Abstract
Objectives
Age-related cognitive changes can be influenced by both brain maintenance (BM), which refers to the relative absence over time of changes in neural resources or neuropathologic changes, and cognitive reserve (CR), which encompasses brain processes that allow for better-than-expected behavioral performance given the degree of life-course-related brain changes. This study evaluated the effects of age, BM, and CR on longitudinal changes over 2 visits, 5 years apart, in 3 cognitive abilities that capture most of age-related variability.
Methods
Participants included 254 healthy adults aged 20–80 years at recruitment. Potential BM was estimated using whole-brain cortical thickness and white matter mean diffusivity at both visits. Education and intelligence quotient (IQ; estimated with American National Adult Reading Test) were tested as moderating factors for cognitive changes in the 3 cognitive abilities.
Results
Consistent with BM—after accounting for age, sex, and baseline performance—individual differences in the preservation of mean diffusivity and cortical thickness were independently associated with relative preservation in the 3 abilities. Consistent with CR—after accounting for age, sex, baseline performance, and structural brain changes—higher IQ, but not education, was associated with reduced 5-year decline in reasoning (β = 0.387, p = .002), and education was associated with reduced decline in speed (β = 0.237, p = .039).
Discussion
These results demonstrate that both CR and BM can moderate cognitive changes in healthy aging and that the 2 mechanisms can make differential contributions to preserved cognition.
Keywords: Cortical thickness, Diffusion MRI, Mean diffusivity, Memory, Reasoning
Age-related cognitive decline has been documented in life-span epidemiological studies (Hartshorne & Germine, 2015; Hughes et al., 2018) with the majority of studies examining only a truncated age range rather than the whole life span (Singh-Manoux et al., 2012). The pattern of age-related changes varies across cognitive abilities (Salthouse, 1998; Tucker-Drob, 2019) in both cross-sectional (Tucker-Drob, 2009; Whitley et al., 2016) and longitudinal (De Vis et al., 2018; Hughes et al., 2018; Singh-Manoux et al., 2012) studies. Processing speed usually exhibits the steepest decline (Salthouse, 2019), whereas vocabulary is well maintained until late adulthood (Singh-Manoux et al., 2011). Longitudinal changes in cognition have been associated with baseline age, indicating that older participants show greater accelerated decline in global cognition (Singh-Manoux et al., 2011), reasoning (De Vis et al., 2018), and memory abilities (Salthouse, 2016). After accounting for age, there is still large variability in the rate of cognitive decline, ranging from rapid decline to even some improvement (Wilson et al., 2009). The concepts of brain maintenance (BM) and cognitive reserve (CR) have been used to explain these individual differences (Stern, 2012). Here we evaluate the differential effect of age, BM, and CR on cognitive decline in a life-span sample of healthy adults initially ranging from age 20 to 80 years old.
According to a recent consensus Framework (Stern et al., 2023), BM can be defined as “the relative absence over time of changes in neural resources or neuropathologic changes as a determinant of preserved cognition in older age” and CR as “a property of the brain that allows for cognitive performance that is better than expected given the degree of life-course-related brain changes and brain injury or disease.” In addition to genetic components, both BM and CR can be influenced by lifetime exposures such that certain life experiences may increase a person’s BM and/or CR (Stern et al., 2020). Life experiences can be indexed with individual difference factors. Here we included years of education and estimated intelligence quotient (IQ), two factors most often examined in the literature as individual difference factors.
Even though BM can take place at any level of the neural structure, from microscopic synaptic architecture to macroscopic measure of cortical thickness (Nyberg et al., 2012), common brain measures for approximating BM include relative changes in grey matter cortical thickness and white matter mean diffusivity. Studies have reported associations between changes in whole-brain and regional brain volumes with changes in global as well as specific domains of cognition (Gorbach et al., 2017; Persson et al., 2016). There is some support for life experiences influencing BM; IQ and education have been related to brain structural measures (Cox et al., 2016; Zarnani et al., 2019). A recent study reported that performance changes in a German intelligence test were associated with changes in both area and thickness of caudal middle frontal gyrus, bank of superior temporal sulcus, and fusiform gyrus (Sele et al., 2021). Another study found that a cross-sectional estimate of BM, quantified by gray matter volume and thickness and white matter tract fractional anisotropy, was correlated with both education and IQ score estimated from the American National Adult Reading Test (NART-IQ; Habeck et al., 2016).
In both cross-sectional and longitudinal studies, several individual difference factors including education and IQ have been shown to moderate the relationship between age-related brain changes and cognitive performance; these factors have thus been hypothesized to contribute to CR. Cross-sectional studies consistently showed the protective effect of CR, mostly approximated with education, in cognitively normal older adults (Singh-Manoux et al., 2011; Wilson et al., 2009; Zahodne et al., 2015). However, except for a few studies (Zahodne et al., 2015), longitudinal studies showed education to be a poor predictor of cognitive decline, both when education was used on its own or as part of a composite score (Karlamangla et al., 2009; Soldan et al., 2017). Other individual difference factors such as IQ and occupational attainment have been reported to exhibit protective effect on the rates of cognitive decline (Then et al., 2015).
While many studies have examined the effects of individual difference factors on cognitive decline, few studies have differentiated the effects of CR from BM (Bertola et al., 2019; Zahodne et al., 2019). Dissociating the effects of BM and CR on cognitive decline necessitates testing the associations between cognitive changes with an individual difference factor while accounting for changes in brain structure, which most studies were lacking. For example, in the recent study by Bertola et al. (2019), the effect of education on memory decline was tested without accounting for any brain changes. Even if longitudinal change in a brain structural measure is not associated with an individual difference factor, individual variability in brain structural changes can still be associated with differential cognitive decline. Thus, controlling for individual differences in the change in brain structural metrics can enable a more accurate delineation of cognitive changes most protected by CR, which is hypothesized to contribute to variability in cognitive decline beyond that accounted for by BM.
The goal of the current study was to determine the roles of age, BM, and CR in the longitudinal performance trajectories of three independent cognitive abilities—episodic memory, reasoning, and processing speed. The study followed a group of healthy adults for 5 years; they initially spanned age 20 to 80 years rather than the truncated age range used in most of the previous studies. Latent change score model (LCSM; Kievit et al., 2018), a robust statistical approach that estimates changes over a comprehensive set of cognitive tests covering the three abilities, was used to examine the cognitive changes. Years of education and NART-IQ (Grober & Sliwinski, 1991) were used as potential factors influencing BM and CR. Even though NART-IQ is likely influenced by years of education, including both factors in the models allowed us to evaluate the factors’ independent effects. To assess BM and CR as potential protective factors in cognitive decline, cortical thickness and white matter mean diffusivity at both baseline and follow-up, along with NART-IQ and education, were included in the LCSM as potential moderators of cognitive change. To explore BM, we tested whether change in brain measures was associated with change in cognition, and whether the change in brain measures was moderated by education or NART-IQ. To explore CR, we tested whether change in cognition was moderated by education or IQ, and then repeated this analysis controlling for the two brain measures. We hypothesized that the rate of cognitive changes would accelerate with older age, and that thicker cortex, lower white matter mean diffusivity, and higher NART-IQ would be associated with less cognitive decline.
Method
Participants
A total of 254 participants were drawn from our ongoing studies at Columbia University Irving Medical Center: The Reference Ability Neural Network study and the CR study (Stern et al., 2014, 2018). The two studies recruited healthy adults from 20 to 80 years old. Please see Supplementary Information S11 for screening procedure and inclusion criteria.
Demographic information for the participants is presented in Table 1. We did not find any systematic difference between the participants who had and had not completed the functional Magnetic Resonance Imaging (fMRI) procedures. Supplementary Table S5 details the missing data. One hundred and seventy-five participants had at least one task measure missing and 79 participants had complete set of 18 task data in the study. Two participants did not receive any MRI scans and were excluded from cortical thickness and mean diffusivity analyses.
Table 1.
Demographic Characteristics (n = 254)
| Age, years | Mean (SD) | 53.1 (17.1) |
| Median [min, max] | 60.0 [20.0, 80.0] | |
| Follow-up interval, years | Mean (SD) | 4.87 (0.635) |
| Median [min, max] | 5.00 [4.00, 7.00] | |
| Sex, n (%) | Females | 142 (55.9%) |
| Males | 112 (44.1%) | |
| Ethnicity | Caucasian | 155 (61.0%) |
| African American | 61 (24.0%) | |
| Others | 38 (15.0%) | |
| Education, years | Mean (SD) | 16.3 (2.40) |
| Median [min, max] | 16.0 [11.0, 24.0] | |
| NART-IQ | Mean (SD) | 117 (8.27) |
| Median [min, max] | 120 [94.2, 131] |
Notes: NART-IQ = IQ score estimated from the American National Adult Reading Test; SD = standard deviation.
Cognitive Tasks
To estimate robust estimation of latent abilities of the three abilities, each ability was estimated with three measures from out-of-scanner and three from in-scanner tasks for a total of 24 measures included in the analysis. Out-of-scanner tasks consisted of traditional paper-and-pencil tests, and in-scanner tasks were neuropsychological tests adapted for computer testing in the fMRI. Details of the tasks can be found in Supplementary Information S11.
MRI Acquisition and Processing
fMRI scans and several structural MRI scans were acquired on a 3.0-T Philips Achieva Magnet. Details of the scan parameters along with the data processing details can be found in Supplementary Information S11. T1 structural data were analyzed in FreeSurfer v5.1 and visually checked and corrected, then the mean whole-brain cortical thickness was calculated for each participant. For diffusion MRI data, analysis was performed with MRtrix3 to quantify whole-brain white matter mean diffusivity. The two brain measures were used in subsequent analyses.
Statistical Analysis
For descriptive statistics, mean, standard deviation, median, minimum, and maximum were reported for continuous variables and frequency and percent were reported for categorical variables. The correlation among demographic variables was examined using Spearman’s correlation and two-sample t test assuming unequal variances between males and females.
Change Point Analysis in Cognitive Abilities
To determine whether there are nonlinear effects in age-related changes in cognitive abilities, we tested whether there were inflection points in the association between age and changes in the abilities by estimating the latent change scores from the LCSM without adjusting for any covariate. The LCSM estimates the changes in latent scores rather than the observed scores. A piece-wise linear regression with one inflection point was performed for the inflection points ranging from 30 to 70 years. To evaluate the fit of the model, we compared the model to a linear regression model without any inflection point, and to the most basic model, a model with only the respective baseline cognitive abilities as covariate. The best inflection point was selected based on Bayesian Information Criterion (BIC).
Testing IQ and Education Moderation Using LCSM
To test whether individual difference factors (IQ—estimated with American National Adult Reading Test—and years of education) were associated with cognitive changes beyond demographic variables, a multiple indicator LCSM (Kievit et al., 2018) was used as depicted in Supplementary Figure S2. We modeled the three abilities in the manner of traditional confirmatory factor analysis as described in previous studies (Stern et al., 2014).
Moderating factors
Given that changes in cognition are modeled with LCSM, any associations between a factor and cognitive change scores are moderating factors of cognitive change over time. Age, sex, education, and IQ were included in the model to test each factor’s moderation of the change scores for each ability. We reported the parameter estimates of associations as well as the overall goodness-of-fit measures for both models (comparative fit index [CFI], Tucker-Lewis index [TLI], root mean square error of approximation [RMSEA]).
Accounting for Potential Effects of BM
To fully understand the effects of CR and BM on changes in cognition, for any individual difference factor showing a moderating effect on cognitive decline, we tested: (1) whether the individual difference factor also moderated brain structural changes, and (2) if the individual difference factor’s moderation of cognitive changes is independent of brain structural measures (using whole-brain cortical thickness and white matter mean diffusivity at two time points).
The first question was examined for each of cortical thickness and mean diffusivity as dependent variables using mixed-effects linear modeling. Each model included age, time (baseline/follow-up), sex, IQ, years of education, time × age, and time × IQ as fixed effects, and a random intercept to account for within-subject correlation due to repeated measurements. Significant time × IQ effect would indicate that IQ moderates changes in brain structure. The second question was addressed by adding cortical thickness and mean diffusivity measures at both baseline and follow-up as time-varying covariates to the LCSM with the regression coefficients at both time points constrained to be the same, as illustrated in Supplementary Figure S2. To stabilize model fitting, the cortical thickness and mean diffusivity measures were standardized using their baseline means and standard deviations.
Results
Demographic Characteristics
Table 1 provides a summary of participant characteristics at baseline. Participants aged 20 to 80 years at baseline were followed for approximately 5 years on average. IQ was correlated with baseline age (ρ = 0.31, p < .001) and with years of education (ρ = 0.51, p < .001). IQ was also higher in males (male: 119 ± 7.5; female: 116 ± 8.7; t(249.94) = −2.58, p = .01) while no sex difference was found for age and education (ps > .1).
Longitudinal Changes in Cognitive Abilities
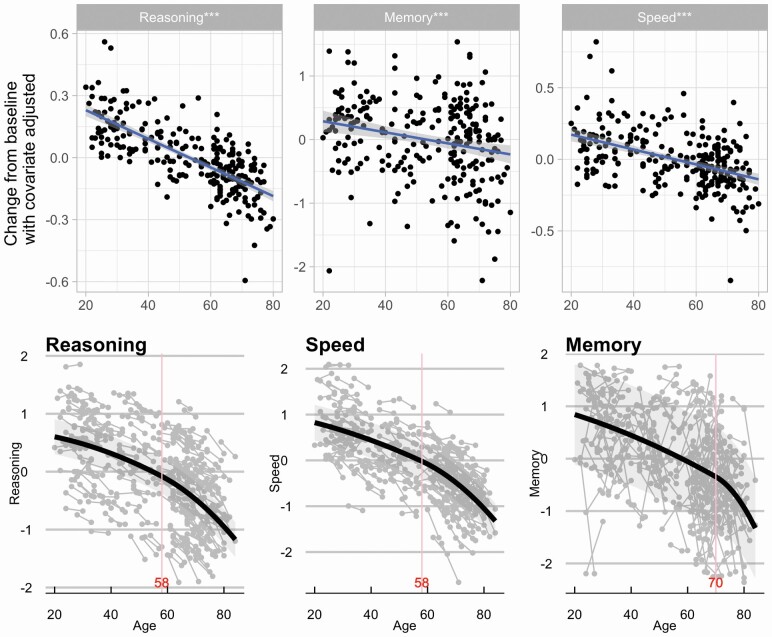
Bottom of Figure 1 shows the aging trajectory for each of the cognitive abilities. As expected, even after controlling for their baseline cognitive abilities, the three cognitive abilities showed decline over time (reasoning: β = −0.81, p < .001; memory: β = −0.15, p = .028; speed: β = −0.68, p < .001).
Figure 1.
(Top) Age moderation on changes in cognitive abilities. For all three abilities, the rate of change decreases with age. All models were adjusted for intelligence quotient, sex, and the two other baseline reference abilities. (Bottom) Performance on each ability at both time points against increasing age. Peak detection and inflection points are shown. Decline in reasoning, speed, and memory were accelerated after the respective inflection points of the quadratic b-spline (vertical lines).
Age Moderation on Changes in Cognitive Abilities
As shown in the top of Figure 1, for all cognitive abilities, we found age moderation of changes in the abilities after adjusting for the respective baseline ability performance. With older age, there was larger decline in reasoning (β = −0.79, p < .001), memory (β = −0.29, p < .001), and speed (β = −0.57, p < .001).
Change Point Analysis of Cognitive Abilities
We further explored whether there is a peak age and inflection point in the rate of change for each ability across the life span. A linear mixed-effect model was tested with each of the cognitive abilities as the dependent variable, age as the fixed effect, and random intercept to account for within-subject correlation due to repeated measurement. For all cognitive abilities, the quadratic trend with one inflection point performed the best as supported by the lowest BIC across all models. As illustrated in the bottom of Figure 1, decline in reasoning and speed showed accelerated decline after age 58 (indicated by vertical line in Figure 1). Memory showed a much later change point, at the age of 70 years, after which decline accelerated steeply.
Moderating Effects of Education and IQ on Changes in Cognitive Abilities
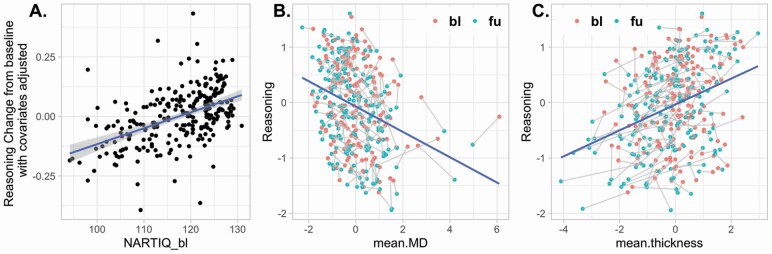
After adjusting for age and baseline abilities, higher IQ was associated with smaller declines in reasoning (β = 0.36, p < .001) and memory (β = 0.14, p = .039), but not in speed (β = 0.02, p = .88). With education being added to the model, education did not show significant moderation effect on changes in any of the cognitive abilities (ps > .05). Statistical details are listed in Supplementary Table S1. Figure 2A illustrates the changes in reasoning plotted against IQ after adjusting for all covariates.
Figure 2.
(A) Effect of intelligence quotient (IQ) on changes in reasoning ability adjusted for age, sex, education, the other two baseline cognitive abilities, and whole-brain cortical thickness and white matter mean diffusivity at both time points. (B and C) Association between whole-brain mean diffusivity and baseline (red dots) and follow-up (green dots) and of mean cortical thickness on changes in the reasoning ability. bl = baseline; fu = follow-up; MD = mean diffusivity; NART-IQ = IQ score estimated from the American National Adult Reading Test.
Differentiating CR and BM as Mechanisms Underlying IQ Moderation
Differential rate of change in cognition could be attributed to CR, BM, or both. We first examined the possibility that IQ moderated changes in cognition via an association with differential structural brain change by testing for the effects of IQ on the two brain measures separately. This was examined in two separate models, with each of the brain measures as outcome, and age, time (baseline and follow-up), sex, IQ, education, time × age, and time × IQ as independent variables. While age was associated with the change in both cortical thickness (β(Time × Age) = 0.172, p < .001) and mean diffusivity (β(Time × Age) = −0.185, p < .001), IQ was not associated with the change in either (p(Time × IQ) > 0.895). Statistical details are listed in Supplementary Table S1. Time × education was not examined because education did not moderate rate of cognitive change in the LCSM in Table 2.
Table 2.
Statistical Results for the Mixed-Effects Linear Model Testing Individual Difference Factors on Brain Structural Measures Across Two Time Points, Baseline and 5-Year Follow-Up
| Mean diffusivity | Cortical thickness | |||||
|---|---|---|---|---|---|---|
| Predictors | β | Standardized CI | p | β | Standardized CI | p |
| Intercept | 0.078 | −0.063 to 0.218 | <.001 | 0.201 | 0.058 to 0.343 | <.001 |
| Time points | −0.042 | −0.121 to 0.037 | .254 | −0.256 | −0.336 to −0.176 | .524 |
| Age | 0.422 | 0.312 to 0.533 | <.001 | −0.389 | −0.502 to −0.277 | <.001 |
| Sex (male) | −0.165 | −0.371 to 0.040 | .114 | −0.176 | −0.384 to 0.033 | .099 |
| NART-IQ | 0.06 | −0.068 to 0.189 | .358 | 0.096 | −0.033 to 0.226 | .145 |
| Education | 0.052 | −0.068 to 0.171 | .395 | −0.052 | −0.171 to 0.067 | .393 |
| Time * Age | 0.172 | 0.089 to 0.254 | <.001 | −0.185 | −0.270 to −0.101 | <.001 |
| Time * NART-IQ | 0.005 | −0.076 to 0.087 | .895 | −0.004 | −0.088 to 0.080 | .930 |
Notes: β = standardized coefficient; CI = confidence interval; NART-IQ = IQ score estimated from the American National Adult Reading Test. Time = baseline and follow-up. Bolded = p <.001.
Even though IQ was not associated with structural changes, individual differences in structural changes might influence cognitive changes, so the structural measures were included in the LCSM (see Supplementary Figure S2). The model estimates are shown in Table 3. Bivariate correlations between the 18 measures of cognitive tests and each of education, NART-IQ, mean diffusivity, and mean thickness are shown in Supplementary Information S3 tables. The estimated loadings for the measures in the LCSM are shown in Supplementary Table S4.
Table 3.
Statistical Parameters for Age and NART-IQ Moderation of Changes in Reference Abilities With Sex, Education, Cortical Thickness, and Mean Diffusivity (MD) as Covariates
| RA | Parameters | Estimate | SE | Z | p Value | 95% LCI | 95% UCI | β |
|---|---|---|---|---|---|---|---|---|
| Reasoning | Intercept* | −0.546 | 0.215 | −2.538 | .011 | −0.967 | −0.124 | −1.837 |
| Age*** | −0.173 | 0.043 | −4.059 | <.001 | −0.256 | −0.089 | −0.565 | |
| Sex | 0.029 | 0.053 | 0.543 | .587 | −0.076 | 0.133 | 0.049 | |
| Education | 0.025 | 0.013 | 1.920 | .055 | −0.001 | 0.050 | 0.205 | |
| BL reasoning*** | −0.219 | 0.050 | −4.416 | <.001 | −0.317 | −0.122 | −0.569 | |
| NART-IQ** | 0.116 | 0.038 | 3.048 | .002 | 0.041 | 0.190 | 0.387 | |
| Thickness** | 0.062 | 0.024 | 2.616 | .009 | 0.016 | 0.109 | 0.082 | |
| MD* | −0.066 | 0.028 | −2.344 | .019 | −0.122 | −0.011 | −0.086 | |
| Speed | Intercept* | −0.548 | 0.223 | −2.460 | .014 | −0.985 | −0.111 | −1.901 |
| Age* | −0.110 | 0.048 | −2.302 | .021 | −0.203 | −0.016 | −0.370 | |
| Sex | −0.099 | 0.057 | −1.741 | .082 | −0.210 | 0.012 | −0.171 | |
| Education* | 0.028 | 0.013 | 2.069 | .039 | 0.001 | 0.054 | 0.237 | |
| BL speed** | −0.153 | 0.053 | −2.905 | .004 | −0.257 | −0.050 | −0.401 | |
| NART-IQ | 0.012 | 0.037 | 0.327 | .743 | −0.060 | 0.084 | 0.042 | |
| Thickness | 0.047 | 0.025 | 1.883 | .060 | −0.002 | 0.096 | 0.063 | |
| MD** | −0.092 | 0.030 | −3.116 | .002 | −0.151 | −0.034 | −0.123 | |
| Memory | Intercept | −0.387 | 0.421 | −0.919 | .358 | −1.213 | 0.439 | −0.514 |
| Age | −0.095 | 0.076 | −1.248 | .212 | −0.244 | 0.054 | −0.122 | |
| Sex | −0.185 | 0.107 | −1.726 | .084 | −0.395 | 0.025 | −0.123 | |
| Education | 0.020 | 0.025 | 0.798 | .425 | −0.030 | 0.070 | 0.067 | |
| BL memory*** | −0.388 | 0.070 | −5.526 | <.001 | −0.526 | −0.251 | −0.467 | |
| NART-IQ | 0.118 | 0.066 | 1.791 | .073 | −0.011 | 0.247 | 0.156 | |
| Thickness*** | 0.143 | 0.042 | 3.368 | .001 | 0.060 | 0.226 | 0.159 | |
| MD | −0.083 | 0.048 | −1.720 | .085 | −0.178 | 0.012 | −0.092 | |
| Fit | CFI | TLI | BIC | RMSEA | 95% LCI | 95% UCL | p Value | |
| 0.8097 | 0.8032 | 13363.92 | 0.0747 | 0.0697 | 0.0796 | <.001 |
Notes: Since the dependent variables are changes in cognition, all associations are moderations of the changes. BIC = Bayesian Information Criterion; BL = baseline; CFI = comparative fit index; LCI = lower confidence interval; NART-IQ = IQ score estimated from the American National Adult Reading Test; RA = reference ability; RMSEA = root mean square error of approximation; SE = standard error; TLI = Tucker–Lewis index; UCI = upper confidence interval.
*p < .05. **p < .01. ***p < .001.
Table 3 shows that the two structural measures moderated cognitive changes. In the presence of age, sex, education, IQ, and mean diffusivity, cortical thickness showed a moderation effect on cognitive changes in reasoning and memory but not in speed. After accounting for covariates including cortical thickness, mean diffusivity showed moderation effects on cognitive changes for reasoning and speed, with each ability showing negative βs, suggesting that better preservation of white matter (lower mean diffusivity) was associated with better cognitive performance. Associations between brain measures and performance for the reasoning ability are illustrated in Figures 2B and C.
With the brain structural measures at both time points in the model (Table 3), IQ moderation of cognitive changes that was observed in the original model remained significant for reasoning (β = 0.387, p = .002) but the moderation effect became marginally significant for memory (β = 0.156, p = .073). Education showed moderating effect on declines for speed only (β = 0.237, p = .039). Results of a supplementary analysis testing the interaction of IQ and brain measures (mean diffusivity and thickness separately) were included in Supplementary Information S6 for completeness.
Sensitivity Analysis
Missing data
The missing data were due to issues with response paddles, MR scanner, participant discomfort, and to disruptions from the coronavirus disease 2019 pandemic. Supplementary Information S5 shows the number of missing data for each test measure and the statistical tests for differences in demographics for participants with and without complete data sets. There was no difference in age, sex, education, NART-IQ, and follow-up interval between participants with and without complete data. Sensitivity analysis results for including only participants with five or less missing test measures (n = 158) are shown in Supplementary Information S7. With the smaller n, effect of NART-IQ was still significant for reasoning when controlling for cortical thickness but became trend level for reasoning and memory when controlling for mean diffusivity. However, the direction and effect sizes remain similar to the results for the full sample.
In- versus out-of-scanner
We also conducted sensitivity analysis by in-scanner and out-of-scanner variables separately. Results are shown in Supplementary Information S8. We further examined the effects of IQ and education in separate models (Supplementary Information S9). We also adjusted for the time elapsed between baseline and follow-up visits (Supplementary Information S10). The findings remain similar to that in the fully adjusted models. There was one outlier who showed a large decline; when we reran the LCSM after removing this participant the results did not change. Overall results were similar.
Collinearity between IQ and education
To ensure the models were not affected by strong collinearity between IQ and education, we examined variance inflation factors for four factors: age = 1.083, IQ = 1.448, education = 1.336, and sex = 1.030. These values showed that the models did not contain too much multicollinearity among these factors, although IQ and education had slightly larger variance inflation factors than age and sex.
Discussion
This paper explored the potential contributions of age, BM, and CR on individual differences in rates of age-related cognitive decline in a life-span sample. As expected, age was related with more rapid decline in memory, reasoning, and processing speed. The results further demonstrated differential contributions of BM and CR to the cognitive abilities. For BM, changes in white matter mean diffusivity moderated differential changes in reasoning and speed; changes in mean cortical thickness moderated differential changes in reasoning and memory. These findings are consistent with the concept of BM in that individual differences in cognitive changes are associated with the degree to which the brain is “maintained.” With regards to CR, after taking individual brain changes into account, IQ was most influential in reasoning changes and was marginally associated with changes in memory, but not in processing speed. The contribution of IQ to differential ability to preserve cognitive function in the presence of age-related brain changes is consistent with the concept of CR. Thus, our data found that both BM and CR metrics contributed to individual differences in the rate of cognitive decline.
Although changes in whole-brain cortical thickness and white matter mean diffusivity were not related to IQ, they were nevertheless related to differential cognitive decline. Our results align well with several previous longitudinal studies, which reported changes in grey and white matter volumes being associated with changes in various cognitive abilities (Gorbach et al., 2017; Persson et al., 2016). Vinke et al. (2018) conducted a large epidemiological study on the aging trajectories of a number of brain structural changes and found that mean diffusivity had the second steepest declining slope among all of the brain measures, after total brain volume. The association between changes in white matter mean diffusivity and changes in two of the three abilities likely indicates the sensitivity of mean diffusivity to early microstructural damage that underlies early age-related cognitive changes. This greater sensitivity to structural decline enables the observation of BM in differential cognitive decline.
We included education in all models and observed a significant relationship between education and changes in the speed ability and a marginally significant association for reasoning. Education has been a commonly debated factor in cognitive decline for healthy aging. Most studies that used education either as the sole factor (Karlamangla et al., 2009; Proust-Lima et al., 2008) or as part of a composite score (Soldan et al., 2017) reported association between education and the level of cognitive performance, but there has been minimal evidence of education’s association with age-related cognitive declines (Lovden et al., 2020). These studies suggest that years of education is not guaranteed to be a good estimator of someone’s CR for healthy older adults. There may be several reasons for this observation. Years of education stabilizes in young adulthood and do not increase with an individual’s experience, as CR might. Neither does years of education capture the qualitative differences in the nature of the educational experience. Apart from the complication that education is still ongoing for younger participants of life-span studies, assessment of education can be inconsistent across individuals and studies, depending on whether the cumulative years of schooling or the equivalent years of education for degrees earned is reported. Also, many samples may include only a limited range of education masking possible associations with cognitive decline, which manifest more clearly in studies of wide education ranges (Zahodne et al., 2015). In contrast, however, many studies have demonstrated that education moderates the effect of Alzheimer’s disease pathology on cognition or clinical disease severity (Stern et al., 1999; Verlinden et al., 2016), although some have argued that this may be partially due to selection effects (Lovden et al., 2020). Our finding of an association between education and speed should be considered with these limitations in mind.
After controlling for structural changes, IQ, another commonly used factor for estimating CR, moderated changes in reasoning and marginally in memory, such that individuals with higher IQ showed slower decline. This is in contrast to education’s association with changes in speed, which suggests that different life experiences may influence cognitive abilities in unique ways. CR is hypothesized to exert protective effects in the presence of age- or disease-related brain changes through providing greater processing efficiency and capacity, more flexibility in solution strategy, and/or through compensatory processes that provide alternative networks when the primary network is damaged (Stern, 2012). Our findings suggest education may influence abilities relying more on processing speed while IQ may influence abilities requiring more reasoning processes.
While the best test of CR is to examine the moderation of a CR proxy on the brain-to-cognition association as stated by the Reserve and Resilience Framework (Stern et al., 2023). However, the Framework allows for two operationalizations of CR: apart from a moderating role of CR implemented via a CR × structure interaction, there can also be a direct influence of CR beyond brain structure, implemented by an additional main effect. We picked the latter and chose to control for brain structure because including the brain structural measures as time-varying covariates enabled us to use the whole sample, whereas using brain changes in the IQ moderation approach would result in a substantial reduction of sample size.
The neurobiological mechanisms contributing to CR’s protective effect are an active area of study (Buss et al., 2021). Our finding that IQ, a measure highly correlated with semantic knowledge, is associated with reduced rate of cognitive decline, combined with the common observation that semantic knowledge increases with age, suggests that some aspects of CR may increase with age: accumulation of semantic knowledge may enable older adults to shift toward reliance on semantic knowledge as their general cognition declines (Spreng & Turner, 2019). Older adults to use their previously acquired knowledge to aid performance of tasks such as episodic memory (Musielak et al., 2014) and decision making (Li et al., 2013). For example, in a task to recall grocery prices, older participants’ prior knowledge allowed them to recall the realistic prices well, which sufficiently minimized age differences in task performance (Castel, 2005). Neurobiologically, Turner and Spreng (2015) proposed the default–executive coupling hypothesis of aging to explain the shift toward the reliance on semantic knowledge based on their observation that the functional coupling between the default mode network and lateral prefrontal regions, which was shown to increase with task demand. However, the semantic knowledge shift can also result in worsening performance when previous knowledge impedes task performance (Spreng & Turner, 2019). Thus, while this theory may help explain some of IQ’s influence on maintaining cognitive performance in older adults, more research is needed to understand how experiences/genetic dispositions leading to higher IQ provides protective effect on cognitive aging.
Understanding how CR and BM factors influence cognitive aging will improve the accuracy in mapping cognitive changes in the typical aging process, and thus enabling more sensitive detection of atypical aging status in clinical care for older adults. For example, if cognitive testing becomes part of older adults’ annual health checkup, deviation from the norm may signal the need for further clinical evaluation. However, for the predicted aging trajectory to become a clinically meaningful tool, a fuller model incorporating more comprehensive set of CR and BM factors, such as additional lifestyle factors and more fine-grain brain measures, respectively, will greatly improve the predictive accuracy of cognitive changes in the aging process. Furthermore, given that the current sample of participants has a mean education of 16 years and NART-IQ of 117, replication is needed in a sample with a greater range of education and IQ levels.
All longitudinal studies are susceptible to practice effects which can confound the observed cognitive changes in this study. However, despite potential practice effects, robust cognitive decline was observed over 5 years for the three abilities across the life span and taking practice factor into account would only further strengthen the main effects. Due to the correlation between cognitive changes and other moderators such as age, education, and IQ, we also could not rule out the possibility of practice effects influencing interaction effects. The decision to use the same test versions across study visits was made after carefully weighing the possibility of mismatch in task difficulty when using different versions of the same tests against the possibility of practice effects confounding the outcomes. The latter was seen as less problematic.
Given the differential patterns observed across the cognitive abilities, future examination of factors contributing to cognitive decline should include multiple cognitive abilities to fully explore the role CR and BM play in the decline of each ability. Without a comprehensive set of cognitive abilities being assessed for age by individual difference factor interactions, effects specific to certain abilities may be missed.
Overall, our findings provide support for BM and CR as mechanisms contributing resilience in cognitive aging and that differential life experiences contribute unique influences on the various cognitive trajectories. While education was found to exert an effect on speed changes, estimated IQ showed robust moderations of the effects of aging on reasoning changes, even after accounting for brain structural changes. The results also demonstrate that BM and CR can both simultaneously moderate the effect of age on the cognitive abilities.
Supplementary Material
Acknowledgments
All deidentified neuropsychological and behavioral data, and neuroimaging summary measures, along with analytical scripts used to produce the results presented in the manuscript will be uploaded to Dryad (datadryad.org). Dryad works with Zenodo (zenodo.org) to simplify data sharing by making the data and associated scripts used in a study easily accessible on a public platform. This study was not preregistered.
Contributor Information
Yunglin Gazes, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Seonjoo Lee, Department of Psychiatry and Biostatistics, Columbia University, New York, New York, USA; Mental Health Data Science, New York State Psychiatric Institute, New York, New York, USA.
Zhiqian Fang, Department of Biostatistics, Columbia University, New York, New York, USA.
Ashley Mensing, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Diala Noofoory, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Geneva Hidalgo Nazario, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Reshma Babukutty, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Bryan B Chen, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Christian Habeck, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Yaakov Stern, Cognitive Neuroscience Division, Department of Neurology, Columbia University Irving Medical Center, New York, New York, USA.
Funding
This work was supported by the National Institute of Aging (grant numbers K01AG051777, R01AG026158, R01AG038465, and R01AG062578-01A1).
Conflict of Interest
None declared.
References
- Bertola, L., Wei-Ming Watson, C., Avila, J. F., Zahodne, L. B., Angevaare, M., Schupf, N., & Manly, J. J. (2019). Predictors of episodic memory performance across educational strata: Multiple-group comparisons. Journal of the International Neuropsychological Society, 25(9), 901–909. doi: 10.1017/s1355617719000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, E. W., Corbett, N. J., Roberts, J. G., Ybarra, N., Musial, T. F., Simkin, D., Molina-Campos, E., Oh, K. J., Nielsen, L. L., Ayala, G. D., Mullen, S. A., Farooqi, A. K., D’Souza, G. X., Hill, C. L., Bean, L. A., Rogalsky, A. E., Russo, M. L., Curlik, D. M., Antion, M. D., & Nicholson, D. A. (2021). Cognitive aging is associated with redistribution of synaptic weights in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 118(8), 1–10. doi: 10.1073/pnas.1921481118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel, A. D. (2005). Memory for grocery prices in younger and older adults: The role of schematic support. Psychology and Aging, 20(4), 718–721. doi: 10.1037/0882-7974.20.4.718 [DOI] [PubMed] [Google Scholar]
- Cox, S. R., Dickie, D. A., Ritchie, S. J., Karama, S., Pattie, A., Royle, N. A., Corley, J., Aribisala, B. S., Valdes Hernandez, M., Munoz Maniega, S., Starr, J. M., Bastin, M. E., Evans, A. C., Wardlaw, J. M., & Deary, I. J. (2016). Associations between education and brain structure at age 73 years, adjusted for age 11 IQ. Neurology, 87(17), 1820–1826. doi: 10.1212/WNL.0000000000003247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis, J. B., Peng, S. L., Chen, X., Li, Y., Liu, P., Sur, S., Rodrigue, K. M., Park, D. C., & Lu, H. (2018). Arterial-spin-labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4-year longitudinal study. Journal of Magnetic Resonance Imaging, 48(2), 449–458. doi: 10.1002/jmri.25938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach, T., Pudas, S., Lundquist, A., Oradd, G., Josefsson, M., Salami, A., de Luna, X., & Nyberg, L. (2017). Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiology of Aging, 51, 167–176. doi: 10.1016/j.neurobiolaging.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Grober, E., & Sliwinski, M. (1991). Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology, 13(6), 933–949. doi: 10.1080/01688639108405109 [DOI] [PubMed] [Google Scholar]
- Habeck, C., Razlighi, Q., Gazes, Y., Barulli, D., Steffener, J., & Stern, Y. (2016). Cognitive reserve and brain maintenance: Orthogonal concepts in theory and practice. Cerebral Cortex, 27, 3962–3969. doi: 10.1093/cercor/bhw208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne, J. K., & Germine, L. T. (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological Science, 26(4), 433–443. doi: 10.1177/0956797614567339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, M. L., Agrigoroaei, S., Jeon, M., Bruzzese, M., & Lachman, M. E. (2018). Change in cognitive performance from midlife into old age: Findings from the Midlife in the United States (MIDUS) Study—Erratum. Journal of the International Neuropsychological Society, 24(8), 891–891. doi: 10.1017/s1355617718000887 [DOI] [PubMed] [Google Scholar]
- Karlamangla, A. S., Miller-Martinez, D., Aneshensel, C. S., Seeman, T. E., Wight, R. G., & Chodosh, J. (2009). Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology, 170(3), 331–342. doi: 10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit, R. A., Brandmaier, A. M., Ziegler, G., van Harmelen, A. L., de Mooij, S. M. M., Moutoussis, M., Goodyer, I. M., Bullmore, E., Jones, P. B., Fonagy, P., Consortium, N., Lindenberger, U., & Dolan, R. J. (2018). Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Developmental Cognitive Neuroscience, 33, 99–117. doi: 10.1016/j.dcn.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Baldassi, M., Johnson, E. J., & Weber, E. U. (2013). Complementary cognitive capabilities, economic decision making, and aging. Psychology and Aging, 28(3), 595–613. doi: 10.1037/a0034172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden, M., Fratiglioni, L., Glymour, M. M., Lindenberger, U., & Tucker-Drob, E. M. (2020). Education and cognitive functioning across the life span. Psychological Science in the Public Interest, 21(1), 6–41. doi: 10.1177/1529100620920576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musielak, C., Giraudeau, C., Chasseigne, G., & Mullet, E. (2014). To what extent does the existence of functional relations in a learning setting change the pattern of differences between younger and older adults’ performances?. Experimental Aging Research, 40(4), 455–476. doi: 10.1080/0361073X.2014.926776 [DOI] [PubMed] [Google Scholar]
- Nyberg, L., Lovden, M., Riklund, K., Lindenberger, U., & Backman, L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Persson, N., Ghisletta, P., Dahle, C. L., Bender, A. R., Yang, Y., Yuan, P., Daugherty, A. M., & Raz, N. (2016). Regional brain shrinkage and change in cognitive performance over two years: The bidirectional influences of the brain and cognitive reserve factors. Neuroimage, 126, 15–26. doi: 10.1016/j.neuroimage.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust-Lima, C., Amieva, H., Letenneur, L., Orgogozo, J. M., Jacqmin-Gadda, H., & Dartigues, J. F. (2008). Gender and education impact on brain aging: A general cognitive factor approach. Psychology and Aging, 23(3), 608–620. doi: 10.1037/a0012838 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (1998). Independence of age-related influences on cognitive abilities across the life span. Developmental Psychology, 34(5), 851–864. doi: 10.1037//0012-1649.34.5.851 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (2016). Continuity of cognitive change across adulthood. Psychonomic Bulletin and Review, 23(3), 932–939. doi: 10.3758/s13423-015-0910-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse, T. A. (2019). Trajectories of normal cognitive aging. Psychology and Aging, 34(1), 17–24. doi: 10.1037/pag0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sele, S., Liem, F., Merillat, S., & Jancke, L. (2021). Age-related decline in the brain: A longitudinal study on inter-individual variability of cortical thickness, area, volume, and cognition. Neuroimage, 240, 118370. doi: 10.1016/j.neuroimage.2021.118370 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux, A., Kivimaki, M., Glymour, M. M., Elbaz, A., Berr, C., Ebmeier, K. P., Ferrie, J. E., & Dugravot, A. (2012). Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. BMJ, 344, d7622. doi: 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux, A., Marmot, M. G., Glymour, M., Sabia, S., Kivimaki, M., & Dugravot, A. (2011). Does cognitive reserve shape cognitive decline?. Annals of Neurology, 70(2), 296–304. doi: 10.1002/ana.22391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan, A., Pettigrew, C., Cai, Q., Wang, J., Wang, M. C., Moghekar, A., Miller, M. I., Albert, M., & Team, B. R. (2017). Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiology of Aging, 60, 164–172. doi: 10.1016/j.neurobiolaging.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N., & Turner, G. R. (2019). The shifting architecture of cognition and brain function in older adulthood. Perspectives on Psychological Science, 14(4), 523–542. doi: 10.1177/1745691619827511 [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11(11), 1006–1012. doi: 10.1016/s1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Albert, M., Barnes, C. A., Cabeza, R., Pascual-Leone, A., & Rapp, P. R. (2023). A framework for concepts of reserve and resilience in aging. Neurobiology of Aging, 124, 100–103. doi: 10.1016/j.neurobiolaging.2022.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Albert, S., Tang, M. X., & Tsai, W. Y. (1999). Rate of memory decline in AD is related to education and occupation: Cognitive reserve?. Neurology, 53(9), 1942–1947. doi: 10.1212/wnl.53.9.1942 [DOI] [PubMed] [Google Scholar]
- Stern, Y., Arenaza-Urquijo, E. M., Bartres-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., Ewers, M., Franzmeier, N., Kempermann, G., Kremen, W. S., Okonkwo, O., Scarmeas, N., Soldan, A., Udeh-Momoh, C., Valenzuela, M., Vemuri, P., Vuoksimaa, E., & the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, 16(9), 1305–1311. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Gazes, Y., Razlighi, Q., Steffener, J., & Habeck, C. (2018). A task-invariant cognitive reserve network. Neuroimage, 178, 36–45. doi: 10.1016/j.neuroimage.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Habeck, C., Steffener, J., Barulli, D., Gazes, Y., Razlighi, Q., Shaked, D., & Salthouse, T. (2014). The Reference Ability Neural Network Study: Motivation, design, and initial feasibility analyses. Neuroimage, 103, 139–151. doi: 10.1016/j.neuroimage.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then, F. S., Luck, T., Luppa, M., Konig, H. H., Angermeyer, M. C., & Riedel-Heller, S. G. (2015). Differential effects of enriched environment at work on cognitive decline in old age. Neurology, 84(21), 2169–2176. doi: 10.1212/WNL.0000000000001605 [DOI] [PubMed] [Google Scholar]
- Tucker-Drob, E. M. (2009). Differentiation of cognitive abilities across the life span. Developmental Psychology, 45(4), 1097–1118. doi: 10.1037/a0015864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob, E. M. (2019). Cognitive aging and dementia: A life-span perspective. Annual Review of Developmental Psychology, 1(1), 177–196. doi: 10.1146/annurev-devpsych-121318-085204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, G. R., & Spreng, R. N. (2015). Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: The default–executive coupling hypothesis of aging. Journal of Cognitive Neuroscience, 27(12), 2462–2476. doi: 10.1162/jocn_a_00869 [DOI] [PubMed] [Google Scholar]
- Verlinden, V. J. A., van der Geest, J. N., de Bruijn, R. F. A. G., Hofman, A., Koudstaal, P. J., & Ikram, M. A. (2016). Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimer’s & Dementia, 12(2), 144–153. doi: 10.1016/j.jalz.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Vinke, E. J., de Groot, M., Venkatraghavan, V., Klein, S., Niessen, W. J., Ikram, M. A., & Vernooij, M. W. (2018). Trajectories of imaging markers in brain aging: The Rotterdam Study. Neurobiology of Aging, 71, 32–40. doi: 10.1016/j.neurobiolaging.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Whitley, E., Deary, I. J., Ritchie, S. J., Batty, G. D., Kumari, M., & Benzeval, M. (2016). Variations in cognitive abilities across the life course: Cross-sectional evidence from Understanding Society: The UK Household Longitudinal Study. Intelligence, 59, 39–50. doi: 10.1016/j.intell.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. S., Hebert, L. E., Scherr, P. A., Barnes, L. L., Mendes de Leon, C. F., & Evans, D. A. (2009). Educational attainment and cognitive decline in old age. Neurology, 72(5), 460–465. doi: 10.1212/01.wnl.0000341782.71418.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne, L. B., Ajrouch, K. J., Sharifian, N., & Antonucci, T. C. (2019). Social relations and age-related change in memory. Psychology and Aging, 34, 751–765. doi: 10.1037/pag0000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne, L. B., Stern, Y., & Manly, J. J. (2015). Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology, 29(4), 649–657. doi: 10.1037/neu0000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnani, K., Nichols, T. E., Alfaro-Almagro, F., Fagerlund, B., Lauritzen, M., Rostrup, E., & Smith, S. M. (2019). Discovering markers of healthy aging: A prospective study in a Danish male birth cohort. Aging, 11(16), 5943–5974. doi: 10.18632/aging.102151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.