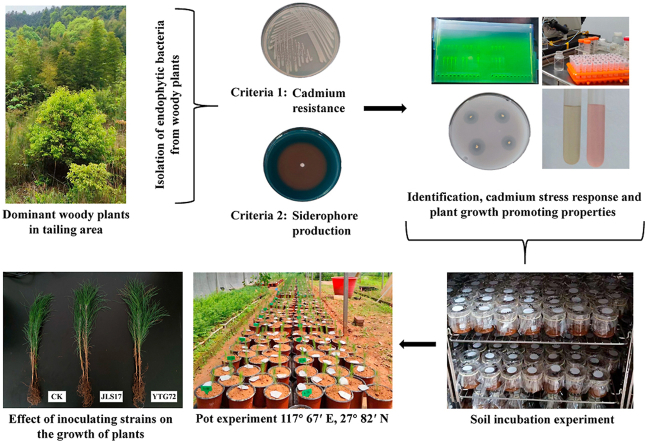
Abstract
Endophyte-assisted phytoremediation is an emerging technique for soil heavy metals (HMs) remediation and has become a research focus in the world because of the benefits of endophytes on plant growth and uptake of HMs. In this study, multifunctional endophytic bacteria strains were isolated and screened, and the feasibility of these strains for soil cadmium (Cd) remediation was investigated by soil incubation experiments and pot experiments. All endophytic bacteria were isolated from the roots of woody plants grown on Cd-contaminated soil. Seven endophytic bacteria strains had capacities to tolerate Cd toxicity and produce siderophores, and sequence analysis of the 16S rRNA gene classified these strains as belonging to the genera Burkholderia, Pseudomonas, Pantoea, and Herbaspirillum. All strains were able to produce hydroxamate siderophores (32.40%–91.49%) and had three or more plant growth promoting properties such as phosphorus solubilization, nitrogen fixation, indole acetic acid and 1-aminocyclopropane-1-carboxylate deaminase production. They were all strongly resistant to Cd2+ toxicity, with the minimum inhibitory concentration in LB medium ranging from 1.5 mM to 9.0 mM. Except for strain Burkholderia contaminans JLS17, other strains showed decreasing removal rates within continuously elevated Cd2+ concentration of 10–100 mg L−1. Compared with the uninoculated treatment, the inoculation of strains B.contaminans JLS17, Pseudomonas lurida JLS32, and Pantoea endophytica JLS50 effectively increased the concentration of acid-soluble Cd and decreased the concentration of reducible, oxidizable, and residual Cd in the soils of different Cd contamination levels. In pot experiments, inoculation of strains JLS17 and YTG72 significantly (p < 0.05) promoted the growth of above-ground parts and root system of slash pine (Pinus elliottii) under Cd stress. This study provides a valuable biological resource for endophyte-assisted phytoremediation and a theoretical basis for the application of endophytic bacteria for remediation of Cd-contaminated soil.
Keywords: Cadmium contamination, Cadmium resistance, Endophytic bacterium, Endophyte-assisted phytoremediation, Siderophore, Plant growth promotion
Graphical abstract
Highlights
-
•
Seven Cd-tolerant and siderophore-producing endophytic strains were isolated.
-
•
Strain JLS17 effectively removed Cd2+ from medium with high Cd2+ concentrations.
-
•
Strains JLS17, JLS32 and JLS50 activated Cd in the soil.
-
•
Strains JLS17 and YTG72 promoted the growth of Pinus elliottii under Cd stress.
-
•
Candidate strains can be used as potential remediation agents for the phytoremediation.
1. Introduction
Cadmium (Cd) is an important alloying element used in batteries, electroplate, electronic equipment and aerospace materials, and it often occurs in most zinc ores as a by-product of zinc production [1]. However, this element is nonessential for humans, animals and plants. Cd is recognized as one of the most toxic heavy metals (HMs) in the world due to its ability to induce oxidative stress in cells, leading to damage to lipids, proteins and DNA [2]. Cd enters the soil through human activities such as mining, metal smelting, and agriculture producing, then is absorbed by plants, and continues to accumulate through the food chain to humans, thus posing a serious health threat [3]. The current situation of soil Cd contamination is not optimistic, and it was reported that the over standard rate of Cd in Chinese soil was 7.0% [4,5]. Cd contamination has raised a global concern due to its strong migration, resistance to degradation and high toxicity [3].
The decontamination of HMs-contaminated soil is an urgent necessity in the world. The current remediation methods for soil HMs contamination mainly include physical remediation, chemical remediation and bioremediation [6]. Compared with physical and chemical remediation, bioremediation has the advantages of high efficiency, economy and ecological sustainability [7]. As the only effective bioremediation method used for soil improvement and reduction of soil HMs toxicity in mining areas, phytoremediation, which is defined as a low-cost remediation technology, uses plants to reduce the concentration of HMs in soil [6]. Nevertheless, phytoremediation is still limited by the slow growth of plant and long remediation time, as a consequence, microbe-assisted phytoremediation has emerged [8]. The microbe-assisted phytoremediation commonly catalyzes the conversion of HMs to non-toxic forms or direct adsorption of HMs ions via different surface interactions both outside and inside the microbial cells, such as ion exchange, chelation, complexation and physisorption [9]. In this way, the bioavailability of HMs in the soil is altered and the biotoxicity is reduced [9]. Microorganisms can also have an impact on plant growth dynamics. The plant growth promoting (PGP) properties possessed by microorganisms, such as phosphorus solubilization, nitrogen fixation, siderophore and indole acetic acid (IAA) production, which can improve the utilization of soil nutrients by plants and promote plants growth. In addition, microorganisms are able to regulate metal resistance systems and promote or limit the accumulation of HMs in plants [10].
Among the plant growth promoting bacteria (PGPB), siderophore-producing bacteria (SPB) are of particular interest. Siderophores are small molecules with high affinity for Fe3+ produced by bacteria, fungi and plants [11]. They not only bind specifically to Fe3+ to provide iron nutrition, but also form soluble metal-siderophore chelates with metals such as Al3+, Cu2+, Cd2+, Pb2+ and Zn2+, reducing their free concentration and toxicity [12]. Compared to the commonly used chemically synthesized metal chelator ethylene diamine tetraacetic acid (EDTA), siderophores are degradable in the soil and do not cause subsequent environmental contamination [13]. On the other hand, siderophores not only mitigate the toxicity of HMs and enhance the accumulation of HMs, but also act as a carbon source to promote microbial growth [14,15]. The HMs chelating ability of siderophores is considered to be applicable for phytoremediation, and inoculation with SPB and addition of SPB culture supernatant were verified to increase plant uptake of HMs [16]. For instance, Cd-tolerant SPB Serratia nematodiphila LRE07, Enterobacter aerogenes LRE17, Enterobacter sp. LSE04 and Acinetobacter sp. LSE06 promote the growth of Cd hyperaccumulator Solanum nigrum L. and increased Cd extraction from the soils [17].
Currently, bacteria with HMs remediation potential are mostly found in soil. Compared with plant growth-promoting rhizobacteria, plant growth-promoting endophytic bacteria (PGPEB) have a significant competitive edge due to their close relationship with plants, and many PGPEB are facultative and able to live outside of plant tissues as rhizobacteria [10]. Although there is still a lack of PGPEB strains valuable for metal detoxification, the use of HMs-tolerant PGPEB as assistant materials for phytoremediation may have better potential for application. Consequently, in this study, some siderophore-producing endophytic bacteria with Cd tolerance were isolated from the roots of woody plants in Cd-contaminated tailing areas. Here, we aimed to assess their capacity of tolerance and removal to Cd2+, analyze their effects on the potential role of soil Cd speciation transformation (e.g. dissolution, reduction, oxidation), and evaluate their growth-promoting effects on plants growing under Cd-stressed conditions. The results of this study are expected to provide promising biological resources for phytoremediation of soil Cd contamination.
2. Materials and methods
2.1. Source and sampling of plant materials
Samples were collected in the tailing area in Yushui district, Xinyu city, Jiangxi Province, China (27°36′N, 114°52′E), where phytoremediation experiments were conducted with woody plants such as oriental white oak (Quercus glauca), alder (Alnus cremastogyne), caulis spatholobi (Spatholobus suberectus), indian azalea (Rhododendron simsii), slash pine (Pinus elliottii) and masson pine (Pinus massoniana). Well-grown woody plants were selected as the dominant plants. Three plants with the same growth vigor were randomly selected from each species and their roots, branches and leaves were collected respectively. All samples were placed in individual self-sealing bags, stored at 4 °C and immediately transported to the laboratory for microbial isolation.
2.2. Isolation of endophytic bacteria and qualitative detection of siderophore-producing capacity
Plant materials were rinsed with running water and dried with sterile filter paper. For surface disinfection, weight of 1 g of plants tissue samples were immersed in 75% (v/v) alcohol for 30 s and then 3% sodium hypochlorite solution for 10 min and finally washed 7 times in sterile water. The final rinse water was plated on Luria-Bertan (LB) agar medium (tryptone 10 g L−1, yeast extract 5 g L−1, NaCl 10 g L−1, agar 18 g L−1, pH 7.0) to verify whether the surface disinfection was effective. After then, plant materials were ground into a homogenate in sterile water. A total of 150 μL of appropriate dilutions were plated onto LB agar medium containing 30 mg L−1 Cd2+ and inoculated at 28 °C for 7 d, and single colonies were picked.
Siderophore-producing capacity was examined by using Chrome azurol sulphonate (CAS) medium (chrome azurol 60.5 mg, hexadecyl trimethyl ammonium bromide 72.9 mg L−1, NH4Cl 125 mg L−1, KH2PO4 37.5 mg L−1, NaCl 62.5 mg L−1, 10 μM FeCl3·6H2O, agar 9 g L−1). Twenty-one isolates were inoculated onto CAS medium and cultured at 28 °C for 5 d [18]. The color change from blue to orange around the isolate indicating the capacity to produce siderophore, and these isolates were chosen for further analyses.
2.3. Identification of the isolates by 16S rRNA gene sequencing
The selected Cd-resistance and siderophore-producing endophytic bacteria strains were cultured in LB medium to extract their DNA using Bacterial Genomic DNA Extraction Kit (Bomed Biotechnology Co., Ltd., Beijing, China). The extracted DNA was used as the template and the 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), and the amplifcation system and procedure were described by Chen et al. [18]. The amplified product was sequenced at Bomed Biotechnology Co., Ltd. (Beijing, China). The sequences obtained were compared in EzTaxon (https://www.ezbiocloud.net/) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/) databases, and the type strain with the highest homology was selected. All sequences were aligned using Clustal W and the phylogenetic tree was constructed using the neighbor-joining method, Kimura 2-parameter model and 1000 bootstraps in MEGA 7.0 [19,20].
2.4. Quantitative analysis and type determination of siderophore
The isolates were cultured in iron-free modified sucrose-aspartic acid (MSA) medium (sucrose 20 g L−1, l-asparagine 2 g L−1, K2HPO4 0.5 g L−1, MgSO4·7H2O 0.5 g L−1, pH 7.0) at 28 °C for 48 h at 180 rpm in triplicate. Siderophore production was expressed in the percent siderophore unit (SU), and determined according to Alexander and Zuberer [21] and Wang et al. [16].
FeCl3 test was used to determine hydroxamate-type and catecholate-type siderophore [22]. And Shenker's test was used to determine carboxylate-type siderophore [23].
2.5. Cadmium resistance and cadmium removal of the isolates
The stock solution was prepared with CdCl2·2.5H2O and then filtered through a 0.22 μm sterile filter to remove microorganisms. After the medium was sterilized and cooled, the required amount of sterile stock solution was added to prepare medium with different Cd2+ concentrations.
2.5.1. Effects of Cd2+ on the growth of bacteria
A total of 100 μL of bacterial suspension (5 × 108 CFU mL−1) was inoculated in 10 mL LB medium containing increasing concentrations of Cd2+ (0–10 mM) in triplicate. Cultures were incubated at 28 °C and 180 rpm for 72 h. The bacterial growth was measured by the absorbance at 600 nm (OD600) using UV/visible spectrophotometer (Persee TU-1901, China). The lowest Cd2+ concentration which completely inhibited bacterial growth was considered as the minimum inhibitory concentration (MIC).
2.5.2. Cd2+ removal in growth media
A total of 100 μL of bacterial suspension (5 × 108 CFU mL−1) was inoculated in 10 mL LB medium with Cd2+ concentrations of 10, 25, 50 and 100 mg L−1 in triplicate. Cultures were incubated at 28 °C and 180 rpm for 96 h. After centrifugation at 8000 rpm for 20 min, the supernatant was collected and filtered through a 0.22 μm sterile filter. The Cd2+ concentrations in the filtrate were quantified using inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer ELAN DRC-e, USA). The Cd2+ removal rate (RR) was calculated as follows: , where Cs is the Cd2+ concentration of the supernatant, and Cr is the Cd2+ concentration of the sterile LB medium.
2.6. PGP characteristics of the isolates
Phosphate solubilization was assessed by the formation of a halo around the colony. A volume of 1 μL of bacteria was inoculated in solid Pikovskaya (PVK) medium (glucose 10 g L−1, (NH4)2SO4 0.5 g L−1, MgSO4·7H2O 0.3 g L−1, NaCl 0.3 g L−1. CaCO3 1 g L−1, KCl 0.3 g L−1, FeSO4·7H2O 0.03 g L−1, MnSO4·H2O 0.03 g L−1, Ca3(PO4)2 2 g L−1, agar 18 g L−1, pH 7.0) and incubated for 7 d at 28 °C in triplicate. The phosphate solubilization capacity of the strain was quantified by the phosphate solubilization index (PSI) [24].
IAA production was measured by colorimetric assay. The isolates were inoculated in liquid LB medium supplemented with 300 mg L−1 of l-tryptophan and incubated in the dark at 28 °C and 180 rpm for 48 h in triplicate. Then, the content of IAA in the cultures was determined according to the method of Patten and Glick [25].
Both nitrogenase and 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity of the isolates were determined in triplicate by double antibody sandwich assay according to the enzyme-linked immunosorbent assay (ELISA) kit instructions (Sino Best Biological Technology Co., Ltd., Shanghai, China).
2.7. Cd speciation analysis in contaminated soil inoculated with candidate strains
Considering the Cd resistance, siderophore-producing capacity and PGP properties, the strains JLS17, JLS32, JLS50 and YTG72 were selected for soil incubation experiment. The soil used in this experiment was collected from the uncontaminated area around the mine area in Yushui District, Xinyu City, Jiangxi (total Cd concentration was 0.244 mg kg−1). To prepare soils with low (3 mg kg−1), medium (6 mg kg−1) and high (9 mg kg−1) Cd contamination levels, 2 mm sieved air-dried soil was spiked with CdCl2·2.5H2O solution and mixed thoroughly and aged at room temperature for 30 d, during which time the soil was maintained at 60% of the maximum soil water holding capacity.
Experiments were conducted in sterile conditions in 150 mL beakers with 50 g of sterilized soil (sterilized at 121 °C for 2 h). The soils were inoculated with 10 mL LB cultures (5 × 108 CFU mL−1) or sterile cultures in triplicate at 30 °C in triplicate. Sterile water was replenished to the soil every 2 d depending on the reduction in sample mass. The samples were collected after 30 d and analyzed with the modified BCR three-step sequential extraction method [26].
2.8. Pot experiment on the plant growth promotion under Cd stress inoculated with candidate strains
In order to verify the plant growth-promoting effect of the candidate strains, strain JLS17 with the highest Cd tolerance and strain YTG72 with the most PGP properties were selected as potted test strains, and Pinus elliottii, a indigenous tree species with Cd remediation potential screened in the previous study [27], was used as the inoculated plant. P. elliottii annual seedlings were provided by the Experimental Center of Subtropical Forestry, Chinese Academy of Forestry, Jiangxi Province, China, with a height of 15.2 cm and a stem diameter of 1.60 mm. The method of soil preparation and the level of Cd contamination were the same as in Section 2.7 above. Each pots (15 cm in height and 12 cm in diameter) contained 2 kg of soil (1.82 g kg−1 total N, 86.33 mg kg−1 available N, 4.34 mg kg−1 available P, 71.26 mg kg−1 readily available K, 12.53 g kg−1 organic matter, pH 4.81) and was planted with one seedling. Three inoculations (JLS17, YTG72 and CK) with four Cd contamination levels (0, 3, 6 and 9 mg kg−1) were set up for a total of 12 treatments. Thirty-six seedlings per treatment were carried out from July to November 2022 in an open-sided greenhouse in Experimental Center of Subtropical Forestry (117°67′E, 27°82′N). A total of 50 mL of 5 × 108 CFU mL−1 was inoculated near the roots of plants in the middle of each month, and four times inoculations were conducted during the experiment in total. The inoculation of sterile cultures was used as control, and plants on soil remained unfertilized.
Plant height and stem diameter were measured before each inoculation. At the end of the experiment, five plants randomly selected from each treatment with average growth were cut and cleaned, and the root system was scanned using a root scanner (Microtek ScanMaker i800 Plus, China), and root length, root surface area, and root volume were analyzed using a root analysis system (Wseen LA-S, China).
2.9. Statistical analysis
The experimental data were processed using Microsoft Excel 2019 and IBM SPSS 26. One-way analysis of variance (ANOVA) was conducted to compare the means of the parameters, with p < 0.05 considered significant for Duncan's test.
3. Results
3.1. Isolation and colony characteristics of bacteria with Cd-resistance and siderophore-producing capacity
A total of 21 bacteria strains were isolated from plant samples, all of which were derived from roots and were able to grow in LB medium with a Cd2+ concentration of 30 mg L−1, and 7 of these strains detected the siderophore production. The colony characteristics of these bacteria were recorded in Table 1. Most of the bacteria were isolated from Quercus glauca, Alnus cremastogyne, Spatholobus suberectus, and Rhododendron simsii, and the colonies were white or light yellow, round, and with moist surface.
Table 1.
Colony characteristics of Cd-tolerant and siderophore-producing isolates grown in LB medium and identification based on their 16S rRNA gene sequences.
| Isolates | Isolated plant species | Shape | Color | Surface state | Accession number | Type strain | Identity (%) |
|---|---|---|---|---|---|---|---|
| JLS17 | Quercus glauca | Round | Yellow | Moist | OQ103353 | Burkholderia contaminans (LMG 23361) | 99.79 |
| JLS30 | Alnus cremastogyne | Round | White | Moist | OQ103392 | Pseudomonas kribbensis (46-2) | 99.86 |
| JLS32 | Quercus glauca | Round | White | Moist | OQ103397 | Pseudomonas lurida (LMG 21995) | 99.72 |
| JLS50 | Spatholobus suberectus | Round | Light yellow | Moist | OQ103354 | Pantoea endophytica (596) | 99.79 |
| JLS69 | Quercus glauca | Round | White | Moist | OQ103355 | Pseudomonas sivasensis (P7) | 99.71 |
| JLS70 | Quercus glauca | Round | White | Moist | OQ103393 | Pseudomonas palleroniana (CFBP 4389) | 99.79 |
| YTG72 | Rhododendron simsii | Round | White | Moist | OQ103356 | Herbaspirillum huttiense subsp. Putei (IAM 15032) | 100.00 |
3.2. Identification of strains by 16S rRNA gene sequences
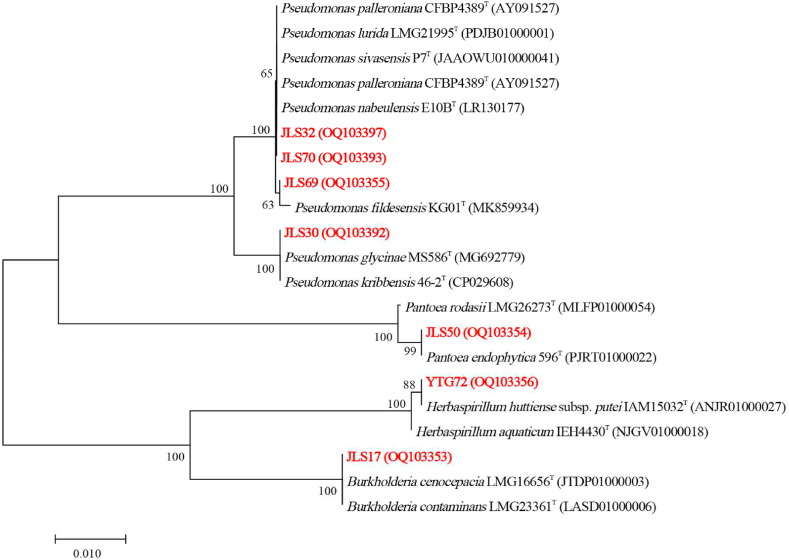
The endophytic strains with good Cd resistance and siderophore-producing capacity were identified, and results are showed in Table 1. Phylogenetic analysis showing close relatives is given in Fig. 1. The 7 strains belonged to 4 genera, with Pseudomonas being the most abundant genus isolated and screened in this study. Among these strains, JLS30, JLS32, JLS69 and JLS70 showed high sequence similarity with Pseudomonas (99.71%–99.86%). The other three strains, JLS17, JLS50 and YTG72, showed high sequence similarity with Burkholderia contaminans (99.79%), Pantoea endophytica (99.79%), and Herbaspirillum huttiense subsp. Putei (100%), respectively.
Fig. 1.
Phylogenetic evolutionary tree based on 16S rRNA sequences, using the Neighborn Joining method, Kimura 2-parameter, and 1000 bootstrap. Genbank accession numbers are indicated in brackets. The bar represents the number of substitutions per 100 nucleotide positions.
3.3. Siderophore-producing characteristics of strains
The siderophore-producing characteristics for all strains are showed in Table 2. Among the 7 strains, the highest siderophore production was from genus Pseudomonas, particularly Pseudomonas kribbensis JLS30 (91.49%). Most strains have high siderophore production with SU values above 70%. In the FeCl3 assay, the maximum absorption peaks of the 7 strains to be tested all appeared at 420–450 nm, while the negative Shenker's test also showed that the types of siderophore produced by all 7 strains were hydroxamate-type.
Table 2.
Cd tolerance and PGP properties of isolated strains.
| Strain | Siderophore |
MIC (mM) | PSI* (λ/λ0) | IAA (μg mL−1) | Nitrogenase activity (U L−1) | ACC deaminase (pg mL−1) | |
|---|---|---|---|---|---|---|---|
| Production (%) | Type | ||||||
| JLS17 | 78.17 ± 0.15 c | Hydroxamate | 9.0 | 1.98 ± 0.17 de | ND | 16.39 ± 2.70 c | 44.64 ± 2.02 c |
| JLS30 | 91.49 ± 0.15 a | Hydroxamate | 3.0 | 2.00 ± 0.10 de | 1.18 ± 0.08 c | 81.32 ± 4.00 a | ND |
| JLS32 | 88.49 ± 0.24 b | Hydroxamate | 4.0 | 2.45 ± 0.38 d | ND | 53.39 ± 7.10 b | 105.17 ± 6.03 b |
| JLS50 | 71.83 ± 0.15 d | Hydroxamate | 1.5 | 4.00 ± 0.16 b | 27.26 ± 0.47 a | ND | 160.21 ± 10.85 a |
| JLS69 | 32.40 ± 1.94 e | Hydroxamate | 2.0 | 6.08 ± 0.57 a | ND | ND | ND |
| JLS70 | 87.93 ± 0.41 b | Hydroxamate | 4.0 | 3.10 ± 0.45 c | ND | 55.02 ± 0.38 b | ND |
| YTG72 | 77.24 ± 0.41 c | Hydroxamate | 3.0 | 1.65 ± 0.21 e | 8.26 ± 0.09 b | 11.08 ± 4.04 c | 115.37 ± 9.37 b |
±, Standard deviation; ND: no detection.
PSI (λ/λ0)*: low (PSI < 2), medium (2 < PSI < 3) and high (PSI > 3) [28].
Mean values denoted by the same letter in columns indicate no significant difference according to Duncan's test at p < 0.05.
3.4. Response of the bacterial strains to cadmium
3.4.1. Effect of Cd2+ on the growth of the bacterial strains
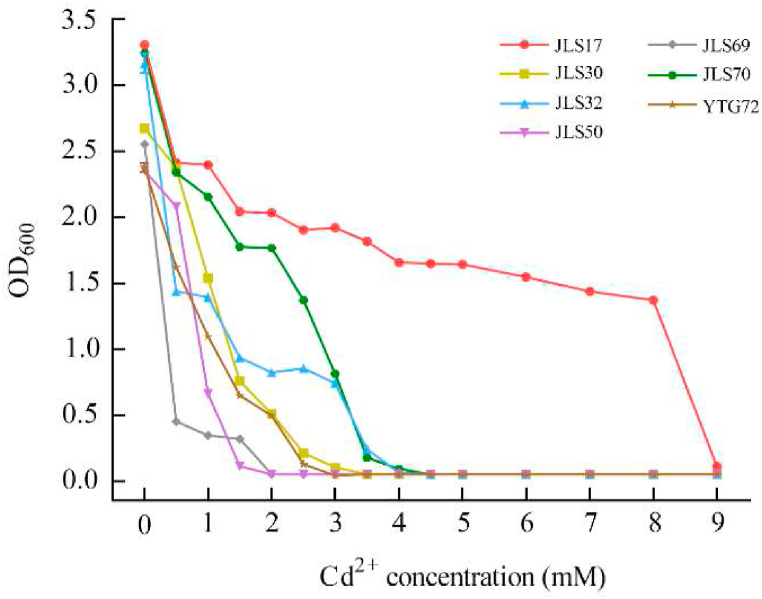
The effect of Cd2+ on the biomass of the strains is shown in Fig. 2. The biomass of 7 strains decreased with the increasing concentration of Cd2+ in the culture medium. All strains showed strong resistance to Cd2+, and they were able to grow at 1 mM of Cd2+, with 6 of them having MICs between 1.5 mM and 4.0 mM. Strain JLS17 was regarded as the most effective Cd-resistant bacteria, which was able to maintain a high biomass at extremely high Cd2+ concentration range (0–8 mM), with a slow decrease in OD600 from 3.306 to 1.373 and a MIC of 9.0 mM.
Fig. 2.
Growth of each strain individually cultured in LB medium with different Cd2+ concentrations.
3.4.2. Removal of Cd2+ by the strains
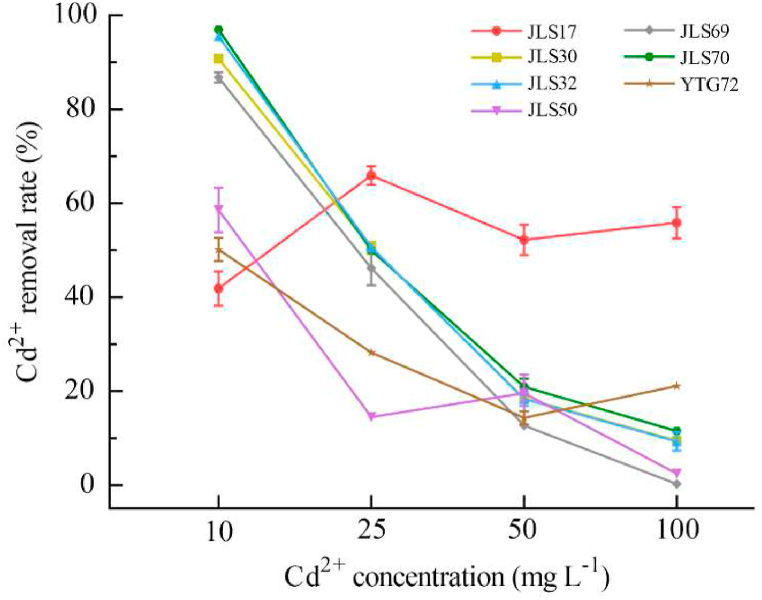
The removal rate of Cd2+ from the growth medium by the strains is shown in Fig. 3. All strains showed high removal rate (41.9%–97.0%) at 10 mg L−1 of Cd2+, among which, strain JLS70 had the strongest ability for Cd2+ removal, and JLS32, JLS30 and JLS69 also removed more than 85%. Except for strain JLS17, the removal rate of Cd2+ by other strains decreased with increasing Cd2+ concentration from 14.5% to 50.7% (25 mg L−1), to 12.8%–19.6% (50 mg L−1). The removal rate of Cd2+ by most strains was less than 10% at 100 mg L−1 of Cd2+. It was worth noting that the removal rate of Cd2+ by strain JLS17 was less affected by the concentration, and the removal rate was always maintained at 41.9%–66.0%. The amount of Cd2+ removed by strain JLS17 was up to 55.9 mg L−1 at the concentration of 100 mg L−1.
Fig. 3.
Cd2+ removal of each strain individually cultured in LB medium with different Cd2+ concentrations. The error bars in the figures represent the standard deviations from the mean.
3.5. PGP properties of strains
The PGP properties for all strains were showed in Table 2. There was a significant (p < 0.05) difference in PGP properties among the 7 strains. All strains had the capacity to solubilize calcium phosphate with PSI ranging from 1.65 to 6.08, and strain JLS 69 had the highest phosphate solubilization capacity. Strains JLS30, JLS50 and YTG72 were able to produce IAA, with strain JLS50 being the most productive (27.26 μg mL−1). The majority proportion (71%) of strains could produce nitrogenase, among which strain JLS32 had the strongest nitrogenase activity (81.32 U L−1). More than half of the strains had ACC deaminase activity, with strain JLS50 having the highest enzyme activity of 160.21 pg mL−1. In brief, all strains had PGP properties, and some of them, such as strain YTG72, JLS17, JLS30, and JLS32 had more than three PGP properties, demonstrating that the tested strains had the potential to promote the growth of plants.
3.6. Effect of inoculating strains on Cd speciation of contaminated soil
After the addition of CdCl2·2.5H2O to the soil, the final soil Cd concentration for each contamination level was 4.216 mg kg−1 (low), 6.596 mg kg−1 (medium) and 9.999 mg kg−1 (high), respectively. The results of the different fractions of Cd concentration in the soil in each treatment are given in Table 3. The concentrations of different fractions of Cd in soil were higher at increased Cd levels. The concentration of four fractions of Cd in the soil were acid-soluble state > reducible state > residual state > oxidizable state. The acid-soluble Cd concentration was the highest, accounting for 90% of the total Cd concentration, and the reducible, oxidizable, and residual Cd accounted for about 5%, 1% and 2%–5% of the total Cd concentration respectively. Compared to the uninoculation treatment (CK), treatment with strain JLS32 significantly (p < 0.05) increased the acid-soluble Cd concentration in all contaminated soil, while strain JLS17 and JLS50 increased the acid-soluble Cd concentration of the soil in low-medium and medium-high levels of soil, respectively. The reducible, oxidizable and residual Cd did not differ much between the treatments with strains in the low contaminated soil. However, when the soil contamination level increased, treatment with strain YTG72 resulted in a decrease in the concentration of reducible Cd and oxidizable Cd in the soil. In addition, the residual Cd concentration in the soil treated with the strain showed a decreasing trend.
Table 3.
Cd concentration (mg kg−1) of each fraction-BCR in soils with three levels of contamination.
| Contamination level | Strain | Acid-soluble | Reducible | Oxidizable | Residual |
|---|---|---|---|---|---|
| Low | JLS17 | 3.822 a | 0.227 ab | 0.053 b | 0.183 b |
| JLS32 | 3.817 a | 0.192 b | 0.054 b | 0.205 ab | |
| JLS50 | 3.682 b | 0.224 ab | 0.052 b | 0.207 ab | |
| YTG72 | 3.718 b | 0.222 ab | 0.055 b | 0.191 ab | |
| CK | 3.665 b | 0.235 a | 0.062 a | 0.212 a | |
| Medium | JLS17 | 5.961 a | 0.367 a | 0.081 c | 0.204 b |
| JLS32 | 5.971 a | 0.359 a | 0.081 c | 0.232 b | |
| JLS50 | 5.968 a | 0.302 b | 0.073 c | 0.296 a | |
| YTG72 | 5.843 b | 0.312 b | 0.095 b | 0.287 a | |
| CK | 5.772 b | 0.367 a | 0.113 a | 0.295 a | |
| High | JLS17 | 8.955 b | 0.549 a | 0.170 bc | 0.273 b |
| JLS32 | 9.230 a | 0.417 c | 0.168 bc | 0.244 c | |
| JLS50 | 9.166 a | 0.441 c | 0.178 ab | 0.254 c | |
| YTG72 | 8.999 b | 0.489 b | 0.164 bc | 0.284 b | |
| CK | 8.981 b | 0.522 a | 0.186 a | 0.308 a |
Mean values denoted by the same letter in columns indicate no significant difference according to Duncan's test at p < 0.05.
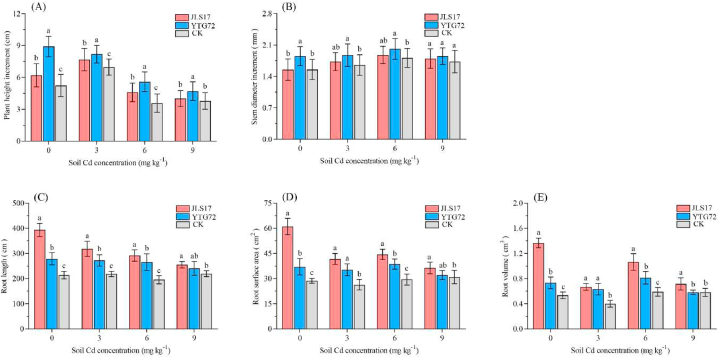
3.7. Effect of inoculating strains on the growth of P. elliottii under Cd stress
The growth indicators of P. elliottii are shown in Fig. 4. Both strains JLS17 and YTG72 showed growth-promoting effects on P. elliottii seedlings in the presence/absence of Cd contamination. Among the inoculation treatments under different Cd contamination levels, strain YTG72 showed more pronounced promotion in terms of plant height increment (17.1%–71.2%) (Fig. 4A) and stem diameter increment (6.9%–19.4%) compared to the control (Fig. 4B), whereas strain JLS17 showed more pronounced promotion in terms of root length (16.1%–84.0%) (Fig. 4C), root surface area (17.5%–113.0%) (Fig. 4D), and root volume (24.1%–153.7%) (Fig. 4E) of P. elliottii. The plant growth-promoting effect of the strain gradually decreased with increasing levels of Cd contamination. These results verified that candidate strains had growth-promoting effects on plants under certain levels of Cd stress.
Fig. 4.
Growth response of P. elliottii seedlings inoculated with strain JLS17 and YTG72. Different lowercase letters represent significant differences at p < 0.05. The error bars in the figures represent the standard deviations from the mean.
4. Discussion
The endophytic bacteria isolated in the study belonged to the Burkholderia, Pseudomonas, Pantoea, and Herbaspirillum genera. These genera are reported to be common bacteria that grow in HMs contaminated soils and plants, which tend to exhibit the tolerance to HMs [10,29]. Siderophore is considered to be an important factor in improving microbial and plant resistance to HMs toxicity [30]. For example, strain Pseudomonas aeruginosa with siderophore-producing capacity had stronger HMs tolerance than the same strain without this capacity, and that siderophores reduced concentrations of metal ions such as Cu2+, Ni2+, Pb2+, and Zn2+ in the strains to varying degrees [31]. When the SU value exceeds 60%, the strain can be considered to have a high siderophore production [32]. Six of the strains in our study had SU values above 70%, indicating their high capacity to produce siderophore. The capacity of endophytic bacteria to produce siderophore depends largely on their hereditary property, for example, most of the siderophore-producing strains in this study belong to the Pseudomonas genus. The difference in the source plant also affects this capacity. The strain H. huttiense JLS72 isolated from R. simsii root had a high production of siderophore (77.24%), while this capacity was not found for the strain H. huttiense AW1 isolated from the rhizosphere of Solanum nigrum by Jiang et al. [33]. Other factors such as external pH, temperature, carbon sources and the presence of other metals can also stimulate or inhibit the production of siderophores [11]. According to the characteristic functional groups, siderophores have been classified as catecholate, hydroxamate, carboxylate, and mixed types, of which hydroxamate being the most common type and more capable of chelating Fe ions [34,35]. All 7 strains produced hydroxamate siderophore, which was reported to can be used for metal binding because of its preferential affinity for Cd ions as compared with Fe ions [14,36]. Some hydroxamate siderophores can effectively leached out Fe–Mn oxide state, organic matter bound state, and residual state Cd, and activated Cd with poor bioavailability in the contaminated soil [15].
An important prerequisite for applying theses strains to remediation of contaminated soil is the resistance to HMs. The negative correlations between the biomass and Cd concentration indicated that Cd induced the growth inhibition of strains. The toxicity of Cd is reflected in its damage to cell membranes and DNA structure, therefore, only a few bacteria can survive in a certain concentration of HMs environment [37]. Nevertheless, these strains showed higher levels of tolerance to cadmium compared to previous studies, with 71.4% of the strains being able to tolerate Cd concentrations above 1.5 mM, and strain B. contaminans JLS17 showed the best tolerance to Cd. Members of the genus Burkholderia is one of the most versatile and adaptable genera, including the most tolerant species to HMs [[38], [39], [40]]. Differences in Cd resistance of strains may be due to the presence of different Cd resistance genes, or having different detoxification mechanisms. For instance, Burkholderia contaminans, Pseudomonas koreensi, and Pantoea sp. possess resistant genes for cadmium (CzcD), which contribute to the export of Cd from the cytoplasm and/or periplasm into the extracellular medium for detoxification [[40], [41], [42]]. In addition, endophytic bacteria can bind to Cd through secreted siderophores, extracellular polysaccharides and metalloproteins to prevent entry into the cell, and some bacteria can also adapt to Cd stress through intracellular isolation and activation of antioxidant systems [43,44].
The removal of metal ions by endophytic bacteria can be affected by different microbial species, metal types and ion concentrations [45]. According to the relevant records, Burkholderia, Pseudomonas, Pantoea, and Herbaspirillum genus of bacteria are Gram-negative bacteria, and these bacteria can accumulate metal ions in the periplasm [[46], [47], [48]]. Also, their cell wall possesses unique major components of lipopolysaccharide and lipoprotein that bind the metal onto the cell envelope [43,49,50]. Most strains showed decreasing removal rates within continuously elevated Cd2+ concentration of 10–100 mg L−1, which may be due to the fact that continuously elevated Cd toxicity reduced the biomass of the strain, and decreased the binding sites and surface area available for Cd2+ [51]. However, the Cd2+ removal by strain JLS17 did not decrease significantly as most strains did, and interestingly, higher concentration seemed to promote the Cd2+ removal. Similar results were found in previous researches. The Burkholderia cenocepacia YG-3 removed Cd up to about 60.0% and did not decrease with increasing initial Cd concentration [43]. And Burkholderia cepacia AL96Co had 80% removal at low Cd concentrations (100 mg L−1) while higher removal of 92.44% at high concentrations (500 mg L−1) [49]. This suggests that strain JLS17 may have different Cd2+ adsorption mechanisms compared to other strains. At low concentrations, the strain may be dominated by intracellular accumulation, and as the concentration increases, the mode of Cd2+ removal by the strain shifts from intracellular accumulation to extracellular adsorption [43]. Furthermore, the enhanced Cd adsorption may be due to an increase in electrostatic interactions, associated with covalent interactions, which is confirmed by a previous report [49,52].
The PGP properties of endophytic bacteria are key factors for microbial-assisted phytoremediation of HMs contaminated soil. Properties such as phosphate solubilization, nitrogen fixation, and production of IAA and ACC deaminase can promote plant growth and contribute to the extraction and transport of metals by plants. Five strains (71.4%) had high phospholysis capacity (PSI > 2). The Cd-tolerant phosphate solubilizing endophytic bacterium Bacillus subtilis VITATJM4 (PSI = 2.14) was reported to promote elevated Cd levels in Pennisetum purpureum accumulating from the soil to its tissues, predominantly in the roots, thereby reducing Cd toxicity [53]. Metal accumulation was also reported to be influenced by soil nitrogen levels, such as Leucaena leucocephala grown on high nitrogen (150 mg N dm−3) showed the greatest accumulation of metals [54]. Nitrogenase activity was detected in more than half of the strains (71.4%), and they might be able to increase the nitrogen content of the soil to promote Cd accumulation in plants. The IAA produced by microorganisms has been certified to promote the growth of roots and stems of metal-stressed plants and to increase the metals accumulation [55,28]. The B. contaminans ZCC found by You et al. [40] produced 10.29 mg L−1 of IAA, whereas in the present study, no IAA production was observed for the similar strain B. contaminans JLS17. This difference may be related to the type of soil and host plant [18]. The ACC deaminase produced by bacteria can reduce the level of ethylene produced in plants induced by Cd stress, which helps alleviate stress damage in plants under a variety of stress conditions [56]. The ACC deaminase activity was detected in some tested strains, indicating that they may mitigate Cd toxicity to plants under Cd stress. According to Carlos et al. [57], the ACC deaminase-producing strain Serratia K120 had higher ACC deaminase activity in the presence of HMs and protected Helianthus annuus from growth inhibition caused by HMs in the soil. The endophytic bacteria of the Burkholderia, Pseudomonas, Pantoea, and Herbaspirillum genera such as strains JLS17, JLS32, JLS 50, YTG72 can dissolve phosphorus or can produce IAA, ACC deaminase, nitrogenase, which may contribute to the phytoremediation of contaminated sites.
Considering the potential unclear synergistic or antagonistic effects between different strains, a single inoculation was used to verify the growth-promoting effect of PGPEB on plants. Indeed, the pot experiment verified part of our assumptions that the inoculation treatment effectively promoted the growth of P. elliottii seedlings with/without Cd stress. The strains were both beneficial in helping P. elliottii resist Cd stress, even in the soil where Cd was activated. It is noteworthy that strain YTG72 mainly promoted the growth of above-ground parts, while strain JLS17 mainly promoted the growth of root system, indicating that there are differences in the way strains promote plant growth. With similar PGP properties, this difference may be related to the secondary metabolites, hormones, and various signal compounds secreted by strains [58]. How the candidate strains mitigate the toxic effects of Cd on P. elliottii (e.g. biomass allocation, nutrient uptake, and antioxidant system feedback) and what effects they have on Cd accumulation in P. elliottii remain to be elucidated in the next studies.
The first three fractions especially for the acid-soluble state of HMs extracted by modified BCR signified potential bioavailability of metals in soils [32]. The percentages of acid-soluble Cd in each control (with the addition of sterile medium) in this study were around 90%, which was much higher than the previous studies [32,59]. The main reasons may be that the artificially added Cd ions, which are more chemically reactive. Moreover, by measuring soil pH, the soils of different contamination levels exhibited strong acidity, with pH of 4.89 (CK), 4.85 (low), 4.77 (medium), and 4.74 (high), thus leading to a greater percentage of acid-soluble Cd in the soil. Inoculation with endophytic bacteria could alter the speciation of Cd in contaminated soil after 30 d incubation. Strains JLS17, JLS32, and JLS50 were able to promote the increase of acid-soluble Cd concentration at different contamination levels, and their metabolites may have solubilized the metals and alter the speciation of metals [60]. It has been reported that siderophore-producing bacteria may increase the solubility of HMs, and the production of IAA and phosphate solubilization also acidify the soil microenvironment, leading to a decrease in soil pH and an increase in the acid-soluble Cd concentration [16]. In addition, some tested strains may have metal metabolizing enzymes that solubilize the insoluble form metals into a soluble form [61]. Wang et al. [32] found the acid-soluble Cd transformed into the oxidizable Cd by inoculation with the endophytic bacterium Serratia marcescens PRE01, which may be attributed to the strong biosorption of Cd by strain. However, compared to the uninoculated control, the Cd concentration of the reducible, oxidizable and residual fractions decreased and the Cd concentration of the acid-soluble fraction increased in each treatment, such a trend indicates that strains JLS17, JLS32, and JLS50 are mainly solubilizing Cd in altering the Cd speciation. The application of bacteria with dissolved HMs can effectively improve the HMs bioavailability and help promote the uptake of HMs by plants [62].
5. Conclusions
Seven strains of Cd-tolerant and hydroxamate siderophores-producing endophytic bacteria were isolated from roots of selected dominant woody plants in Cd-contaminated tailing areas. Sequence analysis of the 16S rRNA gene classified the isolated strains as belonging to the genera Burkholderia, Pseudomonas, Pantoea, and Herbaspirillum. These strains had good tolerance and removal of Cd2+ from culture medium, especially strain JLS17. Besides siderophores production, all strains had two or more PGP properties and pot experiments demonstrated also that both strains JLS17 and YTG72 had growth-promoting effects on P. elliottii. Inoculation of soil with strains JLS17, JLS32 and JLS50 increased the acid-soluble Cd concentration and reduced the concentration of other insoluble Cd in soils with different levels of contamination, indicating that the strains had the ability to activate soil Cd. Therefore, these strains are expected to be applied as remediation agents for endophyte-assisted phytoremediation of Cd-contaminated soils to regreen mines. The related mechanisms and the effect of the strains on plant Cd uptake still need to be further investigated and elucidated in the study.
Data availability statement
Data will be made available on request.
Additional information
Supplementary content related to this article has been published online at [URL].
CRediT authorship contribution statement
Yanglong Li: Investigation, Data curation, Formal analysis, Methodology, Writing – original draft. Shumeng Wei: Formal analysis, Software. Xiangteng Chen: Investigation. Yuhong Dong: Methodology, Data curation. Mansheng Zeng: Resources. Chaowu Yan: Resources. Lingyu Hou: Validation. Ruzhen Jiao: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the horizontal project “Screening and evaluation of highly enriched plants for remediation of heavy metal contaminated soil” from Standard Technology Engineering (Qingdao) Company Limited.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e17661.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sharma R.K., Archana G. Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl. Soil Ecol. 2016;107:66–78. doi: 10.1016/j.apsoil.2016.05.009. [DOI] [Google Scholar]
- 2.Gallego S.M., Pena L.B., Barcia R.A., Azpilicueta C.E., Iannone M.F., Rosales E.P., Zawoznik M.S., Groppa M.D., Benavides M.P. Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ. Exp. Bot. 2012;83:33–46. doi: 10.1016/j.envexpbot.2012.04.006. [DOI] [Google Scholar]
- 3.Zheng Y.T., Xiao C.Q., Chi R. Remediation of soil cadmium pollution by biomineralization using microbial-induced precipitation: a review. World J. Microbiol. Biotechnol. 2021;37:208. doi: 10.1007/s11274-021-03176-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y.J., Liu C., Zhou D.M., Chen H.M. A Critical view on the status quo of the farmland soil environmental quality in China: discussion and suggestion of relevant issues on Report on the national general survey of soil contamination. J. Agro-Environ. Sci. 2014;33:1465–1473. doi: 10.11654/jaes.2014.08.001. [in Chinese] [DOI] [Google Scholar]
- 5.Liu X.J., Tian G.J., Jiang D., Zhang C., Kong L.Q. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ. Sci. Pollut. Res. Int. 2016;23:17941–17952. doi: 10.1007/s11356-016-6968-7. [DOI] [PubMed] [Google Scholar]
- 6.Luo Z.B., He J.L., Polle A., Rennenberg H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016;34:1131–1148. doi: 10.1016/j.biotechadv.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Mahar A., Wang P., Ali A., Awasthi M.K., Lahori A.H., Wang Q., Li R.H., Zhang Z.Q. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol. Environ. Saf. 2016;126:111–121. doi: 10.1016/j.ecoenv.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y.R., Zhang X.Y., Deng J.Y., Zhao Q.Q., Xu H. Lead and cadmium-induced oxidative stress impacting mycelial growth of Oudemansiella radicata in liquid medium alleviated by microbial siderophores. World J. Microbiol. Biotechnol. 2012;28:1727–1737. doi: 10.1007/s11274-011-0983-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J.Y., Li P., Meng D.L., Gu Y.B., Zheng Z.Y., Yin H.Q., Zhou Q.M., Li J. Isolation, characterization and inoculation of Cd tolerant rice endophytes and their impacts on rice under Cd contaminated environment. Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.113990. [DOI] [PubMed] [Google Scholar]
- 10.Begum N., Afzal S., Zhao H.H., Lou L.Q., Cai Q.S. Shoot endophytic plant growth-promoting bacteria reduce cadmium toxicity and enhance switchgrass (Panicum virgatum L.) biomass. Acta Physiol. Plant. 2018;40:170. doi: 10.1007/s11738-018-2737-1. [DOI] [Google Scholar]
- 11.Roskova Z., Skarohlid R., McGachy L. Siderophores: an alternative bioremediation strategy? Sci. Total Environ. 2022;819 doi: 10.1016/j.scitotenv.2022.153144. [DOI] [PubMed] [Google Scholar]
- 12.Schalk I.J., Hannauer M., Braud A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011;13:2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 13.Dimkpa C.O., Merten D., Svatos A., Buchel G., Kothe E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009;107:1687–1696. doi: 10.1111/j.1365-2672.2009.04355.x. [DOI] [PubMed] [Google Scholar]
- 14.Khan A., Gupta A., Singh P., Mishra A.K., Ranjan R.K., Srivastava A. Siderophore-assisted cadmium hyperaccumulation in Bacillus subtilis. Int. Microbiol. 2020;23:277–286. doi: 10.1007/s10123-019-00101-4. [DOI] [PubMed] [Google Scholar]
- 15.Yi S.W., Li F., Wu C., Wei M., Tian J., Ge F. Synergistic leaching of heavy metal-polycyclic aromatic hydrocarbon in co-contaminated soil by hydroxamate siderophore: role of cation-pi and chelation. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127514. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Huang W., Li Y.Q., Yu F.B., Penttinen P. Isolation, characterization, and evaluation of a high-siderophore-yielding bacterium from heavy metal-contaminated soil. Environ. Sci. Pollut. Res. Int. 2022;29:3888–3899. doi: 10.1007/s11356-021-15996-8. [DOI] [PubMed] [Google Scholar]
- 17.Chen L.A., Luo S.L., Xiao X.A., Guo H.J., Chen J.L., Wan Y., Li B., Xu T.Y., Xi Q.A., Rao C. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl. Soil Ecol. 2010;46:383–389. doi: 10.1016/j.apsoil.2010.10.003. [DOI] [Google Scholar]
- 18.Chen J.Q., Zhao G.Y., Wei Y.H., Dong Y.H., Hou L.Y., Z Jiao R. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci. Rep. 2021;11:9081. doi: 10.1038/s41598-021-88635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson J.D., Higgins D.G., Gibson T.J., Clustal W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander D.B., Zuberer D.A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils. 1991;12:39–45. doi: 10.1007/bf00369386. [DOI] [Google Scholar]
- 22.Baakza A., Vala A.K., Dave B.P., Dube H.C. A comparative study of siderophore production by fungi from marine and terrestrial habitats. J. Exp. Mar. Biol. Ecol. 2004;311:1–9. doi: 10.1016/j.jembe.2003.12.028. [DOI] [Google Scholar]
- 23.Csaky T.Z. On the estimation of bound hydroxylamine in biological materials. Acta Chem. Scand. 1948;2:450–454. doi: 10.3891/acta.chem.scand.02-0450. [DOI] [Google Scholar]
- 24.Morales A., Alvear M., Valenzuela E., Castillo C.E., Borie F. Screening, evaluation and selection of phosphate-solubilising fungi as potential biofertiliser. J. Soil Sci. Plant Nutr. 2011;11:89–103. doi: 10.4067/s0718-95162011000400007. [DOI] [Google Scholar]
- 25.Patten C.L., Glick B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauret G., Lopez-Sanchez J.F., Sahuquillo A., Rubio R., Davidson C., Ure A., Quevauviller P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999;1:57–61. doi: 10.1039/a807854h. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.L., Wang C.Q., Yan C.W., Liu S.W., Chen X.T., Zeng M.S., Dong Y.H., Jiao R.Z. Heavy metal concentrations and accumulation characteristics of dominant woody plants in iron and lead−zinc tailing areas in Jiangxi, Southeast China. Forests. 2023;14:846. doi: 10.3390/f14040846. [DOI] [Google Scholar]
- 28.Minari G.D., Saran L.M., Constancio M.T.L., da Silva R.C., Rosalen D.L., de Melo W.J., C Alves L.M. Bioremediation potential of new cadmium, chromium, and nickel-resistant bacteria isolated from tropical agricultural soil. Ecotoxicol. Environ. Saf. 2020;204 doi: 10.1016/j.ecoenv.2020.111038. [DOI] [PubMed] [Google Scholar]
- 29.Abou-Shanab R.A.I., van Berkum P., Angle J.S. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere. 2007;68:360–367. doi: 10.1016/j.chemosphere.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Tamariz-Angeles C., Huaman G.D., Palacios-Robles E., Olivera-Gonzales P., Castaneda-Barreto A. Characterization of siderophore-producing microorganisms associated to plants from high-Andean heavy metal polluted soil from Callejon de Huaylas (Ancash, Peru) Microbiol. Res. 2021;250 doi: 10.1016/j.micres.2021.126811. [DOI] [PubMed] [Google Scholar]
- 31.Braud A., Geoffroy V., Hoegy F., Mislin G.L.A., Schalk I.J. Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Environ. Microbiol. Rep. 2010;2:419–425. doi: 10.1111/j.1758-2229.2009.00126.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Lin H., Dong Y.B., He Y.H., Liu C.J. Isolation of vanadium-resistance endophytic bacterium PRE01 from Pteris vittata in stone coal smelting district and characterization for potential use in phytoremediation. J. Hazard Mater. 2018;341:1–9. doi: 10.1016/j.jhazmat.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 33.Jiang M., Zhang D., Zhi Y.E., Wulan E., Zhou P. Isolation, screening and identification of plant growth promoting rhizobacteria to enrich cadmium accumulation in Solanum nigrum L. Microbiol. China. 2019;46:2231–2240. doi: 10.13344/j.microbiol.china. 180654. [in Chinese] [DOI] [Google Scholar]
- 34.Khan A., Singh P., Srivastava A. Synthesis, nature and utility of universal iron chelator - siderophore: a review. Microbiol. Res. 2018;212:103–111. doi: 10.1016/j.micres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Boukhalfa H., Crumbliss A.L. Chemical aspects of siderophore mediated iron transport. Biometals. 2002;15:325–339. doi: 10.1023/a:1020218608266. [DOI] [PubMed] [Google Scholar]
- 36.Hamidpour M., Karamooz M., Akhgar A., Tajabadipour A., Furrer G. Adsorption of cadmium and zinc onto micaceous minerals: effect of siderophore desferrioxamine B. Pedosphere. 2019;29:590–597. doi: 10.1016/s1002-0160(17)60384-9. [DOI] [Google Scholar]
- 37.Liu C.J., Lin H., Dong Y.B., Li B., Wang L. Identification and characterization of plant growth-promoting endophyte RE02 from Trifolium repens L. in mining smelter. Environ. Sci. Pollut. Res. Int. 2019;26:17236–17247. doi: 10.1007/s11356-019-04904-w. [DOI] [PubMed] [Google Scholar]
- 38.Compant S., Nowak J., Coenye T., Clement C., Barka E.A. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol. Rev. 2008;32:607–626. doi: 10.1111/j.1574-6976.2008.00113.x. [DOI] [PubMed] [Google Scholar]
- 39.Dourado M.N., Martins P.F., Quecine M.C., Piotto F.A., Souza L.A., Franco M.R., Tezotto T., Azevedo R.A. Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann. Appl. Biol. 2013;163:494–507. doi: 10.1111/aab.12066. [DOI] [Google Scholar]
- 40.You L.X., Zhang R.R., Dai J.X., Lin Z.T., Li Y.P., Herzberg M., Zhang J.L., Al-Wathnani H., Zhang C.K., Feng R.W. Potential of cadmium resistant Burkholderia contaminans strain ZCC in promoting growth of soy beans in the presence of cadmium. Ecotoxicol. Environ. Saf. 2021;211 doi: 10.1016/j.ecoenv.2021.111914. [DOI] [PubMed] [Google Scholar]
- 41.Legatzki A., Grass G., Anton A., Rensing C., Nies D.H. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 2003;185:4354–4361. doi: 10.1128/JB.185.15.4354-4361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayangbenro A.S., Babalola O.O., Aremu O.S. Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere. 2019;231:113–120. doi: 10.1016/j.chemosphere.2019.05.092. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Zhang X., Liu X.M., Huang Z.L., Niu S.Q., Xu T., Zeng J.R., Li H., Wang T.F., Gao Y. Physiological, biochemical and proteomic insight into integrated strategies of an endophytic bacterium Burkholderia cenocepacia strain YG-3 response to cadmium stress. Metallomics. 2019;11:1252–1264. doi: 10.1039/c9mt00054b. [DOI] [PubMed] [Google Scholar]
- 44.Dourado M.N., Souza L.A., Martins P.F., Peters L.P., Piotto F.A., Azevedo R.A. Burkholderia sp. SCMS54 triggers a global stress defense in tomato enhancing cadmium tolerance. Water Air Soil Pollut. 2014;225:2159. doi: 10.1007/s11270-014-2159-7. [DOI] [Google Scholar]
- 45.Sun L.N., Guo Y.X., Hou X.T., Zhuang J., Yang Z.Z., Chen Z.J., Tian W. Effect of cadmium-tolerant and immobilizing bacteria on cadmium uptake in different wheat cultivars. J. Agro-Environ. Sci. 2020;39:1878–1887. doi: 10.11654/jaes.2020-0291. [in Chinese] [DOI] [Google Scholar]
- 46.Arvind G., Sood S., Rahi P., Thakur R., Chauhan S., Chadha I.C.N. Diversity analysis of diazotrophic bacteria associated with the roots of tea (Camellia sinensis (L.) O. Kuntze) J. Microbiol. Biotechnol. 2011;21:545–555. doi: 10.4014/jmb.1012.12022. [DOI] [PubMed] [Google Scholar]
- 47.Blair J.M.A., Richmond G.E., Piddock L.J.V. Multidrug efflux pumps in gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014;9:1165–1177. doi: 10.2217/fmb.14.66. [DOI] [PubMed] [Google Scholar]
- 48.Cruz D., Cisneros R., Benitez A., Zuniga-Sarango W., Pena J., Fernandez H., Jaramillo A. Gram-negative bacteria from organic and conventional agriculture in the hydrographic basin of Loja: quality or pathogen reservoir? Agronomy. 2021;11:2362. doi: 10.3390/agronomy11112362. [DOI] [Google Scholar]
- 49.Oyetibo G.O., Ilori M.O., Obayori O.S., Amund O.O. Equilibrium studies of cadmium biosorption by presumed non-viable bacterial strains isolated from polluted sites. Int. Biodeterior. Biodegrad. 2014;91:37–44. doi: 10.1016/j.ibiod.2014.03.004. [DOI] [Google Scholar]
- 50.Khan Z., Nisar M.A., Hussain S.Z., Arshad M.N., Rehman A. Cadmium resistance mechanism in Escherichia coli P4 and its potential use to bioremediate environmental cadmium. Appl. Microbiol. Biotechnol. 2015;99:10745–10757. doi: 10.1007/s00253-015-6901-x. [DOI] [PubMed] [Google Scholar]
- 51.Oyetibo G.O., Ilori M.O., Obayori O.S., Amund O.O. Metal biouptake by actively growing cells of metal-tolerant bacterial strains. Environ. Monit. Assess. 2015;187:525. doi: 10.1007/s10661-015-4731-z. [DOI] [PubMed] [Google Scholar]
- 52.Puranik P.R., Paknikar K.M. Biosorption of lead, cadmium, and zinc by Citrobacter strain MCM B-181: characterization studies. Biotechnol. Prog. 1999;15:228–237. doi: 10.1021/bp990002r. [DOI] [PubMed] [Google Scholar]
- 53.Viji A.S., Antony B.T., Wagh M.S., Osborne W.J. Bioremoval of cadmium by co-cultivated bacterial strains, Bacillus paramycoides and Bacillus subtilis, in a pilot-scale phyto- and rhizoremediation approach. Int. J. Environ. Sci. Technol. 2021;19:7565–7574. doi: 10.1007/s13762-021-03540-7. [DOI] [Google Scholar]
- 54.Rangel W.M., Thijs S., Janssen J., Longatti S.M.O., Bonaldi D.S., Ribeiro P.R.A., Jambon I., Eevers N., Weyens N., Vangronsveld J. Native rhizobia from Zn mining soil promote the growth of Leucaena leucocephala on contaminated soil. Int. J. Phytoremediation. 2017;19:142–156. doi: 10.1080/15226514.2016.1207600. [DOI] [PubMed] [Google Scholar]
- 55.Fassler E., Evangelou M.W., Robinson B.H., Schulin R. Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS) Chemosphere. 2010;80:901–907. doi: 10.1016/j.chemosphere.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 56.Ali S., Charles T.C., Glick B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014;80:160–167. doi: 10.1016/j.plaphy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Carlos M.H.J., Stefani P.V.Y., Janette A.M., Melani M.S.S., Gabriela P.O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 2016;188-189:53–61. doi: 10.1016/j.micres.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Bhat B.A., Tariq L., Nissar S., Islam S.T., UI Islam S., Mangral Z., Ilyas N., Sayyed R.Z., Muthusamy G., Kim W., Dar T.U. The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 2022;133:2717–2741. doi: 10.1111/jam.15796. [DOI] [PubMed] [Google Scholar]
- 59.Akkajit P., DeSutter T., Tongcumpou C. Fractionation of Cd and Zn in Cd-contaminated soils amended by sugarcane waste products from an ethanol production plant. J. Soils Sediments. 2013;13:1057–1068. doi: 10.1007/s11368-013-0691-5. [DOI] [Google Scholar]
- 60.Praburaman L., Park S.H., Cho M., Lee K.J., Ko J.A., Han S.S., Lee S.H., Kamala-Kannan S., Oh B.T. Significance of diazotrophic plant growth-promoting Herbaspirillum sp. GW103 on phytoextraction of Pb and Zn by Zea mays L. Environ. Sci. Pollut. Res. Int. 2017;24:3172–3180. doi: 10.1007/s11356-016-8066-2. [DOI] [PubMed] [Google Scholar]
- 61.Narayanan M., Kumarasamy S., Ranganathan M., Kandasamy S., Kandasamy G., Gnanavel K. Enzyme and metabolites attained in degradation of chemical pesticides β Cypermethrin by Bacillus cereus. Mater. Today: Proc. 2020;33:3640–3645. doi: 10.1016/j.matpr.2020.05.722. [DOI] [Google Scholar]
- 62.Narayanan M., Ranganathan M., Kandasamy G., Kumarasamy S. Evaluation of interaction among indigenous rhizobacteria and Vigna unguiculata on remediation of metal-containing abandoned magnesite mine tailing. Arch. Microbiol. 2021;203:1399–1410. doi: 10.1007/s00203-020-02115-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.