Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia. Tax, the viral protein, is thought to be crucial in the development of the disease, since it transforms healthy T cells in vitro and induces tumors in transgenic animals. We examined the effect of Tax activity on the growth of the interleukin-2 (IL-2)-dependent T-cell line CTLL-2. Stable expression of Tax in CTLL-2 transformed cell growth from being IL-2 dependent to IL-2 independent. Tax stimulated transcription through NF-κB and the cyclic AMP-responsive element-like sequence in the HTLV-1 promoter. The finding of Tax mutants segregating these two pathways suggested that the NF-κB pathway was essential for IL-2-independent growth of CTLL-2 cells while the CRE pathway was unnecessary. However, both pathways were necessary for another transformation-related activity (colony formation in soft agar) of CTLL-2/Tax. Our results show that Tax has at least two distinct activities on T cells, and suggest that Tax plays a crucial role in IL-2-independent T-cell transformation induced by HTLV-1, in addition to its well-known IL-2-dependent cell transformation.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia, which is characterized by a dysregulated proliferation of T cells (14, 29, 45). HTLV-1 transforms normal T cells in vitro (26, 42). Two types of T-cell lines are transformed by the virus; the first proliferates in an interleukin-2 (IL-2)-dependent manner, while the other proliferates in an IL-2-independent manner (35). The viral nonstructure protein Tax is involved in the IL-2-dependent transformation step, since it transforms normal T cells in the presence of IL-2 (2, 13). However, the exact mechanism through which HTLV-1 transforms normal T cells in an IL-2-independent manner is not well understood.
Tax has multiple functions. For example, it activates the transcription of various cellular genes, such as proto-oncogenes (c-fos, c-jun, fra-1, and c-myc) and genes encoding growth factors (IL-2, IL-6, transforming growth factor-β, OX40 ligand, and granulocyte-macrophage colony-stimulating factor) and their receptors (α-chain of IL-2 receptor, OX40) (8, 10, 11, 15, 20, 23, 27, 40, 43, 45). Tax also represses the transcription of several cellular genes such as those encoding DNA polymerase β and Bax (5, 16). In addition, it stimulates the activity of several kinase enzymes such as cyclin-dependent kinase (CDK) and protein kinase C (18, 38).
In the activities described above, Tax interacts with various cellular proteins such as the transcription factors SRF (CArG binding protein) and CREB (cyclic AMP-responsive element [CRE]-binding protein), IκB (an inhibitor of NF-κB/Rel transcription factors), MEKK1 (kinase of IκB kinase), and INK-4, an inhibitor of CDK (12, 36, 38, 41, 44, 46). These interactions mediate Tax-dependent activation of transcription via CRE (CREB), CArG (SRF), κB element (IκB), and CDK kinase (INK-4). Tax also associates with other cellular proteins such as mitotic checkpoint protein MAD1 and cyclin D (17, 28).
In the present study, we investigated whether Tax is involved in IL-2-independent transformation of T cells by HTLV-1. For this purpose, we introduced the tax gene into a mouse IL-2-dependent T-cell line, CTLL-2, to examine the growth property of these cells in the absence of IL-2. There are two well-known Tax mutants, TaxM22 and Tax703(M47). The latter activates NF-κB-dependent transcription but not CRE-dependent transcription, whereas the reverse is true for TaxM22. Using these two mutants, we also investigated the involvement of these two pathways in the transformation (IL-2-independent proliferation and colony formation in soft agar) of CTLL-2 cells by Tax.
MATERIALS AND METHODS
Plasmids.
The wild-type tax and tax mutant genes were cloned into pHβPr.1-neo, which has a β-actin promoter for protein expression in vivo as well as a neomycin resistance gene for G418 selection (21, 22). The Tax mutants TaxM22 (33) and Tax703(M47) (1, 22, 32) have been previously characterized, and their substituted amino acids (position in parentheses) are Thr(130)Leu to SerAla for TaxM22 and Leu(319)Leu to ArgSer for Tax703. HTLV LTR-luc and κB-luc are luciferase expression plasmids regulated by the HTLV-1 long terminal repeat (LTR) promoter containing Tax-inducible CREs (21-bp repetitive sequence) and a pentamer of the κB element from the IL-2Rα gene (CAGGGGAATCTC), respectively (37).
Cell culture and cell growth assay.
CTLL-2 is a mouse IL-2-dependent T-cell line. TL-Su, HUT102, MT-2, ILT-Hod, ILT-Oot, ILT-MC, ILT-Mat, ILT-Kan2, and ILT-Koy are human HTLV-1-transformed T-cell lines (3, 34). MT-2, HUT102, and TL-Su were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) (RPMI-FCS). ILT cell lines were cultured in RPMI-FCS with 1 nM IL-2. CTLL-2 was cultured in RPMI-FCS with 1 nM IL-2 and 2-mercaptoethanol. To examine cell growth, 105 cells were washed twice with phosphate-buffered saline (PBS) and suspended in RPMI-FCS. They were then cultured in a 24-well at 37°C. The cell number was counted by the trypan blue staining method.
Establishment of CTLL-2 cell lines expressing Tax.
To establish CTLL-2 cell lines expressing Tax proteins, CTLL-2 cells were washed twice with PBS and once with K-PBS (30.8 mM NaCl, 120.7 mM KCl, 8.1 mM Na2HPO4, 1.46 mM KH2PO4), and suspended in K-PBS. Cells (107) in K-PBS were mixed with 30 μg of Tax or Tax mutant expression plasmid in an electroporation cuvette, incubated on ice for 10 min, and then pulsed with a Electroporator (Bio-Rad) at 320 V and 950 μF. In the next step, the cells were placed on ice for 10 min, addition of FCS was added (to a final concentration of 10%), and the cells were further incubated at room temperature for 10 min. Then the cells were seeded in 24-well plates and cultured in RPMI-FCS containing IL-2. At 24 h after electroporation, G418 (0.4 mg/ml) was added, and the cells were cultured for about 4 to 6 weeks. G418-resistant cells were screened for the expression of Tax protein by Western blot analysis, and cells expressing Tax were further cloned by the limiting-dilution method.
Western blotting.
Cell lysates prepared from CTLL-2 or HTLV-1-transformed T-cell lines were resolved by electrophoresis on 10% polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Amersham). The blots were incubated with a mouse monoclonal anti-Tax antibody (Taxy-8) (39) and then washed and incubated with anti-mouse immunoglobulin conjugated with horseradish peroxidase. Proteins recognized by the antibodies were visualized with the enhanced chemiluminescence Western blotting detection system (Amersham).
Luciferase assay.
CTLL-2 cells were washed twice with PBS and suspended in RPMI 1640 medium. The cells were mixed with luciferase plasmid (10 μg) and Tax expression plasmid (20 μg), and DEAE dextran (500 μg/ml) was added. They were then incubated at 37°C for 30 min with occasional mixing. After the cells were washed with RPMI 1640, they were cultured for 48 h, a cell lysate was prepared, and the luciferase activity in the lysate was determined with a luminometer.
EMSA.
For the preparation of nuclear extract, 107 cells were washed with PBS containing 1 mM Na3VO4 and 5 mM NaF. The cells were then treated with 0.2% Nonidet P-40 in lysis buffer containing 20 mM HEPES (pH 7.9), 20 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, and 1 μg of aprotinin per ml. After centrifugation, the pellets were further treated at 4°C for 30 min with lysis buffer supplemented with 420 mM NaCl and 20% glycerol and then subjected to centrifugation. The resulting supernatant was used as the nuclear extract in an electrophoretic mobility shift assay (EMSA). Nuclear extract (10 μg) was preincubated for 15 min on ice with 1 μg of poly(dI-dC) in 20 μl of a binding buffer containing 13 mM HEPES (pH 7.9), 65 mM NaCl, 0.15 mM EDTA, 8% glycerol, and 1 mM dithiothreitol. Approximately 1 ng of labeled oligonucleotide was added to the reaction mixture, which was then further incubated for 15 min at 25°C. The formed complexes were separated from the unbound probe by electrophoresis in a 5% polyacrylamide gel containing 0.5× TBE, and 2.5% glycerol, and the gel was dried and exposed to X-ray film. A double-stranded synthetic oligonucleotide corresponding to the NF-κB binding site (top strand, AGCTTTGGGAAATTCCTCGGGTGGTAC) from the interferon gene was labeled with [γ-32P]ATP by using polynucleotide kinase and was used as the κB-site probe.
Analysis of apoptosis.
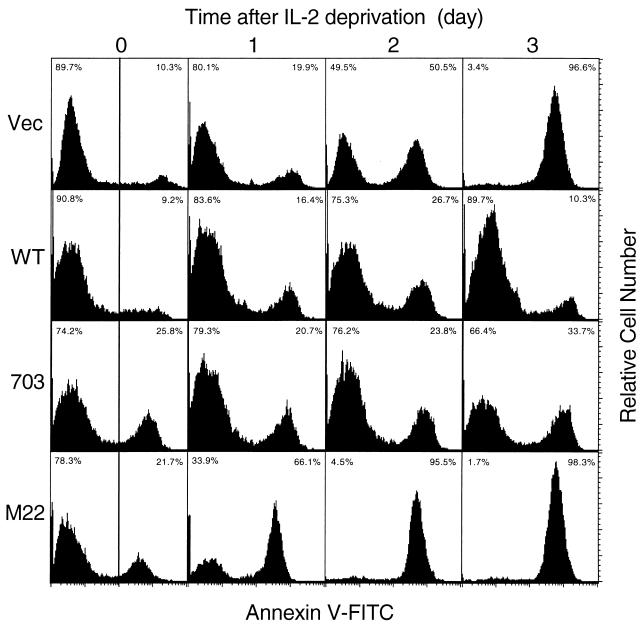
The CTLL-2/Vec, CTLL-2/WT, CTLL-2/703, and CTLL-2/M22 cell lines were washed twice with PBS and then cultured in the absence of IL-2 for 0 to 3 days. The cells were washed twice with cold PBS and suspended in a binding buffer provided by the manufacturer (R & D). They were then mixed with fluorescein-conjugated annexin V and propidium iodine reagent and incubated for 15 min at 20 to 25°C in the dark. After fivefold dilution with the binding buffer, the cells were analyzed for staining for annexin V on a FACScalibur.
Assay of colony formation in soft agar (CFSA).
Cells (103) were suspended in RPMI-FCS containing 0.4% agarose (SeaPlaque; FMC), which was first melted by heating. The suspension (1 ml) was then poured onto a base layer consisting of 2 ml of RPMI 1640 containing 0.53% agarose in a six-well plate. After 2 weeks, the number of colonies (each containing >100 cells) was counted. The colony number was expressed as the percentage of colonies in 103 inoculated cells.
RESULTS
Tax converts cell growth of CTLL-2 from IL-2 dependent to IL-2 independent.
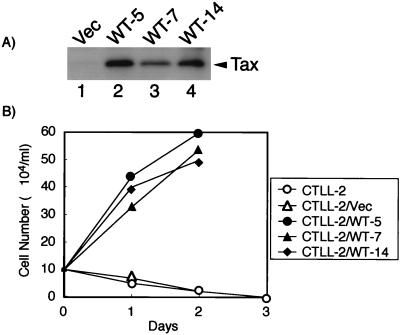
CTLL-2 is a mouse T-cell line, and the cells are dependent on IL-2 for their proliferation. CTLL-2 cells were transfected with either a Tax-expressing plasmid or a vector plasmid and cultured in the presence of IL-2 and G418 for selection. Selected clones were first examined for the expression of Tax protein by Western blot analysis. Tax protein was detected in three representative clones transfected with the tax plasmid (WT-5, WT-7, and WT-14) but not in those transfected with the vector plasmid (Fig. 1A). Such CTLL-2 clones were then cultured in the absence of IL-2, and cell growth was examined by trypan blue exclusion. Similar to parental CTLL-2 cells, cells transfected with vector plasmid (CTLL-2/Vec) rapidly died in the absence of IL-2 whereas all three CTLL-2 cell lines that expressed Tax showed a continuous proliferation (Fig. 1B). These three clones were maintained in the absence of IL-2 for more than 5 months. Thus, Tax can modulate the growth properties of CTLL-2 cells from being IL-2 dependent to being IL-2 independent. The culture supernatant of these CTLL-2/WT cells did not support the growth of parental CTLL-2 cells in the absence of IL-2 and did not contain detectable levels of IL-2, as confirmed by enzyme-linked immunosorbent assay analysis (data not shown). Thus, IL-2-independent proliferation of CTLL-2/WT cells was not due to IL-2 or other cytokines that might have been induced by Tax.
FIG. 1.
Tax induces IL-2-independent proliferation of CTLL-2 cells. (A) Expression of Tax protein in CTLL-2/WT cells. Cell lysates were prepared from CTLL-2 cells transfected either with the vector plasmid (lane 1) or with expression plasmids encoding TaxWT (lanes 2 to 4). The expression of Tax proteins in these lysates was measured by Western blot analysis with anti-Tax antibody (Taxy-8). (B) Cell growth analysis of CTLL-2 cells expressing Tax protein in the absence of IL-2. CTLL-2, CTLL-2/Vec, and three CTLL-2 clones expressing Tax protein were cultured in the absence of IL-2. Cell growth was measured by trypan blue staining.
Function of Tax responsible for IL-2-independent transformation of CTLL-2 cells.
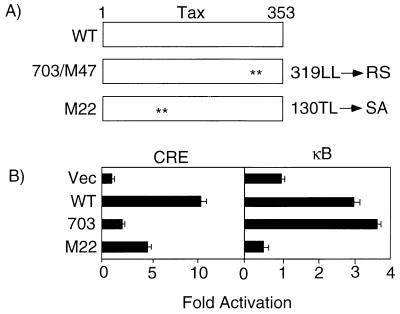
Tax activates transcription via the κB element and CRE in the HTLV-1 LTR and in cellular genes (45). TaxM22 and Tax703(M47) are substitution mutants of Tax, originally described by Smith et al. (32, 33) (Fig. 2A), which can segregate the activities of Tax to the κB element and CRE in various types of cells (1, 22, 32, 33). The wild-type Tax (TaxWT) and the two Tax mutant plasmids were transiently transfected into CTLL-2 cells together with Luc (luciferase) reporters containing either CRE or the κB element. Expression of TaxWT stimulated Luc activity from two reporters with either CRE or the κB element (Fig. 2). Tax703 activated the κB element more than TaxWT did, whereas it caused only a negligible activation of CRE. In contrast, TaxM22 activated CRE and produced only a slight inhibition of the κB element. The activation (fold induction) by Tax of the κB element in CTLL-2 cells was low relative to that reported previously with other cell lines such as the T-cell line Jurkat. IL-2 also activated NF-κB activity in T-cell lines including CTLL-2 (see Fig. 4, lane 4). Thus, the activation by Tax of the κB element may be partially masked by the activation of this element by IL-2. Indeed, Tax and Tax703 but not TaxM22 activated the κB element much more efficiently in Jurkat cells than in CTLL-2 cells (data not shown). In this regard, it was difficult to measure the activation by Tax of the κB element in IL-2-deprived CTLL-2 cells, since these cells died within 3 days.
FIG. 2.
trans-activation phenotype of TaxWT and its mutants. (A) Schematic structures of TaxWT and its mutants. The position of amino acid substitution in each mutant is indicated. (B) Luciferase assay. Reporter Luc plasmids with a CRE (LTR-luc) or κB element were transfected into CTLL-2 cells together with expression plasmids encoding Tax or its indicated mutants. Luc activity in the harvested cells was measured as described in Materials and Methods. Fold activation shows the ratio of Luc activity in cells cotransfected with the expression plasmid encoding Tax or its mutant relative to that of cells cotransfected with the vector plasmid. The average Luc activity of three experiments is presented. Data are representative of three reproducible independent experiments.
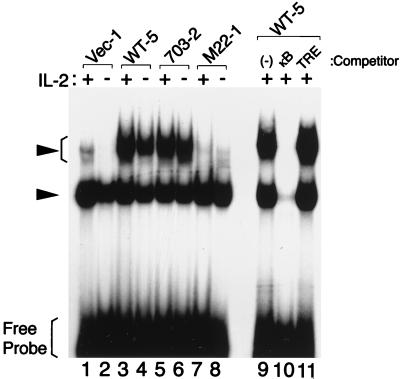
FIG. 4.
NF-κB activity in CTLL-2 cells expressing Tax or its mutant. Nuclear extracts were prepared from CTLL-2 cells transfected with the indicated tax mutant plasmids. The extracts were then incubated with an NF-κB binding site labeled with [γ-32P]ATP in the absence (lanes 1 to 8) or presence of homologous oligonucleotides (lane 10) or an unrelated one (TRE, lane 11). The formed complex was separated by polyacrylamide gel electrophoresis (5% polyacrylamide). Arrows indicate the specific complex.
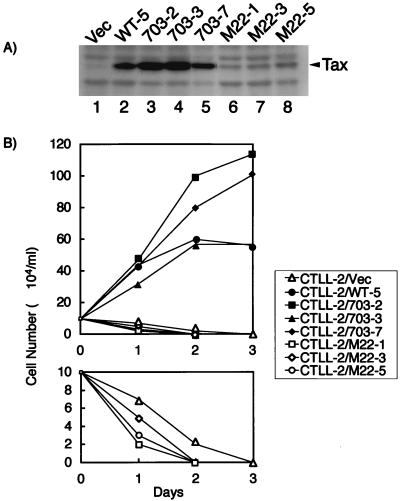
CTLL-2 cells expressing these two Tax mutants were established in the presence of IL-2. Western blotting analysis showed that the expression of Tax703 was the same as or greater than that of TaxWT in CTLL-2 cells whereas the expression of TaxM22 was less than that of TaxWT (Fig. 3A). Subsequently, CTLL-2 cells expressing these Tax mutant proteins were cultured in the absence of IL-2 and their cell growth properties were then examined. Like TaxWT, all three CTLL-2 cell lines expressing Tax703 proliferated in the absence of IL-2 (Fig. 3B) and were cultured in the absence of IL-2 for more than 5 months. In contrast, all three CTLL-2/M22 cell lines rapidly died in the absence of IL-2, and cell death occurred earlier than in CTLL-2/Vec cells (Fig. 3B). These results suggested that the NF-κB pathway is essential for IL-2-independent transformation of CTLL-2 by Tax and that the CRE pathway is not essential for this activity.
FIG. 3.
Pathway of Tax responsible for IL-2-independent proliferation of CTLL-2 cells. (A) Expression of Tax proteins in CTLL-2 cells. Cell lysates were prepared from CTLL-2 cells transfected either with vector plasmid (pHβPr.1-neo) or with expression vectors encoding TaxWT or the indicted Tax mutant proteins. The expression of Tax proteins in these lysates was measured by Western blot analysis with anti-Tax antibody (Taxy-8). (B) Cell growth analysis of CTLL-2 cells expressing each Tax mutant. CTLL-2 clones expressing the indicated Tax mutant were cultured in the absence of IL-2, and cell growth was measured by trypan blue staining.
Tax-mediated activation of NF-κB in CTLL-2 cells.
We next measured the binding activity of the κB element (NF-κB activity) in CTLL-2 cells expressing Tax by using EMSA. Consistent with the Luc assay, CTLL/WT-5 and CTLL/703-2 showed a higher NF-κB activity (slow-migrating complex) than did CTLL/Vec in both the absence and presence of IL-2 (Fig. 4). The Tax-induced complex was specific to the κB element, since it was inhibited by homologous κB oligonucleotides but not by unrelated TRE (TPA-responsive element) from the collagenase gene (Fig. 4). The fast-migrating band was also specific to the κB element, but the complex was not induced by Tax. Thus, the slow-migrating complex was associated with IL-2-independent proliferation (Fig. 3). Similar results were obtained with other Tax expressing CTLL-2 clones. In contrast, CTLL/M22-1 showed a lower NF-κB activity than did CTLL/Vec in the presence of IL-2. Inhibition of NF-κB activity by TaxM22 was consistent with that of the κB reporter by TaxM22 (Fig. 2). In addition, absence of IL-2 reduced NF-κB activity in CTLL-2 cells. Thus, reduced NF-κB activity was associated with CTLL-2 cell death.
Inhibition of apoptosis in CTLL-2 cells expressing Tax.
In the next step, we examined the extent of apoptosis in CTLL-2/Vec and CTLL-2 cells that expressed Tax proteins by using the annexin V staining method. Annexin V binding to cells is a marker of the early stages of apoptosis. Apoptosis of 10.3% of the CTLL-2/Vec cells was detected on day 0. Culture of cells in the absence of IL-2 resulted in a rapid increase in the population of apoptotic cells till day 3, and 96.6% of cells stained with annexin V on day 3 (Fig. 5). On the other hand, there was only a slight increase in apoptotic CTLL-2/WT (from 9.2 to 10.3%) and CTLL-2/703 (from 25.8 to 33.7%) cells during a 3-day culture in the absence of IL-2. Therefore, Tax and Tax703 proteins inhibited apoptosis of CTLL-2 cells in the absence of IL-2. Annexin staining experiments also showed that apoptosis was less prevalent in CTLL-2/WT-5 cells than in CTLL-2/703-2 cells. Thus, both these Tax proteins induced IL-2-independent proliferation of CTLL-2, but TaxWT produced a limited degree of apoptosis compared with Tax703. Unlike CTLL-2 cells expressing TaxWT and Tax703, apoptosis of cells that expressed TaxM22 occurred much faster than did apoptosis of CTLL-2/Vec cells. These results were consistent with those of the cell growth analysis (Fig. 3). In addition, more apoptotic cells were detected in CTLL-2/M22 than CTLL-2/Vec cells even in the presence of IL-2 (Fig. 5, compare M22 with Vec on day 0). These results indicated that TaxM22 enhanced apoptosis of CTLL-2 in the absence as well as the presence of IL-2.
FIG. 5.
Tax and Tax703 inhibit apoptosis induced by IL-2 deprivation in CTLL-2 cells. CTLL-2, CTLL-2/WT, CTLL-2/703, and CTLL-2/M22 cells were cultured in the absence of IL-2 for the indicated time intervals. They were then incubated with fluorescein isothiocyanate-conjugated annexin, and staining was measured with a FACScalibur. Numbers represent the percentages of unstained cells (left) and those stained with annexin V (right).
CFSA of CTLL-2 cells expressing Tax.
CFSA is a transformation assay for various types of cells including lymphocytes (31). We examined CFSA activity of CTLL-2 cells that expressed Tax protein, and the results are summarized in Table 1. CTLL-2 and CTLL-2/M22 cells produced only a few colonies in the absence of IL-2, but all three CTLL-2/WT cells reproducibly formed colonies (0.43 to 16.4%). On the other hand, only two of three CTLL-2/703 clones in the first series of experiments and one in the second series of experiments showed CFSA activity, and the colony numbers (0.17 to 0.27%) were smaller than for CTLL/WT (0.8 to 7.4%). Thus, CFSA activity of CTLL-2 cells expressing Tax703 was lower than for those expressing TaxWT, although both TaxWT and Tax703 induced IL-2-independent proliferation of CTLL-2 cells.
TABLE 1.
CFSA activity of CTLL-2 cells expressing Tax protein
| CTLL-2 strain | CFSA (%)a in:
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| Vec | 0 | 0 |
| M22-1 | 0 | 0 |
| M22-3 | 0 | 0 |
| M22-5 | 0 | 0 |
| WT-5 | 2.4 | 2.0 |
| WT-7 | 0.8 | 1.1 |
| WT-14 | 6.7 | 7.4 |
| 703-2 | 0.17 | 0.15 |
| 703-3 | 0.27 | 0 |
| 703-7 | 0 | 0 |
Percentage of colony numbers in 103 inoculated cells.
Cell growth of HTLV-1-transformed T-cell lines in the absence of IL-2.
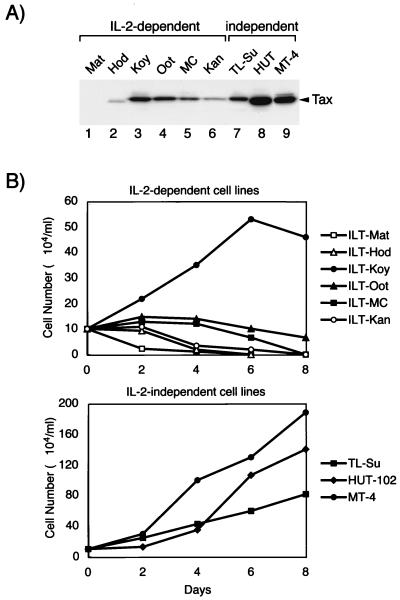
Two types of T-cell lines are transformed by HTLV-1; one is IL-2 dependent, and the other is IL-2 independent. Since Tax induced IL-2-independent proliferation of CTLL-2 cells, we next examined the involvement of Tax in IL-2-independent transformation of T cells by HTLV-1. Six IL-2-dependent and three IL-2-independent cell lines were examined for their expression of Tax as well as for cell growth characteristics in the absence of IL-2. ILT-Koy cells expressed Tax most abundantly among the six IL-2-dependent cell lines and proliferated even in the absence of IL-2 for 6 days, but their number started to decrease on day 8 (Fig. 6) and most died after 3 to 6 weeks (data not shown). Tax could not be detected in ILT-Mat cells by Western blot analysis, and only low levels of expression were detected by Northern blot analysis (data not shown). After deprivation of IL-2, ILT-Mat was the quickest to die among the six IL-2-dependent cell lines. The other four cell lines (ILT-Hod, ILT-Kan2, ILT-Oot, and ILT-MC), which expressed detectable levels of Tax but lower levels than did ILT-Koy, did not grow in the absence of IL-2, although they survived longer than ILT-Mat. Thus, the duration of survival of these cell lines in the absence of IL-2 correlated with the level of expression of Tax in these cells. Three IL-2-independent cell lines expressed relatively high levels of Tax. However, in two IL-2-dependent cell lines (ILT-Koy and ILT-Oot), the level of expression of Tax was equivalent to that in an IL-2-independent cell line (TL-Su), suggesting that even a sufficient level of Tax could not induce IL-2-independent transformation of T-cells by HTLV-1. All three IL-2-independent cell lines showed persistent growth in the absence of IL-2 (Fig. 6B). TL-Su expressed Tax less abundantly and proliferated more slowly than the other two cell lines. Thus, the growth rate of these three IL-2-independent cell lines in the absence of IL-2 correlated with the amount of Tax being expressed by these cells.
FIG. 6.
Tax expression is higher in IL-2-independent T-cell lines than in IL-2-dependent cells. (a) Expression of Tax proteins in HTLV-1-transformed T-cell lines. Cell lysate was prepared from six IL-2-dependent and three IL-2-independent virus-transformed cell lines. The expression of Tax in these cell lines was measured by Western blot analysis as described in Materials and Methods. (b) Cell growth analysis of HTLV-1-transformed T-cell lines in the absence of IL-2. The indicated cell lines were washed twice with PBS and then cultured in the absence of IL-2 for 8 days. Viable-cell numbers were counted by trypan blue staining. Data are representative of three independent reproducible experiments.
DISCUSSION
The major finding of the present study was that Tax, in addition to transforming normal T cells in an IL-2-dependent manner, stimulated the cell growth machinery of T-cell lines in the absence of IL-2. Tax converted the cell growth characteristics of the mouse T-cell line from IL-2 dependent to IL-2 independent (Fig. 1). The duration of cell survival and cell growth of HTLV-1-infected T-cell lines in the absence of IL-2 correlated with the level of expression of Tax in these cells (Fig. 6). Our results suggest that Tax plays a critical role in IL-2-independent transformation of T cells by HTLV-1.
Tax enhanced continuous IL-2-independent proliferation of CTLL-2 cells, but it failed to do so in HTLV-1-transformed T-cell lines (Fig. 6). In this regard, Miyazaki et al. (25) have shown that Tax does not modify the cell growth property of a pro-B-cell line from being IL-3 dependent to IL-3 independent and that persistent IL-3-independent growth requires interaction between Tax and either Myc or Lck oncoproteins. Hence, it is possible that CTLL-2 and IL-2-independent HTLV-1-transformed T-cell lines also activate a cellular gene(s) that interacts with Tax to induce IL-2-independent proliferation of T cells.
Several investigators have attempted to establish T-cell lines expressing a large amount of Tax and failed to do so or selected ones expressing a small amount of Tax. The difficulty may be due to the ability of Tax to induce apoptosis of T cells (7). On the other hand, the CTLL-2/Tax cells used in the present study expressed a large amount of Tax, the extent of which was equivalent to that of HTLV-1-transformed T-cell lines (data not shown). Thus, CTLL-2 cells may be resistant to apoptosis induced by Tax.
At least two distinct activities are necessary for IL-2-independent proliferation of CTLL-2 cells; one is the inhibition of apoptosis induced by IL-2-deprivation (Fig. 5), and the other is promotion of the cell cycle (5, 24). The results of the present study suggested that inhibition of apoptosis of CTLL-2 cells by Tax is mediated by activation of NF-κB in the following manner (Fig. 3). Tax and Tax703, which are activators of NF-κB, inhibit apoptosis of CTLL-2 induced by lack of IL-2 and allow the cells to proliferate in an IL-2-independent manner (Fig. 3). Apoptosis of CTLL-2 induced by deprivation of IL-2 is associated with a reduction in NF-κB activity (Fig. 4). On the other hand, Tax-induced enhancement of the cell cycle in CTLL-2 cells may not be mediated via activation of NF-κB but, rather, may be due to activation of CDKs. This argument is based on previous studies showing that CDKs are key regulators of the pathways that promote cell cycle and that Tax stimulates their activity by interacting with their inhibitor (INK4) in fibroblasts (19, 38) and in Tax-transformed human T-cell lines (30). It is noteworthy, however, that Tax has a variety of functions other than those discussed here. Thus, further analysis is required to determine the exact function involved in Tax-induced IL-2-independent proliferation of CTLL-2.
TaxM22 was expressed less strongly in CTLL-2 cells than were TaxWT and Tax703 (Fig. 3A). This may suggest that TaxM22, if expressed in sufficient amounts, may induce IL-2-independent proliferation of CTLL-2. However, TaxM22 induced a more rapid apoptosis of CTLL-2 cells in the absence of IL-2, indicating that TaxM22 cannot induce IL-2-independent proliferation of CTLL-2 even when expressed in sufficient amounts.
Our results suggested that the IL-2-independent growth-stimulatory activity of Tax in T cells is dependent on Tax more than is the IL-2-dependent transforming activity (Fig. 6). Among IL-2-dependent HTLV-1-infected T-cell lines, ILT-Mat, with a low level of Tax, rapidly died in the absence of IL-2 whereas other cell lines were resistant to apoptosis to an extent dependent on the level of expression of Tax (Fig. 6). The results of annexin staining experiments also showed that ILT-Mat cells were sensitive to apoptosis induced by lack of IL-2 whereas other cell lines expressing detectable amounts of Tax (as determined by Western blot analysis) were resistant to apoptosis (data not shown). Thus, inhibition of apoptosis by activation of Tax may represent a mechanism for cell survival of not only CTLL-2 cells but also human T-cell lines.
TaxM22 stimulated apoptosis of CTLL-2 (Fig. 5). There are at least two mechanisms to explain this phenomenon. TaxM22 inhibited the activity of NF-κB in CTLL-2 cells in the presence of IL-2 (Fig. 2 and 4), and reduced NF-κB activity was associated with apoptosis of CTLL-2 cells after deprivation of IL-2 (Fig. 4). Thus, TaxM22-induced inhibition of NF-κB may be responsible for the stimulation of apoptosis. Alternatively, the CRE pathway activated by TaxM22 may stimulate apoptosis of CTLL-2 cells (Fig. 2).
IL-2 induces the expression of antiapoptotic genes bcl-2 and bcl-X, and this induction is thought to play a role in the inhibition of apoptosis of IL-2-dependent T cells (24). Tax does not, however, induce the expression of bcl-2 and bcl-X genes in a pro-B-cell line (25), and HTLV-1-transformed IL-2-independent T-cell lines do not express the bcl-2 gene more strongly than IL-2-dependent ones do (3). Thus, Tax may inhibit apoptosis of CTLL-2 and HTLV-1-transformed T-cell lines in a manner different from IL-2.
Another major finding of the present study was the identification of a novel transformation-related activity of Tax (CFSA) in CTLL-2 cells and the finding that such activity was different from that of inducing IL-2-independent proliferation of CTLL-2 cells. Tax703, which exerts an IL-2-independent transformation activity equivalent to TaxWT (Fig. 3), showed 10-fold less CFSA activity than TaxWT did. Inhibition of apoptosis (also called anoikis) is postulated as a mechanism of CFSA of transformed cell lines that originated from fibroblasts (9). Inhibition of apoptosis may also play a role in CFSA of CTLL-2 cells, since apoptosis of CTLL-2/WT cells was less extensive than that of CTLL-2/703 cells (Fig. 5). It should, however, be noted that the high rate of apoptosis of CTLL-2/703 cells was not inhibited by IL-2 (data not shown). Thus, this apoptosis is likely to be distinct from that of CTLL-2 cells induced by deprivation of IL-2.
Our results obtained with Tax mutants suggested that the CFSA activity of Tax is mediated by activities that are diminished in Tax703 (Fig. 2 and Table 1). Thus, it seems that the CRE pathway is required for CFSA. In addition, Tax but not Tax703 activates the transcription via the CArG box enhancer, and the pathway is associated with the transformation of rat embryo fibroblasts by Tax together with the v-Ras oncogene (22). Thus, the CArG box pathway is also another possible candidate for mediating CFSA of CTLL-2/Tax cells.
The present studies identified two distinct transformation-related activities of Tax in CTLL-2 cells, and these two activities were different from each other and from IL-2-dependent transformation activity. Thus, analysis of the respective roles of these activities in T-cells will advance our understanding of the transformation of T cells by HTLV-1.
ACKNOWLEDGMENTS
Y.I. and T.T. contributed equally to this study.
We thank K. Matsumoto, W. C. Greene, K. Shimotohno, T. Akagi, and J. Fujisawa for providing plasmids TaxM22, Tax703, HTLV-1 LTR-luc, and κB-luc. We also thank F. G. Issa (Word-Medex, Sydney, Australia) for careful reading and editing of the manuscript.
This work was supported in part by the Ministry of Education, Science, Sports and Culture of Japan, CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation, and a grant provided by the Ichiro Kanehara Foundation.
REFERENCES
- 1.Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2078. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- 2.Akagi T, Shimotohno K. Proliferative response of Tax 1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J Virol. 1993;67:1211–1217. doi: 10.1128/jvi.67.3.1211-1217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai M, Kannagi M, Matsuoka M, Sato T, Yamamoto N, Fujii M. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T-cell lines derived from human T-cell leukemia virus type 1 associated myelopathy/tropical spastic paraparesis. AIDS Res Hum Retroviruses. 1998;14:261–267. doi: 10.1089/aid.1998.14.261. [DOI] [PubMed] [Google Scholar]
- 4.Arima N, Kuziel W A, Grdina T A, Greene W C. IL-2-induced signal transduction involves the activation of nuclear NF- kappa B expression. J Immunol. 1992;149:83–91. [PubMed] [Google Scholar]
- 5.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 6.Broome H E, Dargan C M, Bessent E F, Krajewski S, Reed J C. Apoptosis and Bcl-2 expression in cultured murine splenic T cells. Immunology. 1995;84:375–382. [PMC free article] [PubMed] [Google Scholar]
- 7.Chlichlia K, Busslinger M, Peter M E, Walczak H, Krammer P H, Schirrmacher V, Khazaie K. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene. 1997;14:2265–2272. doi: 10.1038/sj.onc.1201070. [DOI] [PubMed] [Google Scholar]
- 8.Cross S L, Feinberg M B, Wolf J B, Holbrook N J, Wong-Staal F, Leonard W J. Regulation of the human interleukin-2 receptor α chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 9.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–1029. [PubMed] [Google Scholar]
- 11.Fujii M, Sassone-Corsi P, Verma I M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 13.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type I. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeang K T, Widen S G, Semmes O T, Wilson S H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 17.Jin D Y, Spencer F, Jeang K T. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 18.Lindholm P F, Tamami M, Makowski J, Brady J N. Human T-cell lymphotropic virus type 1 Tax 1 activation of NF-kappa B: involvement of the protein kinase C pathway. J Virol. 1996;70:2525–2532. doi: 10.1128/jvi.70.4.2525-2532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low K G, Dorner L F, Fernando D B, Grossman J, Jeang K T, Comb M J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J Virol. 1997;71:1956–1962. doi: 10.1128/jvi.71.3.1956-1962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama M, Shibuya H, Harada H, Hatakeyama M, Seiki M, Fujita T, Inoue J, Yoshida M, Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-I-encoded p40x and T3/Ti complex triggering. Cell. 1987;48:343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Akashi K, Shibata H, Yutsudo M, Hakura A. Single amino acid substitution (58Pro→Ser) in HTLV-I tax results in loss of ras cooperative focus formation in rat embryo fibroblasts. Virology. 1994;200:813–815. doi: 10.1006/viro.1994.1248. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Shibata H, Fujisawa J I, Inoue H, Hakura A, Tsukahara T, Fujii M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura S, Ohtani K, Numata N, Niki M, Ohbo K, Ina Y, Gojobori T, Tanaka Y, Tozawa H, Nakamura M, Sugamura K. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol Cell Biol. 1991;11:1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki T, Liu Z J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Perlmutter R M, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki T, Liu Z J, Taniguchi T. Selective cooperation of HTLV-1-encoded p40tax-1 with cellular oncoproteins in the induction of hematopoietic cell proliferation. Oncogene. 1996;12:2403–2408. [PubMed] [Google Scholar]
- 26.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 27.Nagata K, Ohtani K, Nakamura M, Sugamura K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line. J Virol. 1989;68:3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuveut C, Low K G, Maldarelli F, Schmitt I, Majone F, Grassmann R, Jeang K T. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol Cell Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt I, Rosin O, Rohwer P, Gossen M, Grassmann R. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J Virol. 1998;72:633–640. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 33.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1995;9:2324. doi: 10.1101/gad.4.11.1875. . (Erratum.) [DOI] [PubMed] [Google Scholar]
- 34.Sugamura K, Fujii M, Kannagi M, Sakitani M, Takeuchi M, Hinuma Y. Cell surface phenotypes and expression of viral antigens of various human cell lines carrying human T-cell leukemia virus. Int J Cancer. 1984;34:221–228. doi: 10.1002/ijc.2910340213. [DOI] [PubMed] [Google Scholar]
- 35.Sugamura K, Hinuma Y. Human retroviruses: HTLV-I and HTLV-II. In: Levy J A, editor. The Retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 399–435. [Google Scholar]
- 36.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki T, Hirai H, Murakami T, Yoshida M. Tax protein of HTLV-1 destabilizes the complexes of NF-kappa B and I kappa B-alpha and induces nuclear translocation of NF-kappa B for transcriptional activation. Oncogene. 1995;10:1199–1207. [PubMed] [Google Scholar]
- 38.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka Y, Yoshida A, Tozawa H, Shida H, Nyunoya H, Shimotohno K. Production of a recombinant human T-cell leukemia virus type-I trans-activator (tax 1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax 1 antigen. Int J Cancer. 1991;48:623–630. doi: 10.1002/ijc.2910480423. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya H, Fujii M, Niki T, Tokuhara M, Matsui M, Seiki M. Human T-cell leukemia virus type 1 Tax activates transcription of the human fra-1 gene through multiple cis elements responsive to transmembrane signals. J Virol. 1993;67:7001–7007. doi: 10.1128/jvi.67.12.7001-7007.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe M, Muramatsu M, Hirai H, Suzuki T, Fujisawa J, Yoshida M, Arai K, Arai N. HTLV-I encoded Tax in association with NF-kappa B precursor p105 enhances nuclear localization of NF-kappa B p50 and p65 in transfected cells. Oncogene. 1993;8:2949–2958. [PubMed] [Google Scholar]
- 42.Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita I, Katamine S, Moriuchi R, Nakamura Y, Miyamoto T, Eguchi K, Nagataki S. Transactivation of the human interleukin-6 gene by human T-lymphotropic virus type 1 Tax protein. Blood. 1994;84:1573–1578. [PubMed] [Google Scholar]
- 44.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-1 oncoprotein tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]