Abstract
Objectives
To explore the effect of dexmedetomidine (DEX) on postoperative nausea and vomiting (PONV) in adult patients after general anaesthesia.
Design
Systematic review and meta-analysis.
Eligibility criteria for selecting studies
Randomised controlled trials (RCTs) comparing the efficacy of DEX with placebo or a single drug on PONV in adult patients after general anaesthesia.
Data sources
We searched the PubMed, the Web of Science, the Cochrane Library and Embase (1 January 2000 to 30 June 2022) to select the relevant RCTs.
Data analysis
All the relevant data were analysed by using RevMan V.5.4. Heterogeneity was tested for each outcome, and random-effect or fixed-effect models was selected according to the level of heterogeneity. The primary outcome was the incidence of PONV. The secondary outcomes were the incidence of bradycardia, perioperative opioid consumption, extubation time and the length of hospitalisation.
Results
A total of 18 trials involving 2018 patients were included in this meta-analysis. Notably, 15 updated studies were not involved in the previous meta-analysis. The incidence of PONV in DEX group was lower than that in the control group (OR=0.49, 95% CI: 0.36 to 0.67) and the perioperative opioid consumption in DEX group was also decreased significantly (standard mean difference (SMD)=−1.04, 95% CI: −1.53 to −0.54). Moreover, the length of hospitalisation (SMD=−2.29, 95% CI: −4.31 to −0.28) and the extubation time (SMD=−0.75, 95% CI: −1.26 to −0.25) in DEX group were shorter. Whereas, more number of patients receiving DEX might increase the occurrence of bradycardia (OR=1.60, 95% CI: 1.13 to 2.27).
Conclusions
DEX could decrease the occurrence of PONV in adult patients under general anaesthesia and promote the recovery after surgery. However, DEX might increase the occurrence of bradycardia.
PROSPERO registration number
CRD 42022341548.
Keywords: anaesthetics, adult anaesthesia, adult surgery, adverse events
STRENGTHS AND LIMITATIONS OF THIS STUDY
An up-to-date assessment of the effectiveness of dexmedetomidine (DEX) on postoperative nausea and vomiting.
We excluded studies that DEX compared with opioids agents in our meta-analysis to eliminate the effect of opioids on postoperative nausea and vomiting.
The main limitation of this review was that varied quality and heterogeneity of included studies might affect the certainty of meta-analysis.
Introduction
Postoperative nausea and vomiting (PONV), as a familiar negative events after operation, is known as nausea, vomiting or retching within 1 day after operation, which may be due to the effect of anaesthetics on the emetic control centre in the medulla oblongata.1 The incidence of PONV is about 30% and even rising to 60%–80% in high-risk populations. PONV, an extreme poor medical experience for patients undergoing general anaesthetic surgery, leads to many adverse influences including stomach discomfort, dehydration, water-electrolyte disorders, wound dehiscence, oesophageal injury, reflux and aspiration, which extend the time of hospitalisation and increase the medical costs.2 Fortunately, prophylactic antiemetic agents could decrease the happening of PONV. However, these drugs produce some side effects including headache, restlessness, dry mouth, hypotension and cardiovascular complications, which limit their use in some cases.3 Therefore, exploring suitable drugs and methods to prevent and treat PONV is necessary.
Dexmedetomidine (DEX), as a new adrenal α2 receptor agonist with high selectivity, has sedation, hypnosis and analgesia effects without respiratory depression, which is widely used in perioperative period. These characteristics have enabled DEX to be a multifunctional drug in the presentments of numerous negative events during anaesthesia. For the last few years, the effect of DEX on PONV attracted increasing attention from anaesthesiologists. One clinical study reported that postoperative administration of DEX, as patient-controlled analgesia regimen, produced early antiemetic effects.4 Another research indicated that intravenous DEX could prevent the occurrence of PONV in adult patients after laparoscopic hysterectomy.5 While different results were observed in the similar articles,6 7 it is still disputed whether intraoperative use of DEX can ameliorate the occurrence of PONV in patients after general anaesthesia.
As far as we know, no updated analysis of the data about the effect of DEX on PONV was performed during general anaesthesia. Therefore, in order to obtain the most recent proof, we thoroughly evaluated the effect of intraoperative use of DEX on PONV in adult patients experiencing general anaesthesia according to the results from the 18 randomised controlled trials (RCTs) in our meta-analysis.
Methods
Patient and public involvement
No patient involved.
Registration
This meta-analysis was prepared by following the criteria as outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysesguidelines8 (online supplemental document 1). The meta-analysis was registered on PROSPERO (registry number: CRD 42022341548).
bmjopen-2022-067102supp001.pdf (124KB, pdf)
Search strategy
Two investigators independently searched for articles published in PubMed, the Web of Science, Embase and the Cochrane Library. The complete search strategy protocol is shown in online supplemental document 2. In order to ensure the contemporary practice, the literature was searched from 1 January 2000 to 30 June 2022.
bmjopen-2022-067102supp002.pdf (74.5KB, pdf)
Inclusion and exclusion criteria
The inclusion criteria were in accordance with patient–intervention–comparison–outcome:
Patients: adult participants undergoing general anaesthetic surgery.
Intervention: received a single or continuously administered intravenous dose of intraoperative DEX.
Comparison: received a single or continuously administered intravenous injection of placebo or comparator.
Outcomes: the incidence of PONV and bradycardia, the perioperative opioid consumption, the extubation time and the length of hospitalisation.
The reviews, abstracts, case reports or duplicates were excluded. Additionally, some RCTs meeting the following criteria were also excluded: (1) drug/drugs (including DEX) versus combinational drugs; (2) DEX compared with opioids agents; (3) adult patients undergoing surgery under local or spinal–epidural anaesthesia; and (4) full text not available.
Data extraction and analysis
All information of the articles was collected independently by two researchers using standardised forms. Any problems were decided by a third author in order to discuss and reach an agreement. The corresponding data were collected: first author, type of surgery, publication year, number of patients, administrations for patients, the incidence of PONV and bradycardia, the perioperative opioid consumption, the extubation time and the length of hospitalisation. A standardised Excel file was used to save the extracted data. And all the data were pooled together. Studies were excluded when the primary outcome was not clearly reported with quantifiable data or it was not possible to extract and calculate the appropriate data from the published results.
Risk-of-bias assessment
In accordance with the Cochrane risk-of-bias tool,9 the risk-of-bias in the included articles were evaluated by two authors independently (figure 1). According to the following criteria: bias from selection, performance, detection, attrition, reporting and other, we reviewed and scored each study as ‘high’, ‘unclear’ and ‘low’.
Figure 1.
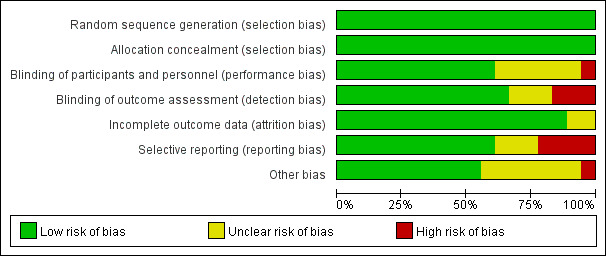
The risk-of-bias of included studies.
Statistical analysis
We used the Review Manager V.5.4 software to perform statistical analysis. For dichotomous data, we calculated ORs with 95% CIs. And when the outcome was expressed using varied approaches, we used standard mean difference (SMD) and 95% CIs to analyse the continuous data. We used the I-square (I2) test to evaluate the heterogeneity of included studies. A random-effects model was chosen when I2≥50%, otherwise a fixed-effect model was selected. Funnel plots were used for quality assessment of bias. And the sensitivity analysis was performed by removing these studies and observing the consistency for this meta-analysis involving at least 10 trials.
Results
Study selection
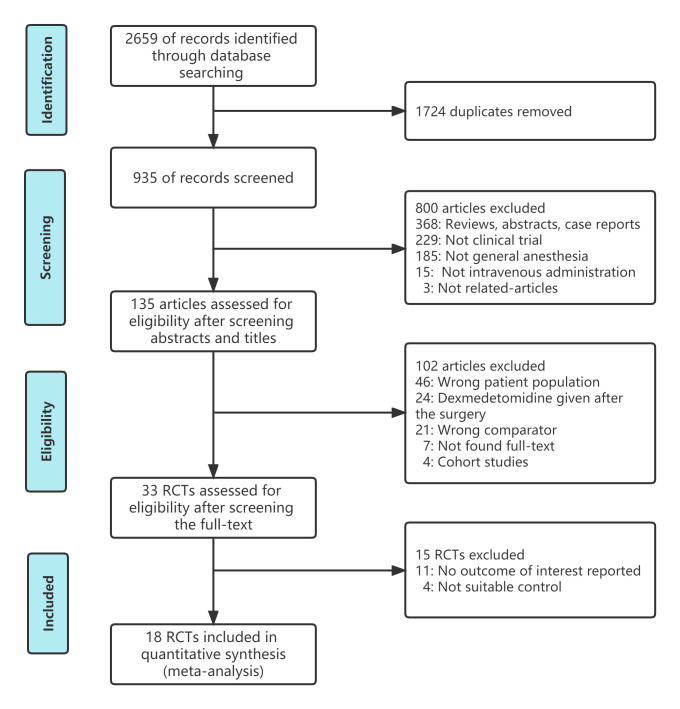
The procedure of article screening, selection of articles and the causes for exclusion are displayed in the flow diagram (figure 2). The initial search included 2659 documents, and after taking out the duplicates and checking the abstracts and titles, 33 trials were considered potentially eligible. After carefully reading the full-text studies, 18 studies were eventually included, of which 15 studies were new articles appearing after the previously published meta-analyses.
Figure 2.
Flow diagram of the inclusion and exclusion process. RCTs, randomised controlled trials.
Study characteristics
The main characteristics of the 18 articles are summed up in table 1. Sixteen articles in the included studies investigated the efficacy of DEX compared with saline, two trials examined the efficacy of DEX compared with clonidine and dexamethasone, respectively. The 18 articles including a total of 2018 patients in this meta-analysis were published from 2015 to 2021 with sample sizes varying from 19 to 334 participants.
Table 1.
Characteristics of the included trials
| Author | Year | Age (years) | Gender male/female | Sample size | Type of surgery | Administration mode | Comparisons | Numbers of nausea and/or vomiting DEX/control | Scale used for assessing PONV | |
| DEX | Control | |||||||||
| Bakri et al24 | 2015 | 31.1±2.4 | 32.3±2.1 | 15/71 | 43/43 | Laparoscopic cholecystectomy | 1 µg/kg DEX intravenous | 8 mg Dexa intravenous | 9/12 | A VAS of 0– 100 mm, 0 score meant no nausea, while 100 score meant the worst imaginable nausea |
| Peng et al25 | 2015 | 42.3±10.8 | 43.5±11.1 | 33/43 | 38/38 | Craniotomy | Continuous infusion of DEX 0.5 µg/kg/hour intravenous | Equal volume of saline | 16/28 | 0, absent; 1, nausea not requiring treatment; 2, nausea requiring treatment; and 3, vomiting. |
| Chen et al26 | 2016 | 56.67±2.705 | 60.17±1.644 | 29/31 | 30/30 | Laparoscopic resection of colorectal cancer | Loading dose 1 µg /kg DEX, continuous infusion of 0.3 µg /kg/hour intravenous | Equal volume of saline | 6/6 | 0, absent; 1, nausea not requiring treatment; 2, nausea requiring treatment; and 3, vomiting. |
| Bielka et al27 | 2018 | 55 (49–61) | 53 (49–66) | July 53 | 30/30 | Laparoscopic сholecystectomy | Continuous infusion of DEX 0.5 µg/kg/hour intravenous | Equal volume of saline | 2/8 | NR |
| Das et al28 | 2018 | 50.74±9.05 | 47.92±8.08 | NR | 50/50 | Breast cancer surgery | Continuous infusion of DEX 0.6 µg/kg/hour intravenous | Equal volume of saline | 2/8 | NR |
| Wu et al17 | 2019 | 67 | 69 | NR | NR | Laparoscopic radical prostatectomy | Loading dose 1 µg /kg DEX, continuous infusion of 0.5 µg /kg/hour intravenous | Equal volume of saline | 2/10 | NR |
| Bala et al29 | 2019 | 37.2±11.0 | 41±13.4 | 33/27 | 30/30 | Transsphenoidal pituitary surgery | Loading dose 1 µg /kg DEX, continuous infusion of 0.5 µg /kg/hour intravenous | Equal volume of saline | 2/6 | NR |
| Wu et al30 | 2019 | 55.00±5.07 | 54.85±4.96 | 25/15 | 20/20 | Cholangiojejunostomy or resection of tumour | Loading dose 0.5 µg /kg DEX, continuous infusion of 0.5 µg /kg/hour intravenous | Equal volume of saline | 1/3 | NR |
| Bakshi et al31 | 2020 | 51.8±12 | 47.2±13 | 14/26 | 19/21 | Robotic-assisted laparoscopic oncosurgeries | Loading dose 1 µg /kg DEX, continuous infusion of 0.2 µg /kg/hour intravenous | Equal volume of saline | 4/3 | NR |
| Chen and Chen 32 | 2020 | 42.68±7.59 | 43.35±6.82 | 41/36 | 39/38 | Radical resection of gastric cancer | Loading dose 0.6 µg /kg DEX, continuous infusion of 0.4 µg /kg/hour intravenous | Equal volume of saline | 1/3 | NR |
| Asri et al33 | 2020 | 51±12 | 46±16 | 32/10 | 21/21 | Thoracic surgery | Continuous infusion of DEX 0.3 µg/kg/hour intravenous | Equal volume of saline | 0/5 | NR |
| Author |
Year |
Age (years) | Gender male/female |
Number |
Type of surgery |
Administration mode |
Comparisons |
Numbers of nausea and/or vomiting DEX/control |
Scale used for assessing PONV |
|
| DEX | Control | |||||||||
| Pi and Yang 34 | 2021 | 42.15±1.34 | 41.88±1.56 | 162/55 | 121/96 | Thoracoscopic radical resection of lung cancer | Loading dose 0.4 µg /kg DEX, continuous infusion of 0.2 µg /kg/hour intravenous | Equal volume of saline | 0/1 | NR |
| Lu et al35 | 2021 | 70.1±5.8 | 70.4±6.5 | 445/230 | 344/331 | Abdominal Surgery | Loading dose 0.5 µg /kg DEX, continuous infusion of 0.2 µg /kg/hour intravenous | Equal volume of saline | 16/17 | 10-point rating scale 0: no PONV and 10: maximal PONV |
| Bafna et al36 | 2021 | 27.23+7.75 | 27.49+8.68 | 38/32 | 35/35 | Endoscopic sinus surgery | Loading dose 1 µg /kg DEX, continuous infusion of 1 µg /kg/hour intravenous | Loading dose 2 µg /kg clonidine, continuous infusion of 1 µg /kg/hour intravenous | 4/1 | NR |
| Prashantha37 | 2021 | 37.73±7.24 | 35.63±12.61 | 19/61 | 40/40 | Laparoscopic сholecystectomy | DEX IV | Saline intravenous | 5/14 | NR |
| Chen et al38 | 2021 | 55.93±11.14 | 55.64±10.81 | 41/35 | 38/38 | Thoracoscopic pulmonary segmentectomy | 0.6 µg/kg DEX infusion from 10 min before induction | Equal volume of saline | 1/1 | NR |
| Yan and Ti-jun7 | 2021 | 73.2±5.8 | 72.7±4.3 | 42/58 | 50/50 | Total hip arthroplasty | Continuous infusion of DEX 0.3 µg/kg/hour intravenous | Equal volume of saline | 5/4 | NR |
| Chen et al16 | 2021 | 50.76±8.32 | 51.07±9.43 | 44/36 | 40/40 | Intestinal surgery | Loading dose 1 µg /kg DEX, continuous infusion of 0.4 µg /kg/hour intravenous | Equal volume of saline | 1/6 | NR |
DEX, dexmedetomidine; Dexa, dexamethasone; NR, not reported; PONV, postoperative nausea and vomiting; VAS, Visual Analogue Scale.
The association between DEX and PONV
All 18 trials involved the effect of DEX on the incidence of PONV. There was no heterogeneity between the articles (p<0.00001, I2=26%, figure 3), so a fixed-effects model was chosen. The consequences revealed that the occurrence of PONV in DEX group was lower than that in the control group (OR=0.49, 95% CI: 0.36 to 0.67, figure 3), which indicated that DEX notably prevent the happening of PONV in adult patients after general anaesthetic surgery.
Figure 3.
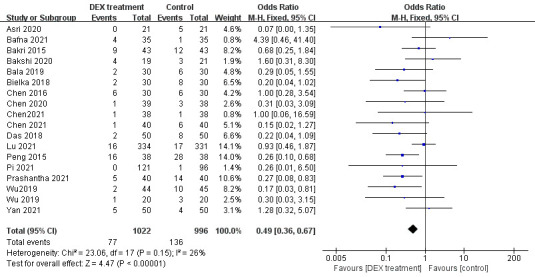
The total effect of DEX on postoperative nausea and vomiting. DEX, dexmedetomidine.
The association between DEX and perioperative opioid consumption
Eight studies assessed the effect of DEX on perioperative opioid consumption. Because of a high heterogeneity (p<0.00001, I2=91%, figure 4), a random-effect model was selected. The consequences of this meta-analysis indicated that the perioperative opioid consumption was lower in DEX group (SMD= −1.04, 95% CI: −1.53 to −0.54, figure 4). Our results suggested that DEX decreased the perioperative opioid consumption significantly.
Figure 4.
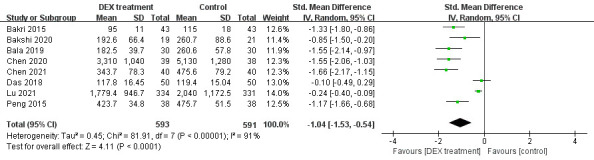
Perioperative opioid consumption in DEX and control group. DEX, dexmedetomidine.
Other recovery outcomes
Four literatures including 200 patients involved the length of hospitalisation. The study heterogeneity was high (p<0.00001, I2=96%, figure 5), so a random-effect model was selected. The consequence found that the length of hospitalisation in DEX group was shorter (SMD= −2.29, 95% CI: −4.31 to −0.28, figure 5). Four trials including 292 subjects referred to the extubation time. A random-effect model was chosen due to high heterogeneity (p=0.004, I2=77%, figure 6). There was a shorter time to extubation in DEX group (SMD= −0.75, 95% CI: −1.26 to −0.25, figure 6). Therefore, meta-analysis of the eight literatures indicated that DEX could accelerate the recovery of patients after anaesthesia.
Figure 5.
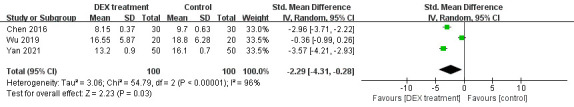
The effect of dexmedetomidine on the length of hospitalisation.
Figure 6.
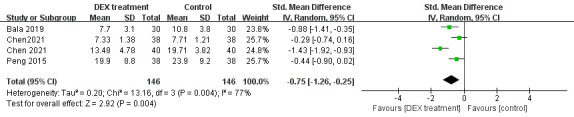
The effect of DEX on the extubation time. DEX, dexmedetomidine.
Side effects
Eight trials described the incidence of bradycardia. A fixed-effect model was selected considering the little heterogeneity (p=0.32, I2=14%, figure 7). Compared with the control group, the number of participants who developed bradycardia in the DEX group was higher (OR=1.60, 95% CI: 1.13 to 2.27, figure 7). The consequences from this meta-analysis revealed that DEX might increase the occurrence of bradycardia.
Figure 7.
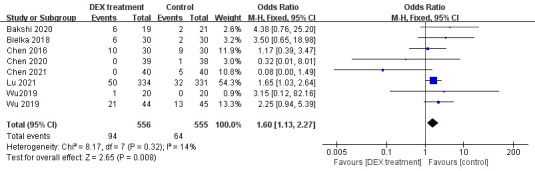
Incidence of bradycardia in DEX and control group. DEX, dexmedetomidine.
Risk of bias
Publication bias of literatures including the incidence of PONV in our meta-analysis was assessed by funnel plots, and no publication bias was found (figure 8). We removed each study one by one for sensitivity analysis and found that the results did not change (online supplemental document 3).
Figure 8.
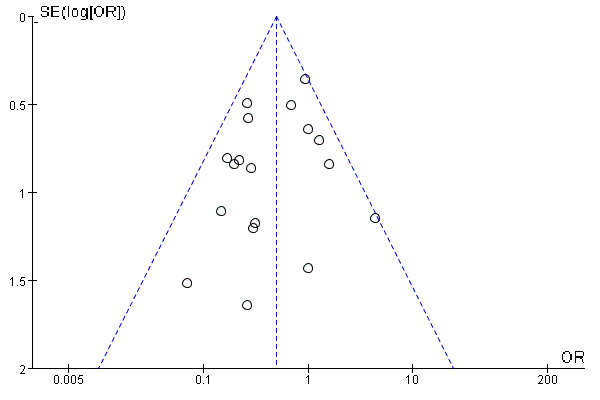
Test for publication bias of the studies included in the incidence of postoperative nausea and vomiting.
bmjopen-2022-067102supp003.pdf (53.6KB, pdf)
Discussion
This present meta-analysis showed that DEX is a potential effective agent for decreasing the incidence of PONV and promoting the recovery of adult patients undergoing general anaesthetic surgery, but it might increase the incidence of bradycardia.
PONV is an unsatisfactory experience and painful adverse event for patients, especially in the first day after surgery. Its incidence is approximately 30% and up to 80% without prevention.1 10 Moreover, some surgical types were associated with the high occurrence of PONV, especially in gynaecological surgery, otolaryngology surgery and neurosurgery.3 There are many risk factors that can increase the incidence of PONV by 20%, respectively, in patients, including anaesthetic factors, surgical factors, female, non-smokers and the medical history of motion sickness and/or PONV.11 These risk factors might also vary with the premedication, anaesthetic technique and postoperative management.12 Among the factors of anaesthesia, general anaesthesia is more likely to cause PONV compared with regional anaesthesia.13 The pathophysiology process of PONV is very elusive. A study suggested that injuries from operation, anaesthesia, visceral nerve stimulation, hypoxia, hypotension and pain were the major irritants, which could trigger the vomiting response when they reach the cortical/thalamic, cerebellar and vestibular nuclei and the chemoreceptor triggering band outside of the blood–brain barrier.14 Although there are multiple methods and drugs to prevent PONV in clinical practice, the efficacy of PONV prophylaxis remains unsatisfactory especially in high-risk patients.1
DEX exerts the anxiolytic, sedative and analgesic effects by reducing the release of norepinephrine induced by α2 adrenergic receptors in the spinal cord and locus coeruleus. However, it could not result in excessive sedation or respiratory depression as the results of accumulation.15 Therefore, DEX was used as an appropriate short-acting sedative for patients under general anaesthesia in perioperative period. Previous articles indicated that DEX reduced the occurrence of PONV, which were similar to our result. For instance, a study reported that DEX administered could decrease the occurrence of PONV in patients experiencing intestinal surgery,16 another study discovered that intraoperative use of DEX could be a valid measure to prevent the PONV in patients after laparoscopic radical prostatectomy.17 But the mechanisms for the effect of DEX on PONV are still obscure. Previous articles reported DEX could decrease the occurrence of PONV by modulating 5-hydroxytryptamine (5-HT) and dopamine release, suppressing the histamine-induced expression of interleukin-6 and reducing sympathetic outflow and total catecholamine release.18 19 So, one of the key mechanisms about the effect of DEX on PONV might be attributable to the regulation of neurotransmitters. Moreover, it is well known to us that the amount of intraoperative opioid use directly influenced the frequency and degree of PONV.13
DEX also can prevent the perioperative stress response by regulating heart rate and blood pressure, however, DEX might produce some adverse events like bradycardia especially in patients with atrioventricular block or hypovolaemia.20 Similar consequence with our article, a meta-analysis of 3638 patients from nine high-quality RCTs reported that DEX could increase the incidence of bradycardia,21 which might be due to presynaptic α2 receptor stimulation by DEX results in decreasing norepinephrine release.
Additionally, it was interesting to find that DEX could shorten the time to extubation in this meta-analysis, which was similar to the result of one previous meta-analysis.22 However, because of the limited data and the high heterogeneity among the studies, the pooled result should be interpreted cautiously and further investigations were needed to support the conclusion.
In fact, there were two previous meta-analyses also reported that DEX could low the occurrence of PONV compared with the control group.12 23 The included population of these two meta-analyses was the children and adults, and one study did not limit the methods of anaesthesia and the administration of DEX. Notably, we mainly focused on the adult patient population under general anaesthesia, and the intervention was perioperative intravenous DEX, which differed from the two previous meta-analyses. Moreover, the RCTs that DEX comparing with opioids agents were excluded in our meta-analysis to eliminate the effect of opioids on PONV. Additionally, our study involved a number of updated RCTs and added some indicators about the recovery after surgery. Ultimately, our results suggested that DEX did decrease the occurrence of PONV, and accelerated the recovery of adult patients after general anaesthesia.
Clinical significance
The results of this meta-analysis might help the doctors and nurses to formulate plans to prevent PONV and offer a new testimony to expand the clinical significance of DEX apart from its conventional usage for sedation and analgesia.
Limitations
There were several limitations to this meta-analysis. First, the included articles did not give consistent doses of DEX, the influence of diverse doses of DEX on PONV in adult patients after general anaesthesia needs to be further explored. Second, the severity degree of PONV was not quantified using a formal scale, so further study is required to explore the effect of DEX on different severity degrees of PONV.
Conclusion
In a word, DEX could decrease the occurrence of PONV in adult patients who experience general anaesthesia, and accelerate postoperative recovery. Thus, DEX can be used as an adjuvant drug for general anaesthesia to prevent the development of PONV in clinical practice. However, it is essential to be vigilant as DEX might increase the occurrence of bradycardia during surgery.
Supplementary Material
Footnotes
Contributors: The author acting as guarantor was JL. WZ and JL were involved in the conception and design of this meta-analysis. NW and ZW conducted the data extraction. HZ and ML contributed to statistical analysis. WZ analysed the data and drafted the manuscript. JH, DY and MZ offered major comments and revised the manuscript. All authors have read and approved the manuscript.
Funding: The work was supported by the Key Research and Development Program of Hebei Province (Grant No. 19277714D). The funding bodies had no role in the design of the study, and will have no role in the data collection, analysis, interpretation of data, and writing of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2020;131:411–48. 10.1213/ANE.0000000000004833 [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa E, Iwamoto R, Hojo T, et al. Cross-sectional study of PONV risk factors for oral surgery after intubated general anesthesia with total intravenous anesthesia. Anesth Prog 2022;69:18–23. 10.2344/anpr-68-03-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elvir-Lazo OL, White PF, Yumul R, et al. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: an updated review. F1000Res 2020;9. 10.12688/f1000research.21832.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li HJ, Liu S, Geng ZY, et al. Adding dexmedetomidine to morphine-based analgesia reduces early postoperative nausea in patients undergoing gynecological laparoscopic surgery: a randomized controlled trial. BMC Anesthesiol 2020;20:11. 10.1186/s12871-019-0928-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Hwang HW, Jeong J-Y, et al. The effect of low-dose dexmedetomidine on pain and inflammation in patients undergoing laparoscopic hysterectomy. J Clin Med 2022;11:2802. 10.3390/jcm11102802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie C, Zhang C, Sun H, et al. Effects of dexmedetomidine on postoperative nausea and vomiting in adult patients undergoing ambulatory thyroidectomy: a randomized clinical trial. Front Med (Lausanne) 2021;8:781689. 10.3389/fmed.2021.781689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan C, Ti-jun D. Effects of intraoperative dexmedetomidine infusion on postoperative delirium in elderly patients undergoing total hip arthroplasty. Int Surg 2021;105:328–35. 10.9738/INTSURG-D-17-00029.1 [DOI] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloor Y, Czarnetzki C, Curtin F, et al. Genetic susceptibility toward nausea and vomiting in surgical patients. Front Genet 2021;12:816908. 10.3389/fgene.2021.816908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu H, Zheng C, Liang B, et al. Mechanism and risk factors of nausea and vomiting after TACE: a retrospective analysis. BMC Cancer 2021;21:513. 10.1186/s12885-021-08253-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X, Zhou M, Feng J-J, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:8450–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Poon Y-Y, Ke T-Y, Hung K-C, et al. Risk factors of postoperative vomiting in the eye of "real-world evidence"-modifiable and clinical setting-dependent risk factors in surgical trauma patients. J Pers Med 2021;11:386. 10.3390/jpm11050386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konno D, Sugino S, Shibata TF, et al. Antiemetic effects of baclofen in a shrew model of postoperative nausea and vomiting: whole-transcriptome analysis in the nucleus of the solitary tract. CNS Neurosci Ther 2022;28:922–31. 10.1111/cns.13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Yu Y, Miao S, et al. Effects of peri-operative intravenous administration of dexmedetomidine on emergence agitation after general anesthesia in adults: a meta-analysis of randomized controlled trials. Drug Des Devel Ther 2019;13:2853–64. 10.2147/DDDT.S207016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Kang Z, Wang Y, et al. The anti-inflammatory effect of dexmedetomidine administration on patients undergoing intestinal surgery: a randomized study. Drugs R D 2021;21:445–53. 10.1007/s40268-021-00368-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Yao H, Cheng N, et al. Determining whether dexmedetomidine provides a reno-protective effect in patients receiving laparoscopic radical prostatectomy: a pilot study. Int Urol Nephrol 2019;51:1553–61. 10.1007/s11255-019-02171-9 [DOI] [PubMed] [Google Scholar]
- 18.Xu S, Hu S, Ju X, et al. Effects of intravenous lidocaine, dexmedetomidine, and their combination on IL-1, IL-6 and TNF-Α in patients undergoing laparoscopic hysterectomy: a prospective, randomized controlled trial. BMC Anesthesiol 2021;21:3. 10.1186/s12871-020-01219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Wang R, Li B, et al. The effect of dexmedetomidine on postoperative nausea and vomiting in patients undergoing thoracic surgery-a meta-analysis of a randomized controlled trial. Front Surg 2022;9:863249. 10.3389/fsurg.2022.863249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra S, Behera BK, Mitra JK, et al. Effect of preoperative dexmedetomidine nebulization on the hemodynamic response to laryngoscopy and intubation: a randomized control trial. Korean J Anesthesiol 2021;74:150–7. 10.4097/kja.20153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin C, Tu H, Jie Z, et al. Effect of dexmedetomidine on delirium in elderly surgical patients: a meta-analysis of randomized controlled trials. Ann Pharmacother 2021;55:624–36. 10.1177/1060028020951954 [DOI] [PubMed] [Google Scholar]
- 22.Pan H, Liu C, Ma X, et al. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth 2019;66:1489–500. 10.1007/s12630-019-01440-6 [DOI] [PubMed] [Google Scholar]
- 23.Jin S, Liang DD, Chen C, et al. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: a PRISMA-compliant meta analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e5770. 10.1097/MD.0000000000005770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakri MH, Ismail EA, Ibrahim A. Comparison of dexmedetomidine and dexamethasone for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean J Anesthesiol 2015;68:254–60. 10.4097/kjae.2015.68.3.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng K, Jin X, Liu S, et al. Effect of intraoperative dexmedetomidine on post-craniotomy pain. Clin Ther 2015;37:1114–21. 10.1016/j.clinthera.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Huang P, Lai L, et al. Dexmedetomidine improves gastrointestinal motility after laparoscopic resection of colorectal cancer. Medicine (Baltimore) 2016;95:e4295. 10.1097/MD.0000000000004295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bielka K, Kuchyn I, Babych V, et al. Dexmedetomidine infusion as an analgesic adjuvant during laparoscopic cholecystectomy: a randomized controlled study. BMC Anesthesiol 2018;18:44. 10.1186/s12871-018-0508-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das R, Das RK, Sahoo S, et al. Role of dexmedetomidine as an anaesthetic adjuvant in breast cancer surgery as a day-care procedure: a randomised controlled study. Indian J Anaesth 2018;62:182–7. 10.4103/ija.IJA_752_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bala R, Chaturvedi A, Pandia MP, et al. Intraoperative dexmedetomidine maintains hemodynamic stability and hastens postoperative recovery in patients undergoing transsphenoidal pituitary surgery. J Neurosci Rural Pract 2019;10:599–605. 10.1055/s-0039-3399402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu F, Duan H, Xie Y. Preventive effects of dexmedetomidine on renal dysfunction and hemodynamic stability in malignant obstructive jaundice patients during peri-operative period. Med Sci Monit 2019;25:6782–7. 10.12659/MSM.916329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakshi SG, Paulin SV, Bhawalkar P. A randomised controlled trial to evaluate the peri-operative role of intraoperative dexmedetomidine infusion in robotic-assisted laparoscopic oncosurgeries. Indian J Anaesth 2020;64:784–9. 10.4103/ija.IJA_664_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Chen X. Dexmedetomidine contributes to reduced anesthesia dosages and improves anesthetic effectiveness in the radical resection of gastric cancer. Int J Clin Exp Med 2020;13:6533–41. [Google Scholar]
- 33.Asri S, Hosseinzadeh H, Eydi M, et al. Effect of dexmedetomidine combined with inhalation of isoflurane on oxygenation following one-lung ventilation in thoracic surgery. Anesth Pain Med 2020;10:e95287. 10.5812/aapm.95287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pi Y, Yang Y. Application value of conventional anesthesia combined with dexmedetomidine in thoracoscopic radical resection of lung cancer. Int J Clin Exp Med 2021;14:1301–8. [Google Scholar]
- 35.Lu Y, Fang P-P, Yu Y-Q, et al. Effect of intraoperative dexmedetomidine on recovery of gastrointestinal function after abdominal surgery in older adults: a randomized clinical trial. JAMA Netw Open 2021;4:e2128886. 10.1001/jamanetworkopen.2021.28886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bafna U, Sharma P, Singhal RK, et al. Comparison of hypotensive properties of dexmedetomidine versus clonidine for induced hypotension during functional endoscopic sinus surgery: a randomised, double-blind Interventional study. Indian J Anaesth 2021;65:579–85. 10.4103/ija.IJA_57_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prashantha K, Usha S. Dexmedetomidine infusion in patients undergoing elective laparoscopic cholecystectomy under general anesthesia: postoperative analgesia and complications. European Journal of Molecular & Clinical Medicine 2021;8:1906–11. [Google Scholar]
- 38.Chen J, Chen B, Chen A. Effects of anesthesia induction using dexmedetomidine in thoracoscopic pulmonary segmentectomy on the safety and protection of lung function. Int J Clin Exp Med 2021;14:565–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067102supp001.pdf (124KB, pdf)
bmjopen-2022-067102supp002.pdf (74.5KB, pdf)
bmjopen-2022-067102supp003.pdf (53.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.