Abstract
Two live attenuated single-deletion mutant simian immunodeficiency virus (SIV) constructs, SIV239Δnef and SIVPBj6.6Δnef, were tested for their abilities to stimulate protective immunity in macaques. During the immunization period the animals were examined for specific immune responses and virus growth. Each construct generated high levels of specific immunity in all of the immunized animals. The SIV239Δnef construct was found to grow to high levels in all immunized animals, with some animals remaining positive for virus isolation and plasma RNA throughout the immunization period. The SIVPBj6.6Δnef was effectively controlled by all of the immunized animals, with virus mostly isolated only during the first few months following immunization and plasma RNA never detected. Following an extended period of immunization of over 80 weeks, the animals were challenged with a pathogenic simian-human immunodeficiency virus (SHIV) isolate, SIV89.6PD, by intravenous injection. All of the SIV239Δnef-immunized animals became infected with the SHIV isolate; two of five animals eventually controlled the challenge and three of five animals, which failed to check the immunizing virus, progressed to disease state before the unvaccinated controls. One of five animals immunized with SIVPBj6.6Δnef totally resisted infection by the challenge virus, while three others limited its growth and the remaining animal became persistently infected and eventually died of a pulmonary thrombus. These data indicate that vaccination with attenuated SIV can protect macaques from disease and in some cases from infection by a divergent SHIV. However, if animals are unable to control the immunizing virus, potential damage that can accelerate the disease course of a pathogenic challenge virus may occur.
Currently the most effective means of vaccination under development by the human immunodeficiency virus (HIV) scientific research community is attenuated virus. The studies of attenuated lentiviruses have been limited to animal models (2, 9, 13, 27, 31, 38, 47) and a few naturally occurring cases in the human population (10, 22, 26). All of the naturally attenuated viruses isolated from both humans and macaques have been reported to cause little or no pathogenicity in the infected hosts (22, 26), although reversion to pathogenic virus has been reported (46). Attenuated isolates, constructed by molecular biologic techniques (9, 13, 33), have been used to immunize adult nonhuman primates with little observed pathology, although recent studies have found that they can cause disease in newborn macaques (4, 48). The attenuated-virus vaccine strategy has been tested and proven to be very effective at protecting juvenile to adult animals from homologous challenge virus (2, 9, 27, 31, 47). Inactivated isolates tested in vivo have included both natural (2, 3, 31) and constructed deletions in the nef coding region (9), and constructs with deletions in the accessory genes vif, vpr, and vpx and in downstream portions of the nef coding regions found in the 3′ long terminal repeat have also been studied (13). As was originally predicted by Daniel et al. (9) during their studies involving the nef gene deletion mutants of simian immunodeficiency virus (SIV), the greater the number of deletions built into the virus accessory genes, the less efficiently the virus grows both in vitro and in vivo, suggesting a lower pathogenicity. This lower growth potential is associated with a reduced immune response and less-effective protection following challenge (13). Evaluation of the immune responses following immunization has revealed that the attenuated virus can induce potent immunity associated with both humoral and cellular responses (18, 47). Upon challenge with homologous and heterologous SIV/HIV-2, animals immunized with nef deletion mutants or mutants with deletions in nef and vpr were usually completely protected from the challenge. Immunized animals infected with the challenge virus were found to limit the replication and survive significantly longer than unimmunized controls; however, this has not always been true for all attenuated-virus isolates (11, 46).
The mechanism of protection induced by attenuated lentiviruses is currently unknown. Immunized animals have been shown to produce strong cellular and humoral responses to the immunizing virus, including cytotoxic T-cell production (18), helper T-cell responses (15), and neutralizing antibodies (18). Unfortunately, no correlates of the protective immunity have been determined. Passive antibody transfer studies recently performed by Almond et al. (3) have not shown any linkage between the antibodies developed following immunization and the observed protection. In addition, recent studies by Langlois et al. (23) have shown that the antiviral antibodies generated by attenuated-SIV-immunized animals fail to neutralize the SIV challenge stocks in vitro. In vivo depletion of lymphocytes with anti-CD57 or anti-CD8 monoclonal antibody treatments did not affect the protective responses of the immunized animals (39). These studies suggest that the mechanism may be viral interference by receptor blocking (45) or the presence of other nonspecific immune mediators, such as cytokines or chemokines (6, 7, 12, 41).
The present studies were designed to focus on the humoral responses generated during immunization and to determine the breadth of the protective response by challenging with a pathogenic simian-human immunodeficiency virus (SHIV) that contains an HIV-1-derived envelope. The challenge virus, SHIV89.6PD, is highly infectious and causes a rapid pathology in macaques (25, 28, 34, 40). It was constructed by using the SIV239 molecular clone as the backbone with the substitution of the HIV-189.6 envelope gene for the SIV239 envelope (35). The construct has recently been described by Reimann et al. (35), and an in vivo-passaged isolate has been derived and cloned (20). This isolate infects macaques when they are challenged either by intravenous injection or application on mucosal surfaces (25, 40) and causes a rapid disease course with a near-total loss of detectable CD4 cells. For the present studies we chose to use two nef-deleted SIV strains, SIV239Δnef (9) and SIVPBj6.6Δnef (33). The results of this study show that these attenuated viruses can totally protect some animals from infection with the SHIV89.6PD and that if infection occurs, the acute-disease course can be altered. However, animals that were unable to control the immunizing viruses were found to have difficulty in controlling the challenge virus and were subsequently found to progress to disease more rapidly than the controls.
MATERIALS AND METHODS
Animals.
Animals used in this study were captive-bred, juvenile rhesus macaques from a breeding colony in the United States or imported pigtailed macaques. All animals were screened and confirmed to be free of antibodies to SIV, simian retrovirus (SRV), and simian T-cell leukemia virus type 1 (STLV-1) and free of isolatable SIV and SRV following culture performed prior to their inclusion in the study. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards.
Virus stocks.
Construction of the SIV239Δnef and SIVPBj6.6Δnef viruses has been described previously (9, 33). The SIV239Δnef virus stock was supplied by Ronald C. Desrosiers. The SIVPBj6.6Δnef construct was supplied by Frank Novembre. The attenuated-virus stocks used for this experiment were grown in CEM×174 cells in an acute infection with harvest at day 12 postinoculation (p.i.). The stocks were filtered through a 0.45-μm-pore-size filter and frozen in aliquots at −165°C. The titers of these stocks were 103 50% tissue culture infective doses (TCID50) per ml, as measured by using log dilutions on CEM×174 cells. The rhesus macaques were inoculated intravenously with 1.0 ml of the undiluted SIV239Δnef stock, and the pigtailed macaques were inoculated intravenously with the SIVPBj6.6Δnef stock. The SHIV89.6 construction has been previously described (35). The pathogenic isolate derivation has been described in detail by Reimann et al. (34). The specific isolate (SHIV89.6PD) used in these studies was derived in CEM×174 cells from culture with the plasma of the second in vivo-passage of the 89.6 isolate (25). The virus stock was produced from acute infection of macaque peripheral blood lymphocytes for 7 days. The titer of the stock was 2.5 × 104 TCID50/ml in CEM×174 cells and 2.5 × 104 50% macaque infectious doses. The animals were challenged with 25 50% infective doses of the SHIV89.6PD construct/ml by intravenous injection.
Virus detection and isolation.
SIV p27 was detected in the plasma of the macaques by using an SIV p27-specific enzyme-linked immunosorbent assay (ELISA) (Coulter Diagnostics, Miami, Fla.). For isolating virus, macaque peripheral blood lymphocytes were isolated by using a 95% Ficoll-Hypaque separation solution (Sigma, St. Louis, Mo.). Approximately 2.5 × 106 cells were placed in 2.5 ml of RPMI 1640 medium containing 10% fetal bovine serum, 0.5% gentamicin, 5.0% glutamine, and 5 mg of phytohemagglutinin-p (PHA-p) (Sigma) per ml. Following 3 days of culture, the supernatant was removed and a portion was tested for the presence of SIV p27 (Coulter Diagnostics). The cells were washed free of PHA, adjusted to 106/ml, and placed in media without PHA-p and with 10% recombinant interleukin-2 (Boehringer Mannheim, Indianapolis, Ind.). An equal number of human 3-day PHA-blasted peripheral blood mononuclear cells (PBMC) were then added. Cultures were readjusted to 106 cells/ml every 3 to 4 days with a 100% medium change and assayed twice per week for the presence of SIV p27. Cultures were held for up to 4 weeks. A culture was designated as positive after positive test results were obtained from two successive specimens.
Flow cytometry.
Peripheral blood lymphocyte subset analysis was performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) with a panel of mouse anti-human monoclonal antibodies to B cells (phycoerythrin-conjugated CD20), T cells (fluorescein isothiocyanate-conjugated CD2), and T-cell subsets (phycoerythrin-conjugated CD4 and fluorescein isothiocyanate-conjugated CD8) (Becton Dickinson). Analysis was performed by a whole-blood-lysis procedure as directed by the manufacturer.
Anti-SIV antibody ELISA.
Circulating SIV antibodies were detected by an HIV-2 ELISA (Genetic Systems, Seattle, Wash.) which is cross-reactive for SIV antibody. The ELISA used a fixed plasma dilution of 1:200. All samples from an individual animal were run at the same time. The assay was run as directed except that the plates were read on a Vmax kinetic ELISA reader (Molecular Devices, Menlo Park, Calif.) at 650 nm. Values of 10−3 optical density (OD) units per min minus background were used as ELISA units.
ELISA measurement of HIV-1 gp160-specific serum IgG binding.
Affinity-purified oligomeric HIV-1 gp160451 (Advanced Bioscience Laboratories, Kensington, Md.) (0.65 μg/ml in phosphate-buffered saline) (PBS) (pH 7.4; 0.01% thimerosal) was coated onto Immulon 2 microtiter plates (Dynatech, Chantilly, Va.), left overnight at 4°C, and assayed as described previously (44). Oligomeric gp160 was affinity purified from cell cultures infected with HIV-1451 as described previously (19). Briefly, plates were washed twice with wash buffer (PBS with 0.1% Tween 20 [pH 7.4]) prior to the incubation with twofold dilutions of macaque serum, diluted in diluent (wash buffer with 5% skim milk [pH 7.4]), for 1 h at 37°C. Plates were washed three times with wash buffer and incubated with horseradish peroxidase-conjugated goat anti-monkey immunoglobulin G (IgG) (Nordick Laboratories, San Clemente, Calif.) (diluted 1:4,000 in serum diluent). After a 1-h incubation at 37°C, plates were washed three times and substrate [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Kirkegaard and Perry, Gaithersburg, Md.] was then added. The reaction was stopped with addition of 0.5% sodium dodecyl sulfate after 30 min at 37°C.
Quantitative PCR of viral RNA.
Circulating levels of viral RNA were determined with an externally controlled PCR assay. Serum samples were rapidly thawed at 37°C, and to each sample an equal volume of 1× PBS containing 5 mg of heat-inactivated bovine serum albumin (Sigma) per ml was added. Samples were centrifuged at 12,000 × g for 30 min at 4°C. The viral pellets were resuspended and lysed in 800 μl of Tri-reagent (Molecular Research Center, Woodland, Tex.) and purified following the manufacturer’s instructions. Quantitative liquid hybridization was carried out by a modification of the method described by Vahey et al. (43) by using SIV gag sequences. The assay employs external standards consisting of cloned templates of a cognate region of SIVsmh4 gag DNA from a plasmid clone, p5′4i (16), obtained from Vannesa Hirsch. SIV gag RNA was amplified by reverse transcriptase-linked PCR. Reverse transcription was carried out in 40 μl of solution containing 7 μl of 5× first-strand buffer (Gibco-BRL), 20 U of RNasin (Promega, Madison, Wis.), 0.4 μl of 25 mM deoxynucleoside triphosphates and 200 U of Superscript RT (Gibco-BRL, Gaithersburg, Md.). Incubation was for 10 min at 45°C. SIV gag DNA was amplified in 100 μl of a solution containing 10 μl of 10× PCR buffer (Perkin Elmer, Norwalk, Conn.), 0.8 μl of 25 mM deoxynucleoside triphosphates (Promega), and 2.5 U of Taq polymerase (Perkin Elmer) with 1.0 mM 5′ primer, 5′-CCCGCAGTAAAGAATTGGATGAC-3′, and 1.0 mM 3′ primer, 5′-ACTTCCAGCAGCCCTGTCTTCT-3′. Amplification was for 24 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 3 min.
The PCR products were detected by liquid hybridization with 32P-labeled oligonucleotide probes for sequences internal to the PCR product. Liquid hybridization was carried out in 40 μl of a solution containing 30 μl of PCR product, 10 μl of labeled probe in 1× Tris-EDTA buffer (10 mM Tris-HCl, 30 mM EDTA [pH 8.0]) at 94°C for 5 min followed by incubation at 55°C for 10 min. The antisense oligonucleotide probe for SIV gag was 5′-GTGTTGGAATTGTGGAAAGGAGGGA-3′. The hybridized products were separated from the unincorporated material with a 10% miniacrylamide gel. The isotopic signal was quantitated by stored phosphor technology (Molecular Dynamics Corp., Sunnyvale, Calif.).
Differential nef RNA PCR assay.
A differential PCR assay was developed to determine the presence of a full nef sequence. The assay was designed to include a pair of primers that bind outside of the 5′ and 3′ ends of the deletions described by Kestler et al. (21). Sequences were obtained from the GenBank database. The reaction conditions were identical to those described above. The paired primers were 5′-GATGGATATCTGCAATCCCCAGGAGGATTA-3′ for the 5′ end and 5′-TAAATCCCTTCCAGTCCCCCCTTTTC-3′ for the 3′ end. The amplified product corresponds to a 299-bp length for the wild-type nef gene and an approximately 166-bp product for the nef deletion mutants. The amplified products were visualized by the liquid hybridization method described above with an antisense probe, 5′-GGGCTTGAGCTCACTCTCTTGTGAGGG-3′.
Virus neutralization assays.
Virus-neutralizing titers were determined with cytopathic effect reduction assays. Cytopathic effect reduction titers were determined as described by Montefiori et al. (29, 30) for SIV and SHIV isolates.
Immunoblots.
SIVMne/E11s (5) was grown in AA2 cells, clarified, and harvested by ultracentrifugation. This virus has significant immunologic cross-reactivity to both SIV239 and SIVPBj antigens. Pelleted virus was subjected to sodium dodecyl sulfate-gel electrophoresis and then transferred to nitrocellulose by the method of Towbin et al. (42). HIV-1 strips were obtained from a commercial source (Cambridge Biotech, Rockville, Md.). Immunoblots were probed with macaque plasma diluted to 1:100. Immune complexes were detected by a goat anti-human IgG (Sigma) by the enhanced chemiluminescence system (Amersham, Arlington Heights, Ill.) with Kodak XAR-5 X-ray film (Kodak, Rochester, N.Y.).
RESULTS
Virus isolation and immune response following SIVΔnef immunization.
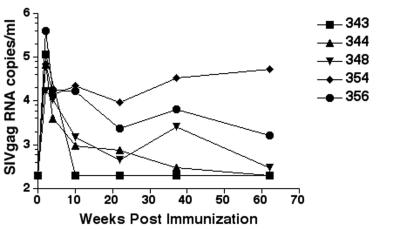
Two nef-deleted viruses, SIV239Δnef and SIVPBj6.6Δnef, were used to immunize two different macaque species, rhesus and pigtailed, respectively. In each virus, the nef gene was altered by deletion of a portion coding only for the nef protein. Within 1 week following an intravenous inoculation, virus was isolated from all the inoculated animals (Table 1). The rhesus macaques receiving the SIV239Δnef stock had moderate to high levels of virus as assessed by numbers of positive cultures (Table 1) and circulating virus RNA levels (Fig. 1). Three of five animals (348, 354, and 356) remained culture positive throughout the period of immunization (more than 80 weeks), while the remaining two animals (343 and 344) were culture positive intermittently during the immunization period. Of the three animals that remained culture positive throughout the immunization period, all were positive for circulating SIV gag RNA at the time of the challenge, and the number of copies ranged from 102 to 103 per ml (Table 2). These results were in contrast to those for the pigtailed macaques immunized with the SIVPBj6.6Δnef, of which only one animal had any positive isolations after 20 weeks, three were isolation positive only during the first 4 weeks of the immunization period, and none had detectable SIV RNA in their plasma at any time during the immunization period.
TABLE 1.
Virus isolation following immunization with SIVΔnef
| Animal no. | Virus isolation at no. of wks p.i.
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 6 | 8 | 10 | 16 | 20 | 24 | 28 | 34 | 38 | 42 | 48 | 58 | 62 | 68 | 80 | 86 | |
| Pigtaileda | |||||||||||||||||||
| 2370 | − | + | + | − | + | + | − | + | − | + | + | + | + | + | |||||
| 2394 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | |||||
| 2574 | − | + | + | − | − | − | − | − | − | − | − | − | − | − | |||||
| 2580 | − | + | + | − | − | − | − | − | − | − | − | − | − | − | |||||
| 2581 | − | + | + | + | − | − | − | + | − | − | − | − | − | − | |||||
| Rhesusb | |||||||||||||||||||
| 343 | − | + | + | + | + | + | − | + | + | − | + | + | − | − | |||||
| 344 | − | + | + | + | + | − | − | − | + | − | − | − | − | − | |||||
| 348 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| 354 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| 356 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
Pigtailed macaques were immunized with SIVPBj6.6Δnef by intravenous injection.
Rhesus macaques were immunized with SIV239Δnef by intravenous injection.
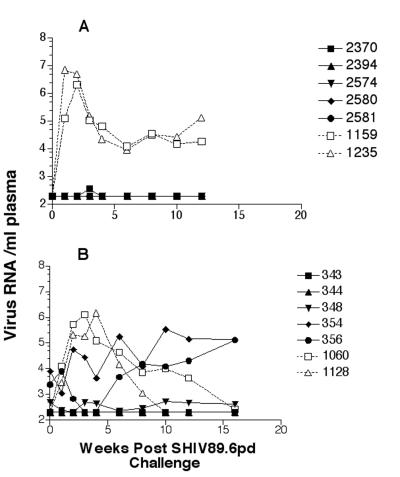
FIG. 1.
Plasma RNA levels following immunization with SIV239Δnef. The lower limit of detection for the assay was 200 copies/ml. The values on the y axis are expressed as log10. Rhesus macaques infected with the pathogenic molecular clone SIV239 produce 105 to 107 RNA copies/ml within 3 weeks of intravenous challenge.
TABLE 2.
Immunologic and virologic characterization for the vaccinated animals on the day of challenge
| Animal no. | Virus status | Virus type | Viral RNA (no. of SIV gag copies/ml of plasma) | Neutralization titera
|
SIV antibody titer (10−3 OD units/ml equivalents) | |
|---|---|---|---|---|---|---|
| SIV | HIV89.6P | |||||
| SIVPBj6.6 | ||||||
| 2370 | +/−b | Δnef | <200 | <4 | <4 | 39 |
| 2394 | − | <200 | <4 | <4 | 33 | |
| 2574 | − | <200 | <4 | <4 | 43 | |
| 2580 | − | <200 | <4 | <4 | 27 | |
| 2581 | − | <200 | <4 | <4 | 30 | |
| SIV239 | ||||||
| 343 | − | <200 | 105 | <4 | 40 | |
| 344 | − | <200 | 72 | <4 | 28 | |
| 348 | + | Δnef | 500 | 191 | <4 | 42 |
| 354 | + | Δnef | 8,200 | 92 | <4 | 27 |
| 356 | + | Δnef | 2,400 | 65 | <4 | 44 |
Neutralization assays were performed with SIVPBj and SHIV89.6P with sera from the pigtailed macaques and with SIV239 and SHIV89.6P with the sera from the rhesus macaques. The neutralization titer is given as the reciprocal of the serum dilution at which 50% of the cells were protected from virus-induced killing. Neutralization titers of SHIV89.6P for challenge controls RQ1060 and RQ1128 at 38 weeks postchallenge were 333 and 4,755, respectively (30). The SIVPBj isolate has proven difficult to neutralize when the antibody titer is high for sera from long-term survivors of virulent SIVPBj infections.
Macaque 2370 was isolation positive at one month previous to challenge date and isolation negative on the challenge date.
To confirm that the immunized animals were infected with nef-deleted virus, PCR assays were performed on DNA from fresh PBMC and RNA from viral pellets from virus-isolation-positive culture supernatants. The assay was designed to amplify the entire nef gene and determine size differences. In all cases the animals were found to be infected with an isolate that was a nef-deletion-containing virus (data not shown).
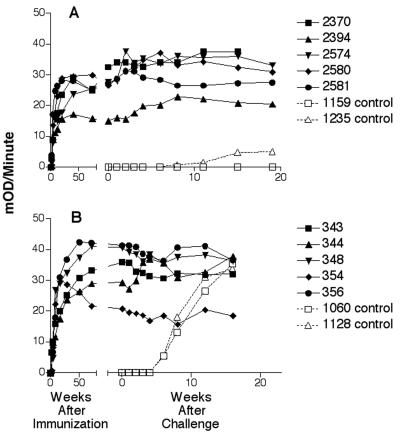
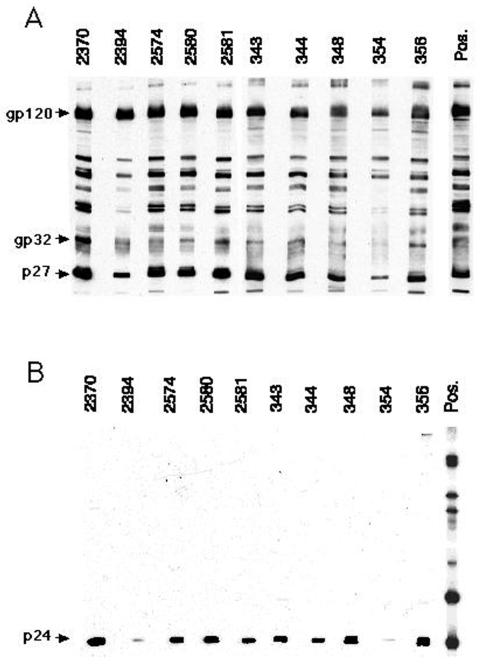
Humoral immune responses were detected in all animals within 3 weeks of immunization; the level of antibody increased to moderate to high levels during the immunization period (Fig. 2). Immunoblot analysis showed that all animals developed broad responses that included binding to all of the major SIV proteins, while no antibody cross-reactive to HIV-1 envelope proteins was observed by immunoblotting. Some cross-reactivity to HIV-1 p24 protein was observed (Fig. 3); however, this protein is not coded for by the challenge virus. Low-level cross-reactivity to HIV-1 envelope protein was observed by ELISA (Table 3). Interestingly, the animals immunized with the SIVPBj6.6Δnef isolate generated higher titers for the HIV envelope protein than did the animals immunized with SIV239Δnef. As revealed by neutralization assays performed on the day of challenge, sera from the SIV239Δnef-immunized animals were calculated to have titers against SIV239 between 65 and 191 but were uniformly negative toward SHIV89.6PD (Table 2). Although all the SIVPBj6.6Δnef-immunized animals had moderate to high antibody titers, all were negative for SIVPBj neutralization, and SHIV89.6PD-specific neutralization was also found to be negative.
FIG. 2.
Antibody responses following immunization with attenuated virus and after challenge with SHIV89.6PD. Levels of antibody are expressed as kinetic units (10−3 OD units/min) on the y axis for each individual animal. Solid symbols are data for animals immunized with attenuated virus. A, responses of animals immunized with the SIVPBj6.6Δnef isolate; B, responses of animals immunized with SIV239Δnef. Open symbols are data for control animals. The break in the x axis demarcates the period prior to challenge (to the left) and that following challenge (to the right), at week 92 p.i. for panel A and at week 86 for panel B.
FIG. 3.
Immunoblot analysis of serum collected from attenuated-virus-immunized animals on the day of challenge. Reactivities to SIVmne/e11s-derived antigens (A) and HIV-1IIIB-derived antigens (B) are shown.
TABLE 3.
Summary of ELISA for HIV envelope responses prior to challenge with SHIV89.6PD
| Monkey no. | Vaccineb | HIV gp160 titerc |
|---|---|---|
| 2370 | PBjΔnef | 1,600 |
| 2394 | PBjΔnef | 100 |
| 2574 | PBjΔnef | 200 |
| 2580 | PBjΔnef | 400 |
| 2581 | PBjΔnef | 200 |
| 1159 | None | <50 |
| 1235 | None | <50 |
| 343 | 239Δnef | <50 |
| 344 | 239Δnef | <50 |
| 348 | 239Δnef | 400 |
| 354 | 239Δnef | 50 |
| 356 | 239Δnef | <50 |
| RQ1060 | None | <50 |
| RQ1128 | None | <50 |
| RQ1060a | 102,000 | |
| RQ1128a | 409,600 |
Sera were collected at 16 weeks after challenge with SHIV89.6PD.
PBjΔnef, SIVPBj6.6Δnef; 239Δnef, SIV239Δnef.
The reciprocal of the highest serum dilution which is greater than two times the mean pooled preimmunization OD plus two standard deviations of the mean.
Virus isolation and immune response after SIV89.6PD challenge.
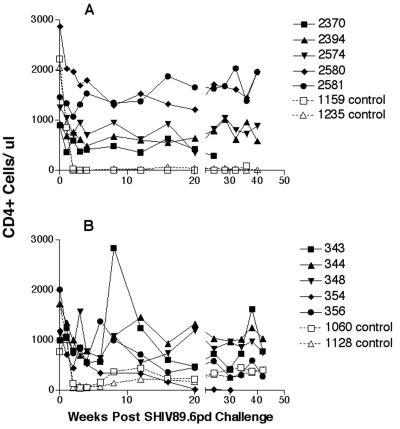
SHIV89.6PD grows to high titers in vivo in macaques following intravenous challenge, causing a rapid decline in detectable number of circulating CD4+ cells in naive macaques (34). We challenged the immunized macaques with 25 TCID50 of the SHIV89.6PD stock via the intravenous route. These challenges were performed in the immunized rhesus and pigtailed macaques at 86 and 92 weeks, respectively. Two control animals per immunization group were also inoculated in a similar fashion. All animals were monitored for virus isolation (Table 4) and circulating CD4+ cell loss (Fig. 4). As expected, the controls showed a rapid decline in circulating CD4+ cells by 2 weeks p.i. (Fig. 4). This rapid decline was absent in all of the vaccinated animals during the early follow-up period, even in those confirmed to be infected with the SHIV89.6PD. An inguinal lymph node biopsy specimen was collected at 2 weeks postchallenge from each animal, and following the preparation of a single-cell suspension of the tissues, virus isolation and lymphocyte subpopulation determinations were performed (Table 5). The results confirmed the findings for the peripheral blood, except that one animal, animal 2581, had SHIV isolated from the inguinal lymph node biopsy specimen collected at 2 weeks but was never found to show detectable circulating infected PBMC. The biopsy specimens from immunized animals showed moderate or no change in % CD4 or CD4:CD8 ratio from prechallenge levels in lymph nodes. The controls had a near-total loss of detectable CD4+ cells in the lymph nodes by 2 weeks p.i. During the follow-up period, the infected control rhesus macaques had a slow rebound in CD4+ cell numbers (Fig. 4) and each developed moderate to high levels of SIV antibody (Fig. 2). This was in contrast to the control pigtailed macaques that had no recovery of CD4+ cells and developed few or no SIV-specific antibodies.
TABLE 4.
Isolation of virus from SIVΔnef-immunized animals following SHIV89.6PD challenge
| Animal no. | Virus isolation at no. of wks postchallenge
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 28 | 32 | 34 | 36 | 38 | 40 | 42 | 45 | 48 | 49 | 56 | |
| 2370 | − | + | + | + | − | − | + | + | + | + | + | Eutha | ||||||||||||||
| 2394 | − | + | + | − | − | + | + | − | − | − | + | − | − | − | − | − | + | − | ||||||||
| 2574 | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||||
| 2580 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||||
| 2581 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||||||
| 1159C | − | + | + | + | + | + | + | + | + | + | + | + | + | Eutha | ||||||||||||
| 1235C | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| 343 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | ||||||||
| 344 | − | + | + | + | + | − | − | − | − | − | + | + | − | − | − | − | − | − | ||||||||
| 348 | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | ||||||||
| 354 | + | + | + | + | + | + | + | + | + | + | + | + | Eutha | |||||||||||||
| 356 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | Eutha | ||||||||
| 1060C | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| 1128C | − | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | + | ||||||||
Animal euthanized due to disease onset.
FIG. 4.
Levels of circulating CD4+ lymphocytes in peripheral blood during the first 40 weeks following SHIV challenge. Solid symbols are data for vaccinated animals, and open symbols are data for control animals. A, levels for animals immunized with the SIVPBj6.6Δnef isolate; B, levels for animals immunized with SIV239Δnef.
TABLE 5.
Lymph node lymphocyte populations as determined by flow cytometry
| SIVPBjnef-deleted-virus-immunized macaque
|
SIV239nef-deleted-virus-immunized macaque
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal no. | Virus isolation | Percentage of total lymphocytesc
|
CD4:CD8 ratio | Animal no. | Virus isolation | Percentage of total lymphocytesc
|
CD4:CD8 ratio | ||||||
| CD20+ | CD2+ | CD4+ | CD8+ | CD20+ | CD2+ | CD4+ | CD8+ | ||||||
| 2370 Prea | − | 19 | 73 | 45 | 27 | 1.67 | 343 Pre | + | 9 | 87 | 57 | 28 | 2.04 |
| 2370 Postb | + | 21 | 66 | 41 | 28 | 1.46 | 343 Post | + | 14 | 82 | 46 | 28 | 1.64 |
| 2394 Pre | + | 17 | 78 | 61 | 18 | 3.39 | 344 Pre | + | NDd | ND | ND | ND | |
| 2394 Post | + | 22 | 69 | 55 | 18 | 3.06 | 344 Post | + | 18 | 68 | 35 | 27 | 1.30 |
| 2574 Pre | − | 27 | 66 | 47 | 21 | 2.24 | 348 Pre | + | 24 | 68 | 45 | 26 | 1.73 |
| 2574 Post | + | 37 | 49 | 35 | 21 | 1.67 | 348 Post | + | 28 | 67 | 30 | 29 | 1.03 |
| 2580 Pre | − | 26 | 66 | 48 | 20 | 2.40 | 354 Pre | + | 26 | 64 | 43 | 28 | 1.54 |
| 2580 Post | − | 29 | 60 | 45 | 20 | 2.25 | 354 Post | + | 23 | 50 | 35 | 30 | 1.17 |
| 2581 Pre | − | 18 | 78 | 58 | 20 | 2.90 | 356 Pre | + | 18 | 77 | 46 | 30 | 1.53 |
| 2581 Post | + | 21 | 73 | 57 | 20 | 2.85 | 356 Post | + | 34 | 58 | 22 | 31 | 0.71 |
| 1159 Pre | − | 15 | 81 | 63 | 18 | 3.50 | 1060 Pre | − | 11 | 83 | 54 | 30 | 1.80 |
| 1159 Post | + | 40 | 59 | 0.7 | 49 | 0.01 | 1060 Post | + | 21 | 59 | 1.6 | 54 | 0.03 |
| 1235 Pre | − | 16 | 80 | 60 | 20 | 3.00 | 1128 Pre | − | 14 | 79 | 58 | 22 | 2.64 |
| 1235 Post | + | 37 | 55 | 0.4 | 55 | 0.01 | 1128 Post | + | 29 | 49 | 1.3 | 46 | 0.03 |
Pre, lymph node collected prior to SHIV89.6PD challenge.
Post, lymph node collected 2 weeks after SHIV89.6PD challenge.
Value equals the percentage of total lymphocytes gated by forward scatter versus side scatter differentiation.
ND, not done.
As detailed in Table 4, virus was isolated from the peripheral blood cells of all of the animals in the SIV239Δnef immunization group by 1 week postchallenge; all of these isolates contained a nef coding region, indicating an infection with the SHIV. Four of these five animals were persistently infected with a nef-containing virus at all time points following challenge. Animal 344 was isolation positive for a nef-containing virus for up to 6 weeks p.i. and then became consistently isolation negative. The two control animals were isolation positive at all time points from one week p.i. until 36 weeks p.i., when macaque 1128 became intermittently positive. By week 16 p.i., animal 354 was showing signs of significant CD4+ cell loss, and this animal was euthanized during week 28 p.i. with lymphopenia, including a circulating CD4+ cell count of 4 cells per μl, anorexia, and severe diarrhea. Animal 356 was euthanized at week 56 with severe pancreatitis associated with a large abscess involving both the pancreas and spleen. Animal 348 was euthanized at week 79 with anorexia, >50% loss from starting weight, neutrophilia, a CD4+ cell count of 200/μl, and fibromatosis involving the entire peritoneum. This animal was tested at the time of necropsy for the presence of SRV and was found to be negative by serology and by coculture with Raji cells.
The animals immunized with the SIVPBj6.6Δnef were all virus isolation negative at the challenge time, although one, animal 2370, was isolation positive at the immediately previous time point, 4 weeks before the challenge. Following the challenge with SHIV89.6PD, three of five animals showed a nef-containing virus within 1 week postchallenge. The remaining two animals were negative for virus isolation from the peripheral blood throughout the postchallenge period (Table 3). Animal 2581 had SHIV isolatable from the lymph node at 2 weeks p.i. (Table 5) but was never found to have SHIV in circulating PBMC. All five pigtailed macaques were negative for circulating p27 at all time points postchallenge. Two of the four infected animals had virus isolations during the 2 to 8 weeks following the challenge and remained isolation negative thereafter. The count of CD4+ lymphocytes declined during the first week following challenge for all animals, but that for the immunized animals remained within the normal range for pigtailed macaques (Fig. 4). The two controls, animals 1159 and 1235, became consistently culture positive by 1 week p.i. and were p27 antigen positive (0.14 and 1.64 ng/ml of serum, respectively) at week 2. Their circulating CD4+ lymphocyte counts dropped to less than 10 cells/μl by week 3 (Fig. 4), and only low levels of antibodies were detectable for either animal (Fig. 2). The lymph node biopsy specimens showed a near-total loss of CD4+ cells (Table 5). Monkey 1159 was euthanized at week 36 with anemia and lymphopenia, including a total loss of CD4+ cells, severe neutrophilia, severe wasting, and absence of detectable circulating antibodies. Animal 1235 was euthanized during week 77 p.i. while being treated for a staphylococcal septicemia. One of the immunized animals, animal 2370, was considered persistently infected and remained isolation positive at most time points; this animal died at week 24 with a pulmonary thrombosis, with no evidence of pneumonia and infarction.
SIV RNA in plasma.
Circulating SIV gag RNA was detected in three of the five SIV239Δnef-immunized animals and none of the five SIVPBj6.6Δnef-immunized animals at the time of challenge (Table 2 and Fig. 5). Following challenge with the SHIV89.6PD, all three SIV239Δnef RNA-positive, immunized rhesus macaques and one SIVPBj6.6Δnef-immunized pigtailed macaque had circulating viral RNA (Fig. 5) that contained an undeleted nef gene. The assay results for the remaining animals were all below the level of detection (<200 copies/ml). SIV RNA was easily detected in all of the control animals by 1 week postchallenge, and these levels peaked by 2 to 4 weeks. The controls for both groups had high levels of circulating RNA (>106 RNA copies/ml) (Fig. 5). The overall levels were observed to be higher in the naive pigtailed macaques than in the rhesus macaques. In addition, the pigtailed macaques developed a higher sustained level of RNA, whereas by 16 weeks postchallenge the levels for both control rhesus macaques were at or near the lower limit of detection of the assay.
FIG. 5.
Early plasma virus RNA levels following challenge with SHIV89.6PD. The lower limit of detection for the assay was 200 copies/ml. Solid symbols are data for vaccinated animals, and open symbols are data for control animals. A, levels for animals immunized with the SIVPBj6.6Δnef isolate; B, levels for animals immunized with SIV239Δnef. The values on the y axis are expressed as log10.
DISCUSSION
This study was initially designed to determine the breadth and limits of protection associated with the immunity developed following immunization with attenuated lentiviruses. To achieve this we chose to challenge with a pathogenic SHIV that contained an HIV-1 envelope and the SIV239 gag-pol. This SHIV isolate contains the envelope gene from the HIV-189.6 isolate, which is classified as a primary isolate with dual tropism for both lymphocytes and monocytes as defined by recent studies by Rucker et al. (36) showing that it can bind the CCR2b, CCR3, CCR5, and CXCR4 coreceptors. Following in vivo passage a highly pathogenic SHIV, SHIV89.6PD, was isolated (34, 35). The choice of a SHIV as a challenge virus for our study was made due to the recent finding that attenuated-virus immunization could very effectively protect animals from infection with homologous strains of SIV and nonpathogenic SHIV without any obvious correlate of protection (2, 9, 13, 27, 31, 37, 38, 47). Additional studies involving passive transfer of antibodies generated following immunization and the removal of CD8+ lymphocytes by monoclonal antibodies have also been inconclusive as to their protective values (3, 39). Currently no definitive correlation has been made as to the means of protection, leading some investigators to speculate that the protection may be due to nonspecific immunity or to viral interference (31).
Our goal for this study was to determine if attenuated virus could protect against a pathogenic virus containing a highly diverse envelope and, if protection was observed, whether it could be attributed to specific immunity. In addition, we were interested in determining if animals that were not protected from infection had an altered disease course. We chose to use two attenuated isolates, SIV239Δnef and SIVPBj6.6Δnef, to compare differences in growth and immunizing potential. The SHIV challenge virus contains the gag-pol portion of the SIV239 isolate, so that the SIV239Δnef is totally homologous in sequence to the gag-pol portion of the challenge virus, while the SIVPBj isolate varies up to 10% in the gag-pol region. The findings of this study demonstrate that the immunizing attenuated viruses stimulated effective broadly protective responses in some animals. These responses do not appear to induce a sterilizing immunity, except in the case of animal 2580, but appear to be very effective at blocking the acute pathogenicity of the SHIV89.6PD. The mechanism of protection is unlikely to be associated with specific envelope antibodies since the SHIV envelope is highly diverse and neutralizing antibodies were absent at the time of challenge. This corroborates the findings of Almond et al. (3), which showed that passive antibody treatments with immune serum from attenuated-virus-immunized animals did not protect from homologous pathogenic challenge, and of Langlois et al. (23), which showed that sera from attenuated-virus-immunized macaques failed to neutralize the challenge viruses. In addition, the protection does not appear to be associated with a viral receptor interference mechanism (45), since the animals that were replicating virus at a high level at the time of challenge were more susceptible to SHIV infection and disease than others that had little or no documented virus replication. This is emphasized by our finding that the immunizing virus SIVPBj6.6Δnef, which had limited growth potential in the immunized macaques, was more protective than the more-efficiently growing SIV239Δnef. The protective responses observed may be associated with gag-specific responses or possibly with nonspecific immune responses, such as the presence of β-chemokines or interferons. These responses are not sufficient to inhibit infection with the challenge virus but may act to decrease and possibly block its spread.
The results presented indicate that although the immunizing viruses are attenuated in phenotype, they are not totally avirulent in all hosts. Previous studies by Whatmore et al. (46) have shown that nef deletion does not eliminate pathogenicity in macaques. More recently, even a triple-deletion mutant has been shown to cause some pathology in neonates (4, 48). Our results support this finding, in that some immunized animals failed to control the attenuated virus. This is highlighted by the very effective protective responses observed in most SIVPBj6.6Δnef-immunized animals as contrasted to the SIV239Δnef-immunized group, which appear to be related to the overall growth of the immunizing virus. As was observed, the SIVPBj6.6Δnef as the immunizing virus was effectively controlled in all but one animal following the early period of immune development. In addition, the circulating RNA from the SIVPBj6.6Δnef was not detected in any animals during the immunization period. These animals very effectively blocked the SHIV infection and/or growth; one in five was completely protected and three in five controlled the SHIV after challenge. The SIVPBj6.6Δnef isolate has been used to infect an additional 15 animals, and circulating RNA was detected in only 1 animal, at a level of 103 RNA copies/ml, during the early infection (data not shown). This indicates that the isolate is significantly more attenuated than its wild-type parent strain, which grows to high levels in pigtailed macaques (24, 32). This is in contrast to the observations that all SIV239Δnef-immunized animals had circulating viral RNA following immunization and that three of these five animals were unable to control virus growth during the >80-week period of immunization. Following challenge, all three of these SIV239Δnef-immunized animals failed to control the SHIV infection and all died prior to the controls, suggesting an enhanced disease course due to undetected immune system damage caused by the immunizing virus.
The variation of the responses in the two types of macaques, rhesus and pigtailed, towards the challenge virus is of interest. These macaques are commonly used in SIV and/or SHIV research, and the pigtailed macaque has been shown to be more susceptible to most SIV and some HIV-1 isolates than the rhesus macaque (1, 14, 17, 24). These studies indicate that this is also the case for the SHIV89.6PD isolate. The virus infected both macaque types, and the patterns of early growth and pathogenicity are similar, with rapid virus growth and a rapid loss of circulating and tissue CD4+ cells. Overall differences can be observed beginning at the early time points, when higher virus loads are observed in the pigtailed macaques. By a few months postchallenge the differences become quite apparent, with CD4+ cells returning, antibodies developing, and virus RNA levels dropping in the control rhesus macaques, while the pigtailed macaques had no apparent CD4+ cell rebound or humoral response toward the virus. The control pigtailed macaques regained few, if any, circulating CD4+ cells and generated only minimal levels of SHIV antibodies, and both controls died during the study period. This indicates that the SHIV89.6PD isolate is more pathogenic in pigtailed macaques and makes the observed protective responses induced by the SIVPBj6.6Δnef even more significant.
Use of attenuated HIV constructs has recently been proposed for inclusion in human clinical trials (8). The specific constructs would have deletions additional to those in the viruses reported here. Each additional mutation has been shown to cause the virus to grow less efficiently and to induce a diminished immune response (13). Our findings suggest that a window of opportunity exists for a safe and effective attenuated-lentivirus vaccine to work. This window is limited first by the potential of the immunizing virus to cause damage to the immune system and second by the level of the immunity generated following vaccination. The width of this opening is currently unknown. Our findings indicate that single-deletion mutants should not be used, due to the potential for the immunizing virus to cause immune system damage and the potential for enhanced disease upon contact with a pathogenic virus. The findings of Baba et al. (4) and Wyand et al. (47) indicate that the deletions of nef, nre, and vpr do not eliminate the potential safety problems in neonatal macaques, and this phenomenon could also apply to immunosuppressed or immunocompromized individuals. Desrosiers et al. (13) have developed mutants with four and five deletions, each having the expected loss in growth potential in vitro. When a four-deletion mutant was used in vivo, it generated low-level infections with low or nondetectable immune responses (13). Protection studies involving the four-deletion mutant have not been reported in macaques to date, but the limited immune response developed suggests that it will not generate sufficiently high levels of protection. The five-deletion mutant failed to attain measurable growth in vitro. These studies indicate that additional methods of attenuation or combinations of deletions should be tested and that their potential use in developing vaccine candidates should be scrutinized for both safety and generated immunity in animal models prior to their use in humans.
ACKNOWLEDGMENTS
We thank Ronald C. Desrosiers of the New England Primate Center for supplying the SIV239Δnef isolate, Frank Novembre of the Yerkes Primate Center for supplying SIVPBj6.6Δnef, and David Montefiori of Duke University for performing the virus neutralization assays reported in these studies.
These studies were sponsored by a Department of the Army cooperative agreement, DAMD17-93-V-3004.
REFERENCES
- 1.Agy M B, Frumkin L R, Corey L, Coombs R W, Wolinsky S M, Koehler J, Morton W R, Katze M G. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992;257:103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- 2.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 3.Almond N, Rose J, Sangster R, Silvera P, Stebbings R, Walker B, Stott E J. Mechanisms of protection induced by attenuated simian immunodeficiency virus. 1. Protection cannot be transferred with immune serum. J Gen Virol. 1997;78:1919–1922. doi: 10.1099/0022-1317-78-8-1919. [DOI] [PubMed] [Google Scholar]
- 4.Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste R E, Roodman S T, Hill R W, Knott W B, Ribas J L, Lewis M G, Eddy G A. Infectivity of titered doses of simian immunodeficiency virus clone E11S inoculated intravenously into rhesus macaques (Macaca mulatta) J Med Primatol. 1994;23:83–88. doi: 10.1111/j.1600-0684.1994.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J. Novel campaign to test live HIV vaccine. Science. 1997;277:1035. doi: 10.1126/science.277.5329.1035. [DOI] [PubMed] [Google Scholar]
- 9.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 10.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 11.Denesvre C, Le Grand R, Boissin-Cans F, Chakrabarti L, Hurtrel B, Vaslin B, Dormont D, Sonigo P. Highly attenuated SIVmac142 is immunogenic but does not protect against SIVmac251 challenge. AIDS Res Hum Retroviruses. 1995;11:1397–1406. doi: 10.1089/aid.1995.11.1397. [DOI] [PubMed] [Google Scholar]
- 12.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y M, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gartner S, Liu Y, Polonis V, Lewis M G, Elkins W R, Hunter E A, Jing M, Corts K, Eddy G A. Adaption of HIV-1 to pig-tailed macaques. J Med Primatol. 1994;23:155–163. doi: 10.1111/j.1600-0684.1994.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 15.Giavedoni L, Ahmad S, Jones L, Yilma T. Expression of gamma interferon by simian immunodeficiency virus increases attenuation and reduces postchallenge virus load in vaccinated rhesus macaques. J Virol. 1997;71:866–872. doi: 10.1128/jvi.71.2.866-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch V M, Dapolito G, McGann C, Olmsted R A, Purcell R H, Johnson P R. Molecular cloning of SIV from sooty mangabey monkeys. J Med Primatol. 1989;18:279–285. [PubMed] [Google Scholar]
- 17.Hirsch V M, Johnson P R. Pathogenesis of experimental SIV infection of macaques. Semin Virol. 1992;3:175–183. [Google Scholar]
- 18.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalyanaraman V S, Rodriguez V, Veronese F, Rahman R, Lusso P, DeVico A L, Copeland T, Oroszlan S, Gallo R C, Sarngadharan M G. Characterization of the secreted, native gp120 and gp160 of the human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1990;6:371–380. doi: 10.1089/aid.1990.6.371. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson G B, Halloran M, Li J, Park I W, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 23.Langlois A J, Desrosiers R C, Lewis M G, Kewalramani V N, Littman D R, Zhou J Y, Manson K, Wyand M S, Bolognesi D P, Montefiori D C. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J Virol. 1998;72:6950–6955. doi: 10.1128/jvi.72.8.6950-6955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis M G, Zack P M, Elkins W R, Jahrling P B. Infection of rhesus and cynomolgus macaques with a rapidly fatal SIV (SIVSMM/PBj) isolate from sooty mangabeys. AIDS Res Hum Retroviruses. 1992;8:1631–1639. doi: 10.1089/aid.1992.8.1631. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y C, Brosio P, Lafaile M, Li J, Collman R G, Sodroski J, Miller C J. Vaginal transmission of chimeric simian human immunodeficiency viruses in rhesus macaques. J Virol. 1996;70:3045–3050. doi: 10.1128/jvi.70.5.3045-3050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marthas M L, Ramos R A, Lohman B L, Van Rompay K K A, Unger R E, Miller C J, Banapour B, Pedersen N C, Luciw P A. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller C J, Marthas M, Greenier J, Lu D, Dailey P J, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239Delta3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 30.Montefiori D C, Reimann K A, Wyand M S, Manson K, Lewis M G, Collman R G, Sodroski J G, Bolognesi D P, Letvin N L. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol. 1998;72:3427–3431. doi: 10.1128/jvi.72.4.3427-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 32.Novembre F J, Johnson P R, Lewis M G, Anderson D C, Klumpp S, McClure H M, Hirsch V M. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993;67:2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novembre F J, Lewis M G, Saucier M M, Yalley-Ogunro H, Brennan T, McKinnon K, Bellah S, McClure H M. Deletion of the nef gene abrogates the ability of SIVsmmPBj to induce acutely lethal disease in pigtail macaques. AIDS Res Hum Retroviruses. 1996;12:727–736. doi: 10.1089/aid.1996.12.727. [DOI] [PubMed] [Google Scholar]
- 34.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W Y, Montefiori D C, Lee-Parritz D E, Lu Y C, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y J, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stahl-Hennig C, Dittmer U, Nisslein T, Petry H, Jurkiewicz E, Fuchs D, Wachter H, Matz-Rensing K, Kuhn E M, Kaup F J, Rud E W, Hunsmann G. Rapid development of vaccine protection in macaques by live-attenuated simian immunodeficiency virus. J Gen Virol. 1996;77:2969–2981. doi: 10.1099/0022-1317-77-12-2969. [DOI] [PubMed] [Google Scholar]
- 39.Stebbings R, Stott J, Almond N, Hull R, Lines E, Silvera P, Sangster R, Corcoran T, Rose J, Cobbold S, Gotch F, McMichael A, Walker B. Mechanisms of protection induced by attenuated simian immunodeficiency virus. II. Lymphocyte depletion does not abrogate protection. AIDS Res Hum Retroviruses. 1998;14:1187–1198. doi: 10.1089/aid.1998.14.1187. [DOI] [PubMed] [Google Scholar]
- 40.Steger K K, Dykhuizen M, Mitchen J L, Hinds P W, Preuninger B L, Wallace M, Thomson J, Montefiori D C, Lu Y C, Pauza C D. CD4+-T-cell and CD20+-B-cell changes predict rapid disease progression after simian-human immunodeficiency virus infection in macaques. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor M D, Korth M J, Katze M G. Interferon treatment inhibits the replication of simian immunodeficiency virus at an early stage: evidence for a block between attachment and reverse transcription. Virology. 1998;241:156–162. doi: 10.1006/viro.1997.8964. [DOI] [PubMed] [Google Scholar]
- 42.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahey M, Birx D L, Michael N L, Burke D S, Redfield R R. Assessment of gag DNA and genomic RNA in peripheral blood mononuclear cells in HIV-infected patients receiving intervention with a recombinant gp160 subunit vaccine in a phase I study. AIDS Res Hum Retroviruses. 1994;10:649–654. doi: 10.1089/aid.1994.10.649. [DOI] [PubMed] [Google Scholar]
- 44.VanCott T C, Veit S C D, Kalyanaraman V, Earl P, Birx D L. Characterization of a soluble, oligomeric HIV-1 gp160 protein as a potential immunogen. J Immunol Methods. 1995;183:103–117. doi: 10.1016/0022-1759(95)00038-c. [DOI] [PubMed] [Google Scholar]
- 45.Volsky D J, Simm M, Shahabuddin M, Li G, Chao W, Potash M J. Interference to human immunodeficiency virus type 1 infection in the absence of downmodulation of the principal virus receptor, CD4. J Virol. 1996;70:3823–3833. doi: 10.1128/jvi.70.6.3823-3833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whatmore A M, Cook N, Hall G A, Sharpe S, Rud E W, Cranage M P. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69:5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyand M S, Manson K H, Lackner A A, Desrosiers R C. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat Med. 1997;3:32–36. doi: 10.1038/nm0197-32. [DOI] [PubMed] [Google Scholar]