Abstract
Objective
To systematically review the proportions of infants with early exposure to antenatal corticosteroids but born at term or late preterm, and short term and long term outcomes.
Design
Systematic review and meta-analyses.
Data sources
Eight databases searched from 1 January 2000 to 1 February 2023, reflecting recent perinatal care, and references of screened articles.
Eligibility criteria for selecting studies
Randomised controlled trials and population based cohort studies with data on infants with early exposure to antenatal corticosteroids (<34 weeks) but born at term (≥37 weeks), late preterm (34-36 weeks), or term/late preterm combined.
Data extraction and synthesis
Two reviewers independently screened titles, abstracts, and full text articles and assessed risk of bias (Cochrane risk of bias tool for randomised controlled trials and Newcastle-Ottawa scale for population based studies). Reviewers extracted data on populations, exposure to antenatal corticosteroids, and outcomes. The authors analysed randomised and cohort data separately, using random effects meta-analyses.
Main outcome measures
The primary outcome was the proportion of infants with early exposure to antenatal corticosteroids but born at term. Secondary outcomes included the proportions of infants born late preterm or term/late preterm combined after early exposure to antenatal corticosteroids and short term and long term outcomes versus non-exposure for the three gestational time points (term, late preterm, term/late preterm combined).
Results
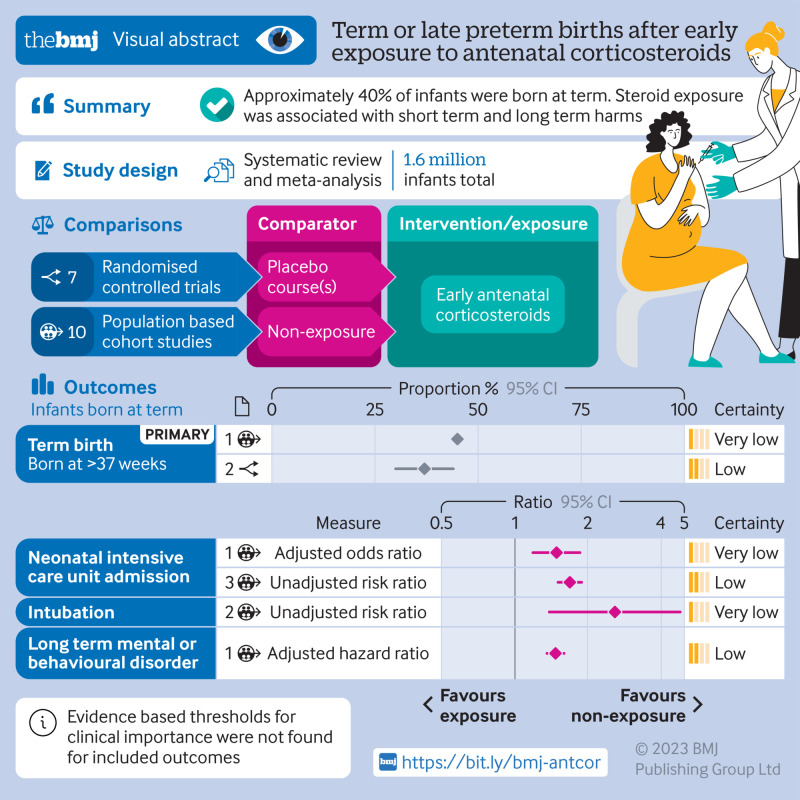
Of 14 799 records, the reviewers screened 8815 non-duplicate titles and abstracts and assessed 713 full text articles. Seven randomised controlled trials and 10 population based cohort studies (1.6 million infants total) were included. In randomised controlled trials and population based data, ∼40% of infants with early exposure to antenatal corticosteroids were born at term (low or very low certainty). Among children born at term, early exposure to antenatal corticosteroids versus no exposure was associated with increased risks of admission to neonatal intensive care (adjusted odds ratio 1.49, 95% confidence interval 1.19 to 1.86, one study, 5330 infants, very low certainty; unadjusted relative risk 1.69, 95% confidence interval 1.51 to 1.89, three studies, 1 176 022 infants, I2=58%, τ2=0.01, low certainty), intubation (unadjusted relative risk 2.59, 1.39 to 4.81, absolute effect 7 more per 1000, 95% confidence interval from 2 more to 16 more, one study, 8076 infants, very low certainty, one study, 8076 infants, very low certainty), reduced head circumference (adjusted mean difference −0.21, 95% confidence interval −0.29 to −0.13, one study, 183 325 infants, low certainty), and any long term neurodevelopmental or behavioural disorder in population based studies (eg, any neurodevelopmental or behavioural disorder in children born at term, adjusted hazard ratio 1.47, 95% confidence interval 1.36 to 1.60, one study, 641 487 children, low certainty).
Conclusions
About 40% of infants exposed to early antenatal corticosteroids were born at term, with associated adverse short term and long term outcomes (low or very low certainty), highlighting the need for caution when considering antenatal corticosteroids.
Systematic review registration
PROSPERO CRD42022360079.
Introduction
Although preterm birth (<37 weeks’ gestation) is the leading cause of death in newborns and young children,1 a single course of corticosteroids during the antenatal period is one of the most powerful interventions for reducing neonatal mortality and morbidity.2 3 4 5 6 Antenatal corticosteroids, administered for pregnancies at increased risk of preterm birth (24-34 weeks+6 days) accelerate maturation of fetal organs, especially the lungs.7 8 9 10 However, some fetuses exposed to antenatal corticosteroids exceed expectations and are born at term (≥37 weeks). Even infants born during the late preterm period (34-36 weeks) may not benefit from early antenatal corticosteroids (<33 weeks+6 days).11 The administration of antenatal corticosteroids during the late preterm period (34-36 weeks) has been commonly adopted in the US since 2016,12 but less frequently in Canada and Europe.3 13 This unnecessary exposure to antenatal corticosteroids has been an issue for more than 50 years, as the first randomised controlled trial published in 1972 found that 30% of infants exposed to early antenatal corticosteroids were born at term.14 Guidelines from the UK4 and North America,15 however, have recently recommended tracking the proportion of pregnancies with unnecessary early exposure to antenatal corticosteroids (ie, births at term). Indeed, the UK guideline advocates that the proportion of infants born at term after early exposure to antenatal corticosteroids should be ideally 0%.4 The resulting balancing metric can “discourage clinicians from overusing antenatal corticosteroids” and support clinicians in critically assessing those pregnancies that should receive antenatal corticosteroids.15 In the past, it may have been incentivised for “providers to use antenatal corticosteroids liberally.”15
Literature based on animal studies has shown that exposure to antenatal corticosteroids is associated with altered fetal brain development, including a 15% reduction in cortical surface16 17; reduced neurons in the hippocampus,18 an area involved in memory and learning; loss of microtubule associated proteins in the frontal neocortex19; reduced neuronal plasticity20; and impaired regulation of the hypothalamic-pituitary-adrenal axis.21 22 More recently is growing about the long term neurodevelopmental risks to infants born at term after exposure to antenatal corticosteroids.11 Also, long term cardiovascular, renal, and metabolic impacts have been noted in both animals23 24 and humans.25 26 27 28 29 30
We conducted a systematic review and meta-analysis to understand the proportions of infants exposed to early antenatal corticosteroids who were born at term, late preterm, or term/late preterm combined, and short term and long term outcomes, and outcomes in pregnant people.
Methods
The systematic review was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (version 6.1)31 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.32 Our protocol was published in the PROSPERO registry (CRD42022360079).
Data sources
After consultation with a research librarian, we searched eight databases from 1 January 2000 to 1 February 2023: Ovid Medline, Ovid Embase, PsycInfo, Cochrane CENTRAL, Cumulative Index of Nursing and Allied Health Literature, Web of Science, ClinicalTrials.gov, and the first 275 results on Google Scholar (see supplementary eAppendix 1). Also, we reviewed references of screened studies for additional records.
Eligibility
We considered studies that included exposure to antenatal corticosteroids defined as “early exposure,” specifically occurring before 34 weeks+6 days of gestation (an upper limit in recent clinical practice guidelines)13 33 for infants born at term (≥37 weeks), or before 33 weeks+6 days for infants born either late preterm (34-36 weeks) or term/late preterm (combined from ≥34 weeks). Both gestational periods were considered as “mistimed” exposures to antenatal corticosteroids. Outcomes for infants born at term, late preterm, or term/late preterm combined were presented separately. We excluded studies in which antenatal corticosteroids were administered during the late preterm period as we focused on mistimed steroids, not steroids administered according to the criteria of the Antenatal Late Preterm Steroids (ALPS) randomised controlled trial (34-36 weeks),34 a practice widely adopted in the US,12 although less frequently in Canada and Europe.3 13 Unless studies only considered singleton pregnancies, we excluded studies that reported the proportion of infants born at term or late preterm but not the number because the proportion of infants born at term could not be calculated. Supplementary eAppendix 2 lists the excluded studies and reasons for exclusion. We included studies that captured births in 2000 or later, because of relevance to current antenatal and postnatal care. No language restrictions were applied.
Our primary outcome was the proportion of infants born at term (≥37 weeks, including post-term), with early exposure to antenatal corticosteroids. Secondary outcomes included the proportions of infants born late preterm (34-36 weeks) or term/late preterm (combined from ≥34 weeks) and the short term and long term outcomes versus no exposure for all three gestational time points (see supplementary eAppendix 3).
We considered randomised controlled trials and only population based cohort studies to reduce selection bias. Population based cohort studies were defined as ones including either all births in a particular geographical area or a sample representative of all births in a predefined population.35 36 We excluded studies that included exclusively populations of infants in neonatal intensive care units as they underrepresent term or late preterm births. We also excluded other observational study designs and other publication types as they would not include or represent all births within an area (to the same degree as population based cohort studies), which is important when generalising results to the overall population.37
Data screening and extraction
Two reviewers (KN and AG or KN and YW) independently screened titles and abstracts and full texts, extracted data, and assessed bias and certainty of the evidence. A piloted data extraction form was used to collect data on the characteristics of study populations, details of exposure to antenatal corticosteroids, and outcomes. For studies with overlapping populations for a specific outcome, we evaluated the largest cohort. Reviewers resolved discrepancies through discussion, with an additional reviewer (SDM) involved as necessary.
We assessed the risk of bias for population based studies using an outcome specific modified Newcastle-Ottawa scale (for proportions, short term outcomes, and long term outcomes a maximum of 13, 12, and 13 stars, respectively, could be awarded based on confounders and covariates, see supplementary eAppendix 4).38 The Newcastle-Ottawa scale was selected for its ability to distinguish variations in quality based on number of confounders and covariates38 and its reliability compared with the ROBINS-I (Risk Of Bias In Non-randomised Studies - of Interventions) tool.39 For randomised controlled trials, we used the Cochrane risk of bias (RoB) 2.0 tool.31 We utilised the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool to assess certainty of evidence as high, moderate, low, or very low based on the GRADE domains (risk of bias, inconsistency, imprecision, indirectness, publication bias, and other considerations).40
Data analysis and synthesis
We analysed randomised and non-randomised data separately. The proportion of infants born at term after early exposure to antenatal corticosteroids was defined as the number of infants born at term divided by the number of infants exposed early to antenatal corticosteroids. Adjusted and unadjusted data were analysed separately, with emphasis on adjusted data in population based cohort studies. When feasible, we conducted a random effects meta-analysis for weighted proportions41 and short term or long term outcomes.31 We determined risk ratios, odds ratios, and mean differences, along with corresponding 95% confidence intervals. A two sided P value of <0.05 was considered statistically significant. We used the I2 and τ2 statistics to determine the total study variability in effect estimates and the between study variability, respectively.31 Pooled weighted proportions (using inverse variance) were calculated in R version 4.0.3 (Meta Package; R Foundation for Statistical Computing)42; other outcomes were estimated using Review Manager version 5.4.1 (Cochrane Community, UK).43
We planned subgroup analyses to compare outcomes between a specified gestational age at exposure to antenatal corticosteroids versus an unspecified one (assuming that as is typical according to guidelines, exposure to antenatal corticosteroids occurred “early”), betamethasone versus dexamethasone, male versus female infant, and a single course (either two doses 12 mg betamethasone 24 hours apart or four doses 6 mg dexamethasone 12 hours apart)4 versus multiple courses of early antenatal corticosteroids. We conducted post hoc sensitivity analyses to understand purely population based data that included all births—that is, we excluded samples that were representative of the area but did not include all births.
Patient and public involvement
No patients or members of the public were involved in the design, conduct, or reporting of the study, or in the dissemination of findings. During their clinical routine, however, several of the authors have regular contact with pregnant patients during which the benefits and potential harms of antenatal corticosteroids are commonly discussed. The experiences from these interactions have been taken into account during the planning, conduct, and reporting of this systematic review. In addition, the study was not funded, limiting our ability to adequately reimburse pregnant patients for their input.
Results
Of 14 799 records (excluding 5984 duplicates) identified, we assessed 8815 titles and abstracts and 713 full text articles. Seven 17 studies met the inclusion criteria: 10 population based studies with >1 663 450 children and seven randomised controlled trials with 4315 children (fig 1, table 1, and table 2).44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 The reviewers were in agreement on 98% of the included and excluded articles. All the population based studies and all except two of the randomised controlled trials were from high income countries (Australia, Canada, Finland, France, New Zealand, and the US),44 45 46 47 48 49 50 51 52 53 54 56 58 59 60 one randomised controlled trial was from a middle income country (Iran),57 and one international randomised controlled trial was from low and middle income countries (Bangladesh, India, Kenya, Nigeria, and Pakistan).55
Fig 1.
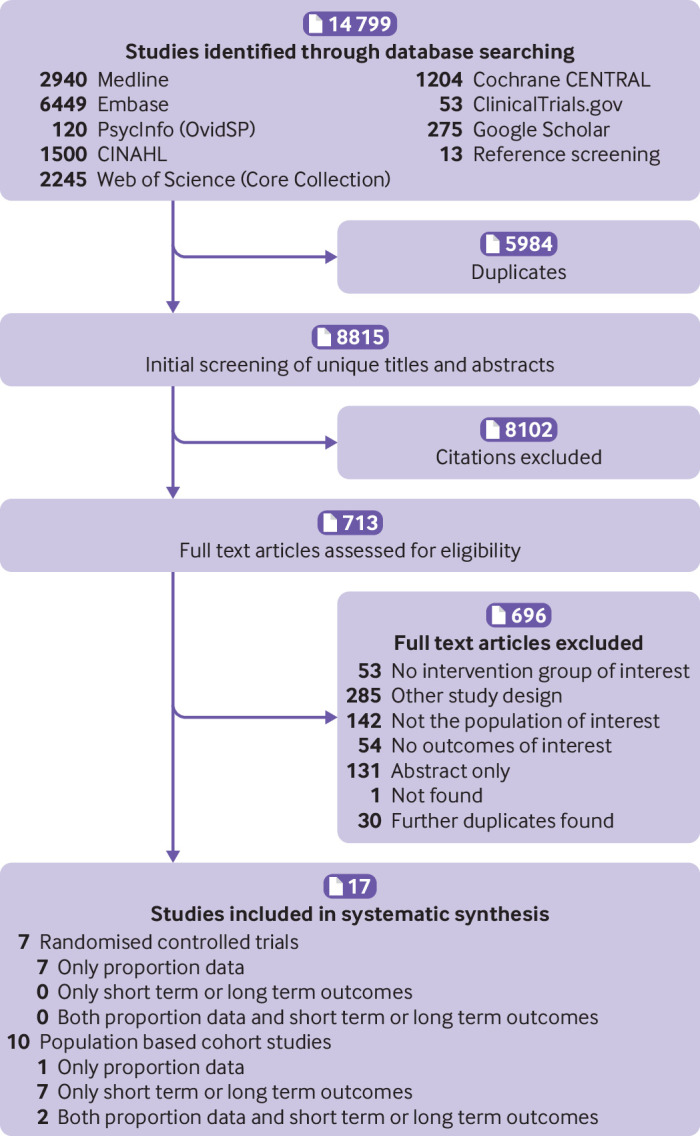
Flow diagram of study selection
Table 1.
Characteristics of included population based studies in a systematic review and meta-analysis on term, late preterm, and term/late preterm births after early exposure to antenatal corticosteroids
| Studies by gestation and outcomes (country) | Inclusion criteria | ACS treatment (type of ACS) | Risk of bias assessment (NOS): score (total score) |
|---|---|---|---|
| Born at term | |||
| Proportions of infants: | |||
| Raikkonen 2020 (Finland)44 | • Singleton live births between 2006 and 2017 • Infants with maternal and child identification codes for data linkage and data on gestational age • Infants survived until end of first year of life |
NS*† | 7 (13) |
| Short term outcomes: | |||
| Diguisto 2020 (France)45‡ | • Singleton or multiple births between 2000 and 2013 • Gestational age <34 weeks when ACS were administered |
NS* | 10§; 9¶; 8**; 7¶ (12) |
| McKinzie 2021 (USA)46†† | • Singleton births between 2012 and 2019 | NS (betamethasone)* | 8§; 7** (12) |
| Rodriguez 2019 (Finland)47 | • Singleton births with gestational age >24 weeks between 2006 and 2010 | NS* | 7§** (12) |
| Melamed 2019 (Canada)48 | • Singleton live births between 2006 and 2011 | NS*† | 8** (12) |
| Raikkonen 2022 (Finland)49 | • Singleton live births between 2006 and 2017 • Infants with maternal and child identification codes for data linkage and data on gestational age • Infants survived until end of first year of life |
NS*† | 7** (12) |
| Long term outcomes: | |||
| Melamed 2019 (Canada)48 | • Singleton live births between 2006 and 2011 | NS*† | 10§; 8** (13) |
| Raikkonen 2022 (Finland)49 | • Singleton live births between 2006 and 2017 • Infants with maternal and child identification codes for data linkage and data on gestational age • Infants survived until end of first year of life |
NS*† | 9§; 7** (13) |
| Raikkonen 2020 (Finland)44 | • Singleton live births between 2006 and 2017 • Infants with maternal and child identification codes for data linkage and data on gestational age • Infants survived until end of first year of life |
NS*† | 9§; 7** (13) |
| Osteen 2022 (USA)50†† | • Singleton births between 2012 and 2014 • Pregnancy at risk of preterm labour • Children ≥5 years old with encounter in electronic medical record after 5th birthday |
NS (betamethasone or dexamethasone)a | 8§; 7** (13) |
| Born late preterm | |||
| Proportion of infants: | |||
| Malloy 2012 (USA)51 | • Singleton or multiple births with infants between 22 and 36 weeks’ gestational age and born in 2007 • Only included records with information on ACS use |
NS* | 6 (13) |
| Short term outcomes: | |||
| Malloy 2012 (USA)51 | • Singleton or multiple births with infants between 22 and 36 weeks’ gestational age and born in 2007 • Only included records with information on ACS use |
NS* | 7§; 6** (12) |
| Long term outcomes: | |||
| Aviram 2022 (Canada)52 | • Singleton live births between 2006 and 2011 • Gestational age <34 weeks when ACS were administered |
NS† | 10‡; 8** (13) |
| Born at term/late preterm (combined) | |||
| Proportion of infants: | |||
| Razaz 2015 (Canada)53 | • Singleton or multiple live births between 1988 to 2012 | 1 course or 1 dose (NS)* | 6 (13) |
| Short term outcomes: | |||
| Rodriguez 2019 (Finland)47 | • Singleton births with gestational age >24 weeks between 2006 and 2010 | NS* | 7§** (12) |
ACS=antenatal corticosteroids; NOS=Newcastle-Ottawa scale; NS=not specified.
Newcastle-Ottawa scale used for population based studies: A maximum of 12 points (star system) could be awarded when assessing risk of bias for short term outcomes, a maximum of 13 points (stars) could be awarded when assessing risk of bias for long term outcomes or proportion outcomes.
Although gestational age when ACS were administered is not specified, definition of early exposure to ACS is likely met. Early exposure to ACS was defined as ACS administered <34 weeks+6 days of gestation for infants born at term, and <33 weeks+6 days for infants born late preterm or term/late preterm (combined).
Number of courses of ACS was not specified but a single course is likely provided given reference to guideline recommendations.
Sample representative of population: All live term births in 175 French maternity units in every region of France.
Risk of bias assessment for adjusted short term or long term outcomes. Studies are ordered by risk of bias assessment for adjusted outcomes when available, but if not available are ordered by risk of bias assessment for unadjusted outcomes.
Risk of bias assessments differ based on the outcome. Head circumference has fewer points awarded compared with the other outcomes.
Risk of bias assessment for unadjusted short term or long term outcomes.
Sample representative of population: Singleton term births within Indiana University Health hospitals.
Table 2.
Characteristics of included randomised controlled trials in a systematic review and meta-analysis on term, late preterm, and term/late preterm births after early exposure to antenatal corticosteroids
| Studies by gestation and outcomes (country) | Inclusion criteria | ACS treatment (type of ACS) | Risk of bias assessment (Cochrane RoB*) in order R, D, Mi, Me, S, O |
|---|---|---|---|
| Proportion of infants | |||
| Born at term: | |||
| Schmitz 2022 (France)54 | • Singleton pregnancy at risk of preterm birth between 2017 and 2019 • Gestational age <32 weeks at trial entry • Received 1 dose of ACS before trial entry • Age ≥18 years |
1 course betamethasone v placebo | +, + ,+, +, + , + |
| WHO ACTION-1 Trial Collaborators 2020 (international)55 | • Singleton or multiple pregnancy between 2017 and 2019 • Gestational age 26-33+6 weeks at trial entry • No previous ACS exposure but planned or expected birth ≤48 hours |
1 course dexamethasone with one potential repeat course if birth did not occur after 7 days v placebo | +, +, +, +, +, + |
| Crowther 2006 (Australia and New Zealand)56 | • Single, twin, or triplet pregnancy between 1998 and 2004 • Gestational age <32 weeks at trial entry • Received 1 complete course of ACS ≥7 days previously and remained at risk of preterm birth • No contraindication to further corticosteroids |
Repeat dose betamethasone v placebo | + ,+ ,+, + ,+ , + |
| Danesh 2012 (Iran)57 | • Singleton pregnancy at risk of preterm birth in 2011 • Gestational age 24-34 weeks at trial entry • 16-45 years of age at high risk of preterm labour with intact membranes or PPROM • Residence in Isfahan, low Bishop Score (≤5), non-smoker, and hospital admission ≥3 days |
1 course betamethasone v 1 course dexamethasone | +, + ,+, + ,?, ? |
| Born late preterm: | |||
| Schmitz 2022 (France)54 | • Singleton pregnancy at risk of preterm birth between 2017 and 2019 • Gestational age <32 weeks at trial entry • Received 1 dose of ACS before trial entry • Age ≥18 years |
1 course betamethasone v placebo | + ,+ ,+, +, +, + |
| Crowther 2006 (Australia and New Zealand)56 | • Single, twin, or triplet pregnancy between 1998 and 2004 • Gestational age <32 weeks at trial entry • Received 1 complete course of ACS ≥7 days previously and remained at risk of preterm birth • No contraindication to further corticosteroids |
Repeat dose betamethasone v placebo | + ,+ ,+, + ,+, + |
| Peltoniemi 2007 (Finland)58 | • Singleton or multiple pregnancy between 2001 and 2005 • Gestational age <34 weeks at trial entry • Received 1 complete course ≥7 days before trial entry and preterm birth was imminent • High risk of spontaneous delivery <48 hours indicated by cervical opening ≥3 cm, regular contractions, or PPROM |
1 repeat dose betamethasone v placebo | +,+, +, +, ?, ? |
| Born at term/late preterm (combined): | |||
| Schmitz 2022 (France)54 | • Singleton pregnancy at risk of preterm birth between 2017 and 2019 • Gestational age <32 weeks at trial entry • Received 1 dose of ACS before trial entry • Age ≥18 years |
1 course betamethasone v placebo | +, +, +, +, +, + |
| McEvoy 2010 (USA)59 | • Singleton or twin pregnancy between 2001 and 2007 • Gestational age <34 weeks at trial entry • Received 1 complete course of ACS ≥14 days previously and remained at risk of preterm birth |
1 repeat course betamethasone v placebo | +, +, +, +, +, + |
| Garite 2009 (USA)60 | • Singleton or twin pregnancy between 2003 and 2008 • Gestational age 25-32+6 weeks at trial entry • Received 1 complete course of ACS ≥14 days before enrolment and <30 weeks • Recurrent or continued risk of preterm birth within next 7 days |
1 repeat course betamethasone or dexamethasone v placebo | +, +, +, +, +, + |
| Crowther 2006 (Australia and New Zealand)56 | • Single, twin, or triplet pregnancy between 1998 and 2004 • Gestational age <32 weeks at trial entry • Received 1 complete course of ACS ≥7 days ago and remained at risk of preterm birth • No contraindication to further corticosteroids |
Repeat dose betamethasone v placebo | +, +, +, +, +, + |
ACS=antenatal corticosteroids; PPROM=preterm premature rupture of membranes; RoB=risk of bias; WHO=World Health Organization.
Cochrane RoB 2 used for randomised controlled trials: +=low risk of bias; −=high risk of bias; ?=some concerns about bias; D=bias due to deviations from intended interventions; Me=bias in measurement of outcome; Mi=bias due to missing outcome data; O=overall risk in bias; R=bias arising from randomisation process; S=bias in selection of reported results.
Most of the study outcomes were at a low risk of bias but had low or very low levels of certainty based on GRADE. Ratings (star system) on the modified Newcastle-Ottawa scale ranged from 6 or 7 out of 13 for proportion outcomes, 6-10 out of 12 for short term outcomes, and 7-10 out of 13 for long term outcomes (see supplementary eTable 1 and eAppendix 4). Five of the randomised controlled trials were rated as low risk of bias for all included proportion outcomes,54 55 56 59 60 whereas two had concerns about selective reporting of results (table 2).57 58
Outcomes for infants born at term
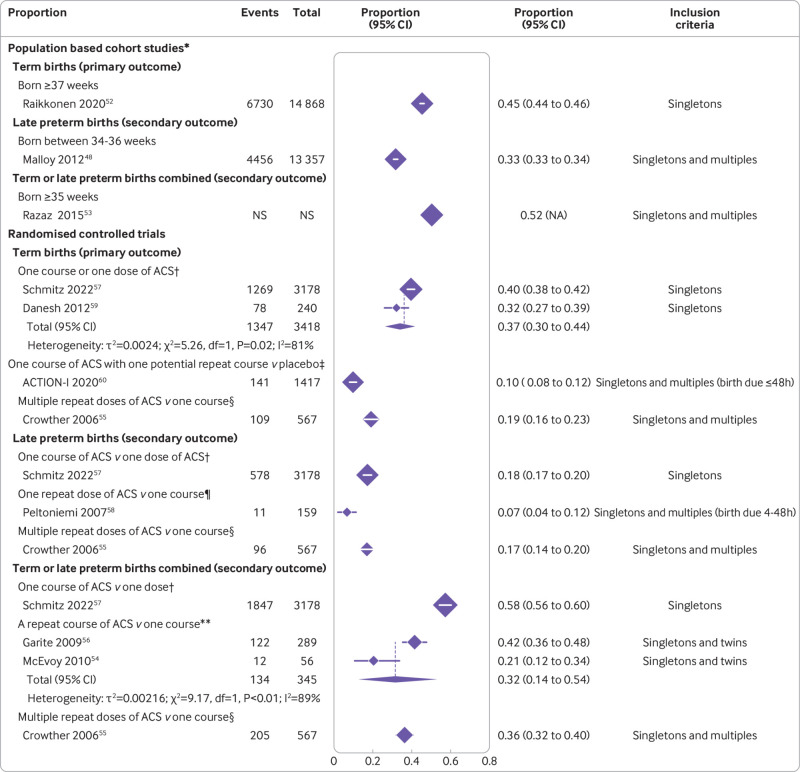
In the population based studies, 45% of infants with early exposure to antenatal corticosteroids were born at term (95% confidence interval 44% to 46%, one study, including only singletons, 14 868 infants, very low certainty) (table 3 and fig 2).44 In the randomised controlled trials, 37% of singletons with early exposure to antenatal corticosteroids were born at term (30% to 44%, two randomised controlled trials with a single course of antenatal corticosteroids, 3418 infants, I2=81%, τ2=0.002, low certainty) (table 3 and fig 2).54 57 Studies including only singletons had higher rates of term births than studies including twins or triplets; the last two populations have high rates of prematurity. In randomised controlled trials that considered repeat courses of early antenatal corticosteroids, the proportion of infants born at term was lower: 10% (8% to 12%, one randomised controlled trial including singletons, twins, and triplets or more with birth expected in <48 hours, 1417 infants, moderate certainty)55; and 19% (16% to 23%, one randomised controlled trial including multiple repeat doses of antenatal corticosteroids for singletons, twins, or triplets at continued risk of preterm birth ≥7 days after the last dose, 567 infants, moderate certainty), fig 2).56
Table 3.
Key proportion, short term, outcomes, and long term outcomes in a systematic review and meta-analysis on term, late preterm, and term/late preterm births after early exposure to antenatal corticosteroids
| Outcome by effect measure and size (95% CI) [absolute effect (95% CI)]* | Study design (No of studies) | ACS group (No/Total No or Total No) | Unexposed group (No/Total No or Total No) | GRADE certainty of evidence |
|---|---|---|---|---|
| Proportion outcomes | ||||
| Born at term: | ||||
| Proportion (%): 45 (44 to 46)† | Population based cohort (n=1)49 | 6730/14 868 | NA | Very low |
| Proportion (%): 37 (30 to 44)† | Randomised controlled trials (n=2)54 57 | 1347/3418 | NA | Low |
| Born late preterm: | ||||
| Proportion (%): 33 (33 to 34)‡ | Population based cohort (n=1)51 | 4456/13 357 | NA | Very low |
| Proportion (%): 18 (17 to 20)† | Randomised controlled trial (n=1)54 | 578/3178 | NA | High |
| Born at term/late preterm (combined): | ||||
| Proportion (%): 52 (NA)‡ | Population based cohort (n=1)53 | NS | NA | Very low |
| Proportion (%): 58 (56 to 60)† | Randomised controlled trial (n=1)54 | 1847/3178 | NA | High |
| NICU admission | ||||
| Born at term: | ||||
| Adjusted odds ratio: 1.49 (1.19 to 1.86)§ | Population based cohort (n=1)46 | 179/1459 | 247/3871 | Very low |
| Unadjusted relative risk: 1.69 (1.51 to 1.89)¶ [36 more per 1000 (27 more to 47 more per 1000)] | Population based cohort (n=3)46 48 49 | 1204/13 612 | 61 156/1 162 410 | Low |
| Born late preterm: | ||||
| Unadjusted relative risk: 2.25 (1.37 to 3.71) [234 more per 1000 (69 more to 508 more per 1000)] | Population based cohort (n=2)51 52 | 3330/7145 | 36 926/197 088 | Very low |
| Born at term/late preterm (35+0-37+6 weeks) (combined): | ||||
| Unadjusted relative risk: 1.50 (1.35 to 1.66) [140 more per 1000 (98 more to 185 more per 1000)] | Population based cohort (n=1)47 | 288/685 | 959/3425 | Low |
| Born at term/late preterm (≥35 weeks) (combined): | ||||
| Unadjusted relative risk: 1.48 (1.35 to 1.64) [67 more per 1000 (49 more to 89 more per 1000)] | Population based cohort (n=1)47 | 419/2031 | 1411/10 155 | Low |
| Neonatal death | ||||
| Born late preterm: | ||||
| Adjusted odds ratio: 0.69 (0.47 to 1.01)** | Population based cohort (n=1)51 | 29/4456 | 602/174 109 | Very low for both results |
| Unadjusted relative risk: 1.88 (1.30 to 2.73) [3 more per 1000 (1 more to 6 more per 1000)] | ||||
| Intubation | ||||
| Born at term: | ||||
| Unadjusted relative risk: 2.59 (1.39 to 4.81)¶ [7 more per 1000 (2 more to 16 more per 1000)] | Population based cohort (n=1)47 | 15/1346 | 29/6730 | Very low |
| Born at term/late preterm (35+0-37+6 weeks) (combined): | ||||
| Unadjusted relative risk: 2.02 (1.25 to 3.25) [17 more per 1000 (4 more to 37 more per 1000)] | Population based cohort (n=1)47 | 23/685 | 57/3425 | Low |
| Born at term/late preterm (>35 weeks) (combined): | ||||
| Unadjusted relative risk: 2.21 (1.51 to 3.23) [10 more per 1000 (4 more to 19 more per 1000)] | Population based cohort (n=1)47 | 38/2031 | 86/10 155 | Low |
| Head circumference at birth (cm) | ||||
| Born at term: | ||||
| Adjusted mean difference: −0.21 (−0.29 to −0.13)†† | Population based cohort (n=1)47 | 1279 | 182 046 | Low |
| Unadjusted mean difference: −0.25 (−0.31 to −0.19) | Population based cohort (n=1)47 | 1730 | 236 117 | Very low |
| Born at term/late preterm (35+0-37+6 weeks) (combined): | ||||
| Adjusted mean difference: -0.43 (−0.55 to −0.31)†† | Population based cohort (n=1)47 | 647 | 14 070 | Low |
| Unadjusted mean difference: -0.47 (−0.59 to −0.35) | Population based cohort (n=1)47 | 849 | 18 217 | Low |
| Any long term mental or behavioural disorder | ||||
| Born at term: | ||||
| Adjusted hazard ratio: 1.47 (1.36 to 1.60)‡‡ | Population based cohort (n=1)44 | 598/6730 | 40 051/634 757 | Low |
| Born late preterm: | ||||
| Adjusted hazard ratio: 1.12 (1.05 to 1.20)§§ | Population based cohort (n=1)52 | 1156/2689 | 8581/22 979 | Low |
ACS=antenatal corticosteroids; NA=not applicable; NICU=neonatal intensive care unit; NS=not specified.
Bold font represents statistically significant harm in infants exposed to corticosteroids versus non-exposed.
Absolute effects reported when feasible (ie, when absolute values in each group were available).
Includes only singletons exposed to a single course or dose of ACS.
Includes singletons and multiples (eg, twins) exposed to a single course of ACS.
Adjusted for maternal age, race, insurance status, diabetes mellitus, asthma, hypertensive disorder of pregnancy, and estimated gestational age at delivery.
Includes infants born post term (≥42 weeks of gestation).
Adjusted for: maternal age, education, gravidity, race, singleton pregnancy, mode of delivery, infant sex, birth weight, NICU admission, surfactant administration, and assisted ventilation.
Adjusted for maternal age, social economic status, cohabitation, height, pre-pregnancy weight, and pre-pregnancy body mass index; number of previous pregnancies, miscarriages, induced abortions, and ectopic pregnancies; medical risk factors (ovum donation, insemination, glucose test performed, pathological glucose test, insulin treatment); self-reported smoking; admission to hospital for high blood pressure; and infant sex.
Adjusted for maternal age, parity, mode of delivery, smoking during pregnancy, pre-pregnancy body mass index, premature rupture of membranes, (international statistical classification of diseases and related health problems, 10th revision code O42), gestational diabetes, hypertension in pregnancy, any lifetime mental disorder diagnosis, child sex, Apgar score, admission to NICU, birth weight, and gestational age at birth.
Suspected neurocognitive disorder; adjusted for maternal age, gestational age at birth, parity, income, chronic hypertension, pregestational diabetes, hypertensive complications, gestational diabetes, preterm premature rupture of membranes, induction of labour, mode of delivery, infant sex, birth weight <10th percentile, five minute Apgar score <7, resuscitation at birth, and admission to NICU.
Fig 2.
Proportions of infants born at term, late preterm, or term/late preterm combined from population based cohort studies and randomised controlled trials. *Likely exposed to a single course of ACS without ACS administered late preterm. †Proportion born after a single course or single dose of ACS. ‡Proportion born after a single course of ACS with one potential repeat course. §Proportion born after multiple repeat doses of ACS. ¶Proportion born after a single additional dose of ACS. **Proportion born after a repeat course of ACS. ACS=antenatal corticosteroids; CI=confidence interval; df=degrees of freedom; IV=inverse variance; multiples=twins, triplets, or higher order; NA=not applicable; NS=not specified
Early exposure to antenatal corticosteroids in infants born at term was associated with some adverse adjusted secondary short term outcomes in population based studies, including admission to a neonatal intensive care unit (adjusted odds ratio 1.49, 95% confidence interval 1.19 to 1.86, one study, 5330 infants, very low certainty, table 3),46 being small for gestational age (1.78, 1.48 to 2.14, one study, 5330 infants, low certainty, see supplementary eFigure 1),46 and reduced anthropometric measurements at birth (eg, reduced head circumference, adjusted mean difference −0.21 cm, 95% confidence interval −0.29 to −0.13 cm, one study, 183 325 infants, low certainty, table 3 and fig 1, also see supplementary eFigure 1).47 Table 3 highlights some key outcomes that were determined to be important based on clinical expertise of the team, which involved maternal-fetal medicine (high risk obstetrics) and neonatology. Most other short term outcomes were either non-significant or unadjusted but significant, such as admission to the neonatal intensive care unit (unadjusted relative risk 1.69, 95% confidence interval 1.51 to 1.89, absolute effect 36 more per 1000 (95% confidence interval 27 more to 47 more per 1000), three studies, 1 176 022 infants, I2=58%, τ2=0.01, low certainty, table 3),46 48 49 intubation (1.77, 0.82 to 3.78, absolute effect 4 more per 1000 (1 fewer to 15 more per 1000), two studies, 13 406 infants, I2=65%, τ2=0.20, very low certainty, table 3),46 47 hypoglycaemia requiring treatment (2.12, 1.63 to 2.76, absolute effect 35 more per 1000 (20 more to 55 more per 1000), one study, 5330 infants, low certainty, see supplementary eFigure 1),46 and antibiotic treatment (1.48, 1.16 to 1.90, absolute effect 18 more per 1000 (6 more to 34 more per 1000), one study, 8076 infants, very low certainty, see supplementary eFigure 1).47 In a sensitivity analysis (ie, keeping studies that included all births in an area), in infants born at term after early exposure to antenatal corticosteroids, the risk of intubation was increased (2.59, 1.39 to 4.81, absolute effect 7 more per 1000 (2 more to 16 more per 1000), one study, 8076 infants, very low certainty, see supplementary eFigure 2).47 Prespecified subgroup analyses did not explain heterogeneity (supplementary eFigure 2). Randomised controlled trial’s lacked data on short term outcomes stratified for term birth.
From term born infants, exposure to early antenatal corticosteroids versus non-exposure was associated with adverse long term outcomes in four population based studies (1 174 248 infants, supplementary eFigure 1),44 48 49 50 including 19 out of 26 adjusted long term outcomes44 48 49 50 (eg, significantly increased risk of any mental or behavioural disorder in children born at term, adjusted hazard ratio 1.47, 95% confidence interval 1.36 to 1.60, one study, 641 487 children, low certainty, table 3, supplementary eFigure 1).44 Regarding other long term outcomes for children born at term in the population based studies, data were unadjusted (very low certainty, supplementary eFigure 1), or for the randomised controlled trials were lacking.
Outcomes for infants born late preterm
About one in three infants exposed to early antenatal corticosteroids was born late preterm between 34 and 36 weeks (33% (95% confidence interval 33% to 34%, one population based study of singletons or multiples (eg, twins), 13 357 infants, very low certainty), table 3, fig 2).51 In the randomised controlled trial data, 18% of infants exposed to early antenatal corticosteroids were born late preterm (95% confidence interval 17% to 20%, one randomised controlled trial of a single course versus a single dose of antenatal corticosteroids in singletons alone, 3178 infants, high certainty, table 3, fig 2).54 In the randomised controlled trial data of repeat antenatal corticosteroids, the proportion of infants born late preterm varied between 7% (95% confidence interval 4% to 12%, one randomised controlled trial of a single repeat dose of antenatal corticosteroids in singletons, twins, or triplets expected to be born in <48 hours, 159 infants, low certainty),58 and 17% (14% to 20%, one randomised controlled trial of multiple repeat doses of antenatal corticosteroids in singletons, twins, or triplets, 567 infants, moderate certainty), fig 2).56
After early exposure to antenatal corticosteroids and late preterm birth, there was a trend towards a reduction in neonatal death (adjusted odds ratio 0.69, 95% confidence interval 0.47 to 1.01, one study, 178 565 infants, very low certainty, table 3, supplementary eFigure 3).51 Conversely, the same study also showed an increased risk of admission to the neonatal intensive care unit51 (unadjusted relative risk 2.25, 95% confidence interval 1.37 to 3.71, absolute effect 234 more per 1000, 95% confidence interval from 69 more to 508 more per 1000, two studies, 204 233 infants, I2=100%, τ2=0.13, very low certainty, table 3, supplementary eFigure 4; no adjusted data available).51 52
For children born late preterm (34-36 weeks), early exposure to antenatal corticosteroids was associated with adverse long term outcomes (neurocognitive disorders, adjusted hazard ratio 1.12, 95% confidence interval 1.05 to 1.20, 25 668 children, low certainty, as well as visual or audiometry testing, table 3, supplementary eFigure 3).52
Outcomes for infants born at term/late preterm (combined)
More than half of infants with early exposure to antenatal corticosteroids were born at term/late preterm (≥35 weeks) combined (52%, one population based study of singletons, twins, or triplets, very low certainty, table 3, fig 2)53; similarly, in the randomised controlled trial data, 58% of singletons were born at term/late preterm combined (95% confidence interval 56% to 60%, one randomised controlled trial of a single course versus single dose of antenatal corticosteroids, 3178 infants, high certainty, table 3, fig 2).54 In the randomised controlled trial data of repeat courses of antenatal corticosteroids versus one course, one third of singletons and twins were born at term/late preterm combined (32%, 95% confidence interval 14% to 54%, two randomised controlled trials of a single repeat course, 345 infants, I2=89%, τ2=0.02, very low certainty59 60; 36%, 32% to 40%, one randomised controlled trial of multiple repeat doses, 567 infants, moderate certainty, fig 2).56
For infants born 35-37 weeks+6 days, one population based study found some adverse adjusted short term outcomes associated with early exposure to antenatal corticosteroids versus non-exposure (including reduced head circumference, adjusted mean difference −0.43 cm, 95% confidence interval −0.55 to −0.31 cm, one study, 14 717 infants, low certainty, table 3, supplementary eFigure 5).47 For infants born at term/late preterm (≥35 weeks) combined, the risk of admission to a neonatal intensive care unit increased significantly (unadjusted relative risk 1.48, 95% confidence interval 1.35 to 1.64, absolute effect 67 more per 1000, 95% confidence interval from 49 more to 89 more per 1000, one study, 12 186 infants, low certainty, table 3, supplementary eFigure 5).47 The findings for other adverse short term outcomes were either non-significant or unadjusted but significant, of which some are important (eg, ≥35 weeks: intubation, unadjusted relative risk 2.21, 95% confidence interval 1.51 to 3.23, absolute effect 10 more per 1000, 95% confidence interval from 4 more to 19 more per 1000, one study, 12 186 infants, low certainty, table 3 47; blood transfusion, 5.00, 1.76 to 14.24, absolute effect 3 more per 1000, 95% confidence interval from 1 more to 9 more per 1000, one study, 12 186 infants, low certainty47; and antibiotic treatment, 1.70, 1.47 to 1.98, absolute effect 43 more per 1000, 95% confidence interval from 29 more to 60 more per 1000, one study, 12 186 infants, low certainty47; supplementary eFigure 5). None of the randomised controlled trials had data on short term or long term outcomes stratified for birth at term/late preterm combined. In subgroup analysis by infant sex, both male and female infants exposed to early antenatal corticosteroids versus non-exposure and born at term/late preterm combined (35-37 weeks+6 days) showed lower five minute Apgar scores and an increased risk of hospital admission beyond the first seven days of life (one population based study, supplementary eFigure 6).47
Outcomes in pregnant people
No studies reported on prespecified outcomes in pregnant people.
Discussion
In our systematic review, we found that approximately 40% of infants with early exposure to antenatal corticosteroids were born at term (low/very low certainty), with increased risks of neonatal intensive care admission, intubation, hypoglycaemia requiring treatment, smaller head circumference, and adverse long term neurodevelopmental outcomes (low/very low certainty). Similar outcomes were noted for the more than half of infants with early exposure to antenatal corticosteroids who were born at term/late preterm combined. For infants born very prematurely, antenatal steroids save potentially lives and severe morbidity but as pregnancy progresses to term, the benefits shift to risks. Furthermore, no studies reported on prespecified outcomes in pregnant people.
Meaning of the study
We explored biologically plausible outcomes, as the current dosing of antenatal corticosteroids leads to high levels that are undesirable owing to potential toxicity.11 Glucocorticoids can affect up to 20% of the transcriptome, influencing fetal development and programming.61 Infants born at term or late preterm, in addition to having received antenatal corticosteroids, are exposed to a surge of endogenous steroids.62 In line with our finding of increased admission to neonatal intensive care, the 2006 Cochrane systematic review noted that infants randomly allocated to early antenatal corticosteroids but who were born after 36 weeks had a relative risk of death of 3.25 compared with those allocated to placebo (95% confidence interval 0.99 to 10.66, two studies, 498 infants,63 these data have not been updated in subsequent Cochrane reviews). The ALPS randomised controlled trial, examining steroids administered between 34 and 36 gestational weeks (ie, a later gestational window than the trials in the 2006 Cochrane), found no differences in neonatal death (2 infants (0.1%) in the betamethasone group versus 0 infants in the placebo group: P=0.50).34 Infants with early exposure to antenatal corticosteroids and born at term had reduced anthropometric measurements at birth (eg, reduced head circumference); antenatal corticosteroids inhibit insulin-like growth factors and placental lactogen, which are essential for fetal growth.64 Head circumference is a surrogate measure of fetal brain growth65 and brain volume66; smaller head circumferences even within the normal range are associated with an increased risk of intellectual disability.65 Alterations of the hypothalamic-pituitary-adrenal axis, which can occur with exposure to steroids,67 regulates stress response and are associated with neurodevelopmental impairment,68 69 which we described in this systematic review. Metabolically, exposure to antenatal steroids increases glucose concentrations and resultant insulin concentrations, thereby increasing the risk of hypoglycaemia. Antenatal corticosteroids may directly result in long term adversity (as indicated by animal studies and by biological effects in humans, such as alteration to the hypothalamic-pituitary-adrenal axis); be a marker for disease severity (eg, mothers with pre-eclampsia who received early antenatal corticosteroids are more ill than those who give birth after the gestational window of antenatal corticosteroids use); or both.
The high proportions of infants born at term or late preterm after early exposure to antenatal corticosteroids underscores both the challenges in predicting preterm birth and the tradition of administering antenatal corticosteroids relatively “liberally.”15 Tracking the proportion of infants born at term after early antenatal corticosteroids can assist to “discourage clinicians from overusing antenatal corticosteroids.”15 In line with a less liberal approach to the use of antenatal corticosteroids is the fact that researchers in the disciplines of obstetrics and paediatrics are studying whether short term benefits can be maintained with lower doses of steroids.54 70
We explored factors affecting the proportions of infants born at term or late preterm after early exposure to antenatal corticosteroids. Compared with the randomised controlled trials of a single course of antenatal corticosteroids, the lower proportions of births at term, late preterm, or at term/late preterm combined in the randomised controlled trials of repeat steroids are not a sign of better timing because by definition the initial course was inopportune. Secondly, as >50% of twins are born preterm71 72 and 95% of triplets are born preterm,73 as expected the studies that included twins or triplets had lower proportions of births at term or late preterm than studies including only singletons. Finally, the two randomised controlled trials with noticeably lower rates of mistimed antenatal corticosteroids (10% of births at term55 and 7% late preterm)58 than the 40% seen in many studies had much more stringent inclusion criteria of “imminent” preterm birth within 48 hours, not reflective of most current clinical practice guidelines.
Strengths and weaknesses of this review
Our study has several strengths. We conducted a comprehensive search of eight databases, without language restrictions, to understand a clinically important matter of birth at term after the use of early antenatal corticosteroids.15 We emphasised population based studies as they reflect generalisable, real world data that best address the proportion of births at term after exposure to early antenatal corticosteroids, and this study design has lower risks for selection bias than other observational study designs.37 Although randomised controlled trial data can include more selected samples in specific settings, both population based and randomised controlled trial data yielded generally consistent results for infants born at term after exposure to early antenatal corticosteroids (∼40%) and infants born at term/late preterm combined (>50%). To decrease the impact of potential confounding in the non-randomised data, we concentrated on adjusted analyses. One included large population based cohort explored a robust way to deal with potential confounders by analysing consecutive sibling pairs (those who received antenatal corticosteroids and those who did not) and still found significant increases in adverse neurodevelopmental outcomes.44 Three included population based studies used a different robust method to address potential confounding by limiting the study population (both in those exposed to antenatal corticosteroids and in those not exposed) to pregnancies at risk of preterm labour, and still found an association between early exposure to antenatal corticosteroids and outcomes such as small for gestational age,46 birth length <5th percentile,45 and childhood weight <10th percentile.50
Limitations of our study include that compared with randomised controlled trials, population based studies are prone to bias and may have lower certainty evidence. However, gestational age at birth in randomised controlled trials would be a post-randomisation variable and thus would require considerable caution. For this reason it is possible that short term or long term outcomes from randomised controlled trials were not reported specifically for children born at term or late preterm. Thus, observational data from population based studies were required, and residual confounding cannot be ruled out. However, animal studies that have little or no confounding suggest potential toxicity with antenatal corticosteroids at similar doses to those used clinically in humans and have found many of the same impacts now beginning to be explored in human data.11 Our planned subgroup analyses of prespecified outcomes by type of steroid and infant sex could not be conducted owing to lack of data, and these are areas for future research.
Relation to other published reviews on the topic
A previous scoping review in which the literature review ended in November 2021, reported reduced anthropometric measurements and neurodevelopmental harm for infants born at term.74 Our current synthesis of long term outcomes includes additional studies49 50 and updates our previous systematic review and meta-analysis, the literature review for which ended in October 2021.75 Unlike the previous study, however, this current study focused on the proportions of infants born at term (or late preterm) after early exposure to antenatal corticosteroids and also included short term outcomes in the infants. Although the interval from administration of antenatal corticosteroids to delivery was outside the scope of our research question, a recent systematic review examining such intervals was unable to determine the optimal timing.76
Unanswered questions and future research
Further research with long term follow-up in randomised controlled trials will be critical for term or late preterm infants, and in the meantime potentially for children outside of randomised controlled trials. One study involving teacher assessment of children aged 12 years of a parent who had been randomised to antenatal corticosteroids at term gestation, found a doubling of the risk of being in the lower quartile of academic ability compared with children whose parent received placebo (8.5% v 17.7%, P=0.03).77 Hence, long term follow-up data after randomisation to early antenatal corticosteroids requires investigation as this is the time period when corticosteroids are most commonly administered.3 13 Owing to a lack of available data, we were unable to identify if specific clinical indications for antenatal corticosteroids are more likely to be associated with term births; an important area for future research.
Conclusions
In this systematic review and meta-analysis we found that about 40% of infants exposed to early antenatal corticosteroids were born at term (low or very low certainty evidence) and showed increased risks of admission to neonatal intensive care, intubation, hypoglycaemia requiring treatment, reduced head circumference, and increased neurodevelopmental or cognitive outcomes (low or very low certainty evidence). To decrease the proportions of infants exposed to steroids who are born at term, better prediction tools for preterm birth and enhanced criteria for the administration of antenatal corticosteroids may be required, along with a less liberal approach to the use of antenatal corticosteroids.15
What is already known on this topic
Antenatal steroids remain one of the most powerful interventions to reduce neonatal mortality and morbidity in infants born very early, with benefits well documented in randomised controlled trials for >50 years
Predicting preterm birth is, however, challenging—infants exposed to early antenatal corticosteroids (<34 weeks’ gestation) may be born at term (≥37 weeks), making the exposure unnecessary and a potential risk
Understanding the proportion of infants unnecessarily exposed to antenatal corticosteroids and their outcomes are important to inform clinical practice and guidelines
What this study adds
In this systematic review of both population based studies and randomised controlled trials, ∼40% of infants were born at term after exposure to early antenatal corticosteroids (low/very low certainty)
Early exposure to steroids was associated with increased risks of admission to neonatal intensive care, intubation, hypoglycaemia requiring treatment, reduced anthropometric measurements, and increased long term neurodevelopmental or cognitive risks (low/very low certainty)
Given the proportions of exposed infants being born at term after exposure to early antenatal corticosteroids and associated short term and long term concerns, caution might be warranted when using antenatal corticosteroids
Acknowledgments
We thank Jo-Anne Petropoulous (MLIS, Health Sciences Library, McMaster University) for assistance in developing the search strategies, Gabriel Shapiro (Department of Epidemiology, Biostatistics and Occupational Health, McGill University) for editorial assistance, and Jasper Bain, Raeesah Mohammed, and Rhea Varguise (all from the Department of Obstetrics and Gynaecology, McMaster University) for administrative support with this systematic review.
Web extra.
Extra material supplied by authors
Supplementary information: eTable 1, eFigures 1-6, eAppendices 1-4, and eReferences
Contributors: All authors were involved in the design of the study. SDM and KEM conceived the idea for this systematic review. KN, AG, and YW screened records, completed data analysis, assessed risk of bias and certainty, and presented findings. KN drafted the initial manuscript. MB, EVA, KEM, and SDM provided critical revisions of the manuscript based on important intellectual content, and SDM supervised the project. KN, AG, YW, and SDM are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: SDM is supported by a Tier II Canadian Institutes of Health Research (CIHR) Canada Research Chair (950-229920). CIHR was not involved in any aspect of this study. The authors declare they have no sources of financial support for this project.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The guarantors (KN, AG, YW, and SDM) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patients and public communities: An abstract was presented at the Society of Obstetricians and Gynaecologists of Canada Annual Clinical and Scientific Conference on 8 June 2023. The findings will be disseminated to the public through social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
The corresponding author (SDM) should be contacted for any requests.
References
- 1. Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health 2022;6:106-15. 10.1016/S2352-4642(21)00311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2020;12:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sweet DG, Carnielli VP, Greisen G, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023;120:3-23. 10.1159/000528914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stock SJ, Thomson AJ, Papworth S, Royal College of Obstetricians and Gynaecologists . Antenatal corticosteroids to reduce neonatal morbidity and mortality: Green-top Guideline No. 74. BJOG 2022;129:e35-60. 10.1111/1471-0528.17027 [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Preterm labour and birth. 2022. NICE guideline, No 25 . https://www.nice.org.uk/guidance/ng25 [PubMed]
- 6. Daskalakis G, Pergialiotis V, Domellöf M, et al. European guidelines on perinatal care: corticosteroids for women at risk of preterm birth. J Matern Fetal Neonatal Med 2023;36:2160628. 10.1080/14767058.2022.2160628 [DOI] [PubMed] [Google Scholar]
- 7. Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol 1996;14:599-607. 10.1165/ajrcmb.14.6.8652188 [DOI] [PubMed] [Google Scholar]
- 8. Taeusch HW, Jr, Brown E, Torday JS, Nielsen HC. Magnitude and duration of lung response to dexamethasone in fetal sheep. Am J Obstet Gynecol 1981;140:452-5. 10.1016/0002-9378(81)90044-2 [DOI] [PubMed] [Google Scholar]
- 9. Schwab M, Roedel M, Anwar MA, et al. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. J Physiol 2000;528:619-32. 10.1111/j.1469-7793.2000.00619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garafova A, Kornanova E, Chovancova D, et al. Relationships between antenatal corticosteroids and catecholamine blood pressure support in neonates: considering of maternal stress-related diseases. Stress 2020;23:694-9. 10.1080/10253890.2020.1806227 [DOI] [PubMed] [Google Scholar]
- 11. Jobe AH, Kemp M, Schmidt A, Takahashi T, Newnham J, Milad M. Antenatal corticosteroids: a reappraisal of the drug formulation and dose. Pediatr Res 2021;89:318-25. 10.1038/s41390-020-01249-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Committee on Obstetric Practice . Committee Opinion No. 713: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol 2017;130:e102-9. 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 13. Skoll A, Boutin A, Bujold E, et al. No. 364-Antenatal corticosteroid therapy for improving neonatal outcomes. J Obstet Gynaecol Can 2018;40:1219-39. 10.1016/j.jogc.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 14. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515-25. 10.1542/peds.50.4.515 [DOI] [PubMed] [Google Scholar]
- 15. Hamm RF, Combs CA, Aghajanian P, Friedman AM, Society for Maternal-Fetal Medicine (SMFM). Electronic address: smfm@smfm.org. Patient Safety and Quality Committee . Society for Maternal-Fetal Medicine Special Statement: Quality metrics for optimal timing of antenatal corticosteroid administration. Am J Obstet Gynecol 2022;226:B2-10. 10.1016/j.ajog.2022.02.021 [DOI] [PubMed] [Google Scholar]
- 16. Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol 1999;94:213-8. [DOI] [PubMed] [Google Scholar]
- 17. Tsiarli MA, Rudine A, Kendall N, et al. Antenatal dexamethasone exposure differentially affects distinct cortical neural progenitor cells and triggers long-term changes in murine cerebral architecture and behavior. Transl Psychiatry 2017;7:e1153. 10.1038/tp.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noorlander CW, Tijsseling D, Hessel EV, et al. Antenatal glucocorticoid treatment affects hippocampal development in mice. PLoS One 2014;9:e85671. 10.1371/journal.pone.0085671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol 2003;547:117-23. 10.1113/jphysiol.2002.025700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noorlander CW, Visser GH, Ramakers GM, Nikkels PG, de Graan PN. Prenatal corticosteroid exposure affects hippocampal plasticity and reduces lifespan. Dev Neurobiol 2008;68:237-46. 10.1002/dneu.20583 [DOI] [PubMed] [Google Scholar]
- 21. Dunn E, Kapoor A, Leen J, Matthews SG. Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol 2010;588:887-99. 10.1113/jphysiol.2009.182139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol 2002;172:71-81. 10.1677/joe.0.1720071 [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci 2010;17:186-95. 10.1177/1933719109351098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sloboda DM, Moss TJ, Li S, et al. Hepatic glucose regulation and metabolism in adult sheep: effects of prenatal betamethasone. Am J Physiol Endocrinol Metab 2005;289:E721-8. 10.1152/ajpendo.00040.2005 [DOI] [PubMed] [Google Scholar]
- 25. Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137-42. 10.1042/cs0980137 [DOI] [PubMed] [Google Scholar]
- 26. Nixon PA, Washburn LK, Michael O’Shea T, et al. Antenatal steroid exposure and heart rate variability in adolescents born with very low birth weight. Pediatr Res 2017;81:57-62. 10.1038/pr.2016.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelly BA, Lewandowski AJ, Worton SA, et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics 2012;129:e1282-90. 10.1542/peds.2011-3175 [DOI] [PubMed] [Google Scholar]
- 28. South AM, Nixon PA, Chappell MC, et al. Antenatal corticosteroids and the renin-angiotensin-aldosterone system in adolescents born preterm. Pediatr Res 2017;81:88-93. 10.1038/pr.2016.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finken MJ, Keijzer-Veen MG, Dekker FW, et al. Dutch POPS-19 Collaborative Study Group . Antenatal glucocorticoid treatment is not associated with long-term metabolic risks in individuals born before 32 weeks of gestation. Arch Dis Child Fetal Neonatal Ed 2008;93:F442-7. 10.1136/adc.2007.128470 [DOI] [PubMed] [Google Scholar]
- 30. Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005;365:1856-62. 10.1016/S0140-6736(05)66617-2 [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2019. 10.1002/9781119536604. [DOI] [Google Scholar]
- 32. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finnish Medical Society. Duodecim Current Care-Premature Birth. Accessed 4 Aug 2022. https://www.kaypahoito.fi/hoi50089
- 34. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. NICHD Maternal–Fetal Medicine Units Network . Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med 2016;374:1311-20. 10.1056/NEJMoa1516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gellman MD. Encyclopedia of behavioral medicine. Springer, 2020. 10.1007/978-3-030-39903-0. [DOI] [Google Scholar]
- 36. Szklo M. Population-based cohort studies. Epidemiol Rev 1998;20:81-90. 10.1093/oxfordjournals.epirev.a017974 [DOI] [PubMed] [Google Scholar]
- 37. Canova C, Cantarutti A. Population-based birth cohort studies in epidemiology. Int J Environ Res Public Health 2020;17:5276. 10.3390/ijerph17155276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed 4 Aug 2022. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 39. Zhang Y, Huang L, Wang D, Ren P, Hong Q, Kang D. The ROBINS-I and the NOS had similar reliability but differed in applicability: A random sampling observational studies of systematic reviews/meta-analysis. J Evid Based Med 2021;14:112-22. 10.1111/jebm.12427 [DOI] [PubMed] [Google Scholar]
- 40. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380-2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 41. Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol 2021;21:189. 10.1186/s12874-021-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. R: A Language and Environment for Statistical Computing. Version 4.0.3. R Foundation for Statistical Computing; 2020. Accessed 1 May 2022. https://www.R-project.org
- 43. Review Manager (RevMan). Version 5.4. The Cochrane Collaboration; 2020.
- 44. Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA 2020;323:1924-33. 10.1001/jama.2020.3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diguisto C, Arthuis C, Couderchet J, et al. Impact of antenatal corticosteroids on head circumference of full-term newborns: A French multicenter cohort study. Acta Obstet Gynecol Scand 2020;99:1147-54. 10.1111/aogs.13839 [DOI] [PubMed] [Google Scholar]
- 46. McKinzie AH, Yang Z, Teal E, et al. Are newborn outcomes different for term babies who were exposed to antenatal corticosteroids? Am J Obstet Gynecol 2021;225:536.e1-536.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodriguez A, Wang Y, Ali Khan A, Cartwright R, Gissler M, Järvelin M-R. Antenatal corticosteroid therapy (ACT) and size at birth: A population-based analysis using the Finnish Medical Birth Register. PLoS Med 2019;16:e1002746. 10.1371/journal.pmed.1002746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melamed N, Asztalos E, Murphy K, et al. Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population-based study. BMJ Open 2019;9:e031197. 10.1136/bmjopen-2019-031197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Räikkönen K, Gissler M, Tapiainen T, Kajantie E. Associations between maternal antenatal corticosteroid treatment and psychological developmental and neurosensory disorders in children. JAMA Netw Open 2022;5:e2228518. 10.1001/jamanetworkopen.2022.28518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osteen SJ, Yang Z, McKinzie AH, et al. Long-term childhood outcomes for babies born at term who were exposed to antenatal corticosteroids. Am J Obstet Gynecol 2023;228:80.e1-6. 10.1016/j.ajog.2022.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malloy MH. Antenatal steroid use and neonatal outcome: United States 2007. J Perinatol 2012;32:722-7. 10.1038/jp.2012.22 [DOI] [PubMed] [Google Scholar]
- 52. Aviram A, Murphy K, McDonald S, et al. Antenatal corticosteroids and neurodevelopmental outcomes in late preterm births. Arch Dis Child Fetal Neonatal Ed 2022;107:250-5. 10.1136/archdischild-2021-322152 [DOI] [PubMed] [Google Scholar]
- 53. Razaz N, Skoll A, Fahey J, Allen VM, Joseph KS. Trends in optimal, suboptimal, and questionably appropriate receipt of antenatal corticosteroid prophylaxis. Obstet Gynecol 2015;125:288-96. 10.1097/AOG.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 54. Schmitz T, Doret-Dion M, Sentilhes L, et al. BETADOSE trial study group. Groupe de Recherche en Obstétrique et Gynécologie . Neonatal outcomes for women at risk of preterm delivery given half dose versus full dose of antenatal betamethasone: a randomised, multicentre, double-blind, placebo-controlled, non-inferiority trial. Lancet 2022;400:592-604. 10.1016/S0140-6736(22)01535-5 [DOI] [PubMed] [Google Scholar]
- 55. Oladapo OT, Vogel JP, Piaggio G, et al. WHO ACTION Trials Collaborators . Antenatal dexamethasone for early preterm birth in low-resource countries. N Engl J Med 2020;383:2514-25. 10.1056/NEJMoa2022398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS, Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group . Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet 2006;367:1913-9. 10.1016/S0140-6736(06)68846-6 [DOI] [PubMed] [Google Scholar]
- 57. Danesh A, Janghorbani M, Khalatbari S. Effects of antenatal corticosteroids on maternal serum indicators of infection in women at risk for preterm delivery: A randomized trial comparing betamethasone and dexamethasone. J Res Med Sci 2012;17:911-7. [PMC free article] [PubMed] [Google Scholar]
- 58. Peltoniemi OM, Kari MA, Tammela O, et al. Repeat Antenatal Betamethasone Study Group . Randomized trial of a single repeat dose of prenatal betamethasone treatment in imminent preterm birth. Pediatrics 2007;119:290-8. 10.1542/peds.2006-1549 [DOI] [PubMed] [Google Scholar]
- 59. McEvoy C, Schilling D, Peters D, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. Am J Obstet Gynecol 2010;202:544.e1-9. 10.1016/j.ajog.2010.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garite TJ, Kurtzman J, Maurel K, Clark R, Obstetrix Collaborative Research Network . Impact of a ‘rescue course’ of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol 2009;200:248.e1-9. 10.1016/j.ajog.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 61. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 2013;132:1033-44. 10.1016/j.jaci.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update 2016;22:240-59. [DOI] [PubMed] [Google Scholar]
- 63. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006;(3):CD004454. [DOI] [PubMed] [Google Scholar]
- 64. Braun T, Husar A, Challis JR, et al. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta 2013;34:407-15. 10.1016/j.placenta.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 65. Aagaard K, Matthiesen NB, Bach CC, Larsen RT, Henriksen TB. Head circumference at birth and intellectual disability: a nationwide cohort study. Pediatr Res 2020;87:595-601. 10.1038/s41390-019-0593-3 [DOI] [PubMed] [Google Scholar]
- 66. Bolduc FV, Shevell MI. Corrected head circumference centiles as a possible predictor of developmental performance in high-risk neonatal intensive care unit survivors. Dev Med Child Neurol 2005;47:766-70. 10.1017/S001216220500160X [DOI] [PubMed] [Google Scholar]
- 67. McGowan PO, Matthews SG. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology 2018;159:69-82. 10.1210/en.2017-00896 [DOI] [PubMed] [Google Scholar]
- 68. Wood CE, Keller-Wood M. The critical importance of the fetal hypothalamus-pituitary-adrenal axis. F1000Res 2016;5:F1000 Faculty Rev-115. 10.12688/f1000research.7224.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol 2014;10:391-402. 10.1038/nrendo.2014.73 [DOI] [PubMed] [Google Scholar]
- 70.McDonald S, Murphy K. SNACS collaborators. Single Dose of Antenatal Corticosteroids for Pregnancies at Risk of Preterm Delivery (SNACS). Accessed Feb 24, 2023. https://www.clinicaltrials.gov/ct2/show/NCT05114096
- 71. Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol 2010;203:305-15. 10.1016/j.ajog.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 72. Michaluk A, Dionne M-D, Gazdovich S, Buch D, Ducruet T, Leduc L. Predicting preterm birth in twin pregnancy: was the previous birth preterm? A Canadian experience. J Obstet Gynaecol Can 2013;35:793-801. 10.1016/S1701-2163(15)30835-5 [DOI] [PubMed] [Google Scholar]
- 73. Kalikkot Thekkeveedu R, Dankhara N, Desai J, Klar AL, Patel J. Outcomes of multiple gestation births compared to singleton: analysis of multicenter KID database. Matern Health Neonatol Perinatol 2021;7:15. 10.1186/s40748-021-00135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sarid EB, Stoopler ML, Morency A-M, Garfinkle J. Neurological implications of antenatal corticosteroids on late preterm and term infants: a scoping review. Pediatr Res 2022;92:1225-39. 10.1038/s41390-022-02135-3 [DOI] [PubMed] [Google Scholar]
- 75. Ninan K, Liyanage SK, Murphy KE, Asztalos EV, McDonald SD. Evaluation of Long-term Outcomes Associated With Preterm Exposure to Antenatal Corticosteroids: A Systematic Review and Meta-analysis. JAMA Pediatr 2022;176:e220483. 10.1001/jamapediatrics.2022.0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McDougall ARA, Aboud L, Lavin T, et al. Effect of antenatal corticosteroid administration-to-birth interval on maternal and newborn outcomes: a systematic review. EClinicalMedicine 2023;58:101916. 10.1016/j.eclinm.2023.101916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed 2013;98:F195-200. 10.1136/archdischild-2012-303157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: eTable 1, eFigures 1-6, eAppendices 1-4, and eReferences
Data Availability Statement
The corresponding author (SDM) should be contacted for any requests.