Abstract
Cnidarians are regarded as one of the earliest-diverging animal phyla. One of the hallmarks of the cnidarian body plan is the evolution of a free-swimming medusa in some medusozoan classes, but the origin of this innovation remains poorly constrained by the fossil record and molecular data. Previously described macrofossils, putatively representing medusa stages of crown-group medusozoans from the Cambrian of Utah and South China, are here reinterpreted as ctenophore-grade organisms. Other putative Ediacaran to Cambrian medusozoan fossils consist mainly of microfossils and tubular forms. Here we describe Burgessomedusa phasmiformis gen. et sp. nov., the oldest unequivocal macroscopic free-swimming medusa in the fossil record. Our study is based on 182 exceptionally preserved body fossils from the middle Cambrian Burgess Shale (Raymond Quarry, British Columbia, Canada). Burgessomedusa possesses a cuboidal umbrella up to 20 cm high and over 90 short, finger-like tentacles. Phylogenetic analysis supports a medusozoan affinity, most likely as a stem group to Cubozoa or Acraspeda (a group including Staurozoa, Cubozoa and Scyphozoa). Burgessomedusa demonstrates an ancient origin for the free-swimming medusa life stage and supports a growing number of studies showing an early evolutionary diversification of Medusozoa, including of the crown group, during the late Precambrian–Cambrian transition.
Keywords: cambrian explosion, cnidarian, medusae
1. Introduction
Cnidarians (Medusozoa, Polypodiozoa + Myxozoa, Anthozoa) represent a morphologically diverse and ecologically important group of animals in modern oceans and freshwater ecosystems [1]. One of the most fascinating adaptations to emerge within this group, is the ability to transition from sessile polyp to a muscular swimming bell (medusa phase) in Medusozoa (Cubozoa, Scyphozoa, Staurozoa and Hydrozoa). The evolution of this life cycle probably occurred once, in the common ancestor of all medusozoans [2], and was later lost in many groups, although convergent evolution of a medusa stage in different medusozoan lineages has also been suggested [3].
Cnidarians are thought to represent one of the earliest-diverging branches of animals, making them key for understanding the origin of Bilateria (e.g. [4–6]), but the origin and early evolution of medusozoans, and of the medusa stage in particular, remains poorly constrained by the fossil record. The late Ediacaran to early Cambrian fossil record suggests an early burst of cnidarian diversification, including microscopic forms, putative soft-bodied impressions and mineralized tubular fossils interpreted as stem-group medusozoans, or as members of particular medusozoan lineages [7 and references herein]. The tubular conulariids, for example, have been compared to Staurozoans (e.g. [8]) or extant coronate scyphozoans (e.g. [9]), and more recently have been allied with olivooids and Pseudooides as stem-group medusozans [7]. Conulariids have a fossil record spanning the terminal Ediacaran to the Triassic period (e.g. [10,11]). Thus far, no conulariid medusae are known and new evidence suggests that a free-living medusa life stage might have been lost in at least some species [12]. Microscopic olivooid fossils dating back to 535 million years ago may point to the evolutionary origin of swimming medusae, however these forms are encased within a periderm, and likely could not sustain any form of dynamic locomotion [13].
Recognizing cnidarian body fossils, and in particular putative macroscopic medusoids lacking hard parts, remains challenging for several reasons. The cnidarian body-plan has a very low preservation potential in general [14,15] and planktonic medusae, in particular, are much less likely to be buried and preserved compared to benthic organisms including polyps [16,17]. In addition, cnidarians have evolved complex life cycles, which renders recognizing ontogenetic stages of the same species in the fossil record challenging. Medusozoan body fossils are rare, though they appear more frequently in Lagerstätten deposits [16]. Late Cambrian impressions evidence the early evolution of large free-swimming medusoids [18], but since few characters are preserved, connections to known medusozoan groups remain difficult to establish with certainty, concealing the evolutionary significance of these fossils. Over the past two decades, both the Cambrian Marjum Formation in Utah [19] and Chengjiang Lagerstätte in South China [20] have yielded rare and poorly preserved body fossils which have been interpreted as medusoids based on their bell-like bodies and putative externally projecting ‘tentacles’. Herein, we reinterpret these ‘tentacles’ as ctenophore comb rows and thus reject a medusozoan affinity for these fossils (see Results and discussion). Another possible medusa body fossil has been briefly reported from the Qingjiang Formation [21], however, its anatomy is also more reminiscent to a group of problematic fossils purported to be related to the origin of ctenophores [22] and it is yet to be described. Later Palaeozoic medusoid fossils are often regarded as crown-group medusozoans [16,23], although limited preservation of medusozoan synapomorphies continues to be a challenge for phylogenetic analyses.
2. Material and methods
(a) . Fossil material
The fossil material studied here is deposited in the collections of Invertebrate Palaeontology at the Royal Ontario Museum, Toronto (ROMIP). Some of this material, was first mentioned as representing probable medusoids in an unpublished Master's thesis focused on the palaeoecology of the Raymond Quarry fauna, but was never described [24]. For the purposes of this study, some specimens were mechanically prepared to remove matrix that covered anatomical features. Specimens were photographed under different lighting conditions including cross-polarized lighting.
(b) . Phylogenetic analysis
The phylogenetic analysis was based on a previously published character matrix for both fossil and extant Medusozoa and their close relatives [7,25] (see nexus file in electronic supplementary material, data). The dataset is composed of 106 taxa and 347 discrete characters, all unordered, with Choanoflagellata used as the outgroup. Bayesian phylogenetic analyses were performed using MrBayes v.3.2.7 x64. Tree searches followed an Mkv + Γ model with two runs of four chains sampling every 100 generations during two runs for up to 100 000 000 Markov chain Monte Carlo generations, with a stop value set to 0.01 and burn-in of 25%. As previously implemented with this dataset, a partial backbone constraint was used to enforce the monophyly of extant clades, based on separate molecular results [7,25]. The results were summarized as a majority rule consensus tree. In order to better characterize uncertainty of the phylogenetic position of Burgessomedusa, we then performed monophyly tests on the set of posterior trees, following Moysiuk & Caron [26]. R code is available as an electronic supplementary material file and results of the monophyly tests are summarized in electronic supplementary material, table S1. In order to test the sensitivity of our analyses to our bell shape interpretations, we repeated all analyses for a dataset in which characters 317 (Adult medusoid shape: bell pyramidal, cubic, actinuloid) and 318 (Shape of horizontal cross-section of the medusa: circular, four-part symmetry) were coded as ambiguous.
(c) . Morphometrics
Data on umbrella dimensions of extant medusozoans were taken from previously published literature [27]. Two outlier data points (800, 0.25), (1200, 0.25) were omitted from the graph for clarity. Only fossil specimens preserved laterally, and showing a complete bell were measured.
3. Systematic palaeontology
Phylum Cnidaria Verrill, 1865
Subphylum Medusozoa Peterson, 1979
Burgessomedusa phasmiformis gen. et sp. nov.
Genus: urn:lsid:zoobank.org:act:EB7812B9-CDFC-48E0-8D59-618D5A755904
Species: urn:lsid:zoobank.org:act:F9088364-1683-4B19-BF8A-87F306FA40D4
(a) . Etymology
Genus is a compound name with Burgess referring to the locality, the Burgess Shale, and medusa (Latin) referring to the clade Medusozoa. Species is a compound name with phasma (Greek) and forma (Latin), in reference to the ghostly figure of the umbrella.
Type material: Holotype, ROMIP 65781_1 (figures 1a, 2a; electronic supplementary material, figure S1). Other material: 181 specimens reposited at the Royal Ontario Museum, Canada (electronic supplementary material, table).
Figure 1.

Size variations and general morpho-anatomical details of Burgessomedusa phasmiformis gen. et sp. nov. (a) Holotype ROMIP65781.1 (close-up in figure 2a). (b) ROMIP65782.2–3, with putative gonads (close-up in figure 2b). (c), ROMIP65783.1, with putative gonads. (d) ROMIP65784, with putative stomach cavity. e,f, specimens with putative gonads ROMIP65785 (e), ROMIP65786 (f). (g) ROMIP65787, with a contracted umbrella. (h) ROMIP65788, with putative gonads (close-up in figure 2e). (i) ROMIP65114.1–3. (j) ROMIP65789. (k) ROMIP65790.1–2. Abbreviations: bm, bell margin; go, gonads; man, manubrium; st, stomach cavity; ten, tentacles. Scale = 2 cm.
Figure 2.
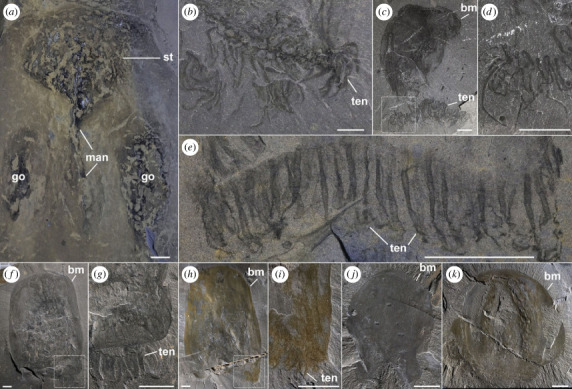
Morphological details of Burgessomedusa phasmiformis gen. et sp. nov. (a) Close-up of stomach cavity, manubrium, and gonads, ROMIP65781.1. (b) Close-up of tentacles ROMIP65782.2. (c,d) specimen showing disarticulated tentacles (close up in d), ROMIP65791. (e) Close-up of tentacles showing equidistant interspaces, ROMIP65788. (f,g) ROMIP65792, with short tentacles (close up in g) placed under the oral umbrella margin. (h,i), ROMIP65793, with tentacle remnants (close up in i). (j) ROMIP65794, specimen with irregular umbrella margin. (k) ROMIP65795.1, specimen showing tetraradial symmetry. All abbreviations are as in figure 1. Scales = 1 cm.
(b) . Horizon and locality
Middle Cambrian (Wulian), Burgess Shale, Raymond Quarry Shale Member, Raymond Quarry (British Columbia, Canada) [28].
(c) . Preservation
As is typical with Burgess Shale fossils, specimens are preserved as flat, carbonaceous compressions and are buried at different angles along bedding planes as a result of rapid burial. The majority of specimens are preserved laterally and some are obliquely preserved but no specimens can be confidently interpreted to be preserved vertically, oral or aboral sides up. This suggests that like most other large macroscopic Burgess Shale species (e.g. [29]), the specimens were mainly buried along their long axis in hydrodynamically stable orientations. Specimens typically show the umbrella, tentacles, and, more rarely, internal soft tissues, for example, gonads (e.g. figures 1c,e,f, 2a; electronic supplementary material, figures S3, S6). Disarticulation of external labile structures (for example, tentacles) is observed with oblique and a minority of laterally preserved specimens. In most cases, either tentacles are dissociated from the umbrellas (e.g. figure 2c; electronic supplementary material, figure S17a), or specimens show no clear evidence of tentacles (e.g. figure 2j,k; electronic supplementary material, figure S17b–f). In the latter case, it is possible that the tentacles are still concealed and folded below the umbrella margin, but groups of tentacles with their marginal tissues may also have detached due to decay and transport, as shown in modern decay experiments [15]. The umbrella surface texture varies from smooth to mottled, presumably as a result of differential decay processes (e.g. figure 1a–c,f,g,i; electronic supplementary material, figures S1–S3, S6, S12, S15). Similar mottled textures occur in other animals preserved from the same locality, such as Anomalocaris [30], suggesting a similar taphonomic cause. The mottled textures tend to be expressed only on large specimens, and above internal organs, including the gut and body cavity in Anomalocaris, and the gonads or stomach area in Burgessomedusa. Clusters of three or more specimens show comparable states of soft tissue preservation, with some clusters consisting of similar-sized specimens and others made up of individuals of different sizes (e.g. electronic supplementary material, figures S1–S3, S14–16).
(d) . Diagnosis
Medusoid with a tetraradial bell-shaped umbrella with width reaching approximately 40% of umbrella height. Over 90 finger-like tentacles along the oral margin, reaching approximately 15% of umbrella height in length. Stomach cavity located at apex of the umbrella, occupying approximately 30% of the body area. Manubrium extending up to two-thirds the length of the umbrella. Gonads elongate and ovoid, occupying approximately 45% of the umbrella height, located along umbrella corners, but internally positioned approximately halfway between umbrella margins and manubrium.
(e) . Description
The outline of the umbrella is often oblong (approx. 2.5 times taller than wide in the largest specimens) tapering to a smooth, domed apex (e.g. figure 1a,c,i), though the umbrellas of some specimens are cuboidal (e.g. figure 1f), especially the smallest individuals, are more hemispherical (e.g. figure 1d,k). The bell surface is divided longitudinally into a variable number of alternating textured and smooth longitudinal zones. Either four subequal longitudinal zones consisting of two textured and two smooth surfaces (e.g. figure 1f), or two textured and three smooth surfaces (one central, and two lateral; e.g. figure 1a; electronic supplementary material, figures S17f) may be observed, depending on burial orientation. The textured surfaces are seemingly preserved above internal soft tissues which appear much darker than the rest of the fossil (e.g. figures 1f, 2k; electronic supplementary material, figures S6, S12, S15). Since only half of the bell is visible in these compressed specimens, we interpret that there are likely four textured zones, representing the four corners of the umbrella, suggesting tetraradial symmetry. When the umbrella is preserved with one of the four sides parallel to bedding, only two corners are visible (overlapping the gonads) with a smooth area in between and portions of the smooth areas of the two juxtaposing sides also visible along the umbrella margins (e.g. figure 1a; electronic supplementary material, figure S17f).
The oral half of the umbrella appears to be more flexible, as indicated by specimens with oral margins following a sinusoidal curvature (e.g. figures 1b,i, 2e; electronic supplementary material, figures S2c,d, S11). The oral margin itself is smooth without any conspicuous ridges or overhanging lappet-like features (e.g. figure 2f,g,k; electronic supplementary material, figures S2c,d, S17k).
Up to 90 or more tentacles, extrapolated to the full body based on the exposed fossil surface, are located along the oral umbrella margin (e.g. figure 2e; electronic supplementary material, figure S11). The tentacles are evenly spaced and seem to originate from the inner side of the umbrella margin, inserted orthogonally (figure 2f–i). Each tentacle measures ca. 15% of the umbrella height and tapers distally to a smooth point (e.g. figure 2b–e). Tentacles have smooth external margins, and their random orientation suggests that they were highly flexible (e.g. figure 2b–e).
Internal anatomical structures are preserved as dark carbonaceous compressions. Some specimens show a large, hemispherical carbonaceous area that occupies the top third of the umbrella height, and up to a fifth of the visible umbrella surface area (e.g. figures 1 and 2a), which we interpret as the stomach cavity. The bottom of the stomach cavity is connected to a thin, columnar structure which is located within the central axis of the umbrella and extends to about half the umbrella height, which we interpret as the manubrium (figures 1 and 2a). Adjacent to the manubrium, there are four other oblong dark carbonaceous patches parallel to the umbrella, which we interpret as gonads (e.g. figure 1a,c,g, 2a; electronic supplementary material, figures S1, S3, S6). The margins of the gonads are not well-defined and may range from 20% to 80% of the umbrella length. Despite the variation in size among specimens, the position of these gonads is consistent.
4. Results and discussion
Burgessomedusa appears very similar in gross morphology to crown-group medusozoans, although it is difficult to relate directly to any known groups. Burgessomedusa is morphologically different from extant stauromedusans [31] because it lacks a stalk or a peduncle, and its tentacles are not clustered. The cuboidal shape and tetraradial symmetry of the umbrella of Burgessomedusa are reminiscent to extant cubozoans, but a definite cubozoan affinity cannot be established due to the lack of key morphological characteristics [8,32] such as: (1) four ‘pedalia’ at the corners of the umbrella from which the tentacles emerge; (2) an extension of the umbrella called a velarium which is located at the inner space of the oral margin, similar to the velum of hydrozoans; (3) frenula (structures connecting the velarium to the inside of the bell); and (4) developed rhopalia for vision and balance.
In Burgessomedusa, the tentacle shape and placement along the oral umbrella margin are more comparable to those in extant scyphozoans and hydrozoans. Tentacle attachment in both Burgessomedusa and scyphozoans differs from that in cubozoans in that scyphozoan tentacles attach along the entirety of the oral umbrella margin rather than only to distinct pedalia [32]. Burgessomedusa notably shares the numerous oral marginal tentacles with scyphozoans of the paraphyletic order Semaeostomeae, however, unlike these forms, oral arms or radial canals could not be observed in our material. In addition, the texture of the tentacles is smooth, with no indication of the banded structures that are common in scyphozoans. The presence of marginal tentacles and absence of suctorial mouths with proximally fused oral arms in Burgessomedusa make affinities with the order Rhizostomeae unlikely. Coronatae possess a coronal groove, which is also not present in any of the specimens, rendering affinities with this group equally doubtful. The umbrella shape in Burgessomedusa also differs from most scyphozoans. Like any large fossil specimens from the Burgess Shale, Burgessomedusa tends to be preserved on its hydrodynamically stable axis, parallel to the oral-aboral axis. This is in large part because of the oblong nature of their umbrellas. With some exceptions, scyphozoans tend to be preserved with their oral or aboral sides up instead [16], implying that they are wider than tall, although the mode of entombment of fossils from the Burgess Shale differs from other fossil sites, including mass shoaling event-type fossil deposits [18]. Taken together, a scyphozoan affinity appears uncompelling. Comparisons with hydrozoan medusae are less warranted based on the cuboid-shaped umbrella and the lack of a velum in Burgessomedusa.
Our majority rule consensus tree from a constrained Bayesian phylogenetic analysis recovers Burgessomedusa as a stem cubozoan (figure 3; electronic supplementary material, figures S18–S19) with moderate support (66% of trees). Further examination of the set of posterior trees with monophyly tests reveals that the next most strongly supported placements are within either the stem groups of Acraspeda [33] (9%) or of the clade comprising Cubozoa and Scyphozoa (7%) with other possible affinities represented by a minority of trees (electronic supplementary material, table S1). The three best-supported topologies remain stable even when characters relating to the cubic bell shape are coded as ambiguous, although the level of certainty decreases such that Burgessomedusa collapses into a polytomy with the extant medusozoan clades in the consensus tree (electronic supplementary material, figures S20–S21, table S1). Significantly, only a small fraction of trees (4% assuming cubic shape interpretation, 7% with ambiguous coding) support a position in the medusozoan stem group. Thus, despite some uncertainty, Burgessomedusa can be regarded with confidence as a crown-group medusozoan and most likely as a stem-group cubozoan or a stem-group acraspedan.
Figure 3.
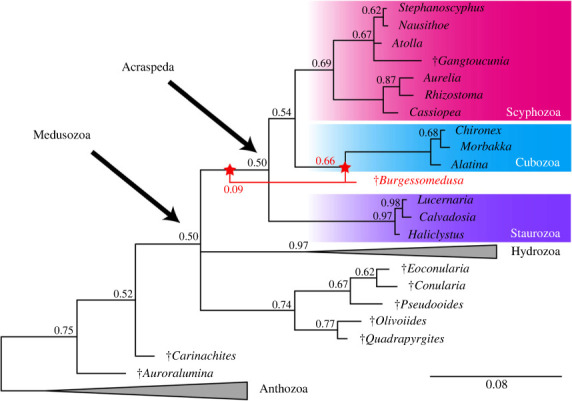
Phylogenetic position of Burgessomedusa phasmiformis gen. et sp. nov. Bayesian phylogenetic analysis (347 characters, 106 taxa, Mkv + Γ model) showing the position of Burgessomedusa phasmiformis gen. et sp. nov. within Medusozoa. Numbers indicate the posterior probabilities; scale bar indicates the average number of substitutions per site. Nodes with 100% posterior probability are not labelled. Red lines indicate alternative placements for Burgessomedusa.
Burgessomedusa sheds little light as to the morphology of the ancestral medusozoan, but provides a minimum age constraint for the evolution of large swimming medusae by at least the middle Cambrian (Wulian), supporting an early origination of medusae [13,34]. Fossil evidence (e.g. [9–11,35]), and recent phylogenomic studies suggest a polypoid-first diversification within the Medusozoa [33,36,37]. According to this hypothesis, the transition from sessile polyps to pelagic medusae required the acquisition of several key characteristics, such as thicker mesoglea, a larger body size, the loss of biomineralised structures and a stalk, specialised tentacles, the diversification of nematocysts, and the acquisition of sense organs, to list a few [38]. However, the distinct taphonomic bias against the preservation of medusoid life stages [17] and paucity of direct ontogenetic evidence (except for the possible ephyra stage of Olivooides [13,39]) should necessarily be considered before this hypothesis becomes more widely accepted. In addition, other molecular studies suggest the possibility that a medusa stage might have already been present in the ancestral cnidarian and was lost in anthozoans [40,41].
(a) . Ecological interpretations
Extant cnidarians exhibit predatory lifestyles (and some parasitism) [1], which may be influenced by both morphological and physiological constraints imposed by the shape and size of the umbrella [27]. Extant medusozoans may be prolate (i.e. torpedo-like) or oblate (i.e. saucer-like), with smaller species exhibiting more variability in shapes compared to the largest species, which are only oblate [27]. Cubozoans and Burgessomedusa are most comparable in terms of their relatively large size range and the prolate shape of their umbrellas (figures 4 and 5) suggesting that the former may be reasonable functional analogues. Extant cubozoans forage in habitats with high prey densities, utilizing their velarium for bursts of speed, their highly developed rhopalia for balance and to observe their surroundings [27]. Juveniles (less than 4 cm) use mainly jet-propulsion and larger individuals move mainly by rowing [42]. Burgessomedusa lacks rhopalia and there is no evidence of a velum or velarium-like structure to restrict the umbrellar opening for jet propulsion. Since most Burgessomedusa specimens are large (greater than 4 cm) and similar in shape to large modern cubozoans, a rowing propulsion as a main mode of locomotion seems plausible. Whether they could orient themselves to particular prey fields like modern cubozoans do is unknown ([42] and references therein). Burgessomedusa specimen clusters may reflect snapshots of blooming, which has been documented in Palaeozoic deposits as preserved mass strandings [18], or seasonal patterns of benthic migrations comparable to what is known in some modern scyphozoans [43]. In the context of the Burgess Shale and owing to the exceptional state of preservation observed and cooccurrence with benthic fauna such as Ottoia, Vauxia and Haplophrentis, most specimens were probably buried alive very quickly by mudflows, indirectly suggesting that these medusae must have lived along the benthos for some periods of time. One specimen is also preserved with both Leanchoilia and Olenoides within its umbrella space (electronic supplementary material, figure S17g,h). If not coincidental, this positioning would suggest that Burgessomedusa was a nektobenthic predator capable of capturing large, motile prey, thus sharing ecospace in the Burgess Shale palaeocommunity with giant arthropod predators such as Anomalocaris [30] and chaetognaths [44]. Burgessomedusa adds to the complexity and diversity of Cambrian marine ecosystems and demonstrates that large predatory niches were not exclusively occupied by large arthropods during the early evolutionary burst of animals.
Figure 4.
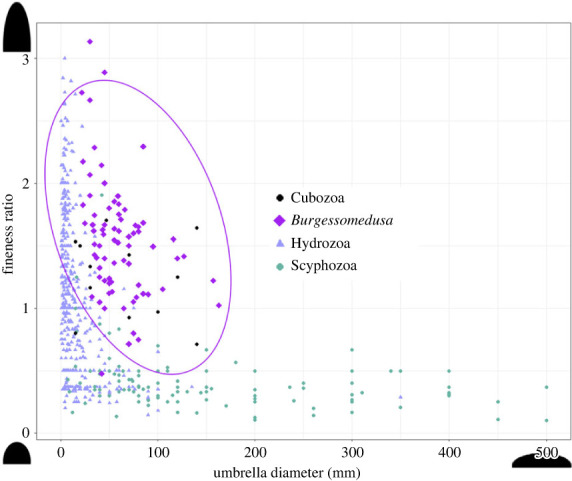
Medusozoan umbrella morphospace based on umbrella diameter and height. Fineness ratio is umbrella height divided by the diameter. Colour based on the medusozoan class: light purple = Hydrozoa; black = Cubozoa; grey green = Scyphozoa; purple = Burgessomedusa phasmiformis gen. et sp. nov. Data on swimming umbrella dimensions from [27].
Figure 5.

Life reconstruction showing a cluster of Burgessomedusa phasmiformis gen. et sp. nov. swimming above the benthos. This reconstruction is based on the Raymond Quarry Burgess Shale community with clusters of Vauxia sponges represented in the foreground. Artwork by C. McCall.
(b) . Reinterpretation of macroscopic Cambrian medusoid body fossils as ctenophores
Ctenophores share their gross morphology with medusozoan jellyfish and are often mistaken for them: both have transparent and gelatinous bodies and some of each have tentacles. However, modern ctenophores differ from jellyfish in that they have biradial symmetry, eight longitudinal ctene rows along the body, colloblasts and cydippid larvae with a pair of long tentacles which can be absent in many species [45]. Cambrian stem-group ctenophores are typically reported with multiples of 8 comb rows with distinctive cushion plates, organic skeletons, aboral sense organs and oral skirts (e.g. [46,47]) but tentacles are not present. Medusozoan marginal tentacles arise from the oral margin of the umbrella, while the comb rows of ctenophores encircle the entire length of the body, which is typically preserved in fossil ctenophores (electronic supplementary material, figure S7a–d).
Fossils from Utah [19] and Chengjiang [20] were described as putative medusozoans based on the supposed presence of external tentacles with transverse bands that resemble nematocyst bands [19], putative umbrella outlines, and possible rhopalia [20], although the Chengjiang species was initially interpreted as a ctenophore [48]. Unfortunately, descriptions are based on a small number of specimens, most of them poorly preserved or incomplete, which makes interpretations more difficult. We argue that the relative position and morphology of the putative tentacles on the body more closely resembles the comb rows of ctenophores and other problematic fossils [22].
Specimens from the Marjum Formation, Utah, supposedly represented three crown-group medusozoan classes [19]: Scyphozoa, Cubozoa and Hydrozoa (electronic supplementary material, figures S7e–k, S8). These medusozoan interpretations are problematic for several reasons:
-
(1)
Putative ‘tentacles’ appear nearly parallel to each other, unlike flexible medusozoan tentacles (e.g. figure 2).
-
(2)
The transverse lines of the ‘tentacles’ appear to be paired and have a clear demarcation through the middle, more akin to the comb rows of both extant and fossil ctenophores (electronic supplementary material, figure S7a–d).
-
(3)
There is a clear preferential preservation of the transverse lines along ‘tentacles’, like the cushion plates in described Cambrian ctenophores (e.g. electronic supplementary material, figures S7a–d).
-
(4)
There is evidence of remnants of tissues between ‘tentacles’, suggestive of a partial preservation of the ctenophore body as seen in other Cambrian ctenophores (electronic supplementary material, figure S7a–d). This shows that the putative ‘tentacles’ extend along the majority of the ‘umbrella’ above the previously proposed ‘umbrella margin’, which is analogous to how ctenophore comb rows are positioned in both fossil and extant ctenophores.
Yunannoascus haikouensis from China is another medusozoan-like fossil which was initially interpreted as a stem ctenophore [48], but was more recently revised as a pelagic medusozoan [20] (electronic supplementary material, figure S9). Only a single fragmented specimen of this species is known. Arguments against a ctenophoran affinity were that the ‘tentacles’ are separated by host rock, rather than being distributed on the body, putative rhophalia were present, and the ‘tentacles’ originate from the umbrella margin, rather than along the entire bell length. Like the Utah material, the ‘tentacles’ do appear to be separated by the host rock, although again, this separation is likely taphonomic. Each transverse element appears to be connected to a longitudinal element (electronic supplementary material, figure S9b), which might be equivalent to putative comb row nerves that are similarly preserved in other fossil ctenophores [46]. The twisted appearance of the ‘tentacles’ does not provide counterevidence, since other fossil ctenophore taxa were shown to have twisted and irregular comb rows in more decayed material (e.g. Xianoascus canadensis [47]). In addition, other proposed medusozoan-diagnostic structures such as putative marginal lappets and manubrium are poorly preserved and highly likely to be decay-related or compressional artefacts instead. Marginal lappet-like structures are also found in fossil ctenophores, where they are referred as oral skirts (e.g. in Thalassostaphylos elegans [46]). Other medusozoan characters described by the authors are also doubtful, considering the incompleteness and poor state of preservation of this specimen. A full redescription of the Utah and Chinese material is out of the scope of this study.
5. Conclusion
Burgessomedusa phasmiformis gen. et sp. nov. from the Burgess Shale provides a minimal age estimate for the timing of evolution of large macroscopic medusae in crown-group medusozoans, suggesting that complex life cycles with a medusa stage in this clade likely evolved during the Cambrian explosion [13,34,49]. Previously described putative Cambrian jellyfish body fossils from Burgess Shale-type deposits in China [20] and USA [19] are reinterpreted as ctenophores. Burgessomedusa most likely represents either a stem-cubozoan or stem-acraspedan. The prolate, box-like shape of the bell provides indirect evidence of rowing propulsion and predatory habit based on cubozoan analogues. Burgessomedusa shows that pelagic Cambrian ecosystems were not uniquely dominated by large arthropod predators such as Anomalocaris [30] and Titanokorys [50], but that they also harboured a diversity of other predators including chaetognaths [44,51], ctenophores [47] and jellyfish.
Acknowledgements
We thank three anonymous reviewers for their constructive comments. The fossil material from the ROM was collected under different Parks Canada Research and Collecting permits, mainly between 1991 and 1997. We thank C. McCall for the life reconstruction, M. Akrami for collection support, A. Izquierdo-López and H. Osawa for providing feedback on phylogenetic analyses and discussions and S. Scharf for editorial suggestions. We also thank L. Parry and F. Zhao for providing high resolution images of Cambrian ctenophore fossils from Utah and China, respectively. The Utah material described by Cartwright et al. (2007) [19] was unavailable for this study. This is the Royal Ontario Museum Burgess Shale project number 95.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The data are provided in electronic supplementary material [52]. Phylogenetic code is provided within the .nex file (electronic supplementary material, data) which can be accessed using any text reading software such as Notepad++. R code is provided within the .R file.
Authors' contributions
J. Moon: formal analysis, investigation, methodology, writing—original draft, writing—review and editing; J.-B.C.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing; J. Moysiuk: formal analysis, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This work was supported by doctoral fellowships from the University of Toronto (Department of Ecology and Evolutionary Biology) to J. Moon and by a NSERC Discovery Grant to J.-B.C. (no. 341944). J. Moysiuk was additionally supported by an Ontario Graduate Scholarship.
References
- 1.Giribet G, Edgecombe GD. 2020. 8. Cnidaria. In The invertebrate tree of life, pp. 55-79. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Kraus JE, Fredman D, Wang W, Khalturin K, Technau U. 2015. Adoption of conserved developmental genes in development and origin of the medusa body plan. Evodevo 6, 23. ( 10.1186/s13227-015-0017-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvini-Plawen LV. 1978. On the origin and evolution of the lower Metazoa. J. Zool. Syst. Evol. Res. 16, 40-87. ( 10.1111/j.1439-0469.1978.tb00919.x) [DOI] [Google Scholar]
- 4.Laumer CE, et al. 2019. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. R. Soc. B 286, 20190831. ( 10.1098/rspb.2019.0831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laumer CE, Gruber-Vodicka H, Hadfield MG, Pearse VB, Riesgo A, Marioni JC, Giribet G. 2018. Support for a clade of Placozoa and Cnidaria in genes with minimal compositional bias. Elife 7, e36278. ( 10.7554/eLife.36278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins AG. 1998. Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence. Proc. Natl Acad. Sci. USA 95, 15 458-15 463. ( 10.1073/pnas.95.26.15458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn FS, Kenchington CG, Parry LA, Clark JW, Kendall RS, Wilby PR. 2022. A crown-group cnidarian from the Ediacaran of Charnwood Forest, UK. Nat. Ecol. Evol. 6, 1095-1104. ( 10.1038/s41559-022-01807-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques AC, Collins AG. 2004. Cladistic analysis of Medusozoa and cnidarian evolution. Invertebr. Biol. 123, 23-42. ( 10.1111/j.1744-7410.2004.tb00139.x) [DOI] [Google Scholar]
- 9.Van Iten H, de Moraes Leme J, Simões MG, Marques AC, Collins AG. 2006. Reassessment of the phylogenetic position of conulariids (?Ediacaran-Triassic) within the subphylum medusozoa (phylum cnidaria). J. Syst. Paleontol. 4, 109-118. ( 10.1017/S1477201905001793) [DOI] [Google Scholar]
- 10.Leme JM, Van Iten H, Simões MG. 2022. A New conulariid (Cnidaria, Scyphozoa) from the terminal Ediacaran of Brazil. Front. Earth Sci. 10, 777746. ( 10.3389/feart.2022.777746) [DOI] [Google Scholar]
- 11.Van Iten H, Leme JM, Pacheco MLAF, Simões MG, Fairchild TR, Rodrigues F, Galante D, Boggiani PC, Marques AC. 2016. Origin and early diversification of phylum Cnidaria: Key macrofossils from the Ediacaran system of North and South America. In The cnidaria, past, present and future: the world of medusa and her sisters (eds Goffredo S, Dubinsky Z), pp. 31-40. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 12.Van Iten H, Hughes NC, John DL, Gaines RR, Colbert MW. In press. Conulariid soft parts replicated in silica from the Scotch Grove Formation (lower Middle Silurian) of east-central Iowa. J. Paleontol. ( 10.1017/jpa.2023.6) [DOI] [Google Scholar]
- 13.Wang X, Vannier J, Yang X-G, Leclère L, Ou Q, Song X, Han J. 2022. Muscle systems and motility of early animals highlighted by cnidarians from the basal Cambrian. Elife Sci. 11, e74716. ( 10.7554/eLife.74716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcmahon S, Tarhan LG, Briggs DEG. 2017. Decay of the sea anemone Metridium (actiniaria): implications for the preservation of cnidarian polyps and other soft-bodied diploblast-grade animals. Palaios 32, 388-395. [Google Scholar]
- 15.Adler-Ohde L. 2013. The taphonomy of soft-bodied cnidarians. Dublin: University College Dublin. [Google Scholar]
- 16.Young GA, Hagadorn JW. 2011. The fossil record of cnidarian medusae. Palaeoworld 19(3–4, Sp. Iss. SI), 212-221. [Google Scholar]
- 17.Young GA, Hagadorn JW. 2020. Evolving preservation and facies distribution of fossil jellyfish: a slowly closing taphonomic window. Bollettino della Società Paleontologica Italiana 59, 186. [Google Scholar]
- 18.Hagadorn JW, Dott RH Jr, Damrow D. 2002. Stranded on a Late Cambrian shoreline: Medusae from central Wisconsin. Geology 30, 147-150. (). [DOI] [Google Scholar]
- 19.Cartwright P, Halgedahl SL, Hendricks JR, Jarrard RD, Marques AC, Collins AG, Lieberman BS. 2007. Exceptionally preserved jellyfishes from the Middle Cambrian. PLoS ONE 2, e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Hu S, Cartwright P, Zhao F, Ou Q, Kubota S, Wang X, Yang X. 2016. The earliest pelagic jellyfish with rhopalia from Cambrian Chengjiang Lagerstätte. Palaeogeogr. Palaeoclimatol. Palaeoecol. 449, 166-173. ( 10.1016/j.palaeo.2016.02.025) [DOI] [Google Scholar]
- 21.Fu D, et al. 2019. The Qingjiang biota—A Burgess Shale-type fossil Lagerstätte from the early Cambrian of South China. Science 363, 1338-1342. ( 10.1126/science.aau8800) [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Vinther J, Parry LA, Wei F, Green E, Pisani D, Hou X, Edgecombe GD, Cong P. 2019. Cambrian sessile, suspension feeding stem-group ctenophores and evolution of the comb jelly body plan. Curr. Biol. 29, 1112-1125.e2. ( 10.1016/j.cub.2019.02.036) [DOI] [PubMed] [Google Scholar]
- 23.Young GA, Rudkin DM, Dobrzanski EP, Robson SP, Nowlan GS. 2007. Exceptionally preserved Late Ordovician biotas from Manitoba, Canada. Geology 35, 883-886. ( 10.1130/g23947a.1) [DOI] [Google Scholar]
- 24.Devereux M.G. 2001. Palaeoecology of the middle Cambrian Raymond Quarry fauna, Burgess Shale, British Columbia, 196 p. Unpublished MSc thesis, University of Western Ontario, London, Canada. [Google Scholar]
- 25.Zhang G, Parry LA, Vinther J, Ma X. 2022. Exceptional soft tissue preservation reveals a cnidarian affinity for a Cambrian phosphatic tubicolous enigma. Proc. R. Soc. B 289, 20221623. ( 10.1098/rspb.2022.1623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moysiuk J, Caron J-B. 2022. A three-eyed radiodont with fossilized neuroanatomy informs the origin of the arthropod head and segmentation. Curr. Biol. 32, 3302-3316.e2. ( 10.1016/j.cub.2022.06.027) [DOI] [PubMed] [Google Scholar]
- 27.Costello JH, Colin SP, Dabiri JO. 2008. Medusan morphospace: phylogenetic constraints, biomechanical solutions, and ecological consequences. Invertebr. Biol. 127, 265-290. ( 10.1111/j.1744-7410.2008.00126.x) [DOI] [Google Scholar]
- 28.Fletcher TP, Collins D. 1998. The Middle Cambrian Burgess Shale and its relationship to the Stephen Formation in the southern Canadian Rocky Mountains. Can. J. Earth Sci. 35, 413-436. ( 10.1139/e97-120) [DOI] [Google Scholar]
- 29.Whittington HB. 1985. The Burgess Shale. New Haven, CT: Yale University Press. [Google Scholar]
- 30.Daley AC, Edgecombe GD. 2014. Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88, 68-91. ( 10.1666/13-067) [DOI] [Google Scholar]
- 31.Miranda LS, Hirano YM, Mills CE, Falconer A, Fenwick D, Marques AC, Collins AG. 2016. Systematics of stalked jellyfishes (Cnidaria: Staurozoa). PeerJ 4, e1951. ( 10.7717/peerj.1951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly M, et al. 2006. The Phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 1668, 127-182. ( 10.5281/zenodo.180149) [DOI] [Google Scholar]
- 33.Kayal E, Bentlage B, Sabrina Pankey M, Ohdera AH, Medina M, Plachetzki DC, Collins AG, Ryan JF. 2018. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 18, 68. ( 10.1186/s12862-018-1142-0) [DOI] [Google Scholar]
- 34.Wang X, et al. 2020. An intermediate type of medusa from the early Cambrian Kuanchuanpu Formation, South China. Palaeontology 63, 775-789. ( 10.1111/pala.12483) [DOI] [Google Scholar]
- 35.Cunningham JA, et al. 2015. Critical appraisal of tubular putative eumetazoans from the Ediacaran Weng'an Doushantuo biota. Proc. R. Soc. B 282, 20151169. ( 10.1098/rspb.2015.1169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zapata F, et al. 2015. Phylogenomic analyses support traditional relationships within Cnidaria. PLoS ONE 10, e0139068. ( 10.1371/journal.pone.0139068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalturin K, et al. 2019. Medusozoan genomes inform the evolution of the jellyfish body plan. Nat. Ecol. Evol. 3, 811-822. ( 10.1038/s41559-019-0853-y) [DOI] [PubMed] [Google Scholar]
- 38.Han J, Zhang X, Komiya T. 2016. Integrated evolution of cnidarians and oceanic geochemistry before and during the Cambrian Explosion. In The cnidaria, past, present and future: The world of medusa and her sisters (eds Goffredo S, Dubinsky Z), pp. 15-29. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 39.Dong X-P, Cunningham JA, Bengtson S, Thomas C-W, Liu J, Stampanoni M, Donoghue PCJ. 2013. Embryos, polyps and medusae of the Early Cambrian scyphozoan Olivooides. Proc. R. Soc. B 280, 20130071. ( 10.1098/rspb.2013.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold DA, et al. 2019. The genome of the jellyfish Aurelia and the evolution of animal complexity. Nat. Ecol. Evol. 3, 96-104. ( 10.1038/s41559-018-0719-8) [DOI] [PubMed] [Google Scholar]
- 41.Leclère L, et al. 2019. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat. Ecol. Evol. 3, 801-810. ( 10.1038/s41559-019-0833-2) [DOI] [PubMed] [Google Scholar]
- 42.Colin SP, Costello JH, Katija K, Seymour J, Kiefer K. 2013. Propulsion in Cubomedusae: mechanisms and utility. PLoS ONE 8, e56393. ( 10.1371/journal.pone.0056393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ceh J, Gonzalez J, Pacheco AS, Riascos JM. 2015. The elusive life cycle of scyphozoan jellyfish: metagenesis revisited. Sci. Rep. 5, 12037. ( 10.1038/srep12037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briggs DEG, Caron JB. 2017. A large Cambrian chaetognath with supernumerary grasping spines. Curr. Biol. 27, 2536-2543.e1. ( 10.1016/j.cub.2017.07.003) [DOI] [PubMed] [Google Scholar]
- 45.Giribet G., Edgecombe G.D. 2020. 3. Ctenophora. In The invertebrate tree of life, pp. 23-32. Princeton, NJ: Princeton University Press. [Google Scholar]
- 46.Parry LA, Lerosey-Aubril R, Weaver JC, Ortega-Hernández J. 2021. Cambrian comb jellies from Utah illuminate the early evolution of nervous and sensory systems in ctenophores. iScience 24, 102943. ( 10.1016/j.isci.2021.102943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway Morris S, Collins D. 1996. Middle Cambrian ctenophores from the Stephen Formation, British Columbia, Canada. Philos. Trans. R. Soc. London, Ser. B 351, 279-308. [Google Scholar]
- 48.Hu S, Steiner M, Zhu M, Erdtmann BD, Luo H, Chen L, Weber B. 2007. Diverse pelagic predators from the Chengjiang Lagerstätten and the establishment of modern-style pelagic ecosystems in the early Cambrian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 307-316. [Google Scholar]
- 49.Wang X, Han J, Vannier J, Ou Q, Yang X, Uesugi K, Sasaki O, Komiya T. 2017. Anatomy and affinities of a new 535-million-year-old medusozoan from the Kuanchuanpu Formation, South China. Palaeontology 60, 853-867. ( 10.1111/pala.12320) [DOI] [Google Scholar]
- 50.Caron J-B, Moysiuk J. 2021. A giant nektobenthic radiodont from the Burgess Shale and the significance of hurdiid carapace diversity. R. Soc. Open Sci. 8, 210664. ( 10.1098/rsos.210664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vannier J, Steiner M, Renvoisé E, Hu SX, Casanova JP. 2007. Early Cambrian origin of modern food webs: evidence from predator arrow worms. Proc. R. Soc. B 274, 627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moon J, Caron J-B, Moysiuk J. 2023. A macroscopic free-swimming medusa from the middle Cambrian Burgess Shale. Figshare. ( 10.6084/m9.figshare.c.6743107) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Moon J, Caron J-B, Moysiuk J. 2023. A macroscopic free-swimming medusa from the middle Cambrian Burgess Shale. Figshare. ( 10.6084/m9.figshare.c.6743107) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [52]. Phylogenetic code is provided within the .nex file (electronic supplementary material, data) which can be accessed using any text reading software such as Notepad++. R code is provided within the .R file.


