Abstract
Background
Blood platelets are mediators of atherothrombotic disease and are regulated by complex sets of genes. Association studies in European ancestry populations have already detected informative platelet regulatory loci. Studies in other ancestries can potentially reveal new associations because of different allele frequencies, linkage structures, and variant effects.
Objectives
To reveal new regulatory genes for platelet count (PLT).
Methods
Genome-wide association studies (GWAS) were performed in 20,218 Bangladeshi and 9198 Pakistani individuals from the Genes & Health study. Loci significantly associated with PLT underwent fine-mapping to identify candidate genes.
Results
Of 1588 significantly associated variants (P < 5 × 10−8) at 20 loci in the Bangladeshi analysis, most replicated findings in prior transancestry GWAS and in the Pakistani analysis. However, the Bangladeshi locus defined by rs946528 (chr1:46019890) did not associate with PLT in the Pakistani analysis but was in the same linkage disequilibrium block (r2 ≥ 0.5) as PLT-associated variants in prior East Asian GWAS. The single independent association signal was refined to a 95% credible set of 343 variants spanning 8 coding genes. Functional annotation, mapping to megakaryocyte regulatory regions, and colocalization with blood expression quantitative trait loci identified the likely mediator of the PLT phenotype to be PIK3R3 encoding a regulator of phosphoinositol 3-kinase (PI3K).
Conclusion
Abnormal PI3K activity in the vessel wall is already implicated in the pathogenesis of atherothrombosis. Our identification of a new association between PIK3R3 and PLT provides further mechanistic insights into the contribution of the PI3K pathway to platelet biology.
Keywords: Bangladesh, blood platelets, cardiovascular diseases, genome-wide association study, phosphatidylinositol 3-kinases
Essentials
-
•
Understanding which genes control platelet count provides insights into atherothrombosis.
-
•
To identify new platelet genes, we analyzed data from Bangladeshi and Pakistani populations.
-
•
Most platelet genes identified in our analysis overlapped with those in other populations.
-
•
A new region containing the PIK3R3 gene was linked to platelet count in Bangladeshis.
1. Introduction
Circulating platelet count (PLT) is an independent predictor of morbidity and mortality from multiple cardiovascular and inflammatory disorders, including atherothrombosis [[1], [2], [3]], but is influenced by complex sets of interacting genes (h2 > 0.3-0.8) [4,5] that may be different between ancestries [1,6]. Understanding the genetic basis of PLT offers important insights into the pathophysiology of platelet-mediated cardiovascular disease and disparities in health outcomes between populations [7,8].
Large genetic association studies for PLT and other blood cell traits have historically been restricted to European (EUR) populations [9]. However, utilization of non-EUR populations can reveal novel genetic associations because of differences in allele frequency, linkage disequilibrium (LD) structure, and variant effects driven by environmental selection pressures and genetic drift [10]. Transancestry and ancestry–specific genome-wide association studies (GWAS) have already exploited this to reveal multiple new loci for PLT [1,6]. Here, we extend this approach by performing a GWAS in a UK collection of individuals from Bangladesh alongside a comparator population of individuals from Pakistan.
2. Methods
Analyses were performed on the July 2021 data release of the Genes & Health study [11] in accordance with ethical approval from the London South-East NRES Committee of the Health Research Authority (14/LO/1240). The analysis populations comprised predominantly first-generation British Bangladeshi and British Pakistani adults genotyped using the Illumina Infinium Global Screening Array v3 chip.
Phenotype data were derived from linked electronic health records, which included blood cell counts measured using a Sysmex XE-2100 analyzer (Sysmex, Kobe, Japan). PLT for each individual was defined as the mean of all PLTs recorded in the electronic health records. PLT were adjusted for sex, age, height, and weight, and rank-based inverse normal transformation was applied. Associations between PLT and variants imputed using the TOPMed-r2Minimac4 1.5.7 Imputation Server were calculated using BOLT-LMM v2.3.6 using the first 10 genetic principal components as covariates. Index variants were defined as those with the lowest P value within a genome-wide significant (P < 5 × 10−8) locus. Conditional analyses were performed at each significantly associated locus using the index variant as a covariate to detect the presence of secondary association signals. The contributions of associated variants from each locus to PLT were evaluated further by comparing allele frequencies and effect sizes between the Bangladeshi and Pakistani populations. We tested for colocalization of variants in genomic regions associated with PLT in previous transancestry GWAS and in relevant subpopulations [1] to detect whether the Bangladeshi index variants were likely to represent the same or different association signals. Variants in the Bangladeshi association interval defined by the index variant rs946528 underwent functional annotation using Ensembl Variant Effect Predictor (VEP) to identify their likely effect on coding genes, mapping to epigenetic data from megakaryocytes, and colocalization analysis with whole blood expression quantitative trait loci (eQTLs) (Supplementary Methods).
3. Results and Discussion
The characteristics of the 20,218 Bangladeshi and 9198 Pakistani individuals in the analysis populations are summarized in Supplementary Table S1. The mean PLT was lower in the Bangladeshi (mean, 266.4 × 109/L) than in the Pakistani populations (271.5 × 109/L; P = 1.16 × 10−10; Supplementary Fig. S1). Although small case series have shown that the frequency of thrombocytopenia (PLT <150 × 109/L) is higher in residents of the Eastern Indian subcontinent compared to other regions [12], we were unable to detect an increase in thrombocytopenia in British Bangladeshis (Supplementary Table S1). Single nucleotide polymorphism (SNP)-based heritability for PLT was 26.9% in the Bangladeshis and 25.3% in Pakistanis.
The genomic inflation factors in the Bangladeshi and Pakistani populations were 1.096 and 1.047, respectively, indicating adequate control of population stratification. In the Bangladeshi population, 1588 variants were associated with PLT at a genome-wide significance threshold of P < 5 × 10−8, assigned to 20 different loci (Figure 1, Table). All 20 Bangladeshi index variants occurred at approximately the same frequency in other ancestries in the 1000 Genomes Continental genomes dataset with the exception of rs149810016 in GFI1B that was present only in the South Asian (SAS) dataset (Supplementary Table S2). Conditional analysis of the variants at each locus revealed that 2 loci had secondary signals of association (P < 5 × 10−8). These were rs3846855 and rs653178, which mapped to GGNBP1 and ATXN2, respectively (Supplementary Fig. S2A), both of which were associated with PLT in the transancestry or ancestry-specific GWAS of Chen et al. [1], and rs653178 was in LD with the index variant for that region.
Figure 1.
Genomic associations with platelet count in the Bangladeshi population (n = 20,292). (A) Manhattan plot showing 20 loci in which the index variant has a probability of association above the genome-wide significance threshold of P < 5 × 10−8 (red line). Selected loci are annotated with gene names identified using variant effect predictor most severe consequence option. The inset figure is the quantile-quantile plot of genome-wide association study P values. (B) Minor allele frequency (MAF), effect size (beta), and phenotypic variation explained (PVE) for each of the 1588 platelet count–associated variants identified in the Bangladeshi population compared to the same variants in the Pakistani population. For the Bangladeshi locus on chromosome 1, variants in the association interval (r2 > 0.5) of the index variant rs964528 are indicated by the red dots.
Table.
Platelet count–associated index variants identified in Bangladeshi individuals (n = 20,218) and colocalization with prior transancestry and ancestry–specific genome-wide association studies [1].
| Bangladeshi index variants |
Posterior probability of a common shared association signal (H4) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chromosomal position | rsID | Gene(s) with VEP most severe consequence | Coded/alternate allele | Alternate allele frequency | Beta (SE) | P value | Transancestry | SAS | EAS |
| chr1:46019890 | rs946528 | MAST2 | C/T | 0.58 | 0.067 (0.011) | 2.1 × 10−10 | 2.8% | 4.0% | 34.7% |
| chr1:247549001 | rs41315846 | GCSAML | T/C | 0.41 | 0.063 (0.011) | 1.7 × 10−8 | 100.0% | - | - |
| chr2:31258101 | rs592039 | EHD3 | G/A | 0.85 | 0.106 (0.016) | 4.7 × 10−11 | 99.7% | - | - |
| chr2:159926221 | rs1877194 | Intergenic | A/G | 0.46 | −0.056 (0.010) | 4.7 × 10−8 | 52.6% | 4.5% | 11.1% |
| chr3:56815721 | rs1354034 | ARHGEF3 | T/C | 0.50 | 0.093 (0.010) | 3.2 × 10−20 | 100.0% | - | - |
| chr4:6889792 | rs11734132 | Intergenic | G/C | 0.19 | 0.079 (0.014) | 1.1 × 10−8 | 99.6% | - | - |
| chr4:110027510 | rs80079941 | Intergenic | G/C | 0.18 | −0.118 (0.013) | 4.0 × 10−19 | <1% | 68.5% | 98.8% |
| chr5:66710497 | rs59596869 | MAST4 | C/T | 0.13 | 0.089 (0.015) | 4.6 × 10−9 | <1% | 53.6% | 9.6% |
| chr6:33575632 | rs210139 | BAK1 | A/C | 0.71 | 0.103 (0.011) | 3.9 × 10−21 | 30.8% | 95.8% | 98.0% |
| chr6:135100038 | rs34164109 | HBS1L | C/T | 0.11 | 0.130 (0.017) | 3.2 × 10−15 | 96.7% | - | - |
| chr7:106700379 | rs342244 | Intergenic | T/G | 0.35 | −0.069 (0.011) | 2.7 × 10−10 | 90.3% | - | - |
| chr8:105570896 | rs4734879 | ZFPM2 | A/G | 0.32 | −0.103 (0.012) | 6.3 × 10−18 | 99.4% | - | - |
| chr9:4788616 | rs35797651 | RCL1 | C/G | 0.35 | 0.084 (0.011) | 6.5 × 10−14 | 96.1% | - | - |
| chr9:132987359 | rs149810016 | GFI1B | C/A | 0.02 | −0.284 (0.038) | 6.6 × 10−14 | <1% | <1% | 6.2% |
| chr10:63267383 | rs7098181 | JMJD1C | G/T | 0.48 | 0.085 (0.010) | 2.6 × 10−17 | 98.6% | - | - |
| chr12:111411711 | rs7309325 | SH2B | G/T | 0.41 | 0.077 (0.010) | 2.8 × 10−14 | <1% | 97.2% | 88.9% |
| chr14:103098397 | rs61007561 | EXOC3L | A/AG | 0.29 | 0.072 (0.011) | 2.1 × 10−10 | 98.1% | - | - |
| chr17:35563315 | rs55910622 | SLFN14 | G/T | 0.07 | 0.124 (0.020) | 4.2 × 10−10 | 57.3% | 97.6% | 0.1% |
| chr18:23141009 | rs11082304 | CABLES1 | G/T | 0.46 | −0.057 (0.010) | 2.1 × 10−8 | 100.0% | - | - |
| chr20:58999408 | rs163787 | CTSZ | A/G | 0.80 | 0.076 (0.013) | 1.4 × 10−8 | <1% | 97.7% | 0.0% |
Bangladeshi index variants were defined as those with the lowest P value within each associated locus. Chromosomal positions are expressed relative to the GRCh38 genome assembly with the coded/alternate alleles on the positive strand. Genes were assigned to each index variant by annotating with VEP and selecting the gene with the most severe functional consequence. Effect size (beta), SE, and probability of association are shown for each Bangladeshi index variant. Data are also presented for a colocalization analysis generated using the coloc( ) R package in which the platelet count associations for all variants within 500 kB of the Bangladeshi index variants were compared in the transancestry genome-wide association study population. If there was a low posterior probability of colocalization (<80%), then colocalization was tested against the SAS and EAS genome-wide association study analysis population [1].
EAS, East Asian; SAS, South Asian; VEP, variant effect predictor.
In the smaller Pakistani population, there were 68 PLT-associated variants at 5 loci (Supplementary Fig. S3, Supplementary Table S3). The allele frequencies, effect sizes, and phenotypic variation explained (PVE; the contribution that each variant makes to the spread of PLT in the population) of the PLT-associated variants in the Bangladeshi population were in most cases similar to those in the Pakistani population. However, for the Bangladeshi index variant rs946528 (chr1:46019890), variants in LD with the index variant (defined as r2 ≥0.5) were conspicuous because of a disproportionately greater effect size on PLT in the Bangladeshi compared with the Pakistani population, resulting in greater PVE. Allele frequencies between the Bangladeshi and Pakistani study populations were similar (Figure 1B).
In order to explore whether the PLT loci identified in the Bangladeshi GWAS replicated associations in the largest-to-date transancestry meta-GWAS of blood cell traits comprising 721,201 individuals [1], we performed colocalization analyses for sets of variants within 500 kB of each of the 20 Bangladeshi index variants (Table, Supplementary Table S6). For 11 of the Bangladeshi index variants, there was a high posterior probability (PP) that the association signal with PLT in the transethnic GWAS was driven by the same variant (PP of H4 >0.8). A further 5 variants had a similarly high likelihood of colocalization with PLT-associated variants in either the SAS or East Asian (EAS) ancestry–specific GWAS populations [1] (Table and Supplementary Table S6).
Of the remaining 4 Bangladeshi index variants that did not colocalize with previous PLT-associated variants, rs149810016 and rs59596869 mapped by VEP to GFI1B and to MAST4, respectively, which are PLT-associated genes identified from other index variants in the meta-GWAS by Chen et al. [1] rs1877194 was annotated by VEP to an intergenic region but is adjacent to the PLT-associated LY75-C302. These findings suggest that these association signals potentially indicate new regulatory mechanisms for PLT mediated through their respective annotated genes. The final remaining Bangladeshi locus with index variant rs946528 uniquely showed a single association signal with PLT (Supplementary Fig. S2B), no colocalization with PLT-associated variants in the transancestry and EAS or SAS GWAS (Table, Supplementary Table S6) and was not annotated to PLT genes identified in the meta-GWAS.
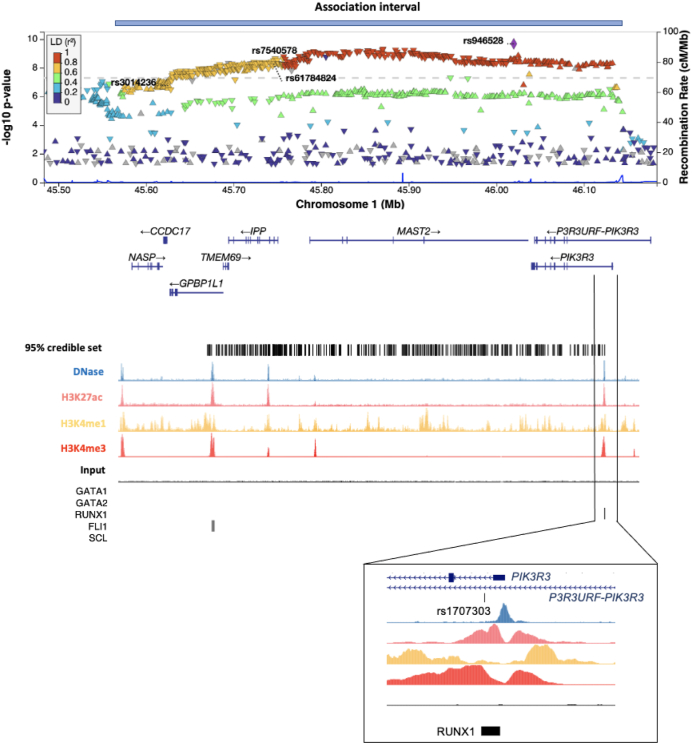
The locus containing Bangladeshi index variant rs946528 comprised a 562 kB region (chr1:45575428-46137676) of low recombination containing a 95% credible set of 343 variants (Figure 2). This region did contain other variants associated with PLT in the EAS ancestry–specific GWAS performed by Chen et al. [1] and 2 other NHGRI-EBI–cataloged GWAS that were restricted to EAS populations (Supplementary Figure S4, Supplementary Table S4) [13,14]. All of these variants were >200 kB from rs946528 and since none colocalized with variants in the rs946528 region, this likely represents a novel PLT-associated signal.
Figure 2.
Detailed view of the Bangladeshi rs946528 association interval. LocusZoom plot of associations in the 562 kB association interval for the chromosome 1 index variant rs946528, defined as variants within r2 ≥ 0.5 calculated using linkage disequilibrium (LD) reference data from the Bengali from Bangladesh 1000 Genomes dataset. Variants with P > .05 are excluded to enable visualization of the recombination peaks. Indicated variants are the index rs946528 and the 3 variants associated with platelet count in prior East Asian ancestry–specific genome-wide association studies. Beneath and aligned with this are the 8 UCSC Genome Browser–annotated genes within the interval; positions of the 95% credible set of 343 variants and annotated regions of epigenetic activity from CD34−, CD41+, and CD42+ megakaryocytes, the progenitor cell for circulating platelets, which were provided by the BLUEPRINT Epigenomics Project [25]. Megakaryocyte chromatin immunoprecipitation sequencing (ChIP-seq) data are also shown indicating relevant transcription factor binding sites [16]. The expanded view shows the relationship between the significantly associated variant rs1707303 and a putative regulatory region surrounding the first exon of the candidate gene PIK3R3.
Variants in LD (r2 > 0.5) with rs946528 span 8 UCSC Genome Browser–annotated genes (Figure 2, Supplementary Table S5). Among the genes contained within this region, only PIK3R3 was represented in both the platelet transcriptome and proteome catalogs within the PlateletWeb functional genomics database [15]. Moreover, among all variants in the 95% credible set, only rs1707303 in intron 1 of PIK3R3 overlapped with a prominent area of epigenetic activity, suggesting that this variant lies within a regulatory region (Figure 2). At least part of this epigenetic activity may be accounted for by the immediate adjacency of rs1707303 to a consensus binding site for RUNX1, a critical transcription factor necessary for differentiation and maturation of platelet-forming megakaryocytes [16]. The rs946528 association signal colocalized with cis-eQTLs in whole blood for MAST2 and IPP with posterior probabilities of 45.2% and 31.4%, respectively, below the threshold of 80% usually considered indicative of a shared causal variant (Supplementary Fig. S5, Supplementary Table S5). Neither MAST2 nor IPP are expressed in platelets and neither has a plausible biological role in platelet production [15].
In this first reported GWAS for a hematological trait in a Bangladeshi population, several lines of evidence suggested that the chromosome 1 locus defined by the index variant rs946528 represented a novel association signal with PLT. First, the rs946528 haplotype had disproportionately larger PVE estimates in the Bangladeshi than in the Pakistani population, for which data collection and analysis methods were identical. This finding was driven by differences in variant effect size between the Bangladeshi and Pakistani populations and not by minor allele frequency. Although variants within this locus also drove an association with PLT in a large prior transancestry and EAS–specific GWAS [1], colocalization analysis suggested that this was through different causal variants and that the driver variant in the Bangladeshi population was in a distant genomic location and likely linked to a different gene. It was noteworthy that the Bangladeshi rs946528 association window also included variants that replicated findings from other GWAS for PLT in previous EAS populations but which have not been previously annotated [1,13,14]. By contrast, the other PLT-associated loci in the Bangladeshi and Pakistani GWAS replicated prior transancestry GWAS, and in most cases, they were linked to genes already implicated in platelet biology [1]. Replication of the rs946528 association interval as an apparently ancestral EAS PLT-associated locus was unsurprising given that modern Bangladeshi populations are predominantly of SAS ancestry but with significant EAS and South-East Asian admixture [17]. This confirmatory discovery in the Bangladeshi population highlights the value of ancestry-specific GWAS in providing additional insights into the architecture of complex loci associated with population traits that complement transancestry or large EUR population GWAS [1,9].
One significant challenge with the rs946528 locus is that the single independent association signal for PLT was attributable to 95% credible set of 343 variants in a haplotype block containing 8 annotated genes. Considering orthogonal evidence from several sources, PIK3R3 was identified as the most likely candidate mediator of the PLT phenotype. This was primarily because the PIK3R3 intron 1 variant rs1707303 was unique within the rs946528 haplotype in that it mapped to an area with multiple epigenetic signals indicating a PIK3R3 regulatory region most likely related to a consensus binding site for RUNX1, a critical megakaryocyte transcription factor [16]. PIK3R3 is further supported as a candidate mediator of the PLT phenotype because it was the only gene at this locus to be expressed within platelets and because it encodes phosphatidylinositol 3-kinase regulatory subunit gamma (PIK3R3; Uniprot Q92569). In an interactome analysis using the STRING database [18], PIK3R3 has 10 first order interactions with high confidence (>0.7 score), all with proteins that are also represented in the platelet proteome (Figure 3) [18]. The PIK3R3 interactors are enriched for proteins with the biological process Gene Ontology terms GO:0014065 phosphatidylinositol 3-kinase signaling (strength, 2.54; false discovery rate, 1.04 × 10−13) and GO:0048009 insulin-like growth factor receptor signaling pathway (strength, 2.61; false discovery rate, 8.08 × 10−6) [19], both implicated in mediating megakaryocyte maturation and platelet production [[20], [21]].
Figure 3.
Interaction network for PIK3R3. The nodes represent PIK3R3 and interacting genes designated within Platelet Web [25] as present in the platelet proteome (blue) and in both the proteome and transcriptome (yellow). The edges indicate physical interactions between the encoded proteins. Only STRING database v11.5 first order interactions with confidence scores of >0.7 are shown [19].
Potential limitations of this study are that the association between the rs946528 locus and PLT has not been replicated in an exclusively Bangladeshi population and that the datasets used for variant imputation and colocalization analyses have necessarily been derived from EUR populations. However, despite this, the otherwise compelling evidence that PIK3R3 is a candidate mediator of PLT remains significant as the encoded protein PIK3R is also a mediator of vascular smooth muscle proliferation and neointimal formation in atherogenesis [22,23], which are potentially tractable to therapies that target the PI3K pathway [24]. Complementary functional characterization of the PIK3R3 locus and testing associations with cardiovascular disease outcomes are required to complete evaluation of this locus. The GWAS summary statistics (available via the NHGRI-EBI GWAS Catalog) have future value in fine mapping other loci and for development of polygenic risk scores.
Appendix
The current members of the Genes & Health Research Team (in alphabetical order by surname) are Shaheen Akhtar, Mohammad Anwar, Elena Arciero, Omar Asgar, Samina Ashraf, Gerome Breen, Raymond Chung, Charles J. Curtis, Shabana Chaudhary, Maharun Chowdhury, Grainne Colligan, Panos Deloukas, Ceri Durham, Faiza Durrani, Fabiola Eto, Sarah Finer, Ana Angel Garcia, Chris Griffiths, Joanne Harvey, Teng Heng, Qin Qin Huang, Matt Hurles, Karen A. Hunt, Shapna Hussain, Kamrul Islam, Ben Jacobs, Ahsan Khan, Amara Khan, Cath Lavery, Sang Hyuck Lee, Robin Lerner, Daniel MacArthur, Daniel Malawsky, Hilary Martin, Dan Mason, Mohammed Bodrul Mazid, John McDermott, Sanam McSweeney, Shefa Miah, Sabrina Munir, Bill Newman, Elizabeth Owor, Asma Qureshi, Samiha Rahman, Nishat Safa, John Solly, Farah Tahmasebi, Richard C. Trembath, Karen Tricker, Nasir Uddin, David A. van Heel, Caroline Winckley, and John Wright.
Acknowledgments
The Genes & Health Research Team thank Social Action for Health, Centre of The Cell, members of our Community Advisory Group, and staff who recruited and collected data from volunteers. We thank the National Institute for Health Research National Biosample Centre (UK Biocentre), the Social Genetic & Developmental Psychiatry Centre (King’s College London), Wellcome Sanger Institute, and Broad Institute for sample processing, genotyping, sequencing, and variant annotation. We also thank Barts Health NHS Trust, NHS Clinical Commissioning Groups (City and Hackney, Waltham Forest, Tower Hamlets, Newham, Redbridge, Havering, and Barking and Dagenham), East London NHS Foundation Trust, Bradford Teaching Hospitals NHS Foundation Trust, Public Health England (especially David Wyllie), Discovery Data Service/Endeavour Health Charitable Trust (especially David Stables), and NHS Digital for GDPR-compliant data sharing backed by individual written informed consent. Most of all, we thank all of the volunteers participating in Genes & Health.
Funding
K.B. is funded by a GW4-CAT Wellcome Trust Fellowship (216277/Z/19/Z). For open access, the author has applied a CC BY public copyright license to any author-accepted manuscript arising from this submission. Genes & Health is/has recently been core-funded by Wellcome (WT102627 and WT210561), the Medical Research Council (UK) (M009017), Higher Education Funding Council for England Catalyst, Barts Charity (845/1796), Health Data Research UK (for London substantive site), and research delivery support from the NHS National Institute for Health Research Clinical Research Network (North Thames). Genes & Health is/has recently been funded by Alnylam Pharmaceuticals, Genomics PLC, and a Life Sciences Industry Consortium of Bristol-Myers Squibb Company, GlaxoSmithKline Research and Development Limited, Maze Therapeutics Inc, Merck Sharp & Dohme LLC, Novo Nordisk A/S, Pfizer Inc, and Takeda Development Centre Americas Inc. D.V. is a member of the Health Protection Research Unit in Chemical and Radiation Threats and Hazards, a partnership between Public Health England and Imperial College London, which is funded by the National Institute for Health Research.
Ethics statement
The Genes and Health study was approved by the London South-East NRES Committee of the Health Research Authority (14/LO/1240).
Author contributions
K.B. performed the analyses and cowrote the manuscript. L.F. and D.v.H. contributed to the analysis. D.V. codesigned the analyses. A.D.M. conceived the study and cowrote the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Neil Zakai
Dragana Vuckovic and Andrew D. Mumford contributed equally to this study.
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100175
Contributor Information
Kate Burley, Email: kate.burley@bristol.ac.uk.
Genes & Health Research Team@EastLondonGenes:
Shaheen Akhtar, Mohammad Anwar, Elena Arciero, Omar Asgar, Samina Ashraf, Gerome Breen, Raymond Chung, Charles J. Curtis, Shabana Chaudhary, Maharun Chowdhury, Grainne Colligan, Panos Deloukas, Ceri Durham, Faiza Durrani, Fabiola Eto, Sarah Finer, Ana Angel Garcia, Chris Griffiths, Joanne Harvey, Teng Heng, Qin Qin Huang, Matt Hurles, Karen A. Hunt, Shapna Hussain, Kamrul Islam, Ben Jacobs, Ahsan Khan, Amara Khan, Cath Lavery, Sang Hyuck Lee, Robin Lerner, Daniel MacArthur, Daniel Malawsky, Hilary Martin, Dan Mason, Mohammed Bodrul Mazid, John McDermott, Sanam McSweeney, Shefa Miah, Sabrina Munir, Bill Newman, Elizabeth Owor, Asma Qureshi, Samiha Rahman, Nishat Safa, John Solly, Farah Tahmasebi, Richard C. Trembath, Karen Tricker, Nasir Uddin, David A. van Heel, Caroline Winckley, and John Wright
Supplementary material
References
- 1.Chen M.H., Raffield L.M., Mousas A., Sakaue S., Huffman J.E., Moscati A., et al. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell. 2020;182 doi: 10.1016/j.cell.2020.06.045. 1198–213.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Msaouel P., Lam A.P., Gundabolu K., Chrysofakis G., Yu Y., Mantzaris I., et al. Abnormal platelet count is an independent predictor of mortality in the elderly and is influenced by ethnicity. Haematologica. 2014;99:930–936. doi: 10.3324/haematol.2013.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shameer K., Denny J.C., Ding K., Jouni H., Crosslin D.R., de Andrade M., et al. A genome- and phenome-wide association study to identify genetic variants influencing platelet count and volume and their pleiotropic effects. Hum Genet. 2014;133:95–109. doi: 10.1007/s00439-013-1355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans D.M., Frazer I.H., Martin N.G. Genetic and environmental causes of variation in basal levels of blood cells. Twin Res. 1999;2:250–257. doi: 10.1375/136905299320565735. [DOI] [PubMed] [Google Scholar]
- 5.Vuckovic D., Bao E.L., Akbari P., Lareau C.A., Mousas A., Jiang T., et al. The polygenic and monogenic basis of blood traits and diseases. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.008. 1214–31.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schick U.M., Jain D., Hodonsky C.J., Morrison J.V., Davis J.P., Brown L., et al. Genome-wide association study of platelet count identifies ancestry-specific loci in Hispanic/Latino Americans. Am J Hum Genet. 2016;98:229–242. doi: 10.1016/j.ajhg.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Rifai M., Cainzos-Achirica M., Kanaya A.M., Kandula N.R., Dardardi Z., Joshi P.H., et al. Discordance between 10-year cardiovascular risk estimates using the ACC/AHA 2013 estimator and coronary artery calcium in individuals from 5 racial/ethnic groups: comparing MASALA and MESA. Atherosclerosis. 2018;279:122–129. doi: 10.1016/j.atherosclerosis.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury R., Alam D.S., Fakir, Adnan S.D., Naheed A., Tasmin I., et al. The Bangladesh Risk of Acute Vascular Events (BRAVE) study: objectives and design. Eur J Epidemiol. 2015;30:577–587. doi: 10.1007/s10654-015-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astle W.J., Elding H., Jiang T., Allen D., Ruklisa D., Mann A.L., et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167:1415–1429.e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y.R., Keating B.J. Trans-ethnic genome-wide association studies: advantages and challenges of mapping in diverse populations. Genome Med. 2014;6:91. doi: 10.1186/s13073-014-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finer S., Martin H.C., Khan A., Hunt K.A., MacLaughlin B., Ahmed Z., et al. Cohort profile: East London Genes & Health (ELGH), a community-based population genomics and health study in British Bangladeshi and British Pakistani people. Int J Epidemiol. 2020;49:20–21i. doi: 10.1093/ije/dyz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali S., Ghosh K., Daly M.E., Hampshire D.J., Makris M., Ghosh M., et al. Congenital macrothrombocytopenia is a heterogeneous disorder in India. Haemophilia. 2016;22:570–582. doi: 10.1111/hae.12917. [DOI] [PubMed] [Google Scholar]
- 13.Sakaue S., Kanai M., Tanigawa Y., Karjalainen J., Kurki M., Koshiba S., et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53:1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai M., Akiyama M., Takahashi A., Matoba N., Momozawa Y., Ikeda M., et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet. 2018;50:390–400. doi: 10.1038/s41588-018-0047-6. [DOI] [PubMed] [Google Scholar]
- 15.Boyanova D., Nilla S., Birschmann I., Dandekar T., Dittrich M. PlateletWeb: a systems biologic analysis of signaling networks in human platelets. Blood. 2012;119:e22–e34. doi: 10.1182/blood-2011-10-387308. [DOI] [PubMed] [Google Scholar]
- 16.Tijssen M.R., Cvejic A., Joshi A., Hannah R.L., Ferreira R., Forrai A., et al. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev Cell. 2011;20:597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordaux R., Weiss G., Saha N., Stoneking M. The northeast Indian passageway: a barrier or corridor for human migrations? Mol Biol Evol. 2004;21:1525–1533. doi: 10.1093/molbev/msh151. [DOI] [PubMed] [Google Scholar]
- 18.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valet C., Severin S., Chicanne G., Laurent P.A., Gaits-Iacovoni F., Gratacap M.P., et al. The role of class I, II and III PI 3-kinases in platelet production and activation and their implication in thrombosis. Adv Biol Regul. 2016;61:33–41. doi: 10.1016/j.jbior.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Chen S., Hu M., Shen M., Wang S., Wang C., Chen F., et al. IGF-1 facilitates thrombopoiesis primarily through Akt activation. Blood. 2018;132:210–222. doi: 10.1182/blood-2018-01-825927. [DOI] [PubMed] [Google Scholar]
- 22.Li G., Xie N., Yao Y., Zhang Y., Guo J., Feng Y., et al. Identification of PI3K regulatory subunit p55gamma as a novel inhibitor of vascular smooth muscle cell proliferation and neointimal formation. Cardiovasc Res. 2015;105:75–85. doi: 10.1093/cvr/cvu235. [DOI] [PubMed] [Google Scholar]
- 23.Madeddu P., Kraenkel N., Barcelos L.S., Siragusa M., Campagnolo P., Oikawa A., et al. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol. 2008;28:68–76. doi: 10.1161/ATVBAHA.107.145573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durrant T.N., Hers I. PI3K inhibitors in thrombosis and cardiovascular disease. Clin Transl Med. 2020;9:8. doi: 10.1186/s40169-020-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens J.H., Stunnenberg H.G. BLUEPRINT: mapping human blood cell epigenomes. Haematologica. 2013;98:1487–1489. doi: 10.3324/haematol.2013.094243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.