Abstract
This study examined the extent to which secure base script knowledge—reflected in the ability to generate narratives in which attachment-relevant events are encountered, a clear need for assistance is communicated, competent help is provided and accepted, and the problem is resolved—is associated with mothers’ electrophysiological, subjective, and observed emotional responses to an infant distress vocalization. While listening to an infant crying, mothers (N = 108, M age = 34 years) lower on secure base script knowledge exhibited smaller shifts in relative left (vs. right) frontal EEG activation from rest, reported smaller reductions in feelings of positive emotion from rest, and expressed greater levels of tension. Findings indicate that lower levels of secure base script knowledge are associated with an organization of emotional responding indicative of a less flexible and more emotionally restricted response to infant distress. Discussion focuses on the contribution of mothers’ attachment representations to their ability to effectively manage emotional responding to infant distress in a manner expected to support sensitive caregiving.
Infant crying is a potent vocalization that evolved to signal distress and elicit an immediate caregiving response from parents to help soothe the infant, making it an integral component of the parent-child relationship (Bowlby, 1982). Mounting evidence suggests that the quality of mothers’ caregiving in response to infant distress has important implications not only for the quality of the parent-child relationship but also for children’s social and emotional development. Specifically, infants whose mothers respond less sensitively to their distress are at heightened risk for attachment insecurity, less optimal affective and physiological regulation, impaired social competence, and behavioral problems (Conradt & Ablow, 2010; Leerkes, 2011; Leerkes, Blankson, & O’Brien, 2009; McElwain & Booth-LaForce, 2006). Parents’ emotional responding to infant signals is thought to organize caregiving behavior (Dix, 1991), and in recent years, there has been growing interest in how mothers’ emotional responding to infant crying might contribute to the quality of their caregiving. For example, studies have found that mothers who exhibit less optimal physiological and emotional responding to infant crying interact less sensitively with their infant (Killeen & Teti, 2012; Leerkes, 2010; Leerkes, Parade, & Gudmundson, 2011). These data provide insight into the specific challenges mothers face when confronted with infant distress, suggesting that some mothers might have difficulty responding to infant distress because they are unable to effectively manage their own physiological and emotional responses. Despite such evidence, the factors that contribute to mothers’ emotional responding to infant crying remain unclear. Drawing on attachment theory (Bowlby, 1982), the current study examined the role of mothers’ attachment representations in organizing their physiological, subjective, and observed emotional responding to infant crying.
Adult Attachment Representations and Infant Crying
By adulthood, early attachment-related experiences involving individuals’ ability to use parents as a secure base from which to explore the environment and as a haven of safety when distressed become internalized in the form of representations that guide responding within close interpersonal relationships (Bowlby, 1982). Such representations have been assessed by developmental psychologists with the Adult Attachment Interview (AAI; George, Kaplan, & Main, 1984–1996), a semistructured interview in which adults are asked to describe their relationships with their parents in childhood and to provide support for their descriptions in the form of autobiographical memories. The narratives produced within the context of the AAI are coded for coherence, or the extent to which they are internally consistent without being emotionally overwrought. The Attachment Script Assessment (ASA; Waters & Rodrigues-Doolabh, 2004) is another measure of adult attachment used by developmental psychologists in which adults are asked to provide stories using word prompts designed to elicit attachment-relevant narratives. Narratives produced within the context of the ASA are coded for secure base script knowledge, or the extent to which they are organized around a secure base script in which attachment-relevant events are encountered, a clear need for assistance is communicated, competent help is provided and accepted, and the problem is resolved (Waters & Waters, 2006). Common to both the AAI and the ASA is that narratives are implicitly evaluated by trained coders for adults’ access to a secure attachment-relevant script or an understanding that effective attachment relationships serve the complementary functions of the provision of a secure base of exploration and a safe haven in times of uncertainty (see Roisman, 2009). In addition to such conceptual convergence, coherence in the AAI and secure base script knowledge in the ASA are moderately to strongly correlated (rs = .50–.60; Waters & Waters, 2006), leading some scholars to argue that the ASA might serve as a cost-effective alternative to the AAI (Waters & Waters, 2006).
Individual differences in the extent to which adults have access to a well-defined secure base script are believed to reflect distinct emotional and cognitive organizations of prior secure base experiences that are carried forward into future interpersonal interactions and guide responding when confronted with attachment-relevant challenges (Bowlby, 1982; Waters & Waters, 2006). Accordingly, it might be expected that mothers’ attachment representations contribute to organizing their emotional responding to infant crying. More specifically, Bowlby (1982) argued that the attachment system is a biobehavioral stress regulatory system with a key feature of insecurity being the inability to flexibly manage intense emotion and stress without becoming overwhelmed. Thus, mothers’ attachment representations might influence their affective response patterns to infant crying, such that limited access to a well-defined secure base script might impair mothers’ ability to effectively manage their emotional response in a manner that might support sensitive responding.
Recent studies of the impact of adult attachment on nonparents’ responding to infant cry vocalizations provide preliminary support for this claim. Specifically, one study found that college students (none of whom were parents) lower on secure base script knowledge exhibited greater electrodermal reactivity from rest, an indicator of inhibition (Fowles, 1980, 1988; Gray, 1975), when listening to infant crying (Groh & Roisman, 2009), a pattern of findings that Ablow, Marks, Feldman, and Huffman (2013) replicated in a sample of expectant mothers. Similarly, Riem, Bakermans-Kranenburg, van IJzendoorn, Out, and Rombouts (2012) found that nonparents lower on coherence of mind as assessed by the AAI exhibited heightened amygdala activation (a region of the brain linked with threat detection and the experience of fear) to infant crying. Although based on samples of nonparents, such evidence suggests that adults’ representations of attachment-relevant experiences might play an important role in contributing to individual differences in mothers’ emotional responding to infant distress.
The current study extends this prior research by investigating the contribution of adults’ attachment representations to multilevel indicators of emotional responding to infant crying in a sample of mothers. Current perspectives on parenting emphasize that multiple indicators of parents’ emotional response (physiological responses, experienced emotion, and observed behavior) are best studied simultaneously (Teti & Cole, 2011), because aspects of emotion vary in the extent to which they are visible and under conscious control (Tsai & Levenson, 1997). Accordingly, in the current study we focus on electrophysiological, subjective, and observed indicators of mothers’ emotional responding to infant crying that, as described below, are important predictors of the quality of mothers’ caregiving behavior.
EEG alpha asymmetry
Over three decades of research on the association between EEG asymmetry and emotional responding has demonstrated that specific patterns of EEG asymmetry measured in the alpha frequency band over frontal and parietal brain regions are linked with emotional processing (Coan & Allen, 2004; Heller, 1993). Moreover, developmental scholars have recently extended such research to parent–child relationships and found that mothers’ emotional responding as reflected in EEG asymmetries when confronted with infant distress contributes to their sensitive-responsiveness observed during interactions with their infant (Killeen & Teti, 2012). These findings provide evidence not only for the organizing role of emotion in parenting but also for EEG asymmetry as a useful indicator of the emotional processes that contribute to parenting behavior.
Frontal EEG alpha asymmetry.
In a review of over 70 empirical investigations, Coan and Allen (2004) concluded that resting asymmetries in frontal EEG activity are implicated in traitlike dispositions to respond to emotional stimuli and that state-related asymmetries in EEG activation are implicated in change in emotional state. More specifically, investigations of EEG asymmetry at rest have addressed questions regarding whether an individual’s resting EEG asymmetry is associated with a traitlike tendency to respond to emotional events in expected ways. Such studies have provided evidence that individuals exhibiting greater relative left (vs. right) frontal EEG asymmetry at rest report more intense feelings of positive, approach-oriented emotion (i.e., happiness and amusement) to positively valenced stimuli, whereas individuals exhibiting greater relative right (vs. left) frontal EEG asymmetry at rest report more intense feelings of negative, withdrawal-oriented (i.e., sadness or fear) emotion to negatively valenced stimuli (Wheeler, Davidson, & Tomarken, 1993).
In contrast to investigations of resting EEG asymmetries, investigations of frontal EEG asymmetries in response to emotional stimuli examine context-specific patterns of EEG activation when confronted with emotional stimuli. Investigations employing a range of emotional stimuli, including emotional films (Davidson, Ekman, Saron, Senulis, & Friesen, 1990), (un)pleasant odors (Kline, Blackhart, Woodward, Williams, & Schwartz, 2000), and emotional questions (Ahern & Schwartz, 1985), have demonstrated that stimuli designed to evoke positive, approach-oriented emotion (i.e., joy or happiness) elicit a pattern of greater relative left frontal EEG activation and stimuli designed to evoke negative, withdrawal-oriented emotion (i.e., sadness, disgust, or fear) elicit a pattern of greater relative right frontal EEG activation.
Moreover, according to the capability model of individual differences in frontal EEG asymmetry (Coan, Allen, & McKnight, 2006), EEG activation in response to emotional stimuli reflects the degree to which individuals have the capacity to respond to or inhibit a response to emotional stimuli. Especially relevant to the current investigation, mothers have been found to vary in the extent to which they exhibit dynamic shifts from rest in frontal EEG asymmetry when viewing their distressed infant, which in turn had implications for not only their reported feelings of emotion but also how sensitively they interacted with their infant. Specifically, mothers who exhibited smaller (vs. larger) shifts from rest in frontal EEG asymmetry toward greater relative right activation in response to their infants’ distress reported feeling less concerned and worried when viewing a video recording of their distressed infant and were also less emotionally available when interacting with their infant (Killeen & Teti, 2012). Drawing on the capability model of EEG asymmetry, Killeen and Teti (2012) argued that these shifts in frontal EEG asymmetry reflect mothers’ capacity to empathically engage their infants’ emotional states, suggesting that the smaller shifts in EEG asymmetry exhibited by some mothers reflect a less empathic emotional response to their infants’ distress that ultimately undermines their ability to sensitively interact with their child. In addition, Killeen and Teti (2012) found that mothers’ EEG asymmetry at rest was not associated with their emotional availability, suggesting that state-related emotional tendencies in response to infant emotional displays (but not traitlike emotional tendencies) are especially important contributors to the quality of mothers’ caregiving.
In the current study, we extended this prior work by exploring the contribution of mothers’ attachment representations to their frontal EEG activation when confronted with infant crying. Because the extent to which individuals’ access to a secure base script might influence their ability to flexibly engage in attachment-relevant contexts, mothers lower on secure base script knowledge would be expected to have a limited capacity to empathically engage infant distress. As such, and consistent with evidence from Killeen and Teti (2012), we hypothesized that secure base script knowledge would be associated with shifts in frontal EEG asymmetry when confronted with infant crying (but not resting EEG asymmetry). Specifically, mothers lower on secure base script knowledge were expected to exhibit smaller shifts from rest in frontal EEG asymmetry toward greater relative right activation in response to infant crying, reflecting a restricted emotional response to infant distress.
Parietal EEG alpha asymmetry.
Whereas frontal EEG asymmetries reflect emotional valence, posterior EEG asymmetries are theorized to reflect emotional arousal (Heller, 1993). Converging evidence from behavioral, hemodynamic, psychophysiological, and lesion studies suggests that arousal is modulated by the right posterior brain region (see Heller, 1993; Heller, Nitschke, & Miller, 1998). Although considerably fewer studies have focused on posterior EEG asymmetry than frontal EEG asymmetry, investigations of parietal EEG asymmetry have provided evidence that both resting parietal EEG asymmetry and parietal EEG asymmetry in response to emotional stimuli are linked with emotional arousal. For example, parietal EEG asymmetry at rest has been linked with a clinical measure of posttraumatic stress disorder, with individuals suffering from greater levels of posttraumatic stress disorder arousal symptoms exhibiting greater relative right parietal EEG activity at rest (Metzger et al., 2004). Moreover, when listening to emotional narratives designed to elicit anxious arousal, anxious individuals exhibited an increase in right parietal EEG activation from rest (Heller, Nitschke, Etienne, & Miller, 1997), providing evidence that shifts in parietal EEG asymmetry in response to emotional stimuli reflect changes in emotional arousal.
Access to a secure base script is believed not only to contribute to individuals’ ability to flexibly engage attachment-relevant challenges but to do so without becoming over-aroused or overwhelmed. Thus, although we are not aware of any study examining links between parietal EEG asymmetry in response to infant emotional cues and sensitivity, given the conceptual convergence between secure base script knowledge and the emotional processes reflected in parietal EEG asymmetry, we also investigated whether variation in secure base script knowledge is associated with shifts in (but not resting) parietal EEG asymmetry when confronted with infant distress. Specifically, we hypothesized that, when listening to an infant crying, mothers lower on secure base script knowledge would exhibit greater shifts from rest in parietal EEG asymmetry, because such responding would be indicative of heightened emotional arousal to infant crying.
Reported feelings of emotion
In addition to electrophysiological indicators of emotional responding, we examined mothers’ reports of their positive and negative emotion. Studies of the association between mothers’ subjective emotional responding to infant emotional signals and the quality of parent–child relationships have primarily focused on mothers’ feelings of negative emotion in response to infant distress. Negative parental emotion has been argued to undermine sensitive caregiving because it increases parents’ focus on their own parenting goals and behavior, ultimately interfering with parents’ ability to sensitively respond to their child’s needs (Dix, 1991). Mothers who report feeling greater levels of negative emotion to infant crying respond less sensitively to their own infant’s distress (Leerkes, 2010), and mothers who report greater levels of anger, in particular, to infant crying have been found to engage in punitive and minimizing responding to their infant’s distress, which in turn predicted greater levels of infant avoidant attachment behavior (Leerkes et al., 2011).
In addition to parents’ negative emotion, Dix (1991) argued that parents’ positive emotion is integral to the functioning of parent–child relationships. Although positive emotion is generally considered to facilitate sensitive caregiving, both positive and negative emotions that are poorly matched to the specific situational demands of the caregiving context may undermine sensitive caregiving (Dix, 1991). Providing support for this claim, mothers who reported stronger feelings of positive emotion (e.g., amusement) in response to infant crying were found to report more negative attributions about why the infant was crying (e.g., spoiled or difficult temperament) and to report experiencing greater levels of emotional rejection by their parents in childhood (Leerkes & Siepak, 2006). We hypothesized that mothers lower on secure base script knowledge would be less likely to appropriately match shifts in the emotional context, resulting in greater increases in their feelings of negative emotion and smaller decreases in their feelings of positive emotion from rest in response to infant crying.
Observed facial expressions
Parents’ outward expression of emotion in response to infant signals provides a direct window for infants into the emotional state of the parents and influences the dynamics of parent–child interactions (Dix, 1991). Whereas mothers’ expression of positive emotion elicits positive emotion from infants, mothers’ negative and blunted affect elicits negative emotion and distress from infants (Cohn & Tronick, 1983; Lay, Waters, & Park, 1989; Zekoski, O’Hara, & Wills, 1987). Moreover, mothers’ expressed emotion within contexts designed to elicit infant distress (e.g., parent–child separation and reunion) has been found to organize their infants’ emotional expression. Specifically, mothers who displayed greater levels of tense facial features (e.g., compressed lips), indicative of emotional suppression, were found to have infants who, over the course of the first 2 years of life, came to express greater levels of tension within such contexts (Malatesta et al., 1989), suggesting that a mother’s expression of tension within distressing contexts has negative implications for her infant’s emotional development. Insecure–avoidant infants who are believed to minimize emotion within attachment-relevant contexts have been found to exhibit tense facial expressions indicative of emotional suppression during reunion episodes of the Strange Situation procedure (Ainsworth, Blehar, Waters, & Wall, 1978; Malatesta et al., 1989). Given such evidence, we examined the contribution of mothers’ attachment representations to their observed tension when listening to infant crying. Paralleling insecure infants, we hypothesized that mothers lower on secure base script knowledge would express more tension when listening to infant crying.
Method
The sample for this study consisted of 108 mothers drawn from two subsamples of mothers and their children. Participants in both subsamples were recruited to participate in two laboratory visits. This study focused on one of these laboratory visits in which only mothers participated. The mother laboratory visits were similar across subsamples. Participants were recruited via advertisements in local childcare centers and community centers and through advertisements in university newsletters to faculty and staff. Participants (a) were administered the ASA (Waters & Rodrigues-Doolabh, 2004) while being digitally recorded, (b) listened to an audiotaped recording of infant crying while being physiologically monitored and videorecorded, and (c) reported how strongly they felt a range of emotions at rest and after listening to the audiotaped recording. Participants were compensated $50 after completing the laboratory visit. A total of 142 participants were administered the protocol described above, but participants were excluded for the following, non-mutually exclusive reasons: having conditions implicated in altered brain structure and/or function (e.g., being left-handed, endorsing moderate to severe head injury, n = 18), and/or having impediments to either the collection or the reduction of the physiological data (e.g., video equipment malfunctioned, excessive muscle artifact, or reference electrode malfunctioned, n = 18), resulting in the final sample of 108 mothers. Excluded participants did not differ from those included in analyses on demographic variables, secure base script knowledge, electrophysiological responding, or subjective emotional responding. As would be expected given that excessive facial movements contribute to muscle artifact in EEG data, excluded participants exhibited greater levels of tension when listening to infant crying than participants included in analyses, t (139) = 3.20, p < .05. However, the direction and significance of findings involving the observed tension data reported below remained the same when all participants were included in analyses.
Mothers were on average 34.31 years of age (SD = 5.23, range = 22–46 years) and their children (56 female) were on average 38.49 months of age (SD = 13.76, range = 18–83 months). Mothers were heterogeneous in terms of ethnicity (63.9% European American, 15.7% Asian, 9.3% African American, 4.6% Hispanic, and 6.5% mixed/other). Median reported family income was $71,000 to $80,000 per year, and ranged from less than $10,000 to over $100,000 per year. Mothers’ education ranged from 1 (high school degree) to 4 (advanced degree) with a mean level of 3.44 (SD = 0.74).
Stimuli
The audio recording of infant crying used here is the same one described in Groh and Roisman (2009). As reported there, 20 research assistants listened to a large set of potential audio recordings of infant crying. The audio recording selected for this study was unanimously viewed as a prototypical expression of infant distress. The average fundamental frequency of the infant crying vocalization was 360.06 Hz (SD = 58.41). The amplitude at which participants listened to the infant vocalization was equated across participants by holding the volume of the audio recordings constant (peak amplitude of each cry averaged 89.51 decibels, SD = 1.85).
Apparatus
Visual.
Remotely controlled, high-resolution color video cameras recorded the participants’ facial expressions during the study. Cameras were partially hidden from participants’ view inside a media storage cabinet.
Electrophysiological.
A system consisting of two Pentium computers, Snapmaster Data Acquisition System (2000), and James Long, Inc., bioamplifiers (Caroga Lake, NY) was used to acquire continuous recordings of participants’ physiological responses at rest and while listening to the audio recording of infant crying. EEG was recorded with a custom-designed Electro-Cap International, Inc., 16-channel cap with tin electrodes spaced equidistantly, extending inferiorly to the F7/F8 ring of the International 10–20 System. For the purpose of this study, EEG was collected from 14 scalp sites (F3, F4, F7, F8, C3, C4, P3, P4, P7, P8, O1, O2, A1, and A2). However, because the specific focus of this study was on frontal and parietal EEG asymmetry, results are reported for two frontal scalp sites (F3 and F4) and two parietal scalp sites (P3 and P4). All EEG sites were referenced online to Cz, and Afz served as the ground. Electrodes were placed above and below the left eye (the lower electrode served as the reference) and near the outer canthus of each eye (the right electrode served as the reference) and recorded vertical and horizontal electrooculograms (EOG) for offline eye-movement artifact rejection. Electrode impedances were maintained below 10 kΩ for EEG sites and below 20 kΩ for EOG sites. Half-power amplifier bandpass was 0.3 to 100 Hz, and data were digitized at 1024 Hz.
Procedure
After completing online questionnaires including a demographic questionnaire, mothers were invited to a laboratory designed as a comfortable living room environment where they completed the ASA. Next, physiological sensors measuring brain activity (EEG) and eye movements (EOG) were attached to the participant. Other sensors were attached to participants, but relevant data are not reported here. Following a brief habituation period, participants were asked to rest completely, clearing their mind of all thoughts, feelings, and emotions, for a total of 4 min, during which a resting baseline was acquired. According to standard practice in acquiring baseline spectral EEG data (Allen, Coan, & Nazarian, 2004; Tomarken & Davidson, 1994; Towers & Allen, 2009), the 4-min baseline was broken into eight 30-s blocks in which the participants were asked to have their eyes either open and focused on a cross in front of them or closed (order counterbalanced). After the resting baseline, participants completed the Emotional Experience Questionnaire (EEQ) on which they recorded their current emotional state.
Next, participants were instructed that they would hear a recording of an infant delivered through headphones and that they should close their eyes, listen carefully to the infant, and try to think of how they would respond if the infant were their own child. Participants listened to an audio recording of an infant crying for 3 min. Participants also listened to an audio recording of an infant laughing, which was presented in a manner counterbalanced with the infant cry stimulus. Although participants were asked to remain as still as possible, many of them laughed when listening to the positively valenced vocalization, resulting in muscle artifact in the EEG data and a substantial number of participants (~20%) with missing EEG. Thus, data from this condition are not reported on here. (As in Groh and Roisman, 2009, no significant associations between secure base script knowledge and emotional responding emerged in the laughter condition.) After listening to the audio recording of an infant crying, participants described their emotional state while they listened to the recording using the EEQ.
Measures
ASA.
The narrative-based ASA is a word-prompt method used to assess participants’ awareness of and access to a secure base script (Waters & Rodrigues-Doolabh, 2004). Participants were given a card with the title of each story and a list of 12 words (subdivided into three columns). They were told that by scanning down the columns, they would be provided with a general outline for what the story is supposed to be about. They were asked to develop the best possible story that they could tell, that the story should be about a page in length if it were written down, and to include as much detail and information as possible. Participants were informed that they did not have to include all the words, that they could change the order of the words or change the words themselves. The narratives were recorded and later transcribed verbatim for coding. Participants were asked to tell six stories (order counterbalanced), three of which concerned children’s relationships (Baby’s Morning, Doctor’s Office, and Trip to Park) and three of which concerned adult relationships (Jane and Bob’s Camping Trip, The Accident, and An Afternoon Shopping). The two adult stories (Jane and Bob’s Camping Trip and The Accident) and two child stories (Baby’s Morning and Doctor’s Office) designed to tap secure base script knowledge were coded. Average narrative length measured in number of words was comparable to other studies (M = 297.25, SD = 154.52; e.g., Vaughn, Verissimo, et al., 2006).
Narratives were coded by the first two authors on a 7-point scale for the extent to which they were organized around a secure base script using the method developed by Waters and Rodrigues-Doolabh (2004). A secure base script is one in which there is a bid for help, the bid is recognized and help is offered, the help is useful in overcoming the problem, and the situation ultimately returns to normal. Narratives that receive the highest score (7) clearly show this structure. Conversely, narratives that receive the lowest score (1) lack the secure base structure and can include bizarre details (e.g., Sue dies in The Accident, and Mike is overwhelmed by sadness). Scores of secure base script knowledge (M = 3.95, SD = 1.15) were similar to those found in previous studies (e.g., Groh & Roisman, 2009; Vaughn, Waters, et al., 2006).
Secure base script knowledge has been operationalized across studies by taking the mean of the scores for the four stories, and as in prior research, secure base script knowledge was moderately to strongly correlated across stories in this data set (rs = .54–.64). Accordingly, a composite score was created by averaging scores across narratives, and this composite score was used in all analyses (α = 0.85). Two independent coders coded the narratives, and interrater reliability for the composite score was calculated on 30% of the narratives on which the coders overlapped. Interrater reliability was high (intraclass correlation coefficient = 0.90). Note that both coders have demonstrated reliability on this measure with the fourth author of this paper, who has published data based on the ASA (e.g., Bost et al., 2006). Secure base script knowledge was standardized for all analyses.
Subjective emotions.
The EEQ assessed how strongly participants felt 25 different emotions at rest and after listening to infant crying on a 9-point Likert scale (0 = no emotion, 8 = the most emotion you have felt in your life). A principal components analysis with varimax rotation was conducted on change from rest to the crying condition items. Results revealed that these emotion terms comprised two reliable factors when listening to infant crying: change in positive emotion (contentment, happiness, pride, relief, and satisfaction) and change in negative emotion (anger, calm [reversed scored], pain, sadness, shame, and tension). The Cronbach αs for the aggregates were 0.77 and 0.71, respectively. All changes in subjective emotion variables were skewed. To correct for this, the skewed variables were natural logarithm transformed. All change in subjective emotion variables were then standardized, and these standardized values were used for all analyses.
Observed behavior.
Participants’ facial expressions while listening to infant crying were coded offline by two independent coders on scales reflecting tension (i.e., strong and prolonged tense facial features [e.g., furrow brows and compressed lips]), positive affect (i.e., relaxed facial features), and emotion regulation (i.e., maintenance of relaxed facial features and, if tension displayed, rapid return to relaxed facial features). Tension, positive affect, and emotion regulation were coded on 5-point scales across the 3-min crying condition. Interrater reliability was acceptable (intraclass correlation coefficients = 0.64–0.73, M = 0.69) on all scales. All scales were submitted to a principal components analysis with varimax rotation. The eigenvalues indicated that a one-component solution reflecting tension while listening to infant crying (M = 2.16, SD = 0.89) best accounted for the data, and was composed of tension, positive affect (reversed scored), and emotion regulation (reversed scored) in response to infant crying (α = 0.95). The composite scale was standardized and used in all analyses reported below. The observational coding manual is available upon request from the first author.
EEG data reduction
EEG signals were measured during the rest period and while listening to the audio recording of infant crying. Using James Long, Inc. (2000), software, EEG signals were rereferenced offline to a computed average-mastoid reference (A1, A2). Gross movement, eye blink, and other artifacts were removed manually. If artifact appeared in one channel, data from all channels were removed. However, if artifact appeared in one channel throughout the entire condition, all data from that channel were removed so as not to lose data from the other channels. All participants had at least 1 min of EEG data per condition, and the average percentage of rejected epochs per participant during the rest condition and the infant crying condition was comparable to values reported in the literature (rest condition, M = 39%, range = 11%–74%; infant crying condition, M = 22%, range = 0%–67%; e.g., Coan & Allen, 2003).
Data were epoched into 0.5-s artifact-free epochs with 50% overlap to compensate for the loss of data due to the imposition of a Hamming window prior to spectral analysis. A fast Fourier transform, using a Hamming window, transformed data to power spectra, and the average power spectrum for each condition (rest or infant crying) was obtained. Total power within the alpha frequency band (8–13 Hz) expressed in microvolts squared was extracted for each condition. Each condition comprised at least 120 epochs, and prior research has demonstrated that acceptable estimates of internal consistency can be achieved with as few as 100 epochs (Towers & Allen, 2009). To normalize the distribution of alpha power prior to statistical analyses, raw alpha power from each electrode site during the resting period and while listening to infant crying was natural logarithm transformed. It is important to note that alpha power is inversely related to EEG activity. Thus, greater alpha power values indicate less EEG activity (Davidson, 1988).
Statistical analyses
We examined the influence of the following covariates: mother age, mother ethnicity, income, child age, and ASA narrative length. Because the direction and significance of effects did not change when controlling for these covariates, they were not included in the analyses presented below. EEG data were missing for some participants because artifact was present in the channel throughout one of the conditions. Specifically, during the rest condition, F3 and F4 data were missing for one participant (<1%). During the crying condition, two participants were missing F3 data and two participants were missing F4 data (<5%). Missing data were imputed using a single imputation equation modeling algorithm. Descriptive data for dependent variables are presented in Table 1. Of note, EEG values were comparable to those reported elsewhere in the literature (for a review, see Allen et al., 2004; Davidson, 1988).
Table 1.
Means and standard deviations for dependent variables during the rest and infant crying conditions
| Rest |
Infant Crying |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Alpha power | ||||
| Left frontal (F3) | 3.64 | 0.90 | 3.76 | 0.94 |
| Right frontal (F4) | 3.67 | 0.90 | 3.79 | 0.94 |
| Left parietal (P3) | 3.65 | 0.98 | 3.81 | 1.02 |
| Right parietal (P4) | 3.67 | 0.96 | 3.83 | 1.01 |
| Reported feelings of emotion | ||||
| Positive emotion | 0.98 | 0.59 | −0.12 | 0.64 |
| Negative emotion | −0.08 | 0.75 | 0.87 | 0.45 |
| Observed tension | — | — | 2.16 | 0.89 |
Note: The alpha power is in microvolts squared. Lower alpha power values indicate greater EEG activity. F3, F4, P3, and P4, the scalp electrode sites used in recording the EEG values in the frontal (F) and parietal (P) brain regions. The values for alpha power and reported feelings of emotion reflect values after being natural logarithm transformed.
Results
Secure base script knowledge and electrophysiological responding to infant crying
To account for the nested structure of the electrophysiological data (e.g., EEG activity nested within condition and hemisphere), hierarchical linear modeling (version 6.0; Bryk & Raudenbush, 1992) was used. Specifically, a two-level model was used in which (a) condition (rest or infant crying) and hemisphere (left or right) were entered as within-subject variables at Level 1, (b) secure base script knowledge was entered as a between-subject variable at Level 2, and (c) all possible interactions were entered. This analytic approach is in accordance with current suggestions to analyze EEG data within a general linear model framework with hemisphere and condition entered as factors (vs. using asymmetry scores), so that the contribution of each hemisphere to the EEG asymmetry can been examined (Allen et al., 2004). Separate analyses were conducted for frontal (F3/F4) and parietal (P3/P4) electrode sites.
Frontal EEG activity.
Focusing first on findings specific to the association between secure base script knowledge and change in frontal right (vs. left) EEG activity from rest to the infant crying condition, as seen in Table 2, results revealed significant main effects for hemisphere, condition, and secure base script knowledge. There was more left (vs. right) EEG activity, and EEG activity was greater during the rest (vs. crying) condition at frontal electrode sites. A significant main effect of secure base script knowledge was found, indicating that across conditions and hemispheres, secure base script knowledge was negatively associated with EEG activity at frontal electrode sites. Relevant to hypotheses, results revealed that the significant main effect of secure base script knowledge was qualified by a significant three-way interaction among secure base script knowledge, condition, and hemisphere.
Table 2.
Results of hierarchical linear model analyses with secure base script knowledge predicting natural logarithm transformed EEG alpha power during the rest and infant crying conditions at left and right frontal (F3, F4) and parietal (P3, P4) electrodes
| Fixed Effect | b | SE | t Ratio | Approximate df | p |
|---|---|---|---|---|---|
| Frontal electrodes | |||||
| Intercept | 3.529 | 0.090 | 39.144 | 106 | .000 |
| SBSK | 0.164 | 0.084 | 1.954 | 106 | .053 |
| Hemisphere | 0.016 | 0.005 | 3.372 | 424 | .001 |
| SBSK × Hemisphere | 0.001 | 0.004 | 0.287 | 424 | .774 |
| Condition | 0.124 | 0.029 | 4.209 | 424 | .000 |
| SBSK × Condition | −0.017 | 0.030 | −0.563 | 424 | .574 |
| Condition × Hemisphere | −0.002 | 0.002 | −0.873 | 424 | .384 |
| SBSK × Condition × Hemisphere | −0.004 | 0.002 | −1.993 | 424 | .047 |
| Parietal electrodes | |||||
| Intercept | 3.493 | 0.097 | 36.076 | 106 | .000 |
| SBSK | 0.172 | 0.098 | 1.755 | 106 | .082 |
| Hemisphere | 0.011 | 0.013 | 0.858 | 424 | .392 |
| SBSK × Hemisphere | 0.010 | 0.012 | 0.891 | 424 | .374 |
| Condition | 0.164 | 0.032 | 5.068 | 424 | .000 |
| SBSK × Condition | −0.001 | 0.034 | −0.021 | 424 | .984 |
| Condition × Hemisphere | −0.000 | 0.006 | −0.002 | 424 | .998 |
| SBSK × Condition × Hemisphere | −0.007 | 0.006 | −1.145 | 424 | .253 |
Note: SBSK, Secure base script knowledge.
Following up this significant three-way interaction, simple slopes analysis (Aiken & West, 1991; Preacher, Curran, & Bauer, 2006) indicated that, as expected, although secure base script knowledge was not associated with relative right (vs. left) EEG activity at rest (simple slope = −0.005, SE = 0.007, t = −0.762, p = .45), secure base script knowledge was significantly associated with right (vs. left) EEG activity during the crying condition (simple slope = −0.013, SE = 0.006, t = −2.073, p < .05; see Figure 1). More specifically, examination of descriptive statistics above and below the median of secure base script knowledge indicated that individuals lower on secure base script knowledge exhibited similar decreases in left and right EEG activity from rest to the infant crying condition, whereas individuals higher on secure base script knowledge exhibited larger decreases in EEG activity in the left hemisphere in comparison to the right hemisphere (see Table 3). These data indicate that lower (vs. higher) levels of secure base script knowledge are associated with smaller shifts in right (vs. left) EEG activation from rest in response to infant crying, due to smaller decreases in relative left frontal EEG activation from rest to the infant crying condition. In addition, the explained proportion of variance when adding secure base script knowledge to the model was .03, indicating that 3% of the variation in EEG activity was explained by condition, hemisphere, secure base script knowledge, and the interactions among these variables.
Figure 1.
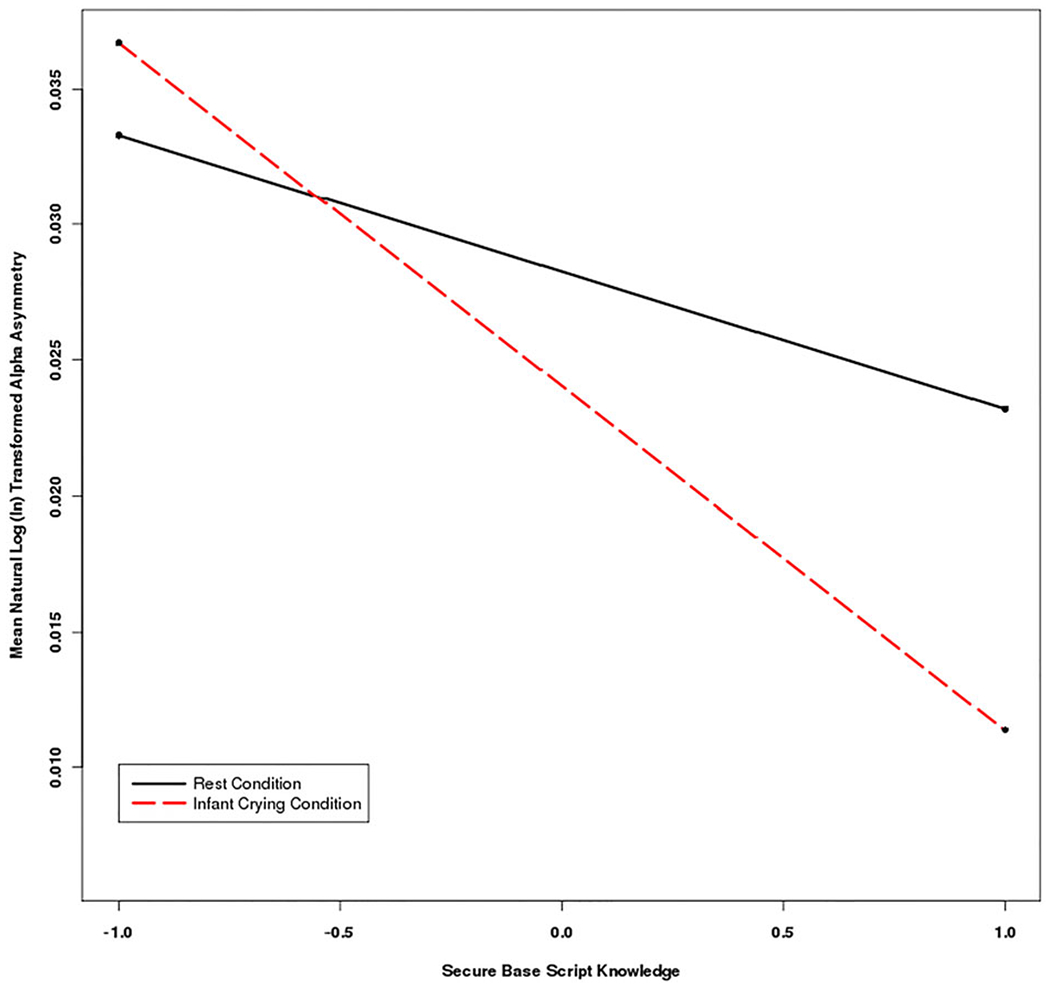
(Color online) Mean natural logarithm transformed EEG alpha asymmetry during the rest and infant crying conditions for frontal electrode sites (F3 and F4) for individuals ±1 SD above and below the mean for secure base script knowledge. Higher alpha asymmetry values indicate more relative left (vs. right) EEG activity. Mothers lower on secure base script knowledge exhibited less change in left (vs. right) frontal EEG alpha power from rest to the infant crying condition.
Table 3.
Natural logarithm transformed EEG alpha values for left and right frontal electrode sites (F3, F4) during the rest and infant crying conditions above and below the median of secure base script knowledge
| Left Frontal (F3) |
Right Frontal (F4) |
|||
|---|---|---|---|---|
| Condition | M | SD | M | SD |
| Below the median of SBSK | ||||
| Rest | 3.55 | 0.93 | 3.58 | 0.94 |
| Infant crying | 3.67 | 0.92 | 3.70 | 0.92 |
| Above the median of SBSK | ||||
| Rest | 3.72 | 0.87 | 3.76 | 0.85 |
| Infant crying | 3.86 | 0.96 | 3.87 | 0.95 |
Note: SBSK, Secure base script knowledge.
Parietal EEG activity.
Findings specific to the association between secure base script knowledge and change in parietal right (vs. left) EEG activity from rest to the infant crying condition revealed a significant main effect for condition, indicating less EEG activity across hemispheres during the crying (vs. rest) condition at parietal electrodes (see Table 2). Secure base script knowledge was not found to be significantly associated with EEG activity or to interact with condition and/or hemisphere in predicting EEG activity during the crying (vs. rest) condition at the parietal electrodes. The explained proportion of variance when adding secure base script knowledge to the model was .02.
Secure base script knowledge and subjective emotional responding to infant crying
Correlations were used to examine the association between secure base script knowledge and change in subjective emotional responses from rest to infant crying. Secure base script knowledge was not significantly associated with change in reported feelings of negative emotion from rest when listening to infant crying, r (106) = .01, p = .96. However, as hypothesized, secure base script knowledge was significantly and negatively associated with change in reported feelings of positive emotion from rest when listening to infant crying, r (106) = −.22, p < .05; individuals below the median on secure base script knowledge reported smaller changes in feelings of positive emotion from rest (M = −0.90, SD = 0.65) than those above the median on secure base script knowledge (M = −1.29, SD = 0.83) when listening to infant crying.
Secure base script knowledge and observed responding to infant crying
Correlations were also used to examine the association between secure base script knowledge and tension when listening to infant crying. As hypothesized, secure base script knowledge was significantly and negatively associated with tension when listening to infant crying, r (106) = −.27, p < .01, indicating that individuals lower (vs. higher) on secure base script knowledge displayed significantly more tension when listening to infant crying.
Electrophysiological, subjective, and observed responding to infant crying
We also investigated the associations among electrophysiological, subjective, and observed responding to infant crying. Findings indicated that change in reported feelings of positive emotion from rest to the infant crying condition were not significantly associated with tension to infant crying, r (106) = .13, p = .18. As seen in Table 4, shifts in frontal EEG activation from rest to the infant crying condition were not significantly associated with change in reported feelings of positive emotion from rest or tension to infant crying.
Table 4.
Results of hierarchical linear model analyses with change in natural logarithm transformed reported feelings of positive emotion from rest to the infant crying condition and tension to infant crying predicting natural logarithm EEG alpha power during the rest and infant crying conditions at left and right frontal (F3, F4) electrodes
| Fixed Effect | b | SE | t Ratio | Approximate df | p |
|---|---|---|---|---|---|
| Positive emotion | |||||
| Intercept | 3.714 | 0.087 | 42.882 | 106 | .000 |
| PE | −0.006 | 0.092 | −0.065 | 106 | .948 |
| Hemisphere | 0.016 | 0.005 | 3.371 | 424 | .001 |
| PE × Hemisphere | −0.001 | 0.004 | −0.150 | 424 | .881 |
| Condition | 0.124 | 0.029 | 4.226 | 424 | .000 |
| PE × Condition | −0.032 | 0.022 | −1.434 | 424 | .153 |
| Condition × Hemisphere | −0.002 | 0.002 | −0.870 | 424 | .385 |
| PE × Condition × Hemisphere | 0.003 | 0.002 | 1.506 | 424 | .134 |
| Tension | |||||
| Intercept | 3.529 | 0.091 | 38.572 | 106 | .000 |
| Tension | −0.019 | 0.090 | −0.210 | 106 | .834 |
| Hemisphere | 0.016 | 0.005 | 3.412 | 424 | .001 |
| Tension × Hemisphere | −0.008 | 0.004 | −1.769 | 424 | .077 |
| Condition | 0.124 | 0.030 | 4.215 | 424 | .000 |
| Tension × Condition | −0.023 | 0.030 | −0.766 | 424 | .444 |
| Condition × Hemisphere | −0.002 | 0.002 | −0.873 | 424 | .384 |
| Tension × Condition × Hemisphere | 0.004 | 0.002 | 1.751 | 424 | .080 |
Note: PE, Change in reported feelings of positive emotion from rest to the infant crying condition.
Discussion
Mothers’ sensitive responding to infant distress has important implications for children’s social and emotional development (Conradt & Ablow, 2010; Leerkes, 2011; Leerkes et al., 2009; McElwain & Booth-LaForce, 2006). Mounting evidence from investigations of mothers’ emotional responding to infant distress has demonstrated that a key contributor to observations of mothers’ sensitive-responsiveness is how they emotionally respond to infant crying (Killeen & Teti, 2012; Leerkes, 2010; Leerkes et al., 2011), suggesting that mothers must first manage their own emotional response to infant distress to provide high-quality care to their offspring. Such evidence might ultimately inform more targeted interventions aimed at addressing the specific emotional challenges parents face within specific caregiving contexts that undermine sensitive caregiving and, in turn, negatively impact their children’s social and emotional competence. However, which parents are at risk for such emotional challenges, and thus those who might benefit the most from such targeted interventions, remains unclear. In the current study, we found convergent evidence across multiple levels of analysis that mothers lower on secure base script knowledge exhibited patterns of electrophysiological, subjective, and observed responding to infant crying indicative of a restricted emotional response, providing evidence that mothers’ attachment representations play a role in helping to organize their emotional responding to infant distress. Below we elaborate further on these findings by channel of emotional response.
Electrophysiological responding to infant crying
As expected, mothers’ secure base script knowledge was associated with shifts in relative right (vs. left) frontal EEG activation from rest when listening to infant crying, but not with resting right (vs. left) frontal EEG activity. According to attachment theory (Bowlby, 1982), the attachment system is activated by specific contexts expected to threaten one’s sense of security. Thus, variation in adults’ attachment representations would be expected to be associated with their emotional responding within attachment-relevant contexts, but not with traitlike dispositions toward specific affective states. A key feature of security is the ability to flexibly engage both positive and negative emotion given situational demands without becoming overwhelmed. Evidence from numerous investigations has implicated resting frontal EEG asymmetry in traitlike emotional tendencies toward positive versus negative affect (Coan & Allen, 2004). In contrast, asymmetries in frontal EEG activation in response to emotional stimuli have been implicated in context-specific emotional responding (Coan & Allen, 2004), with individual differences in such asymmetries indicative of individuals’ capacity for affective engagement (Coan et al., 2006). Thus, in line with attachment theory, evidence from the current study suggests that variation in attachment is associated with statelike changes reflective of a greater capacity to emotionally engage with infant distress, but not with a particular tendency toward positive or negative affect regardless of context.
The findings from the current investigation also indicate that, as expected, mothers lower (vs. higher) on secure base script knowledge exhibited smaller shifts in relative right (vs. left) frontal EEG activation from rest when listening to infant crying. As noted above, variation in the extent to which individuals exhibit shifts in frontal EEG activation when confronted with emotional stimuli reflect individuals’ capacity for emotional engagement, with smaller shifts indicative of a limited capacity to engage and even a tendency to inhibit engagement in particular emotional states (Coan et al., 2006). Thus, the smaller shifts from rest in relative right (vs. left) frontal EEG activation when listening to infant crying exhibited by mothers lower on secure base script knowledge is indicative of a restricted and perhaps inhibited emotional response when confronted with infant distress. Such findings converge with those from a study of nonparents, in which individuals’ lower secure base script knowledge were found to exhibit a pattern of autonomic physiological reactivity suggestive of emotional inhibition when listening to infant crying (Groh & Roisman, 2009). Taken together, findings from these studies suggest that individuals lower on secure base script knowledge are characterized by a pattern of physiological responding indicative of a restricted emotional response when listening to infant crying.
Prior research has provided evidence that shifts in frontal EEG asymmetry to infant distress are also associated with observations of maternal sensitivity. Specifically, Killeen and Teti (2012) found that mothers who exhibited smaller shifts toward greater relative right frontal EEG activation from rest when confronted with infant distress interacted less sensitively with their infants. It is important to note that in their study, Killeen and Teti (2012) reported on change in mothers’ frontal EEG asymmetry scores from rest to the infant distress condition; thus, mothers’ asymmetry scores to infant distress cannot be interpreted without reference to resting asymmetry levels. Accordingly, shifts toward greater relative right frontal EEG asymmetry from rest do not necessarily indicate more right (vs. left) frontal EEG activation to infant distress, but instead reflect the extent to which individuals changed in relative right (vs. left) frontal EEG activation from their resting right (vs. left) frontal EEG activity. Similar to findings from Killeen and Teti (2012), we found that mothers with less secure base script knowledge exhibited less change in their relative right (vs. left) frontal EEG activation from rest when listening to infant crying. Evidence from across these studies indicates that mothers’ secure base script knowledge contributes to their capacity for affective responding when confronted with infant distress, which in turn has implications for the quality of their caregiving.
We also examined which hemisphere contributed to shifts in relative right (vs. left) frontal EEG activation and found that mothers lower on secure base script knowledge exhibited a smaller decrease in relative left (vs. right) frontal EEG activation from rest than did mothers higher on secure base script knowledge. Evidence from numerous investigations has implicated greater relative left frontal EEG activation in response to emotional stimuli with positive, approach-oriented emotion (e.g., exuberance or joy) and greater relative right frontal EEG activation with negative emotion that may or may not have a withdrawal component (e.g., sadness or fear; Coan & Allen, 2004). Accordingly, mothers lower on secure base script knowledge exhibited a pattern of electrophysiological activity indicative of smaller decreases in a more positive, exuberant emotional state. Although positive emotion is generally considered conducive to parenting, Dix (1991) has argued that parents’ positive emotion might undermine parenting when not matched to the caregiving context. Thus, in the context of infant distress, the smaller decreases in a positive emotional state exhibited by mothers lower on secure base script knowledge might reflect a less affectively attuned response to infant distress.
Subjective and observed emotional responding to infant crying
In addition to influencing frontal EEG activation to infant crying, we found that mothers’ attachment representations were associated with their subjective emotional responding. Specifically, mothers lower on secure base script knowledge reported smaller reductions in their feelings of positive emotion from rest when confronted with infant crying. In line with claims that positive emotion is disruptive to parent–child interactions when it is contextually out of place (Dix, 1991), greater reported feelings of positive emotion to infant distress have been linked with affectively insensitive responding to infant crying and reports of having experienced emotional rejection from parents in childhood (Leerkes & Siepak, 2006). In light of such theory and evidence, and paralleling the electrophysiological data in the current study, the smaller reductions in feelings of positive emotion reported by mothers lower on secure base script knowledge is suggestive of a more restricted, less affectively matched response to infant crying.
Regarding mothers’ observed emotional responding to infant distress, mothers lower on secure base script knowledge exhibited more tension to infant crying than did mothers higher on secure base script knowledge. More specifically, when confronted with infant distress, mothers lower on secure base script knowledge exhibited more evidence of tense facial expressions, including furrowed brows and compressed lips. These emotional expressions have been implicated in general feelings of distress and emotional suppression (Jonas, 1986; Malatesta et al., 1989; Malatesta & Izard, 1984). Given such evidence, findings from the current study indicate that when confronted with infant distress, mothers lower on secure base script knowledge express emotion reflective of heightened emotional distress and efforts to suppress their emotional response. Such emotional responding suggests that these mothers find managing their own emotional response to infant distress particularly difficult. Moreover, these findings build on prior evidence that insecure–avoidant infants, who minimize attachment-relevant emotion, express stronger levels of tension when confronted with attachment-relevant challenges (Malatesta et al., 1989) by providing evidence that similar patterns of emotional responding characterize mothers’ emotional responding to attachment-relevant challenges in ways consistent with the content of their attachment representations.
Electrophysiological, subjective, and observed responding to infant crying
In the current study, mothers’ electrophysiological, subjective, and observed responding to infant crying were not significantly associated. Although it remains unclear why these channels of emotional response were not significantly correlated, one possibility might be that different channels of response capture different aspects of emotional responding that range in the extent to which they are under conscious control (Tsai & Levenson, 1997); thus, mothers’ electrophysiological, subjective, and observed responding might provide complementary, yet unique insight into different aspects of their emotional response to infant crying. In addition, there might be methodological constraints that limit the strength of associations among these channels of response. For example, excessive facial movements contribute to artifact in the EEG signal. Thus, EEG data are unavailable during the point at which the strongest association between electrophysiological and observed responding might be expected.
It should also be noted that these findings indicate that mothers’ shifts in frontal EEG asymmetry do not account for their change in reported feelings of positive emotion and expressed tension to infant crying, a pattern of findings not unprecedented in the literature. For example, Riem et al. (2012) found that although adults’ attachment representations were associated with multiple channels of emotional responding to infant crying, the channels of emotional response were not associated with each other. Given such growing evidence of a gap between brain activation and behavioral responding, further research is needed to uncover why multilevel indicators of responding within the same context are not more strongly associated.
Despite such findings, evidence from this study highlights the importance of incorporating multiple channels of emotional response into developmental research. For example, the findings that mothers lower on secure base script knowledge exhibited smaller shifts in frontal EEG asymmetry and smaller changes in reported feelings of positive emotion to infant crying might be interpreted as evidence that such mothers did not find an unfamiliar infant crying particularly arousing. However, inconsistent with such an interpretation, these mothers also exhibited greater levels of tension to infant crying, suggesting that the cry stimulus was a salient emotional cue for them. Incorporating multiple channels of responding into the current study provided a clearer understanding of the emotional profiles of mothers when confronted with infant distress. Such findings suggest that mothers are emotionally aroused by infant crying, and that their attachment representations contribute to variation in the quality of their emotional response, with mothers lower on secure base script knowledge exhibiting convergent evidence across multiple channels of response of a restricted emotional response.
Limitations
This study was designed with the goal of not only examining how secure base script knowledge is associated with emotional responding to infant distress but also extending prior research on the autonomic correlates of adult attachment (Beijersbergen, Bakermans-Kranenburg, van IJzendoorn, & Juffer, 2008; Dozier & Kobak, 1992; Groh & Roisman, 2009; Holland & Roisman, 2010; Roisman, 2007; Roisman, Tsai, & Chiang, 2004) to a more direct measure of neurophysiological responding. Accordingly, this study provided the first evidence that adult attachment is associated with electrophysiological responding when confronted with an infant attachment-relevant challenge. That said, infant crying was not found to elicit an overall change in frontal EEG asymmetry from rest. Although such evidence is somewhat surprising given the intense emotional nature of infant crying, this result is not inconsistent with prior work. For example, theory-consistent links between individual differences in psychopathology and change in EEG asymmetry from rest to an emotional stimulus in the absence of a main effect of stimulus on change in EEG asymmetry have been found (Heller et al., 1997).
In addition, in light of Heller’s (1993) neuropsychological model of emotion, in which parietal hemispheric brain activity has been implicated in arousal, we hypothesized that variation in secure base script knowledge would be associated with change in parietal EEG asymmetry from rest when listening to infant crying. Although findings from this study did not support this hypothesis, variation in adult attachment has been found to be more consistently associated with autonomic reactivity specific to inhibitory processes, but not general autonomic arousal (i.e., electrophysiological reactivity, but not heart rate; Groh & Roisman, 2009; Roisman et al., 2004). Thus, adult attachment may be more clearly linked with particular types of physiological arousal that are especially relevant to the motivational strategies reflected in individual differences in adult attachment (e.g., inhibition), but not with more general indicators of physiological arousal.
Finally, we did not find that variation in secure base script knowledge was associated with changes in subjective feelings of negative emotion. Some evidence suggests that negative emotional responding to infant distress, if motivated by mothers’ concern for the infant, is associated with more sensitive responding to infant distress (Leerkes, 2010). Thus, an interesting avenue for future research would be to investigate whether secure base script knowledge is associated with different types of negative emotional responding to infant distress (e.g., child oriented [feeling sad for the infant] vs. parent oriented [feeling upset with the infant]).
Conclusions
In the current study, mothers lower on secure base script knowledge exhibited patterns of electrophysiological, subjective, and observed responding when listening to infant crying indicative of a less flexible and more emotionally restricted response to infant distress. Coherence across these multilevel indicators of emotional responding documents the role of secure base scripts in contributing to mothers’ ability to manage their own physiological and emotional responses to infant distress that, in turn, contribute to mothers’ sensitive caregiving and children’s interpersonal development (Killeen & Teti, 2012; Leerkes et al., 2011; Malatesta et al., 1989). Taken together, findings from this study provide insight into the emotional assets and deficits that mothers might bring to specific caregiving contexts as a function of their attachment history, and future research of this kind might help clarify risk and resilience factors within parent–child relationships that undermine or promote children’s development.
Acknowledgments
This work was supported in part by a postdoctoral fellowship provided by the National Institute of Child Health and Human Development (T32-HD07376) through the Center for Developmental Science, University of North Carolina at Chapel Hill (to A.M.G.); funds from the Research Board at the University of Illinois at Urbana–Champaign (to K.B.); funds from the Family Resiliency Center at the University of Illinois at Urbana–Champaign (to N.M. and G.I.R.); and funds from the USDA National Institute of Food and Agriculture (ILLU-793-362; to N.M.).
References
- Ablow JC, Marks AK, Feldman SS, & Huffman LC (2013). Associations between first-time expectant women’s representations of attachment and their physiological reactivity to infant cry. Child Development, 84, 1373–1391. [DOI] [PubMed] [Google Scholar]
- Ahern GL, & Schwartz GE (1985). Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia, 23, 745–755. [DOI] [PubMed] [Google Scholar]
- Aiken SL, & West SG (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage. [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, & Wall S (1978). Patterns of attachment: A psychological study of the Strange Situation. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Allen JJB, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67, 183–218. [DOI] [PubMed] [Google Scholar]
- Beijersbergen MD, Bakermans-Kranenburg MJ, van IJzendoorn MH, & Juffer F (2008). Stress regulation in adolescents: Physiological reactivity during the Adult Attachment Interview and conflict interaction. Child Development, 79, 1707–1720. [DOI] [PubMed] [Google Scholar]
- Bost KK, Shin N, McBride BA, Brown GL, Vaughn BE, Coppola G, et al. (2006). Maternal secure base scripts, children’s attachment security, and mother–child narrative styles. Attachment and Human Development, 8, 241–260. [DOI] [PubMed] [Google Scholar]
- Bowlby J (1982). Attachment and loss: Vol. 1. Attachment. New York: Basic Books. [Google Scholar]
- Bryk AS, & Raudenbush SW (1992). Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage. [Google Scholar]
- Coan JA, & Allen JJB (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40, 106–114. [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–49. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, & McKnight PE (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JF, & Tronick EZ (1983). Three-month-old infants’ reactions to simulated maternal depression. Child Development, 54, 185–193. [PubMed] [Google Scholar]
- Conradt E, & Ablow J (2010). Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development, 33, 251–265. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1988). EEG measures of cerebral asymmetry: Conceptual and methodological issues. International Journal of Neuroscience, 39, 71–89. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, & Friesen WV (1990). Approach–withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. Journal of Personality and Social Psychology, 58, 330–341. [PubMed] [Google Scholar]
- Dix T (1991). The affective organization of parenting: Adaptive and maladaptive processes. Psychological Bulletin, 110, 3–25. [DOI] [PubMed] [Google Scholar]
- Dozier M, & Kobak R (1992). Psychophysiology in attachment interviews: Converging evidence for deactivating strategies. Child Development, 63, 1473–1480. [PubMed] [Google Scholar]
- Fowles D (1980). The three arousal model: Implications of Gray’s two-factor learning model for heart rate, electrodermal activity, and psychopathy. Psychophysiology, 17, 87–104. [DOI] [PubMed] [Google Scholar]
- Fowles D (1988). Physiology and psychopathology: A motivational approach. Psychophysiology, 25, 373–391. [DOI] [PubMed] [Google Scholar]
- George C, Kaplan N, & Main M (1984–1996). Adult Attachment Interview protocol. Unpublished manuscript, University of California, Berkeley. [Google Scholar]
- Gray J (1975). Elements of the two-process theory of learning. New York: Academic Press. [Google Scholar]
- Groh AM, & Roisman GI (2009). Adults’ autonomic and subjective emotional responses to infant vocalizations: The role of secure base script knowledge. Developmental Psychology, 45, 889–893. [DOI] [PubMed] [Google Scholar]
- Heller W (1993). Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology, 7, 476–489. [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, & Miller GA (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106, 376–385. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, & Miller GA (1998). Lateralization in emotion and emotional disorders. Current Directions in Psychological Science, 7, 26–32. [Google Scholar]
- Holland AS, & Roisman GI (2010). Adult attachment security and young adults’ dating relationships over time: Self-reported, observational, and physiological evidence. Developmental Psychology, 46, 552–557. [DOI] [PubMed] [Google Scholar]
- Jonas R (1986). A component analysis of the emotionality of type A behavior pattern. Unpublished doctoral dissertation, New School for Social Research, New York. [Google Scholar]
- Killeen LA, & Teti DM (2012). Mothers’ frontal EEG asymmetry in response to infant emotion states and mother–infant emotional availability, emotional experiences, and internalizing symptoms. Development and Psychopathology, 24, 9–12. [DOI] [PubMed] [Google Scholar]
- Kline JP, Blackhart GC, Woodward KM, Williams SR, & Schwartz GER (2000). Anterior electroencephalographic asymmetry changes in elderly women in response to a pleasant and an unpleasant odor. Biological Psychology, 52, 241–250. [DOI] [PubMed] [Google Scholar]
- Lay K, Waters E, & Park KA (1989). Maternal responsiveness and child compliance: The role of mood as a mediator. Child Development, 60, 1405–1411. [PubMed] [Google Scholar]
- Leerkes EM (2010). Predictors of maternal sensitivity to distress. Parenting: Science and Practice, 10, 219–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM (2011). Maternal sensitivity during distress tasks: A unique predictor of attachment security. Infant Behavior and Development, 34, 443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Blankson A, & O’Brien M (2009). Differential effects of maternal sensitivity to infant distress and nondistress on social–emotional functioning. Child Development, 80, 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Parade SH, & Gudmunson JA (2011). Mothers’ emotional reactions to crying pose risk for subsequent attachment security. Journal of Family Psychology, 25, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, & Siepak KJ (2006). Attachment linked predictors of women’s emotional and cognitive responses to infant distress. Attachment and Human Development, 8, 11–32. [DOI] [PubMed] [Google Scholar]
- Malatesta CZ, Culver C, Tesman JR, Shepard B, Fogel A, Reimers M, et al. (1989). The development of emotion expression during the first two years of life. Monographs of the Society for Research in Child Development, 54, 1–104. [PubMed] [Google Scholar]
- Malatesta CZ, & Izard CE (1984). The facial expression of emotion: Young, middle-aged, and older adult expressions. In Malatesta CZ & Izard CE (Eds.), Emotion in adult development (pp. 253–273). Beverly Hills, CA: Sage. [Google Scholar]
- McElwain NL, & Booth-LaForce C (2006). Maternal sensitivity to infant distress and nondistress as predictors of infant–mother attachment security. Journal of Family Psychology, 20, 247–255. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pitman RK, et al. (2004). PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. Journal of Abnormal Psychology, 113, 324–329. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, van IJzendoorn MH, Out D, & Rombouts SARB (2012). Attachment in the brain: Adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment and Human Development, 14, 533–551. [DOI] [PubMed] [Google Scholar]
- Roisman GI (2007). The psychophysiology of adult attachment relationships: Autonomic reactivity in martial and premarital interactions. Developmental Psychology, 43, 39–53. [DOI] [PubMed] [Google Scholar]
- Roisman GI (2009). Adult attachment: Toward a rapprochement of methodological cultures. Current Directions in Psychological Science, 18, 122–126. [Google Scholar]
- Roisman GI, Tsai JL, & Chiang K-HS (2004). The emotional integration of childhood experience: Physiological, facial expressive, and self-reported emotional response during the Adult Attachment Interview. Developmental Psychology, 40, 776–789. [DOI] [PubMed] [Google Scholar]
- Teti DM, & Cole PM (2011). Parenting at risk: New perspectives, new approaches. Journal of Family Psychology, 25, 625–634. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, & Davidson RJ (1994). Frontal brain activation in repressers and nonrepressors. Journal of Abnormal Psychology, 103, 339–349. [DOI] [PubMed] [Google Scholar]
- Towers DN, & Allen JJB (2009). A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology, 46, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JL, & Levenson RW (1997). Cultural influences on emotional responding: Chinese American and European American dating couples during interpersonal conflict. Journal of Cross-Cultural Psychology, 28, 600–625. [Google Scholar]
- Vaughn BE, Veríssimo M, Coppola G, Bost KK, Shin N, McBride B, et al. (2006). Maternal attachment script representations: Longitudinal stability and associations with stylistic features of maternal narratives. Attachment and Human Development, 8, 199–208. [DOI] [PubMed] [Google Scholar]
- Vaughn BE, Waters HS, Coppola G, Cassidy J, Bost KK, & Veríssimo M (2006). Script-like attachment representations and behavior in families and across cultures: Studies of parental secure base narratives. Attachment and Human Development, 8, 179–184. [DOI] [PubMed] [Google Scholar]
- Waters HS, & Rodrigues-Doolabh L (2004). Manual for decoding secure base narratives. Unpublished manuscript, State University of New York at Stony Brook. [Google Scholar]
- Waters HS, & Waters E (2006). The attachment working models concept: Among other things, we build script-like representations of secure base experiences. Attachment and Human Development, 8, 185–198. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, & Tomarken AJ (1993). Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology, 30, 82–89. [DOI] [PubMed] [Google Scholar]
- Zekoski EM, O’Hara MW, & Wills KE (1987). The effects of maternal mood on mother–infant interaction. Journal of Abnormal Child Psychology, 15, 361–378. [DOI] [PubMed] [Google Scholar]


