Abstract
Objective:
To evaluate the cross-culture application of the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE) to a cohort of Spanish-speaking patients with temporal lobe epilepsy (TLE) living in the U.S.
Methods:
Eighty-four Spanish-speaking patients with TLE completed neuropsychological measures of memory, language, executive function, visuospatial functioning, and attention/processing speed as part of the Neuropsychological Screening Battery for Hispanics (NeSBHIS). The contribution of demographic and clinical variables to cognitive performance was evaluated. A sensitivity analysis was conducted by examining the base rates of impairment across several impairment thresholds. The IC-CoDE taxonomy was then applied and the base rate of cognitive phenotypes for each cutoff was calculated. The distribution of phenotypes was compared to the published IC-CoDE taxonomy data, which utilized a large, multicenter cohort of English-speaking patients with TLE.
Results:
Across the different impairment cutoffs, memory was the most impaired cognitive domain, with impairments in list learning ranging from 50–78%. Application of the IC-CoDE taxonomy utilizing a −1.5SD cutoff revealed an Intact cognitive profile in 47.6% of patients, Single Domain impairment in 23.8% of patients, Bi-Domain impairment in 14.3% of patients, and Generalized impairment in 14.3% of the sample. This distribution was comparable to the phenotype distribution observed in the IC-CoDE validation sample.
Significance:
We demonstrate a similar pattern and distribution of cognitive phenotypes in a Spanish-speaking epilepsy cohort compared to an English-speaking sample. This suggests stability in the underlying phenotypes associated with TLE and applicability of the IC-CoDE for guiding cognitive diagnostics in epilepsy research that can be applied to culturally and linguistically diverse samples.
Introduction
Epilepsy is the fourth most common neurological disorder, following migraine, dementia, and stroke, affecting approximately 50 million people worldwide and accounting for 0.5% of the global burden of disease1, 2. Cognitive dysfunction is a highly prevalent comorbidity in epilepsy, impacting individuals’ quality of life3–5, and leading to poor psychosocial outcomes6, including lower education7 and occupational attainment8. Given this, understanding the cognitive profiles across and within epilepsy syndromes has been an ongoing research inquiry for over a century. Importantly, a plethora of studies have demonstrated that in the focal epilepsies cognitive dysfunction is less circumscribed than hypothesized by the classical lesion model [e.g., memory impairments in temporal lobe epilepsy (TLE)] and that significant cognitive heterogeneity exists within epilepsy syndromes (for review see Hermann et al.9).
In efforts to better understand this heterogeneity, investigations have focused on identifying patterns of cognitive deficits or cognitive phenotypes9. Across studies in focal epilepsy, three-to-four phenotypes have been identified with stable and reproducible cognitive patterns including a group with generalized impairment, a group with focal deficits, and a third group with intact profiles9–18. This phenotype approach has proven useful for establishing links between distinct neural abnormalities and patterns of cognitive impairment11, 12, 14, 16 and predicting cognitive progression10 and postoperative outcomes18. Despite an extensive literature focused on the neuropsychological syndromes of the epilepsies and the recent advances in cognitive phenotyping, there are no evidence-based criteria for guiding cognitive diagnostics in epilepsy research.
To address this critical gap in epilepsy research, the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE) was developed as a joint initiative between the International League Against Epilepsy (ILAE) Neuropsychology Task Force and the International Neuropsychological Society (for more information see Norman et al.19). The IC-CoDE is a consensus-based classification system that incorporates a five-domain cognitive model (i.e., language, memory, executive function, attention/processing speed, and visuospatial abilities) and provides an operational definition of impairment and an expert consensus-based diagnostic approach19, 20. In the first proof-of-principle study, the IC-CoDE taxonomy was found to be stable and reproducible across six independent epilepsy cohorts of adults with TLE (N=1,409) across the United States, and the distribution of phenotypes were similar to previous findings based on data-driven approaches20, demonstrating its potential utility as a diagnostic tool for research. An important advantage of the IC-CoDE is that it was intentionally developed to be applied to diverse patient cohorts regardless of the tests and normative data used. This approach is critical for the cross-cultural application of such taxonomy to linguistically and culturally diverse samples that have been traditionally underrepresented in epilepsy research.
Spanish-speaking individuals represent the fastest growing linguistically diverse population living in the U.S.21 Importantly, Hispanic/Latinx individuals are disproportionally impacted by the epilepsies22, with Hispanic adults having a twofold higher incidence of epilepsy relative to their Non-Hispanic White counterparts23. Furthermore, Hispanic/Latinx individuals are impacted by disparities in access to care, epilepsy treatments, and epilepsy outcomes7, 24, 25. Disparities in access to neuropsychological services is further exacerbated by the dearth of neuropsychological resources (e.g., culturally-adapted tests, appropriate norms) available for evaluating Spanish-speaking patients26, 27 and the lack of validated diagnostic frameworks28. In epilepsy specifically, Spanish-speaking populations have been underrepresented in all areas of research with only a few studies examining the clinical utility of neuropsychological tests in this population29–32. As the Spanish-speaking population in the U.S. continues to increase, validating neuropsychological frameworks will be critical to address disparities in access to care and advance epilepsy research. To this end, the IC-CoDE initiative aims to apply a consensus-based taxonomy to Spanish-speaking cohorts and examine factors that should be considered when applying the IC-CoDE to non-English speaking patients in order to determine its generalizability and international applicability and to facilitate global research efforts in epilepsy.
In this study, we apply the IC-CoDE to a sample of Spanish-speaking adults with TLE living in the U.S. who completed the Neuropsychological Screening Battery for Hispanics (NeSBHIS) as part of their epilepsy surgery workup. The NeSBHIS was developed to address the dearth of neuropsychological tests available for Spanish-speakers26, 33, and its clinical utility in epilepsy evaluations has been examined in several epilepsy samples29–32. Barr et al., demonstrated that the test battery was sensitive in detecting impairments in cognitive processes commonly observed in patients with epilepsy29. In a follow-up study, Bender et al. examined the construct validity of the NeSBHIS, providing evidence for the battery’s stable structure and validity as a cognitive assessment tool for epilepsy30. Other studies have demonstrated its predictive validity in determining seizure laterality31, 32. Here, we also examine the rates of impairment at the individual test level considering several impairment cut-offs and discuss demographic and cultural factors that are important to consider when applying the IC-CoDE.
Methods
Participants
Data were acquired from a consecutive series of 84 adults (18 years or older) with TLE who had completed a neuropsychological evaluation at the NYU-Langone Comprehensive Epilepsy Center between 2000 and 2012. All participants self-identified as “Hispanic” or “Latino/a/x”, emigrated from Spanish-speaking countries, and requested a Spanish-language assessment. A diagnosis of TLE was confirmed in each case through continuous video/EEG (VEEG) monitoring in accordance with the criteria defined by the ILAE34.
Procedures and measures
The present study was performed according to the policies of the Institutional Review Board of NYU School of Medicine. Data were collected as part of an IRB-approved data registry. Patients completed an inpatient presurgical evaluation, however, all patients were on antiseizure medication (ASM) at the time of testing per testing protocol. Patients were tested individually in one or two sessions totaling approximately 90 minutes. All tests were administered and scored by bilingual (Spanish-English) examiners. The NeSBHIS was designed to evaluate the domains of language, memory, visuospatial functions, mental control (i.e., attention and concentration), and psychomotor speed26, 33. Most measures in the battery were adapted from subtests used internationally by the World Health Organization.
The following measures were selected from the NeSBHIS battery to assess the cognitive domains of language, memory, executive function, visuospatial, and attention and processing speed as outlined by the IC-CoDE taxonomy20. Supplementary Table 1 contains a full description of the tests. Language ability included naming measured with the Pontón-Satz Boston Naming Test (P-S BNT)35 and verbal fluency measured with the Controlled Oral Word Association Test36 (COWAT; letters F-A-S). Memory was evaluated with the delayed scores of the WHO-UCLA Auditory Verbal Learning Test (AVLT)37 and the Rey Osterrieth Complex Figure Test (ROCFT)38. Only the delayed scores were used in this study to enable a direct comparison with the original IC-CoDE sample. The Taylor scoring system was used to score and evaluate the accuracy of the designs produced for the ROCFT39. Attention was measured with the Digit Span subtest from the Escala de Inteligencia Wechsler para Adultos (EIWA)40. Processing speed was measured with the Digit Symbol subtest from the EIWA40 and Color Trails condition 141. Measures of executive function included set-shifting evaluated with Color Trails condition 241 and abstract reasoning evaluated with the Raven’s Standard Progressive Matrices42. Lastly, visuospatial abilities were measured with the Block Design Subtest from the EIWA40 and the copy condition of the ROCFT. All raw scores were converted to z-scores adjusting for sex, age, and education using the normative data in Pontón et al.26
Test Impairment Analysis
Rates of impairment at the individual test level were calculated to compare the cognitive processes/tests that were most sensitive in this sample. Z-scores were classified as impaired or not impaired using ≤ −1.0, ≤ −1.5, and ≤ −2.0 standard deviation (SD) cutoffs. Although the IC-CoDE validation study only included −1.0SD and −1.5SD cutoffs, the diagnostic validity study of the NeSBHIS with an epilepsy sample utilized a −2.0SD impairment cutoff29, so we added this as an additional cutoff for purposes of this study. Base rates were calculated by dividing the number of patients classified as impaired on an individual test to the total number of patients who completed the measure.
Cognitive Phenotype Analyses
The IC-CoDE taxonomy was applied to patients who had at least two tests per cognitive domain as outlined in McDonald et al.20 This resulted in a final sample of 63 patients (75% of the initial sample). The cognitive phenotypes were generated using all three impairment cutoffs (i.e., ≤ −1.0, ≤ −1.5, and ≤ −2.0). There were two tests available for language, memory, executive function, and visuospatial abilities and three tests available for attention and processing speed. To be impaired in a domain, at least two tests per domain had to meet the selected cut-off. The total number of impaired domains was used to characterize the cognitive phenotype for each patient. As described in McDonald et al.20, Single-Domain is defined as impaired in one cognitive domain, Bi-Domain is defined as impaired in two domains, Generalized is defined as impaired in three domains or more domains, and Intact is defined as no impairment in any cognitive domain.
Statistical analyses
Spearman rho correlations were conducted to examine the association between test scores, education, and age. Chi-square goodness of fit tests were used to compare the proportion of patients in each cognitive phenotype in this sample to the IC-CoDE validation sample at each impairment threshold. Analysis of variance (ANOVA) and Fisher’s Exact (FE) tests were conducted to compare clinical, demographic, and cognitive data across the cognitive phenotypes. When results from the ANOVA were significant, group contrasts were assessed using post-hoc pairwise tests with Bonferroni correction. Multiple comparisons were corrected using Benjamini-Hochberg false discovery rate.43
Data Availability Statement
Authors have full access to all study data and take full responsibility for the data, the conduct of the research, the analyses and interpretation of the data, and the right to publish all data.
Results
Patient characteristics
Eighty-four patients were included in the study. Demographic and clinical characteristics are presented on Table 1. Patients ranged in age from 18 to 72 years, with the majority being female, right-handed, and 58.3% having an education of a high school degree equivalent or more. A majority of the sample can be categorized as originating from Mexico (7.1%), South America (Ecuador: 10.7% and Colombia: 8.3%), or the Caribbean (Dominican Republic: 13.1% and Puerto Rico: 9.5%). This distribution is consistent with the population trends among Hispanic immigrants residing in Northeastern United States. Regarding clinical characteristics, age of epilepsy onset ranged from before age 1 to 36 years, with a range of duration of disease from less than one year to 62 years. Approximately 74% of the patients were on ASM polytherapy. The most common pathology was mesial temporal sclerosis (MTS) and on average patients reported approximately 10 seizures per month (range 0.17–30).
Table 1:
Demographics and clinical variables across entire sample
| All TLE |
|
|---|---|
| N | 84 |
| Mean age | 37.81 (12.12) |
| Education | 11.33 (4.05) |
| Age of onset* | 13.96 (9.87) |
| Duration of epilepsy* | 25.37 (14.35) |
| Number of ASM | 1.83 (.787) |
| Seizure frequency per month (n=32) | 9.812 (8.35) |
| Side of seizure onset: L/ R/ B | 33 (39.8%) / 38 (45.8%) / 12 (14.5%) |
| MRI Etiology (n=51) | |
| Mesial temporal sclerosis (MTS) | 18 (35.3%) |
| Temporal lesions (non-MTS) | 8 (15.7%) |
| Mutilobar (including temporal) | 4 (7.8%) |
| Evidence of atrophy | 3 (5.9%) |
| Multifocal | 1 (2%) |
| MRI within normal limits | 17 (33.3%) |
| Sex: Female | 57 (67.9%) |
| Handedness: Right | 73 (88%) |
| Region of origin | |
| North America | 6 (7.1%) |
| Central America | 7 (8.3%) |
| South America | 26 (31%) |
| Caribbean | 20 (23.8%) |
| Unknown | 25 (29.8%) |
83% of patients had data available
ASM: antiseizure medications; TLE: temporal lobe epilepsy; L: left; R: right; B: bilateral
Contribution of demographic variables to cognitive performance
Table 2 displays the Spearman rho correlations between age and education and the raw and z-scores across the neuropsychological measures. For the raw scores, older age was associated with poorer performance on processing speed (Digit Symbol) and visuospatial abilities (ROCFT Copy), and fewer years of education was associated with poorer performance across all neuropsychological measures. Although raw scores were corrected for age, education, and sex, given the small sample sizes of the normative data (N=300), we were interested in examining whether residual effects of these demographic variables remained after applying the normative adjustments. Given the small sample size, scores that were greater than three SDs below or above the mean of the patient group were removed for these analyses. This resulted in the removal of five scores across all measures. Fewer years of education was associated with worse performance on naming (P-S BNT), simple attention (Digit Span), and processing speed (Color Trails 1). Figure 1 illustrates scatterplots of the significant correlations. Given the impact of education on the cognitive scores, as a post-hoc analysis we divided patients into low (i.e., <10 years) and high education (i.e., ≥ 10 years). Approximately 73% of the sample were categorized as having high education. For the low education group, education was not associated with performance on the P-S BNT (ρ=.250, p=.288), Digit Span (ρ =.390, p=.099), or Color Trails 1 (ρ=.435, p=.092). For the high education group, lower scores on the P-S BNT (ρ=.432, p<.001) and Color Trails 1 (ρ=.406, p=.003) were associated with fewer years of education.
Table 2:
Pearson correlations between neuropsychological score and demographic characteristics
| Raw Scores | Corrected Z-scores | |||
|---|---|---|---|---|
|
| ||||
| Age | Education | Age | Education | |
|
|
||||
| Language | rho (p-value) | rho (p-value) | rho (p-value) | rho (p-value) |
| P-S BNT | .137 (.215) | .589 (<.001) | −.137 (.220) | .411 (<.001) |
| Letter Fluency | .144 (.198) | .415 (<.001) | .048 (.670) | .165 (.142) |
| Memory | ||||
| AVLT-Delayed | −.143 (.194) | .273 (.012) | −.142 (.201) | .072 (.521) |
| ROCFT Memory | −.176 (.123) | .461 (<.001) | −.003 (.978) | .207 (.069) |
| Attention/Processing Speed | ||||
| Digit Span Total | .111 (.332) | .471 (<.001) | .135 (.235) | .339 (.002) |
| Digit Symbol | −.341 (.002) | .546 (<.001) | −.112 (.325) | .154 (.176) |
| Color Trails 1 | .122 (.318) | −.506 (<.001) | −.042 (.731) | .329 (.006) |
| Executive Function | ||||
| Color Trails 2 | .156 (.205) | −.486 (<.001) | −.116 (.353) | .240 (.052) |
| Raven’s Matrices | −.225 (.043) | .544 (<.001) | −.165 (.142) | .248 (.027) |
| Visuospatial | ||||
| Block Design | −.142 (.210) | .613 (<.001) | .042 (.710) | .234 (.036) |
| ROCFT Copy | −.271 (.014) | .339 (.002) | −.110 (.330) | .069 (.542) |
P-S BNT: Pontón-Satz Boston Naming Test; AVLT: Auditory Verbal Learning Test; ROCFT: Rey–Osterrieth Complex Figure Test
Bold: Significant with a false-discovery rate correction q* = .018
Italics: Significant at a p-value of .05
Figure 1. Association between education and cognitive scores.
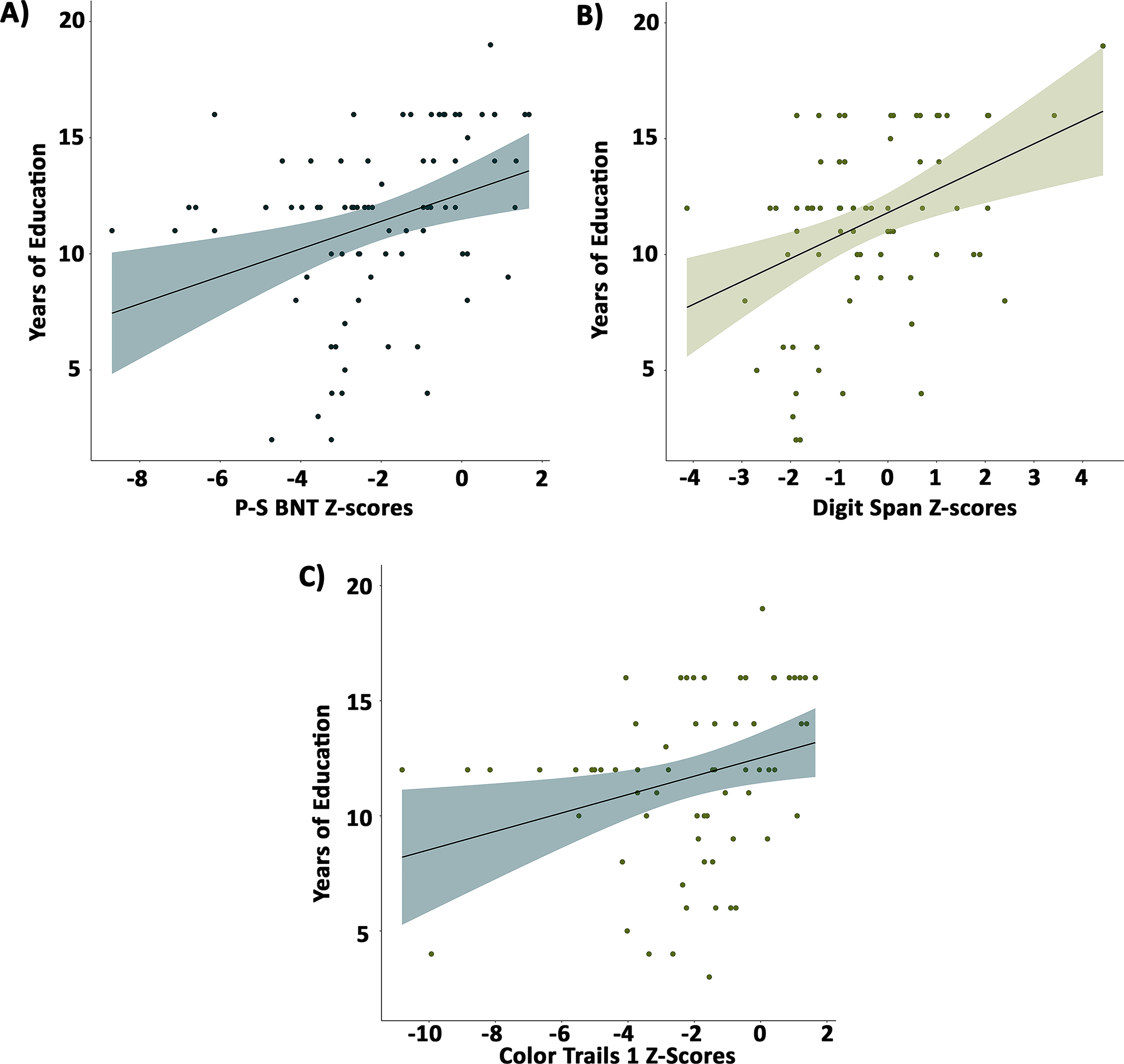
Greater years of education was associated with better performance in (A) naming, (B) simple attention, and (C) processing speed. P-S BNT: Pontón-Satz-Boston Naming Test
Impairment across measures
Figure 2 demonstrates the pattern of impairment across measures using the −1.0SD, −1.5SD, and −2SD cutoffs. Across the cutoffs, cognitive impairment was primarily observed in delayed word recall (AVLT-Delayed; 50%−78.3%), visual memory (ROCFT Memory; 31.7%−65.4%), naming (P-S BNT; 55.1% −64.3%), processing speed (Color Trails 1; 48.3%−63.8%) and set-shifting (Color Trails 2; 39%−51.5%).
Figure 2. Impairment rates at the individual test level for −1.0, −1.5, and −2.0 standard deviation cutoffs.
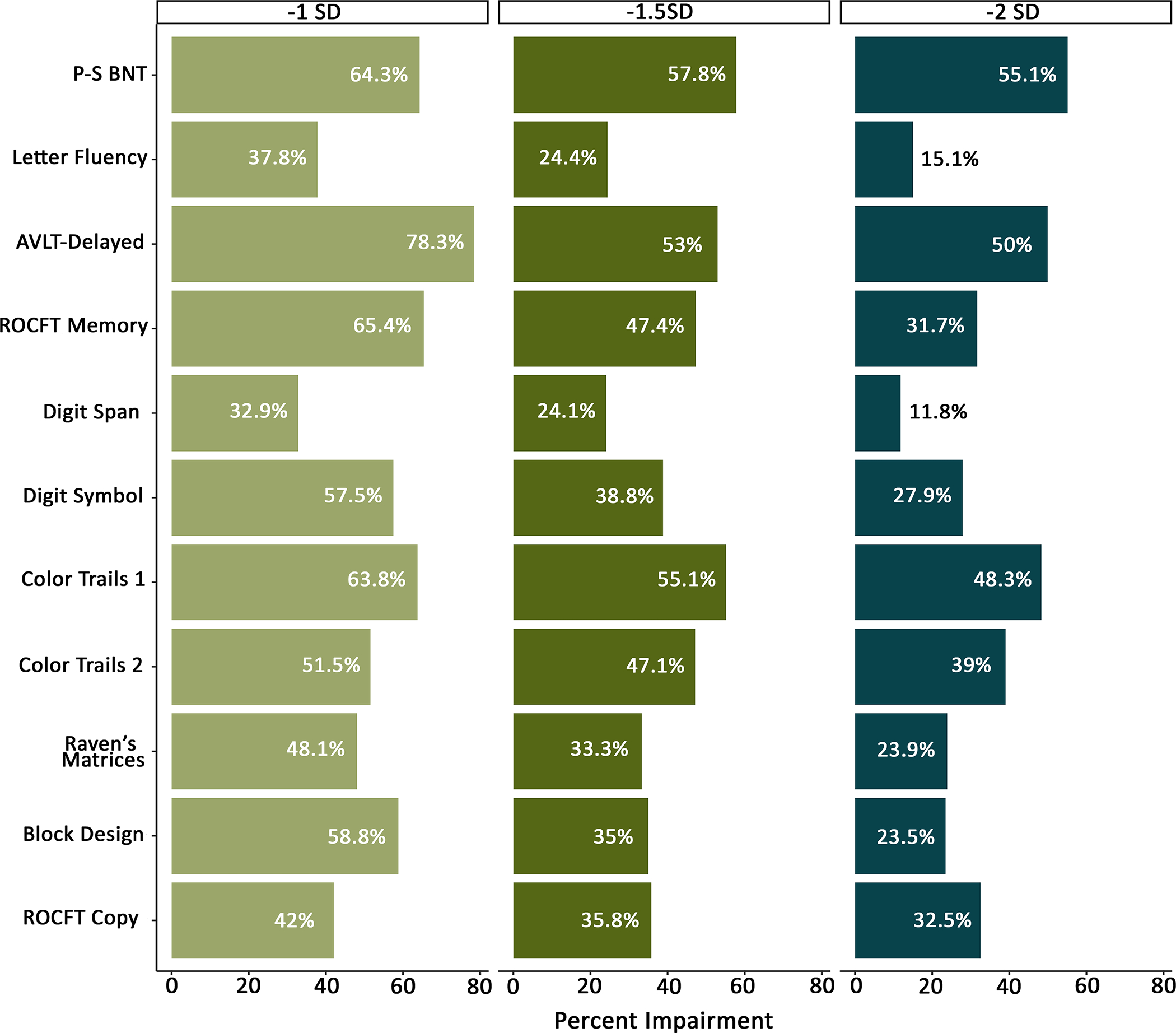
P-S BNT: Pontón-Satz Boston Naming Test; AVLT: Auditory Verbal Learning Test; ROCFT: Rey–Osterrieth Complex Figure Test
Given the effects of education on P-S BNT, Digit Span, and Color Trails 1, we also examined differences in the rates of impairment between the high and low education groups for the ≤ −1.5SD cutoff. The rates of impairment for the PS-BNT (FE=5.31, p=.036) were significantly different between the low education (80%) and high education group (50.8%)
As a post-hoc analysis, we also compared the rates of impairment between males and females for the −1.5 cutoff and found differences in AVLT Delayed (FE= 7.127, p= .010; males: 74.1% vs females: 42.9%) and Digit Span (FE= 5.857, p=.022; males: 41.7% vs females: 16.4%) with males demonstrating higher rates of impairment.
Cognitive phenotypes using the IC-CoDE taxonomy
The distribution of cognitive phenotypes for each cutoff and the distribution of the IC-CoDE validation sample are shown in Figure 3. Using a ≤ −1.0 SD cutoff, 34.9% of the sample demonstrated Generalized impairment with three or more domains impaired; 17.5% demonstrated a Bi-Domain phenotype; 22.2% showed a Single-Domain pattern; and 25.4% showed an Intact profile with no impairments across any of the domains. Using a ≤ −1.5 SD cutoff, a Generalized phenotype was observed in 14.3% of the sample; 14.3% of the sample demonstrated a Bi-Domain phenotype; 23.8% showed a Single-Domain phenotype; and 47.6% showed an Intact profile. Using a ≤ −2 SD cutoff, there were no patients that demonstrated a Generalized phenotype; 6.3% showed a Bi-Domain phenotype; 25.4% demonstrated a Single-Domain phenotype; and 68.3% showed an Intact profile. Post-hoc analyses revealed no differences across phenotype distribution between the low and high education groups for the −1.5 SD cutoff (FE=1.396, p=.652).
Figure 3. Cognitive phenotype distribution across impairment thresholds.
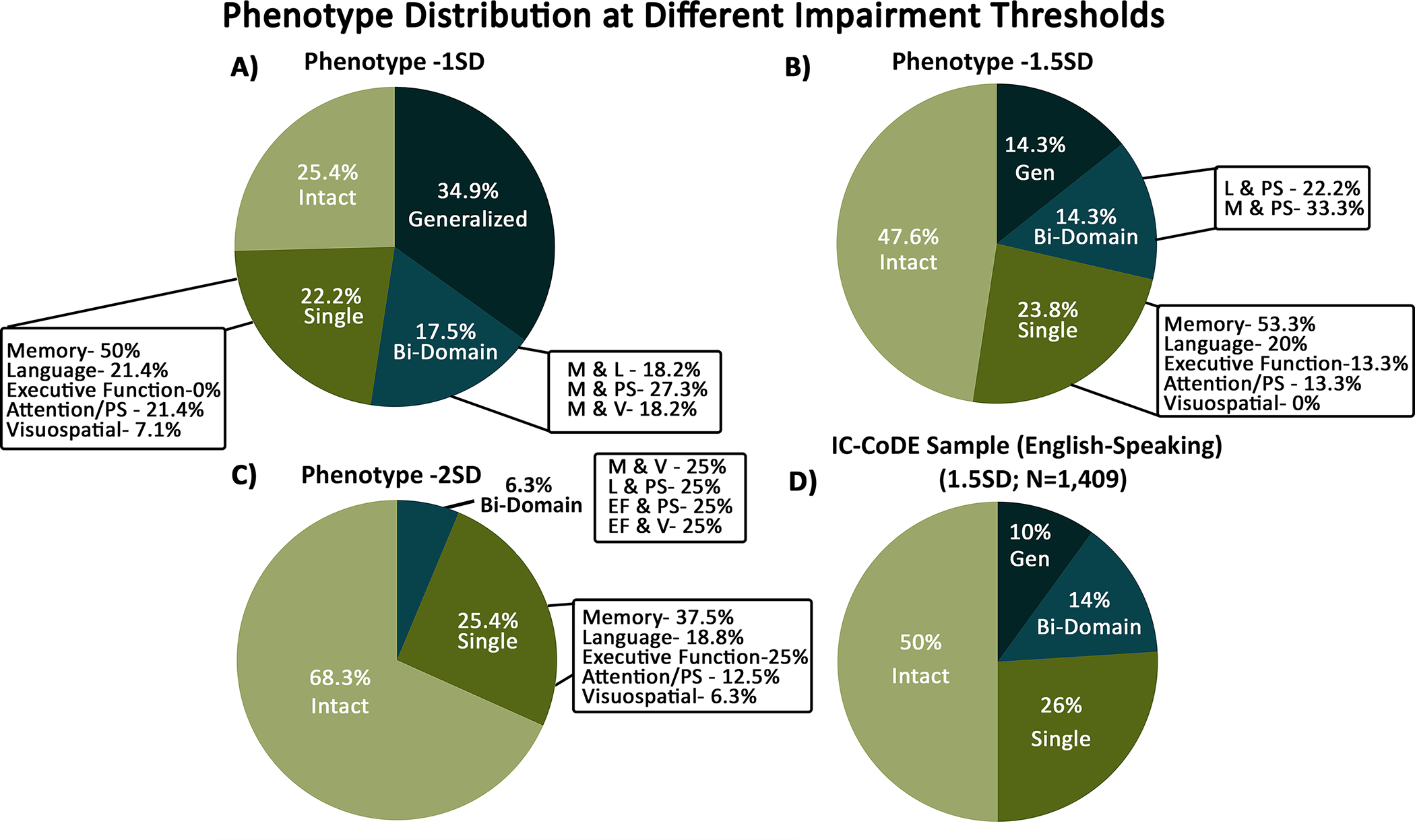
Distribution of phenotypes across (A) −1.0 SD cutoff, (B) −1.5 SD cutoff, and (C) −2.0 SD cutoff in the Spanish-speaking sample. Panel D shows the phenotype distribution of the IC-CoDE validation English-speaking sample utilizing the 1.5SD cutoff (McDonald et al., 2022). For the Bi-Domain distribution, subgroups (i.e., combinations of domains) with less than two patients are not illustrated. For the −2SD cutoff, all subgroups within the Bi-Domain phenotype had less than two patients and are not illustrated. PS: processing speed; M: memory; L: language; V: visuospatial
Comparison to the English-speaking validation sample
Supplementary Table 2 includes differences in comparisons between the weighted averages (i.e., across study sites) of demographic characteristics (i.e., age and education) and clinical characteristics (i.e., onset and duration) of the English-speaking validation sample to our Spanish-speaking sample. There were differences in education, age of onset, and duration, with the Spanish-speaking sample having fewer years of education, earlier age of seizure onset, and shorter duration of disease. There were no differences in age. Given that the −1.5SD cutoff was the suggested cutoff proposed by McDonald et al.20, the phenotype distribution for this cutoff was compared to the English-speaking validation sample. The Spanish-speaking sample demonstrated comparable rates across all the phenotype groups: Generalized phenotype (χ2= 1.218, p=.269), Bi-Domain (χ2= .005, p=.946), Single-Domain (χ2= .125, p=.696), and Intact (χ2= .139, p=.709). The −1.5SD cutoff was selected for subsequent analyses. Furthermore, the distribution of phenotypes was similar to the distribution reported for data-driven approaches (i.e., 21.1% generalized, 30.6% focal, and 43% intact)9, 20.
Demographic, clinical, and cognitive characteristics across phenotypes
Table 3 shows the demographic and clinical characteristics across the cognitive phenotypes. There were differences in the sex distribution across phenotypes, with the Single-Domain group having a lower proportion of females. There were no other differences in clinical and demographic variables.
Table 3:
Clinical and demographic characteristics across cognitive phenotypes
| Generalized | Bi-Domain | Single-Domain | Intact | F | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| N (%) | 9 (14.3%) | 9 (14.3%) | 15 (23.8%) | 30 (47.6%) | ||
| Age | 31.9 (8.24) | 37.3 (13.2) | 33.8 (13.1) | 39.5 (11.2) | 1.436 | .241 |
| Education | 10.1 (4.59) | 12.1 (3.65) | 12.8 (3.06) | 12.03 (3.46) | 1.005 | .397 |
| Onset | 14.8 (6.42) | 10.4 (11.9) | 12.5 (9.91) | 17.00 (9.71) | 1.192 | .323 |
| Duration | 16.4 (6.19) | 25.1 (13.9) | 23.5 (15.5) | 23.6 (13.9) | .460 | .712 |
| # ASMs | 1.77 (.667) | 2.00 (.534) | 1.86 (.833) | 1.96 (.865) | .176 | .912 |
| Seizure frequency (n=25) | 15 (6.00) | 5.67 (5.68) | 13.63 (11.46) | 4.12 (2.19) | 3.615 | .030 |
|
|
||||||
| Fisher’s Exact | p-value | |||||
|
|
||||||
| Sex: Female | 6 (66.7%) | 7 (77.8%) | 6 (40.0%) | 26 (86.7%) | 10.37 | .012 |
| Handedness: R | 8 (100%) | 8 (88.9%) | 13 (86.7%) | 26 (86.7%) | 1.018 | .877 |
| Side of seizure onset | 5.324 | .497 | ||||
| Left | 2 (22.2%) | 3 (33.3%) | 8 (53.3%) | 11 (37.9%) | ||
| Right | 5 (55.6%) | 6 (66.7%) | 6 (40%) | 12 (41.4%) | ||
| Bilateral | 2 (22.2%) | 0 (0%) | 1 (6.7%) | 6 (10.7%) | ||
| MRI Pathology (n=34) | 4.19 | .716 | ||||
| MTS | 2 (40%) | 1 (20%) | 3 (42.9%) | 5 (29.4%) | ||
| Non-MTS | 3 (60%) | 2 (40%) | 2 (28.6%) | 5 (29.4%) | ||
| WNL | 0 (0%) | 2 (40%) | 2 (28.6%) | 7 (41.2%) | ||
ASM: anti-seizure medications; R: right; mesial temporal sclerosis: MTS; WNL: within normal limits
Bold signifies significant with a false discovery rate correction
Discussion
Here, we provide results on the first cross-cultural application of the IC-CoDE in a Spanish-speaking sample of adults with TLE. We also demonstrate for the first time, the rates and distribution of cognitive phenotypes in this Hispanic cohort, which has been a burgeoning interest in epilepsy and other neurological disorders more broadly. Spanish-speaking individuals represent the fastest growing linguistically diverse population in the U.S., and there is a critical need to increase the representation of this population in epilepsy research. Utilizing the NeSBHIS, a neuropsychological battery that has been validated across several epilepsy cohorts, we demonstrate that the pattern of test impairment and the distribution of IC-CoDE-derived phenotypes are similar to previous published IC-CoDE findings20. We also test different impairment cut-offs and demonstrate the importance of exploring demographic factors that may be critical to consider when applying the IC-CoDE to culturally and linguistically diverse samples. Thus, our findings can serve as a model methodology for similar efforts in other languages. Importantly, the IC-CoDE provides a consensus-based cognitive taxonomic system that facilitates research focused on characterizing the presence and patterns of cognitive impairment within and across epilepsy syndromes and examining the underlying neurobiological, clinical, social, and cultural factors associated with the different phenotypes. Unlike other neurological disorders that share a common language for cognitive disorders thus facilitating harmonized research approaches, a common cognitive classification system in epilepsy had yet to be established. Therefore, the IC-CoDE provides a new approach for global communication and collaboration where researchers can pool large datasets across international samples even when different tests were administered in different languages.
Considerations of normative data
The dearth of neuropsychological instruments and normative data for diverse populations has been one of the most significant challenges and limitations to the field of neuropsychology for both clinical practice and research28. Several attempts have been made to develop normative data that provide comprehensive demographic adjustments for racial/ethnic and linguistically diverse groups. The NeSBHIS is one of the most frequently used normative datasets available for Spanish-speaking individuals, providing adjustments for age, education, and sex26. In our sample, years of education was associated with performance across all 11 cognitive measures, and age was associated with performance on two of the 11 measures (i.e., Digit Symbol and ROCFT Copy). After applying the NeSBHIS norms to the sample, the effects of age were corrected; however, the effects of education were corrected for only 8 of the 11 measures. Despite normative adjustments, fewer years of education continued to be associated with lower scores on naming, simple attention, and processing speed. Post-hoc analyses revealed a differential impact of education on cognitive scores based on high (i.e., ≥ 10 years) and low (i.e., <10 years) education, however, no clear pattern emerged. Cross-cultural studies have noted that in culturally and linguistically diverse populations, the relationship between education and neuropsychological performance is curvilinear rather than linear44. Thus, exploring different norms, including samples with a wide range of educational levels (e. g., low literacy) and including more than two neuropsychological measures per domain may provide greater insight into the effects of sociodemographic versus clinical factors on performance.
Importantly, when applying normative data to diverse cohorts, it is important to examine the demographic characteristics of the normative sample. The NeSBHIS normative sample was predominantly (i.e., 62%) Mexican-born Hispanics compared to our current sample which is 7.1% Mexican-born. Therefore, the NeSBHIS standardization sample may not accurately represent our sample introducing intracultural variability and potentially explaining the limited adjustments of the normative data for education. Another possibility is variability in quality of education across the cohort given the heterogeneity in country of origin which could be associated with differences in the education systems across countries. This limitation has been previously reported in other studies with epilepsy cohorts30, 31. Despite these limitations, applying other population norms to interpret test performance has been shown to lead to an overestimation of impairment across numerous of neuropsychological tests45. This is particularly important for the application of the IC-CoDE given that the diagnostic classification is based on impairment across a range of tests. Therefore, to reduce false-positives, we advocate for the use of population-specific demographically-adjusted norms when applying the IC-CoDE to culturally and linguistically diverse populations. Furthermore, reporting the contribution of demographic characteristics to cognitive performance will provide transparency on the generalizability of the results and highlight potential contributors to differences in cognitive phenotype rates across populations when using the IC-CoDE.
Patterns of impairment
Similar to previous IC-CoDE results20 and findings from the cognitive phenotype literature9, our cohort demonstrated impairments across all cognitive domains examined. Widespread cognitive deficits have been a consistent finding in the TLE literature19 and have been shown to be associated with widespread brain abnormalities, including cortical thinning, reduction in white matter integrity, and network disruption19, 46, 47. However, to the best of our knowledge, the are no neuroimaging studies in Spanish-speaking patients living in the U.S. and, given that these patients present with a host of other health-related risk factors impacting brain health, investigating the brain abnormities associated with the extent of cognitive impairment is warranted.
In this cohort, memory was the most commonly impaired domain, diverging from the results of the IC-CoDE validation sample in which language was the most commonly impaired domain20. In our sample, more than half of the sample was impaired in list learning and visual memory demonstrating higher rates of impairment relative to the IC-CoDE sample. Although visual memory tests have been shown to have greater variability in their lateralizing effects and lower sensitivity in detecting medial temporal lobe dysfunction48–51, Smith et al.31 demonstrated that the ROCFT Delay scores were significantly different between Spanish-speaking patients with right and left hemisphere seizure onset and it was a significant contributor to their lateralizing predictive model. Thus, it is possible that the sensitivity of visual memory tests is influenced by cultural and linguistic factors that have not been well studied in the neuropsychology of epilepsy. This highlights the potential subtle differences in cognitive profile characterization across linguistically diverse groups that may be due to differences in the underlying etiologies, the tests administered, the impact of culture and language on the cognitive constructs examined, or other non-epilepsy factors.
When examining impairment at the individual test level, P-S BNT had the highest impairment rates ranging from 55.1% to 64.3% depending on the cutoff used. This pattern is similar to the IC-CoDE validation sample, where we report impairment rates ranging from 53% to 67% for the BNT for −1SD and −1.5SD cutoffs, respectively. Furthermore, in the original diagnostic validation study of the NeSBHIS in epilepsy, Barr et al. 29 reported a 41.4% impairment rate for the P-S BNT using a −2SD cutoff in a heterogenous sample of Hispanic patients with epilepsy. Naming deficits are commonly reported in patients with TLE52; however, rates of impairment with the BNT are thought to be inflated due to limitations of the test that have been globally recognized and that we highlight in McDonald et al.20, including outdated stimuli, skewed distribution, and overestimation of impairment in non-native English speakers. Despite Ponton et al.’s35 important attempts to ensure cultural relevance and appropriateness of the original BNT in Spanish-speaking populations, in cultures with languages comprised of regional dialects (e.g., Spanish in Hispanics), it is highly unlikely to capture all existing correct synonymous options for a test item in confrontation naming test like the BNT27. Thus, it is possible that dialectical differences among our sample are also contributing to the high impairment rates on the P-S BNT. As efforts to apply the IC-CoDE to linguistically diverse samples continue, it is important to take into account the limitation of naming tests and their impact on phenotype classification and to consider other naming tests that have been shown to be more culturally sensitive such as the Multilingual Naming Test (MINT)53.
Application of the IC-CoDE
The application of the IC-CoDE to this sample yielded a similar pattern of phenotypes compared to the IC-CoDE validation sample20 and other findings using data-driven approaches10, 13–15. This suggests that there is stability in the overall pattern of phenotypes identified across cohorts, and now across languages. Importantly, the −1.5SD cutoff suggested by McDonald et al.20 was defined as an optimal cutoff given that it yielded a distribution of phenotypes that was consistent across the different epilepsy centers and approximated the distribution of phenotypes described in the epilepsy literature. This cutoff also appeared to be the optimal cutoff in our Spanish-speaking sample. Notably, the −2SD may have been too stringent given that this cutoff did not capture any generalized impairment. Although the distribution of the phenotypes was similar across both studies, there were within phenotype differences. For example, as noted above memory was the most commonly impaired domain within the Single domain phenotype across all three cutoffs followed by language. The opposite was observed for the original IC-CoDE where language was the most impaired domain followed by memory. For the Bi-Domain phenotype, the distribution of the subgroups for the Spanish sample was restricted to fewer subgroups, whereas the original IC-CoDE sample had six subgroups within the Bi-Domain phenotype for both cutoffs. The differences in the Bi-Domain distribution across studies may be due to a number of factors, including variability in the test batteries, the norms used, and specific patient characteristics. As other sites validate the IC-CoDE, further variability within the Single and Bi-Domain phenotypes groups may emerge which may allow for the examination of factors that are specific to the study versus those are more related to the disorder.
Notably, there were differences in demographic and clinical variables between the Spanish-speaking sample and the IC-CoDE validation sample, specifically the Spanish-speaking sample had fewer years of education and greater disease burden (i.e., earlier age of onset), which have been associated with greater cognitive impairment9. Interestingly, in our sample there were no differences in demographic or disease variables among the phenotype groups. The lack of differences in side of seizure onset across the phenotypes has been a consistent finding in the phenotype literature9, which has been attributed to the network nature of the focal epilepsies leading to more distributed cognitive and behavioral deficits. Although there has been some variability across the clinical profiles associated with different phenotypes12–15, a general pattern has emerged with patients with Generalized impairment demonstrating greater disease burden (e.g., longer duration of epilepsy, higher rates of mesial temporal lobe sclerosis, higher seizure frequency, and greater number of ASM), while those with an Intact phenotype have greater years of education and shorter disease duration. It is possible that given our small sample sizes across phenotypes we were not able to capture more subtle differences in clinical and demographic characteristics across the phenotypes. Another possibility is that given the limited research in this population, it is unclear whether classic epilepsy characteristics are associated with the extent of cognitive impairment as research on this topic has been generalized from less ethnically, racially, culturally, and linguistically diverse cohorts. This is particularly important as Hispanic patients are impacted by epilepsy and non-epilepsy related health disparities, and identifying the unique factors associated with cognitive deficits in this population can better inform research and clinical care.
Limitations and Future Directions
Despite this study being the first attempt at the cross-cultural application of the IC-CoDE, there are several limitations to address in future research. First, our sample was relatively small compared to the IC-CoDE validation sample, which can impact the base rates of cognitive phenotypes and differences in clinical profiles. Given that the NeSBHIS is a widely used tool in epilepsy, the IC-CoDE initiative can provide the infrastructure to develop multi-center collaborations aimed at replicating the current findings and investigating specific cognitive profiles across centers (e.g., single domain memory impairment). Second, there are limitations to the normative data used (e.g., relatively small normative sample), and education continued to be a significant contributor to cognitive performance despite normative adjustment for age, education, and sex. Education being stratified into two educational groups (i.e., < or > 10 years) may be contributing to the limited adjustments for education. Other normative approaches such as regression-based norms or normative data with more refined educational groups can potentially address this issue. Importantly, studies examining the utility of other comprehensive norms available for Spanish-speaking individuals collected in the U.S.54 including the NP-NUMBRS norms45 or that have been collected in countries in Latin America including the collection of norms published in the NeuroRehabilitation 2015 Special Issue “Commonly used Neuropsychological Tests for Spanish Speakers: Normative Data from Latin America”55 are needed. Specifically, it will be important to examine whether U.S. or Latin America collected norms are the most appropriate for Spanish-speaking immigrant populations living in the U.S. Further, studies examining the impact of acculturation and other factors known to impact neuropsychological performance such years living in the U.S. on norms and battery selection and phenotype distribution are warranted. Third, we included two tests per domain given that the NeSBHIS battery has a limited number of tests. Although consensus on the optimal number of tests to include per domain within the IC-CoDE classification system has yet to be stablished, the more tests included the greater probability of finding impairment at the domain level. However, it will be critical to systematically examine the optimal number of tests and which measures are the most sensitive to impairment in this population with a wider range of neuropsychological tests. Further, we only included delayed scores to probe for memory performance in order to follow the same approach as the original IC-CoDE sample and subsequent application in epilepsy17 and other disorders56, 57, however, the learning trials particularly for verbal memory tests can provide additional information that could further identify more defined single-domain phenotypes (e.g., learning impaired versus amnesic phenotype). Lastly, as noted above the NeSBHIS battery provides a limited number of tests per domain (e.g., two measures of language, one measure of verbal memory, two measures of visuospatial abilities). Although the measures included for language, processing speed, and executive function were similar to the original IC-CoDE study, there was less variability in measures of memory and visuospatial abilities. Specifically, the memory domain may have benefited from the inclusion of other measures such as prose recall and associative memory. Given that the NeSBHIS is currently the only battery that has been empirically validated in patients with epilepsy, the clinical utility of other Spanish neuropsychological batteries with a wider range of tests must be first validated in patients with epilepsy in order to deploy their clinical and research use. Despite these limitations, we demonstrate a similar pattern of impairment and phenotypes compared to the IC-CoDE validation sample, suggesting stability of this diagnostic framework that can inform its international applicability.
Supplementary Material
Key points.
We provide results on the first cross-cultural application of the IC-CoDE in a Spanish-speaking sample of adults with temporal lobe epilepsy.
Across the different impairment cutoffs, memory was the most impaired cognitive domain.
The rates and pattern of cognitive phenotypes in this Spanish-speaking sample was similar to previous phenotype findings.
These findings demonstrate that the IC-CoDE approach can be applied to culturally and linguistically diverse samples
Funding:
This research was supported by the National Institute of Health: R01 NS120976 (CRM); 1R01 NS-111022, R01 NS120976, U01 NS093650, and R01 NS117568 (BPH)
Footnotes
Competing Interests: None of the authors has any conflict of interest to disclose.
References
- 1.Bell GS, Neligan A, Sander JW. An unknown quantity--the worldwide prevalence of epilepsy. Epilepsia 2014;55:958–962. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Epilepsy: a public health imperative. 2019.
- 3.Perrine K, Hermann BP, Meador KJ, et al. The relationship of neuropsychological functioning to quality of life in epilepsy. Archives of Neurology 1995;52:997–1003. [DOI] [PubMed] [Google Scholar]
- 4.Giovagnoli AR, Avanzini G. Quality of life and memory performance in patients with temporal lobe epilepsy. Acta Neurologica Scandinavica 2000;101:295–300. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich T, Reyes A, Paul BM, et al. Beyond depression: The impact of executive functioning on quality of life in patients with temporal lobe epilepsy. Epilepsy Res 2019;149:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadr SS, Javanbakht J, Javidan AN, Ghaffarpour M, Khamse S, Naghshband Z. Descriptive epidemiology: prevalence, incidence, sociodemographic factors, socioeconomic domains, and quality of life of epilepsy: an update and systematic review. Arch Med Sci 2018;14:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burneo JG, Jette N, Theodore W, et al. Disparities in epilepsy: report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia 2009;50:2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennum P, Christensen J, Ibsen R, Kjellberg J. Long-term socioeconomic consequences and health care costs of childhood and adolescent-onset epilepsy. Epilepsia 2016;57:1078–1085. [DOI] [PubMed] [Google Scholar]
- 9.Hermann BP, Struck AF, Busch RM, Reyes A, Kaestner E, McDonald CR. Neurobehavioural comorbidities of epilepsy: towards a network-based precision taxonomy. Nat Rev Neurol 2021;17:731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 2007;13:12–20. [DOI] [PubMed] [Google Scholar]
- 11.Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes A, Kaestner E, Bahrami N, et al. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology 2019;92:e1957–e1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elverman KH, Resch ZJ, Quasney EE, Sabsevitz DS, Binder JR, Swanson SJ. Temporal lobe epilepsy is associated with distinct cognitive phenotypes. Epilepsy Behav 2019;96:61–68. [DOI] [PubMed] [Google Scholar]
- 14.Hermann B, Conant LL, Cook CJ, et al. Network, clinical and sociodemographic features of cognitive phenotypes in temporal lobe epilepsy. Neuroimage Clin 2020;27:102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes A, Kaestner E, Ferguson L, et al. Cognitive phenotypes in temporal lobe epilepsy utilizing data- and clinically driven approaches: Moving toward a new taxonomy. Epilepsia 2020;61:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Ramos C, Struck AF, Cook C, et al. Network topology of the cognitive phenotypes of temporal lobe epilepsy. Cortex 2021;141:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrotta K, Reyes A, Kaestner E, et al. Cognitive phenotypes in frontal lobe epilepsy. Epilepsia 2022;63:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baxendale S, Thompson P. The association of cognitive phenotypes with postoperative outcomes after epilepsy surgery in patients with temporal lobe epilepsy. Epilepsy Behav 2020;112:107386. [DOI] [PubMed] [Google Scholar]
- 19.Norman M, Wilson SJ, Baxendale S, et al. Addressing neuropsychological diagnostics in adults with epilepsy: Introducing the International Classification of Cognitive Disorders in Epilepsy: The IC CODE Initiative. Epilepsia Open 2021;6:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald CR, Busch RM, Reyes A, et al. Development and application of the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE): Initial results from a multi-center study of adults with temporal lobe epilepsy. Neuropsychology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passel JS, D’Vera Cohn D. US population projections, 2005–2050: Pew Research Center; Washington, DC, 2008. [Google Scholar]
- 22.Nathan CL, Gutierrez C. FACETS of health disparities in epilepsy surgery and gaps that need to be addressed. Neurology: Clinical Practice 2018;8:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Control CfD Prevention. Living well with epilepsy II; report of the 2003 National Conference on Public Health and Epilepsy: priorities for a public health agenda on epilepsy. 2019.
- 24.Burneo JG, Black L, Knowlton RC, Faught E, Morawetz R, Kuzniecky RI. Racial disparities in the use of surgical treatment for intractable temporal lobe epilepsy. Neurology 2005;64:50–54. [DOI] [PubMed] [Google Scholar]
- 25.Szaflarski M. Social determinants of health in epilepsy. Epilepsy Behav 2014;41:283–289. [DOI] [PubMed] [Google Scholar]
- 26.Ponton MO, Satz P, Herrera L, et al. Normative data stratified by age and education for the Neuropsychological Screening Battery for Hispanics (NeSBHIS): Initial report. J Int Neuropsychol Soc 1996;2:96–104. [DOI] [PubMed] [Google Scholar]
- 27.Bender HA. The neuropsychological assessment of culturally and linguistically diverse epilepsy patients. Handbook on the neuropsychology of epilepsy: Springer, 2015: 317–344. [Google Scholar]
- 28.Rivera Mindt M, Byrd D, Saez P, Manly J. Increasing culturally competent neuropsychological services for ethnic minority populations: A call to action. The Clinical Neuropsychologist 2010;24:429–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barr WB, Bender HA, Morrison C, Cruz-Laureano D, Vazquez B, Kuzniecky R. Diagnostic validity of a neuropsychological test battery for Hispanic patients with epilepsy. Epilepsy Behav 2009;16:479–483. [DOI] [PubMed] [Google Scholar]
- 30.Bender HA, Cole JR, Aponte-Samalot M, et al. Construct validity of the Neuropsychological Screening Battery for Hispanics (NeSBHIS) in a neurological sample. J Int Neuropsychol Soc 2009;15:217–224. [DOI] [PubMed] [Google Scholar]
- 31.Smith JAD, Kirmse R, Van Enkevort E, et al. Improving neuropsychological seizure lateralization in Spanish-speaking people with epilepsy in the US: The need to account for education and demographic differences. Epilepsy Behav 2020;104:106890. [DOI] [PubMed] [Google Scholar]
- 32.Lancman G, Vazquez-Casals GA, Perrine K, Feoli E, Myers L. Predictive value of Spanish neuropsychological testing for laterality in patients with epilepsy. Epilepsy Behav 2012;23:142–145. [DOI] [PubMed] [Google Scholar]
- 33.Ponton MO, Gonzalez JJ, Hernandez I, Herrera L, Higareda I. Factor analysis of the Neuropsychological Screening Battery for Hispanics (NeSBHIS). Appl Neuropsychol 2000;7:32–39. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 35.Pontón M, Satz P, Herrera L, et al. Modified Spanish version of the Boston naming test. The Clinical Neuropsychologist 1992;3:334. [Google Scholar]
- 36.Benton A. des Hamsher K: Multilingual Aphasia Examination. Iowa City. University of Iowa Press, 1976. [Google Scholar]
- 37.Maj M, D’Elia L, Satz P, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Archives of clinical Neuropsychology 1993;8:123–135. [PubMed] [Google Scholar]
- 38.Osterrieth PA. Le test de copie d’une figure complexe; contribution a l’etude de la perception et de la memoire. Archives de psychologie 1944. [Google Scholar]
- 39.Taylor EM. Psychological appraisal of children with cerebral defects. Psychological Appraisal of Children with Cerebral Defects: Harvard University Press, 2013. [Google Scholar]
- 40.Wechsler D. Manual para la Escala de Inteligencia Wechsler para adultos: Psychogical Corporation, 1972. [Google Scholar]
- 41.D’Elia F, Satz P, Uchiyama C. Color trails: Adult form manual. Odessa, FL: Psychological Assessment Resources; 1994;104:521–527. [Google Scholar]
- 42.Raven JC, Court JH. Raven’s progressive matrices and vocabulary scales: Oxford pyschologists Press Oxford, 1998. [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995;57:289–300. [Google Scholar]
- 44.Ostrosky-Solís F, Lozano A. Digit span: Effect of education and culture. International Journal of Psychology 2006;41:333–341. [Google Scholar]
- 45.Rivera Mindt M, Marquine MJ, Aghvinian M, et al. The neuropsychological norms for the US-Mexico border region in Spanish (NP-NUMBRS) project: Overview and considerations for life span research and evidence-based practice. The Clinical Neuropsychologist 2021;35:466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allone C, Lo Buono V, Corallo F, et al. Neuroimaging and cognitive functions in temporal lobe epilepsy: A review of the literature. J Neurol Sci 2017;381:7–15. [DOI] [PubMed] [Google Scholar]
- 47.Stasenko A, Lin C, Bonilha L, Bernhardt BC, McDonald CR. Neurobehavioral and Clinical Comorbidities in Epilepsy: The Role of White Matter Network Disruption. The Neuroscientist 2022:10738584221076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaz SAM. Nonverbal memory functioning following right anterior temporal lobectomy: a meta-analytic review. Seizure 2004;13:446–452. [DOI] [PubMed] [Google Scholar]
- 49.Barr WB, Chelune GJ, Hermann BP, et al. The use of figural reproduction tests as measures of nonverbal memory in epilepsy surgery candidates. J Int Neuropsychol Soc 1997;3:435–443. [PubMed] [Google Scholar]
- 50.Tallarita GM, Parente A, Giovagnoli AR. The visuospatial pattern of temporal lobe epilepsy. Epilepsy Behav 2019;101:106582. [DOI] [PubMed] [Google Scholar]
- 51.Bentvelzen AC, Kessels RP, Badcock NA, Savage G. The impact of right temporal lobe epilepsy on nonverbal memory: Meta-regression of stimulus-and task-related moderators. Neuropsychology Review 2021:1–21. [DOI] [PubMed] [Google Scholar]
- 52.Hamberger MJ. Object naming in epilepsy and epilepsy surgery. Epilepsy & Behavior 2015;46:27–33. [DOI] [PubMed] [Google Scholar]
- 53.Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Bilingualism: language and cognition 2012;15:594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morlett Paredes A, Gooding A, Artiola i Fortuny L, et al. The state of neuropsychological test norms for Spanish-speaking adults in the United States. The Clinical Neuropsychologist 2021;35:236–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guàrdia-Olmos J, Peró-Cebollero M, Rivera D, Arango-Lasprilla JC. Methodology for the development of normative data for ten Spanish-language neuropsychological tests in eleven Latin American countries. NeuroRehabilitation 2015;37:493–499. [DOI] [PubMed] [Google Scholar]
- 56.Matias-Guiu JA, Herrera E, González-Nosti M, et al. Development of criteria for cognitive dysfunction in post-COVID syndrome: the IC-CoDi-COVID approach. Psychiatry Research 2022:115006. [DOI] [PubMed] [Google Scholar]
- 57.Hancock LM, Galioto R, Samsonov A, Busch RM, Hermann B, Matias-Guiu JA. A proposed new taxonomy of cognitive phenotypes in multiple sclerosis: The International Classification of Cognitive Disorders in MS (IC-CoDiMS). Multiple Sclerosis Journal 2022:13524585221127941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Authors have full access to all study data and take full responsibility for the data, the conduct of the research, the analyses and interpretation of the data, and the right to publish all data.


