Abstract
The 34-kDa early-region 4 open reading frame 6 (E4orf6) product of human adenovirus type 5 forms complexes with both the cellular tumor suppressor p53 and the viral E1B 55-kDa protein (E1B-55kDa). E4orf6 can inhibit p53 transactivation activity, as can E1B-55kDa, and in combination these viral proteins cause the rapid turnover of p53. In addition, E4orf6-55kDa complexes play a critical role at later times in the regulation of viral mRNA transport and shutoff of host cell protein synthesis. In the present study, we have further characterized some of the biological properties of E4orf6. Analysis of extracts from infected cells by Western blotting indicated that E4orf6, like E1A and E1B products, is present at high levels until very late times, suggesting that it is available to act throughout the infectious cycle. This pattern is similar to that of E4orf4 but differs markedly from that of another E4 product, E4orf6/7, which is present only transiently. Synthesis of E4orf6 is maximal at early stages but ceases completely with the onset of shutoff of host protein synthesis; however, it was found that unlike E4orf6/7, E4orf6 is very stable, thus allowing high levels to be maintained even at late times. E4orf6 was shown to be phosphorylated at low levels. Coimmunoprecipitation studies in cells lacking p53 indicated that E4orf6 interacts with a number of other proteins. Five of these were shown to be viral or virally induced proteins ranging in size from 102 to 27 kDa, including E1B-55kDa. One such species, of 72 kDa, was shown not to represent the E2 DNA-binding protein and thus remains to be identified. Another appeared to be the L4 100-kDa nonstructural adenovirus late product, but it appeared to be present nonspecifically and not as part of an E4orf6 complex. Apart from p53, three additional cellular proteins, of 84, 19, and 14 kDa were detected by using an adenovirus vector that expresses only E4orf6. The 19-kDa species and a 16-kDa cellular protein were also shown to interact with E4orf6/7. It is possible that complex formation with these viral and cellular proteins plays a role in one or more of the biological activities associated with E4orf6 and E4orf6/7.
Infection of human cells with adenovirus type 5 (Ad5) leads to the expression of several classes of early proteins, induction of cell and viral DNA synthesis, synthesis of late viral proteins, formation of progeny virions, and, finally, cell death. Most of the early events rely in part or in total on products of early region 1A (E1A), which are largely responsible for transactivation of early transcription units, especially early regions 3 and 4 (E3 and E4), and for the induction of cellular DNA synthesis (reviewed in reference 3). Expression of E1A alone is highly toxic to cells, since E1A proteins induce the accumulation and activation of p53, leading to growth arrest and early cell death by apoptosis (11, 27). Recently we have shown that this response in human cells may be related to induction of unscheduled DNA synthesis by E1A, since both p53 accumulation and stimulation of entry into S phase rely on complex formation between E1A proteins and either the RB family of tumor suppressors or the p300/CBP family of histone acetyltransferases (44). In rodent cells expressing E1A, p300 binding appears to be of greater importance (9, 44). Such a response would severely limit the production of viral progeny, and so Ads utilize a variety of strategies to prevent effects induced by p53. p53-dependent apoptosis is blocked by both major products of E1B. The E1B 19-kDa protein (E1B-19kDa) functions by a mechanism similar to the Bcl-2 cellular suppressor of apoptosis and prevents programmed cell death induced by a variety of agents (4, 8, 39, 45, 59). E1B-55kDa targets p53 specifically by complex formation and repression of p53 transactivation activity (52, 53, 60, 61), thus preventing both apoptosis (29, 52) and, presumably, growth arrest. Ads also possess at least two additional mechanisms to inhibit p53. First, the 34-kDa product of open reading frame 6 of E4, termed E4orf6, also binds to and inactivates p53 (13, 37, 43). In addition, E4orf6 interacts with E1B-55kDa and this complex stimulates the rapid turnover of p53 by a mechanism that remains to be established (34, 37, 43, 51). Thus, Ad-infected cells survive activation of p53 sufficiently to generate high levels of progeny.
E4orf6 also plays additional critical roles in productive infection. At later times, viral mRNAs are selectively stabilized and transported to the cytoplasm, where they are efficiently translated to generate high levels of late viral proteins necessary for virion formation (6, 7, 26, 48). Another possibly related event is the shutoff of host cell protein synthesis, which allows selective translation of viral proteins (6, 19). These functions require both E4orf6 and E1B-55kDa, presumably acting as a complex (1, 6, 26, 42). It is now believed that at least one role of E4orf6 in these processes is to target E1B-55kDa to the nucleus and to export it back to the cytoplasm. E4orf6 contains three sequences of importance in this process: a nuclear localization signal, a nuclear export signal, and a nuclear retention signal (12). It is presumed that the complex interacts either directly or indirectly with viral mRNAs that are selected, because E4orf6 appears to localize preferentially in centers of viral replication in the nucleus (14). Additional functions also contribute to host cell shutoff involving viral VA RNAs (40, 54), dephosphorylation of translation initiation factor eIF-4F (23), and a late 100-kDa protein (22).
E4 encodes at least seven products that, except for E4orf6/7, are unrelated to E4orf6. E4orf6/7 shares 58 amino-terminal residues with E4orf6 and functions to promote the expression of E2 through interactions with transcription factor E2F (24, 30, 36, 46). Little is known in detail about the mechanism of action of E4orf6 or the way in which its activity is regulated during lytic infection. To learn more about the biology of E4orf6, we have characterized its synthesis and stability relative to other viral products and determined that it is phosphorylated. In addition, we have found that it interacts with several other viral and cellular proteins and that such complexes may contribute to its biological activity.
MATERIALS AND METHODS
Cells and viruses.
Human p53+ HeLa cells (ATCC CCL-2) and human p53− H1299 cells (33) were cultured on 60-, 100-, or 150-mm-diameter dishes (Corning Glass Works, Corning, N.Y.) in α-modified minimum essential Eagle’s medium (Gibco BRL) or in Dulbecco’s modified Eagle’s medium (Gibco BRL), respectively, supplemented with 10% fetal calf serum (CanSera). The cells were infected with mutant or wild-type (wt) Ad5 at a multiplicity of 35 to 50 PFU per cell, as described previously (53). Such viral stocks were subjected to titer determination on 293 cells (15). The virus used as wt has been described elsewhere (21), although in some cases dl309 (25) was used as wt. Mutant E1B/55K− virus (originally pm2019/2250) does not express E1B-55kDa (32). In one experiment, mutant dl1015, which fails to express E4 products apart from E4orf3 and E4orf4, was used (6). The Ad vectors AdE4orf6, AdE4orf6/7, and AdHis55K used in this study are derivatives of the vectors described previously by Bacchetti and Graham (2a), in which the E1 and E3 regions have been deleted. cDNAs encoding Ad2E4orf6 or Ad2E4orf6/7 were inserted by homologous recombination into a cloning site in the E1 region under the control of the cytomegalovirus promoter, and the resulting vectors were propagated in 293 cells. Because of the absence of the E1A coding region, only E4orf6 or E4orf6/7 is expressed following infection and none of the resident Ad5 vector genes are manifested at detectable levels (43).
Antisera.
E4orf6-specific antibodies were raised in rabbits by using a synthetic peptide corresponding to the carboxy terminus of E4orf6. The peptide (C)HRPILMHDYDSTPM corresponding to amino acid residues 281 to 294 of E4orf6 was synthesized by Fmoc (9-fluoronylmethyloxycarbonyl) solid-phase chemistry. The crude peptide was purified by high-pressure liquid chromatography and the purity was confirmed by analytical high-pressure liquid chromatography and ion-spray mass-spectrometric analysis. A cysteine residue was added at the amino terminus to allow coupling of the peptide through a disulfide bond to keyhole limpet hemocyanin, as described previously (35). This antiserum has been termed E4orf6-C. Antibodies that recognize the amino termini of E4orf6 and E4orf6/7 were raised in rabbits by using a fusion protein consisting of gluthatione S-transferase (GST) fused to amino acid residues 1 to 46 of the amino terminus of E4orf6. This serum has been termed E4orf6-N. E4orf4 antibodies were raised in rabbits by using GST fused to amino residues 86 to 114 at the carboxy terminus of E4orf4. Rabbits were injected subcutaneously at four sites on the back with 200 μg of peptide-keyhole limpet hemocyanin or 500 μg of GST fusion protein in complete Freund’s adjuvant. Booster injections were administered 2 and 3 weeks after initial immunization with the same quantity of antigen in incomplete Freund’s adjuvant. This antiserum has been termed E4orf4-N. Other antisera used in this study included M73 mouse monoclonal antibody, which recognizes the carboxy terminus of E1A proteins (20); 2A6 mouse monoclonal antibody, which recognizes an epitope within the amino-terminal 180 residues of E1B-55kDa (50); 19-C1 polyclonal antiserum raised in rabbits against a synthetic peptide corresponding to the carboxy terminus of E1B-19kDa (31); H2-67 mouse monoclonal antibody against E2-72kDa, a viral DNA-binding protein (5a); and 2100K-1 mouse monoclonal antibody against the L4 100-kDa Ad late nonstructural protein (6a).
Western blotting analysis.
Cell extracts were prepared on ice in lysis buffer A (2% [vol/vol] Nonidet P-40, 10 mM HEPES [pH 7.4], 147 mM KCl, 5 mM MgCl2, 1 mM EGTA) containing 2 μg each of aprotinin, leupeptin, and pepstatin per ml. Total protein was measured by the Bio-Rad protein assay as specified by the manufacturer, and 20 to 40 μg of protein was separated on 0.75-mm-thick sodium dodecyl sulfate (SDS)–12 or 15% polyacrylamide gels with a MINI-PROTEAN II apparatus (Bio-Rad). The proteins were electroblotted onto 0.45-μm-pore-diameter supported nitrocellulose membranes (BA-S85; Schleicher & Schuell) with a semidry apparatus (Millipore) in transfer buffer (10 mM Tris, 96 mM glycine, 20% [vol/vol] methanol) for 1 h at 80 mA per gel. The blots were incubated overnight at 4°C in blocking buffer (20 mM Tris [pH 7.5], 137 mM NaCl, 0.1% [vol/vol] Tween 20, 0.5% calf serum, 5% nonfat dry milk). The blots were incubated with the appropriate dilution of primary antibodies in blocking buffer for 2 h at room temperature and then with a 1:10,000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G or goat anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratories) in the same incubation medium for 1 h. Immunoreactive bands were revealed by using an enhanced chemiluminescence (ECL) Western blotting kit (Renaissance; DuPont-NEN) and Reflection films (NEF-496; DuPont-NEN). Molecular mass determination was performed with the following standards (Mark 12, Novex): myosin (200 kDa), β-galactosidase (116.2 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66.3 kDa), lactate dehydrogenase (36.5 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (21.5 kDa), lysozyme (14.4 kDa), and aprotinin (6 kDa).
Radioactive labeling.
Ad- or mock-infected cells were normally labeled starting from 12 to 18 h postinfection (p.i.) with 200 μCi of [35S]methionine-[35S]cysteine EasyTag Express protein-labeling mix (specific activity, >1,000 Ci/mmol [DuPont-NEN]) per ml or with 0.33 mCi of [32P]orthophosphate (specific activity, 8,500 to 9,120 Ci/mmol; DuPont-NEN) per ml in methionine- and cysteine-free or phosphate-free medium, respectively.
Immunoprecipitation.
Cell extracts were usually prepared in the lysis buffer A, and 9 volumes of buffer B (buffer A without Nonidet P-40) was added over 1 h to reduce the concentration of Nonidet P-40 from 2 to 0.2%. Cell debris was pelleted by centrifugation (18,000 × g for 30 min), and supernatants were precleared with 20 μl of protein A-Sepharose for 2 h at 4°C. The precleared extracts were then subjected to immunoprecipitation overnight with 10 μl of the appropriate antisera and 20 μl of protein A-Sepharose. The beads were washed four times with buffer A containing 0.2% Nonidet P-40. Immunoprecipitated proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) on 18- by 18-cm gels (Bio-Rad) at a constant voltage of 60 V. The gels were stained with Coomassie blue, treated with 2,5-diphenyloxazole in dimethyl sulfoxide, dried, and exposed to Kodak X-Omat AR film at −80°C.
RESULTS
Determination of E4orf6 levels during productive infection.
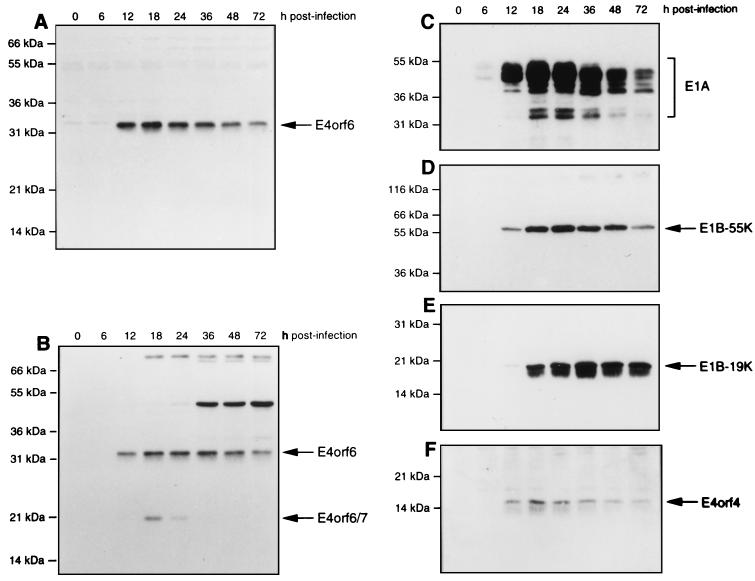
It is known that E4orf6 functions both early and late after infection. To determine the relative levels of E4orf6 and other viral proteins during the infectious cycle, HeLa cells were infected with wt Ad5 and cell extracts were collected at various times, subjected to SDS-PAGE, and then, following transfer, immunoblotted with various specific antisera. Figure 1 shows that with both E4orf6-C serum, which recognizes the carboxy terminus of E4orf6 (Fig. 1A), and E4orf6-N, which recognizes its amino terminus (Fig. 1B), E4orf6 was detectable by at least 12 h p.i. and its levels remained high throughout the infection, with only a modest reduction even by 72 h p.i. Maximum levels were present from 12 to 36 h p.i. Since E4orf6-N serum also recognizes E4orf6/7, Fig. 1B shows that this protein was first detected at low levels by 12 h p.i. and that its levels peaked by 18 h p.i. and then declined rapidly so that by 36 h p.i. no E4orf6/7 was detectable. These results indicated that although these two proteins share 58 amino-terminal residues, E4orf6 is present at much higher levels both early and late after infection. Another E4 product, E4orf4, appeared and persisted in a pattern very similar to E4orf6 (Fig. 1F). The levels of other early viral proteins were also measured for comparison. E1A products were detectable with M73 monoclonal antibody by 6 h p.i. and remained maximal until at least 48 h p.i. (Fig. 1C). E1B-55kDa (Fig. 1D) and E1B-19kDa (Fig. 1E) were detected with 2A6 monoclonal antibody and 19-C1 antipeptide serum, respectively, and were clearly present at low levels by 12 h p.i. The levels of both became maximal by 18 h p.i., after which the level of E1B-19kDa remained constant until 72 h p.i. and that of E1B-55kDa remained constant until at least 48 h p.i.
FIG. 1.
Expression of E4orf6, E4orf6/7, and other Ad proteins in HeLa cells infected with wt Ad5. HeLa cells were infected with wt Ad5, and cell extracts were prepared at various times after infection. Equal amounts of whole-cell protein were separated by SDS-PAGE, and following transfer to nitrocellulose, the levels of E4orf6 and other viral proteins were determined by Western blotting. (A) E4orf6 with E4orf6-C serum. (B) E4orf6 and E4orf6/7 with E4orf6-N serum. (C) E1A proteins with M73 monoclonal antibody. (D) E1B-55kDa with 2A6 monoclonal antibody. (E) E1B-19kDa with 19-C1 serum. (F) E4orf4 levels with E4orf4-C serum. The positions of migration of molecular mass markers are shown on the left, and those of the viral proteins are shown on the right.
Synthesis and stability of E4orf6.
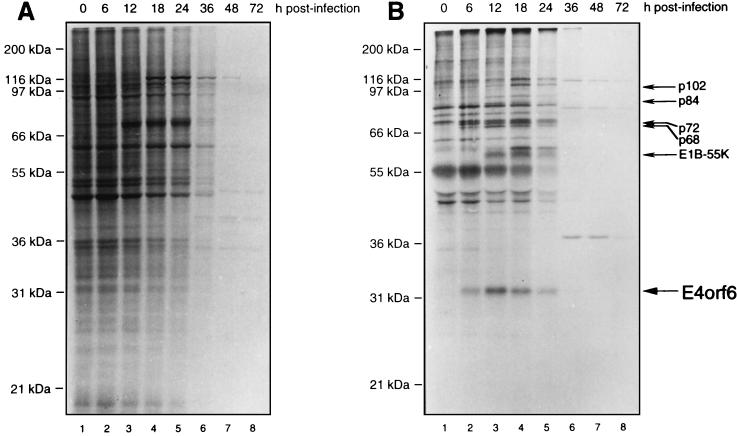
To relate the accumulated levels of E4orf6 to its rate of synthesis, Ad5-infected HeLa cells were pulse-labeled with [35S]methionine-[35S]cysteine for 1 h at various times after infection. Cell extracts were either immunoprecipitated with E4orf6-C serum or analyzed directly by SDS-PAGE. Figure 2A shows the whole-cell pattern. Of note was the clear decline in synthesis of cellular proteins commencing by 36 h p.i., such that essentially no labeled host proteins were detected by 48 h p.i. This reduction in protein synthesis reflects Ad5-induced host cell shutoff. Figure 2B shows that synthesis of E4orf6 as detected in immunoprecipitates commenced by 6 h p.i., was maximal by 12 to 18 h p.i., and then declined sharply until none was apparent by 36 h p.i. Other investigators have reported that levels of cytoplasmic E4 mRNAs decline later in infection (38, 55, 56). It is unclear at present if this effect is due to changes in transcription patterns at later times or if transport and translation of these mRNAs are affected by host cell shutoff, which occurs with similar kinetics. It should be pointed out that in all of the present studies the protein complexes preserved as immunoprecipitates were prepared under mild conditions in buffers containing nonionic detergents. Thus, a number of species in addition to E4orf6, including E1B-55kDa, are also apparent in Fig. 2B. Some of these proteins represent specific E4orf6-binding proteins, which are discussed in more detail below.
FIG. 2.
Time course of host cell shutoff and E4orf6 synthesis in Ad5-infected HeLa cells. HeLa cells were infected with wt Ad5, and at various times after infection they were labeled for 1 h with [35S]methionine-[35S]cysteine and cell extracts were prepared. (A) Analysis of whole-cell protein synthesis. A 5-μg portion of total-cell protein from each sample was analyzed by SDS-PAGE followed by fluorography. (B) Analysis of E4orf6 synthesis. Equal aliquots of the cell extracts shown in panel A were immunoprecipitated with E4orf6-C serum, and immunoprecipitates were analyzed by SDS-PAGE followed by fluorography. The positions of molecular mass markers are shown on the left, and that of E4orf6 is shown on the right.
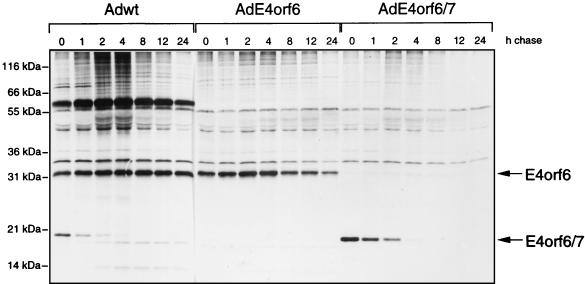
To analyze the stability of E4orf6, HeLa cells were infected either with wt Ad5 or with an Ad vector, AdE4orf6, that expresses E4orf6 under the cytomegalovirus promoter in the absence of other viral products. It should be noted that with such vectors, even though the E1A coding region is absent, we have detected a very low level of expression of resident viral genes equivalent to about 5% or less of wt levels (43); however, such levels are sufficiently low that no biological effect of these products has been apparent (43). These cells were subjected to pulse-chase analysis in which, following a 1-h incubation at 12 h p.i. with [35S]methionine-[35S]cysteine, cells were incubated for various times up to 24 h in medium containing nonradioactive amino acids, extracts were immunoprecipitated with E4orf6-N serum, and precipitates were analyzed by SDS-PAGE. To compare the stability of E4orf6 with that of E4orf6/7, some HeLa cell cultures were also infected with an Ad vector, AdE4orf6/7, that expresses only E4orf6/7 and extracts were immunoprecipitated as above with E4orf6-N serum, which recognizes both E4orf6 and E4orf6/7. Figure 3 shows that the levels of synthesis of E4orf6 in wt Ad5- and AdE4orf6-infected cells were comparable. With wt Ad5, E4orf6 was completely stable for up to 24 h. E4orf6 was also quite stable in AdE4orf6-infected cells, although the level did decline slightly during longer chase periods, suggesting that its association with E1B-55kDa (which is evident as one of several highly stable coprecipitating species in Fig. 3), or some other viral product, may enhance its stability. In contrast, E4orf6/7, which was synthesized at somewhat higher levels in AdE4orf6/7 vector-infected cells, was highly unstable, with a half-life on the order of about 1 h. Thus, in total, the results shown in Fig. 1 to 3 indicated that E4orf6 is synthesized between 6 and at least 24 h p.i. but that overall levels remain high until late times because E4orf6 is highly stable. Conversely, E4orf6/7 is present only transiently at early times because it is highly unstable. These data confirmed that E4orf6 is available at substantial levels throughout the infectious cycle and thus can function maximally at both early and late times.
FIG. 3.
Pulse-chase analysis of E4orf6 and E4orf6/7 proteins in HeLa cells. HeLa cells were infected with wt Ad5, AdE4orf6, or AdE4orf6/7. At 12 h p.i., the cells were labeled for 1 h with [35S]methionine-[35S]cysteine and then incubated further for various times with medium containing an excess of cold methionine. Cell extracts were subjected to immunoprecipitation with E4orf6-N serum, which recognizes both E4orf6 and E4orf6/7, and immunoprecipitates were analyzed by SDS-PAGE followed by autoradiography.
Phosphorylation of E4orf6.
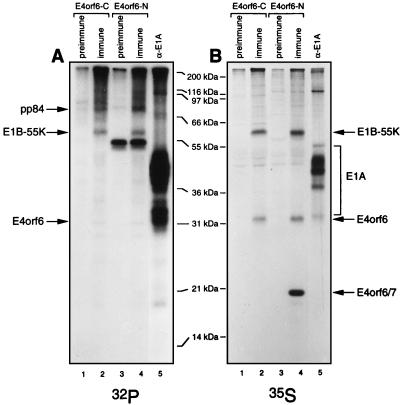
The activities of many proteins, including E1B-55kDa, are regulated by phosphorylation (53). To determine if E4orf6 is phosphorylated, HeLa cells infected by wt Ad5 were labeled with either [32P]orthophosphate or [35S]methionine-[35S]cysteine for 4 h commencing at 15 h p.i. and extracts were immunoprecipitated with either E4orf6-C or E4orf6-N serum or anti-E1A M73 monoclonal antibody and analyzed by SDS-PAGE. Figure 4 shows that E1A products were highly labeled with 32P (Fig. 4A), as expected, because they have been shown by our group to contain at least four or five sites of phosphorylation (58). Figure 4 also shows that E4orf6/7 was not labeled with 32P (Fig. 4A), even though high levels of this product were detected with E4orf6-N serum (Fig. 4B). In the case of E4orf6, low levels of 32P incorporation were evident in precipitates prepared with both sera (Fig. 4A), indicating that one or more sites in E4orf6 must be phosphorylated at a low level. Since E4orf6 and E4orf6/7 share 58 amino-terminal residues, such phosphorylation most probably occurs in another region of E4orf6, although the possibility exists that the presence of residues from the orf7 region prevents phosphorylation. Several potential sites exist in this part of the molecule; however, since the stoichiometry of phosphorylation appears to be quite low, further studies are required to determine its functional significance. It should also be noted that two other phosphoproteins were prominent in precipitates prepared with both E4orf6 sera (Fig. 4A). One corresponded to E1B-55kDa, which is known to be phosphorylated (28, 49) at three carboxy-terminal sites (52, 53). The other phosphoprotein, of about 84 kDa, is discussed further below.
FIG. 4.
Phosphorylation of E4orf6. HeLa cells were infected with wt Ad5 or mock infected, and at 15 h p.i. they were labeled either with [32P]orthophosphate for 4 h (A) or with [35S]methionine-[35S]cysteine for 2 h (B). Cell extracts were immunoprecipitated with E4orf6-C (lanes 1 and 2) or E4orf6-N (lanes 3 and 4) serum or the corresponding preimmune serum or with M73 monoclonal antibody (lanes 5), and the precipitates were analyzed by SDS-PAGE followed by autoradiography. The positions of molecular mass markers are shown in the middle, and those of relevant proteins are shown at the sides.
Detection of E4orf6-binding proteins.
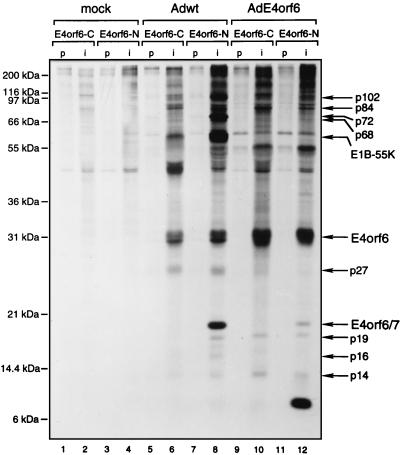
Many Ad5 proteins are known to function in complexes with other viral polypeptides and/or cellular proteins. It has long been known that E4orf6 interacts with both p53 and E1B-55kDa. It is not known if such complexes contain other proteins or if additional novel E4orf6 complexes are formed with viral or cellular proteins. To detect E4orf6-binding proteins, cells were infected with wt Ad5, and after the cells were labeled with [35S]methionine-[35S]cysteine from 18 to 20 h p.i., cell extracts were immunoprecipitated with E4orf6-C (Fig. 5) or E4orf6-N (Fig. 5 and 6) sera and the precipitates were analyzed by SDS-PAGE. Human H1299 cells were used for these studies since they lack p53 and thus p53 and p53-associated proteins will not be present to complicate the analysis. Figure 5 shows that several polypeptides coprecipitated with E4orf6 when E4orf6-specific sera were used (lanes 6 and 8), and these species were not evident in precipitates prepared with preimmune sera (lanes 5 and 7) or in those made from mock-infected cells (lanes 1 to 4). Such species (summarized in Table 1) included proteins that migrated at approximate molecular weights of 102,000 (this protein was evident as two closely migrating species in some preparations, as in Fig. 6, lane 2), 84,000, 72,000, 68,000, 55,000, 27,000, 19,000, and 14,000. A protein of 16 kDa was also detected with the E4orf6-N serum (lane 8) but not with the E4orf6-C serum (lane 6). Other species were also detected, but they appeared to be present nonspecifically or were detected inconsistently over many experiments. We recognize that one or more of the coprecipitating proteins with a molecular mass less than that of the 34-kDa E4orf6 protein could represent degradation products of E4orf6; however, we believe that this is unlikely. Immunoblotting with either E4orf6-C or E4orf6-N serum (such as is shown in Fig. 1) failed to detect these species even upon prolonged exposure of the gels, suggesting that none of these coprecipitating proteins contain either the amino or carboxy terminus of E4orf6. In addition, an abundant protein of about 8 kDa has been detected consistently in immunoprecipitates from AdE4orf6-infected cells with the E4orf6-N serum (Fig. 5, lane 12; Fig. 6A, lane 4) but not with the E4orf6-C serum (Fig. 5, lane 10). This protein is never detected when extracts from Ad5-infected cells are used (Fig. 6, lane 8). This product has also been detected in AdE4orf6-infected cells by immunoblotting with the E4orf6-N serum (data not shown), and thus it must contain the amino terminus of E4orf6. The origin of this 8-kDa protein is still uncertain, but we believe that it represents the product of a novel mRNA formed only with the AdE4orf6 vector and containing the usual splice donor site used to produce E4orf6/7 and an alternative splice acceptor site present in the vector DNA. Its small size suggests that translation must be terminated shortly after the 58-residue amino terminus shared by E4orf6 and E4orf6/7.
FIG. 5.
Detection of E4orf6-binding proteins. p53-null H1299 cells were mock infected (lanes 1 to 4) or infected with either wt Ad5 (lanes 5 to 8) or AdE4orf6 (lanes 9 to 12). Cells were labeled at 18 h p.i. with [35S]methionine-[35S]cysteine for 2 h, and cell extracts were prepared under mild conditions and immunoprecipitated with preimmune (lanes p) or immune (lanes i) E4orf6-C or E4orf6-N serum. The proteins were separated on an SDS–12% polyacrylamide gel, and the labeling pattern was visualized by fluorography. The positions of migration of molecular mass markers are shown on the left, and those of relevant proteins are shown on the right.
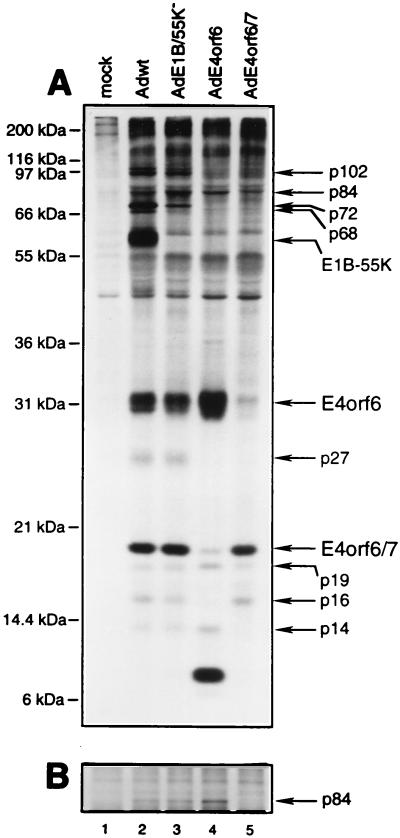
FIG. 6.
Complex formation between E4orf6 and viral or cellular proteins. An experiment similar to that described in the legend to Fig. 5, using H1299 cells and E4orf6-N serum, was performed with mock-infected cells (lane 1) or cells infected with wt Ad5 (lane 2), mutant E1B/55K− (lane 3), AdE4orf6 (lane 4), or AdE4orf6/7 (lane 5). (A) Precipitates were analyzed by SDS-PAGE with gels containing 12% polyacrylamide. (B) Portion of a gel containing similar samples that were analyzed with gels containing 8% polyacrylamide. The positions of migration of molecular mass markers are shown on the left, and those of relevant proteins are shown on the right.
TABLE 1.
Properties of E4orf6- and E4orf6/7-binding proteinsa
| Protein | Origin | Detected withc:
|
Time p.i. (h) first detected | ||||
|---|---|---|---|---|---|---|---|
| wt Ad5 | AdE4orf6 | AdE4orf6/7 | E1B/55K− | ||||
| E4orf6-N | E4orf6-C | ||||||
| p102 (L4 100-kDa) | Viral (nonspecific) | ++ | + | − | − | + | 18 |
| p84 | Cellular | + | + | + | − | + | 12 |
| p72 | Viral | ++ | + | − | − | + | 6 |
| p68 | Viral | ++ | + | − | − | − | 6 |
| E1B-55kDa | Viral | + | + | − | − | − | 12 |
| p53b | Cellular | + | + | + | − | + | ?c |
| p27 | Viral | + | + | − | − | + | ? |
| p19 | Cellular | + | + | + | + | + | ? |
| p16 | Cellular | + | + | − | + | + | ? |
| p14 | Cellular | + | − | + | − | + | ? |
To determine if the coprecipitating proteins are viral or cellular in origin or if their presence requires E1B-55kDa, similar analyses were also performed on H1299 cells infected with Ad vectors that express E4orf6 or E4orf6/7 alone or with Ad5 mutant E1B-55K−, which fails to express E1B-55kDa (Fig. 5 and 6). In most gels, it was difficult to distinguish the p84 protein from a closely migrating nonspecific species, and so the samples shown in Fig. 6A were also analyzed in a gel containing less polyacrylamide, and these results are presented in Fig. 6B. We will first address the origins of these proteins. The p84, p19, p16, and p14 proteins appear to be cellular polypeptides. The p84 and p14 proteins were present in precipitates from cells infected with wt Ad5 (Fig. 5, lanes 6 and 8; Fig. 6, lane 2) and those infected with the AdE4orf6 vector (Fig. 5, lanes 10 and 12; Fig. 6, lane 4) but not those infected with AdE4orf6/7 (Fig. 6, lane 5), indicating that they must be cellular polypeptides that bind to E4orf6 but not E4orf6/7. The p19 protein was present in precipitates prepared using both sera in wt Ad5-infected cells (Fig. 5, lanes 6 and 8; Fig. 6, lane 2) and in those infected by vector AdE4orf6 (Fig. 5, lanes 10 and 12; Fig. 6, lane 4) or vector AdE4orf6/7 (Fig. 6, lane 5). These results indicated that p19 must be a cellular protein that associates with both E4orf6 and E4orf6/7. The p16 species was seen in immunoprecipitates prepared from wt Ad5-infected cells with E4orf6-N serum (Fig. 5, lane 8; Fig. 6, lane 2) but not with E4orf6-C serum (Fig. 5, lane 6). It was also detected with E4orf6-N serum in cells infected with vector AdE4orf6/7 (Fig. 6, lane 5) but not with vector AdE4orf6 (Fig. 5, lane 12; Fig. 6, lane 4). These results suggested that p16 is a cellular protein that associates with E4orf6/7 but not with E4orf6. None of the other species (p102, p72, p68, p55, and p27) was evident when the AdE4orf6 or E4orf6/7 vector was used, suggesting that they represent either viral products or cellular proteins synthesized in response to lytic infection. The p27 species was present at similar levels when either E4orf6-N or E4orf6-C serum was used, suggesting that it represents an E4orf6-binding protein. A protein related to E4orf6 of this approximate size has been reported by others using a 293 cell line derivative expressing E4orf6 (34); however, we have been unsuccessful in detecting this species by immunoblotting with our sera against the amino and carboxy termini of E4orf6 (Fig. 1 and data not shown). For p102, p72, and p68, higher levels were detected with E4orf6-N serum (Fig. 5, lane 8) than with E4orf6-C serum (Fig. 5, lane 6); however, these three proteins were evident with E4orf6-C serum in the time course experiment shown in Fig. 2, indicating that they are E4orf6-binding proteins. It is possible that E4orf6 complexes containing these proteins are not recognized efficiently by the E4orf6-C serum.
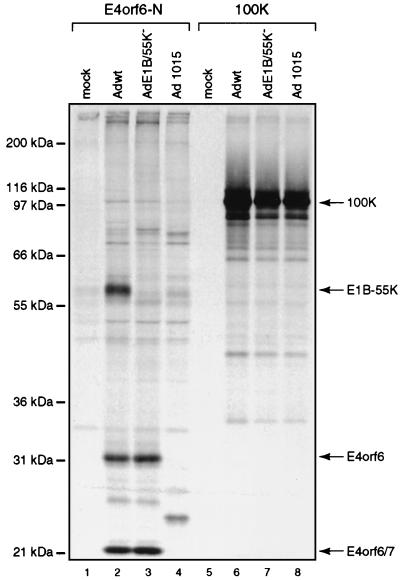
Other information about these binding proteins can be drawn from results shown in Fig. 2 and 4 to 6 and summarized in Table 1. Concerning p102, seen only in infected cells, p102-E4orf6 complexes were first evident at 18 h p.i. (Fig. 2B, lane 4), suggesting that p102 may be a late viral protein. An obvious possibility was the L4 100-kDa late nonstructural protein, which is known to play a role in selective translation of late viral mRNAs (46a). Since E4orf6/E1B-55kDa complexes also participate in this function (6, 19), we studied the interaction between E4orf6 and the L4 100-kDa species directly by using 2100K-1 mouse monoclonal antibodies that immunoprecipitate this late product (6a). H1299 cells were infected with wt Ad5, E1B/55K−, or dl1015, which expresses E4orf3 and E4orf4 but no other E4 product, and following labeling with [35S]methionine-[35S]cysteine, extracts were immunoprecipitated under mild conditions with either E4orf6-N or 2100K-1 antibodies. Figure 7 shows that with both wt Ad5 and E1B/55K−, E4orf6-N serum again coimmunoprecipitated E4orf6 and the series of proteins described above, including p102. Although the other E4orf6-associated proteins were not present with dl1015, p102 was clearly evident, suggesting that it was present nonspecifically. Precipitates prepared with anti-L4 100-kDa protein antibody all contained a prominent species that comigrated with p102. Of particular importance, no E4orf6 was evident in any of these precipitates, even after prolonged exposure of the autoradiographs (data not shown). These results suggested that p102 may represent the L4 100-kDa late viral protein but that it appears to be present nonspecifically and thus does not interact with E4orf6. Both the p72 and p68 proteins, of possible viral origin, were found in association with E4orf6 by 6 h p.i. (Fig. 2B, lane 2), indicating that they must represent early viral or virus-induced polypeptides. We tested the possibility that p72 is the E2 72-kDa DNA-binding protein using H2-67 monoclonal antibody (5a), but found that this was not the case, since these p72 and the E2-72kDa species do not precisely comigrate and no E4orf6 was evident in H2-67 precipitates (data not shown). Complex formation with p72 was independent of E1B-55kDa, as was that of all binding proteins in this study apart from p68, which appeared to require this E1B product, and E1B-55kDa itself (Fig. 6, lane 3). Thus p68 may be present indirectly because it interacts with the 55-kDa product. The p55 binding species is certainly E1B-55kDa, since it was absent with AdE4orf6 (Fig. 5, lanes 10 and 12; Fig. 6, lane 4) and with mutant E1B/55K− (Fig. 6, lane 3). Complex formation with E1B-55kDa was first detected at 12 h p.i. (Fig. 2B, lane 3). p27 also appeared to be of viral origin and to bind to E4orf6. The p84 cellular protein was found to be phosphorylated (Fig. 4, lanes 2 and 4) and to be present in E4orf6 complexes by 12 h p.i. (Fig. 2B, lane 3). The p19 cellular protein was present in precipitates from AdE4orf6- and AdE4orf6/7-infected cells, indicating that it may interact with the 58-residue amino terminus shared by both E4 products. Finally, the p14 cellular protein appeared to associate with E4orf6 whereas the p16 host protein appeared to interact with E4orf6/7.
FIG. 7.
Complex formation between E4orf6 and the L4 100-kDa protein. An experiment similar to that described in the legend to Fig. 5, using H1299 cells and E4orf6-N serum or 2100K-1 anti-L4 100-kDa protein antibody, was performed with mock-infected cells (lanes 1 and 5) or those infected with wt Ad5 (lanes 2 and 6), mutant E1B/55K− (lanes 3 and 7), or dl1015 (lanes 4 and 8). Precipitates were analyzed by SDS-PAGE with gels containing 12% polyacrylamide. The positions of migration of molecular mass markers are shown on the left, and those of relevant proteins are shown on the right.
DISCUSSION
E4orf6 performs critical roles at both early and late stages of Ad infection of human cells. E4orf6 binds to a region toward the carboxy terminus of p53 and inhibits p53 transactivation activity (13). In addition, complex formation with E1B-55kDa results in the rapid turnover of p53, presumably by a mechanism affecting p53 molecules that are also present in these complexes (34, 37, 43, 51). These functions are critical at early times to protect infected cells from growth arrest and apoptosis resulting from the accumulation and activation of p53 induced by E1A proteins (5, 11, 16–18, 27, 44, 47). Late in infection E4orf6-55kDa complexes promote the selective transport of viral mRNAs and shutoff of host protein synthesis to enhance viral yields (1, 2, 6, 10, 19, 26, 41, 42, 48, 49, 57). The studies presented in this report indicated that E4orf6 is produced at early times, and even though its synthesis ceases after 24 h p.i., it is a highly stable protein and persists at high levels to the end of the infectious cycle. Similar results have been obtained by others (10). Thus, ample levels of E4orf6 are available to function during both the early and late phases. The stability of E4orf6 was somewhat greater in wt Ad5-infected cells than in those expressing E4orf6 alone, suggesting that complex formation with E1B-55kDa may enhance its stability. Preliminary studies with the Ad vectors AdE4orf6 and AdHis55K (3a) indicated that no increase in E4orf6 stability occurred, suggesting that proteins other than or in addition to E1B-55kDa may be involved. The overall pattern of accumulation of E4orf6 resembled that of another E4 product, E4orf4, but differed greatly from that of E4orf6/7, which shares 58 amino-terminal residues with E4orf6. E4orf6/7 was seen to be degraded rapidly, so that it was present only transiently during the early phase of the cycle, when it functioned to enhance E2F-dependent expression of E2, which encodes proteins necessary for viral DNA replication. As discussed above, it is unclear why E4orf6 synthesis ceases at later times.
The biological activity of E4orf6 may be regulated in at least two ways. First, we found that E4orf6 is weakly phosphorylated at one or more sites carboxy terminal to residue 58. Mapping of such sites would make it possible to evaluate the role of phosphorylation. Second, E4orf6 may be regulated through complex formation with other cellular or viral proteins. Two E4orf6-binding proteins are already known. E4orf6 interacts with and inhibits p53 (13). It has been suggested that the mechanism of this process may involve E4orf6 interactions that result in the release of TAFII31 and inactivation of the p53 transcription complex (13). E4orf6 also interacts with E1B-55kDa, and such complexes induce the rapid turnover of p53 (34, 37, 43, 51). The mechanism of this process is not understood but appears to require additional components. Later in infection, the E4orf6-55kDa complex functions in the transport of viral mRNAs and the shutoff of host cell protein synthesis. E4orf6 provides a shuttle for E1B-55kDa to enter and exit the nucleus (12, 14); however, it seems likely that additional primate-specific cellular proteins present in E4orf6-55kDa complexes must also contribute to these processes. It would therefore be informative to identify E4orf6-binding proteins.
Our present study has indicated that at least three cellular proteins, in addition to p53, and four or five viral or virally induced proteins interact with E4orf6. As summarized in Table 1, E1B-55kDa was confirmed as an E4orf6-binding protein, but a viral or virally induced p68 polypeptide also required the presence of E1B-55kDa, suggesting that p68 may interact with E4orf6 indirectly via this E1B product. All three cellular species appeared to bind directly to E4orf6. p19 seemed to interact with the amino terminus of E4orf6, since it was also detected with E4orf6/7, which shares a 58-residue amino terminus with E4orf6. The pp84 and p14 cellular proteins presumably interact with another region of E4orf6, since they were not found to interact with E4orf6/7. An additional p16 cellular protein was present when AdE4orf6/7 but not AdE4orf6 was used, suggesting that this protein interacts only with E4orf6/7. It is probable that the p102 viral or virally induced species represents the L4 100-kDa late nonstructural protein (22, 46a); however, our analyses indicated that it is present nonspecifically in immunoprecipitates and in fact does not appear to interact directly with E4orf6. The p72 species was not found to represent the E2 72-kDa DNA-binding protein; however, both p72 and p27 proteins appear to interact with E4orf6. Consistently higher levels of p72 were seen when the amino-terminal E4orf6-N serum was used. This effect may be related to the decreased ability of the carboxy-terminal E4orf6-C serum to interact with E4orf6 bound to these proteins, or it may be because they also bind to E4orf6/7 as well as E4orf6. At present, we are uncertain about the identities of these proteins, apart from E1B-55kDa. What might be the functions of such species? It is likely that additional cellular or viral proteins participate in the degradation of p53 by E4orf6-55kDa complexes. Cellular proteins may be involved in targeting E4orf6 into and out of the nucleus through interactions with the three E4orf6 nuclear targeting sequences. Finally, transport of viral mRNAs and host cell shutoff would appear to require additional proteins involved in binding viral transcripts and other activities required for their efficient transport and translation. Studies to characterize further the identities and binding sites for these E4orf6-associated proteins are under way.
ACKNOWLEDGMENTS
We thank the following colleagues for their invaluable contributions to this work: Tom Shenk for dl309; Ed Harlow for M73 hybridoma cells; Arnie Levine for 2A6 hybridoma cells, and Jane Flint for 2100K-1 antibody.
This work was supported by grants from the Medical Research Council of Canada and the National Cancer Institute of Canada. D.B. and E.Q. were supported by Fellowships or Studentships from the Fonds FRSQ-FCAR-Santé du Québec, and R.C.M. held a Student Research Award from the Glaxo/Burroughs-Wellcome Corporation for a period during this study.
REFERENCES
- 1.Babiss L E, Ginsberg H S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984;50:202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiss L E, Ginsberg H S, Darnell J J. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bacchetti S, Graham F L. Inhibition of cell proliferation by an adenovirus vector expressing the human wild type p53 protein. Int J Oncol. 1993;3:781–788. doi: 10.3892/ijo.3.5.781. [DOI] [PubMed] [Google Scholar]
- 3.Bayley S T, Mymryk J S. Adenovirus E1A proteins and transformation. Int J Oncol. 1994;5:425–444. doi: 10.3892/ijo.5.3.425. [DOI] [PubMed] [Google Scholar]
- 3a.Boivin, D. Unpublished data.
- 4.Boyd J M, Malstrom S, Subramanian T, Venkatesh L K, Schaeper U, Elangovan B, D’Sa-Eipper C, Chinnadurai G. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994;79:341–351. doi: 10.1016/0092-8674(94)90202-x. . (Erratum, 79:1120.) [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite A, Nelson C, Skulimowski A, McGovern J, Pigott D, Jenkins J. Transactivation of the p53 oncogene by E1a gene products. Virology. 1990;177:595–605. doi: 10.1016/0042-6822(90)90525-v. [DOI] [PubMed] [Google Scholar]
- 5a.Branton P E, Evelegh M, Rowe D T, Graham F L, Bacchetti S. Protein kinase and ATP-binding activity associated with the 72-kdalton single-stranded DNA-binding protein from early region 2A of human adenovirus type 5. Can J Biochem Cell Biol. 1985;63:941–952. doi: 10.1139/o85-117. [DOI] [PubMed] [Google Scholar]
- 6.Bridge E, Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 6a.Cepko C L, Sharp P A. Analysis of Ad5 hexon and 100K ts mutants using conformation-specific monoclonal antibodies. Virology. 1983;129:137–154. doi: 10.1016/0042-6822(83)90402-6. [DOI] [PubMed] [Google Scholar]
- 7.Challberg M D, Ketner G. Deletion mutants of adenovirus 2: isolation and initial characterization of virus carrying mutations near the right end of the viral genome. Virology. 1981;114:196–209. doi: 10.1016/0042-6822(81)90265-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Branton P E, Yang E, Korsmeyer S J, Shore G C. Adenovirus E1B 19-kDa death suppressor protein interacts with Bax but not with Bad. J Biol Chem. 1996;271:24221–24225. doi: 10.1074/jbc.271.39.24221. [DOI] [PubMed] [Google Scholar]
- 9.Chiou S-K, White E. p300 binding by E1A cosegregates with p53 induction but is dispensable for apoptosis. J Virol. 1997;71:3515–3525. doi: 10.1128/jvi.71.5.3515-3525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutt J R, Shenk T, Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987;61:543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 12.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B-55kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4282. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 14.Goodrum F D, Shenk T, Ornelles D A. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J Virol. 1996;70:6323–6335. doi: 10.1128/jvi.70.9.6323-6335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham F L, Smiley J, Russel W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 16.Grand R J, Grant M L, Gallimore P H. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology. 1994;203:229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- 17.Grand R J, Lecane P S, Roberts S, Grant M L, Lane D P, Young L S, Dawson C W, Gallimore P H. Overexpression of wild-type p53 and c-Myc in human fetal cells transformed with adenovirus early region 1. Virology. 1993;193:579–591. doi: 10.1006/viro.1993.1166. [DOI] [PubMed] [Google Scholar]
- 18.Grand R J, Owens D, Rookes S M, Gallimore P H. Control of p53 expression by adenovirus 12 early region 1A and early region 1B 54K proteins. Virology. 1996;218:23–34. doi: 10.1006/viro.1996.0162. [DOI] [PubMed] [Google Scholar]
- 19.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harlow E, Franza B J, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985;55:533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison T, Graham F L, Williams J. Host range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977;77:319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- 22.Hayes B W, Telling G C, Myat M M, Williams J F, Flint S J. The adenovirus L4 100-kilodalton protein is necessary for efficient translation of viral late mRNA species. J Virol. 1990;64:2732–2742. doi: 10.1128/jvi.64.6.2732-2742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J T, Schneider R J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991;65:271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- 24.Huang M M, Hearing P. The adenovirus early region 4 open reading frame 6/7 protein regulates the DNA binding activity of the cellular transcription factor, E2F, through a direct complex. Genes Dev. 1989;3:1699–1710. doi: 10.1101/gad.3.11.1699. [DOI] [PubMed] [Google Scholar]
- 25.Jones N, Shenk T. Isolation of adenovirus type 5 host-range deletion mutants defective for transformation of rat embryo cells. Cell. 1979;17:683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 26.Leppard K N, Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989;8:2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 28.Malette P, Yee S-P, Branton P E. Studies on the phosphorylation of the 58,000 dalton early region 1B protein of human adenovirus type 5. J Gen Virol. 1983;64:1069–1078. doi: 10.1099/0022-1317-64-5-1069. [DOI] [PubMed] [Google Scholar]
- 29.Marcellus R C, Teodoro J G, Charbonneau R, Shore G C, Branton P E. Expression of p53 in Saos-2 osteosarcoma cells induces apoptosis which can be inhibited by Bcl-2 or the adenovirus E1B-55 kilodalton protein. Cell Growth Differ. 1996;7:1643–1650. [PubMed] [Google Scholar]
- 30.Marton M J, Baim S B, Ornelles D A, Shenk T. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J Virol. 1990;64:2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGlade C J, Tremblay M L, Yee S P, Ross R, Branton P E. Acylation of the 176R (19-kilodalton) early region 1B protein of human adenovirus type 5. J Virol. 1987;61:3227–3234. doi: 10.1128/jvi.61.10.3227-3234.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLorie W, McGlade C J, Takayesu D, Branton P E. Individual adenovirus E1B proteins induce transformation independently but by additive pathways. J Gen Virol. 1991;72:1467–1471. doi: 10.1099/0022-1317-72-6-1467. [DOI] [PubMed] [Google Scholar]
- 33.Mitsudomi T, Steinberg S M, Nau M M, Carbone D, D’Amico D, Bodner S, Oie H K, Linnoila R I, Mulshine J L, Minna J D, Gazdar A F. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene. 1992;7:171–180. [PubMed] [Google Scholar]
- 34.Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mumby S M, Gilman A G. Synthetic peptide antisera with determined specificity for G protein α or β subunits. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- 36.Neill S D, Hemstrom C, Virtanen A, Nevins J R. An adenovirus E4 gene product trans-activates E2 transcription and stimulates stable E2F binding through a direct association with E2F. Proc Natl Acad Sci USA. 1990;87:2008–2012. doi: 10.1073/pnas.87.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc Natl Acad Sci USA. 1997;94:1206–1211. doi: 10.1073/pnas.94.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevins J R, Ginsberg H S, Blanchard J-M, Wilson M C, Darnell J E. Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979;32:727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen M, Branton P E, Walton P A, Oltvai Z N, Korsmeyer S J, Shore G C. Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus. J Biol Chem. 1994;269:16521–16524. [PubMed] [Google Scholar]
- 40.O’Malley R P, Mariano T M, Siekierka J, Mathews M B. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986;44:391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- 41.Ornelles D A, Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Querido E, Teodoro J G, Branton P E. Accumulation of p53 induced by the adenovirus E1A protein requires regions involved in the stimulation of DNA synthesis. J Virol. 1997;71:3526–3533. doi: 10.1128/jvi.71.5.3526-3533.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. . (Erratum, 89:9974.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raychaudhuri P, Bagchi S, Neill S D, Nevins J R. Activation of the E2F transcription factor in adenovirus-infected cells involves E1A-dependent stimulation of DNA-binding activity and induction of cooperative binding mediated by an E4 gene product. J Virol. 1990;64:2702–2710. doi: 10.1128/jvi.64.6.2702-2710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Riley D, Flint S J. RNA-binding properties of a translational activator, the adenovirus L4 100-kilodalton protein. J Virol. 1993;67:3586–3595. doi: 10.1128/jvi.67.6.3586-3595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Prieto R, Lleonart M, Ramón y Cajal S. Lack of correlation between p53 protein level and sensitivity of DNA-damaging agents in keratinocytes carrying adenovirus E1a mutants. Oncogene. 1995;11:675–682. [PubMed] [Google Scholar]
- 48.Sandler A B, Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989;63:624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarnow P, Sullivan C A, Levine A J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982;120:510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 51.Steegenga W T, Riteco N, Jochemsen A G, Fallaux F J, Bos J L. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 52.Teodoro J G, Branton P E. Regulation of p53-dependent apoptosis, transcriptional repression and cell transformation by phosphorylation of the 55kDa E1B protein of human adenovirus type 5. J Virol. 1997;71:3620–3627. doi: 10.1128/jvi.71.5.3620-3627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teodoro J G, Halliday T, Whalen S G, Takayesu D, Graham F L, Branton P E. Phosphorylation at the carboxy terminus of the 55-kilodalton adenovirus type 5 E1B protein regulates transforming activity. J Virol. 1994;68:776–786. doi: 10.1128/jvi.68.2.776-786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thimmappaya B, Weinberger C, Schneider R J, Shenk T. Adenovirus VA1 RNA is required for efficient translation of viral mRNA at late times after infection. Cell. 1982;31:543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- 55.Tigges M A, Raskas H J. Splice junctions in adenovirus 2 early region 4 mRNAs: multiple splice sites produce 18 to 24 RNAs. J Virol. 1984;50:106–117. doi: 10.1128/jvi.50.1.106-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virtanen A, Gilardi P, Naslund A, LeMoullec J M, Pettersson U, Perricaudet M. mRNAs from human adenovirus 2 early region 4. J Virol. 1984;51:822–831. doi: 10.1128/jvi.51.3.822-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberg D H, Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986;57:833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whalen S G, Marcellus R C, Whalen A, Ahn N G, Ricciardi R P, Branton P E. Phosphorylation within the transactivation domain of adenovirus E1A protein by mitogen-activated protein kinase regulates expression of early region 4. J Virol. 1997;71:3545–3553. doi: 10.1128/jvi.71.5.3545-3553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White E, Sabbatini P, Debbas M, Wold W S, Kusher D I, Gooding L R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor α. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature (London) 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 61.Yew P R, Liu X, Berk A J. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 1994;8:190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]