Abstract
Background.
Extramural venous invasion (EMVI) on baseline MRI is associated with poor prognosis in patients with locally advanced rectal cancer. This study investigated the association of persistent EMVI after total neoadjuvant therapy (TNT) (chemoradiotherapy and systemic chemotherapy) with survival.
Methods.
Baseline MRI, post-TNT MRI, and surgical pathology data from 175 patients with locally advanced rectal cancer who underwent TNT and total mesorectal excision between 2010 and 2017 were retrospectively analyzed for evidence of EMVI. Two radiologists assessed EMVI status with disagreement adjudicated by a third. Pathologic EMVI status was assessed per departmental standards. Cox regression models evaluated the associations between EMVI and disease-free and overall survival.
Results.
EMVI regression on both post-TNT MRI and surgical pathology was associated with disease-free survival (hazard ratio, 0.17; 95% confidence interval (CI), 0.04–0.64) and overall survival (hazard ratio, 0.11; 95% CI, 0.02–0.68). In an exploratory analysis of 35 patients with EMVI on baseline MRI, only 6 had EMVI on pathology compared to 18 on post-TNT MRI; these findings were not associated (p = 0.2). Longer disease-free survival was seen with regression on both modalities compared with remaining positive. Regression on pathology alone, independent of MRI EMVI status, was associated with similar improvements in survival.
Conclusion.
Baseline EMVI is associated with poor prognosis even after TNT. EMVI regression on surgical pathology is common even with persistent EMVI on post-TNT MRI. EMVI regression on surgical pathology is associated with improved DFS, while the utility of post-TNT MRI EMVI persistence for decision-making and prognosis remains unclear.
INTRODUCTION
For locally advanced rectal cancer, intensification and deintensification of neoadjuvant regimens and operative management rely heavily on preoperative risk stratification using MRI.1 Among the high-risk features evaluable on MRI, extramural venous invasion (EMVI) has emerged as a feature of interest and debate. EMVI was first described in pathological specimens as tumor within the large vessels outside the muscularis propria. Its appearance on MRI has since been described in detail by multiple groups.2–6 Several studies have reported that EMVI is associated with risk of synchronous and metachronous distant metastases, shorter disease-free survival (DFS), and local recurrence.7–14 Different sensitivities and specificities have been reported for assessment of EMVI by MRI.15–19 MRI’s level of accuracy in assessing EMVI has implications both for selection of intensive treatment (baseline assessment) and for treatment deintensification such as watch-and wait management (post-neoadjuvant therapy assessment).
The association between EMVI at baseline and poor prognosis has been confirmed by multiple studies, including a 2021 meta-analysis.20 Evaluating the impact of EMVI regression after neoadjuvant therapy has been more challenging. The meta-analysis also found a significantly worse overall survival (OS) and DFS when EMVI is present after neoadjuvant therapy.20 A phase II prospective trial found a high rate of EMVI regression after neoadjuvant chemotherapy, but persistently worse DFS and recurrence compared to those who were EMVI negative at baseline.13 Other studies found no differences in survival between patients with EMVI regression and patients with residual EMVI based on MRI after neoadjuvant chemoradiotherapy8 and no association between EMVI regression and DFS in multivariable analyses.21 A pooled analysis of data from the EXPERT and EXPERT-C trials found that EMVI regression on posttreatment MRI was significantly associated with distant progression-free survival but not overall progression-free survival, local progression-free survival, or OS.22
At our center, the standard treatment for locally advanced rectal cancer is total neoadjuvant therapy (TNT) (chemoradiotherapy and systemic chemotherapy).23 In 2021, two randomized trials showed that TNT significantly increases the likelihood of pathologic complete response and significantly reduces the likelihood of metastasis.24,25 In the recently completed RAPIDO trial, comparing neoadjuvant short-course radiotherapy followed by chemotherapy to the standard of care, baseline EMVI was associated with worse prognosis, and the magnitude of the association seemed to differ by treatment arm.24 Patients in the experimental group who had EMVI on baseline MRI had a 29% probability of disease-related treatment failure compared with 38% for the standard-of-care group, suggesting a possible benefit of TNT for patients with EMVI. The effect of TNT on EMVI has not been examined in detail.
In this study, we evaluated the prognostic significance of baseline EMVI and posttreatment EMVI in patients with locally advanced rectal cancer who underwent TNT and total mesorectal excision. We sought to analyze oncologic outcomes for patients with EMVI at baseline and determine the implications of EMVI regression after TNT. We also sought to determine whether post-TNT assessment of EMVI by MRI or assessment of EMVI by surgical pathology is more closely associated with prognosis, as a means of assessing the reliability of post-TNT MRI for decision-making and prognostication.
METHODS
Patients
Patients with stage II or III rectal cancer treated with TNT and surgical resection at Memorial Sloan Kettering Cancer Center between July 2010 and May 2017 were identified retrospectively using an institutional database. Patients whose records did not include a baseline MRI or a post-TNT MRI were excluded from the study. Clinical, demographic, and survival data were collected from the electronic medical records, including age at diagnosis, clinical TNM classifications and disease stage (according to the 8th edition of the AJCC Cancer Staging Manual), tumor height from the anal verge, neoadjuvant therapy regimen, procedure date, pathologic grade, pathologic perineural invasion, margin status, and pathologic TNM classifications.26 A modified Charlson Comorbidity Index (excluding cancer) was calculated based on the National Cancer Institute’s comorbidity weights.27 Racial and ethnic characteristics were not readily available. The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center.
Treatment and Follow-Up
All patients underwent either neoadjuvant therapy- either systemic chemotherapy followed by chemoradiotherapy or chemoradiotherapy followed by systemic chemotherapy, at the discretion of the treating surgeon and medical oncologist. This TNT protocol has been described previously.23 Systemic chemotherapy typically consisted of eight cycles of leucovorin-fluorouracil-oxaliplatin or five cycles of capecitabine-oxaliplatin. For chemoradiotherapy, the recommended regimen at our institution is 50 to 50.4 Gy with concurrent infusional fluorouracil or oral capecitabine.23 Residual tumor was excised using total mesorectal excision. Postoperative follow-up followed the guidelines of the National Comprehensive Cancer Network.28
EMVI Assessment by MRI
All MRIs employed two-dimensional T2-weighted sequences in the oblique axial plane, sagittal plane, and oblique coronal plane.1 Two abdominal radiologists with expertise in cancer imaging (D.D.B.B. and J.G.P.; 3 and 5 years of post-training experience, respectively) independently reviewed the baseline and post-TNT MRIs for each patient. The post-TNT MRI was performed after the patient finished both parts of neoadjuvant therapy. If a patient underwent more than one post-TNT MRI, the first one following TNT was used for analysis.
EMVI was categorized using the five-point criteria developed by Smith et al.,16 with scores of 0 to 2 considered indicative of EMVI absence and scores of 3 and 4 considered indicative of EMVI presence (Table 1 and Fig. 1A and B). EMVI regression was defined as EMVI absence on post-TNT MRI in patients who had EMVI on baseline MRI. Differences between the assessments of the two radiologists were resolved by a third radiologist (J.L.F.; 14 years of post-training experience) with expertise in cancer imaging.
TABLE 1.
Classification of EMVI on MRI16
| Score | Features | Classification |
|---|---|---|
| 0 | Tumor extension through the rectal wall that is not nodular; no mesorectal vessels near the tumor | No EMVI |
| 1 | Minimal nodular extramural extension, but not in an area near the mesorectal vessels | No EMVI |
| 2 | Tumor extension near the mesorectal vessels, but without vessel expansion or tumor signal | No EMVI |
| 3 | Abnormal tumor signal within the mesorectal vessels adjacent to the tumor, but the vessel caliber and contour is only slightly abnormal | EMVI |
| 4 | Obvious abnormal vessel contour and caliber with tumor signal | EMVI |
FIG. 1.
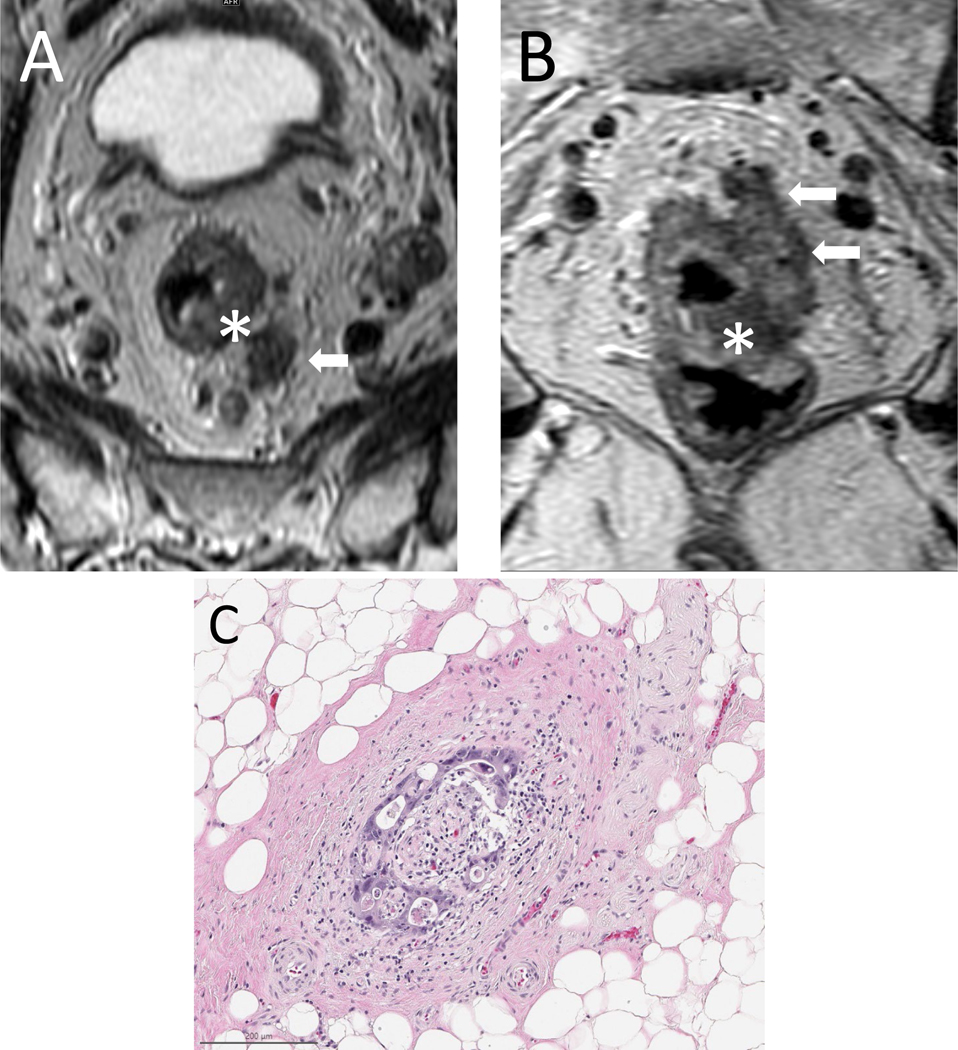
Axial (A) and coronal (B) T2-weighted images of a primary rectal mass (asterisk) with adjacent EMVI extending along the course of mesorectal vessels (arrows) to the left of the primary tumor. (C) Corresponding histopathology.
EMVI Assessment by Surgical pathology
All pathologic data were extracted from pathology reports and/or cancer protocol synoptics based on the guidelines of the College of American Pathologists.29 Hematoxylin-eosin-stained slides and tumor blocks were created per institution protocols at the time of the surgical resection. They were retrospectively reviewed by a gastrointestinal pathologist (M.N.). EMVI was categorized according to the guidelines of the Royal College of Pathologists (United Kingdom).30 Venous invasion was diagnosed when the tumor was seen within an endothelium-lined space surrounded by smooth muscle (Fig. 1C). When a circumscribed tumor nodule was adjacent to a muscularized artery (orphan artery sign), elastin staining (with elastin trichrome) or smooth muscle staining (with desmin or caldesmon) was performed to confirm venous invasion. Presence of either pattern beyond the muscularis propria layer was categorized as EMVI. EMVI regression was defined as EMVI absence on surgical pathology in patients who had EMVI on baseline MRI. The pathologist was blinded to the MRI data.
Statistical Analysis
Differences in characteristics between patients with and without EMVI based on baseline MRI were evaluated using the Wilcoxon rank sum test, Chi-square test, or Fisher exact test. Kaplan-Meier curves were used to compare DFS and OS between the two groups. Multivariable analyses using Cox proportional hazards models were applied to evaluate differences in survival based on EMVI status; hazard ratios (HR) and 95% confidence intervals (CI) are reported. DFS was analyzed as time from total mesorectal excision to recurrence (systemic or local) or death; OS was analyzed from time of total mesorectal excision until death. Observations without an event were censored at last follow-up. For patients who had EMVI at baseline, exploratory subgroup analyses (univariable and multivariable) were performed. Interobserver agreement between radiologists was assessed using Cohen’s kappa coefficient. Statistical analyses were performed using R version 3.6.2 software, with p < 0.05 considered indicative of statistical significance.
RESULTS
We identified 175 patients who met the inclusion criteria. The mean age for the cohort was 53 years; 55% (n = 97) of the patients were men. Most patients (173 of 175) received systemic chemotherapy first, followed by chemoradiotherapy. The median interval from post-TNT MRI to surgery was 3.9 weeks (interquartile range 3.2, range 0.4–120.1 weeks).
Interobserver agreement on EMVI was moderate for baseline MRI and substantial for post-TNT MRI (Cohen’s kappa coefficient, 0.44 [p < 0.0001] and 0.71 [p < 0.0001], respectively; Table S1).31
EMVI on Baseline MRI
Thirty-five of the 175 patients had EMVI on baseline MRI. They did not differ significantly from the 140 patients without EMVI in age, sex, Charlson Comorbidity Index or tumor height from the anal verge, but their tumors were more likely to be of a higher clinical T category (p = 0.006; Table 2). Based on surgical pathology, 29 (83%) of the 35 patients with EMVI on baseline MRI had tumors of pathologic category T3 or T4, compared with 42 (30%) of the 140 patients without EMVI on baseline MRI (p < 0.001; Table 2). Surgical pathology also showed that patients with EMVI on baseline MRI were more likely to have node-positive disease (15 [43%] of 35 patients compared with 33 [24%] of 140 patients; p = 0.022) and perineural invasion (16 [46%] of 35 compared with 20 [15%] of 140; p < 0.001). Three percent of patients with baseline EMVI had a pathologic complete response compared with 19% (p = 0.018). The two groups did not differ significantly in frequency of positive margin (1 [3%] of 35 patients and 2 [1%] of 140 patients; p = 0.5).
TABLE 2.
Characteristics of patients with or without EMVI on baseline MRI
| Characteristic (n = 175) | No. of patients (%) |
p valuea | |
|---|---|---|---|
| Without EMVI (n = 140) | With EMVI (n = 35) | ||
|
| |||
| Ageb | 54 (26–80) yr | 51 (31–75) yr | 0.11 |
| Female sex | 63 (45) | 15 (43) | 0.80 |
| Tumor locationc | 0.30 | ||
| Lower rectum | 64 (46) | 12 (34) | |
| Middle rectum | 67 (48) | 19 (54) | |
| Upper rectum | 9 (6) | 4 (11) | |
| Clinical T classification | 0.006 | ||
| 2 | 11 (8) | 0 | |
| 3 | 122 (87) | 28 (80) | |
| 4 | 7 (5) | 7 (20) | |
| Clinical N classification | 0.40 | ||
| Node negative | 34 (24) | 6 (17) | |
| Node positive | 106 (76) | 29 (83) | |
| Charlson Comorbidity Index | 0.20 | ||
| 0 | 48 (34) | 18 (51) | |
| 1 | 39 (28) | 8 (23) | |
| 2+ | 53 (38) | 9 (26) | |
| Neoadjuvant therapyd | >0.90 | ||
| Consolidation | 2 (1) | 0 | |
| Induction | 138 (99) | 35 (100) | |
| Pathologic T classification | <0.001 | ||
| 0 | 41 (29) | 2 (6) | |
| 1 | 11 (8) | 1 (3) | |
| 2 | 46 (33) | 3 (9) | |
| 3 | 42 (30) | 28 (80) | |
| 4 | 0 | 1 (3) | |
| Pathologic N classification | 0.022 | ||
| Node negative | 107 (76) | 20 (57) | |
| Node positive | 33 (24) | 15 (43) | |
| pCR | 0.018 | ||
| No | 113 (81) | 34 (97) | |
| Yes | 27 (19) | 1 (3) | |
| Tumor gradee | >0.90 | ||
| Well differentiated | 6 (6) | 1 (3) | |
| Moderately differentiated | 82 (83) | 29 (88) | |
| Poorly differentiated | 11 (11) | 3 (9) | |
| Perineural invasione | <0.001 | ||
| No | 115 (85) | 19 (54) | |
| Yes | 20 (15) | 16 (46) | |
| Positive margin | 0.50 | ||
| No | 138 (99) | 34 (97) | |
| Yes | 2 (1) | 1 (3) | |
EMVI extramural venous invasion, pCR pathologic complete response
Pearson chi-square test, Fisher exact test, or Wilcoxon rank sum test.
Mean (minimum–maximum).
Grouped based on centimeters from the anal verge
Consolidation is chemoradiotherapy followed by systemic chemotherapy; induction is systemic chemotherapy followed by chemoradiotherapy.
Numbers reflect missing data
Patients with EMVI on baseline MRI had significantly shorter DFS (HR, 4.24; 95% CI, 2.08–8.66) and OS (HR, 6.04; 95% CI, 1.81–20.10) than patients without EMVI on baseline MRI, based on multivariable analysis controlling for age, sex, clinical T classification, clinical N classification, Charlson Comorbidity Index, and tumor height from the anal verge (Table 3 and Fig. 2). Seventeen (49%) of the 35 patients with EMVI on baseline MRI had a recurrence (1 local and 16 distant), compared with 17 (12%) of the 140 patients without EMVI on baseline MRI (4 local, 12 distant, and 1 both).
TABLE 3.
Multivariable analyses of DFS and OS
| Characteristic | Hazard ratio (95% CI) |
|
|---|---|---|
| DFS | OS | |
|
| ||
| Age | 1.01 (0.95–1.06) | 1.11 (0.99–1.25) |
| Sex | ||
| Female | Reference | Reference |
| Male | 0.97 (0.47–1.99) | 1.22 (0.40–3.71) |
| Clinical T classification | ||
| 2 | Reference | Reference |
| 3 | 2.36 (0.30–18.6) | 0.29 (0.03–2.74) |
| 4 | 4.66 (0.51–42.4) | 0.56 (0.04–7.83) |
| Clinical N classification | ||
| Node negative | Reference | Reference |
| Node positive | 0.31 (0.15–0.66) | 0.62 (0.18–2.08) |
| Charlson Comorbidity Index | ||
| 0 | Reference | Reference |
| 1 | 0.62 (0.21–1.81) | 0.38 (0.05–2.74) |
| 2+ | 0.67 (0.15–3.03) | 0.18 (0.01–5.02) |
| Tumor locationa | ||
| Lower rectum | Reference | Reference |
| Middle rectum | 1.05 (0.50–2.21) | 1.59 (0.42–6.12) |
| Upper rectum | 2.01 (0.65–6.16) | 4.82 (0.84–27.6) |
| EMVI on baseline MRI | ||
| Yes | Reference | Reference |
| No | 4.24 (2.08–8.66) | 6.04 (1.81–20.10) |
DFS disease-free survival, OS overall survival, CI confidence interval, EMVI extramural venous invasion Bold indicates statistical significance
Grouped based on centimeters from the anal verge
FIG. 2.
DFS and OS for patients with EMVI on baseline MRI and patients without EMVI on baseline MRI.
EMVI Regression on Post-TNT MRI or Surgical pathology
Of the 35 patients with EMVI on baseline MRI, 17 (49%) had no EMVI on post-TNT MRI and 29 (83%) had no EMVI on surgical pathology, with no significant association between assessment by post-TNT MRI and assessment by surgical pathology (p = 0.2; Fisher exact test). Of the 17 patients with EMVI regression on post-TNT MRI, 16 (94%) had EMVI regression based on surgical pathology. In contrast, of the 29 patients with EMVI regression on surgical pathology, only 16 (55%) had EMVI regression based on post-TNT MRI.
EMVI Regression and DFS
For exploratory analyses of survival in relation to EMVI, patients were grouped into five cohorts: (i) no EMVI on baseline MRI, post-TNT MRI, and surgical pathology; (ii) EMVI on baseline MRI with EMVI regression on surgical pathology only (persistent EMVI on post-TNT MRI); (iii) EMVI on baseline MRI with regression on post-TNT MRI only (persistent EMVI on surgical pathology); (iv) EMVI on baseline MRI with EMVI regression on both post-TNT MRI and surgical pathology; (v) EMVI on baseline MRI and persistent EMVI on post-TNT MRI and surgical pathology. Four patients without EMVI at baseline, but EMVI on post-TNT MRI (n=1) or surgical pathology (n=3) were excluded from this exploratory analysis aimed at understanding the implications after regression/persistence after baseline EMVI positivity.
EMVI regression on both post-TNT MRI and surgical pathology was associated with longer DFS (HR, 0.17; 95% CI, 0.04–0.64) and OS (HR, 0.11; 95% CI, 0.02–0.68). Of five patients with EMVI on both post-TNT MRI and surgical pathology, four had a recurrence (3 had metastases to the liver and 1 to the bone). Of 13 patients with EMVI regression on surgical pathology but not on post-TNT MRI, only 7 (54%) had a recurrence (6 distant including 3 to the lungs, 1 to the liver, 1 to the retroperitoneal lymph nodes, and 1 to both the liver and lungs; 1 local). Of 16 patients with EMVI regression on both surgical pathology and post-TNT MRI, only 5 (31%) had a recurrence (all distant lung metastases).
Only one patient had EMVI regression on post-TNT MRI but not on surgical pathology. The 13 patients who had EMVI regression on surgical pathology but not on post-TNT MRI had DFS and OS rates that were similar to those of patients who had EMVI regression on both surgical pathology and post-TNT MRI (p = 0.21 and 0.24, respectively) (Fig. 3).
FIG. 3.
DFS for 136 patients without EMVI on baseline MRI, post-TNT MRI, and surgical pathology (bmrEMVI−; *), 1 patient with EMVI on baseline MRI and on surgical pathology but not on post-TNT MRI (bmrEMVI+, ymrEMVI− only; **), 16 patients with EMVI on baseline MRI and no EMVI on both post-TNT MRI and surgical pathology (bmrEMVI+, yp and ymrEMVI−; #), 5 patients with EMVI on baseline MRI, on post-TNT MRI, and on surgical pathology (bmrEMVI+, yp and ymrEMVI+; ##), and 13 patients with EMVI on baseline MRI and post-TNT MRI but not on surgical pathology (bmrEMVI−, ypEMVI− only; ^).
DISCUSSION
Our study provides evidence in support of the negative association of baseline EMVI with DFS and OS in a cohort of patients with locally advanced rectal cancer who underwent TNT, with disease eventually recurring in 17 of the 35 patients who had EMVI on baseline MRI (16 were distant recurrences). Patients with EMVI regression after TNT had significantly longer DFS on average than patients with persistent EMVI. However, their DFS was still shorter on average than DFS in patients who were EMVI negative on baseline MRI, post-TNT MRI, and surgical pathology. EMVI regression was seen in 83% of patients after TNT on surgical pathology, and many of these patients had persistent EMVI by post-TNT MRI criteria. Their DFS and OS were similar to those patients with EMVI regression on both pathology and post-TNT MRI suggesting that MRI may have a high false-positive rate in assessing the persistence of EMVI after TNT.
Our study uniquely explores every possible combination of baseline MRI positivity and post-TNT conversion and the association with survival. In our TNT population, EMVI regression on both post-TNT MRI and surgical pathology was associated with improved DFS. The association between EMVI regression on surgical pathology alone and DFS was comparable, but the study may have been underpowered for detection of a statistically significant effect. Furthermore, we found that in patients with EMVI regression, DFS was still shorter than DFS in patients who did not have EMVI on any modality. This is novel compared to the recent meta-analysis.20 An early study from 2015 similarly found that EMVI on MRI after neoadjuvant chemoradiotherapy was associated with worse DFS.32 Unlike our study, however, they concluded that post-chemoradiotherapy EMVI assessment on MRI was more closely associated with DFS than the pathological assessment. Of note, this study did not analyze survival outcomes in all possible MRI and histopathological combinations.
The differences in EMVI findings between post-TNT MRI and surgical pathology in 14 of 35 patients highlights the difficulty in assessing residual tumor from post-treatment fibrosis on MRI.6,20,32,33 These differences may be due in part to differences in histopathologic definitions and methodologies.6,8,20,34 Another possibility is that in some cases EMVI may in fact be present at the time of post-TNT MRI and is resolved by the time of surgical pathology. This is a possibility given research showing increased rates of pathologic complete response with an increased period from chemoradiation to surgical resection.35
Patients with EMVI on baseline MRI had a significantly lower rate of pathologic complete response and their tumors were significantly more likely to be of a higher pathologic T or N category compared with patients who did not have EMVI on baseline MRI. This was even with 83% of these patients having regression on surgical pathology after TNT. Given that, during the study period, some patients at our institution were offered nonoperative management because they had a clinical complete response to TNT, we would expect our cohort to represent higher-risk patients. However, our study also shows the high-biological potential of tumors with baseline EMVI, with 46% of these patients developing distant metastases. EMVI regression on post-TNT surgical pathology appears to improve survival; however, these patients remain at high risk compared with the baseline EMVI negative cohort. Interestingly, the 19% rate of pathologic complete response in patients without EMVI on baseline MRI is close to the 22.4% pooled rate in a recent meta-analysis of TNT studies.36 Understanding the true implications of regression on long-term survival and the potential added benefits for TNT would require a larger cohort and is an area of future investigation.
Finally, we measured a Cohen’s kappa of 0.44 for the interobserver agreement between radiologists on the baseline MRIs and 0.77 on the post-TNT MRIs. The interobserver agreement varies in the literature and appears dependent on several factors including whether the MRI was before or after neoadjuvant therapy, the statistical analysis used, and the experience of the radiologist.8,19,21,37 A recent review reported a kappa range between 0.372–0.828.38
The results of our study should be interpreted with several limitations in mind. The study design was retrospective and all pathologic analyses of EMVI were performed by a single pathologist. Additionally, some of the MRIs were obtained outside of the institution (23 in total). While this may create heterogeneity, it also reflects real-world practice patterns and adds to the applicability of this work. The analysis of EMVI regression was likely underpowered because of the relatively small sample size. Advanced analyses using whole-mount pathologic specimens and artificial intelligence may help resolve some of the discrepancies between post-TNT MRI and surgical pathology in assessment of EMVI regression.4,39,40 Improving the accuracy of post-TNT assessment of EMVI will aid effective implementation of nonoperative and other treatment deintensification strategies for rectal cancer.
Supplementary Material
Synopsis.
In patients with locally advanced rectal cancer, extramural venous invasion (EMVI) is negatively associated with disease-free and overall survival. EMVI regression after neoadjuvant therapy is associated with improved survival. Posttreatment MRI appears to underestimate EMVI regression.
ACKNOWLEDGMENTS
We thank Arthur Gelmis for his extraordinary editorial contribution to this study.
We thank Jonathan Yuval for his input into this study.
Funding:
Support for this work was provided to Memorial Sloan Kettering Cancer Center by a core grant from the National Cancer Institute (P30 CA008748)
Disclosures:
Dr. Julio Garcia-Aguilar receives honoraria from Johnson & Johnson, Medtronic, and Intuitive Surgical and owns stock in Intuitive Surgical. Dr. J. Joshua Smith has served as a clinical advisor to Guardant Health, Inc and Foundation Medicine Inc. Dr. Martin R. Weiser receives an honorarium from Precisa. Jessica A. Lavery reports salary support from AACR Project GENIE BPC.
REFERENCES
- 1.Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. Mar-Apr 2019;39(2):367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. Mar 2003;90(3):355–364. [DOI] [PubMed] [Google Scholar]
- 3.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. Jun 1980;67(6):439–442. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi P, Rao SX, Zeng MS. Clinical value of MRI-detected extramural venous invasion in rectal cancer. J Dig Dis. Jan 2017;18(1):2–12. [DOI] [PubMed] [Google Scholar]
- 5.Chand M, Palmer T, Blomqvist L, Nagtegaal I, West N, Brown G. Evidence for radiological and histopathological prognostic importance of detecting extramural venous invasion in rectal cancer: recommendations for radiology and histopathology reporting. Colorectal Dis. Jun 2015;17(6):468–473. [DOI] [PubMed] [Google Scholar]
- 6.Ale Ali H, Kirsch R, Razaz S, et al. Extramural venous invasion in rectal cancer: overview of imaging, histopathology, and clinical implications. Abdom Radiol (NY). Jan 2019;44(1):1–10. [DOI] [PubMed] [Google Scholar]
- 7.van den Broek JJ, van der Wolf FSW, Heijnen LA, Schreurs WH. The prognostic importance of MRI detected extramural vascular invasion (mrEMVI) in locally advanced rectal cancer. Int J Colorectal Dis. Oct 2020;35(10):1849–1854. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XY, Wang S, Li XT, et al. MRI of Extramural Venous Invasion in Locally Advanced Rectal Cancer: Relationship to Tumor Recurrence and Overall Survival. Radiology. Dec 2018;289(3):677–685. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui MRS, Simillis C, Hunter C, et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer. Jun 6 2017;116(12):1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. Jun 2014;69(6):619–623. [DOI] [PubMed] [Google Scholar]
- 11.Song KS, Lee DW, Kim B, et al. Differences in prognostic relevance of rectal magnetic resonance imaging findings before and after neoadjuvant chemoradiotherapy. Sci Rep. Jul 11 2019;9(1):10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho MS, Park YY, Yoon J, et al. MRI-based EMVI positivity predicts systemic recurrence in rectal cancer patients with a good tumor response to chemoradiotherapy followed by surgery. J Surg Oncol. Jun 2018;117(8):1823–1832. [DOI] [PubMed] [Google Scholar]
- 13.Patel UB, Brown G, Machado I, et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for primary rectal cancer: long-term results from the GEMCAD 0801 trial. Ann Oncol. Feb 1 2017;28(2):344–353. [DOI] [PubMed] [Google Scholar]
- 14.Gu C, Yang X, Zhang X, et al. The prognostic significance of MRI-detected extramural venous invasion, mesorectal extension, and lymph node status in clinical T3 mid-low rectal cancer. Sci Rep. Aug 29 2019;9(1):12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn B, Lim JS, Kim H, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. May 2015;25(5):1347–1355. [DOI] [PubMed] [Google Scholar]
- 16.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. Feb 2008;95(2):229–236. [DOI] [PubMed] [Google Scholar]
- 17.Koh DM, Smith NJ, Swift RI, Brown G. The Relationship Between MR Demonstration of Extramural Venous Invasion and Nodal Disease in Rectal Cancer. Clin Med Oncol. 2008;2:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TH, Woo S, Han S, Suh CH, Vargas HA. The Diagnostic Performance of MRI for Detection of Extramural Venous Invasion in Colorectal Cancer: A Systematic Review and Meta-Analysis of the Literature. AJR Am J Roentgenol. Sep 2019;213(3):575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhaveri KS, Hosseini-Nik H, Thipphavong S, et al. MRI Detection of Extramural Venous Invasion in Rectal Cancer: Correlation With Histopathology Using Elastin Stain. AJR Am J Roentgenol. Apr 2016;206(4):747–755. [DOI] [PubMed] [Google Scholar]
- 20.Tan JJ, Carten RV, Babiker A, Abulafi M, Lord AC, Brown G. Prognostic Importance of MRI-Detected Extramural Venous Invasion in Rectal Cancer: A Literature Review and Systematic Meta-Analysis. Int J Radiat Oncol Biol Phys. Oct 1 2021;111(2):385–394. [DOI] [PubMed] [Google Scholar]
- 21.Lee ES, Kim MJ, Park SC, et al. Magnetic Resonance Imaging-Detected Extramural Venous Invasion in Rectal Cancer before and after Preoperative Chemoradiotherapy: Diagnostic Performance and Prognostic Significance. Eur Radiol. Feb 2018;28(2):496–505. [DOI] [PubMed] [Google Scholar]
- 22.Sclafani F, Brown G, Cunningham D, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. Aug 2016;27(8):1557–1565. [DOI] [PubMed] [Google Scholar]
- 23.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. Jun 14 2018;4(6):e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. Jan 2021;22(1):29–42. [DOI] [PubMed] [Google Scholar]
- 25.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. May 2021;22(5):702–715. [DOI] [PubMed] [Google Scholar]
- 26.Amin MB, Edge SB, American Joint Committee on C. AJCC Cancer Staging Manual. Eighth edition. ed. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 27.History of the NCI Comorbidity Index. September 24, 2021. Accessed December 17, 2022.
- 28.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. Jul 2018;16(7):874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. Oct 2009;133(10):1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loughrey MQ, Phillip; Shepherd, Neil Dataset for Histopathological Reporting of Colorectal Cancer. 2018; 4:https://www.rcpath.org/uploads/assets/c8b61ba0-ae3f-43f1-85ffd3ab9f17cfe6/G049-Dataset-for-histopathological-reporting-of-colorectal-cancer.pdf. Accessed December 22, 2021.
- 31.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 32.Chand M, Evans J, Swift RI, et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. Mar 2015;261(3):473–479. [DOI] [PubMed] [Google Scholar]
- 33.Felder SI, Feuerlein S, Parsee A, et al. Endoscopic and MRI response evaluation following neoadjuvant treatment for rectal cancer: a pictorial review with matched MRI, endoscopic, and pathologic examples. Abdom Radiol (NY). May 2021;46(5):1783–1804. [DOI] [PubMed] [Google Scholar]
- 34.Dawson H, Kirsch R, Messenger D, Driman D. A Review of Current Challenges in Colorectal Cancer Reporting. Arch Pathol Lab Med. Jul 2019;143(7):869–882. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. Jul 2011;254(1):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrelli F, Trevisan F, Cabiddu M, et al. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. Mar 2020;271(3):440–448. [DOI] [PubMed] [Google Scholar]
- 37.Bae JS, Kim SH, Hur BY, et al. Prognostic value of MRI in assessing extramural venous invasion in rectal cancer: multi-readers’ diagnostic performance. Eur Radiol. Aug 2019;29(8):4379–4388. [DOI] [PubMed] [Google Scholar]
- 38.Inoue A, Sheedy SP, Heiken JP, et al. MRI-detected extramural venous invasion of rectal cancer: Multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging. Aug 9 2021;12(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung JS, Kwak HD, Ju JK. Is Whole-Mount Section in Rectal Cancer Effective for Measuring Lateral Margin? Ann Coloproctol. Jun 2020;36(3):131–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Liang M, Yang Y, Zhang H, Zhao X. Prediction of false-negative extramural venous invasion in patients with rectal cancer using multiple mathematical models of diffusion-weighted imaging. Eur J Radiol. Jun 2021;139:109731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




