Abstract
Background
Recent studies have shown good serological and cellular immune responses in people living with human immunodeficiency virus (PLWH) after receipt of 2 doses of messenger RNAA (mRNA) severe acute respiratory syndrome coronavirus 2 vaccine. Data are missing regarding the response after 3 vaccine doses.
Methods
We followed up a group of PLWH who received 3 doses of the mRNA BNT162b2 vaccine and for whom data of humoral immune response after 2 vaccine doses were available. Patients provided a blood sample 4–6 months after the booster dose. The aim of the study was to measure the serological and cellular response after the third dose and to evaluate factors associated with the vaccine response.
Results
Fifty patients have provided a serum sample for serological evaluation after the booster. The anti–receptor-binding domain (RBD) immunoglobulin (Ig) G titers were higher after the booster with a median delta of 3240 arbitrary units/mL. The median CD4+ T-cell count was 660/μL (interquartile range, 515–958/μL) and had no influence on the antibody level. Factors associated with lower delta included higher CD8+ T-cell count (P = .02) and longer time between the third dose and the blood test (P = .01). Higher anti-RBD IgG titer after the second vaccine (P = .03), as well as a longer interval between second and third doses (P = .031) were associated with higher delta. There was no increase in the median number of activated interferon γ+ and tumor necrosis factor α+ CD4+ T cells after the booster (n = 8).
Conclusions
The anti-RBD IgG level after 3 doses of mRNA BNT162b2 vaccine was higher than the level after 2 doses, suggesting additional value of the booster. Cellular response did not further increase after a booster.
Keywords: PWH, booster, vaccine response
In people living with human immunodeficiency virus , levels of anti–receptor-binding domain immunoglobulin G were higher after a booster vaccine, dose with no increase in the cellular immune response.
The coronavirus disease 2019 (COVID-19) vaccines have been shown to be effective in reducing the risk of severe illness and death, and vaccination is a main strategy to control the pandemic. People living with human immunodeficiency virus (HIV) (PLWH) who acquire severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are at heightened risk for hospitalization and progression to severe disease [1]. Data from clinical studies in the United States, United Kingdom, and Spain have shown that advanced HIV disease and the presence of comorbid conditions are common among the aging HIV population and present risk factors for severe disease [2–4]. Several studies have shown a robust serological and cellular immune responses in PLWH after a primary regimen of 2 doses of messenger RNA (mRNA) SARS-COV-2 vaccines, especially in patients with high CD4+ T-cell count and undetectable HIV viral load [5–10]. However, a study by Antinori et al [11] showed a diminished cell-mediated immune response, as well as a decreased receptor-binding domain (RBD)–binding antibody response, in PLWH with CD4+ T-cell counts <200/μL.
The emergence of new variants of SARS-COV-2, as well as waning immunity over time, led to resurgence of COVID-19 cases in populations that had been vaccinated earlier. On 30 July 2021, the Israeli Ministry of Health approved the use of the third dose (booster) of mRNA BNT162b2 SARS-COV-2 vaccine to cope with disease resurgence. The study by Arbel et al [12] showed 90% mortality rate reduction in patients >60 years old who received a booster ≥ 5 months after the second vaccine dose. Another study from Israel included participants ≥12 years of age who received a booster vaccine and showed an efficacy of 93% against hospital admission, 92% against severe COVID-19, and 81% against death [13]. The study by Eliakim-Raz et al [14] showed a significant increase in immunoglobulin (Ig) G titers 10 and 19 days after the booster dose of mRNA BNT162b2 vaccine in patients >60 years old.
Data on humoral and cellular immune response after the booster dose in PLWH are limited. A study by Lapointe et al [15] showed that the third vaccine dose boosted neutralization to an average 4-fold higher than the peak levels following the second dose. In that particular study, neutralization was examined 1 month after the booster dose, and the majority of patients had high CD4+ T-cell counts and undetectable viral loads. Neutralization was higher in those who received mRNA1273 vaccine as a booster dose.
In a previous study we examined the humoral and cellular immune responses 4–6 months after a primary series of mRNA BNT162b2 SARS-COV-2 vaccine. We showed comparable responses in PLWH and HIV-negative healthcare workers, except for patients with CD4+ T-cell counts <300/μL, who had diminished antibody response.
We now extended our research and examined the humoral and cellular response to the booster vaccine dose in our cohort of PLWH. The aims of the current study were to evaluate humoral and cellular immune responses 4–6 months after the third (booster) dose of mRNA BNT162b2 SARS-COV-2 vaccine and to compare them with responses after the second vaccine dose.
METHODS
Study Population and Study Design
The study patients were recruited during their routine follow-up visits at the Crusaid Kobler AIDS Center, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel. We prospectively followed up a cohort of PLWH who participated in our previous study evaluating the humoral and cellular immune responses 3–6 months after the second vaccine dose (between 28 April and 28 August 2021), and we evaluated their vaccine responses 3–6 months after the booster dose. Patients were eligible for the study if they received 3 vaccine doses and had no self-reported history of COVID-19. Demographic and clinical data were extracted from medical files, including antiretroviral therapy, comorbid conditions, CD4+ T-cell count, and HIV viral load.
Humoral Immune Response
All patients who were included in our previous cohort and met the inclusion criteria of being vaccinated with 3 doses of BNT162b2 Pfizer mRNA vaccine were invited to participate in this study. The antibody level 3–6 months after a booster dose was compared with the level in the same patients 3–6 months after the primary 2-dose vaccination. For this purpose, a commercial automated SARS-CoV-2 IgG assay was used to evaluate antibody response in individuals who had received the BNT162b2 vaccine. The chemiluminescent microparticle immunoassay provided qualitative and quantitative determination of anti–SARS-CoV-2 spike protein RBD IgG antibody levels (anti-RBD IgG) (SARS-CoV-2 IgG II Quant; catalog no. 6S60; Abbott). The result was provided as arbitrary units (AU) per milliliter, as defined by the manufacturer, ranging between 0 to 40 000 AU/mL for anti-RBD IgG (with levels >150 AU/mL considered positive). Anti–nucleocapsid (N) IgG antibodies were examined in all patients to exclude those who might have had asymptomatic or not previously diagnosed COVID-19.
Cellular Immune Response
In addition to testing of the humoral immune response (anti-RBD IgG), PLWH whose cellular vaccine response was evaluated previously were asked to provide a blood sample for evaluation of cellular immunity 3–6 months after the third vaccine dose.
Peripheral Blood Mononuclear Cell Isolation and Stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by means of Ficoll gradient centrifugation. After isolation, cells were stored in liquid nitrogen for later use. The T-cell response was assessed by stimulating donor PBMCs with a pooled complete S-peptide mix in the presence of a protein transport inhibitor (brefeldin A), followed by staining for an activation marker (CD40L) and intracellular cytokines (tumor necrosis factor (TNF] α and IFN-γ).
The complete S-peptide mix used for stimulation is a pool of lyophilized peptides, consisting mainly of 15-mer sequences with 11–amino acid overlap, covering the complete protein coding sequence (amino acids 5–1273) of the SARS-CoV-2 spike glycoprotein (GenBank MN908947.3; protein QHD43416.1; catalog no. 130-127-951; Miltenyi Biotec). Briefly, donor PBMCs were plated in a 96-well plate at a concentration of 0.5–1 × 106 PBMCs/µL and incubated at 37°C and 5% carbon dioxide with 2 µL of complete pooled S-peptide mix, CytoStim as a positive control, or 10% dimethyl sulfoxide in sterile water as a negative control.
After 2 hours, brefeldin A was added to each well, and the cells were incubated for an additional 4 hours. The cells were then stained with viability dye, followed by fixation, permeabilization, and staining for surface markers (CD3, CD20, CD14, CD4, CD8, and CD154) and for TNF-α and interferon (IFN) γ. After staining, samples were acquired by BD FACS Canto II, and 20 000 CD4+ events were collected for each sample. The analysis was performed on gated CD4+ T cells, and the absolute number of activated IFN-γ+ and TNF-α+ cells was recorded and normalized for 1 × 106 CD4+ T cells. To calculate the actual response rate, the absolute number of positive events in the unstimulated negative control was deducted from the absolute number of events in the S-stimulated samples, as shown in the following formula:
Where CD4s represents CD4+ T cells.
HIV-Related Tests
HIV viral load was determined with Cepheid Xpert HIV-1 Viral Load, and a result <40 copies/mL was considered undetectable. CD4+ and CD8+ T-cell counts were determined using flow cytometry analysis of freshly collected peripheral blood (within 4–6 hours after blood sampling).
Statistical Analysis
Categorical variables were summarized as frequency and percentage. Continuous variables were evaluated for normal distribution using histogram. Because all continuous variables were not normally distributed, they were reported as medians with interquartile ranges. Spearman rank correlation coefficients were used to study the association between continuous variables, and Mann-Whitney tests to compare continuous variables between categories.
Fisher exact and χ2 tests were used to compare categorical variables (Fisher exact tests were used when the expected count was <5 cells/µL in ≥20% of the cells; otherwise, χ2 tests were used). P values were adjusted for multiple comparisons using the false discovery method. Data were presented before and after the adjustment. At P < .05 (2 sided), differences were considered statistically significant for all analyses. IBM SPSS Statistics for Window software (version 27; IBM) was used for all statistical analyses.
Consent and Ethical Approval
This study was approved by the Tel-Aviv Sourasky Medical Center's ethical review board (no. TLV-046–20). Patients provided written consent.
RESULTS
Study Population
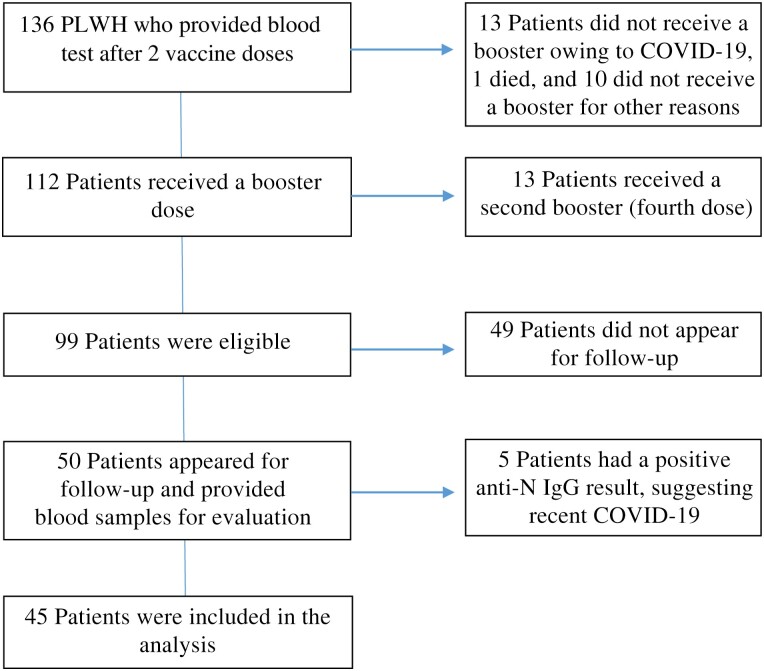
We prospectively followed up a cohort of 136 PLWH who are on active follow-up at the Crusaid Kobler AIDS Center, Tel-Aviv, and participated in our previous study.
Of those patients, 112 (82%) received a booster vaccine and 24 did not, 13 of them owing to symptomatic COVID-19, and the other 11 for other reasons. Thirteen patients had received a second booster (fourth dose) and thus were excluded from the study.
Of the remaining 99 eligible patients, 50 appeared for routine follow-up and provided an additional blood sample. Five patients had a positive anti-N IgG result, suggesting recent COVID-19, and were not included in the analysis (see Figure 1).
Figure 1.
Patient cohort. Abbreviations: COVID-19, coronavirus disease 2019; Ig, immunoglobulin; N, nucleocapsid; PLWH, people living with human immunodeficiency virus.
The patients’ median age (interquartile range [IQR]) was 48 (40–53), and most patients (82%) were male. Their median CD4+ T-cell count (IQR) was 660/μL (515–958/μL), and the HIV viral load was undetectable in 43 patients (96%) . Five patients had CD4+ T-cell counts <350/μL, <200/μL in 1. The vast majority of patients (96%) were receiving integrase inhibitor–based antiretroviral therapy (Table 1).
Table 1.
Baseline Characteristics of Patients With Human Immunodeficiency Virus Receiving 3 Doses of the BNT162b2 Messenger RNA Vaccine
| Characteristic | Patients, No. (%)a |
|---|---|
| Age, median (IQR), y | 48 (40–53) |
| Male sex | 37 (82) |
| Smoking | 11 (24) |
| Diabetes | 0 (0) |
| Hypertension | 1 (2) |
| Ischemic heart disease | 1 (2) |
| Obesity | 1 (2) |
| BMI, median (IQR)b | 23.4 (21.8–28.3) |
| MSM | 30 (67) |
| Heterosexual | 8 (18) |
| People who inject drugs | 2 (4) |
| Time from HIV diagnosis, median (IQR), y | 10 (5–13) |
| Regimen type | |
| II based | 43 (96) |
| NRTI based | 42 (93) |
| NNRTI based | 3 (7) |
| PI based | 3 (7) |
| CD4+ T-cell count, median (IQR), cells/μL | 660 (515–958) |
| HIV viral load | |
| <40 copies/mL | 43 (96) |
| >40 copies, mL | 2 (4) |
| Time, median (IQR), mo | |
| From 3rd vaccine dose | 5.3 (4.7–6) |
| Between 2nd and 3rd doses | 6.8 (6.3–7.3) |
| Anti-RBD IgG, median (IQR) AU/mL | |
| After 2 doses | 995 (604–1452) |
| After 3 doses | 5818 (2339–10 727) |
| Deltac | 3240 (1606.5–9407) |
Abbreviations; AU, arbitrary units; BMI, body mass index; HIV, human immunodeficiency virus; Ig, immunoglobulin; II, integrase inhibitor; IQR, interquartile range; MSM, men who have sex with men; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; RBD, receptor-binding domain.
Data represent no. (%) of patients unless otherwise specified.
BMI calculated as weight in kilograms divided by height in meters squared.
Delta represents the difference between antibody levels after 2 and after 3 vaccine doses.
Humoral Immune Response
Patients received the booster vaccine dose between 1 August 2021 and 5 January 2022, with the vast majority vaccinated in August 2021. All patients received the mRNA BNT162b2 vaccine as a primary series, with an interval of 21 days between the first and the second doses. The same vaccine was used as a booster. The median time (IQR) between the second and third vaccine doses was 6.8 (6.3–7.3) months, and the median time between the third vaccine dose and serological testing was 5.3 (4.7–6) months (see Table 1).
The median anti-RBD IgG level (IQR) after the second vaccine dose was 995 (604–1452) AU/mL, whereas the antibody level after the booster dose was higher, with a median of 5818 (2339–10 727) AU/mL. The median delta (difference between the antibody levels after doses 2 and 3) was 3240 (IQR, 1606.5–9407) AU/mL. No association was observed between the IgG delta and patient age, weight, or years since HIV diagnosis, nor with absolute CD4+ T-cell count or percentage.
Parameters that were associated with lower delta included higher CD8+ T-cell count (P = .02) and longer time between the third vaccine dose and blood test (P = .01). There was a trend for lower delta in patients with a lower CD4/CD8 ratio, but it was not statistically significant (P = .07).
Higher anti-RBD IgG levels after the second vaccine dose, as well as longer time between the second and the third vaccine doses, were associated with higher anti-RBD IgG delta (Table 2). However, after adjustment for multiple comparisons the associations only tended to be significant (Table 2).
Table 2.
Association Between Patient Characteristics and Anti–Receptor-Binding Domain Immunoglobulin G Delta
| Characteristic | Spearman Rank Correlation Coefficient (rs)a | P Value | Adjusted P Value (FDR) |
|---|---|---|---|
| CD4+ T-cell count (absolute) | 0.066 | .67 | .77 |
| CD4+ T cells, % | 0.125 | .41 | .61 |
| CD8+ T-cell count (absolute) | −0.350 | .02b | .08 |
| CD4/CD8 ratio | 0.270 | .07 | .14 |
| Age in years | −0.121 | .43 | .61 |
| Anti-RBD IgG level after 2 doses | 0.324 | .03b | .08 |
| Years since HIV diagnosis | −0.011 | .94 | .94 |
| Time from 3rd dose, months | −0.375 | .01b | .08 |
| Time between 2nd and 3rd doses | 0.325 | .03b | .08 |
| IgG level, median (IQR), AU/mL | |||
| Male patients | 4128 (1737–9407) | .69 | .77 |
| Female patients | 2374 (1212–14 271) |
Abbreviations: FDR, false discovery rate; HIV, human immunodeficiency virus; Ig, immunoglobulin; IQR, interquartile range; RBD, receptor-binding domain.
Values represent rs unless otherwise specified.
Significant at P < .05
Crude comparison of IgG concentrations in male and female patients; P value refers to the comparison between the IgG delta in men and in women.
There was an insufficient number of patients with CD4+ T-cell count <350/μL and only 1 with a count <200/μL. Thus, the association between the very low CD4+ T-cell counts and antibody delta level could not be established. However, all 5 patients with CD4+ T-cell count <350/μL had a higher level of anti-RBD IgG after the booster dose, suggesting a boosting effect even in those who previously showed diminished response.
Cellular Immune Response
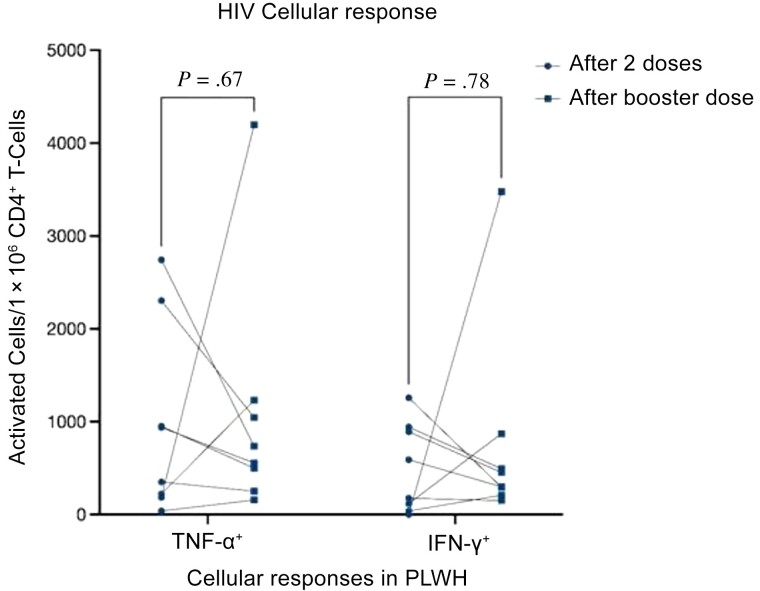
Eight patients whose cellular response was evaluated after the primary vaccine series provided blood samples after the booster dose. Five of them (63%) were male, and 3 (37%) were female.
The median time (IQR) between the second and the third vaccines was 7.28 (6.78–7.77) months, and the median time between the booster date and blood sampling for cellular response was 5.38 (4.63–5.85) months. The median number of TNF-α+ CD4+ T cells was 645 per 1 × 106 CD4+ T cells, which did not differ from the median number after the second vaccine dose. However, the median IFN-γ+ CD4+ T-cell count (IQR) was lower after the third than after the second dose (377 [230–777] vs 384 [69–930] per 1 × 106 CD4+ T cells). After a booster dose an increase in the number of activated IFN-γ+ and TNF-α+ CD4+ T cells was seen in 3 patients, and a decrease in 5. Data did not reach statistical significance (Table 3 and Figure 2). In these 8 patients, the absolute CD4+ T-cell count did not differ, and the anti-RBD-IgG level was significantly higher after a booster dose (median, 7728 vs 1164 AU/mL; P = .01).
Table 3.
Cellular Immune Response in 8 Patients
| Parameter | Response, Median (IQR) | Patients, No. (%) | P Value | |||
|---|---|---|---|---|---|---|
| After 2 Doses | After 3 Doses | Change | Decreased Response | Increased Response | ||
| TNF-α–activated cells/1 × 106 CD4+ T cells | 645 (194–1965) | 645 (313–1383) | −242 (−1057 to–786) |
5 (63) | 3 (37) | .67 |
| IFN-γ–activated cells/1 × 106 CD4+ T cells | 384 (59–930) |
377 (230–777) |
−159 (−447 to 609) |
5 (63) | 3 (37) |
.78 |
| CD4+ T-cell count, cells/μL | 675 (568–922) |
764 (650–961) |
72 (−90 to 105) |
3 (37) | 5 (637) | .48 |
Abbreviations: IFN, interferon; IQR, interquartile range; TNF, tumor necrosis factor.
Figure 2.
Cellular immune response in people living with human immunodeficiency virus. Responses were evaluated by stimulating peripheral blood mononuclear cells with an S-peptide mix. Normalized numbers of cytokine-positive CD4+ T-cells are shown (interferon (IFN) γ+ and tumor necrosis factor [TNF] α+ cells). Samples were collected from 8 patients vaccinated with booster for whom cellular response data were available from the previous study.
DISCUSSION
Our current study shows evidence that the third (booster) dose of BNT162b2 mRNA SARS-CoV-2 vaccine induces a significant (almost 6-fold) increase in anti-RBD IgG in a cohort of PLWH. Previous studies of booster doses in an HIV-negative population showed good clinical efficacy against severe disease, hospitalization, and death, as well as elevation of antibody levels soon after the booster dose.
A recent study by Fidler et al [16] showed higher anti-spike IgG levels after 3 vaccine doses compared with 6 months after the first dose in cohort of PLWH. Both cellular and humoral responses were higher after booster vaccination, including against variants of concern. The number of patients was relatively small, and the vaccine type was mixed (ChAdOx1 nCoV-19 vaccine as a primary regimen with heterogenous booster).
A study by Lapointe et al [15] showed that the third vaccine dose boosted neutralization to an average 4-fold higher than the peak levels following the second dose; in this study vaccine response was evaluated 1 month after the booster. Another study, by Vergori et al [17], showed higher antibody response to mRNA COVID-19 vaccination after the third dose than achieved with the primary 2 doses (>2 log2 difference).
Previous studies had shown that age and sex could potentially influence the antibody vaccine response [18, 19]. Patients in our cohort were relatively young (median age, 48 years), with male predominance. However, we did not find associations between patient age or sex and antibody levels.
Data from published studies showed diminished vaccine response in PLWH with low CD4+ T-cell counts (<350/μL) [10, 11]. In our current study, only 5 patients had CD4+ T-cell counts <350/μL, an insufficient number for statistical analysis. However, all of them had an increase in antibody levels after the booster. We have shown that higher absolute CD8+ T-cell counts, as well as a trend for lower CD4/CD8 ratios, can serve as markers of immune dysregulation in PLWH and were associated with lower anti-RBD IgG delta after the booster.
Interestingly, longer intervals between second and third vaccine doses was associated with higher anti-RBD IgG delta. In a similar way, a study by Dyson et al [20], which estimated the immune response to anthrax vaccine, showed that participants had better humoral and cellular responses with longer intervals between vaccine doses. Further studies with more participants are needed to evaluate this association.
In contrast to the humoral immune response, we failed to show an elevation in cellular immune response after the booster dose. In fact, the median number of activated IFN-γ+ CD4+ T cells was lower after the third dose, and we found a decrease in the number of responding cells in 5 of 8 patients, though the difference did not reach statistical significance. The serological response in this subgroup of patients was significantly higher after the booster. The number of patients was small, but it is an interesting observation.
A study by Maringer et al [21] had shown that booster vaccination led to a significant increase in anti-spike IgG responses, which showed a marked decline 6 months after complete vaccination. In contrast, T-cell responses remained stable over time after complete vaccination, with no significant effect of booster vaccination on T-cell responses.
Another study, by Vergori et al [17], showed that—differently from the antibody production—the T-cell response elicited by the third dose was similar to that achieved after the primary vaccination cycle. This particular study evaluated the humoral and cellular immune responses in PLWH 15 days after the booster and compared them with responses 1 month after the primary vaccination. These data could indicate that boost vaccination is of particular relevance for the amelioration of antiviral antibody activity, whereas robust T-cell immunity is established after complete primary vaccination.
Our study has several limitations. First, the cohort was relatively small and homogenous, with male predominance. The single-center study represents mainly the Tel-Aviv population, with the majority of patients being young men who have sex with men and with a relatively low rate of comorbid conditions. There was an insufficient number of patients with low CD4+ T-cell counts to assess whether such counts could be associated with the antibody level or cellular response. This information is of key importance considering that this particular group of patients had shown a diminished immune response after primary SARS-CoV-2 vaccination schedules. Another limitation is that the study design does not allow the investigation of cellular and humoral immunity kinetics. Finally, we did not have a control group of HIV-negative individuals.
The strengths of our study include the evaluation of both cellular and humoral immune responses in the same cohort of patients over time and a longer time frame of 4–6 months after both the primary schedule and the booster. All of our patients received the BNT162b2 mRNA vaccine, avoiding the possible confounder of vaccine type.
In conclusion, our current study shows a significant increase in humoral response, but not cellular immune response, after a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in PLWH. Higher absolute CD8+ T-cell counts and a trend for lower CD4/CD8 ratios, as well as longer time between the booster vaccine and the blood test, had a negative impact on antibody levels. Higher anti-RBD IgG after primary vaccine schedule and longer intervals between the second and the third vaccine doses were associated with higher antibody deltas.
Acknowledgments
Author contributions . Study concept and study design, patient recruitment, and manuscript writing: L. T., D. H., T. H., and D. T. Serological testing: T. H., R. M., S. A, N. M., and I. L. Cellular immunity testing and supervision: D. H., T. F., and T. H. Manuscript preparation: G. G. and S. L. Laboratory supervision: A. A. Statistical analysis: T. Z. B.
Financial support . This work was supported by the Israeli Ministry of Health, which supplied serological testing kits without cost.
Contributor Information
Luba Tau, Crusaid Kobler AIDS Center, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel; Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
David Hagin, Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel; Department of Allergy and Clinical Immunology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Tal Freund, Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel; Department of Allergy and Clinical Immunology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Tamar Halperin, Microbiological Laboratory, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Amos Adler, Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel; Microbiological Laboratory, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Rotem Marom, Microbiological Laboratory, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Svetlana Ahsanov, Microbiological Laboratory, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Natasha Matus, Microbiological Laboratory, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Inbar Levi, Microbiological Laboratory, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Gal Gerber, Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Shir Lev, Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Tomer Ziv-Baran, Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Dan Turner, Crusaid Kobler AIDS Center, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel; Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
References
- 1. UNAIDS 2020 report. Available at: https://aids2020.unaids.org/report/. Accessed January 2022.
- 2. Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis 2021; 73:e2095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braunstein SL, Lazar R, Wahnich A, Daskalakis DC, Blackstock OJ. Coronavirus disease 2019 (COVID-19) infection among people with human immunodeficiency virus in New York City: a population level analysis of linked surveillance data. Clin Infect Dis 2021; 72:e1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med 2020; 173:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV). Clin Infect Dis 2021; 74:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021; 9:E568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:E474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021; 35:2399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tau L, Turner D, Adler A, et al. SARS-CoV-2 humoral and cellular immune responses of patients with HIV after vaccination with BNT162b2 mRNA COVID-19 vaccine in the Tel-Aviv Medical Center. Open Forum Infect Dis 2022; 9:ofac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antinori A, Cicalini S, Meschi S, et al. Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to COVID-19. N Engl J Med 2021; 385:2413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 398:2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eliakim-Raz N, Leibovici-Weisman Y, Stemmer A, et al. Antibody titers before and after a third dose of the SARS-CoV2 BNT162b2 vaccine in adults aged ≥ 60 years. JAMA 2021; 326:2203–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lapointe HR, Mwimanzi F, Cheung PK, et al. People with human immunodeficiency virus receiving suppressive antiretroviral therapy show typical antibody durability after dual coronavirus disease 2019 vaccination and strong third dose responses. J Infect Dis 2023; 227:838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fidler S, Fox J, Tipoe T, et al. Booster vaccination against SARS-CoV-2 induces potent immune responses in people with human immunodeficiency virus. Clin Infect Dis 2023; 76:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vergori A, Cozzi Lepri A, Cicalini S, et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun 2022; 13:4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020; 71:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graham NR, Whitaker AN, Strother CA, et al. Kinetics and isotype assessment of antibodies targeting the spike-protein receptor binding domain of severe acute respiratory syndrome-coronavirus 2 in COVI-19 patients as a function of age, biological sex and disease severity. Clin Transl Immunol 2020; 9:e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyson EH, Simpson AJH, Gwyther RJ, et al. Serological responses to Anthrax Vaccine Precipitated (AVP) increase with time interval between booster doses. Vaccine 2022; 40:6163–78. [DOI] [PubMed] [Google Scholar]
- 21. Maringer Y, Nelde A, Schroeder SM, et al. Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci Immunol 2022; 7:eadd3899. [DOI] [PMC free article] [PubMed] [Google Scholar]