Key Points
Question
What is the effect among patients with small- or medium-sized vestibular schwannoma of upfront radiosurgery on tumor volume at 4 years vs a wait-and-scan approach with treatment given only when tumor growth was documented radiographically?
Findings
In this randomized clinical trial that included 100 patients, the mean ratio between tumor volume at the trial end and baseline (V4:V0) was 0.87 in the upfront radiosurgery group and 1.51 in the wait-and-scan group, a significant difference.
Meaning
Among patients with small or medium vestibular schwannoma, a treatment strategy consisting of upfront radiosurgery was more effective at reducing tumor volume at 4 years than was the initial wait-and-scan approach.
Abstract
Importance
Current guidelines for treating small- to medium-sized vestibular schwannoma recommend either upfront radiosurgery or waiting to treat until tumor growth has been detected radiographically.
Objective
To determine whether upfront radiosurgery provides superior tumor volume reduction to a wait-and-scan approach for small- to medium-sized vestibular schwannoma.
Design, Setting, and Participants
Randomized clinical trial of 100 patients with a newly diagnosed (<6 months) unilateral vestibular schwannoma and a maximal tumor diameter of less than 2 cm in the cerebellopontine angle as measured on magnetic resonance imaging. Participants were enrolled at the Norwegian National Unit for Vestibular Schwannoma from October 28, 2014, through October 3, 2017; 4-year follow-up ended on October 20, 2021.
Interventions
Participants were randomized to receive either upfront radiosurgery (n = 50) or to undergo a wait-and-scan protocol, for which treatment was given only upon radiographically documented tumor growth (n = 50). Participants underwent 5 annual study visits consisting of clinical assessment, radiological examination, audiovestibular tests, and questionnaires.
Main Outcomes and Measures
The primary outcome was the ratio between tumor volume at the trial end at 4 years and baseline (V4:V0). There were 26 prespecified secondary outcomes, including patient-reported symptoms, clinical examinations, audiovestibular tests, and quality-of-life outcomes. Safety outcomes were the risk of salvage microsurgery and radiation-associated complications.
Results
Of the 100 randomized patients, 98 completed the trial and were included in the primary analysis (mean age, 54 years; 42% female). In the upfront radiosurgery group, 1 participant (2%) received repeated radiosurgery upon tumor growth, 2 (4%) needed salvage microsurgery, and 45 (94%) had no additional treatment. In the wait-and-scan group, 21 patients (42%) received radiosurgery upon tumor growth, 1 (2%) underwent salvage microsurgery, and 28 (56%) remained untreated. For the primary outcome of the ratio of tumor volume at the trial end to baseline, the geometric mean V4:V0 was 0.87 (95% CI, 0.66-1.15) in the upfront radiosurgery group and 1.51 (95% CI, 1.23-1.84) in the wait-and-scan group, showing a significantly greater tumor volume reduction in patients treated with upfront radiosurgery (wait-and-scan to upfront radiosurgery ratio, 1.73; 95% CI, 1.23-2.44; P = .002). Of 26 secondary outcomes, 25 showed no significant difference. No radiation-associated complications were observed.
Conclusion and relevance
Among patients with newly diagnosed small- and medium-sized vestibular schwannoma, upfront radiosurgery demonstrated a significantly greater tumor volume reduction at 4 years than a wait-and-scan approach with treatment upon tumor growth. These findings may help inform treatment decisions for patients with vestibular schwannoma, and further investigation of long-term clinical outcomes is needed.
Trial Registration
ClinicalTrials.gov Identifier: NCT02249572
This randomized clinical trial evaluates whether upfront radiosurgery is more effective in reducing vestibular schwannoma tumor size than treatment delivered only when tumor growth has been detected radiographically.
Introduction
Vestibular schwannomas are benign tumors deriving from the Schwann cells of the vestibular portion of the eighth cranial nerve. They are estimated to represent 8% of all intracranial tumors and are the most common neoplasm of the cerebellopontine angle.1 The hallmark symptoms are ipsilateral hearing loss, tinnitus, dizziness, and imbalance, but large tumors with mass effect and hydrocephalus may present with more severe symptoms. Microsurgery is the treatment of choice in large vestibular schwannomas.2 However, the tumor size at the time of diagnosis has decreased over time, and most newly diagnosed tumors are small or medium sized.3 For these tumors, treatment guidelines recommend either upfront stereotactic radiosurgery or an observational wait-and-scan approach with treatment when tumor growth is detected.2
Stereotactic radiosurgery provides a highly conformal single-fraction radiation dose delivered to the tumor, with a steep-dose gradient falloff outside the target volume. The goal of radiosurgery in vestibular schwannoma management is to prevent further tumor growth. In a 2019 series, long-term tumor growth control was achieved in 94% of patients.4 However, studies have suggested that a substantial portion of untreated tumors may not exhibit growth during the first 10 years after diagnosis.5,6 Accordingly, an initial wait-and-scan approach with serial imaging and treatment upon tumor growth has become a popular alternative to upfront radiosurgery. There remains a lack of randomized trials investigating the efficacy of radiosurgery, and the management of small- and medium-sized vestibular schwannoma is controversial.1,2,7,8
The V-REX (Vestibular Schwannoma, Radiosurgery or Expectation) trial aimed to determine whether upfront radiosurgery is more effective than a wait-and-scan protocol, with treatment initiated only upon growth to reduce the tumor volume during a 4-year observation period.
Methods
Study Design
This trial was an investigator-initiated, randomized, investigator-blinded, superiority clinical trial conducted at the Norwegian National Unit for Vestibular Schwannoma, Haukeland University Hospital. Enrollment began October 28, 2014, and ended October 3, 2017; the final follow-up was October 20, 2021. The study protocol and statistical analysis plan are available in Supplement 1.9 The study complied with the World Medical Association Declaration of Helsinki10 and was approved by the local and institutional regional ethics committees. We report the trial in accordance with the recommendations of Consolidated Standards of Reporting Trials.11 The patients received oral and written information about the trial and provided a written consent form before participation (Supplement 1). All data except personal identification were entered into a password-protected database managed and monitored by a third-party institution (DIPS AS, Oslo, Norway).
Participants
The Norwegian National Unit for Vestibular Schwannoma routinely manages care of all patients with vestibular schwannoma in Norway. We invited all consecutive eligible patients (aged 18-70 years) with a newly diagnosed (<6 months) unilateral vestibular schwannoma with a maximal tumor diameter of less than 2 cm in the cerebellopontine angle as measured on magnetic resonance imaging (MRI) to participate. Key exclusion criteria were severe comorbidity or type 2 neurofibromatosis in the patient or a first-degree relative.12
Randomization
Eligible participants were randomly assigned in a 1:1 ratio to undergo either upfront radiosurgery or follow a wait-and-scan protocol where treatment was given only when tumor growth was documented radiographically (Figure 1). Two study coordinators and a biostatistician conducted the enrollment and randomization process without the involvement of the investigators. We used permuted block randomization with sequentially numbered opaque sealed envelopes (block sizes of 2, 4, and 6) and stratified for age and whether the tumor was extracanalicular or intracanalicular at the time of recruitment.13
Figure 1. Participant Flow in the V-REX Randomized Clinical Trial.
aStratified for age and whether the tumor was extracanalicular or intracanalicular at the time of recruitment.
bA multidisciplinary team, independent from the blinded study investigators, evaluated the participants annually, and chose either radiosurgery or salvage microsurgery in the case of tumor growth.
Interventions and Assessments
Study Schedule
Participants assigned to upfront radiosurgery received treatment within 2 months of randomization (Table 1). All participants underwent the baseline and 4 annual study visits consisting of radiological examination, clinical assessments, and audiovestibular testing carried out by blinded technicians and assessors. In addition, the participants filled out standardized questionnaires addressing patient-reported outcome measures. Participants in the wait-and-scan group with documented tumor growth, according to a multidisciplinary team independent of the blinded investigators, received treatment within 2 months. Study coordinators and blinded investigators did not engage in treatment decisions.
Table 1. Baseline Characteristics of Patients in the V-REX Triala.
| Characteristic | Upfront radiosurgery (n = 48) | Wait and scan (n = 50) |
|---|---|---|
| Age, mean (SD), y | 54 (12) | 54 (10) |
| Sex, No. (%) | ||
| Female | 22 (46) | 19 (38) |
| Male | 26 (54) | 31 (62) |
| Radiological characteristics, No. (%) | ||
| Left-sided tumor | 19 (40) | 19 (38) |
| Intracanalicular tumor | 18 (38) | 16 (32) |
| Tumor volume | ||
| Mean (SD), mm3 | 765 (799) | 514 (588) |
| Median (IQR), mm3 | 362 (206-942) | 328 (190-566) |
| Patient-reported symptoms, No. (%)b | ||
| Hearing loss | 45 (94) | 42 (84) |
| Tinnitus | 36 (75) | 43 (86) |
| Dizziness | 27 (56) | 26 (52) |
| Balance problems | 27 (56) | 19 (38) |
| Fatigue | 29 (60) | 24 (48) |
| Headache | 21 (44) | 25 (50) |
| Facial pain | 3 (6) | 5 (10) |
| Changes in taste | 4 (8) | 2 (4) |
| Clinical findings | ||
| Asymmetric facial sensation, No. (%) | 6 (13) | 3 (6) |
| Absent corneal reflex, No./total (%) | 1/44 (2) | 1/48 (2) |
| House-Brackmann Score, No. (%)c | ||
| I | 48 (100) | 49 (98) |
| II | 0 | 1 (2) |
| III-VI | 0 | 0 |
| Audiometry | ||
| Pure-tone average, mean (SD), dBd | 45 (24) | 38 (20) |
| Word recognition score, mean (SD), %e | 72 (39) | 84 (27) |
| AAO-HNS classification, No./total (%)f | ||
| A | 32/47 (68) | 39/48 (81) |
| B | 2/47 (4) | 2/48 (4) |
| C | 2/47 (4) | 1/48 (2) |
| D | 11/47 (23) | 6/48 (12) |
| Dynamic posturographyg | ||
| Composite equilibrium score, mean (SD) | 65 (14) | 68 (16) |
| Unsteady on posturographyh | 26 (54) | 22 (44) |
| Video nystagmographyi | ||
| Caloric asymmetry, mean (SD), % | 50 (35) | 41 (29) |
| Canal paresis on tumor side | 23 (49) | 26 (54) |
| PANQOL, mean (SD)j | ||
| Total score | 71 (18) | 73 (17) |
| Anxiety | 74 (23) | 78 (24) |
| Facial dysfunction | 89 (14) | 88 (18) |
| General health | 65 (20) | 65 (19) |
| Balance | 75 (26) | 75 (28) |
| Hearing loss | 58 (23) | 59 (26) |
| Energy | 66 (24) | 68 (23) |
| Pain | 67 (35) | 73 (31) |
Abbreviations: AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; PANQOL, Penn Acoustic Neuroma Quality-of-Life.
Percentages may not total 100 because of rounding. All hearing outcomes are from the tumor side.
Patients were interviewed by a blinded investigator and asked to respond “yes” or “no” about experiencing 8 symptoms common among patients with vestibular schwannoma.
The House-Brackmann score is based on clinical assessment of facial nerve palsy. Grade I indicates normal facial nerve function; grade II, slight dysfunction; grade III, moderate dysfunction; grade IV, moderate-severe dysfunction; grade V, severe dysfunction; and grade VI, total paralysis.
The average hearing sensitivity at 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz.
A 10-step scale reporting the percentage of words correctly repeated by the patient.
Hearing grades are based on the pure-tone average and the word recognition score. Grades A and B are considered serviceable hearing, and grades C and D, not serviceable hearing.
A weighted average of 6 independent sensory conditions tested with dynamic posturography (range, 0-100; higher scores indicate better balance performance).
Unsteadiness was defined as a composite score lower than the age-adjusted normative values supplied by the manufacturer.
On-video nystagmography percentage was calculated using the Jongkees formula. Caloric asymmetry exceeding 25% was classified as canal paresis.
The score ranges from 0 to 100, with higher scores indicating a better quality of life. The PANQOL includes a total score (minimal clinically important difference, 11 points) and 7 domain scores.
Radiosurgery
Stereotactic radiosurgery was performed using the Leksell Gamma Knife Perfexion (2014–2019) and the Leksell Gamma Knife Icon (2019–2021) models (Elekta Instrument AB). We prescribed 12 Gy to the tumor margin with a variable isodose line within a range of 40% to 60%. We routinely strove to limit the falloff dose to the brainstem and the modiolus of the cochlea. Details of the radiation dose plans are provided in eTable 1 in Supplement 2.
Radiology
Participants underwent gadolinium-enhanced T1-weighted brain MRI at baseline and annually for 4 years, using the same protocol each time. We used the MAGNETOM Sola 1.5 T (Siemens Healthineers AG) from 2014 to 2016 and the SIGNA Artist 1.5 T (GE Healthcare) from 2016 to 2021. No other scans, such as those taken during radiosurgery procedures, were used for study purposes. A technician not involved in the treatment replaced all written image information before uploading the MRI scans to a trial server allocated for research. The investigator was unable to compare scans from individual patients.
To measure tumor volume, we applied the SmartBrush function in iPlan Brainlab Elements (version 2.4.0, Brainlab AG). The blinded investigator outlined the tumor margin manually on each slice and masked nontumor contrast-enhancing structures such as the jugular bulb. The program software generated a 3-dimensional tumor model based on the selected areas and calculated the tumor volume in cubic centimeters. The segmental outlining was done 4 times on each examination; twice on the axial and twice on the coronal plane series, and the mean of the 4 measurements was registered. In addition, a neuroradiologist without any involvement in the trial, examined the scans as part of the clinical routine. The trial protocol contains a detailed description of the volumetry procedure.
Clinical Assessment
All participants underwent on-site interviews and cranial nerve examinations by a blinded consultant neurologist (see Supplement 1, for complete examination forms). The participants wore scrub hats covering their foreheads to hide any scars from the stereotactic frame. The neurologist asked the participants to respond “yes” or “no” about experiencing 8 symptoms common among vestibular schwannoma patients (hearing loss, tinnitus, dizziness, balance problems, fatigue, headache, facial pain, changes in taste). The examiner used a toothpick to test pinprick sensation and a cotton ball to assess light touch sensation in the facial area supplied by the trigeminal nerve (fifth cranial nerve). A wisp of cotton gently applied onto the cornea was used to evaluate the corneal reflex. The facial nerve (seventh cranial nerve) was evaluated by inspecting facial movement, and the findings were graded according to the House-Brackmann scale (grades I, normal facial nerve function, to VI, total paralysis).14 Additional information on clinical assessments is provided in the trial protocol (Supplement 1).
Audiovestibular Testing
Hearing acuity was measured by tonal and speech audiometry. The pure-tone average is the average value of hearing sensitivity at 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz. The word recognition score is a 10-step scale reporting the percentage of words correctly repeated when administered to the patient through a headset. The outcomes of both tests provided data for grading according to the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) classification system, with grades A and B considered serviceable hearing and grades C and D considered not serviceable hearing. A detailed description is presented in the protocol (Supplement 1).15
Dynamic posturography was performed using the SMART EquiTest (NeuroCom) and the NeuroCom Sensory Organization Test. This protocol is a 6-condition assessment providing information about interactions among the 3 sensory systems (somatosensory, visual, and vestibular systems) contributing to balance performance. The test yields a composite score, a weighted average of the equilibrium score on the 6 different sensory conditions, ranging from 0 to 100; higher scores indicate better balance performance. Unsteadiness was defined as a composite score lower than the age-adjusted normative values supplied by the manufacturer.16 Video nystagmography was performed to test the vestibulo-ocular reflex. Caloric asymmetry percentage was calculated using the Jongkees formula.17 Caloric asymmetry of more than 25% was classified as canal paresis. A detailed description of the audiovestibular tests is presented in the trial protocol (Supplement 1).
Patient-Reported Outcome Measures
The participants responded to the Penn Acoustic Neuroma Quality of Life (PANQOL) scale, a validated vestibular schwannoma–specific quality-of-life assessment battery consisting of 26 questions, each with 5 response alternatives.18 The responses yield a PANQOL total score and 7 domain scores for anxiety, facial function, general health, balance, hearing, energy, and pain . The scores range from 0 to 100, with the higher scores indicating better quality of life. The minimal clinically important difference (MCID) for PANQOL total score is 11 points.19 Additional information on patient-reported outcomes is provided in the trial protocol (Supplement 1).
Outcomes
The primary outcome was the ratio between tumor volume at the trial end at 4 years and baseline (V4:V0). For example, for an individual, a V4:V0 value of 1.5 corresponds to a 50% increase in tumor size.
Secondary outcomes assessed at 4 years were the presence of 8 patient-reported symptoms (hearing loss, tinnitus, dizziness, balance problems, fatigue, headache, facial pain, changes in taste), 3 clinical cranial nerve examinations (corneal reflex, facial sensation, and facial nerve palsy, according to the House-Brackmann Score, grades I-VI),14 3 hearing outcomes from audiometry (pure-tone average, 0-100 dB; word recognition score, 0 to 100%; and AAO-HNS Hearing Classification, grades A-D),15 2 outcomes from posturography (composite equilibrium score, 0-100 and objective unsteadiness defined as a composite score lower than the age-adjusted normative values),16 2 outcomes from video nystagmography (caloric asymmetry, 0 to 100% and canal paresis defined as caloric asymmetry >25%),17 and 8 PANQOL scores (total score, 0-100; MCID, 11; and 7 domain scores, 0-100).18,19 The EuroQol 5-dimension 5-level (EQ-5D) responses and data on health economy outcomes were prespecified and obtained but are not reported herein.
Safety outcomes were the risk of salvage microsurgery and radiation-associated complications, including hydrocephalus, brainstem necrosis, radiation-induced tumors, and malignant transformation of vestibular schwannoma.20,21,22
In post hoc analyses, comparisons of tumor volumes and secondary outcomes at various time points were performed. Furthermore, we examined the likelihood of retaining hearing at the trial end among participants with serviceable hearing at baseline, as defined by the AAO-HNS classification.
Sample Size Calculations
Power calculations were based on a 2-sided t test with a significance level of .05 and a power of 80%.9 We used existing data from our prospectively managed vestibular schwannoma database and performed the power analysis. Because the goal of radiosurgery is to prevent tumor growth, we chose tumor volume change (V4:V0) as a primary outcome and the basis for the power analysis. There is no MCID established for the primary outcome. The estimated difference in means of the V4:V0 ratio was 0.80, and the estimated standard deviations were 0.99 for the upfront radiosurgery group and 1.73 for the wait-and-scan group. With a 1:1 allocation, the analysis yielded a sample size of 100 patients to demonstrate a difference in tumor volume. Hearing acuity was examined as a potential primary outcome. However, the estimated sample size of 600 patients needed to demonstrate difference or similarity in hearing outcomes was considered unfeasible.
Statistical Analysis
Primary and secondary outcomes were analyzed with patients assigned according to their randomization group. The tumor volumes (V0 through V4) and tumor volume ratios (V4:V0) were strongly right-skewed but approximately symmetrical after log transformation. Therefore, these variables were analyzed after first log transforming the values. We report back-transformed results as geometric means and ratios of geometric means. Because the values were approximately symmetrical after log transformation, the geometric mean is similar to the median and can be interpreted in the same way. The primary outcome (V4:V0) was analyzed using the Welch t test on the log scale. We prespecified both volume ratio (V4:V0) and volume-doubling time as primary outcomes in the protocol. There was, however, no simple model based on volume-doubling time (eg, exponential growth) that fit with our existing data. Therefore, we made a blinded decision to remove volume-doubling time as a coprimary outcome.
Secondary outcomes are reported as point estimates of effects with 2-sided 95% CIs. The widths of the intervals are not adjusted for multiplicity. Thus, the intervals may not be used in place of formal hypothesis testing, and no definite conclusions should be drawn from these data. For all continuous outcomes, results are based on longitudinal models, fitted using generalized least squares. For maximum flexibility, the models had an unstructured covariance matrix and separate mean parameters for each combination of time points and randomization group, except for the prerandomization baseline measurements. This model uses data from all patients, including patients with missing data at some time points. Although we expect most missing data to be missing completely at random, this method provides unbiased estimates also under the less strict missing-at-random assumption. For categorical data, we present differences in proportions between groups, along with CIs, calculated using the recommended Newcombe Hybrid Score method.23 The protocol called for reporting differences in categorical data using odds ratio. We decided post hoc to additionally report absolute differences with CIs. The rate of missing data was low, and we report the number of observations for each variable. All tests were 2-sided with α = .05.
Statistical analyses were performed using R Statistical Software (version 4.2.1; R Core Team, 2022). The longitudinal models were fitted using the R package nlme (version 3.1-159; R Core Team, 2023). CIs for categorical data were calculated using the contingencytables package (version 1.0.2; Fagerland M, 2022).
Results
Overall, 142 adult patients with newly diagnosed (<6 months) vestibular schwannoma (<2 cm in the cerebellopontine angle) were screened for eligibility; 100 were enrolled and randomized to either upfront radiosurgery (n = 50) or the wait-and-scan protocol (n = 50; Figure 1). Two patients, both allocated to upfront radiosurgery, declined intervention and formally withdrew from the trial immediately after randomization. The remaining 98 participants completed the trial and were included in the analysis.
The demographic, radiological, and clinical characteristics of all patients at baseline were mostly balanced between the groups (Table 1). The geometric mean tumor volume at the time of diagnosis was 381 mm3 (median, 350 mm3; arithmetic mean, 637 mm3; range, 29-2914 mm3). None of the patients had hydrocephalus. The most frequent symptoms at baseline were hearing loss (89%) and tinnitus (81%). Seventy-five percent had AAO-HNS hearing class A, 49% were unsteady on dynamic posturography, and 52% had canal paresis on the tumor side.
In the upfront radiosurgery group, 3 patients (6%) needed additional treatment 3 years after the intervention because of continued tumor growth; 1 (2%) had repeated radiosurgery, and 2 (4%) received salvage microsurgery (Figure 1). In the wait-and-scan group, 21 patients (42%) received radiosurgery upon tumor growth: 14 after 1 year, 6 after 2 years, and 2 after 3 years. One patient (2%) had salvage microsurgery after 3 years without prior radiosurgery. The remaining 28 patients (56%) had nongrowing tumors and received no active treatment.
Primary Outcome
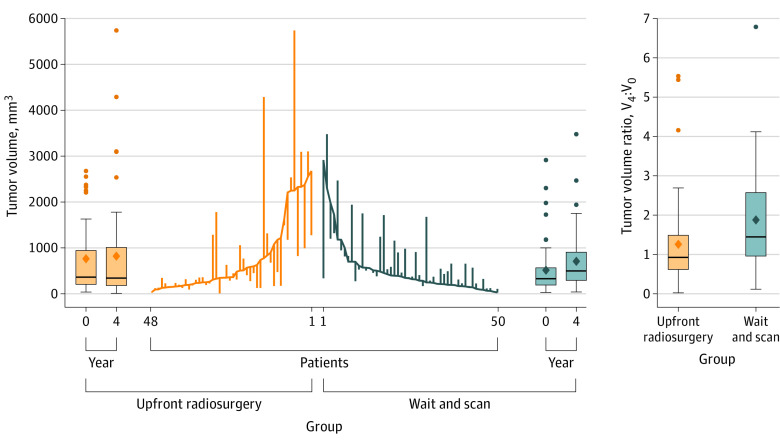
The ratio of tumor volume at 4 years relative to baseline (V4:V0) was significantly lower among those who received upfront radiosurgery (geometric mean, 0.87; 95% CI, 0.66-1.15; IQR, 0.62-1.49; range, 0.02-5.53) than among those who underwent the wait-and-scan approach (geometric mean, 1.51; 95% CI, 1.23-1.84; IQR, 0.96-2.57; range, 0.12-6.79; Figure 2 and Table 2). The wait-and-scan approach to upfront radiosurgery ratio of geometric means was 1.73 (95% CI, 1.23-2.44; P = .002). Volume curves for individual patients, including the timing of treatments, are presented in the eFigure in Supplement 2.
Figure 2. Changes in Tumor Volume in Patients Treated With Upfront Radiosurgery vs the Wait-and-Scan Protocol With Treatment Upon Tumor Growth.
The parallel line plot contains a vertical line for each patient that extends from the baseline tumor volume to the 4-year volume. Descending lines indicate a shrinking tumor. Baseline values are placed in ascending order for the upfront radiosurgery group and descending order for the wait-and-scan group. The ends of the boxes in the box plots are located at the first and third quartiles, with the solid line indicating the median and the diamond indicating the mean. Whiskers extend to the upper and lower adjacent values, the location of the furthest point within a distance of 1.5 interquartile ranges from the first and third. Dots indicate more extreme values.
Table 2. Primary and Secondary Outcomes at 4 Yearsa.
| No. (%) of patients | Difference in means or proportions (95% CI) | Odds ratio (95% CI) | ||
|---|---|---|---|---|
| Upfront radiosurgery (n = 48) | Wait and scan (n = 50) | |||
| Primary outcome | ||||
| Relative tumor volume change (V4:V0) | ||||
| Median (IQR) | 0.92 (0.62 to 1.49) |
1.45 (0.96 to 2.57) |
||
| Geometric mean (95% CI) | 0.87 (0.66 to 1.15) |
1.51(1.23 to 1.84) | Ratio: 1.73 (1.23 to 2.44)b |
|
| Log (V4:V0), mean (SD) | −0.14 (0.97) | 0.41 (0.71) | −0.55 (−0.89 to −0.21)c |
|
| Secondary outcomed | ||||
| Patient-reported symptoms, %e | ||||
| Hearing loss | 45 (94) | 43 (86) | 8 (−5 to 21) | 2.44 (0.60 to 9.05) |
| Tinnitus | 40 (83) | 40 (80) | 3 (−12 to 19) | 1.25 (0.48 to 3.62) |
| Dizziness | 21 (44) | 22 (44) | 0 (−19 to 19) | 0.99 (0.45 to 2.28) |
| Balance problems | 29 (60) | 21 (42) | 18 (−1 to 36) | 2.11 (0.92 to 4.63) |
| Fatigue | 31 (65) | 24 (48) | 17 (−3 to 34) | 1.98 (0.86 to 4.39) |
| Headache | 15 (31) | 8 (16) | 15 (−2 o 31) | 2.39 (0.94 to 6.38) |
| Facial pain | 2 (4) | 5 (10) | −6 (−18 to 5) | 0.39 (0.08 to 2.01) |
| Changes in taste | 11 (23) | 5 (10) | 13 (−2 to 28) | 2.68 (0.90 to 7.36) |
| Clinical examinations | ||||
| Absent corneal reflex | 5 (10) | 6/49 (12) | −2 (−15 to 12) | 0.83 (0.26 to 3.19) |
| Asymmetric facial sensation | 6 (12) | 0/49 (0) | 12 (3 to 25) | Not calculable |
| House-Brackman Score progressionf | 8/47 (17) | 6/48 (12) | 5 (−10 to 19) | 1.44 (0.48 to 4.74) |
| Audiometryg | ||||
| Pure-tone average, mean (SD), dB | 60 (24) | 61 (27) | −2 (−8 to 5) | |
| Word recognition score, mean (SD), % | 42 (38) | 47 (40) | −6 (−19 to 7) | |
| Serviceable hearing | 16 (33) | 20 (40) | −7 (−25 to 12) | 0.75 (0.33 to 1.65) |
| Dynamic posturographyh | ||||
| Composite equilibrium score, mean (SD) | 68 (19) (n = 43) | 72 (15) (n = 42) | −4 (−10 to 2) | |
| Unsteady on posturography, No./total (%) | 15/43 (35) | 11/42 (26) | 9 (−11 to 27) | 1.51 (0.62 to 3.95) |
| Video nystagmographyi | ||||
| No. | 44 | 43 | ||
| Caloric asymmetry, mean (SD), % | 48 (35) | 48 (30) | 0 (−12 to 12) | |
| Canal paresis on tumor side, No./total (%) | 28/44 (64) | 33/43 (77) | −13 (−31 to 6) | 0.53 (0.22 to 1.28) |
| PANQOL, mean (SD)j | ||||
| No. | 47 | 48 | ||
| Total score | 68 (18) | 71 (17) | −2 (−7 to 3) | |
| Anxiety | 80 (21) | 78 (22) | 1 (−6 to 9) | |
| Facial dysfunction | 81 (21) | 85 (17) | −4 (−12 to 3) | |
| General health | 59 (22) | 58 (24) | 1 (−7 to 8) | |
| Balance | 71 (25) | 75 (22) | −3 (−10 to 3) | |
| Hearing loss | 57 (24) | 56 (23) | 1 (−6 to 8) | |
| Energy | 61 (24) | 68 (21) | −7 (−14 to 0) | |
| Pain | 69 (35) | 75 (31) | −7 (−18 to 4) | |
Abbreviation: PANQOL, Penn Acoustic Neuroma Quality of Life.
Percentages may not total 100 because of rounding.
Wait-and-scan to upfront radiosurgery ratio.
Tumor volumes (V0 through V4) and tumor volume ratios (V4:V0) were strongly right-skewed but approximately symmetrical after log transformation. The primary outcome (V4:V0) was analyzed using the Welch t test on the log scale. The Welch t test, P = .002. The log function is the natural logarithm.
Estimates for continuous outcomes are arithmetic means with SDs and are based on longitudinal models with adjustments for random baseline imbalance and any missing data. Estimates for categorical outcomes are counts (%) and differences in percentages, for which the CIs have been calculated using the Newcombe Hybrid Score method. CIs for secondary outcomes are not adjusted for multiplicity; therefore, no definite conclusions can be drawn from these data. All hearing outcomes are from the tumor side.
See the footnotes in Table 1 for the methods used for collecting these data.
Seventeen participants progressed from House-Brackmann grade I to II, and 1 patient progressed to grade III. The patient with House-Brackmann grade II at baseline presented normal facial nerve at all follow-up assessments. For grade definitions, see the footnotes in Table 1.
For definitions of the pure-tone average, the word recognition and serviceable hearing definitions and scores, see the footnotes in Table 1.
For equilibrium measures, see the footnotes in Table 1.
For on-video nystagmography calculations, see the footnotes in Table 1.
For the PANQOL score ranges and measures, see the footnotes in Table 1.
Secondary Outcomes
Of the 26 prespecified secondary outcomes, only reduced facial sensation on clinical examination demonstrated significant difference at the trial end (6 in the upfront radiosurgery group and none in the wait-and-scan group at the trial end; for context, the number with asymmetric facial sensation at baseline were 6 and 3, respectively). The remaining 25 secondary outcomes demonstrated no significant difference (Table 2).
Hearing acuity, according to pure-tone audiometry, declined in both groups during the study period (Figure 3 panels B and C; eTable 2 in Supplement 2). At 4 years, the mean pure-tone average was 60 dB in the upfront radiosurgery group and 61 dB in the wait-and-scan group; the mean deterioration in pure-tone average from baseline to 4-year audiometry was 18 dB in the upfront radiosurgery group and 20 dB in the wait-and-scan group (mean difference, −2 dB; 95% CI, −8 dB to 5 dB). At 4 years, the mean word recognition score was 42 percentage points in the upfront radiosurgery group and 47 percentage points in the wait-and-scan group; the mean reduction in word recognition score was 35 percentage points in the upfront radiosurgery group and 29 percentage points in the wait-and-scan group (mean difference, −6; 95% CI, −19 to 7).
Figure 3. Development of Tumor Volume and Clinical Outcomes From Baseline to Year 4.

Shown are the development of outcomes from baseline to 4-year follow-up. The estimates are geometric means in Panel A and arithmetic means in the other panels, all with 95% CIs, indicated with whiskers. They are based on longitudinal models with adjustments for random baseline imbalance and missing data. A, Shows volumetric tumor growth curves for each group. The increase in tumor volume seen during the first year following radiosurgery may reflect pseudoprogression, a transient volumetric tumor enlargement caused by ischemic infarction and central necrosis. B and C, Pure-tone average and the word recognition score show a reduction in hearing acuity in both groups. D, The composite-equilibrium score showed some increase over time for both groups indicating improved vestibular function. E, The caloric asymmetry slightly increased over time in both groups indicating worsening of canal paresis over time. F, The Penn Acoustic Neuroma Quality-of-Life (PANQOL) total score appeared fairly stable throughout the study period, except for a small dip at year 1 in both groups. Details of changes in continuous outcomes from baseline to trial end are in eTable 2 in Supplement 2.
aThe average hearing sensitivity at 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz.
bA 10-step scale reporting the percentage of words correctly repeated by the patient.
cFor equilibrium score and range definitions, see the footnotes in Table 1.
dFor calculation and measures of caloric asymmetry see the footnotes in Table 1.
eFor the PANQOL score ranges and measures, see the footnotes in Table 1.
Regarding vestibular function, the composite-equilibrium score showed some increase over time for both groups (mean scores at year 4, 68 in the upfront radiosurgery group and 72 in the wait-and-scan group), and the mean change from baseline to year 4 were 1 for the upfront radiosurgery group and 5 for the wait-and-scan group (mean difference, −4; 95% CI, −10 to 2; Figure 3D and eTable 2 in Supplement 2). Caloric asymmetry increased over time (48% in the upfront radiosurgery group and 48% in the wait-and-scan group at 4 years), with a mean absolute change of 15% from baseline to year 4 for both groups (mean difference, 0; 95% CI, −12 to 12; Figure 3E and eTable 3 in Supplement 2).
Neither of the groups demonstrated significant change in the PANQOL total score from baseline to year 4. At year 4, the scores were 68 points for the upfront radiosurgery group and 71 points for the wait-and-scan group; the mean change from baseline to year 4 was −4 (95% CI, −7 to 0) for the upfront radiosurgery group and −1 (95% CI, −5 to 2) for the wait-and-scan group (mean between group difference, −2; 95% CI, −7 to 3; Figure 3F and eTable 2 in Supplement 2).
Safety Outcomes
No deaths or radiation-associated complications occurred during the trial, including hydrocephalus, brainstem necrosis, radiation-induced tumors, or malignant transformation. The risk of salvage microsurgery was low in both groups, 4% in the upfront radiosurgery group and 2% in the wait-and-scan group. With the exception of 1 patient experiencing transient dysphagia, there were no other complications following salvage microsurgery.
Post Hoc Analysis
There was no significant difference in absolute volume after 1 year (geometric mean, 514 mm3 vs 567 mm3; P = .30; Figure 3A). From 2 years onward, the patients receiving upfront radiosurgery had a superior tumor volume reduction at each time point compared with the wait-and-scan group. Differences in tumor volume between the groups at each time point is provided in eTable 3 in Supplement 3 . The absolute tumor volume at 4 years had a geometric mean of 333 mm3 in the upfront radiosurgery group and 533 mm3 in the wait-and-scan group.
In the upfront radiosurgery group, 18 of the 34 patients (53%) with serviceable hearing at baseline per AAO-HNS Hearing Classification system had nonserviceable hearing at the 4-year follow-up. In the wait-and-scan group, the corresponding numbers were 22 of 41 patients (54%). We provide descriptive statistics for all secondary outcomes, including longitudinal correlations, for future sample size calculations in eTable 4 in Supplement 2).
Discussion
In this randomized clinical trial, an initial treatment approach of upfront radiosurgery resulted in significantly greater tumor volume reduction at 4 years than the initial wait-and-scan approach. Out of 26 secondary outcomes, including patient-reported symptoms, clinical examinations, audiovestibular tests, and quality-of-life outcomes, only 1 showed significant difference. Both groups demonstrated similar progressive, unilateral hearing loss. The incidence of salvage treatment was low, and no deaths or radiation-associated complications occurred during the trial.
Although the aim of radiosurgery in vestibular schwannoma is to prevent tumor growth, to our knowledge, no randomized clinical trials have compared the efficacy of upfront radiosurgery with a wait-and-scan approach.2,7 Two prospective nonrandomized, nonblinded studies found that upfront radiosurgery was associated with smaller tumor size.24,25 The results of this trial support these findings and reinforce radiosurgery as an effective treatment modality regarding tumor growth. However, in recent decades, vestibular schwannoma management has evolved to prioritize functional outcomes over a radiographic cure.26 The influence of radiosurgery vs the natural course of disease on cranial nerve deficits, notably hearing, is an area of particular interest and controversy, about which the 2 previous studies conflict.27 Régis et al25 concluded that upfront radiosurgery was associated with hearing preservation, whereas Breivik et al24 found no benefit associated with upfront radiosurgery concerning symptoms and quality of life. A recent retrospective-matched comparison found no statistically significant difference in hearing loss among patients managed with radiosurgery or observation alone.28 Two systematic reviews found 60% hearing preservation during the first 2 to 5 years following radiosurgery among patients with serviceable hearing,29,30 which is comparable with the hearing outcomes found in the most extensive series of patients treated with observation alone.31 In this randomized trial, the hearing loss outcomes were not significantly different between treatment strategies. However, the wide 95% CIs around the difference in serviceable hearing shows that the study was underpowered to provide conclusive evidence on the risk of hearing loss.
The widespread access to brain MRI has led to increased detection of small vestibular schwannomas associated with mild symptoms,3 raising concerns about potential overtreatment.32 In this trial, 56% of the patients in the wait-and-scan group remained untreated after 4 years because there was no evidence of tumor growth. If further trials confirm that postponing radiosurgery does not worsen clinical outcomes, these findings would indeed be supportive of an initial wait-and-scan approach from a health economic perspective.
One major concern raised about the wait-and-scan approach is what happens to patients lost to follow-up.1,25 Although patients in this study mostly had adhered with follow-up, this may not hold for all countries. In a French study, 16% of patients were lost to follow-up within the first year of a wait-and-scan investigation.33 Because radiosurgery clearly reduces the risk of future tumor growth, upfront treatment likely reduces the risk of uncontrolled tumor growth among patients lost to follow-up.
Limitations
This study has several limitations. First, because the goal of radiosurgery is to prevent tumor growth, tumor volume was selected as the primary outcome. However, this is a radiographic parameter and not necessarily a reliable surrogate for clinically impactful treatment. The trial was not powered to detect hearing outcomes because the estimated 600 patients needed to demonstrate such differences was considered unachievable.9 Data addressing clinical hearing outcomes in this trial should, therefore, be interpreted with caution. Second, we included patients whose tumors had a maximum cerebellopontine angle diameter of 2 cm, so our findings may not be valid for larger tumors. Third, with yearly follow-up visits, we may have overlooked acute transient adverse events.34 Fourth, the phenomenon of reversible tumor expansion that occurs in a proportion of cases within the first 3 years after radiosurgery, so-called pseudoprogression, may interfere with the primary outcome.35 Fifth, although a 10-year follow-up of the trial is planned in the 2024-2027 year time frame, no conclusions can be drawn about outcomes beyond the 4-year trial period at this time.
Conclusions
Among patients with newly diagnosed small- and medium-sized vestibular schwannoma, upfront radiosurgery demonstrated a significantly greater tumor volume reduction at 4 years compared with a wait-and-scan approach with treatment initiated upon tumor growth. These findings may help inform treatment decisions for patients with vestibular schwannoma, and further investigation of long-term clinical outcomes is needed.
Protocols, Statistical Analysis Plan, and Administrative Documents
eFigure 1. Volume growth curves for individual patients with timing of interventions
eTable 1. Stereotactic radiosurgery treatment data
eTable 2. Changes in continuous outcomes from baseline (t0) to trial end 9 (t4)
eTable 3. Differences in log tumor volume between treatment groups at each follow-up
eTable 4. Summary statistics for secondary outcomes
Nonauthor Collaborators. The V-REX Trial Investigators
Data Sharing Statement
References
- 1.Carlson ML, Link MJ. Vestibular schwannomas. N Engl J Med. 2021;384(14):1335-1348. doi: 10.1056/NEJMra2020394 [DOI] [PubMed] [Google Scholar]
- 2.Goldbrunner R, Weller M, Regis J, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol. 2020;22(1):31-45. doi: 10.1093/neuonc/noz153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reznitsky M, Petersen MMBS, West N, Stangerup SE, Cayé-Thomasen P. Epidemiology of vestibular schwannomas—prospective 40-year data from an unselected national cohort. Clin Epidemiol. 2019;11:981-986. doi: 10.2147/CLEP.S218670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson S, Kano H, Faramand A, et al. Long term results of primary radiosurgery for vestibular schwannomas. J Neurooncol. 2019;145(2):247-255. doi: 10.1007/s11060-019-03290-0 [DOI] [PubMed] [Google Scholar]
- 5.Reznitsky M, Petersen MMBS, West N, Stangerup SE, Cayé-Thomasen P. The natural history of vestibular schwannoma growth-prospective 40-year data from an unselected national cohort. Neuro Oncol. 2021;23(5):827-836. doi: 10.1093/neuonc/noaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinelli JP, Schnurman Z, Killeen DE, et al. Long-term natural history and patterns of sporadic vestibular schwannoma growth: a multi-institutional volumetric analysis of 952 patients. Neuro Oncol. 2022;24(8):1298-1306. doi: 10.1093/neuonc/noab303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzevic D, Legcevic J, Splavski B, Cayé-Thomasen P. Stereotactic radiotherapy for vestibular schwannoma. Cochrane Database Syst Rev. 2014;(12):CD009897. doi: 10.1002/14651858.CD009897.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson ML, Glasgow AE, Grossardt BR, Habermann EB, Link MJ. Does where you live influence how your vestibular schwannoma is managed? examining geographical differences in vestibular schwannoma treatment across the United States. J Neurooncol. 2016;129(2):269-279. doi: 10.1007/s11060-016-2170-5 [DOI] [PubMed] [Google Scholar]
- 9.Dhayalan D, Tveiten OV, Goplen FK, et al. Comparing the impact of upfront radiosurgery versus expectation in vestibular schwannoma (the V-REX study): protocol for a randomised, observer-blinded, 4-year, parallel-group, single-centre, superiority study. BMJ Open. 2021;11(3):e039396. doi: 10.1136/bmjopen-2020-039396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996;276(8):637-639. doi: 10.1001/jama.1996.03540080059030 [DOI] [PubMed] [Google Scholar]
- 12.Meola A, Chang SD. Bilateral vestibular schwannomas in neurofibromatosis type 2. N Engl J Med. 2018;379(15):1463. doi: 10.1056/NEJMicm1804944 [DOI] [PubMed] [Google Scholar]
- 13.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20(2):187-191. doi: 10.1016/j.jcrc.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 14.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi: 10.1177/019459988509300202 [DOI] [PubMed] [Google Scholar]
- 15.Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology–Head and Neck Surgery Foundation Inc. Otolaryngol Head Neck Surg. 1995;113(3):176-178. doi: 10.1016/S0194-5998(95)70100-1 [DOI] [PubMed] [Google Scholar]
- 16.Pletcher ER, Williams VJ, Abt JP, et al. Normative data for the NeuroCom Sensory Organization Test in US military special operations forces. J Athl Train. 2017;52(2):129-136. doi: 10.4085/1062-6050-52.1.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jongkees LB, Philipszoon AJ. Electronystagmography. Acta Otolaryngol Suppl. 1964;189(suppl 189):1+. [PubMed] [Google Scholar]
- 18.Shaffer BT, Cohen MS, Bigelow DC, Ruckenstein MJ. Validation of a disease-specific quality-of-life instrument for acoustic neuroma: the Penn Acoustic Neuroma Quality-of-Life Scale. Laryngoscope. 2010;120(8):1646-1654. doi: 10.1002/lary.20988 [DOI] [PubMed] [Google Scholar]
- 19.Carlson ML, Tveiten OV, Yost KJ, Lohse CM, Lund-Johansen M, Link MJ. The minimal clinically important difference in vestibular schwannoma quality-of-life assessment: an important step beyond P <. 05. Otolaryngol Head Neck Surg. 2015;153(2):202-208. doi: 10.1177/0194599815585508 [DOI] [PubMed] [Google Scholar]
- 20.Shin M, Ueki K, Kurita H, Kirino T. Malignant transformation of a vestibular schwannoma after Gamma Knife radiosurgery. Lancet. 2002;360(9329):309-310. doi: 10.1016/S0140-6736(02)09521-1 [DOI] [PubMed] [Google Scholar]
- 21.Sughrue ME, Yang I, Han SJ, et al. Non-audiofacial morbidity after Gamma Knife surgery for vestibular schwannoma. Neurosurg Focus. 2009;27(6):E4. doi: 10.3171/2009.9.FOCUS09198 [DOI] [PubMed] [Google Scholar]
- 22.Wolf A, Naylor K, Tam M, et al. Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol. 2019;20(1):159-164. doi: 10.1016/S1470-2045(18)30659-4 [DOI] [PubMed] [Google Scholar]
- 23.Fagerland MWLS, Laake P. Analysis of Contingency Tables. Chapman & Hall/CRC; 2017. doi: 10.1201/9781315374116 [DOI] [Google Scholar]
- 24.Breivik CN, Nilsen RM, Myrseth E, et al. Conservative management or Gamma Knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013;73(1):48-56. doi: 10.1227/01.neu.0000429862.50018.b9 [DOI] [PubMed] [Google Scholar]
- 25.Régis J, Carron R, Park MC, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg. 2010;113(suppl):105-111. doi: 10.3171/2010.8.GKS101058 [DOI] [PubMed] [Google Scholar]
- 26.Chan SA, Marinelli JP, Hahs-Vaughn DL, Nye C, Link MJ, Carlson ML. Evolution in management trends of sporadic vestibular schwannoma in the United States over the last half-century. Otol Neurotol. 2021;42(2):300-305. doi: 10.1097/MAO.0000000000002891 [DOI] [PubMed] [Google Scholar]
- 27.Carlson ML, Vivas EX, McCracken DJ, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on hearing preservation outcomes in patients with sporadic vestibular schwannomas. Neurosurgery. 2018;82(2):E35-E39. doi: 10.1093/neuros/nyx511 [DOI] [PubMed] [Google Scholar]
- 28.Schnurman Z, Gurewitz J, Smouha E, et al. Matched comparison of hearing outcomes in patients with vestibular schwannoma treated with stereotactic radiosurgery or observation. Neurosurgery. 2022;91(4):641-647. doi: 10.1227/neu.0000000000002089 [DOI] [PubMed] [Google Scholar]
- 29.Coughlin AR, Willman TJ, Gubbels SP. Systematic review of hearing preservation after radiotherapy for vestibular schwannoma. Otol Neurotol. 2018;39(3):273-283. doi: 10.1097/MAO.0000000000001672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang I, Aranda D, Han SJ, et al. Hearing preservation after stereotactic radiosurgery for vestibular schwannoma: a systematic review. J Clin Neurosci. 2009;16(6):742-747. doi: 10.1016/j.jocn.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 31.Stangerup SE, Thomsen J, Tos M, Cayé-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol. 2010;31(2):271-275. doi: 10.1097/MAO.0b013e3181c34bda [DOI] [PubMed] [Google Scholar]
- 32.Marinelli JP, Grossardt BR, Lohse CM, Carlson ML. Is improved detection of vestibular schwannoma leading to overtreatment of the disease? Otol Neurotol. 2019;40(6):847-850. doi: 10.1097/MAO.0000000000002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakkouri WE, Kania RE, Guichard JP, Lot G, Herman P, Huy PT. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg. 2009;110(4):662-669. doi: 10.3171/2007.5.16836 [DOI] [PubMed] [Google Scholar]
- 34.Tuleasca C, George M, Faouzi M, et al. Acute clinical adverse radiation effects after Gamma Knife surgery for vestibular schwannomas. J Neurosurg. 2016;125(suppl 1):73-82. doi: 10.3171/2016.7.GKS161496 [DOI] [PubMed] [Google Scholar]
- 35.Albano L, Deng H, Wei Z, Vodovotz L, Niranjan A, Lunsford LD. The longitudinal volumetric response of vestibular schwannomas after Gamma Knife radiosurgery. J Neurosurg. 2022;138(5):1273-1280. doi: 10.3171/2022.7.JNS22812 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocols, Statistical Analysis Plan, and Administrative Documents
eFigure 1. Volume growth curves for individual patients with timing of interventions
eTable 1. Stereotactic radiosurgery treatment data
eTable 2. Changes in continuous outcomes from baseline (t0) to trial end 9 (t4)
eTable 3. Differences in log tumor volume between treatment groups at each follow-up
eTable 4. Summary statistics for secondary outcomes
Nonauthor Collaborators. The V-REX Trial Investigators
Data Sharing Statement