is a traditional medicine used in Vietnam for the management of diverse ailments, such as inflammation, pain, and swelling [10]. The methanol extract of G. leptostachya shows various pharmacological effects, containing anti-inflammatory [10], antibacterial [11], and antioxidant [12] properties. Phytochemical investigation has revealed that phenolics, saponins, steroids, and benzopyran derivatives are the main components of G. leptostachya [13]. Previously, we reported four new dammarane triterpenoid saponins (gouaniasines VII–IX) from G. leptostachya. Moreover, their potential nitric oxide inhibitory activity was also evaluated [14]. However, the underlying mechanisms of inflammation have not yet been discovered. With the goal of identifying natural bioactive substances from G. leptostachya and elucidating their structures, in this study we describe the purification and structural identification of one unreported ceanothane-type triterpenoid glycoside (1), and 10 known metabolites (2-11) from G. leptostachya aerial parts (Fig. 1). The mechanisms underlying the anti-inflammatory properties of newly isolated compound 1 were also evaluated. Additionally, the potential anti-inflammatory properties of extract and fractions from G. leptostachya were the first time reported. Our findings enhance our comprehension of the secondary compounds synthesized by G. leptostachya and offer a logical basis for conducting additional research on the anti-inflammatory properties of this valuable medicinal herb.
Fig. 1. Structures of the isolated compound (1-11) purified from G. leptostachya aerial parts.
Material and Methods
General Experimental Procedures
The Jasco P-1020 polarimeter was used to record optical rotation values, while the JASCO Report 100 infrared spectrophotometer was used to obtain FT-IR spectra. The Bruker Avance III 500 spectrometers were utilized to measure all NMR spectra, with TMS serving as the internal standard. The HR-ESI-MS were obtained from an Agilent 6530 Accurate-Mass Q-TOF LC/MS system. Silica gel (70-230, 230-400 mesh, Merck, USA) and YMC RP-18 resins (75 μm, Fuji Silysia Chemical Ltd., Japan) were used as adsorbents in the column chromatography. Merck KGaA (Germany) supplied the TLC plates (silica gel 60 F254 and RP-18 F254, 0.25 μm), which were detected under UV radiation (254 and 365 nm) and by spraying the plates with 10% H2SO4 followed by heating with a heat gun. All chemicals and reagents were procured from Sigma-Aldrich.
Extraction and Isolation
The dried aerial parts of G. leptostachya, weighing 1.5 kg, were subjected to extraction with 96% EtOH, which was carried out thrice, using 10 L of solvent each time. The extraction was performed overnight at 50°C. The solvent was then evaporated under reduced pressure, which resulted in the formation of a tarry residue of EtOH weighing 148.0 g. This residue was then resuspended in H2O and partitioned successively with n-hexane, EtOAc, and n-BuOH, leading to the isolation of an n-hexane extract weighing 18.5 g, an EtOAc extract weighing 36.6 g, an n-BuOH extract weighing 21.0 g, and a water layer, which were obtained after removing the respective solvents. The obtained extracts can be further purified and characterized for the identification of the bioactive compounds present in the plant material. The detailed isolation process is described in the Supplementary data.
Physical and spectroscopic data of new compound: Gouanioside A (1). White shapeless material,[α]D20 0.1, methanol). The major absorption band in the IR spectrum of 1: νmax 3394, 2984, 1728, 1064, and 1049 cm-1. The detailed NMR data were described at Table 1; HR-ESI-MS m/z 1133.5385 [M-H]- (calcd for C54H85O25-, 1133.5393), and m/z 1152.5779 [M+NH4]+ (calcd for C54H90NO25+, 1152.5796).
Table 1.
1H (500 MHz, CD3OD) and 13C-NMR (125 MHz, CD3OD) spectroscopic data, δ ppm, of compound 1.
| Position | 1 | ||||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
| Aglycon | Sugar | ||||
| 1 | 62.6 | 2.37 d (7.5) | Glc | ||
| 2 | 177.0 | - | 1' | 93.4 | 5.62 d (8.0) |
| 3 | 83.6 | 4.10 d (7.0) | 2' | 77.8 | 3.87 m |
| 4 | 43.7 | - | 3' | 78.8 | 3.42 m |
| 5 | 63.6 | 0.90 s | 4' | 70.8 | 3.45 m |
| 6 | 19.0 | 1.37 m, 1.61 m | 5' | 78.3 | 3.80 m |
| 7 | 35.7 | 1.35 m, 1.45 m | 6' | 62.3 | 3.72 m, 3.84 m |
| 8 | 43.0 | - | Glc | ||
| 9 | 51.9 | 1.52 m | 1'' | 102.6 | 5.05 d (7.5) |
| 10 | 49.5 | - | 2'' | 82.6 | 3.87 m |
| 11 | 26.4 | 1.11 m, 1.68 m | 3'' | 87.6 | 3.69 m |
| 12 | 24.9 | 1.41 m, 1.52 * | 4'' | 71.1 | 3.29 m |
| 13 | 39.1 | 2.28 m | 5'' | 78.2 | 3.40 m |
| 14 | 43.8 | - | 6'' | 63.5 | 3.60 m, 3.93 m |
| 15 | 32.1 | 1.10 m, 1.60 m | Glc | ||
| 16 | 32.5 | 1.40 m, 2.58 m | 1''' | 104.8 | 4.74 (d, 8.0) |
| 17 | 57.9 | - | 2''' | 75.9 | 3.24 m |
| 18 | 50.7 | 1.62 t (11.0) | 3''' | 78.4 | 3.39 m * |
| 19 | 48.4 | 3.01 m | 4''' | 71.4 | 3.34 m |
| 20 | 151.8 | - | 5''' | 77.5 | 3.40 m |
| 21 | 31.4 | 1.39 m, 1.90 m | 6''' | 62.6 | 3.65 m, 3.90 m |
| 22 | 37.5 | 1.50 m, 2.01 m | Glc | ||
| 23 | 32.2 | 1.04 s | 1'''' | 104.8 | 4.59 d (7.5) |
| 24 | 19.7 | 0.92 s | 2'''' | 75.4 | 3.30 m |
| 25 | 14.5 | 1.19 s | 3'''' | 78.4 | 3.39 * |
| 26 | 17.6 | 0.99 s | 4'''' | 71.6 | 3.35 m |
| 27 | 15.2 | 1.02 s | 5'''' | 77.5 | 3.30 * |
| 28 | 176.1 | - | 6'''' | 62.6 | 3.76 m, 3.99 m |
| 29 | 19.5 | 1.71 s | |||
| 30 | 110.3 | 4.62 d (1.5), 4.74 s | |||
*Overlapped signals and chemical shifts may be interchanged
Assignments were determined by DEPT, COSY, HSQC, HMBC, TOCSY, and NOESY experiments.
Plant Material
In November 2015, the aerial portions of G. leptostachya were gathered from Thai Nguyen province, Vietnam and underwent taxonomic identification by Professor Pham Thanh Huyen. The corresponding voucher specimen (TB 10663C) has been stored at the Herbarium of NIMM (Vietnam).
Cell Culture, NO and MTT Assay
The method for inhibiting the production of NO in murine macrophage RAW264.7 cells activated with LPS by the compound was conducted according to the literature [26, 28]. In summary, RAW264.7 cells, procured from American Type Culture Collection (ATCC, TIB-71, USA), were cultivated in DMEM enriched with 10% fetal bovine serum (FBS, 16000-044, Gibco, USA) and 100 U/ml penicillin-streptomycin (Gibco) at 37°C in a CO2 incubator. The detailed method is described in Supplementary information.
Measure the Levels of PGE2 and TNF-α, an ELISA Assay
In order to determine the levels of PGE2 and TNF-α produced by RAW264.7 cells in response to LPS stimulation, an enzyme-linked immunosorbent assay (ELISA) was employed using a kit from R&D Systems (USA). RAW264.7 cells were initially cultured in a 6-well plate at a density of 2 × 106 cells/well for 24 h and were subsequently stimulated with LPS for 12 h after pre-treatment with compound 1 for 1 h. The ELISA assay was conducted according to the manufacturer's instructions.
Western Blot Analysis
The mechanism inflammatory of 1 was conducted to examine the capacity for influencing the expression of COX-2 mRNA using the RT-PCR technique, and assessing COX-2 gene activity through luciferase gene assay, as well as evaluating COX-2 protein expression through Western Blot analysis employing a method described previously [8, 28, 29]. RAW264.7 cells were seeded in 6-well culture plates at a density of 2 × 106 cells/well and treated with lipopolysaccharide (LPS) at a concentration of 1 ng/ml for 16 h, following a pre-incubation with substance 1 for 1 h. The cells' lysate was obtained using a Cell Lysis Buffer from Cell Signaling Technology, following the manufacturer's instructions.
Statistical Analysis
The statistical analysis was performed using the GraphPad Prism 8 software (GraphPad Software, USA). The differences between the group treated with the compound and the LPS-only group were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s test. The level of significance was set at p < 0.01, ***p < 0.001.
Molecular Docking Studies
Molecular docking investigations were conducted by the software AutoDock Vina 1.1.2 following the previously reported publication [39]. In brief, the crystal structure of COX-2 (PDB code, 1PXX) was retrieved from the RCSB Protein Data Bank. The ligands' three-dimensional (3D) structures were prepared utilizing Chem3D Pro and saved as mol files, and the most stable conformer was chosen for the docking study. The protein was prepared by removing water, initial ligands, repairing missing residues, and adding polar hydrogen atoms. The detailed procedure is shown in the Supplementary information.
Results and Discussion
Dry samples of G. leptostachya aerial parts (1.5 kg) were extracted with pure ethanol (10 L × three times). A rotary evaporator was used to remove the solvent, yielding an EtOH residue (148.0 g) that was divided into exyl hydride, ethyl acetate, and butyl alcohol to yield an exyl hydride (18.5 g), ethyl acetate (36.6 g), butyl alcohol (21.0 g), and water extracts, respectively. Bioassay-guided fractionation of the EtOH extract of G. leptostachya, employing various chromatography separation techniques (silica gel; YMC RP-18, and Sephadex LH-20) resulted in the purification of a new saponin (1) and 11 known compounds (2-11, Fig. 1), namely epigouanic acid A (2)[14], lupeol (3) [15], alphitolic acid (4) [16], ceanothenic acid (5) [17], daucosterol (6) [18], and n-butyl-β-D-fructopyranoside (7) [19], quercitrin (8) [20], catechin (9) [21], isoquercitrin (10) [22], and kaempferol-3-O-(6-O-E-caffeoyl)-β-D-galactopyranosyl-(1→2)-α-L-rhamnopyranosid (11) [23]. The identified compounds were validated by matching their nuclear magnetic resonance (NMR) data with the relevant literature.
Substance 1 was a white shapeless material, with αD20 = −8.8 (c 0.1, MeOH) and a molecular formula of C54H86O25, as verified by both ionization modes of HR-ESI-MS, with a deprotonated molecular ion at m/z 1133.5385 [M-H]- (calcd for C54H85O25-, 1133.5393) and quasi-molecular ion at m/z 1152.5779 [M+NH4]+ (calcd for C54H90NO25+, 1152.5796). The infrared (IR) spectra displayed strong signals at 3,394 and 1,728 cm−1, supporting the presence and functionalities of ester and hydroxyl groups. The 1H-NMR data of 1 indicated six methyl resonances at δH: 0.92 (3H, s, H-24), 0.99 (3H, s, H-26), 1.02 (3H, s, H-27), 1.04 (3H, s, H-23), 1.19 (3H, s, H-25), and 1.71 (3H, s, H-29); The signals detected in the heteronuclear single quantum coherence (HSQC) spectrum exhibited associations with the carbon signals that corresponded to them, δC of 19.7, 17.6, 15.2, 32.2, 14.5, and 19.5, respectively. Additionally, four sugar proton signals at the anomeric position were detected at δH 4.59 (d, J = 7.5 Hz, H-1''''), 4.74 (d, J = 8.0 Hz, H-1'''), 5.05 (d, J = 7.5 Hz, H-1''), and 5.62 (d, J = 8.0 Hz, H-1')(Table 1). By utilizing carbon-13 nuclear magnetic resonance (13C-NMR) and heteronuclear single quantum coherence (HSQC) measurements together, a total of 30 signals for the aglycone and 24 signals for the sugar moiety were identified in the resulting spectra. The aglycon of 1 was identified as a ceanothane-type triterpenoid [24], using a comprehensive examination of the 1H- and 13C-NMR data (Table 1). The 1H-1H correlated spectroscopy (COSY) data indicated correlations among H-1/H3, H5/H6/H7, H9/H11/H12/H13/H18/H19/H21/H22, and H15/H16 (Fig. S3 and S19). The HMBC cross-peak from H-1 (2.37) to C-2 (177.0), H-23 (1.04) to C-3 (83.6), and H-29 (1.71), as well as H-30 (4.62, and 4.74) to C-19 (48.4), led to the identification of aglycon of 1 as epiceanothic acid. Furthermore, the acid hydrolysis of 1 was used to establish the definite configuration of the sugar moieties. The stereochemistry of the sugar component of 1 was characterized as β-D-glucose (Supplementary Data) [25, 26]; additionally, observation of cross-peaks between δH 5.62 (H-1', Glc-1) and δC 176.1 (C-28), δH 5.05 (H-1'', Glc-2) and δC 77.8 (Glc-1, C-2'), δH 4.74 (H-1''', Glc-3) and δC 82.6 (Glc-2, C-2''), and δH 4.59 (H-1'''', Glc-4) and δC 87.6 (Glc-2, C-3''') indicated that the β-D-glucopyranosyl-(1→3)-[β-D-glucopyranosyl -(1 → 2)]-β-D-glucopyranosyl -(1 → 2)-β-D-glucopyranosyl moiety was linked to the aglycon C-28 (Table 1). Further clarification of the proton signals corresponding to the sugar moiety of 1 was achieved based on 1H-1H COSY, TOCSY, and HMBC correlations (Supplementary Data). The NMR data of 1H and 13C indicated a similarity in the relative configuration of 1 with epiceanothic acid [24]. This was supported by the experimental results of nuclear Overhauser effect spectroscopy. Indeed, the correlations between H-1 (δH 2.37), H-3 (δH 4.10), and H-23 (δH 1.04) revealed that both H-1 and H-3 are α-oriented (Fig. 2B). Similarly, the connection among H-19 (δH 3.01), and H-29 (δH 1.71), and the absence of association between H-19 and H-18 suggested that H-19 is β-oriented (Fig. 2B). Consequently, the structure of new compound 1 was unambiguously identified as epiceanothic acid 28-O-β-D-glucopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside, and named gouanioside A.
Fig. 2.
(A) Connectivity deduced by the COSY (bold), DEPT, and HSQC spectra, and key HMBC correlations (blue arrows) of 1. (B) Significant NOESY (→) correlations of aglycon of 1. After energy minimization, the threedimensional conformation of substance 1 was generated using the Macromodel program (version 12.5, Schrodinger LLC).
For thousands of years, compounds isolated from natural products have served as powerful alternative treatments and pharmaceuticals [27]. Many inflammatory inhibitors are purified from both medicinal herbs and marine organisms. In particular, the medicinal plants Ecklonia cava, Momordica charantia, and Gymnema sylvestre have been widely used in the treatment of diabetes, showing efficacy, nontoxicity, and few to no side effects [28]. In our continuing search for new anti-inflammatory inhibitors from natural products, the anti-inflammatory properties of G. leptostachya crude EtOH extract, n-hexane fraction, ethyl acetate (EtOAc) fraction, n-BuOH fraction, and aqueous fractions were evaluated in terms of their inhibitory effects on the lipopolysaccharide (LPS)-induced expression of NO production. To avoid cytotoxicity, the effects of the crude extract (EtOH) and fractions on RAW 264.7 cells were examined at concentrations of 1, 5, and 25 μM over 3 days using the MTT method [8]. None of the tested crude extract concentrations or fractions showed significant cytotoxicity, i.e., not even at 25 μM (Fig. 3A). NO is regarded as a pivotal factor in the pathogenesis of inflammation. Thus, NO concentrations were evaluated using the Griess method in this study [25]. All extracts and fractions of G. leptostachya exhibited anti-inflammatory effects, in a concentration-dependent manner (Fig. 3B).
Fig. 3.
(A) Cytotoxic effects of ethanol (EtOH) extract (1, 5, and 25 μg) and fractions on RAW 264.7 cells over 3 days. GLT (EtOH extract), GLH (n-hexane fraction), GLB (BuOH fraction), GLE (EtOAc fraction), and GLW (water fraction), respectively. The positive control utilized in the experiment was Dexamethasone (Dexa). (B) Inhibition of the activation of RAW 264.7 cells by the EtOH extract and fractions in vitro. Different concentrations of the treatment were administered to the cells (1, 5, and 25 μg) of extract and fractions, or with Dexa as the positive control. The levels of nitric oxide (NO) in the culture supernatants were determined using the Griess method, **p < 0.01, ***p < 0.001. Dexamethasone was shown to have stable activity in the experiment.
To avoid cytotoxic effects of the new compound 1, the MTT method was also applied. The results showed no cytotoxicity of new compound 1, even at 25 μM (Fig. S10, Supporting Data). To evaluate the inhibitory properties of new compound 1 on the LPS-induced production of NO in RAW264.7 cells, NO was measured using the Griess reaction.
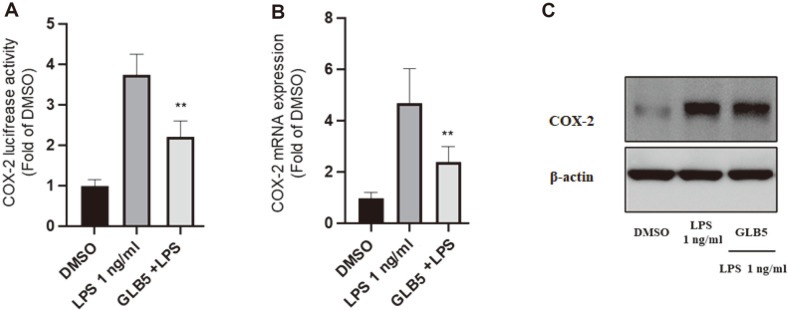
In order to evaluate its effect on NO production, further tests on the anti-inflammatory activities of 1 were carried out by ELISA method. The effects of 1 on mRNA expression levels of pro-inflammatory cytokines (PGE2 and TNF-α) and COX-2 protein were determined in LPS-induced RAW264.7 cell lines using ELISA and Western blot methods. To determine the inhibitory effects of pro-inflammatory cytokines on the expression of PGE2 and TNF-α, LPS-stimulated RAW264.7 cells were treated with compound 1. The experiments were carried out using enzyme-linked immunosorbent assay kits. As shown in Figs. 4A and 4B, 1, 1 showed potent inhibitory effects on both NO and TNF-α, in a concentration-dependent manner. Additionally, 1 reduced TNF-α cell expression at a concentration of 10 μM. The results suggest that 1 significantly inhibits pro-inflammatory cytokines. NO-induced COX-2 expression is mediated partially by the cyclic guanosine monophosphate (cGMP)-dependent pathway. Saponins showed anti-inflammatory effects via regulation of iNOS, COX-2, and other inflammatory factors [28]. The mechanism inflammatory of 1 was conducted to examine the capacity for influencing the expression of COX-2 mRNA using the RT-PCR technique, and assessing COX-2 gene activity through luciferase gene assay, as well as evaluating COX-2 protein expression through Western Blot analysis employing a method described previously [8, 29]. Compound 1 exhibits inhibitory activity against COX-2 luciferase activity (Fig. 5A). Compound 1 demonstrates a significant pharmacological effect in modulating the expression of COX-2 mRNA, resulting in a notable reduction of its cellular abundance (Fig. 5B). Additionally, Compound 1 (10 μM) demonstrated a significant ability to suppress the expression of COX-2 in LPS-activated cells. Therefore, the results suggest that compound 1 exerts an inhibitory effect on the biosynthesis of pro-inflammatory cytokines in macrophages that have been stimulated to an activated state.
Fig. 4.
Inhibitory effects of new compound 1 (GLB5) on lipopolysaccharide (LPS)-stimulated (A) NO, (B) PGE2, and (C) TNF-α production in RAW264.7 macrophages, respectively. RAW264.7 cells (2 × 106 cell/well) were pretreated with compound 1 (GLB5) for 1 h before being stimulated for 12 h with LPS. The NO, PGE2, and TNF-α levels were determined by the Griess method and an enzyme linked immunosorbent assay kit. Data represent five independent experiments and are expressed as the mean ± the standard error of the mean (SEM). Dexamethasone (dexa) was used as the positive control. The experiments were repeated five times. (**p < 0.01, ***p < 0.001). Dexamethasone was shown to have stable activity in the experiment.
Fig. 5.
(A) Effects of compound 1 (GLB5, 10 μM) with the capacity for influencing the expression of COX-2 mRNA using the RT-PCR technique, (B) COX-2 gene activity through luciferase gene assay, and (C) evaluating COX-2 protein expression through Western blot analysis, respectively. β-actin as a loading control. The intensity of the bands was measured using ImageJ software. **p < 0.01 versus the LPS alone group.
To further identify the anti-inflammatory properties of new compound 1 against COX-2 protein expression, molecular docking studies were carried out on 1 and COX-2 protein (PDB ID, 1PXX, Protein Data Bank). The procedure is shown in the Supporting material. The results showed that 1 could bind tightly to the catalytic amino acid residues to inhibit COX-2 protein expression. Compound 1 interacted with catalytic residues for binding, i.e., Phe361(3.0 Å), Asn560(3.18 Å), Ala562(3.06 Å), Ser563(3.31 Å), and Thr561(2.93 Å) (Figs. 6A and 6B). Additionally, compound 1 binds to the active site of COX-2 with binding affinities of –6.3 kcal/mol. The results suggested that the sugar moiety in the chemical structure of saponin could be a crucial factor in inhibiting the COX-2 enzyme. Therefore, the molecular docking simulations displayed strong interactions with COX-2 protein. Taken together, the results imply that compound 1 isolated from G. leptostachya may be useful for the treatment of inflammatory diseases.
Fig. 6. Molecular docking results of compound 1 with the COX-2 protein (PDB: 1PXX).
(A) three-dimensional (3D) docking image of compound 1 was created by Pymol 2.5. (B) two-dimensional (2D) docking image of 1 was created by LigPlot+ 2.2.
Natural bioactive products play an important role in controlling the inflammatory response and are thus important in terms of drug discovery. Saponins, a class of secondary metabolites, are widely distributed in various plant species and have been extensively studied for their potential medicinal plants [30] and marine organisms [31], demonstrate a diverse array of bioactive properties, including anticancer [32], anti-bacterial [33], antifungal [34], anti-inflammatory [32], and antioxidant [35] activities. Triterpene saponin is the main metabolite responsible for the pharmacological effects of Gynostemma pentaphyllum [36], Astragalus membranaceus [37], Korean red ginseng [29], and Momordica charantia [38] extracted from medicinal herbs. Our study showed that compound 1 strongly inhibits the production of NO in LPS-stimulated murine RAW254.7 macrophage cells. Moreover, exposure to 1 (10 μM) elicited a notable reduction in the secretion of PGE2 and TNF-α, along with a marked inhibition of COX-2 protein expression in RAW264.7 cells that were stimulated with LPS. The molecular docking simulation implied the possible mechanism of NO inhibition to be the interactions of a new ceanothane-type triterpenoid saponin with COX-2 protein. In conclusion, this study demonstrated that guanosine A (1), a new triterpene saponin found in the aerial parts of G. leptostachya, may be useful for facilitating the discovery of innovative anti-inflammatory therapeutics and treatments for inflammation-related diseases.
Supplemental Materials
Supplementary data for this paper are available on-line only at http://jmb.or.kr.
Acknowledgments
The authors acknowledge the partial financial support provided by the National Research Foundation of Korea Grant, which is funded by the Korean Government (NRF-2019R1A6A1A03031807 and NRF-2021R1A2C1093814).
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 4.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021;33:127–148. doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell. Mol. Immunol. 2006;3:271–277. [PubMed] [Google Scholar]
- 8.Vinh LB, Jang HJ, Phong NV, Cho K, Park SS, Kang JS, et al. Isolation, structural elucidation, and insights into the antiinflammatory effects of triterpene saponins from the leaves of Stauntonia hexaphylla. Bioog. Med. Chem. Lett. 2019;29:965–969. doi: 10.1016/j.bmcl.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Hung TM, Dang NH, Kim JC, Choi JS, Lee HK, Min B-S. Phenolic glycosides from Alangium salviifolium leaves with inhibitory activity on LPS-induced NO, PGE2, and TNF-α production. Bioog. Med. Chem. Lett. 2009;19:4389–4393. doi: 10.1016/j.bmcl.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 10.Dung TTM, Lee J, Kim E, Yoo BC, Ha VT, Kim Y, et al. Anti-inflammatory activities of Gouania leptostachya methanol extract and its constituent resveratrol. Phytother. Res. 2015;29:381–392. doi: 10.1002/ptr.5262. [DOI] [PubMed] [Google Scholar]
- 11.An NV. Research on plant characteristics, chemical composition, antimicrobial and antifungal effects of Gouania leptostachya. Master thesis, Hanoi University of Pharmacy; 2010. [Google Scholar]
- 12.Batmunkh T, Juan QH, Nga DT, Kyung KE, Ah KY, Wan SY, et al. Free radicals scavenging activity of Mongolian endemic and Vietnamese medicinal plants. Planta Med. 2007;73:P_057. doi: 10.1055/s-2007-986839. [DOI] [Google Scholar]
- 13.Yao C, Zhang SJ, Bai ZZ, Zhou T, LJ X. Two new benzopyran derivatives from Gouania leptostachya DC. var. tonkinensis Pitard. Chin. Chem. Lett. 2011;22:175–177. doi: 10.1016/j.cclet.2010.09.028. [DOI] [Google Scholar]
- 14.Hang NT, Bich Thu NT, Le Ba V, Van On T, Khoi NM, Do TH. Characterisation of four new triterpenoid saponins with nitric oxide inhibitory activity from aerial parts of Gouania leptostachya. Nat. Prod. Res. 2022;36:5999–6005. doi: 10.1080/14786419.2022.2057971. [DOI] [PubMed] [Google Scholar]
- 15.Burns D, Reynolds WF, Buchanan G, Reese PB, Enriquez RG. Assignment of 1H and 13C spectra and investigation of hindered side‐chain rotation in lupeol derivatives. Magn. Reson. Chem. 2000;38:488–493. doi: 10.1002/1097-458X(200007)38:7<488::AID-MRC704>3.0.CO;2-G. [DOI] [Google Scholar]
- 16.Nguyen TTH, Lam KP, Huynh CTK, Nguyen PKP, Hansen PE. Chemical constituents from leaves of Sonneratia alba J.Smith SMITH (Sonneratiaceae) J. Sci. Technol. Dev. 2011;14:11–17. doi: 10.32508/stdj.v14i4.2043. [DOI] [Google Scholar]
- 17.Jou SJ, Chen CH, Guh JH, Lee CN, Lee SS. Flavonol glycosides and cytotoxic triterpenoids from Alphitonia philippinensis. J. Chin. Chem. Soc. 2004;51:827–834. doi: 10.1002/jccs.200400124. [DOI] [Google Scholar]
- 18.Lendl A, Werner I, Glasl S, Kletter C, Mucaji P, Presser A, et al. Phenolic and terpenoid compounds from Chione venosa (sw.) urban var. venosa (Bois Bandé) Phytochemistry. 2005;66:2381–2387. doi: 10.1016/j.phytochem.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Pyo MK, YunChoi HS, Kim YK. Isolation of n-Butyl-B-fructopyranoside rom Gastrodia elata Blume. Nat. Prod. Sci. 2006;12:101–103. [Google Scholar]
- 20.Aderogba MA, Ndhlala AR, Rengasamy KR, Van Staden J. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules. 2013;18:12633–12644. doi: 10.3390/molecules181012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cren-Olivé C, Wieruszeski J-M, Maes E, Rolando C. Catechin and epicatechin deprotonation followed by 13C NMR. Tetrahedron. Lett. 2002;43:4545–4549. doi: 10.1016/S0040-4039(02)00745-1. [DOI] [Google Scholar]
- 22.Kim HY, Moon BH, Lee HJ, Choi DH. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J. Ethnopharmacol. 2004;93:227–230. doi: 10.1016/j.jep.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Xie X, Liu L, Zhang H, Ni F, Wen J, et al. Four new flavonol glycosides from the leaves of Ginkgo biloba. Nat. Prod. Res. 2021;35:2520–2525. doi: 10.1080/14786419.2019.1684282. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Xu L, Qian K, Liu J, Zhang L, Lee K-H, et al. Efficient synthesis and biological evaluation of epiceanothic acid and related compounds. Bioog. Med. Chem. Lett. 2011;21:338–341. doi: 10.1016/j.bmcl.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinh LB, Nguyet NTM, Ye L, Dan G, Phong NV, Anh HLT, et al. Enhancement of an in vivo anti-inflammatory activity of oleanolic acid through glycosylation occurring naturally in Stauntonia hexaphylla. Molecules. 2020;25:3699. doi: 10.3390/molecules25163699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinh LB, Phong NV, Ali I, Dan G, Koh YS, Anh HLT, et al. Identification of potential anti-inflammatory and melanoma cytotoxic compounds from Aegiceras corniculatum. Med. Chem. Res. 2020;29:2020–2027. doi: 10.1007/s00044-020-02613-5. [DOI] [Google Scholar]
- 27.Newman DJ. Natural product based antibody drug conjugates: Clinical status as of November 9, 2020. J. Nat. Prod. 2021;84:917–931. doi: 10.1021/acs.jnatprod.1c00065. [DOI] [PubMed] [Google Scholar]
- 28.Vinh LB, Jang HJ, Phong NV, Dan G, Cho KW, Kim YH, et al. Bioactive triterpene glycosides from the fruit of Stauntonia hexaphylla and insights into the molecular mechanism of its inflammatory effects. Bioorg. Med. Chem. Lett. 2019;29:2085–2089. doi: 10.1016/j.bmcl.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Vinh LB, Lee Y, Han YK, Kang JS, Park JU, Kim YR, et al. Two new dammarane-type triterpene saponins from Korean red ginseng and their anti-inflammatory effects. Bioorg. Med. Chem. Lett. 2017;27:5149–5153. doi: 10.1016/j.bmcl.2017.10.058. [DOI] [PubMed] [Google Scholar]
- 30.Rho T, Jeong HW, Hong YD, Yoon K, Cho JY, Yoon KD. Identification of a novel triterpene saponin from Panax ginseng seeds, pseudoginsenoside RT8, and its antiinflammatory activity. J. Ginseng Res. 2020;44:145–153. doi: 10.1016/j.jgr.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuong NX, Hoang L, Hanh TTH, Van Thanh N, Nam NH, Thung DC, et al. Cytotoxic triterpene diglycosides from the sea cucumber Stichopus horrens. Bioorg. Med. Chem. Lett. 2017;27:2939–2942. doi: 10.1016/j.bmcl.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Patlolla JM, Rao CV. Anti-inflammatory and anti-cancer properties of β-Escin, a triterpene Saponin. Curr. Pharmacol. Rep. 2015;1:170–178. doi: 10.1007/s40495-015-0019-9. [DOI] [Google Scholar]
- 33.Dong S, Yang X, Zhao L, Zhang F, Hou Z, Xue P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd.husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020;149:112350. doi: 10.1016/j.indcrop.2020.112350. [DOI] [Google Scholar]
- 34.Mostafa A, Sudisha J, El-Sayed M, Ito S-i, Ikeda T, Yamauchi N, et al. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem. Lett. 2013;6:274–280. doi: 10.1016/j.phytol.2013.03.001. [DOI] [Google Scholar]
- 35.Sur P, Chaudhuri T, Vedasiromoni J, Gomes A, Ganguly D. Antiinflammatory and antioxidant property of saponins of tea [Camellia sinensis (L) O. Kuntze] root extract. Phytother. Res. 2001;15:174–176. doi: 10.1002/ptr.696. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H-X, Wang Z-Z, Du Z-Z. Sensory-guided isolation and identification of new sweet-tasting dammarane-type saponins from Jiaogulan (Gynostemma pentaphyllum) herbal tea. Food Chem. 2022;388:132981. doi: 10.1016/j.foodchem.2022.132981. [DOI] [PubMed] [Google Scholar]
- 37.Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, et al. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother. Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- 38.Cao TQ, Phong NV, Kim JH, Gao D, Anh HLT, Ngo V-D, et al. Inhibitory effects of cucurbitane-type triterpenoids from Momordica charantia fruit on lipopolysaccharide-stimulated pro-Inflammatory cytokine production in bone marrow-derived dendritic cells. Molecules. 2021;26:4444. doi: 10.3390/molecules26154444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duyen NT, Vinh LB, Phong NV, Khoi NM, Long PQ, Hien TT, et al. Steroid glycosides isolated from Paris polyphylla var. chinensis aerial parts and paris saponin II induces G1/S-phase MCF-7 cell cycle arrest. Carbohydr. Res. 2022;519:108613. doi: 10.1016/j.carres.2022.108613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data for this paper are available on-line only at http://jmb.or.kr.