Abstract
Background
Efanesoctocog alfa is a new class of factor (F) VIII replacement therapy designed to provide high sustained factor levels for longer by overcoming the von Willebrand factor half-life ceiling.
Objectives
To assess the pharmacokinetics and safety of standard half-life (octocog alfa) and extended half-life (rurioctocog alfa pegol) FVIIIs and efanesoctocog alfa.
Methods
This phase 1 study (NCT05042440; EudraCT 2021-000228-37) enrolled previously treated adult men with severe hemophilia A. Patients received sequential single 50-IU/kg doses of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa after appropriate washout periods between each dose.
Results
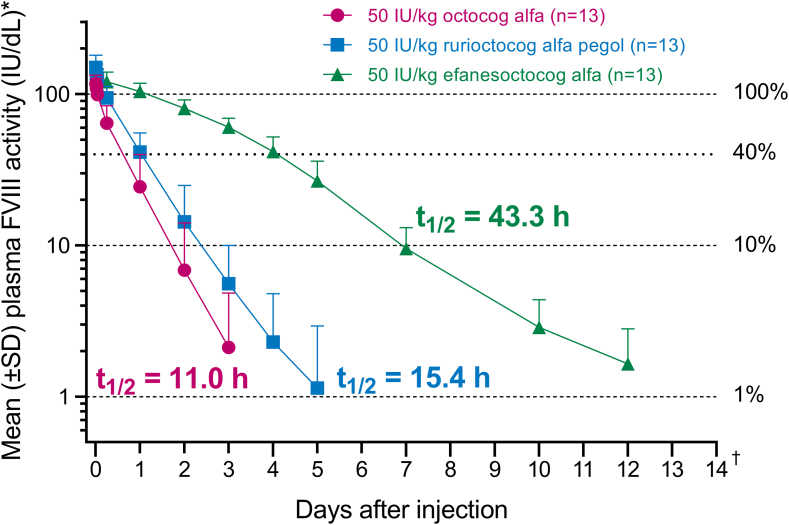
Thirteen participants were enrolled. Geometric mean elimination half-life of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa was 11.0, 15.4, and 43.3 hours, respectively, and area under the FVIII activity-time curve was 1670, 2820, and 10,100 IU × h/dL, respectively. Efanesoctocog alfa maintained mean FVIII activity levels of >40 IU/dL for up to 4 days and at ∼10 IU/dL on day 7. Corresponding times for >40 IU/dL and >10 IU/dL were <1 and <2 days, respectively, for octocog alfa and 1 day and <3 days, respectively, for rurioctocog alfa pegol. No serious treatment-emergent adverse events were reported for efanesoctocog alfa, and no inhibitor development to FVIII was detected.
Conclusion
Efanesoctocog alfa had 3- to 4-fold longer elimination half-life and 3- to 6-fold greater exposure (area under the FVIII activity-time curve, 6.03 and 3.57 folds) than octocog alfa and rurioctocog alfa pegol. Efanesoctocog alfa provided high sustained FVIII activity in the normal-to-near-normal range (>40 IU/dL) for up to 4 days after the dose and at ∼10 IU/dL on day 7.
Keywords: factor VIII, half-life, hemophilia A, pharmacokinetics, von Willebrand factor
Essentials
-
•
Binding of factor (F) VIII to von Willebrand factor (VWF) limits the half-life of recombinant FVIII.
-
•
Efanesoctocog alfa uncouples the binding of recombinant FVIII and VWF to increase the half-life of FVIII.
-
•
We assessed the pharmacokinetics of standard and extended half-life FVIII and efanesoctocog alfa.
-
•
The half-life of efanesoctocog alfa was 3- to 4-fold longer than those of the other 2 recombinant FVIII products.
1. Introduction
People with severe hemophilia A, defined as <1 IU/dL endogenous factor (F) VIII activity, experience frequent bleeds into major joints, soft tissue, and muscles. [1] Prophylaxis with FVIII replacement therapy aims to prevent occurrence of bleeds. Currently available FVIII replacement products are classified as standard half-life (SHL; approximately 12 hours half-life, eg, octocog alfa) [2] or extended half-life (EHL; 14-19 hours half-life, eg, rurioctocog alfa pegol) [[3], [4], [5], [6], [7], [8]]. The half-life of these FVIII replacement products is limited by the chaperone effect of von Willebrand factor (VWF), whereby FVIII is noncovalently bound to VWF, a protein with a half-life of ∼15 hours [9]. Achieving recommended FVIII trough levels of ≥3 to 5 IU/dL [10] with currently available FVIII replacement therapies requires 2 to 3 weekly injections or more [8], which may pose a substantial treatment burden and a barrier to adherence. Furthermore, life-threatening bleeds and joint bleeds still occur with regular prophylaxis, which can have long-term physical and quality-of-life implications for people with severe hemophilia A [[11], [12], [13], [14]]. Maintaining high sustained factor levels in the mild hemophilia (ie, >5 IU/dL) or even normal-to-near-normal range (ie, >40 IU/dL) may be required to increase protection from all bleeds [15], preserve joint health, and improve physical function in order to achieve health equity [16] and, potentially, a “hemophilia-free mind.” [17]
Efanesoctocog alfa (BIVV001) is a new class of FVIII replacement therapy uniquely designed to increase recombinant FVIII half-life in part by decoupling FVIII from endogenous VWF [18] to provide high sustained FVIII activity levels during the once-weekly dosing. Efanesoctocog alfa is composed of a single recombinant FVIII protein, the Fc domain of immunoglobulin G1, the D´D3 domain of VWF (FVIII binding domains), and 2 XTEN polypeptides [18]. Nonclinical and clinical data demonstrate that the half-life of efanesoctocog alfa is VWF independent [[18], [19], [20], [21], [22], [23]]. In a phase 1 repeat-dose study (EU Clinical Trials Register: 2018-001535-51), once-weekly efanesoctocog alfa at 50 IU/kg had a half-life of up to 41 hours and provided high sustained FVIII activity in the normal-to-near-normal range (ie, >40 IU/dL) for most of the week and ∼10 IU/dL on day 7 [20]. This pharmacokinetics (PK) profile was confirmed in the recently completed phase 3 XTEND-1 study (NCT04161495), which evaluated the efficacy, safety, and PK of efanesoctocog alfa in previously treated persons with severe hemophilia A [21,23]. In XTEND-1, prophylaxis with once-weekly efanesoctocog alfa was well tolerated and provided superior bleed protection compared with prior FVIII prophylaxis [21,23]. The primary objective of the current study was to compare the half-life of efanesoctocog alfa, octocog alfa (SHL), and rurioctocog alfa pegol (EHL) after a single intravenous injection. Secondary objectives were to compare additional PK parameters and evaluate the safety and tolerability of efanesoctocog alfa. Due to FVIII binding to endogenous VWF for all previously developed recombinant FVIII molecules and interindividual variability in VWF plasma levels and clearance (CL), recombinant FVIII replacement products have high interindividual PK variability [[24], [25], [26], [27]]. Thus, an intrapatient comparison of the PK of each FVIII product was chosen for this study.
2. Methods
2.1. Study design and population
This was a phase 1, single-center, single-arm, nonrandomized, open-label, fixed-sequence PK comparison study of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa (NCT05042440; EudraCT 2021-000228-37). Previously treated male participants (18-65 years old) with documented severe hemophilia A (defined as <1 IU/dL endogenous FVIII) were enrolled. Key eligibility criteria were ≥150 exposure days of prior FVIII treatment with any recombinant and/or plasma-derived FVIII and/or cryoprecipitate products and platelet count of ≥100,000 cells/μL at screening. Participants known to be HIV positive were required to have a CD4 lymphocyte count of >200 cells/mm3 and a viral load of <400 copies/mL before enrollment. Key exclusion criteria were history of a positive FVIII inhibitor test or a positive inhibitor test at screening (defined as ≥0.6 Bethesda Units/mL); clinical signs or symptoms of decreased response to FVIII; clinically significant liver disease that would make the participant unsuitable for enrollment (eg, cirrhosis, portal hypertension, or acute hepatitis); serious active bacterial, fungal, or viral infection (other than chronic hepatitis or HIV) within 30 days of screening; other known coagulation disorder(s); history of hypersensitivity or anaphylaxis associated with any FVIII product; and major surgery within 8 weeks of screening. All participants provided written informed consent before enrollment. The study was conducted in accordance with ethical principles derived from international guidelines, including the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Conference on Harmonization Good Clinical Practice guidelines, and applicable local laws and regulations.
The total study duration was approximately 67 days and comprised a screening period, octocog alfa dosing period, rurioctocog alfa dosing period, efanesoctocog alfa dosing period, and safety observation period (Figure 1). Participants underwent screening (up to 28 days), which included a washout period of at least 4 to 5 days depending on their current FVIII therapy. Participants received sequential administration of single 50-IU/kg doses of octocog alfa, rurioctocog alfa, and efanesoctocog alfa, each followed by PK sampling and appropriate washout periods prior to the next dose (Figure 1). Participants underwent safety observation throughout the study, including the 28 days after efanesoctocog alfa administration.
Figure 1.
Study design. ∗The washout duration before the administration of octocog alfa could have varied but was at least 4 days for participants whose most recent FVIII replacement was a standard half-life and at least 5 days for participants whose most recent FVIII replacement was an extended half-life. †The dosing periods for octocog alfa and rurioctocog alfa pegol included pharmacokinetic sampling and washout after each dose. Samples for FVIII inhibitor testing were obtained at screening, before administering each recombinant FVIII product, and at 14 and 28 days after efanesoctocog alfa was administered. EHL, extended half-life; FVIII, factor VIII; PK, pharmacokinetic; rFVIII, recombinant factor VIII; SHL, standard half-life.
2.2. Study outcomes
The primary endpoint was the half-life of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa, as assessed using the 1-stage activated partial thromboplastin time clotting assay. Secondary endpoints included the following PK parameters: area under the activity-time curve extrapolated to infinity (AUC0–inf), maximum activity (Cmax), CL, mean residence time, volume of distribution at steady state, and incremental recovery (IR). Safety assessments included occurrence of adverse events (AEs), serious AEs, and clinically significant laboratory abnormalities (including inhibitor to FVIII development) during each treatment period.
2.3. Statistical analysis
PK parameters (Cmax, AUC, elimination half-life [t1/2z], CL, volume of distribution at steady state, IR, and mean residence time) were computed and analyzed by a noncompartmental method (Phoenix WinNonlin, version 8.1) using baseline-corrected FVIII activity. FVIII activity was measured using the activated partial thromboplastin time–based 1-stage clotting assay with Actin FSL and validated using human plasma FVIII standard. The lower limit of quantitation was 0.5 IU/dL for octocog alfa, 0.6 IU/dL for rurioctocog alfa pegol, and 1 IU/dL for efanesoctocog alfa. Baseline-corrected FVIII activity and PK parameters were summarized by treatment group using descriptive statistics. Log-transformed t1/2z, Cmax, AUC, and CL were analyzed with a linear model with fixed terms for treatment and a random term for participant using the SAS PROC MIXED procedure. Point estimates and 90% CIs were provided as an exploratory analysis. Safety was analyzed descriptively, and AEs were coded according to the Medical Dictionary for Regulatory Activities, version 24.1.
3. Results and Discussion
Thirteen participants were enrolled, and all completed the study. All 13 participants were included in the PK and safety populations. Mean (SD) age at enrollment was 36.8 (6.5) years, and mean (SD) weight was 87.8 (18.9) kg. The median (range) number of bleeds in the 12 months before screening was 36 (2-80). Prestudy FVIII regimens included prophylaxis for 3 participants (23.1%) and on-demand therapy for 10 participants (76.9%).
Geometric mean (coefficient of variation percentage) t1/2z of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa was 11.0 hours (39%), 15.4 hours (35%), and 43.3 hours (24%), respectively (Table 1). Single-dose efanesoctocog alfa (50 IU/kg) maintained mean FVIII activity levels of >40 IU/dL for up to 4 days and ∼10 IU/dL on day 7. Corresponding times for octocog alfa were <1 and <2 days, and those for rurioctocog alfa pegol were 1 day and <3 days (Figure 2). Efanesoctocog alfa had lower CL (0.17-fold [90% CI, 0.15-0.19] and 0.28-fold [90% CI, 0.25-0.32]), longer t1/2z (3.94-fold [90% CI, 3.47-4.48] and 2.82-fold [90% CI, 2.48-3.20]), and higher exposure (AUC, 6.03-fold [90% CI, 5.32-6.83] and 3.57-fold [90% CI, 3.15-4.05]) than octocog alfa and rurioctocog alfa pegol, respectively (Table 2). While AUCinf was increased for efanesoctocog alfa compared with those for octocog alfa and rurioctocog alfa pegol, Cmax and, therefore, IR were similar for all 3 FVIII products (Table 1).
Table 1.
Geometric mean (coefficient of variation percentage; first row) and arithmetic mean (SD; second row) for the pharmacokinetic parameters of sequential single-dose octocog alfa (50 IU/kg), rurioctocog alfa pegol (50 IU/kg), and efanesoctocog alfa (50 IU/kg).
| PK parametera,b Geometric mean (CV%) Mean (SD) |
Octocog alfa (SHL) (n = 13) |
Rurioctocog alfa pegol (EHL) (n = 13) |
Efanesoctocog alfa (n = 13) |
|---|---|---|---|
| t1/2z (h)c | 11.0 (39) | 15.4 (35) | 43.3 (24) |
| 11.7 (4.55) | 16.3 (5.63) | 44.4 (10.4) | |
| AUCinf (IU × h/dL) | 1670 (41) | 2820 (31) | 10,100 (15) |
| 1820 (748) | 2950 (905) | 10,200 (1560) | |
| CL (mL/h/kg) | 3.0 (45) | 1.8 (33) | 0.5 (19) |
| 3.26 (1.48) | 1.86 (0.618) | 0.503 (0.0974) | |
| Cmax (IU/dL) | 118 (12) | 148 (20) | 139 (12) |
| 119 (14.7) | 151 (30.3) | 140 (16.9) | |
| IR (IU/dL per IU/kg) | 2.4 (12) | 3.0 (20) | 2.8 (12) |
| 2.37 (0.295) | 3.02 (0.605) | 2.80 (0.338) | |
| MRT (h) | 13.2 (46) | 19.2 (38) | 62.7 (15) |
| 14.6 (6.76) | 20.4 (7.73) | 63.4 (9.41) | |
| Tmax (h)d | 0.17 (0.17-0.50) | 0.17 (0.17-1.00) | 0.17 (0.17-1.00) |
| Vss (mL/kg) | 39.5 (17) | 33.9 (19) | 31.1 (11) |
| 40.1 (6.82) | 34.5 (6.60) | 31.3 (3.44) |
AUCinf, area under the activity-time curve extrapolated to infinity; CL, clearance; Cmax, maximum FVIII activity; CV, coefficient of variation; EHL, extended half-life; IR, incremental recovery; MRT, mean residence time; PK, pharmacokinetic; SHL, standard half-life; t1/2z, elimination half-life; Tmax, time to maximum FVIII activity; Vss, volume of distribution at steady state.
Baseline-corrected factor VIII activity was estimated using the 1-stage activated partial thromboplastin time–based clotting assay.
PK sampling was performed over a period of 3, 5, and 14 days after the administration of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa, respectively.
Primary endpoint.
Median (minimum-maximum).
Figure 2.
Mean (SD) plasma baseline-corrected factor (F) VIII activity-time profile. ∗Units of IU/dL are equivalent to percent FVIII activity. †Mean baseline-corrected FVIII activity for efanesoctocog alfa was below the lower limit of quantitation by 14 days after the dose. The lower limit of quantitation for octocog alfa was 0.5 IU/dL, that for rurioctocog alfa was 0.6 IU/dL, and that for efanesoctocog alfa was 1 IU/dL. t1/2, half-life.
Table 2.
Comparison of the pharmacokinetic parameters of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa.
| PK parametersa | Efanesoctocog alfa vs | Fold change | 90% CI |
|---|---|---|---|
| t1/2z (h) | Octocog alfa (SHL) | 3.94 | 3.47-4.48 |
| Rurioctocog alfa pegol (EHL) | 2.82 | 2.48-3.20 | |
| Cmax (IU/dL) | Octocog alfa (SHL) | 1.18 | 1.10-1.26 |
| Rurioctocog alfa pegol (EHL) | 0.94 | 0.88-1.01 | |
| AUCinf (IU × h/dL) | Octocog alfa (SHL) | 6.03 | 5.32-6.83 |
| Rurioctocog alfa pegol (EHL) | 3.57 | 3.15-4.05 | |
| CL (mL/h/dL) | Octocog alfa (SHL) | 0.17 | 0.15-0.19 |
| Rurioctocog alfa pegol (EHL) | 0.28 | 0.25-0.32 |
AUCinf, area under the activity-time curve extrapolated to infinity; CL, clearance; Cmax, maximum FVIII activity; EHL, extended half-life; PK, pharmacokinetic; SHL, standard half-life; t1/2z, elimination half-life.
PK sampling was performed over a period of 3, 5, and 14 days after the administration of octocog alfa, rurioctocog alfa pegol, and efanesoctocog alfa, respectively.
No treatment-emergent AEs (TEAEs) were reported during the octocog alfa or rurioctocog alfa pegol treatment period. During the efanesoctocog alfa treatment period (including the 28-day observation period), 3 TEAEs were reported in 1 participant, including headache, cough, and a positive SARS-CoV-2 test. No serious or severe TEAEs were reported during the entire study period, and no TEAEs led to permanent study discontinuation. Inhibitor development to FVIII was not detected, and there were no reports of serious hypersensitivity, anaphylaxis, or vascular thrombotic events. No potentially clinically significant abnormalities were reported in the octocog alfa or rurioctocog alfa pegol treatment period. During the efanesoctocog alfa treatment period, 1 participant had white blood cell parameters of a monocyte count of >0.7 × 109/L and an eosinophil count of >0.5 × 109/L or greater than the upper limit of normal (if the upper limit of normal was >0.5 × 109/L). Both were detected 14 days after administration of efanesoctocog alfa and resolved by the end of the study.
Individualized PK-guided prophylaxis is often used for FVIII replacement products in the treatment of hemophilia A [10,28], at least, in part, because of the wide interindividual variability in FVIII PK [28]. It is known that interindividual and intraindividual variations in circulating VWF levels and clearance of VWF affect FVIII half-life [[25], [26], [27]]. In the current study, PK variability was lower with efanesoctocog alfa than with the other 2 recombinant FVIII products; this is evidenced by the lower coefficient of variation for t1/2z, AUC, and CL observed with efanesoctocog alfa than those observed with octocog alfa and rurioctocog alfa pegol (Table 1). Thus, uncoupling recombinant FVIII from VWF may not only provide high sustained FVIII activity levels but also reduce PK variability, as demonstrated with efanesoctocog alfa, thereby increasing the predictability of the FVIII profile over time. The substantially improved half-life and predictable PK of efanesoctocog alfa allow the use of a standard once-weekly dose of 50 IU/kg for prophylaxis and a single 50-IU/kg dose for the treatment of bleeding episodes and reduce the need for monitoring FVIII activity levels [21]. A simplified weekly dosing regimen may further reduce barriers to optimal hemophilia prophylaxis and improve adherence.
4. Conclusions
The geometric mean t1/2z of efanesoctocog alfa was 43.3 hours, which was 3- to 4-fold longer than those of the SHL and EHL recombinant FVIII products. Efanesoctocog alfa (50 IU/kg) provided high sustained factor activity in the normal-to-near-normal range (ie, >40 IU/dL) for up to 4 days after the dose and at ∼10 IU/dL on day 7. No serious TEAEs were reported for efanesoctocog alfa, and no inhibitor development to FVIII was detected. These results support the potential of efanesoctocog alfa prophylaxis to improve bleed protection, joint health, and quality of life with once-weekly dosing.
Acknowledgments
Medical writing support was provided by Sarah Meadows, MRes, and Francis Golder, BVSc, PhD, of Fishawack Communications Ltd, part of Fishawack Health. This work was carried out at the Specialized Hospital for Active Treatment of Hematological Diseases, Clinical Hematology Clinic, Sofia, Bulgaria.
Funding
The study was funded by Sanofi and Sobi.
Ethics statement
All participants provided written informed consent before enrollment. The study was conducted in accordance with ethical principles derived from international guidelines, including the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Conference on Harmonization Good Clinical Practice guidelines, and applicable local laws and regulations.
Author contributions
T.L. and M.Z. contributed to data acquisition. C.J. and S.K. contributed to data analysis. All authors contributed to study concept and/or design, data interpretation and development of the manuscript as well as approved the final paper.
Relationship Disclosure
T.L. has received research funding to conduct clinical trials from Sanofi and Sobi. A.W., C.J., M.Z., and S.K. are employees of Sanofi and hold shares/stock options in the company.
Footnotes
Handling Editor: Dr Johnny Mahlangu
References
- 1.Mannucci P.M., Tuddenham E.G. The hemophilias—from royal genes to gene therapy. N Engl J Med. 2001;344:1773–1779. doi: 10.1056/nejm200106073442307. [DOI] [PubMed] [Google Scholar]
- 2.Baxalta (Takeda) Advate United States prescribing information. 2018. https://www.shirecontent.com/PI/PDFs/ADVATE_USA_ENG.pdf [accessed August 4, 2022]
- 3.Mahlangu J., Powell J.S., Ragni M.V., Chowdary P., Josephson N.C., Pabinger I., et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123:317–325. doi: 10.1182/blood-2013-10-529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiede A., Brand B., Fischer R., Kavakli K., Lentz S.R., Matsushita T., et al. Enhancing the pharmacokinetic properties of recombinant factor VIII: first-in-human trial of glycoPEGylated recombinant factor VIII in patients with hemophilia A. J Thromb Haemost. 2013;11:670–678. doi: 10.1111/jth.12161. [DOI] [PubMed] [Google Scholar]
- 5.Coyle T.E., Reding M.T., Lin J.C., Michaels L.A., Shah A., Powell J. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost. 2014;12:488–496. doi: 10.1111/jth.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konkle B.A., Stasyshyn O., Chowdary P., Bevan D.H., Mant T., Shima M., et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126:1078–1085. doi: 10.1182/blood-2015-03-630897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxalta (Takeda) Adynovate United States prescribing information. 2018. https://www.shirecontent.com/PI/PDFs/ADYNOVATE_USA_ENG.pdf [accessed August 4, 2022]
- 8.Lambert T., Benson G., Dolan G., Hermans C., Jiménez-Yuste V., Ljung R., et al. Practical aspects of extended half-life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9:295–308. doi: 10.1177/2040620718796429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pipe S.W., Montgomery R.R., Pratt K.P., Lenting P.J., Lillicrap D. Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A. Blood. 2016;128 doi: 10.1182/blood-2016-04-713289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava A., Santagostino E., Dougall A., Kitchen S., Sutherland M., Pipe S.W., et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26:1–158. doi: 10.1111/hae.14046. 3rd edition. [DOI] [PubMed] [Google Scholar]
- 11.Olivieri M., Kurnik K., Pfluger T., Bidlingmaier C. Identification and long-term observation of early joint damage by magnetic resonance imaging in clinically asymptomatic joints in patients with haemophilia A or B despite prophylaxis. Haemophilia. 2012;18:369–374. doi: 10.1111/j.1365-2516.2011.02682.x. [DOI] [PubMed] [Google Scholar]
- 12.Gooding R., Thachil J., Alamelu J., Motwani J., Chowdary P. Asymptomatic joint bleeding and joint health in hemophilia: a review of variables, methods, and biomarkers. J Blood Med. 2021;12:209–220. doi: 10.2147/jbm.s304597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gualtierotti R., Solimeno L.P., Peyvandi F. Hemophilic arthropathy: current knowledge and future perspectives. J Thromb Haemost. 2021;19:2112–2121. doi: 10.1111/jth.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren B.B., Thornhill D., Stein J., Fadell M., Ingram J.D., Funk S., et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the joint outcome continuation study. Blood Adv. 2020;4:2451–2459. doi: 10.1182/bloodadvances.2019001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Den Uijl I.E., Fischer K., Van Der Bom J.G., Grobbee D.E., Rosendaal F.R., Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17:41–44. doi: 10.1111/j.1365-2516.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 16.Skinner M.W., Nugent D., Wilton P., O’Mahony B., Dolan G., O’Hara J., et al. Achieving the unimaginable: health equity in haemophilia. Haemophilia. 2020;26:17–24. doi: 10.1111/hae.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumb E., Hermans C. Living with a “hemophilia-free mind” – the new ambition of hemophilia care? Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seth Chhabra E., Liu T., Kulman J., Patarroyo-White S., Yang B., Lu Q., et al. BIVV001, a new class of factor VIII replacement for hemophilia A that is independent of von Willebrand factor in primates and mice. Blood. 2020;135:1484–1496. doi: 10.1182/blood.2019001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konkle B.A., Shapiro A.D., Quon D.V., Staber J.M., Kulkarni R., Ragni M.V., et al. BIVV001 fusion protein as factor VIII replacement therapy for hemophilia A. N Engl J Med. 2020;383:1018–1027. doi: 10.1056/NEJMoa2002699. [DOI] [PubMed] [Google Scholar]
- 20.Lissitchkov T., Willemze A., Katragadda S., Rice K., Poloskey S., Benson C. Efanesoctocog alfa for hemophilia A: results from a phase 1 repeat-dose study. Blood Adv. 2022;6:1089–1094. doi: 10.1182/bloodadvances.2021006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Drygalski A., Chowdary P., Kulkarni R., Susen S., Konkle B., Oldenburg J., et al. Efficacy, safety, and pharmacokinetics of once-weekly efanesoctocog alfa (BIVV001) prophylaxis in previously treated patients with severe hemophilia A: results from the phase 3 XTEND-1 study. Abstract presented at: The International Society on Thrombosis and Hemostasis (ISTH) Annual Congress; July 2022; London. 2022. https://abstracts.isth.org/abstract/efficacy-safety-and-pharmacokinetics-of-once-weekly-efanesoctocog-alfa-bivv001-prophylaxis-in-previously-treated-patients-with-severe-hemophilia-a-results-from-the-phase-3-xtend-1-study/ [accessed September 30, 2022]
- 22.Staber J.M., Lissitchkov T., Konkle B.A., Shapiro A.D., Quon D.V., Kulkarni R., et al. Efanesoctocog alfa half-life and clearance are independent of von Willebrand factor in severe hemophilia A: a post hoc analysis from phase 1/2a studies. Blood. 2021;138:1035. doi: 10.1182/blood-2021-148534. [DOI] [Google Scholar]
- 23.Von Drygalski A., Chowdary P., Kulkarni R., Susen S., Konkle B.A., Oldenburg J., et al. Efanesoctocog alfa prophylaxis for patients with severe hemophilia A. N Engl J Med. 2023;388:310–318. doi: 10.1056/NEJMoa2209226. [DOI] [PubMed] [Google Scholar]
- 24.Abrantes J.A., Solms A., Garmann D., Nielsen E.I., Jönsson S., Karlsson M.O. Relationship between factor VIII activity, bleeds and individual characteristics in severe hemophilia A patients. Haematologica. 2020;105:1443–1453. doi: 10.3324/haematol.2019.217133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsheikh E., Lavin M., Heck L.A., Larkin N., Mullaney B., Doherty D., et al. Heterogeneity in the half-life of factor VIII concentrate in patients with hemophilia A is due to variability in the clearance of endogenous von Willebrand factor. J Thromb Haemost. 2023;21:1123–1134. doi: 10.1016/j.jtha.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Swystun L.L., Ogiwara K., Rawley O., Brown C., Georgescu I., Hopman W., et al. Genetic determinants of VWF clearance and FVIII binding modify FVIII pharmacokinetics in pediatric hemophilia A patients. Blood. 2019;134:880–891. doi: 10.1182/blood.2019000190. [DOI] [PubMed] [Google Scholar]
- 27.Turecek P.L., Johnsen J.M., Pipe S.W., O’Donnell J.S. Biological mechanisms underlying inter-individual variation in factor VIII clearance in haemophilia. Haemophilia. 2020;26:575–583. doi: 10.1111/hae.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ar M.C., Vaide I., Berntorp E., Björkman S. Methods for individualising factor VIII dosing in prophylaxis. Eur J Haematol Suppl. 2014;76:16–20. doi: 10.1111/ejh.12370. [DOI] [PubMed] [Google Scholar]