Abstract
Background
Invasive fungal infections carry a substantial risk of mortality and morbidity. Azole antifungals are used in the treatment of such infections; however, their extensive use can lead to the emergence of antifungal resistance and increased costs to patients and healthcare systems. The aim of this study is to evaluate trends in these antifungals use and costs.
Methods
The secular and regional trends of outpatient azole antifungals were analyzed using Medicare Part D Prescriber Public Use Files for the years 2013–2020. The total days supply (TDS), total drug cost (TDC) per 100 000 enrollees, and cost per day (CPD) were evaluated.
Results
The azole antifungal TDS for Medicare Part D enrollees increased by 12% between 2013 and 2020, and increases were noted for each azole. Southern US regions had the highest TDS, with Arizona having the highest TDS among US states in 2020. Cost analysis showed that TDC of all azoles has increased by 93% over the years, going up from $123 316 in 2013 to $238 336 per 100 000 enrollees in 2020. However, CPD showed an increase only for fluconazole and isavuconazole, with CPD of $1.62 per day and $188.30 per day, respectively.
Conclusions
Combined azole antifungal prescriptions TDS increased among Medicare Part D enrollees. The trend in CPD was mixed, whereas overall costs consistently increased over the same period. Such findings provide an insight into the impact of azole antifungal prescriptions, and increasing use could foreshadow more antifungal resistance. Continued studies to evaluate different prescribers’ trends are warranted.
Keywords: antifungal stewardship, healthcare cost, Medicare Part D
Invasive fungal infections are associated with detrimental health outcomes and high costs affecting the healthcare system and patients worldwide [1]. Antifungal use over the years has risen globally, possibly secondary to numerous factors, including the emergence of antifungal resistance, the rise in the immunocompromised population at risk of invasive fungal infection, and more widespread recognition of endemic mycoses [2–4]. In the United States, the overall cost of antifungals in community settings has risen [5], which could fiscally affect patients and the healthcare systems. Previous studies showed notable regional variability in the outpatient prescription patterns, with the southern states displaying higher rates of antifungal prescriptions in outpatient settings and antibiotic utilization among Medicare Part D enrollees [6, 7].
In recent years, there have been reports of azole-resistant Aspergillus species, with most clinical cases reported from Europe [8, 9]. However, one report showed the presence of resistant strains in the US environment, and the resistance was attributed to agricultural use of fungicides [10]. In addition to azole-resistant molds, yeasts that are resistant to multiple antifungals have been reported with increasing frequency in recent years, especially with the emergence of Candida auris, which is commonly resistant to multiple antifungal therapies [11]. Recent data show continued growth in C auris cases, increasing 44% in 2019 and 95% in 2021, with the majority (86%) resistant to azoles and an alarming trend of echinocandin resistance increase by 3-fold in 2021 [12]. There is also increased reporting of azole resistance in other non-albicans Candida spp, secondary to azole use [13], including hospital-acquired azole nonsusceptible Candida parapsilosis [14]. This overall pattern signals a future of increasing nosocomial spread of fungal pathogens that harbor resistance to multiple antifungal agents.
Azole antifungals are widely used in the treatment of invasive fungal infections. This group of antifungal agents includes several drugs that vary in their antifungal spectrum and cost [15], and, despite that, reports of fungal pathogens resistant to multiple azoles continue to emerge [13, 16]. Consequently, there is a need to track the prevalence of azole exposure and resistance rates to understand their potential impact on clinical outcomes and the broader public health landscape. The aim of this study is to describe the secular and geographical patterns of azole antifungal prescriptions and costs using Medicare Part D prescribers’ data.
METHODS
The publicly available Medicare Part D Prescriber Public Use File (PUF) data for 2013–2020 was used in this study [17]. Prescribers’ data from all states and the District of Columbia were included. Prescribing providers listed as locations “XX” = “Unknown”, “AA” = “Armed Forces Central/South America”, “AE” = “Armed Forces Europe”, “AP” = “Armed Forces Pacific”, “AS” = “American Samoa”, “GU” = “Guam”, “MP” = “Northern Mariana Islands”, “PR” = “Puerto Rico”, “VI” = “Virgin Islands”, and “ZZ” = “Foreign Country” were excluded.
Azole antifungal records were extracted (Supplement Materials) using the “Gnrc_Name” and “Brnd_Name” variables. Different formulations of the same medication were presented in aggregate because the database does not include formulation or route of administration. Claims per enrollees for each state for all medications and azole antifungal medications were extracted from the datasets. Total claims “Tot_Clms”, total day supply “Tot_Day_Suply”, and cost “Tot_Drug_Cst” for each azole antifungal were extracted and adjusted by dividing those values by the corresponding year's Medicare Part D enrollees per state, including only 50 states and the District of Columbia, data acquired from “Kaiser Family Foundation Medicare enrollment” dataset [18].
The total days supply (TDS) is the aggregate number of days for the prescribed medication, and the total drug cost (TDC) was defined as the aggregate drug cost paid for all associated costs related to that medication, which included ingredient cost, dispensing fee, and sales tax based on the amounts paid by the Part D plan, Medicare beneficiary, government subsidies, and any other third-party payers [19].
Data for TDS and TDC were presented per 100 000 enrollees as the TDS or TDC divided by enrollees by state and year × 100 000. The cost per day (CPD) of the different azoles was determined as TDC divided by TDS. In addition, TDS and TDC were calculated per each state and regionally using the US census division and year for all azoles combined and each azole antifungal agent separately. Medicare Part D data provided the TDS and TDC for each prescriber record.
Statistical Analysis
Descriptive statistics were performed by year, region/division, state, and azole antifungal type for TDS, TDC, and CPD. Correlation between the years and TDC or TDS was evaluated using Pearson's correlation. The average total days’ supply and cost per 100 000 enrollees were measured and mapped by states using the Python “matplotlib.pyplot” package [20]. The CPD was presented by year as the minimum, 10th, 25th, median, 75th, and 90th percentile, and maximum values—median is a descriptor metric similar to a minimum, 75th percentile, and others. The median CPD was considered the most reliable estimate of azole cost per day. Microsoft Excel, Python 3.8, SAS version 9.4 (SAS Institute, Cary, NC), and Stata (College Station, TX) 16.1/IC were used in the analysis of the data and creation of figures.
Patient Consent Statement
The study was deemed exempt by the institutional review board of the University of Arizona because the study used public data that included prescribers’ information, and no human subjects were involved or identified in the study.
RESULTS
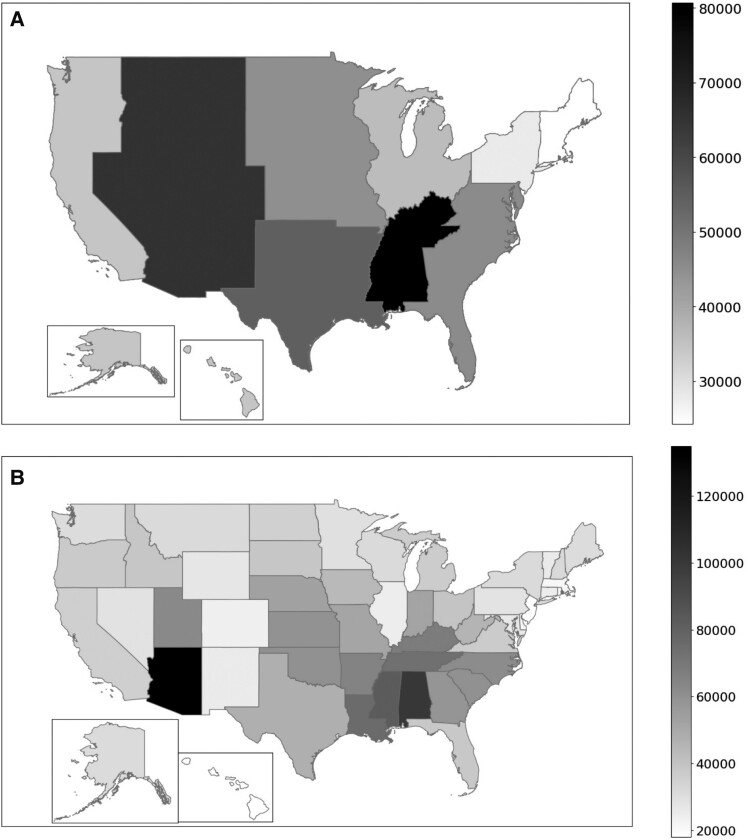
Over the study period, Medicare Part D enrollment increased from 34 199 317 in 2013 to 44 317 390 in 2020. The antifungal azoles TDS and TDC showed varying trends over the years and between US regions and states. In 2020, as seen in Figure 1A and Supplementary Table 1, the East South Central region of the United States used the highest TDS of azoles at 80 664 per 100 000 enrollees, followed by the West Mountain region with 65 533 per 100 000 enrollees. There was also considerable variation within each region. At the state level, TDS was the highest in Arizona for 2020, with 134 992 per 100 000 enrollees, followed by Alabama, with 100 671 per 100 000 enrollees (Figure 1B, Supplementary Table 2). Considering individual azoles, there was a state difference in 2020 TDS, with Arizona having the highest TDS per 100 000 enrollees for all azole antifungals, except voriconazole. For fluconazole, Arizona had 121 687 TDS per 100 000 enrollees, followed by Alabama with 98 212 and Mississippi with 81 451. For itraconazole, Arizona had 7720, followed by Ohio with 2722, and Nebraska with 2415 per 100 000 enrollees. Arizona also led with posaconazole TDS with 2740, followed by California with 1414 and New York with 1152 per 100 000 enrollees. Arizona claimed the highest TDS for isavuconazole with 1421, followed by Nebraska and California with 1110 and 777 per 100 000 enrollees, respectively. Finally, for voriconazole, TDS was the highest in New Mexico with 3664, followed by New York and Texas with 3438 and 2029 per 100 000 enrollees, respectively.
Figure 1.
Regional differences in azole total days supply (TDS) per 100 000 Medicare Part D enrollees for 2020. (A) shows data for each census division, whereas (B) shows TDS by state. The TDS per 100 000 enrollees is presented, according to the legend.
Prescription Days Supply Trend
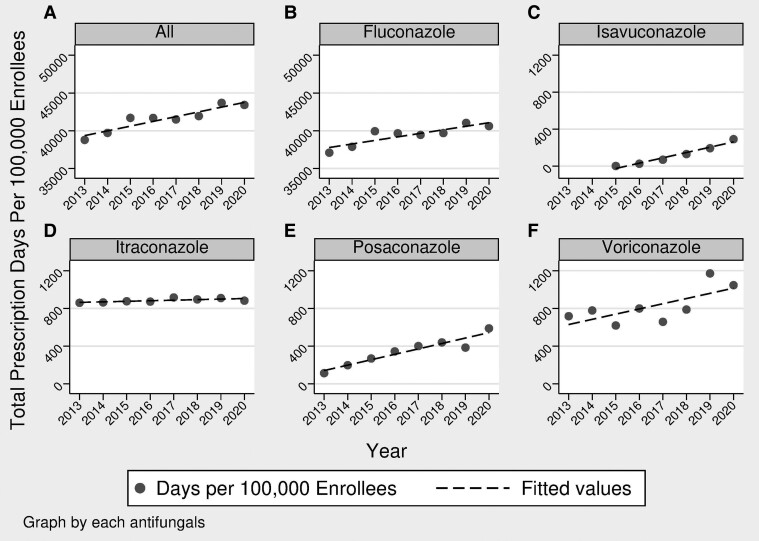
The annual trend of TDS per enrollees has been on the rise over the studied period, as seen in Figure 2. For posaconazole, the trend was reported from 2015 to 2020 because the delayed-release tablet was released early in 2014. Of note, isavuconazole was first approved in March 2015, so isavuconazole was only available for part of 2015; therefore, the trend analysis compared 2020 to 2016. Moreover, it should be noted that the voriconazole generic formulation was approved in 2011 [21]. The TDS per 100 000 enrollees for combined azole antifungals increased in 2020 by 11.95% compared to 2013 with a strong correlation (r = 0.93, P = .0009). The trend for each antifungal azole showed a similar pattern, with TDS per 100 000 enrollees for fluconazole rising by 9.48%, itraconazole by 2.7%, and voriconazole by 45.9% from 2013 to 2020. Posaconazole rose by 118.9% from 2015 to 2020, and isavuconazole rose by 1050.6% from 2016 to 2020. All of these antifungal azole TDS increases had a statistically significant correlation with year, except for itraconazole.
Figure 2.
Azole total days supply (TDS) per 100 000 enrollees for 2013 through 2020 showing trends over the years. (A) All triazole antifungals rose by 11.95%, (B) fluconazole rose by 9.48%, (C) isavuconazole rose by 1050.6%, (D) itraconazole rose by 2.7%, (E) posaconazole rose by118.9%, and (F) voriconazole rose by 45.9%. Besides posaconazole, which was analyzed over 2015–2020, and isavuconazole, which was analyzed over 2016–2020, all other panels show analysis over 2013–2020.
Cost Analysis
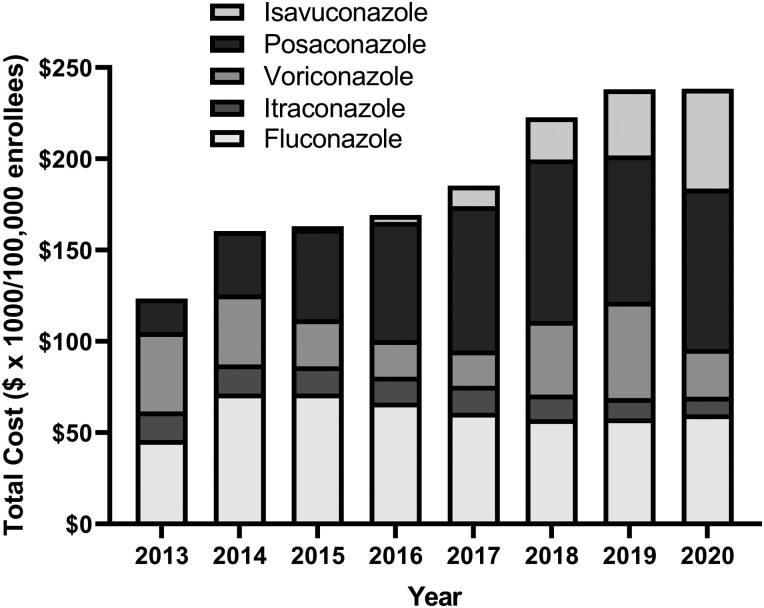
Over the years, there was an uptrend in all azoles TDC per 100 000 enrollees, as seen in Figure 3. All azoles combined trended from $123 316 per 100 000 enrollees in 2013 to $238 336 per 10 000 enrollees, which equated to a 93.3% increase (r = 0.97, P = .0001). Cost per 100 000 enrollees’ rise differed for individual azoles; from 2013 to 2020, fluconazole expenditures rose by 30.6%, itraconazole decreased by 38.2%, and voriconazole decreased by 39.6% between 2013 and 2020. For posaconazole, TDC per 100 000 enrollees increased by 76.8% between 2015 and 2020, and isavuconazole increased by 1333% between 2016 to 2020. The TDC for itraconazole, posaconazole, and isavuconazole showed strong correlations that were statistically significant with the corresponding year, r = −0.91 (P = .0015), r = 0.90 (P = .0021), and r = 0.92 (P = .0013), respectively.
Figure 3.
The total cost expressed in dollars × 1000 of azoles per 100 000 enrollees over the years. The transition from posaconazole suspension to delayed released tablets in 2014, and isavuconazole was introduced in 2015.
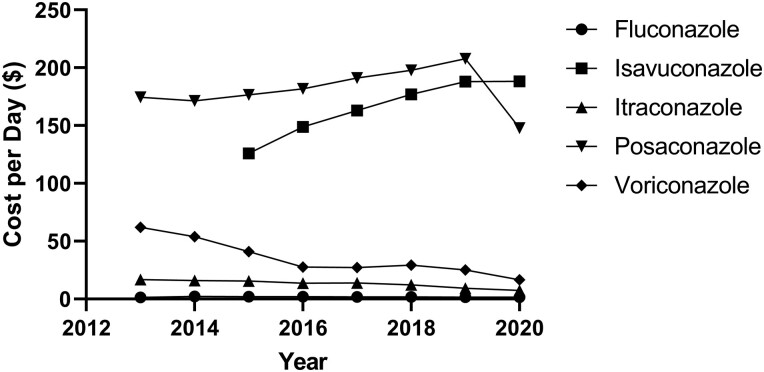
Cost per day analysis showed the highest median cost increase was for isavuconazole, with $188.30 CPD, and fluconazole was the lowest at $1.62 per day, as seen in Table 1. Besides fluconazole and isavuconazole, which showed an increase in the CPD over the years, all of the other agents had a decrease in the CPD over the studied period, as seen in Figure 4.
Table 1.
Cost Per Day of Individual Azoles Based on Medicare Claims Payments and List Costs for the Typical Daily Dose
| Azole | Median Cost/Day 2020 |
Average Annual Inflation Rate 2013*–2020 |
Typical Dose | WAC Cost Per Day Based On Typical Dose |
|---|---|---|---|---|
| Fluconazole | $1.62 | 2.00% | 200 mg every 24 hours | $0.41–3.00 |
| Isavuconazole | $188.30 | 8.37% | 372 mg every 24 hours | $214 |
| Itraconazole | $7.40 | −11.01% | 200 mg every 12 hours | $6.66–30.88 |
| Posaconazole | $147.75 | −4.05% | 300 mg every 24 hours | $48.50–60.00 |
| Voriconazole | $16.64 | −17.11% | 200 mg every 12 hours | $15.00–41.00 |
Abbreviations: WAC, wholesale acquisition cost.
NOTES: *Inflation rate was calculated from 2014 to 2020 for posaconazole because the delayed release tablet was approved in November of 2013. Inflation rate for isavuconazole was from 2015 to 2020 given the approval of that drug in 2015. A negative value for inflation rate denotes costs trending downward. The WAC (March 18, 2023) was obtained from the Red Book, Micromedex. The inflation rate is calculated as [(2020 cost/Original Cost)1/y −1] × 100%, where y is the time interval in years.
Figure 4.
Median cost per day of individual azoles determined from Medicare Claims versus typical dose/formulation and reported wholesale acquisition cost (WAC). The WAC was determined using Red Book Online. IBM Micromedex (https://www.micromedexsolutions.com). Truven Health Analytics/IBM Watson Health; 2023. Accessed 1 April 2023.
DISCUSSION
In this study, the analysis of Medicare Part D providers’ prescription dataset showed that azole antifungals TDS per 100 000 enrollees continued to increase over the years, but TDC and CPD for individual agents showed variable trends. There were also regional differences in the patterns of TDS for the azole antifungal prescriptions, with southern regions of the United States having higher prescription rates and costs than others.
There was an increase in the number of days per 100 000 enrollees for total azole prescriptions among Medicare Part D enrollees in the southern US regions, which was reported in a previous study evaluating outpatient antifungal prescriptions [7]. The reasons behind the regional variations observed in antifungal agent utilization are likely multifactorial, stemming from differences in types of fungal infection epidemiology, including those associated with mucocutaneous fungal infections. Moreover, a recent report has shown that outpatient antifungal prescriptions are more commonly prescribed by specialists in Obstetrics and Gynecology and Dermatology [7]; however, we did not evaluate this hypothesis in our study.
Another aspect that could have affected triazole antifungal prescription patterns is the updates in guidelines for the treatment of invasive fungal infections that coincided with the time of the US Food and Drug Administration approval of different new triazole antifungals [22–24], which may have affected the utilization of some of those antifungals, such as the use of isavuconazole as an alternative therapy for invasive aspergillosis, and the favoring of echinocandins for the treatment of invasive candidiasis to other azoles might have affected the trend and geographical distribution of triazole use.
The study also showed that the overall azole antifungal prescription days adjusted by the state's Medicare Part D enrollees was the highest in Arizona, as shown in the 2020 map. Although previous reports reflect a similar pattern of regional variation of antifungal or antibiotics prescriptions, this is the first report attributing the highest azole prescription rate to Arizona [6, 7]. In contrast to previous studies, our evaluation of azole antifungal utilization relied on measuring total days of prescribed azoles rather than claims per individual, because it better reflects community exposure. In addition, we adjusted for differences in Medicare Part D enrollees across various states. Such observation of high azole antifungal use in some US regions and states can be related to prolonged therapies for endemic mycoses, such as coccidioidomycosis, as seen in Arizona, which is one of the main endemic regions for this fungal infection [24]. Some patients require azole treatment for the remaining lifespan or for several years in cases of coccidioidomycosis involving meningitis or solid organ transplant (SOT) [13, 24, 25]. However, we did not observe a similar trend in California, which is another US state known for endemic coccidioidomycosis. One explanation for such discrepancy is that the proportion of Medicare Part D enrollees who receive azole therapy to the total number of enrollees in both states are different, taking into account that coccidioidomycosis is more endemic to the southern part of California and the at-risk population is much smaller than in Arizona [26]. A state-level analysis of azole antifungal prescriptions among the southwestern US states may miss complicated regional differences in azole prescriptions. These findings are important, because prolonged exposure to azole antifungals can lead to the emergence of resistant fungi [8, 9], as previously reported in the rise of non-albicans Candida spp among SOT in Arizona [13]. Although the coccidioidomycosis endemicity may explain the higher azole antifungal TDS, it does not explain the high TDS of other regions, like the south central one, which is driven mainly by Alabama, and other endemic mycoses like histoplasmosis [3] may not explain the differences, because fluconazole is not recommended for treatment of histoplasmosis. Another possible explanation could be related to tertiary centers that are specialized in treating complicated fungal infections, resistant fungi, and other endemic mycoses and differences in specialties prescribing outpatient antifungals [3, 7]. In addition, increased antibiotic use with associated increased risk for candidiasis could provide one explanation [6]. Other factors related to social and health disparities involving this region may lead to increased use of antifungal against certain fungal infections; as reported before that, candidemia cases are the highest in the south central and south Atlantic regions among 9 of the evaluated sites [27]. Another trend noted in this study is the azole-specific antifungal prescriptions differences between states. Besides voriconazole, all types of azole antifungals TDS were high among Arizona Medicare Part D enrollees, which may be explained by the dermatologic adverse effects associated with voriconazole, especially in areas with high sun exposure like Arizona [28], which may deter the prolonged prescription of voriconazole in southern states [25, 29]. However, despite the aforementioned risk of voriconazole adverse effects, in 2020, New Mexico and Texas had the highest TDS per enrollees. We also observed increased CPD of some of the azoles, whereas others had a reduction in cost over the years, reflected by both TDC and CPD. These differences reflect changes in the reformulation of older drugs or the introduction of new medications, such as the introduction of posaconazole, delayed-release tablets in 2014, and the introduction of isavuconazole in 2015 [30, 31]. In addition, we did not evaluate changes in individual azole antifungals per each state over the years to check whether specific antifungal use was associated with certain geographical regions that may have driven the increase of the use over the years. Finally, these trends of increased costs and prescriptions of antifungal agents have been observed in a previous study that analyzed dermatologists’ Medicare Part D prescriptions from 2013 to 2018. The study found that dermatologists’ prescription claims of terbinafine and ciclopirox increased during that period [32].
The study has several limitations, one of which is that it reflects Medicare Part D enrollees’ populations, which represent selection bias because only selected members of an elderly population enroll in the optional Medicare Part D prescription plans. This population may be at greater risk than the general population for fungal infections. In addition, the regional analysis is based on the prescribers’ National Plan & Provider Enumeration System (NPPES) listed state, which cannot distinguish between prescribers who work in different states and their corresponding NPPES listed address, which may create a bias in the regional prescriptions. Finally, the study analyzed data for outpatient use of azoles, which reflects less than half of the total azoles used in the United States, usually related to hospitalized patients [5, 33].
The study has several strengths. It included large Medicare Part D data spanning 8 years, providing a more comprehensive understanding of the secular trend and geographic distribution of azole antifungal prescriptions. In addition, the observation included adjusted prescribers’ data by the corresponding year of Medicare Part D enrollees, which is important to avoid biases related to population distribution between US regions and states. Finally, the cost data were evaluated using per enrollee and per day cost to look at cost inflation over the years using 2 different methods.
CONCLUSIONS
In conclusion, there was an increase in the clinical use of azole antifungals over the past years, accompanied by a more prominent increase in the total costs of these medications. Furthermore, novel therapeutic agents exhibit noteworthy enhancements and enhanced convenience but remain relatively expensive. These findings are important to consider from cost-effectiveness and potential regionally associated azole fungal resistance emergence in the United States. Future studies to evaluate utilization using other insurance or prescription data to evaluate the use of antifungals are warranted.
Supplementary Material
Acknowledgments
Contributor Information
Mohanad M Al-Obaidi, Division of Infectious Diseases, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Luis Ostrosky-Zeichner, Division of Infectious Diseases, University of Texas Medical School at Houston, USA, Houston, Texas.
David E Nix, Department of Pharmacy Practice and Science, University of Arizona, Tucson, Arizona, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Stop neglecting fungi. Nat Microbiol 2017; 2:17120. doi: 10.1038/nmicrobiol.2017.120 [DOI] [PubMed] [Google Scholar]
- 2. Jorgensen LN, Heick TM. Azole use in agriculture, horticulture, and wood preservation—is it indispensable? Front Cell Infect Microbiol 2021; 11:730297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazi PB, Sahrmann JM, Olsen MA, et al. The geographic distribution of dimorphic mycoses in the United States for the modern era. Clin Infect Dis 2023; 76:1295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pathadka S, Yan VKC, Neoh CF, et al. Global consumption trend of antifungal agents in humans from 2008 to 2018: data from 65 middle- and high-income countries. Drugs 2022; 82:1193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fitzpatrick MA, Suda KJ, Evans CT, Hunkler RJ, Weaver F, Schumock GT. Influence of drug class and healthcare setting on systemic antifungal expenditures in the United States, 2005–15. Am J Health Syst Pharm 2017; 74:1076–83. [DOI] [PubMed] [Google Scholar]
- 6. Arizpe A, Reveles KR, Aitken SL. Regional variation in antibiotic prescribing among Medicare Part D enrollees, 2013. BMC Infect Dis 2016; 16:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benedict K, Tsay SV, Bartoces MG, Vallabhaneni S, Jackson BR, Hicks LA. Outpatient antifungal prescribing patterns in the United States, 2018. Antimicrob Steward Healthc Epidemiol 2022; 1:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prigitano A, Esposto M, Romanò L, Auxilia F, Tortorano A. Azole-resistant Aspergillus fumigatus in the Italian environment. J Glob Antimicrob Resist 2019; 16:220–4. [DOI] [PubMed] [Google Scholar]
- 9. Lestrade PP, Meis JF, Arends JP, et al. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses 2016; 59:101–7. [DOI] [PubMed] [Google Scholar]
- 10. Hurst SF, Berkow EL, Stevenson KL, Litvintseva AP, Lockhart SR. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J Antimicrob Chemother 2017; 72:2443–6. [DOI] [PubMed] [Google Scholar]
- 11. Osei Sekyere J. Candida auris: a systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018; 7:e00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyman M, Forsberg K, Sexton DJ, et al. Worsening spread of Candida auris in the United States, 2019 to 2021. Ann Intern Med 2023; 176:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Obaidi MM, Marquez J, Afghan A, Zangeneh TT. Effect of coccidioidomycosis prophylaxis in selecting non-Candida albicans species amongst solid organ transplant recipients in Arizona. Mycoses 2023; 66:237–41. [DOI] [PubMed] [Google Scholar]
- 14. Alcoceba E, Gomez A, Lara-Esbri P, et al. Fluconazole-resistant Candida parapsilosis clonally related genotypes: first report proving the presence of endemic isolates harbouring the Y132F ERG11 gene substitution in Spain. Clin Microbiol Infect 2022; 28:1113–9. [DOI] [PubMed] [Google Scholar]
- 15. Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: which drug and when? Med Mycol 2019; 57(Supplement_2):S168–78. [DOI] [PubMed] [Google Scholar]
- 16. Howard SJ, Cerar D, Anderson MJ, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 2009; 15:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CMS . Medicare Part D prescribers. Available at: https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers. Accessed 8 February 2023.
- 18. KFF. Medicare beneficiaries enrolled in part D coverage . Available at: https://www.kff.org/other/state-indicator/medicare-beneficiaries-enrolled-in-part-d-coverage/?currentTimeframe=2&selectedRows=%7B%22states%22:%7B%22all%22:%7B%7D%7D,%22wrapups%22:%7B%22united-states%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed 1 April 2023.
- 19. CMS. Medicare part D prescribers—by provider and drug data dictionary. Available at: https://data.cms.gov/resources/medicare-part-d-prescribers-by-provider-and-drug-data-dictionary. Accessed 8 February 2023.
- 20. Matplotlib: a 2D graphics environment. Comput Sci Eng 2007; 9:90–5. [Google Scholar]
- 21. Mylan . Mylan launches first generic version of Vfend® tablets. Available at: https://investor.mylan.com/static-files/30fac70d-3014-486c-a4cf-40acef34407e. Accessed 15 June 2023.
- 22. Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 63:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galgiani J, Ampel N, Blair J, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 2016; 63:e112–46. [DOI] [PubMed] [Google Scholar]
- 25. Truong CN, Nailor MD, Walia R, Cherrier L, Nasar A, Goodlet KJ. Universal lifelong fungal prophylaxis and risk of coccidioidomycosis in lung transplant recipients living in an endemic area. Clin Infect Dis 2021; 4:1966–71. [DOI] [PubMed] [Google Scholar]
- 26. Sondermeyer Cooksey GL, Nguyen A, Vugia D, Jain S. Regional analysis of coccidioidomycosis incidence—California, 2000–2018. MMWR Morb Mortal Wkly Rep 2020; 69:1817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsay S, Mu Y, Williams S, et al. Burden of candidemia in the United States, 2017. Clin Infect Dis 2020; 71:e449–53. [DOI] [PubMed] [Google Scholar]
- 28. Epaulard O, Villier C, Ravaud P, et al. A multistep voriconazole-related phototoxic pathway may lead to skin carcinoma: results from a French nationwide study. Clin Infect Dis 2013; 57:e182–8. [DOI] [PubMed] [Google Scholar]
- 29. Al-Obaidi MM, Nematollahi S, Nix DE, Zangeneh TT. Remarks on the universal lifelong coccidioidomycosis prophylaxis in lung transplant recipients. Clin Infect Dis 2022; 74:1885–6. [DOI] [PubMed] [Google Scholar]
- 30. FDANews . FDA approves new antifungal drug from Astellas. Available at: https://www.fdanews.com/articles/170357-fda-approves-new-antifungal-drug-from-astellas. Accessed 4 July 2023.
- 31. MERCK . FDA approves Merck's NOXAFIL® (posaconazole) injection (18 mg/mL) for intravenous use. Available at: https://www.merck.com/news/fda-approves-mercks-noxafil-posaconazole-injection-18-mg-ml-for-intravenous-use/. Accessed 7 April 2023.
- 32. Wang Y, Lipner S. Analysis of utilization, cost and, prescription trends of onychomycosis medications among Medicare patients. J Am Acad Dermatol 2022; 86:440–42. [DOI] [PubMed] [Google Scholar]
- 33. Vallabhaneni S, Baggs J, Tsay S, Srinivasan AR, Jernigan JA, Jackson BR. Trends in antifungal use in US hospitals, 2006–12. J Antimicrob Chemother 2018; 73:2867–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.