Abstract
Bacillus thuringiensis Q1, isolated from the eutrophic waters of the Haihe River in Tianjin, possesses remarkable algae dissolving character. We determined the lytic effect of B. thuringiensis Q1 fermentation broth, and it proved to be pH- and temperature-stable. Then, we investigated the structure of the algicidal compound by high performance liquid chromatography, gas chromatography tandem quadrupole mass spectrometry and fourier transform infrared spectroscopy, and identified as purine-derived C12H15O5N5. To further understand B. thuringiensis Q1, we performed genome sequencing and analysis. The genome was 5341610 bp, with 35.31% GC content. Some elements involved in algicidal activity, such as quorum sensing pathway and ABC transporter were predicted. Our results reveal that B. thuringiensis Q1 can be used for biological control of harmful algal blooms.
Keywords: Bacillus thuringiensis Q1, Algicidal compound, Anabaena flos-Aquae, Genome
1. Introduction
With recent and rapid development of industrial and agricultural production, water eutrophication has become a serious problem, resulting in large-scale, frequent algal bloom outbreaks in coastal waters. Currently, more than half of China's lakes and reservoirs are in a eutrophication state. According to an investigation of 25 lakes distributed all over the country, more than 90% had a eutrophication trend. The dominant, and most serious algal species are cyanobacteria [1], that secrete a wide range of cyanotoxins. Some cyanobacteria, such as anabaena produce potent neurotoxins such as anatoxin-a, which cause wild mammals and humans to die within minutes [2]. Furthermore, more than 40% of cyanobacteria produce toxins, many of which are still unknown [3].
To prevent algal blooms, it is vital to find a safe and effective method to inhibit microalgae growth. Among current methods, biological control is relatively safe and economical. Some plant, animal and microorganism metabolites can inhibit microalgae. Using algicidal bacteria to control algal blooms has the advantages of safety, efficiency and economy. Therefore, it has been considered as potentially the ideal way to control algal blooms [4,5].
Several algicidal bacteria that are able to inhibit algal cells, have been isolated, such as Bacillus sp, Brevibacillus sp, Streptomyces and Pseudomonas sp [[6], [7], [8], [9]]. Algicidal bacteria can cleave algal cell membranes directly [10], and some natural substances extracted from algicidal bacteria can inhibit algal growth. Increasingly, more chemical compounds from algicidal bacteria have been found and some of them exhibit stability to protease, acid, base and heat [6]. Dissolution of algae is achieved through the action of various compounds, and algicidal bacteria may adapt their action on microalgae cells by releasing a combination of compounds. Active compounds, such as pyoluterorins, are highly toxic against cyanobacteria, but less toxic to eukaryotic microalgae [[7], [11]]. This new finding suggests that algae dissolving substances exhibit species-specificity.
It has been reported that lasso peptides, derived from a bacterium, inhibit Microcystis aeruginosa growth by destroying the thylakoid membranes and downregulating the expression of photosynthetic genes [12]. Secreted compounds from Marinobacter sp. could cause lipid peroxidation of algal cells and destruction of the photosynthetic system [[13], [14]]. Ortho-tyrosine and urocanic identified from Bacillus sp could damage Heterosigma akashiw mitochondrial membranes [15]. However, only a few algicidal compound molecular structures have been elucidated.
We previously isolated an algicidal bacterium from eutrophic waters in the Haihe river of Tianjin, which was then identified as Bacillus thuringiensis (named B. thuringiensis Q1). B. thuringiensis Q1 could effectively dissolve M. aeruginosa and Anabaena flos-aquae by inhibiting chloroplast formation [16,17]. Here, we further investigate the algicidal effect of B. thuringiensis Q1, identify the algicidal compound, and sequence the strains genome.
2. Materials and methods
2.1. Microbes and media
The algicidal bacterium B. thuringiensis Q1, stored in the Bioengineering laboratory of Tianjin Tianshi College, was cultured in LB medium. A. flos-aquae FACHB-1255, purchased from the Wuhan Institute of aquatic biology, Chinese Academy of Sciences, was cultured in BG11 medium.
Seed culture: medium Beef extract 3.0 g/L, peptone 10.0 g/L, NaCl 5.0 g/L, pH 7.2–7.4.
Fermentation medium: soybean meal powder 1.0 g/L, yeast extract 1.5 g/L, peptone 0.5 g/L, CaCl2 0.25 g/L, K2HPO4 0.43 g/L, ZnSO4 0.2 g/L, MgSO4 0.2 g/L, MnSO4 0.25 g/L, FeSO4 0.25 g/L, pH 8.0.
BG11 medium:C₆H₈O₇ 0.3 g/L,C₆H₈FeNO₇ 0.3 g/L,C₂H₈N₂ 0.05 g/L,NaNO330 g/L,K2HPO4 0.78 g/L,MgSO4·7H2O 1.5 g/L, CaCl2·2H2O 1.9 g/L,Na2CO3 2 g/L,H3BO3 2.86 g/L, MnCl2·4H2O 1.81 g/L, ZnSO4·7H2O 0.222 g/L,Na2MnO4·2H2O 0.391 g/L ,Co(NO3)2·6H2O 0.049 g/L.
2.2. Environmental stability of extracellular algae-lysing substances
The B. thuringiensis Q1 fermentation broth was filtered to remove the cells. The filtrates were treated with different pH buffers (pH = 4, 6, 7, 8, 10, 12) or freeze-thaw cycles (1–5 times). Then the treated filtrates were added (10% V/V) to the A. aquatic grown to the logarithmic-phase in BG11 medium and the A. aquatic cell cultivation was continued for another 9-days (25 °C, 2000lux white 12 h:12 h). Chlorophyll a content was measured as described [18], and OD560 values were determined to measure growth.
2.3. Purification of the algicidal activity Q1
B. thuringiensis Q1 was cultured in seed medium at 37 °C with shaking at 180 rpm for 12 h. A10% bacterial solution was transferred into 100 mL fermentation medium, and cultured at 37°Cwith 180 rpm for 20 h. The fermentation broth was centrifuged at 4 °C and 3000×g for 15min to remove B. thuringiensis Q1 cells. The supernatant was concentrated by the rotary evaporation method and then filtered through a 0.2 μm filter membrane. The filtrate was separated and purified by macroporous resin D101. 50 mL of macroporous resin was added into a suitable chromatographic column and washed repeatedly with distilled water to loosen and expand the macroporous resin.
Compounds were eluted by ethanol solution (ethanol: water = 7:3, V/V), with a flow rate of 0.5 mL/min. Each 50-drop was collected into a 15-mL tube, and a total of 30 tubes were collected. The algae-lysing activity of each collected substance was independently determined. The algae-lysing activity of Q1was added to the growing A. aquatica culture in the logarithmic-phase, which was incubated at 25 °C under illumination (2000lux white 12 h:12 h).
2.4. Isolation of the algicidal compound
Separation of alginolytic active elution components (No. 1–7) selected in 2.3 was achieved by Reverse high pressure preparative liquid chromatographic separation. Full wavelength scanning gave absorption maxima between 245 nm and 267.5 nm, and the final selected area was subjected to liquid chromatography at 254 nm. Separation conditions of reverse high performance liquid chromatography: chromatographic column: SymmetryPrep C18 (19 × 150 mm, 7 μm). 95%water and 5%acetonitrile were used as the mobile phase, the flow rate, 0.5 mL/min; a wavelength of 254 nm. The sample was filtered through a 0.22 μm microporous membrane, and the injection volume was 25 μL.
2.5. Structural identification of the algicidal compound
We collected the siderophore corresponding to the peak, dehydrated it via lyophilization and analyzed it using ESI-MS(Waters 3100 Mass Detector). ESI-MS working conditions: 120 °C source temperature, 450 °C desolvation temperature, capillary voltage 3.0 KV, desolvation gas flow: 850 L/Hr, 50–1500 mass range. We analyzed the ESI-MS peak and calculated the molecular weight of the algicidal compound. Functional groups of the algicidal compound were characterized by fourier transform infrared spectroscopy (FT-IR, PERKIN-ELMER Spectrum 65) [19].
2.6. Complete genome sequencing, assembly and annotation
Genomic DNA was extracted with a DNA extraction kit, using a Nandrop2000 Spectrophotometer (Thermo Scientific, USA) to test extracted DNA purity and quality (UV A260/280). Qubit and agarose gel electrophoresis was used to test sample quality. The database was built and Gene Denovo Biotechnology Co., Ltd. was commissioned to conduct the whole genome sequencing project. The next-generation Illumina Miseq sequencing platform as well as third-generation PacBio RSII sequencing platform) were used to scan and sequenced the Q1 genome. Raw data from the illumine platform were filtered using FASTP (version 0.20.0) [20]. After filtering, resulted clean reads were used to correct the genome sequences to improve the assembly quality and determine the final genome sequences using Pilon (version 1.23) software [21].
Prediction of tRNA genes, rRNA genes and other non-coding RNAs genes were achieved by tRNAcsan-SE V13.1 software, RNAmmer software and Rfamdatabase, respectively. Protein sequences of the predicted genes were compared with those of Glimmer 3.02. Using the prediction genomic information, a genome circle map was drawn. The predicted gene sequence was compared with Cluster of Orthologous Groups of proteins (COG), Kyoto Encyclopedia of Genes and Genomes (KEGG), Swiss-Prot, non-redundant protein sequence (Nr) and other functional database by BLAST. Additional annotation was carried out base on the Pathogen Host Interactions (PHI), and Virulence Factors of Pathogenic Bacteria (VFDB) databases.
3. Results
3.1. The B. thuringiensis Q1 lytic effect on algal cell morphology
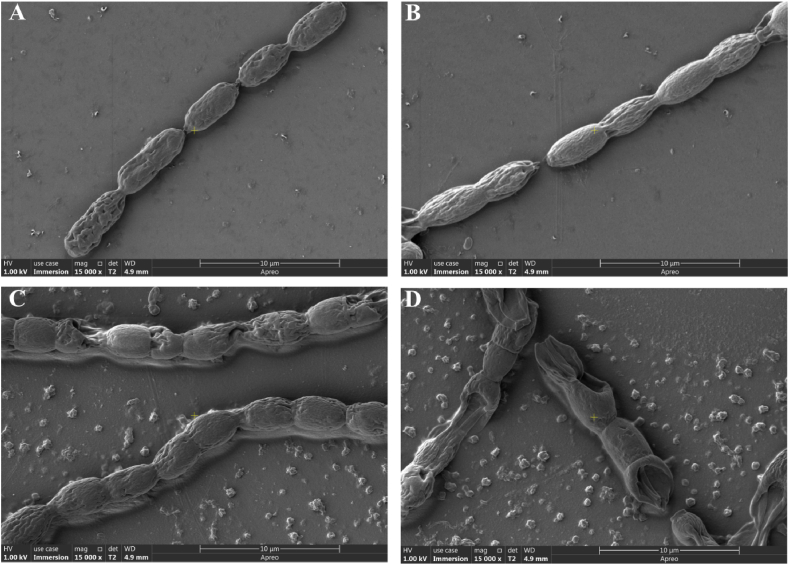
General features of B. thuringiensis Q1 previously included an algicidal effect on A. flos-aquae [16]. To evaluate the lytic effect of B. thuringiensis Q1, its filter fermentation broth was added to the logarithmic phase of the A. flos-aquae cells. A. flos-aquae cells not treated with fermentation broth exhibited normal morphology (Fig. 1A). In comparison, the algal filaments elongated and began to crack after 8 h treatment (Fig. 1B). Alga cells expanded, and were lysed after 32 h (Fig. 1C), resulting in the leaking of intracellular contents. After 48 h, all the cells were completely disrupted (Fig. 1D). These results indicate that active compounds in the fermentation broth secreted by B. thuringiensis Q1 killed A. flos-aquae cells.
Fig. 1.
Cellular morphology of algal cells exposed to B. thuringiensis Q1 fermentation broth. A: A. flos-aquae cells without treatment; B–D: A. flos-aquae cells treated after 8 h, 36 h and 48 h.
3.2. Stability of extracellular alginolytic compounds
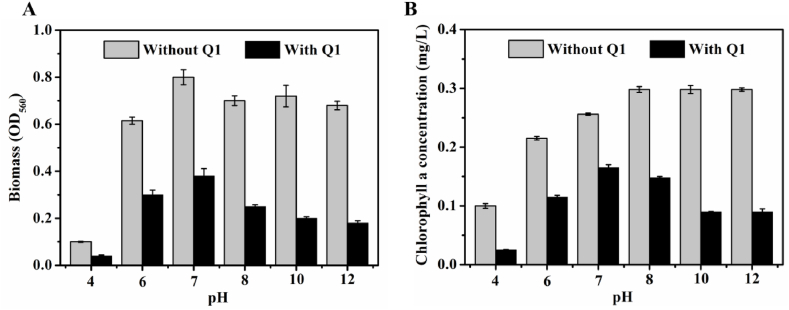
To further validate algicidal compound stability, filtered fermentation broths of B. thuringiensis Q1 treated with different pH (pH = 4, pH = 6, pH = 7, pH = 8, pH = 10, pH = 12) were added to A. flos-aquae at the exponential period. All the pH-treated broths inhibited A. flos-aquae cell growth and algae-lysing activities were observed even when pH was as low as 4 and as high 12, indicating that the algicidal compounds were pH-stable and were not enzymes or proteins (Fig. 2A). In our previous study, we found that the green A. flos-aquae become yellow after B. thuringiensis Q1 fermentation broth treatment 11], indicating that the algicidal compounds inhibited chlorophyll formation. Therefore, chlorophyll a concentrations of treated A. flos-aquae cells were monitored, and were consistent with biomass changes (Fig. 2B).
Fig. 2.
Effects of pH-treated alginolytic compounds biomass (A) and chlorophyll a concentration (B) of A. flos-aquae cells.
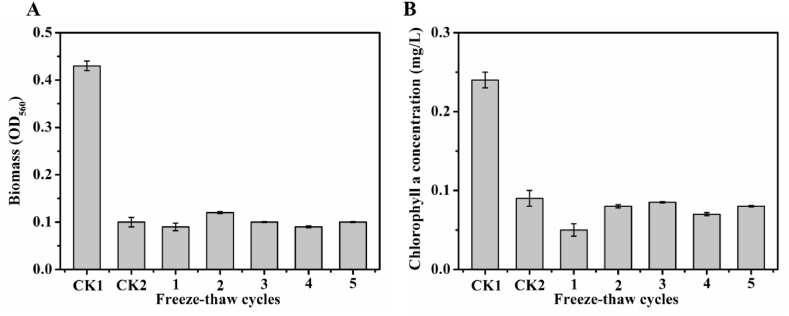
In our previous study, we identified the heat-stability of alginolytic compounds [16]. To investigate their low-temperature-stability, we applied repeated freeze-thaw treatment (once, twice, three, four, and five times) and then investigated algae-lysing activities on A. flos-aquae cells. A. flos-aquae cell growth was significantly affected by B. thuringiensis Q1 fermentation broth (Fig. 3A). Moreover, low-temperature treatment of the broth did not affect its algae-lysing activity, and algae inhibition rate was up to 78.80% even when the broth was freeze-thaw treated five times. Similarly, impaired chlorophyll a formation was observed in the A. flos-aquae cells with addition of the broths(Fig. 3B). These results indicate that the alginolytic compounds were low-temperature-stable.
Fig. 3.
Effects of low-temperature-treated alginolytic compounds biomass (A) and chlorophyll a concentration (B) on A. flos-aquae cells. CK1: A. flos-aquae cells without B. thuringiensis Q1 fermentation broth addition; CK2: A. flos-aquae cells with the addition of non-freeze-thaw-treated broth; 1–5: A. flos-aquae cells with the addition of freeze-thaw-treated (1–5 times) broth.
3.3. Purification of alginolytic compounds
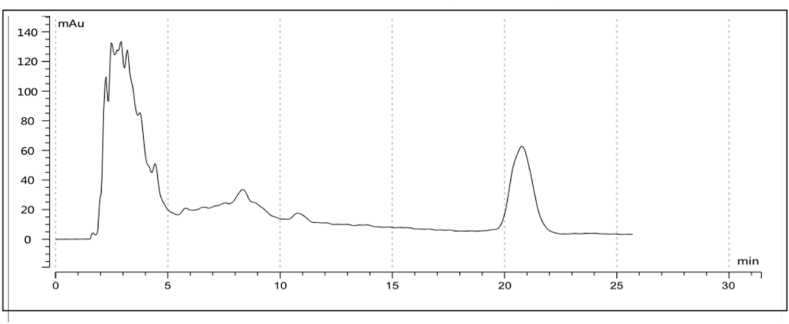
The filtrated B. thuringiensis Q1 fermentation broth was adsorbed with macroporous resin D101, eluted and collected in 30 tubes, and then dried in an oven at 80 °C. After drying, it was dissolved in 4 mL sterile water for activity tests. The activity test showed that chlorophyll a content in tube-5 decreased significantly (Fig. 4). Algicidal compounds were then further separated by reverse high performance liquid chromatography (HPLC) and a single absorption peak (No. 1) appeared at approximately 20-min (Fig. 5).
Fig. 4.
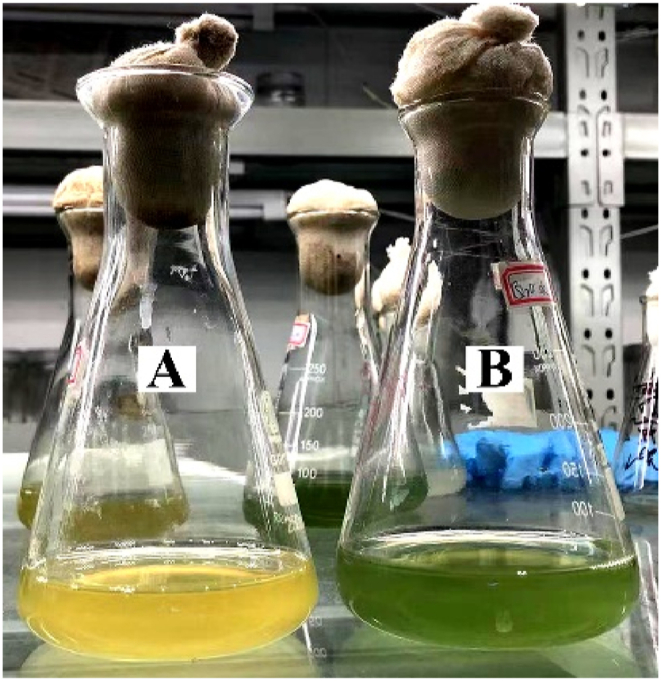
Algae-lysing activity of crude exact by macroporous resin D101. A: addition of crude exact; B: non-addition of crude exact.
Fig. 5.
HPLC elution curve of compound in tube-5.
3.4. Separation of algicidal compound structure
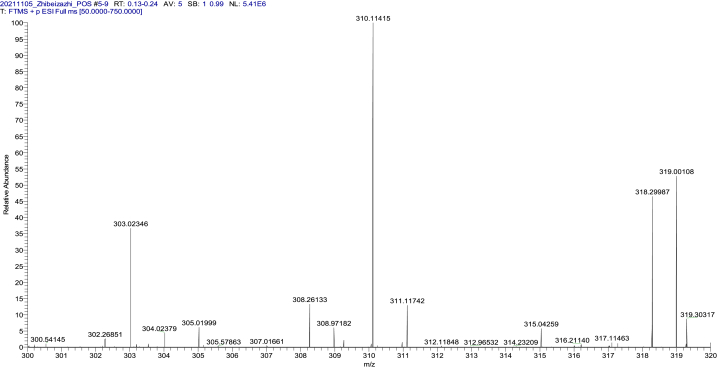
We collected the No. 1 chromatographic peak elution, freeze dried and concentrated it, then further identified it by mass spectrometry analysis. A main peak of 310.25 mass-to-charge ratio with a positive charge ([M+H])+ was maintained, indicating that the algicidal compound is single and with a molecular weight of 309.0 Da (Fig. 6).
Fig. 6.
ESI-MS spectrum of algae-lysing substance.
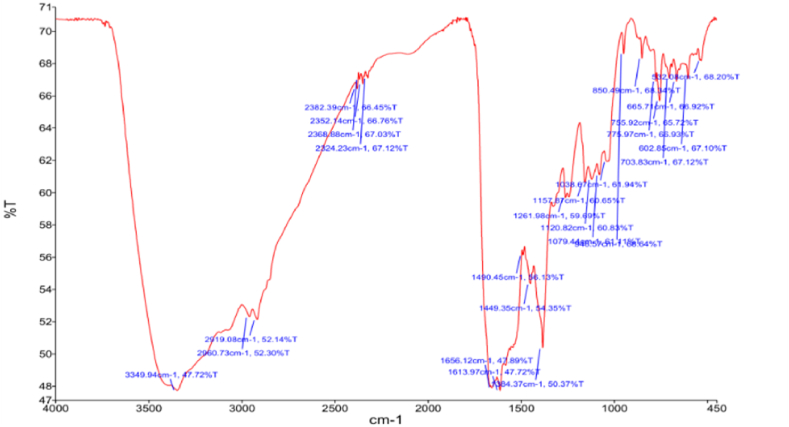
To further reveal the structure of the algicidal compound, fourier transform infrared spectroscopy was employed. The substance spectrum shows an obvious characteristic band located at 2960 cm−1 (Fig. 7), which was attributed to the CH3 group. The compound displayed a strong band at 2919 cm−1 due to CH2 stretching vibration. Furthermore, a band at 1656 cm−1 was polyene; at 1613 cm−1, 1490 cm−1 and 1449 cm−1 were benzene nuclei; at 1384 cm−1 was CH3 bending vibration; at 1261 cm−1 was C–O absorption, or absorption of branched chains with different C; 1157 cm−1, and at 1120 cm−1,1079 cm−1 and 1038 cm−1 were OH stretching vibrations, respectively (Table 1). Comparing the above data with reported literature values, we identified the compound as C12H15O5N5 (2-[(2-Aceramido-6-oxo-6,9-dihydro-1H-purin-9-yl)methoxy]ethyl acetate) (Fig. 8), indicating that it was synthesized from purine.
Fig. 7.
The FT-IR spectra of algicidal compound.
Table 1.
Analysis of band position of infrared spectrum.
| Infrared absorption band/cm−1 | functional group |
|---|---|
| 2960 | -CH3 stretching vibration |
| 2919 | -CH2 stretching vibration |
| 1656 | polyene |
| 1613,1490,1449 | benzene nucleus |
| 1384 | -CH3 bending vibration |
| 1261 | C–O absorption, or absorption of branched chains with different C |
| 1157,1120,1079,1038 | -OH stretching vibration |
Fig. 8.
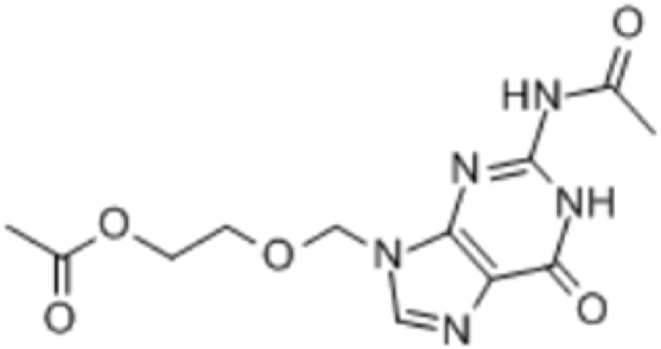
Chemical structure of algae-lysing substance.
3.5. Genomic characteristics of Q1
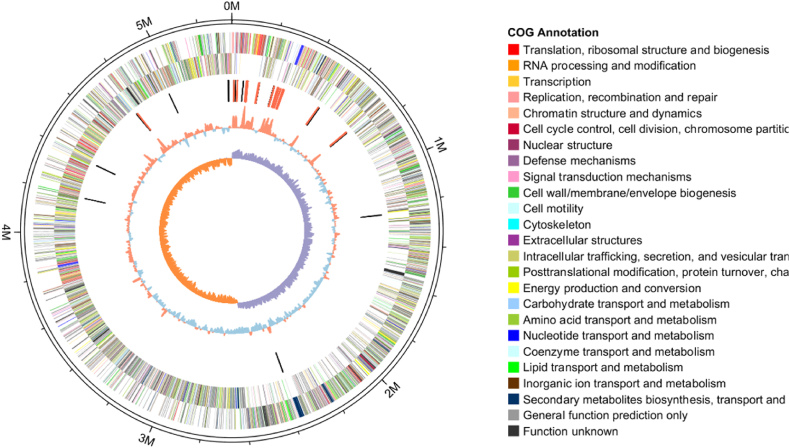
Whole genome sequencing and analysis revealed that B. thuringiensis Q1 is 53416110bp with 35.31% GC content (Fig. 9, Table 2). The genome sequence was deposited in the National Center for Biotechnology Information with the accession number CP090016-CP090018. We predicted the assembled genome and 5136 coding sequences (CDS) were obtained. Total CDS length was 4354326 bp, while 207 were RNAs. By predicting non-coding RNA, 42 rRNAs (5S + 16S + 23S), 108 tRNAs and 8 sRNAs were obtained (Table 2). The genome had 2 plasmids, 2 CRISPR structures and two prophages. Protein projections showed that a total of 362 proteins contained signal peptides, 141transmembrane proteins and 221 secretory proteins.
Fig. 9.
B. thuringiensis Q1 circular genome map. From the outside to the center: label of genome size; the second and the third circle represents CDSs on the forward strand and CDSs on the reverse strand. Different colors represent different COG functional classifications. The fifth circle is tRNA and rRNA in the genome. CDSs are depicted in different colors according to COG annotation. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Bacillus thuringiensis Q1 genome statistics.
| Category | number |
|---|---|
| Genome size | 5341610 |
| DNA G + C (%) | 35.31% |
| Total genes | 5136 |
| Total number of RNA genes | 207 |
| rRNA genes | 42 |
| 5SrRNA | 14 |
| 16SrRNA | 14 |
| 23SrRNA | 14 |
| number of tRNA | 108 |
| sRNA | 8 |
| Transposon PSI | 9 |
| CRISPR | 2 |
| Prophage | 2 |
Based on the result of KEGG analysis, the genome including 778 genes globe and overview maps, 243 genes carbohydrate metabolism, 150 genes membrane transport, 81 genes nucleotide metabolism. 56 gene clusters were found to encode for purine metabolism. Based on KEGG analysis results, the genome included 778 globe and overview map genes, 243 carbohydrate metabolism genes, 150 membrane transport genes, and 81 nucleotide metabolism genes. 56 gene clusters were found to encode purine metabolism.
4. Discussion
Bacteria lyse algae cells either directly by invading them or indirectly by excretion of extracellular compounds [22]. In a previous study, we found that B. thuringiensis Q1 fermentation broth could inhibit Microcystis aeruginosa and A. flos-aquae, but could not inhibit green algae [16]. Bacteria can selectively degrade the microalgae cell wall, which is related to its thickness [23]. Extracellular substances secreted by bacteria including chemicals, lipids, peptides and enzymes can kill microalgae. It was reported that Bacillus sp. could damage M. aeruginosa cell morphology and destroy its DNA by releasing algae lysate and bacillamide [[24], [25], [26]]. In the present study, the B. thuringiensis Q1 extracellular metabolite showed strong algicidal activity against A. flos-aquae by breaking the cell structure. The algicidal compound was identified as novel, purine-derived, (2-[(2-Aceramido-6-oxo-6,9-dihydro-1H-purin-9-yl) methoxy] ethyl acetate, and was shown to be pH- and low-temperature- stable.
The complete B. thuringiensis Q1 genome was sequenced and registered. KEGG functional annotation showed that there were multiple metabolic pathways related to purine. The quorum sensing pathway mediates inter-species communication by producing small extracellular signal molecules, which could regulate antialgal activity of the B. subtillis strain JA [27]. Bioinformatics analysis confirmed the presence of a quorum sensing pathway in B. thuringiensis Q1. We also found that there was biosynthesis of many other secondary metabolites, such as monobactam, streptomycin, penicillin, cephalosporin, carbapenem and prodigiosin. ABC transporter was a kind of multidrug efflux system. To understand the adaptability of B. thuringiensis Q1 to the marine environment, gene features were analyzed. The data revealed 115 genes to be ABC transporters, which function in molecule and ion transport of peptide, multidrug, metal ion, ribose, amino acid, molybdate, nitrate, phosphate and antimicrobial. ABC transporters are important in environmental adaptation and bacterial survival, and may be involved in the release of antialgal compounds. Prodigiosin was a compound with algicidal activity in Hahella sp [[28], [29]]. According to the CAZy data B. thuringiensis Q1 contained one polysaccharide lyase and 155 glycoside hydrolases. Some bacterial extracellular enzymes were identified as involved in algicidal activity, such as glycoside hydrolases and polysaccharide lyases, which can be induced by the presence of microalgae [[10], [30]], while disrupting the cell wall.
Author contribution statement
Jingcheng Qiao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chenglin Zhang: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
The data that has been used is confidential.
Additional information
No additional information is available for this paper.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgements
This study was supported by Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, Tianjin Key Laboratory of Industrial Microbiology. Tianjin University of Science and Technology, Tianjin, (2020KF010) China.
References
- 1.Tian Yan, Xiao-Dong, Tan Zhi-jun, et al. Toxic effects, mechanisms, and ecological impacts of harmful algal blooms in China. Harmful Algae. 2022;(111) doi: 10.1016/j.hal.2022.102148. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y., Zhang Z., Wang M., Shi X., Zhou Y., Zhou Y., Kong Y. Inactivation of harmful Anabaena flos-aquae by ultrasound irradiation: cell disruption mechanism and enhanced coagulation. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105254. [DOI] [PubMed] [Google Scholar]
- 3.Sivonen K., Jones G. vol. 1. 1999. Cyanobacterial toxins. Toxin cyanobacteria in water:a guide to their public health consequences, Monitoring and Management; pp. 43–112. [Google Scholar]
- 4.Fitagerald G.P. Bactericidal and algicidal properties of some algicides for swimming pools. J. Appl. Microbiol. 1959;7:205–211. doi: 10.1128/am.7.4.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal Mili, Yesankar Prena J., Dwivedi Ajay, et al. Biotic control of harmful algal blooms (HABs): a brief review. J. Environ. Manag. 2020;(268) doi: 10.1016/j.jenvman.2020.110687. [DOI] [PubMed] [Google Scholar]
- 6.Liu Fen, Zhu Shun ni, Qin Lei, et al. Isolation, Identification of algicidal bacteria and contrastive study on algicidal properties against Microcystis aeruginosa. Biochem. Eng. J. 2022;(185) doi: 10.1016/j.bej.2022.108525. [DOI] [Google Scholar]
- 7.Chen Shuhe, Haga Miyu, Imai Ichiro, et al. Function of the algicidal bacterium Pseudomonas sp. Go58 isolated from the biofilm on a water plant, and its active compounds, pyoluteorins. Sci. Total Environ. 2023;(872) doi: 10.1016/j.scitotenv.2023.162088. [DOI] [PubMed] [Google Scholar]
- 8.Li Hong-Wei, Xiang Yi-Zhou, Zhang Man, et al. A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: isolation, purification, identification, antibacterial and antibiofilm activity. Food Sci. Technol. 2021;140 doi: 10.1016/j.lwt.2020.110826. [DOI] [Google Scholar]
- 9.Su Jun feng, Shao Si cheng, Huang Ting lin, et al. Algicidal and denitrification characterization of Acinetobacter sp J25 against Microcystis aeruginosa and microbial community in eutrophic landscape water. Mar. Pollut. Bull. 2016;107:233–239. doi: 10.1016/j.marpolbul.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 10.Demuez M., Gonzalez-Fernandez C., Ballesteros M. Algicidal microorganisms and secreted algicides: new tools to induce microalgal cell disruption. Biotechnol. Adv. 2015;(33):1615–1625. doi: 10.1016/j.biotechadv.2015.08003. [DOI] [PubMed] [Google Scholar]
- 11.Inaba Nobuharu, Traner Vera L., Onishi Yuka, et al. Algicidal and growth-inhibiting bacteria associated with seagrass and macroalgae beds in Puget Sound, WA, USA. Harmful Algae. 2017;62:136–147. doi: 10.1016/j.hal.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Yan-Hua, Cai Zhong-Hua, Cheng Ke-Ke, et al. Naturally occurring lasso peptides as algicidal agents against Microcystis aeruginosa. J. Clean. Prod. 2002;(381) doi: 10.1016/j.jclepro.2022.135136. [DOI] [Google Scholar]
- 13.Du Wenjun, Ding Ning, Renjun W. Characterization of cell death in harmful Karnia mikimotoi under algicidal activity of Marinobacter sp. O-7. J. Sea Res. 2023;(191) [Google Scholar]
- 14.Yang Yufeng, Xiaojuan Hu, Zhang Jun, et al. Community level physiological study of algicidal bacteria in the phycospheres of Skeletonema costatum and Scrippsiella trochoidea. Harmful Algae. 2013;28:88–96. doi: 10.1016/j.hal.2013.05.015. [DOI] [Google Scholar]
- 15.Quan Honglin, Zhang Yuan, Yin Pinghe, Zhao Ling. Effects of two algicidal substances , ortho-tryrosine and urocanic acid, on the growth and physiology of Heterosoigma akashiwo. Environ. Pollut. 2021;(284) doi: 10.1016/j.envpol.2021.117004. [DOI] [PubMed] [Google Scholar]
- 16.Qiao Jing-cheng, Cheng-lin, Zhang, Yue-chao, Ma Analysis and identification of algicidal effect of algae-lysing bacteria Q1 from Haihe river. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2019;40(1 Jan):112–118. [Google Scholar]
- 17.Qiao Jing-cheng, Zhang Jian-hui, Wang Guo-wei, et al. Optimization of fermentation conditions of algae-lysing bacteria Q1 to Microcystis aeruginosa by using response surface methodology. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2020;41(3):50–55. [Google Scholar]
- 18.Peng-fei L.I., Sun Xin, Yang Li, et al. Optimization of extraction protocol of chlorophyII a from algae. CIESC J. 2019;70(9):3421–3429. doi: 10.11949/0438-1157.20190149. [DOI] [Google Scholar]
- 19.Ma Guizhen, Wu Shanjie, Fu Hongrun, Bao Zenghai, et al. Isolation and structure elucidation of antifungal metabolites from marine actinomycete BM-2. Chin. J. Biol. Control. 2014;30(3):393–401. [Google Scholar]
- 20.Chen S., Zhou Y., Chen Y., et al. bioRxiv; 2018. Fastp: An Ultra-fast All-In-One FASTQ Preprocessor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker B.J., Abeel T., Shea T., Priest M., Abouelliel A., et al. Pilon: an intergrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9(11) doi: 10.1371/journal.Pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Li Z., Su J., Tian Y., et al. Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol. Control. 2010;52:123–130. doi: 10.1016/j.biocontrol.2009.10.004. [DOI] [Google Scholar]
- 23.Lenneman E.M., Wang P., Barney B.M. Potential application of algicidal bacteria for improved lipid recovery with specific algae. FEMS Microbiol. Lett. 2014;354:102–110. doi: 10.1111/1574-6968.12436. [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Yang C., Chi Y., et al. Algicidal characterization and mechanism of Bacillus licheniformis Sp34 against Microcystis aeruginosa in dianchi lake. J. Basic Microbiol. 2019;59(11):1112–1124. doi: 10.1002/jobm.201900112. [DOI] [PubMed] [Google Scholar]
- 25.Jeong S.Y., Ishida K., Ito Y., Okada S., Murakami M., Bacillamide a. Novel Algicide from the marine bacterium, Bacillus sp.SY-1, against the harmful dinoflagellate. Cpchlodinium polykrikoides. Tetrahedron Lett. 2003;44(43):8005–8007. doi: 10.1002/chin.200406207. [DOI] [Google Scholar]
- 26.Manage P.M., Kawabata Z., Nakano S. Algicidal effect of the bacterium Alcaligenes denitrificans on Microcystis spp. Aquat. Microb. Ecol. 2000;22(2):111–117. doi: 10.3354/ame022111. [DOI] [Google Scholar]
- 27.Zhang Sheng-Jie, Du Xiao-Peng, Zhu Jian-Ming, et al. The complete genome sequence of the algicidal bacterium Bacillus subtilis strain JA and the use of quorum sensing to evaluate its antialgal ability. Biotechnol. Rep. 2020;25 doi: 10.1016/j.btre.2020.e00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia rong Feng, Hu Zhong, Wang Hui. Complete genome sequence of Hahella sp. KA22, a prodigiosin-producing algicidal bacterium. Mar. Genomics. 2019;47 doi: 10.1016/j.margen.2019.04.003. [DOI] [Google Scholar]
- 29.Liu Xiaobo, He Jie, Liao Chunli. Optimization of cryoprotectants to harvest high active cells of a Senedesmus-lysing Enterobacter sp. during long-term preservation. Algal Res. 2016;13:298–302. [Google Scholar]
- 30.Butcher Monica, Puiu Daniela, Romagnoli Mark, et al. Rapidly fatal infection with Bacillus cereus/thuringiensis: genome assembly of the responsible pathogen and consideration of possibly cons tributing toxins. Diagn. Microbiol. Infect. Dis. 2021;(101) doi: 10.1016/j.diagmicrobio.2021.115534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.