Abstract
Background
Typically, animal models studying gastrointestinal microbiotas compromised in early life have employed either germ-free animals or mice treated with a cocktail of antibiotics. Such studies intend to mimic scenarios of infants born by caesarean section and/or subjected to antibiotic treatment. However, the antibiotics used in these studies are rarely prescribed to infants. Therefore, an early life model was developed in which the murine gastrointestinal microbiota was severely disrupted by clindamycin treatment.
Results
In this mouse model, we investigated the extent supplementation with a synbiotic mixture of prebiotics, being scGOS/lcFOS with the human milk oligosaccharide 2’-Fucosyllactose (2’-FL), in combination with or without single strain or mix of “infant type” bifidobacteria, can rescue an antibiotic-compromised microbiota. Shotgun metagenomic sequencing showed that the microbiota was severely disrupted by the clindamycin challenge. No recovery was observed 3 weeks post-challenge in the scGOS/lcFOS/2’FL group, while the group that received the synbiotic treatment of scGOS/lcFOS/2’-FL with Bifidobacterium breve NRBB01 showed partial recovery. Strikingly in the scGOS/lcFOS/2’-FL group receiving the mixture of bifidobacteria resulted in a recovery of the microbiota disruption. Histological analyses showed that the clindamycin-treated animals at the end of the experiment still suffered from mild oedema and villi/colonic crypt irregularities which was ameliorated by the synbiotic intervention.
Conclusion
Our study demonstrates that supplementation of synbiotic mixture of scGOS/lcFOS/2’-FL in combination with a specific mix of infant-type bifidobacterial strains is able to partially revive an antibiotic-perturbed gastrointestinal microbiota.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-023-01595-x.
Introduction
During the first 1000 days following birth, the developing microbiota plays a pivotal role in orchestrating long-term health [1–4]. Gastrointestinal tract (GIT) microbiota maturation during this vulnerable period is driven by an appropriate succession of microbes that confer resilience to perturbations [3, 4]. Different environmental factors such as C-section, preterm birth and antibiotic administration can drastically alter this microbial consortium and may confer long-term health issues [5–7]. Ensuring the appropriate compositional balance of the microbiota is considered an effective measure in maintaining GI health. Bifidobacteria are among the keystone GIT colonizers during early infancy and exert positive health benefits on their host. Following birth, bifidobacteria typically become the dominant species in the colon of breast-fed infants, with “infant type” bifidobacteria specifically abundant in this ecological niche [1, 8, 9]. Human milk oligosaccharides (HMOs) act as important carbohydrate substrates creating this ecological niche [1, 10, 11]. However, there is much we still do not understand about specific host and microbiota interaction capabilities of bifidobacteria. As a result, there is ever-increasing interest from a scientific and applied perspective to study bifidobacteria, and their interactions within the microbiota and their host.
Antibiotic administration is common in the first 6 months after birth [12]. A study in the USA found that 75% of infants had been treated with an antibiotic within the first 48 h following birth [13]. Antibiotic treatment, C-section, preterm birth, and diet drastically alter the GI microbiota (including the prevalence and abundance of bifidobacteria [14, 15]). Further, GI microbiota disruption has been linked to developmental alterations, including allergic asthma, impaired growth, obesity, and metabolic disease [5, 16–19]. Clindamycin is a broad-spectrum lincosamide antibiotic, commonly used to treat infections by anaerobic pathogens in both infants and adults, this includes Staphylococcus aureus [20] and Bacteroides fragilis [21, 22]. In the case of breast-feeding mothers treated with clindamycin, substantial levels of the antibiotic are present in breast milk [23, 24]. It is still unclear whether the use of prebiotics, probiotics or synbiotics confer microbial resilience against the negative impact of antibiotic exposure on the infant GI microbiota. Synbiotics [25], employ both prebiotics (e.g., HMO 2′-Fucosyllactose (2′-FL), galacto-oligosaccharides (scGOS) and fructo-oligosaccharides (FOS)), and probiotics (e.g., Bifidobacterium spp.). Given the huge cost associated with antibiotic treatment of disease and adverse effects on health and development, understanding mechanisms which orchestrate potential microbiota resilience and recovery is crucial.
Several studies examine the restorative and beneficial properties of probiotics following disruption of the GI microbiota [26–29]. While there is reported efficacy of single probiotic strains, such as reduction of intestinal inflammation with B. bifidum JCM1254 [27], there is some evidence to suggest that a multi-strain probiotic or synbiotic treatment can restore a compromised microbiota [30, 31]. In fact Suez et al. [26] reported based on animal model and human studies that post-multi-antibiotic administration of a multi-strain probiotic based on infant-derived bacteria comes at a trade-off of delayed autochthonous microbiota re-establishment (at least 5 months following the probiotic treatment [26]). None of these studies assessed the impact of synbiotics and thus, the aim of our work was to assess a synbiotic mixture of prebiotics and bifidobacteria to rescue a compromised or perturbed microbiota in early life. Here, we use conventional mice in which the microbiota had been compromised by the commonly applied infant antibiotic clindamycin and aimed to examine if and how synbiotic treatment may be able to support host GI microbiota recovery.
Materials and methods
Probiotic strains
The mixture of strains was selected after extensive, in-house screening of various individual Bifidobacterium strains and mixtures thereof based on several criteria. First of all, only strains were selected of the “infant” type, with excellent safety profiles and gastrointestinal survival, and their ability to grow on GOS/FOS/HMO’s. The mixture of the two B. breve strains (NRBB01 and NRBB57), who are phylogenetically and phenotypically distinct from each other within the B. breve species was selected based on previous work [32], as was B. bifidum CNCM-I 4319 [33]. It was this mixture that showed the strongest synergy based on growth when cultivated on 2’-FL and scGOS/lcFOS/2’-FL. This synergy in growth appeared to be based on cross-feeding activities, resulting in enhanced growth as also indicated by increased levels of (bifido)bacterial metabolites, like acetate, L-lactate, and 1,2-propanediol formed as for the individual strains. Not all “infant” type bifidobacterial strains show these synergies, for instance when B. infantis was grown in combination with a B. breve strain on 2’-FL even an antagonism was seen on growth of B. infantis.
Strains selected for this study (Table S1) were Bifidobacterium breve NRBB01, B. breve NRBB57 and B. bifidum CNCM I-4319. Introduction of plasmids into bifidobacterial strains by electroporation was performed as described previously [34, 35] with plasmids each containing a particular selective marker: pSKEM (erythromycin resistance in NRBB01), pPKCM (chloramphenicol resistance to NRBB57) or pDM1 (spectinomycin resistance to CNCM I-4319) [36, 37]. Transformants were incubated anaerobically at 37 °C until an optical density (OD600nm) of ~ 1 was reached. Bacterial cells were then harvested by centrifugation (4052 × g for 20 min) and washed twice with pre-warmed (37 °C) PBS (37 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4). After the final wash, cells were gently resuspended in 20% glycerol: 80% PBS solution (vol/vol) to obtain a stock with CFU/mL of ~ 1 × 109. 1 mL aliquots of this cell suspension were transferred to sterile 2 mL screw cap tubes and frozen at − 80 °C. Stocks were assessed for viable count and confirmed to have remained at ~ 1 × 109 CFU/mL. The bifidobacterial mix was made as described above with the exception that equal volumes of each strain were mixed before glycerol stocks were aliquoted. This mix was plated to confirm the CFU equalled ~ 1 × 109 CFU/mL.
Animals
Three-week old female C57BL/6 mice were obtained from Envigo, UK. Mice were randomly housed in individually ventilated cages (each holding 5 mice) with enrichment materials (cardboard tubes, nesting material, chew sticks), and allowed to acclimatise for 7 days prior to the start of experimentation. Animals were fed when first received and ad libitum throughout the whole study with (AIN93G diet [38] or the AIN93G + scGOS/lcFOS/2’-FL) enriched diet, manufactured by SSNIFF (Table S2). The mouse holding room was maintained at 21 ± 1 °C with humidity of 55 ± 10% and a 12-h/12-h light/dark cycle. All efforts were made throughout the experiments to minimize animal suffering and to reduce the number of animals used.
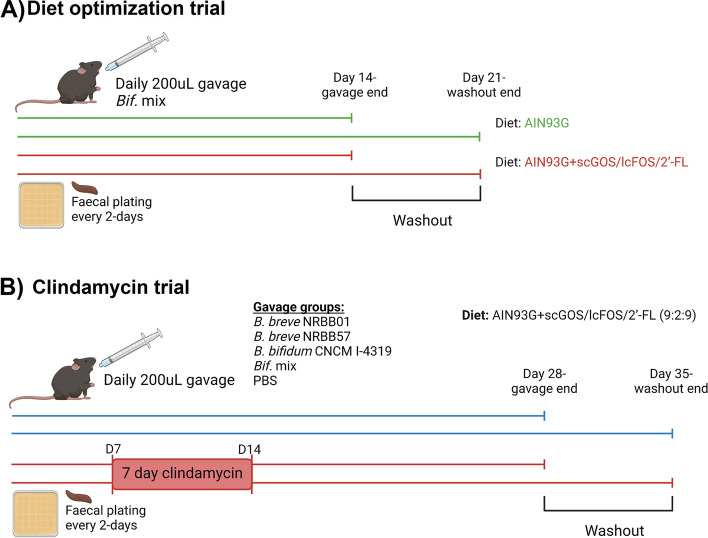
Optimization trial
Mice were fed the AIN93G control diet [38] or the AIN93G:scGOS/lcFOS/2’-FL (9:1:2) prebiotic diet (2.4% w/w carbohydrate substitution; Table S2). In this trial (n = 7/group replicated twice (each group consisted of 3 cages (total of 14 mice) in which one cage housed 4 mice while the other cages housed 5 mice); Fig. 1A), we aimed to optimize a feed enriched with a specific prebiotic mixture (scFOS/lcFOS and 2’-FL) to support growth of the supplemented probiotic strains B. breve NRBB01, B. breve NRBB57 and B. bifidum CNCM I-4319 in order to enhance their level of colonisation. Animals received a daily oral gavage, of 200 µL of the 1 × 109 CFU stock mixture of three bifidobacterial strains, NRBB01, NRBB57 and CNCM I-4319 for 14 days. Replicate groups of animals were additionally left for a final 7 days of bifidobacterial “washout” before being culled (n = 7/group). Mice were fed the AIN93G diet [38] or AIN93G:scGOS/lcFOS/2’-FL (9:1:2; Table S2) for the duration of the trial. To determine colonization levels of the autochthonous and administered bifidobacteria, fecal samples were collected every 2 days into 1 mL PBS containing 0.05% cysteine. Samples were weighed, resuspended, and diluted fourfold and 5 µL quantities of the dilutions were spot plated onto selective reinforced clostridium agar with mupirocin (200 µg/mL; RCA-MUP) with appropriate antibiotic selection (Table S1) for each respective strain. Plates were incubated anaerobically at 37 °C after which bifidobacterial viable counts were performed (expressed in CFU/g). DNA from fecal and colony samples were used as a template for standard PCR using strain-specific primers for strain validation during the trial (Table S3). Mice were culled at the end of the trial for GIT tissue (small intestine, cecum and colon) and content (small intestine and caecum) collection which were stored at − 80 °C for subsequent analysis (qPCR and HPLC).
Fig. 1.
A Diet Trial outline, animals (n = 7/group) received daily the bifidobacterial strain mix (B. breve NRBB01, B. breve NRBB57 and B. bifidum CNCM I-4319) by oral gavage for 14 days while being fed either the AIN93G control diet (green) or the prebiotic diet (AIN93G + scGOS/scFOS/2’-FL; red) before being culled. A washout period of 7 days was used in replicate groups before being culled to determine bifidobacterial strain colonization. B Clindamycin Trial outline, Animals were gavaged daily for 28 days while being fed the prebiotic diet. Five groups of animals (n = 10/group) were used for this purpose (PBS gavage control, B. breve NRBB01, B. breve NRBB57, B. bifidum CNCM I-4319 or Bif. mix), of which half of the animals received antibiotic treatment. From day 7 to day 14 animals in the antibiotic challenge arm (red) were given 250 mg L−1 of clindamycin as part of the water supply. A cohort of animals were culled 2 weeks post-antibiotic challenge. A second cohort (same five groups, n = 10/group) was given a further 7 days as a gavage washout before being culled to determine bifidobacterial strain colonization. Image created with BioRender
Clindamycin trial
The clindamycin trial (Fig. 1B) involved daily gavage with 200 µL of 1 × 109 CFU of an individual strain or the mixture of bifidobacterial strains. Mice were fed a modified AIN93G supplemented with scGOS/lcFOS/2’-FL diet with an increased amount of 2’-FL (4% w/w carbohydrate substitution with scGOS/lcFOS/2’-FL (9:2:9), see Supplementary Table S2). Five groups (n = 10/group; 5 animals/cage) of animals were used for this purpose (PBS gavage vehicle control, B. breve NRBB01, B. breve NRBB57, B. bifidum CNCM I-4319 or the Mix of the 3 strains). Five animals from each group (of 10 animals) received antibiotic treatment. Mice received a daily administration, by oral gavage, with respective bifidobacteria stocks for 28 days, and on day 7 the antibiotic treatment (clindamycin) was administered in the water supply (250 mg/L; [39]). Faecal samples were collected as above every 2 days to assess total and strain-specific Bifidobacterium levels. At the end of the trial animals were culled for the collection of colon, caecum, and small intestine content/tissue for analysis of CFU/g of content and stool, qPCR of host factors from tissue, HPLC of GI content and tissue for histological purposes. The experiment was repeated as described above with the only modification being that animals were left for an additional 7 days to monitor bifidobacterial “washout” before being culled at day 35 after which the same samples were collected for analysis as outlined above.
Collection of samples for HPLC metabolite analysis
Ileum and cecum contents were collected into pre-weighed Eppendorf tubes with 300 µL high-performance liquid chromatography (HPLC) grade H2O (Sigma-Aldrich, Germany). Weights of contents were calculated and the tubes vortexed until contents were completely resuspended. Resuspensions were then centrifuged at 13,000 rpm for 20 min at room temperature and supernatant transferred to High Recovery Vials (Agilent Technologies, Ireland). Carbohydrates and metabolites (glucose, lactose, lactate, acetic acid, formic acid, propionate and ethanol) were determined by an Agilent 1200 HPLC system with a refractive index detector (Agilent Technologies, Ireland). A REZEX 8µ 8%H, Organic Acid Column 300 × 7.8 mM (Phenomenex, USA) is used with 0.01N H2SO4 as the elution fluid, at a flow rate of 0.6 mL/min. The temperature of the column was maintained at 65 °C. Substrate and end-product peaks were identified by comparison of their retention times with standards of pure compounds and known concentrations.
GIT tissue collection and preparation for histology
Approximately 1–1.5 cm of ileum and terminal colon segments from each mouse were collected post-mortem from the Clindamycin Trial at day 29 and 35. All tissues were fixed for 2.5 h in methacarn (methanol-Carney) fixative (60% (v/v) dry methanol, 30% (v/v) chloroform, 10% (v/v) glacial acetic acid [40, 41]). The tissues were automatically processed by a Leica Tissue Processor TP1020 Histokinette (Leica Biosystems, UK). Tissue samples were then embedded in paraffin blocks using a Sakura Tissue-Tek TEC5CMA-1 Tissue Embedding Station (Sakura Finetek, USA). Tissue segments were oriented for longitudinal and transversal sectioning. Five micro meters of tissue sections were made using a Leica RM2135 Rotary Microtome (Leica Biosystems, UK).
Histological staining and scoring
Paraffin-embedded sections were deparaffinised using Clear-Advantage Xylene Substitute (Polyscience, USA) and rehydrated through an ethanol gradient. Haematoxylin and Eosin (H&E) staining for epithelium integrity was performed immediately after deparaffinisation by a Haematoxylin and Eosin Stain Kit (Vector Laboratories, USA) following the manufacturer’s instructions. In addition, replicate slide sections were stained with Alcian Blue/Periodic acid–Schiff (PAS) staining following the manufacturer’s instructions (Polyscience, USA) to visualise mucosal variations. Slides were then imaged using an Olympus BX51 upright microscope and sections were scored as described in previous work [42–45]. All scoring was blinded to experimental conditions with reference to the group only fed the prebiotic diet (no bifidobacterial gavage) to evaluate gross changes compared to control samples in (1) gland number (colon) or villus structure (small intestine), (2) oedema and at the cellular level with (3) goblet cell number, (4) mucus thickness, and (5) acid/neutral mucin distribution from crypts to lumen.
RNA extraction from tissue
Total RNA was isolated from approximately 1–1.5 cm of mid colon harvested at respective end points and stored in RNAlater at − 80 ℃. Following thawing and removal of the RNAlater, tissue was lysed by bead beating in lysis buffer, followed by RNA purification using the GenElute™ Mammalian Total RNA miniprep kit (Sigma-Aldrich, Germany) as per manufacturer’s protocol instructions. RNA was quantified using a Nanodrop and 1 μg was then converted to cDNA with the ReadyScript™ cDNA Synthesis Mix (Sigma-Aldrich) as per manufacturer’s instructions. RT-qPCR was performed, with primers targeting specific genes of interest (Table S3). Briefly, 1 μL of cDNA was added to 10 μL of power KiCqStart SYBR Green qPCR ReadyMix (Sigma-Aldrich) with 0.5 μL of each forward and reverse primer pair (5 pmol/μL) and made up to a final volume of 20 μL with molecular grade H2O. qPCR was performed using the LightCycler 480 System (Roche Diagnostics International AG, Switzerland) following conditions: 95 °C for 5 min, followed by 45 cycles at 95 °C for 10 s, and annealing/extension at 60 °C for 1 min. Relative expression was calculated against β-actin using the 2-ΔΔCT method.
DNA extraction, sequencing, and analysis
Faecal gDNA was extracted using QIAmp Fast DNA Stool Mini Kit (Qiagen) following manufacturer’s instructions, with one modification being a 3-min bead beating step incorporated at the start of the protocol. Paired-end metagenomic shotgun sequencing (MGS) was completed at the Australian Centre of Ecogenomics (ACE) and consisted of QC, indexing, quantification, normalisation, pooling, and sequencing on a 2 × 300 bp V3 MiSeq. Resulting data was demultiplexed and uploaded to One Codex (One Codex, USA [46]) for taxonomic assignment (https://www.onecodex.com/). The One Codex database consists of ~ 115,000 complete microbial genomes, assembled from both of public and private sources. The mouse genome was included in the initial process to screen out host reads. Samples were annotated against the One Codex database through three sequential steps: (1) k-mer based classification (k = 31); (2) artifact filtering; and (3) species-level abundance estimation. For data analysis One Codex Application Program Interface (API; v1) embedded within Jupyter notebook (v0.9.6) with python library (v3.8.3) was used. The relative abundance of each microbial species was estimated based on the depth and coverage of sequencing across every available reference genome. Here plots of relative taxonomic abundance, alpha diversity values (Shannon, Chao1, and Simpson), beta diversity with principal co-ordinate analysis (PCoA) on Bray–Curtis distances, and Spearman’s correlation as a heatmap were all generated. Alpha diversity values were subsequently imported into GraphPad Prism (v9.3.1) for graphing.
Data analysis and statistics
All animal sample size calculations were performed using G*Power3 [47] with a default significance level (α) of 0.05 and power (1-ß) of 0.80. Effect sizes (d and F) were calculated (https://www.psychometrica.de/) based on data obtained from previous unpublished research. Bifidobacterial CFUs were standardised per gram of material and the resulting data was then used for statistical comparison (t test) employing GraphPad Prism (v9.3.1). Alpha diversity repeated measures were used to assess statistical significance for each group at each timepoint in comparison to its respective starting timepoint at Day -7, these were completed with GraphPad. Statistical analysis of beta-diversity was performed using a permutational multivariate analysis of variance (PERMANOVA) and analysis of similarities (ANOISM) using Scikit-bio software library [48] within the One Codex application. Results generated from qPCR of host immune and epithelial barrier function were analysed using Kruskal–Wallis test in GraphPad. Histological measurements produced from Fuji (v1.14.0) were also analysed using Kruskal–Wallis and one-way ANOVA within GraphPad.
Results
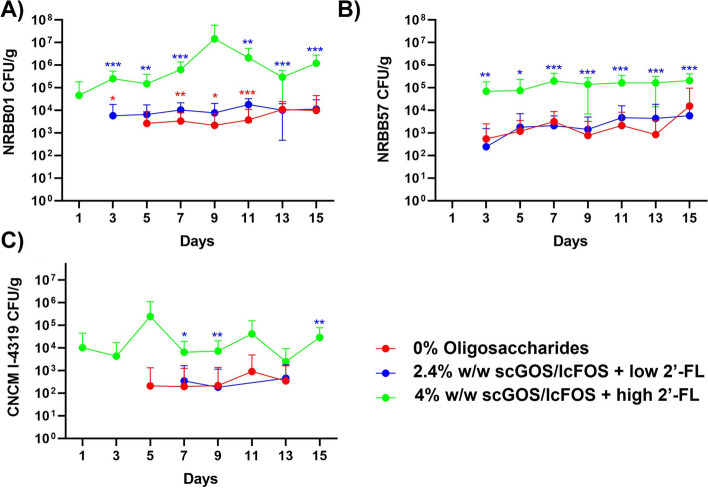
A diet with a high dose of 2’-FL and scGOS/lcFOS improves transient bifidobacterial strain colonization
The control diet (AIN93G) included either no scGOS/lcFOS/2’-FL or a 2.4% w/w carbohydrate substitution during the optimization trial, this was subsequently increased to 4% w/w (Table S2) during the clindamycin challenge trial. The increase to 4% scGOS/lcFOS/2’-FL was determined after the optimization trial colonization rates for B. breve NRBB57 and B. bifidum CNCM I-4319 were not significantly increased when compared to the control diet. Figure 2 compares the results of the optimization trial (0% and 2.4% scGOS/lcFOS/2’-FL) and the first 15 days of the clindamycin trial during which animals were fed a 4% scGOS/lcFOS/2’-FL prebiotic diet and treated with the bifidobacterial mix. Faecal plate counts for each individual probiotic strain within the mix treatment revealed that the diet enriched with 4% scGOS/lcFOS/2’-FL resulted in the most significant synergistic impact on the colonization levels for all 3 strains. Of interest, none of the strains were able to remain/persist within the host following the washout period; in fact, without clindamycin treatment all bifidobacterial counts returned to zero (or undetectable) after 48 h from the final gavage. Bifidobacterial plating of the ileum, cecum, and colon tissue/content for each respective cull day (24 h post-final gavage or 7 days post-gavage) yielded CFU/g results for only the autochthonous bifidobacterial community (data not shown).
Fig. 2.
Specific faecal bifidobacterial counts recovered by selective antibiotic plating. Results of 15 days of optimization and clindamycin trial of animals that were supplemented with with/out GOS/FOS prebiotic diet either enriched with low or high dose of 2’-FL and the bifidobacterial mixture. A B. breve NRBB01, B B. breve NRBB57, and C B. bifidum CNCM-I 4319. Values equal to zero are not shown, n = 10 (NB. bifidobacterial mix animals not administered with clindamycin are only shown in this graph). Individual values represent the mean (± SD) produced from replicate plating of 10 animals and significance level (multiple t test): *, p value < 0.05; **, p value < 0.01; ***, p value < 0.001
Clindamycin murine model—bifidobacterial colonization
Next, we aimed to assess colonisation of autochthonous Bifidobacterium with/out the 7-day antibiotic challenge. Total Bifidobacterium counts recovered from plated faecal material for the duration of the Clindamycin Trial were significantly affected by the antibiotic challenge (Fig. 3). There is a clear depreciation of the autochthonous Bifidobacterium population, which still had not returned to baseline 3 weeks post-antibiotic treatment. All groups were fed the 4% scGOS/lcFOS/2’-FL diet and it is worth noting that the presence of these bifidogenic carbohydrates was not sufficient to recover the indigenous bifidobacterial community 3 weeks post-antibiotic. No significantly different metabolic end products were observed in the HPLC data generated from ileum and caecum contents (Figures S1 and S2).
Fig. 3.
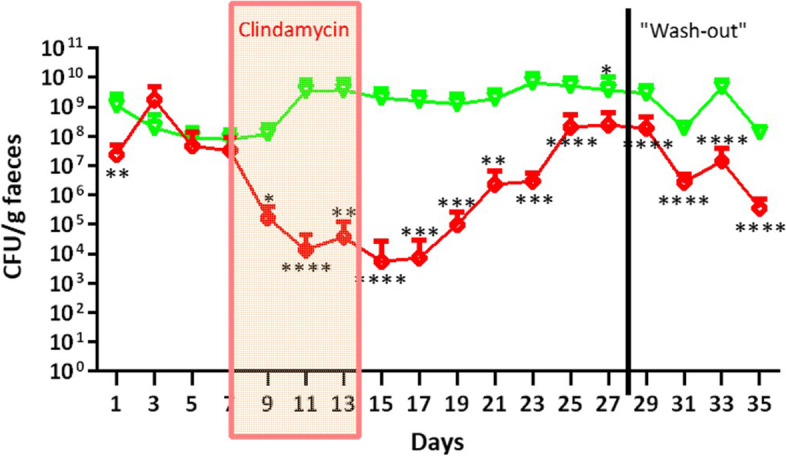
Total Bifidobacterium CFU/g faecal plating for mice treated with scGOS/lcFOS/2’-FL diet only. (Red) denotes mice received clindamycin challenge from day 7–14, where (Green) shows mice abstained from antibiotic challenge. Values are plotted on a log10 axis and values equal to zero are not shown, n = 10. Individual values represent the mean (± SD) produced from replicate plating of 10 animals and significance level (multiple t test): *, p value ≤ 0.05; **, p value ≤ 0.01; ***, p value ≤ 0.001; ****, p value ≤ 0.0001
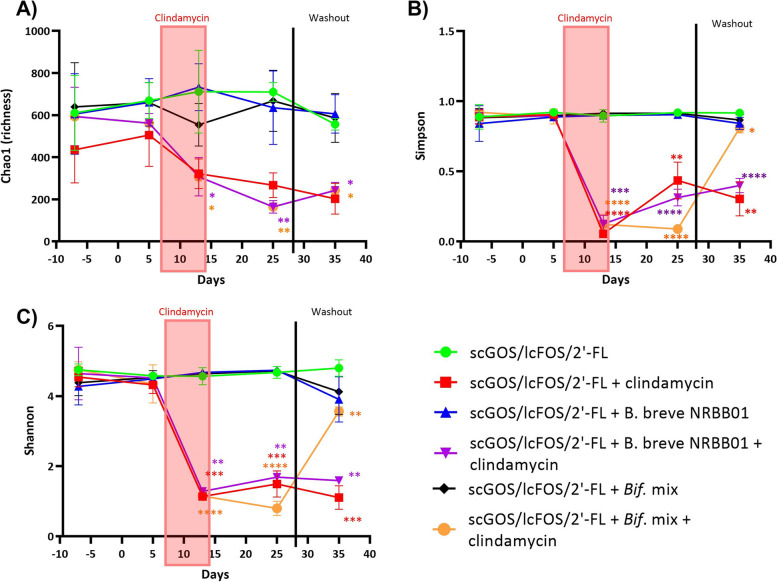
MGS reveals significant recovery of the microbiota upon synbiotic treatment following antibiotic challenge
A subset of samples (n = 5/group) from the clindamycin trial were selected to represent the longitudinal shift of the microbiota across the model. Groups were provided the 4% scGOS/lcFOS/2’-FL diet only, or synbiotic mixture of scGOS/lcFOS/2’-FL diet and B. breve NRBB01, or bifidobacterial mix were selected for microbiome sequencing. Based on faecal counts of total bifidobacteria (Fig. 3), timepoints selected included those considered to represent “major events” that affected the microbiota. These included the day of arrival from the supplier (pre-diet; day -7), 14 days following prebiotic diet (day 5), end of antibiotic challenge (day 13), end of gavage period and 14 days post-antibiotics (day 25), and finally end of the “washout” period (day 35). MGS from mouse faecal pellets gDNA, from 30 mice across 5 timepoints, resulted in a total of 1.3 billion reads passing filtering with a median of 14,027,447 ± 3,091,316 reads/sample. Shannon, Chao1, and Simpson Index, markers of microbiota alpha diversity, demonstrated a steep decrease in diversity upon antibiotic treatment. Interestingly, alpha diversity was only restored (returned to baseline values; Simpson and Shannon) 21 days post-antibiotic exposure for animals that received scGOS/lcFOS/2’-FL + bifidobacterial mix, this was not observed in groups that were fed with only the prebiotic or synbiotic formulation with single strain B. breve NRBB01 (Fig. 4).
Fig. 4.
Alpha diversity metrics. A Chao1 (aka evenness), B Simpson, and C Shannon. Individual values represent the mean (± SD) produced from 5 animals/group and statistical comparison are completed between timepoint and its respective starting timepoint at day -7 (significance color corresponds to group). Significance level (multiple t test): *, p value ≤ 0.05; **, p value ≤ 0.01; ***, p value ≤ 0.001; ****, p value ≤ 0.0001
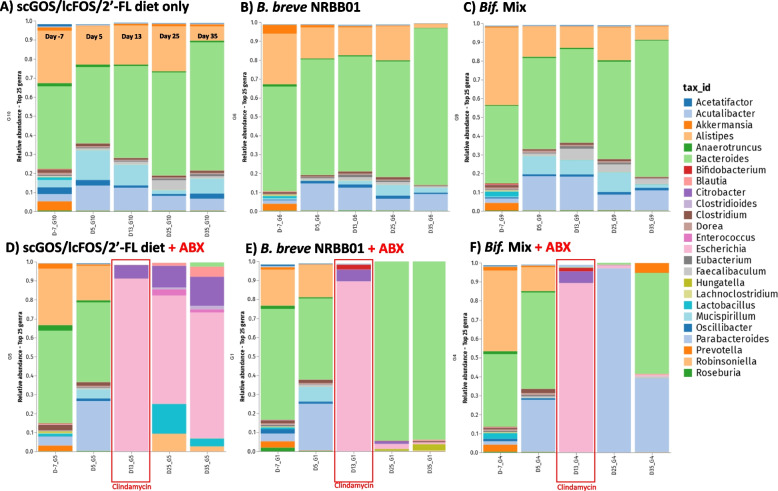
In animals not treated with clindamycin, taxonomic composition profiles (Fig. 5A; Figure S3 and S4) revealed that supplementation of scGOS/lcFOS/2’-FL to the diet most notably affects the relative abundance of Parabacteroides and Mucispirillum and groups fed with synbiotics generally share and maintain a consistent profile predominately of Alistipes, Bacteroides and Parabacteroides (Fig. 5B, C). These data, along with non-significant alterations in alpha-diversity from day -7, suggest that addition of bifidobacteria to the scGOS/lcFOS/2’-FL diet did not result in a significant impact on the host microbiota composition in the absence of antibiotic challenge.
Fig. 5.
Relative abundance of the top 25 genera at day -7, 5, 13, 25, and 35 across the 3 intervention groups supplemented with A scGOS/lcFOS/2’-FL diet only B scGOS/lcFOS/2’-FL and B. breve NRBB01 and, C scGOS/lcFOS/2’-FL and the Bifidobacterium strain Mix without clindamycin treatment. Groups treated with clindamycin between day 7 and 14 (marked by red box) include groups supplemented with D scGOS/lcFOS/2’-FL diet only, E scGOS/lcFOS/2’-FL and B. breve NRBB01, and F scGOS/lcFOS/2’-FL and the Bifidobacterium strain mix. N = 5/group; unassigned genera have been excluded, Figure S3 details the taxonomic composition for individual animals with unassigned genera included
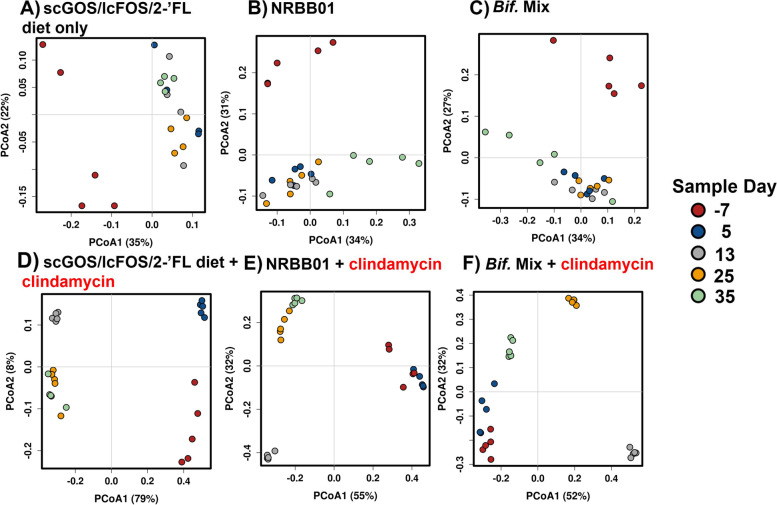
In all groups challenged with clindamycin the microbiota exhibited a dramatic shift towards a community dominated by Citrobacter and Escherichia observed at day 13 (Figure S4C and S4F, respectively). Twenty-one days following treatment completion the composition of the scGOS/lcFOS/2’-FL diet group only still retained a similar microbial profile (Fig. 5D), accompanied by an increase of Lactobacillus, Enterobacter, Enterococcus and Clostridioides (Figure S4). These results suggest the supplementation with scGOS/lcFOS/2’-FL alone was not enough to restore a normal/balanced microbiota. In contrast, animals treated with clindamycin and supplemented with synbiotic B. breve NRBB01 and scGOS/lcFOS/2’-FL became re-colonised and dominated by Bacteroides, after treatment. This was maintained even after the “washout” period (Fig. 5E and Figure S4B). Within this same group, upon cessation of B. breve NRBB01 gavage, Hungatella hathewayi appears to fill the ecological niche. However, the most striking result was the synbiotic bifidobacterial mix treatment (Fig. 5F and Figure S3F), by day 25 animals were predominately re-colonized by Parabacteroides (Figure S4H), and after the “washout” period (day 35) the microbiota appeared more diverse with a recovery of genera present before challenge (day 5), namely Parabacteroides, Bacteroides, and Akkermansia being the most prominent. Beta diversity for each group over the time course confirmed this observation with animals provided synbiotic bifidobacterial mixture 21 days post-antibiotic challenge (day 35) cluster closer with those of baseline animals (day 5, Fig. 6F; ANOISM p = 0.001 and PERMANOVA p = 0.001) than any other clindamycin group. The PCoA also shows that the non-antibiotic groups cluster closely together once animals commence the AIN93G-scGOS/lcFOS/2’-FL diet, compared to day − 7 (day animals are received; Fig. 6A, C). Of the challenge group, the prebiotic only group (Fig. 6D; ANOISM p = 0.001 and PERMANOVA p = 0.001) demonstrated the effect clindamycin has on microbial diversity with animals forming distinct clusters away from baseline (day − 7 and 5) following challenge (day 13) and the shift away from baseline is maintained for the duration of the trial (day 25 and 35). In comparison, clindamycin-administered mice treated with B. breve NRBB01 synbiotic (Fig. 6E; ANOISM p = 0.001 and PERMANOVA p = 0.001) harbour distinct profiles from day 25 and 35, post-antibiotic, which clusters away from baseline. Individual statistical comparisons (PERMANOVA) between each timepoint for each respective group are detailed in Table S4. Collectively, our results demonstrate that the synbiotic effect of the bifidobacterial mix + scGOS/lcFOS/2’-FL supplemented diet was the most effective treatment for initiating a recovery of the microbiota. While beta diversity clustering suggests that 21 days post-antibiotic challenge (day 35) microbiota begins to resemble the profile before clindamycin perturbation (day 5) the difference is still significant (PERMANOVA p = 0.011). Future work will extend the monitoring period to determine if and when this synbiotic mix is capable of total host gut microbiota restoration in comparison to no synbiotic intervention.
Fig. 6.
PCoA plots of beta diversity for non-antibiotic treated groups A scGOS/lcFOS/2’-FL diet only, B scGOS/lcFOS/2’-FL + B. breve NRBB01, and C scGOS/lcFOS/2’-FL + Bifidobacterium Mix. Antibiotic challenged groups include D scGOS/lcFOS/2’-FL only, E scGOS/lcFOS/2’-FL + B. breve NRBB01, and F GOS/FOS/2’-FL + Bifidobacterium Mix. Data points are colour coded by faecal collection day -7 (animal arrival), day 5 (1 week on scGOS/lcFOS/2’-FL diet), 13 (end of clindamycin challenge), 25 (2 weeks post-clindamycin), and 35 (3 weeks post clindamycin)
Antibiotic effects on the host and mitigation of these effects by a synbiotic
Clindamycin administration resulted in increased cecum length 2 weeks post-antibiotic challenge (day 29), compared to non-antibiotic groups (Figure S5). The 35-Day cohort cecum dimensions were reduced in comparison to 29-day, but still had not returned to “baseline” (Supplementary Figure S5). Increased cecum length is likely due to water retention [49], observed oedema caused by immune influx (see below [50]), and aggravation of the compromised microbiota as evidenced by our MGS. Microscopic evaluation of intestinal tissue was performed to assess epithelial integrity (Fig. 7A–D) and acidic and neutral mucin distribution (Fig. 7E–H). Ileum histology show mild oedema and villi/colonic crypt irregularities following clindamycin treatment, which was less pronounced for animals provided synbiotic formulation scGOS/lcFOS/2’-FL + B. breve NRBB01 or the scGOS/lcFOS/2’-FL + bifidobacterial mix. In the colon, at day 29, clindamycin increased colonic folds, oedema and distribution of neutral mucin staining in the scGOS/scFOS/2’-FL group.
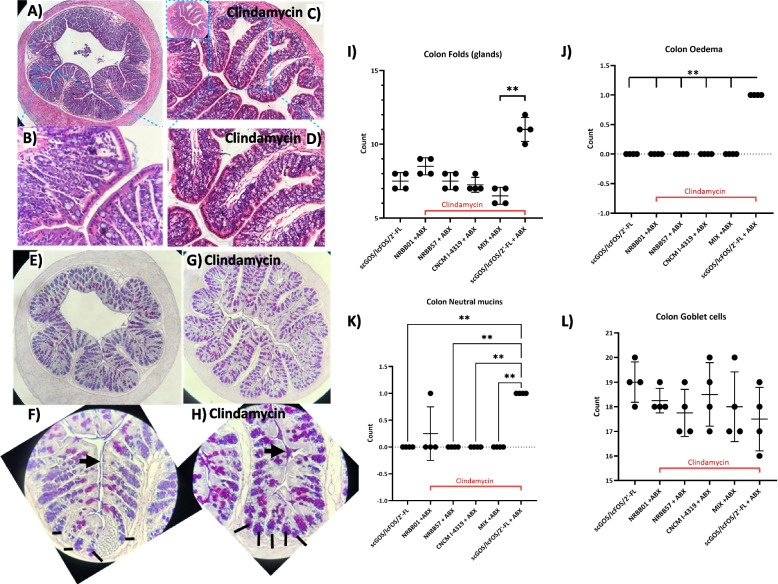
Fig. 7.
Clindamycin induced intestinal changes remain in mice 14 days after cessation (day 29). A–D Changes in histopathological appearance by H&E staining are evident in prebiotic diet only groups without (A, B) and with clindamycin treatment (C, D) with increased colonic glands and oedema at crypt and colonic fold base. E–H Alcian Blue/PAS staining of neutral (pink-red), acidic (blue), mixed (purple) mucins reveal increased staining of neutral mucins following antibiotic treatment (G, H) throughout the colon. This is evident from the crypt to fold tip (apex), see arrows highlighting mucus staining and lines illustrating the colonic crypt (F, H). Histological scoring of the I colonic folds number (glands), J oedema, K neutral mucin staining, L and goblets cells were measured with at least 5 per mouse compared to the prebiotic diet only group. Data are expressed as means ± SD. n = 4 per group. Mean values were significantly different between the groups: ****P < 0.0001
While there was no evident change in goblet cell numbers or mucus thickness, neutral mucin distribution changed from the apical tip of the colonic folds in the non-clindamycin group fed 4% scGOS/scFOS/2’-FL to being distributed throughout the colonic fold from the crypt base to apical tip (Fig. 7F–H). This effect was abrogated by synbiotic scGOS/lcFOS/2’-FL + bifidobacterial mix supplemented groups after clindamycin therapy. No discernible difference between the scGOS/lcFOS/2’-FL only and clindamycin treated group was evident at day 35 by microscopy (Figure S6).
Pro-inflammatory (TNF-α, IL-1β, IFN-g, IL-6), anti-inflammatory (IL-10) cytokines (Figure S7–S8) and gut integrity (Muc2, CLDN-1, CLDN-2, DEFA5) host markers (Figure S9–S10) were assessed from colon tissue collected from antibiotic trial endpoints (day 29 endpoint was collected after the last gavage or day 35 endpoint which represented a 7-day Bifidobacterium treatment washout). Animals which had not received antibiotic challenge did not exhibit significant changes in IL-1β, IL-6, IL-10, TNF-α, and IFN-g expression between prebiotic only group and bifidobacterial synbiotic groups. Clindamycin increased IL-1β at 29-day endpoint for animals given only the scGOS/lcFOS/2’-FL diet, while both scGOS/lcFOS/2’-FL + B. breve NRBB01 and scGOS/lcFOS/2’-FL + B. bifidum CNCM-I 4319 reduced IL-1β expression (Figure S7A). IL-6 was decreased after synbiotic feeding in clindamycin with GOS/FOS/2’-FL + B. breve NRBB01 (day 29) and B. breve NRBB57 (day 35) having the most pronounced effect (Figure S7B and E). TNF-α appears to also be increased by clindamycin treatment at day 35, possibly because of increased abundance of “pathogenic” species, and synbiotic treatment with B. bifidum CNCM I-4319 was most notable at suppressing production of this cytokine at the 35-day endpoint (Figure S8A and C). Our results also suggest IL-10 is induced by the administered synbiotic with bifidobacterial strain mix at day 29 (Figure S7C). However, in synbiotic treatment groups at day 35 there was a significant decrease for B. bifidum CNCM-I 4319 (Figure S7F). In comparison to clindamycin, animals that were fed only the scGOS/lc/FOS/2’-FL diet had decreased IFN-g versus animals not given antibiotic or bifidobacterial gavage (Figure S8B and D). When comparing IFN-g in groups provided synbiotic treatment there was a marginal increase in animals administered B. bifidum CNCM-I 4319 or the Bifidobacterium mix at day 29, and at day 35 B. breve NRBB01 and B. breve NRBB57 were also increased compared to antibiotic scGOS/lcFOS/2’-FL group (Figure S8B and D). Muc2 relative expression was decreased at day 29 for synbiotic bifidobacterial mix clindamycin-treated animals (Figure S9A). DEFA-5 and CLDN-2 were unremarkable for all animals and timepoints (Figure S9 and S10). However, transmembrane tight junction protein claudin-1 (Cldn-1) was increased in the scGOS/lcFOS/2’-FL + clindamycin group at 29-day endpoint (Figure S10). This increase was significant to not only the non-clindamycin group (B. breve NRBB01), but also the synbiotic treatments B. breve NRBB57, B. bifidum CNCM I-4319 and the bifidobacterial mix at day 29 (Figure S10A), these values returned to “baseline” at day 35 (Figure S10C).
Genera level correlations to host factors
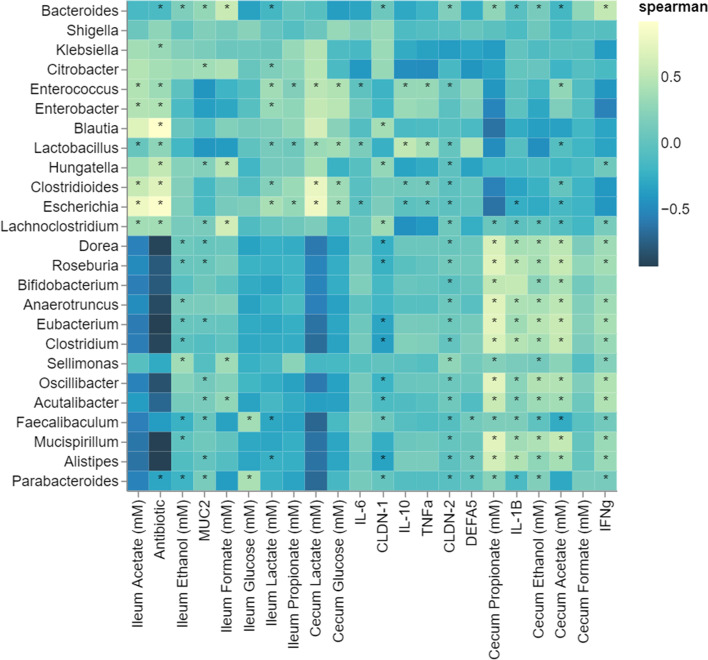
Finally, we conducted Spearman correlations of the MGS data and the metabolomic, histological and qPCR results of host immune and epithelial markers for the day 35 endpoint. The host and metabolic data for matched animals with reported MGS were only included in this analysis (Fig. 8). Overall, this analysis depicts 2 distinct profiles which reflect the taxonomic composition in animals with and without antibiotic challenge. Genera negatively associated with clindamycin were associated with metabolite production (propionate, ethanol, acetate, and formate) in the cecum. Conversely, genera positively associated with clindamycin are correlated with ileum metabolites. This analysis also demonstrates that there is a positive relationship between microbial genera Escherichia, Clostridioides, Enterococcus, and Lactobacillus and TNF-α, these genera were also associated with the clindamycin treatment and there is a striking enrichment of these genera upon antibiotic challenge (Fig. 5D–F).
Fig. 8.
Associations (Spearman correlation) between the top 25 genera identified of all samples and host factors (histological scores, barrier function, immune marker expression, and short-chain fatty acids from the cecum and ileum)
Discussion
The restorative properties of synbiotics for compromised GI microbiota because of premature birth/delivery method has been previously demonstrated [29, 51]; however, the ability of different synbiotics to restore an antibiotic-challenged microbiota remains unclear. This study aimed to assess a synbiotic mixture of prebiotics and infant type bifidobacteria for its ability to rescue a compromised or perturbed microbiota in early life (i.e., C-section, preterm, or antibiotics). We report that murine GIT microbiota resilience can be supported by synbiotic supplementation of a prebiotic mix, (scGOS/lcFOS) enriched with HMO (2’-FL), in combination with one or more infant-type bifidobacterial strains upon challenge with clindamycin.
Previous studies in animals and humans attempting to recover antibiotic perturbed microbiotas with probiotic formulations [26, 27] concluded that microbial re-establishment could likely be delayed as a result of the probiotic administration [26]. However, most patients are unlikely to receive multiple antibiotics as “standard” treatment. In addition to the substantial microbiota remodelling that occurs as a result of antibiotics, there is increased risk of secondary GIT bacterial infection, one example being C. difficile-associated diarrhoea [52, 53]. C. difficile is more common in formula-fed infants [54–56], while C. difficile-associated diarrhoea may be considered rare, although increasing, it is often associated with antibiotic use [57]. The clindamycin animal model we employed is representative of an infantile compromised microbiota as a result of environmental challenges such as antibiotic use, mode of delivery, and birth prematurity. Previous work demonstrated that clindamycin negatively affects the Bacteroidetes phylum, whereas the Firmicutes phylum remains largely unchanged in relative abundance, while Proteobacteria expands dramatically [58]. We observed a similar result with the remodelled microbiota profile appearing adverse or even potentially pathogenic, dominated by Escherichia coli, Clostridioides difficile, Citrobacter spp., and Shigella spp.
Following birth, the GIT microbiota from vaginally born infants typically consists of Bacteroides, Bifidobacterium, Parabacteroides, and some Escherichia/Shigella species [2] and disruptions of this community early in life has been shown to have long lasting negative health effects, such as asthma, obesity, metabolic diseases, neurocognitive development, neonatal morbidity, and mortality [5, 15, 59]. While previous studies in both animal models and new-borns have reported on the protective benefits of commensal strains against pathogenic challenge [14, 28, 58, 60–62], few have also commented on the recovery of the microbiota [31, 63–65]. To address this, we first worked to improve colonization of our bifidobacterial strains by dietary supplementation [38] with scGOS/lcFOS/2’-FL. Previous research demonstrated positive effects of supplementing infant formula with GOS and FOS, including reduced occurrence of gastroenteritis and potential reduction of respiratory infections [66, 67], modulation of the immune response [68–70], and suppression of C. difficile [71]. 2’-FL is the dominant HMO present in most lactating mothers and benefits the host through reducing necrotising enterocolitis in preterm birth (NEC) by increasing endothelial nitric oxide synthase to enhance intestinal blood flow [72–75]. Particular members of Bifidobacterium genus dominate breast-fed babies (such as B. longum subsp. infantis and B. bifidum) and encode enzymatic machinery necessary to utilize HMOs [76, 77], and as such HMOs are used as a selective and supportive carbohydrate [78]. Indeed, we observed that supplementing the control diet with 4% scGOS/lcFOS/2’-FL improved the colonization efficiency of each of strains. Addition of scGOS/lcFOS/2’-FL alone was not sufficient to restore the GI microbiota following antibiotic challenge. Synbiotic treatment of scGOS/lcFOS/2’-FL and B. breve NRBB01 resulted in a partially recovered profile, shifting from Proteobacteria dominated to a recovery and domination of Bacteroides from antibiotic completion until the end of the trial. Although alpha diversity did not return to baseline, there was a marked suppression of E. coli, C. difficile, Citrobacter, and Shigella spp. Similar promotion of Bacteroidetes was reported by Crouzet L, Derrien M, Cherbuy C, Plancade S, Foulon M, Chalin B, van Hylckama Vlieg JET, Grompone G, Rigottier-Gois L and Serror P [79] with Lactobacillus paracasei supplementation. Promisingly, we found that the synbiotic with bifidobacterial mix, while initially dominated by Proteobacteria after the antibiotic period, subsequently shifted to a more diverse profile. While we observed a partial recovery of the microbiota this may be a result of testing human-adapted Bifidobacterium in animal model and/or the premature termination of the trial before a full recovery could be observed. However, we can conclude that treatment with a synbiotic consisting of scGOS/lcFOS/2’-FL and single strain B. breve NRBB01 is only partly sufficient to promote recovery of the microbiota, while the effect of the synbiotic mixture of scGOS/lcFOS/2’-FL, B. breve NRBB01, B. breve NRBB57, and B. bifidum CNCM I-4319 was stronger.
Regarding the host response to clindamycin, long-term disruption to intestinal function was still evident 14 days post-antibiotic, with colonic oedema still evident and increased neutral mucin secretion. Effects of antibiotics on mucin and mucus secretion has been previously observed, suggesting increased neutral mucins allow pathogenic gram-negative bacteria to thrive [43, 44]. The restoration to histological normal appearance by synbiotic supplementation supports the trend towards restoration of the GIT microbiota. RT-qPCR analysis of the synbiotic treatment groups during clindamycin indicated increased IL-10 and IFN-g, suggestive of T cell recruitment, and possibly even T regulatory cells induction, possibly in response to the “pathogenic” microbiota shift observed following treatment. Host factor DEFA-5 is abundant in neutrophil granules and is also found in mucosal epithelium, including the intestine, we observed no significant impact on this from either clindamycin or synbiotic treatment. It should also be noted that our dosage of bifidobacterial strains was relatively high (1 × 109 CFU) for the animal model and translation to human infants; however, it did allow us to confirm that there were no adverse host physiological responses to this synbiotic combination. Overall, the animal model of clindamycin facilitates high throughput cost effective assessment of synbiotics modulating capacity of the microbiota and induction of host epithelial changes. Future studies using this model could include a sample point closer to antibiotic challenge and conduct immunohistochemistry to assess recruitment and infiltration of immune cells. Furthermore, it will be very interesting to investigate specific individual variations within treatment groups to examine the effects of coprophagic activity within co-housed mice and microbiota recovery. Next steps for our research will see the validation of the synbiotic efficacy within human infants at times of microbiota vulnerability (i.e., antibiotic challenge, premature birth, caesarean birth).
Concluding remarks
Previous work employing single [27] and multi-strain [26] probiotics have demonstrated that formulations and type/combination of antibiotics dictates the success of baseline microbiota re-establishment and conferral of host benefits. We aimed to investigate potential effects of prebiotics alone and synbiotics on a disturbed microbiota to restore the GI microbiota. Our study supports a rapid and profound effect on partial microbial restoration with a bifidobacterial mixed synbiotic. Future work should aim to examine whether this synbiotic formulation can restore the microbiota under alternative circumstances (e.g., different antibiotics, pre-term birth).
Supplementary Information
Additional file 1: Table S1. Strains used in this study. Table S2. Diet formulation manufactured by SSNIFF (www.ssniff.com). Table S3. Primers used in this study. Table S4. PERMANOVA statistics between microbiota sequence timepoints of beta diversity matrices for each treatment group. N=5/group. Figure S1. Ileum content HPLC VFA results for A) groups culled on Day 29, and B) Replicate groups culled on Day 35. Figure S2. Cecum content HPLC VFA results for A) groups culled on Day 29, and B) replicate groups culled on Day 35. Figure S3. Relative abundance of the top 25 genera at Day -7, 5, 13, 25 and 35 across the 3 intervention groups supplemented with A)scGOS/lcFOS/2’-FL and B.breve NRBB01 B) scGOS/lcFOS/2’-FL and the Bifidobacterium strain Mix and C) scGOS/lcFOS/2’-FL diet only, without clindamycin treatment. Groups treated with clindamycin between Day 7-14 include groups supplemented with D) scGOS/lcFOS/2’-FL and B. breve NRBB01 E) scGOS/lcFOS/2’-FL and the Bifidobacterium strain Mix and F) scGOS/lcFOS/2’-FL diet only. N=5/group. Figure S4. Line plot of the relative abundance of key genera A) Akkermansia, B) Bacteroides, C) Citrobacter, D) Clostridioides, E) Enterococcus, F) Escherichia, G) Lactobacillus, H) Parabacteroides at Days -7, 5, 13, 25 and 35. Each line represents a treatment group with and without antibiotic treatment (clindamycin) between Day 7-14 (B. breve NRBB01, Bifidobacterium strain Mix and scGOS/lcFOS/2’-FL diet only), without clindamycin treatment. Clindamycin treatment occurred between Day 7-14; n=5/group. Figure S5. Cecum dimensions for replicate groups A (29 Day) and B (35 Day) demonstrate a difference between groups treated with and without antibiotics. Cecums of animals receiving clindamycin treatment are larger in length, which is more noticeable for Group A (cull Day 29) which was culled 2 weeks post antibiotic treatment (n = 5). Group B (cull Day 35) had an n=1 and PBS+ABX control was not imaged. Therefore, these results are only observational and not appropriate for statistical analysis. Figure S6. Histological scoring found no significant differences, at Day 35 (3 weeks post antibiotic), of the colonic folds number (glands), edema, neutral mucin staining, and goblets cells (I-L) were measured with at least 5 per mouse. Data are expressed as means ± SD. n= 4 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S7. Immune markers A) IL-1b, B) lL-6, and C) IL-10 qPCR relative expressions at Day 29 and D) IL-1b, E) lL-6, and F) IL-10 at Day 35. Data are expressed as Log 2 transformed ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S8. Immune markers A) TNF-α and B) IFN-g qPCR relative expressions at Day 29 and C) TNF-α D) IFN-g at Day 35. Data are Log 2 transformed ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S9. Epithelial barrier markers A) Muc2, and B) DEFA5 at Day 29 qPCR relative expressions and C) Muc2, D) DEFA5 at Day 35. Data is Log 2 transformed ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S10. Epithelial barrier markers A) CLDN-1 and A) CLDN-2 qPCR relative expressions at Day 29 and C) CLDN-1, D) CLDN-2 at Day 35. Log 2 transformed data as ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001.
Acknowledgements
We would like to acknowledge the fantastic technical and customer support of One Codex.
Authors’ contributions
ECH planned and performed research and wrote the paper; CMH, NC, RSB, JMK performed research; KvL, KBA, JK, JM, and DvS planned and supervised research and edited the manuscript.
Funding
This publication has emanated from research supported in part by a research grant from Science Foundation Ireland (SFI) under Grant Number SFI/12/RC/2273_P2 and a research grant from Danone Nutricia Research.
Availability of data and materials
Raw sequence data is available and has been deposited at NCBI SRA under the BioProject accession number PRJNA732472.
Declarations
Ethics approval and consent to participate
All experiments were conducted in accordance with the European Directive 2010/63/EU and approved by the Animal Experimentation Ethics Committee of University College Cork and Health Products Regulatory Authority under HPRA Project Licence AE19130/P110.
Consent for publication
Not applicable.
Competing interests
RSB, KvL, KBA and JK are all employed by Danone Nutricia Research.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
John MacSharry and Douwe van Sinderen are joint senior and corresponding authors.
Contributor Information
John MacSharry, Email: j.macsharry@ucc.ie.
Douwe van Sinderen, Email: d.vansinderen@ucc.ie.
References
- 1.Timmerman HM, Rutten NBMM, Boekhorst J, Saulnier DM, Kortman GAM, Contractor N, Kullen M, Floris E, Harmsen HJM, Vlieger AM, et al. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci Rep. 2017;7:8327. doi: 10.1038/s41598-017-08268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, Olin A, Wang J, Mikes J, Tan Z, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184:3884–3898.e3811. doi: 10.1016/j.cell.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J Clin Med. 2021;10(3):459. [DOI] [PMC free article] [PubMed]
- 5.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, et al: Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. [DOI] [PubMed]
- 7.Yassour M, Vatanen T, Siljander H, Hämäläinen A-M, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, et al: Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8:343ra381–343ra381. [DOI] [PMC free article] [PubMed]
- 8.De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149:73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Makino H. Bifidobacterial strains in the intestines of newborns originate from their mothers. Biosci Microbiota Food Health. 2018;37:79–85. doi: 10.12938/bmfh.18-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, Zhuo N, Wang H, Wang L, Wu D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. 2020;10:15792. doi: 10.1038/s41598-020-72635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitaoka M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv Nutr (Bethesda, Md) 2012;3:422S–429S. doi: 10.3945/an.111.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogawski ET, Platts-Mills JA, Seidman JC, John S, Mahfuz M, Ulak M, Shrestha SK, Soofi SB, Yori PP, Mduma E, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ. 2017;95:49–61. doi: 10.2471/BLT.16.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca SN, Ehrenkranz RA, Baltimore RS. Epidemiology of antibiotic use in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 1994;15:156–162. doi: 10.2307/30145554. [DOI] [PubMed] [Google Scholar]
- 14.Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol. 2014;98:6051–6060. doi: 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 15.Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs Res. 2016;65:76–88. doi: 10.1097/NNR.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- 17.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al: The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017;81(4) [DOI] [PMC free article] [PubMed]
- 19.Uzan-Yulzari A, Turta O, Belogolovski A, Ziv O, Kunz C, Perschbacher S, Neuman H, Pasolli E, Oz A, Ben-Amram H, et al. Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization. Nat Commun. 2021;12:443. doi: 10.1038/s41467-020-20495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daum RS: Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. NEJM. 2007;357:380–390. [DOI] [PubMed]
- 21.Douglas RL, Kislak JW. Treatment of Bacteroides fragilis bacteremia with clindamycin. J Infect Dis. 1973;128:569–571. doi: 10.1093/infdis/128.4.569. [DOI] [PubMed] [Google Scholar]
- 22.Tomioka S, Kobayashi Y. Bacteriological studies on Bacteroides fragilis infections and treatment with clindamycin for intravenous injection. Jpn J Antibiot. 1977;30:30–35. [PubMed] [Google Scholar]
- 23.Noti A, Grob K, Biedermann M, Deiss U, Bruschweiler BJ. Exposure of babies to C15–C45 mineral paraffins from human milk and breast salves. Regul Toxicol Pharmacol. 2003;38:317–325. doi: 10.1016/S0273-2300(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda S, Mori S, Azuma S. Clinical evaluation of clindamycin in gyneco-obstetrics. Chemotherapy (Tokyo). 1969;17:899–900. [Google Scholar]
- 25.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018;174:1406–1423.e1416. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 27.Ojima MN, Gotoh A, Takada H, Odamaki T, Xiao J-Z, Katoh T, Katayama T. Bifidobacterium bifidum suppresses gut inflammation caused by repeated antibiotic disturbance without recovering gut microbiome diversity in mice. Front Microbiol. 2020;11:1349. doi: 10.3389/fmicb.2020.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Yang F, Wu Q, Gao J, Liu W, Liu C, Guo X, Suwal S, Kou Y, Zhang B, et al. Protective effects of bifidobacterial strains against toxigenic clostridium difficile. Front Microbiol. 2018;9:888–888. doi: 10.3389/fmicb.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chua MC, Ben-Amor K, Lay C, Neo AGE, Chiang WC, Rao R, Chew C, Chaithongwongwatthana S, Khemapech N, Knol J, Chongsrisawat V. Effect of synbiotic on the gut microbiota of cesarean delivered infants: a randomized, double-blind, multicenter study. J Pediatr Gastroenterol Nutr. 2017;65:102–106. doi: 10.1097/MPG.0000000000001623. [DOI] [PubMed] [Google Scholar]
- 30.Tap J, Ruppé E, Derrien M: 2.14 - The human gut microbiota in all its states: from disturbance to resilience. In Comprehensive Gut Microbiota. Edited by Glibetic M. Oxford: Elsevier; 2022:161–178.
- 31.Korpela K, Salonen A, Vepsäläinen O, Suomalainen M, Kolmeder C, Varjosalo M, Miettinen S, Kukkonen K, Savilahti E, Kuitunen M, de Vos WM. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6:182. doi: 10.1186/s40168-018-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bottacini F, Morrissey R, Esteban-Torres M, James K, van Breen J, Dikareva E, Egan M, Lambert J, van Limpt K, Knol J, et al. Comparative genomics and genotype-phenotype associations in Bifidobacterium breve. Sci Rep. 2018;8:10633. doi: 10.1038/s41598-018-28919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín R, Bottacini F, Egan M, Chamignon C, Tondereau V, Moriez R, Knol J, Langella P, Eutamene H, Smokvina T, van Sinderen D. The infant-derived bifidobacterium bifidum strain CNCM I-4319 strengthens gut functionality. Microorganisms. 2020;8(9):1313. doi: 10.3390/microorganisms8091313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoedt EC, Bottacini F, Cash N, Bongers RS, van Limpt K, Ben Amor K, Knol J, MacSharry J, van Sinderen D. Broad purpose vector for site-directed insertional mutagenesis in Bifidobacterium breve. Front Microbiol. 2021;12:624. doi: 10.3389/fmicb.2021.636822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoedt EC, Bongers RS, Bottacini F, Knol J, MacSharry J, van Sinderen D. Bifidobacterium Transformation. Methods Mol Biol. 2021;2278:13–19. doi: 10.1007/978-1-0716-1274-3_2. [DOI] [PubMed] [Google Scholar]
- 36.O′Connell Motherway M, Watson D, Bottacini F, Clark TA, Roberts RJ, Korlach J, Garault P, Chervaux C, van Hylckama Vlieg JET, Smokvina T, van Sinderen D: Identification of restriction-modification systems of Bifidobacterium animalis subsp. lactis CNCM I-2494 by SMRT Sequencing and Associated Methylome Analysis. PLOS ONE. 2014;9:e94875. [DOI] [PMC free article] [PubMed]
- 37.Cronin M, Knobel M, O'Connell-Motherway M, Fitzgerald GF, van Sinderen D. Molecular dissection of a bifidobacterial replicon. Appl Environ Microbiol. 2007;73:7858–7866. doi: 10.1128/AEM.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 39.Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, Mastroeni P, Scott P, Raisen C, Mottram L, et al. Antibiotic treatment of clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immunol. 2009;77:3661–3669. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rousseau K, Swallow DM. Mucin methods: genes encoding mucins and their genetic variation with a focus on gel-forming mucins. Methods Mol Biol. 2012;842:1–26. doi: 10.1007/978-1-61779-513-8_1. [DOI] [PubMed] [Google Scholar]
- 41.Puchtler H, Waldrop FS, Meloan SN, Terry MS, Conner HM. Methacarn (methanol-Carnoy) fixation. Histochemie. 1970;21:97–116. doi: 10.1007/BF00306176. [DOI] [PubMed] [Google Scholar]
- 42.Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, Boekschoten MV, Savelkoul HFJ, De Vos P, Dekker J, Wells JM. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep. 2019;9:1437. doi: 10.1038/s41598-018-35228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun. 2011;79:1536–1545. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao C, Zhang Q, Zeng W, Liu G, Shao H. The effect of antibiotic cocktails on host immune status is dynamic and does not always correspond to changes in gut microbiota. Appl Microbiol Biotechnol. 2020;104:4995–5009. doi: 10.1007/s00253-020-10611-1. [DOI] [PubMed] [Google Scholar]
- 45.Kamphuis JBJ, Mercier-Bonin M, Eutamène H, Theodorou V. Mucus organisation is shaped by colonic content; a new view. Sci Rep. 2017;7:8527. doi: 10.1038/s41598-017-08938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minot SS, Krumm N, Greenfield NB: One Codex: a sensitive and accurate data platform for genomic microbial identification. bioRxiv 2015:027607. 10.1101/027607.
- 47.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 48.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, et al: SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature methods. 2020;17:261–272. [DOI] [PMC free article] [PubMed]
- 49.Savage DC, Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968;128:97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Håkansson Å, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt ML, Karlsson C, Jeppsson B, Cilio CM, Ahrné S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15:107–120. doi: 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lay C, Chu CW, Purbojati RW, Acerbi E, Drautz-Moses DI, de Sessions PF, Jie S, Ho E, Kok YJ, Bi X, et al. A synbiotic intervention modulates meta-omics signatures of gut redox potential and acidity in elective caesarean born infants. BMC Microbiol. 2021;21:191. doi: 10.1186/s12866-021-02230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878–888. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]
- 53.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penders J. Thijs C Fau - Vink C, Vink C Fau - Stelma FF, Stelma Ff Fau - Snijders B, Snijders B Fau - Kummeling I, Kummeling I Fau - van den Brandt PA, van den Brandt Pa Fau - Stobberingh EE, Stobberingh EE: Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 55.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE: Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243:141–147. [DOI] [PubMed]
- 56.Penders J, Stobberingh EE, van den Brandt PA, van Ree R, Thijs C. Toxigenic and non-toxigenic Clostridium difficile: determinants of intestinal colonisation and role in childhood atopic manifestations. Gut. 2008;57:1025. doi: 10.1136/gut.2007.143214. [DOI] [PubMed] [Google Scholar]
- 57.Elward A, Brady MT, Bryant K, Dasti M, Fauerbach L, Irwin KL, Iwamoto M, Kuntz G, Brian Leas M, Milstone A, et al: Centers for disease control and prevention. Clostridioides difficile in Neonatal Intensive Care Unit Patients: A Systematic Review. (Prevention CfDCa ed. Atlanta: Healthcare Infection Control Practices Advisory Committee (HICPAC); 2018.
- 58.You JS, Yong JH, Kim GH, Moon S, Nam KT, Ryu JH, Yoon MY, Yoon SS. Commensal-derived metabolites govern Vibrio cholerae pathogenesis in host intestine. Microbiome. 2019;7:132. doi: 10.1186/s40168-019-0746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cotten CM. Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr. 2016;28:141–149. doi: 10.1097/MOP.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collins JW, Akin AR, Kosta A, Zhang N, Tangney M, Francis KP, Frankel G. Pre-treatment with Bifidobacterium breve UCC2003 modulates Citrobacter rodentium-induced colonic inflammation and organ specificity. Microbiology. 2012;158:2826–2834. doi: 10.1099/mic.0.060830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen B, Taibi A, Villa RC, Lee S-H, Sagaidak S, Comelli ME. Effects of Bifidobacterium bifidum in Mice Infected with Citrobacter rodentium. Microorganisms. 2019;7(2):51. doi: 10.3390/microorganisms7020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun B, Song M, Park D-J, Oh S. Beneficial Effect of Bifidobacterium longum ATCC 15707 on Survival Rate of Clostridium difficile Infection in Mice. Korean J Food Sci Anim Resour. 2017;37:368–375. doi: 10.5851/kosfa.2017.37.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grazul H, Kanda LL, Gondek D. Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut microbes. 2016;7:101–114. doi: 10.1080/19490976.2016.1138197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia Rodenas CL, Lepage M, Ngom-Bru C, Fotiou A, Papagaroufalis K, Berger B. Effect of Formula Containing Lactobacillus reuteri DSM 17938 on Fecal Microbiota of Infants Born by Cesarean-Section. J Pediatric Gastroenterol Nutr. 2016;63(6):681–687. doi: 10.1097/MPG.0000000000001198. [DOI] [PubMed] [Google Scholar]
- 65.Korpela K, Salonen A, Virta LJ, Kumpu M, Kekkonen RA, de Vos WM. Lactobacillus rhamnosus GG Intake Modifies Preschool Children’s Intestinal Microbiota, Alleviates Penicillin-Associated Changes, and Reduces Antibiotic Use. PLoS ONE. 2016;11:e0154012. doi: 10.1371/journal.pone.0154012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, Lionetti P, Cinquetti M, Iacono G, Amarri S, Guarino A. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: an observational study. Clin Nutr. 2009;28:156–161. doi: 10.1016/j.clnu.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–2424. doi: 10.1093/jn/137.11.2420. [DOI] [PubMed] [Google Scholar]
- 68.Scholtens PA, Alliet P, Raes M, Alles MS, Kroes H, Boehm G, Knippels LM, Knol J, Vandenplas Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J Nutr. 2008;138:1141–1147. doi: 10.1093/jn/138.6.1141. [DOI] [PubMed] [Google Scholar]
- 69.Bakker-Zierikzee AM, Tol EA, Kroes H, Alles MS, Kok FJ, Bindels JG. Faecal SIgA secretion in infants fed on pre- or probiotic infant formula. Pediatr Allergy Immunol. 2006;17:134–140. doi: 10.1111/j.1399-3038.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 70.van Hoffen E, Ruiter B, Faber J, M'Rabet L, Knol EF, Stahl B, Arslanoglu S, Moro G, Boehm G, Garssen J. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy. 2009;64:484–487. doi: 10.1111/j.1398-9995.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 71.Huet F, Abrahamse-Berkeveld M, Tims S, Simeoni U, Beley G, Savagner C, Vandenplas Y, Hourihane JOB. Partly Fermented Infant Formulae With Specific Oligosaccharides Support Adequate Infant Growth and Are Well-Tolerated. J Pediatr Gastroenterol Nutr. 2016;63:e43–e53. doi: 10.1097/MPG.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azagra-Boronat I, Massot-Cladera M, Knipping K, Van't Land B, Stahl B, Garssen J, Rodríguez-Lagunas MJ, Franch À, Castell M, Pérez-Cano FJ. Supplementation with 2'-FL and scGOS/lcFOS ameliorates rotavirus-induced diarrhea in suckling rats. Front Cell Infect Microbiol. 2018;8:372–372. doi: 10.3389/fcimb.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 74.Zhou W, Jiang H, Wang L, Liang X, Mao X. Biotechnological production of 2'-fucosyllactose: a prevalent fucosylated human milk oligosaccharide. ACS Synth Biol. 2021;10:447–458. doi: 10.1021/acssynbio.0c00645. [DOI] [PubMed] [Google Scholar]
- 75.Autran CA, Schoterman MH, Jantscher-Krenn E, Kamerling JP, Bode L. Sialylated galacto-oligosaccharides and 2'-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br J Nutr. 2016;116:294–299. doi: 10.1017/S0007114516002038. [DOI] [PubMed] [Google Scholar]
- 76.Benítez-Páez A, Moreno FJ, Sanz ML, Sanz Y. Genome structure of the symbiont bifidobacterium pseudocatenulatum CECT 7765 and gene expression profiling in response to lactulose-derived oligosaccharides. Front Microbiol. 2016;7:624–624. doi: 10.3389/fmicb.2016.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lawson MAE, O’Neill IJ, Kujawska M, Gowrinadh Javvadi S, Wijeyesekera A, Flegg Z, Chalklen L, Hall LJ. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020;14:635–648. doi: 10.1038/s41396-019-0553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salli K, Hirvonen J, Siitonen J, Ahonen I, Anglenius H, Maukonen J. Selective utilization of the human milk oligosaccharides 2′-fucosyllactose, 3-fucosyllactose, and difucosyllactose by various probiotic and pathogenic bacteria. J Agric Food Chem. 2021;69:170–182. doi: 10.1021/acs.jafc.0c06041. [DOI] [PubMed] [Google Scholar]
- 79.Crouzet L, Derrien M, Cherbuy C, Plancade S, Foulon M, Chalin B, van Hylckama Vlieg JET, Grompone G, Rigottier-Gois L, Serror P. Lactobacillus paracasei CNCM I-3689 reduces vancomycin-resistant Enterococcus persistence and promotes Bacteroidetes resilience in the gut following antibiotic challenge. Sci Rep. 2018;8:5098. doi: 10.1038/s41598-018-23437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Strains used in this study. Table S2. Diet formulation manufactured by SSNIFF (www.ssniff.com). Table S3. Primers used in this study. Table S4. PERMANOVA statistics between microbiota sequence timepoints of beta diversity matrices for each treatment group. N=5/group. Figure S1. Ileum content HPLC VFA results for A) groups culled on Day 29, and B) Replicate groups culled on Day 35. Figure S2. Cecum content HPLC VFA results for A) groups culled on Day 29, and B) replicate groups culled on Day 35. Figure S3. Relative abundance of the top 25 genera at Day -7, 5, 13, 25 and 35 across the 3 intervention groups supplemented with A)scGOS/lcFOS/2’-FL and B.breve NRBB01 B) scGOS/lcFOS/2’-FL and the Bifidobacterium strain Mix and C) scGOS/lcFOS/2’-FL diet only, without clindamycin treatment. Groups treated with clindamycin between Day 7-14 include groups supplemented with D) scGOS/lcFOS/2’-FL and B. breve NRBB01 E) scGOS/lcFOS/2’-FL and the Bifidobacterium strain Mix and F) scGOS/lcFOS/2’-FL diet only. N=5/group. Figure S4. Line plot of the relative abundance of key genera A) Akkermansia, B) Bacteroides, C) Citrobacter, D) Clostridioides, E) Enterococcus, F) Escherichia, G) Lactobacillus, H) Parabacteroides at Days -7, 5, 13, 25 and 35. Each line represents a treatment group with and without antibiotic treatment (clindamycin) between Day 7-14 (B. breve NRBB01, Bifidobacterium strain Mix and scGOS/lcFOS/2’-FL diet only), without clindamycin treatment. Clindamycin treatment occurred between Day 7-14; n=5/group. Figure S5. Cecum dimensions for replicate groups A (29 Day) and B (35 Day) demonstrate a difference between groups treated with and without antibiotics. Cecums of animals receiving clindamycin treatment are larger in length, which is more noticeable for Group A (cull Day 29) which was culled 2 weeks post antibiotic treatment (n = 5). Group B (cull Day 35) had an n=1 and PBS+ABX control was not imaged. Therefore, these results are only observational and not appropriate for statistical analysis. Figure S6. Histological scoring found no significant differences, at Day 35 (3 weeks post antibiotic), of the colonic folds number (glands), edema, neutral mucin staining, and goblets cells (I-L) were measured with at least 5 per mouse. Data are expressed as means ± SD. n= 4 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S7. Immune markers A) IL-1b, B) lL-6, and C) IL-10 qPCR relative expressions at Day 29 and D) IL-1b, E) lL-6, and F) IL-10 at Day 35. Data are expressed as Log 2 transformed ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S8. Immune markers A) TNF-α and B) IFN-g qPCR relative expressions at Day 29 and C) TNF-α D) IFN-g at Day 35. Data are Log 2 transformed ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S9. Epithelial barrier markers A) Muc2, and B) DEFA5 at Day 29 qPCR relative expressions and C) Muc2, D) DEFA5 at Day 35. Data is Log 2 transformed ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001. Figure S10. Epithelial barrier markers A) CLDN-1 and A) CLDN-2 qPCR relative expressions at Day 29 and C) CLDN-1, D) CLDN-2 at Day 35. Log 2 transformed data as ± SD; n= 5 per group. Mean values were significantly different between the groups: *P<0.05, **P<0.01, *** P<0.001.
Data Availability Statement
Raw sequence data is available and has been deposited at NCBI SRA under the BioProject accession number PRJNA732472.