Abstract
The clear cell subtype of kidney cancer encompasses most renal cell carcinoma cases and is associated with the loss of von Hippel-Lindau gene function or expression. Subsequent loss or mutation of the other allele influences cellular stress responses involving nutrient and hypoxia sensing. Autophagy is an important regulatory process promoting the disposal of unnecessary or degraded cellular components, tightly linked to almost all cellular processes. Organelles and proteins that become damaged or that are no longer needed in the cell are sequestered and digested in autophagosomes upon fusing with lysosomes, or alternatively, released via vesicular exocytosis. Tumor development tends to disrupt the regulation of the balance between this process and apoptosis, permitting prolonged cell survival and increased replication. Completed trials of autophagic inhibitors using hydroxychloroquine in combination with other anticancer agents including rapalogues and high dose Interleukin 2 have now been reported. The complex nature of autophagy and the unique biology of clear cell renal cell carcinoma warrant further understanding to better develop the next generation of relevant anticancer agents.
Keywords: Renal cell carcinoma, autophagy, HMGB1, HIF, VHL, angiogenesis
“Life is an equilibrium state between synthesis and degradation of proteins.”; “Even now we have more questions than when I started.”
– 2016 Physiology and Medicine Nobel Laureate for Autophagy, Yoshinori Ohsumi
The initial review of clear cell renal cell carcinoma (ccRCC) and autophagy identified the importance of the disease’s unique properties as well their relation to autophagic modulation [1]. The report emphasized the link between autophagy and cellular variations of the disease, such as the altered oxygen-sensing machinery as well as immunotherapy response and mitochondrial metabolism. Clinical trials evaluating the effectiveness of autophagic modulation in conjunction with other anti-tumor therapies have indicated the potential utility of such neoadjuvant therapies for RCC. Lymphocytes can also induce cell-mediated autophagy as a means of promoting cancer cell survival [2]. Such reports advocate the importance of autophagy research for identification and development of novel therapeutic targets. ccRCC has multiple unique disease characteristics that allow for the development of targeted therapies focusing on both the tumor and the associated endothelium. Understanding the mechanisms and details of autophagy in the context of RCC will aid enable novel treatment options for this unique disease.
GENERAL MECHANISMS OF AUTOPHAGY AND RENAL INJURY
The vHL gene is largely responsible for tumor suppression through its role in oxygen sensing and protein degradation. Following translation, the vHL protein (pvHL) acts as an E3 ligase in a complex to ubiquitinate hypoxia-inducing factors (HIF), targeting them for degradation. In the presence of oxygen, HIFα subunits are hydroxylated at proline residues to which pvHL binds [3].These HIF proteins are ubiquitinated and undergo proteasomal degradation to maintain cellular homeostasis even in the absence of hypoxia [4]. In ccRCC (clear cell renal cell carcinoma), the vHL gene is deleted in cells residing within the proximal renal tubule. Complete loss of the gene can occur due to primary hereditary loss or via inactivating mutation(s) in one allele followed by a somatic mutation of the other allele within individual renal tubule cells. Bi-allelic loss of the gene on chromosome 3 promotes the development of ccRCC tumors. Carcinoma progression results from loss of tumor suppressor function mediated by vHL on HIF proteins, as well as, alternate pro-tumor modulation of the hypoxia pathway [5].
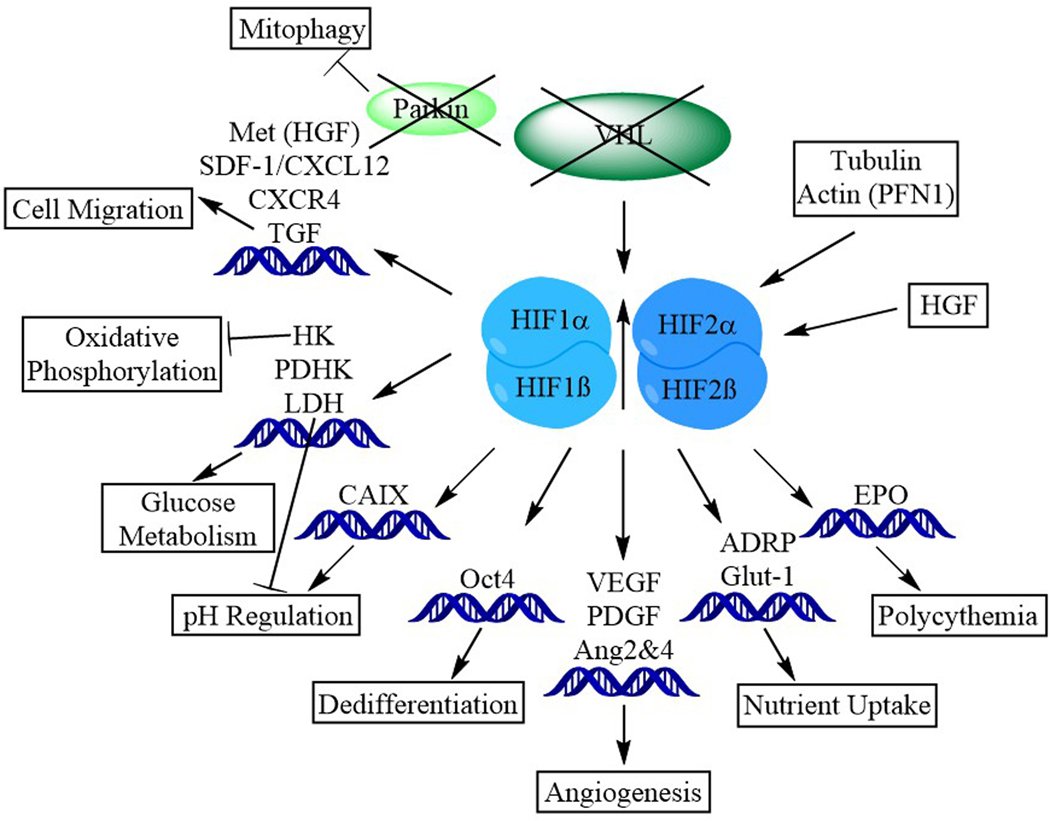
Under conditions of vHL deficiency, the cell accumulates HIFα subunits [5]. These subunits then dimerize with HIFβ and translocate into the nucleus where they serve as transcriptional mediators. It is in this case, lack of vHL protein mimics a low oxygen state and the cell responds to faux hypoxic conditions. [6] by altering its cytoskeleton and releasing proangiogenic factors. HIFα promotes tumor growth (Figure 1) through its control of several genes involved in cell division, promotion of the formation of new blood vessels, and stimulating the production of red blood cells. Specific components of the hypoxia response pathway, including gene activation favoring tumor development, are responsible for promoting the development of ccRCC [6].
Figure 1. Clear cell renal cancer is characterized by acquired or inherited defects in two multi-subunit E3 ligases.
The loss of functional parkin decreases mitophagy and, especially in the case of HIF stabilization, contributes to profound changes in cell biology. In conjunction with the influence of HGF and cytoskeletal molecules like actin and tubulin, VHL-dependent HIF stabilization leads to downstream transcriptional modulation in cell migration, oxidative phosphorylation, glucose metabolism, pH regulation, cellular differentiation, angiogenesis, nutrient uptake, and emergence of EPO-driven erythrocytosis.
Mutated ccRCC cells upregulate various tumor-promoting genes in response to the physiological stress of vHL loss. Normally the hypoxia pathway is activated in the setting of low extracellular oxygen levels, whereas these cells activate the pathway due to the loss of pvHL and accumulation of HIFs. This allows HIF to travel to the nucleus where it upregulates genes intended to increase oxygen delivery and minimize oxygen use within the cell. Stress signaling, including the physiological stress to the cells of the emergent ccRCC, is linked to activation of autophagy pathways in various settings [7]. Autophagy constitutes a process of programmed cellular survival. The transitions associated with cellular self-degradation allows the cell to catabolize intracellular components and recycle nutrients required for extended survival [1]. These ‘faux hypoxic’ conditions characteristic of ccRCC contribute to cellular dysfunction in the oxygen-sensing and autophagic pathways.
Another important factor in the progression of ccRCC is the common activation of the epithelial-mesenchymal transition (EMT) [8]. This transition transdifferentiates epithelial cells into a mesenchymal phenotype, important for its effect on altering cell-cell interaction and promoting migration. Epithelial cells of the kidney change from cells rooted by the basal membrane to elongated fibroblasts to more migratory and stem cell-like with less cell-to-cell contact [8]. EMT cells are also more resistant to chemotherapy due to enhanced autophagy. Induction of the EMT phenotype is associated with higher autophagic flux in cultured RCC cells [8].
Elevated cellular stress in ccRCC is also sensed by mitochondria which serve as rheostats to regulate downstream cell fate pathways. Autophagy is a highly evolutionarily-conserved catabolic process that predates the emergence of the metacaspases and apoptosis, serving as the most primitive cell intrinsic means to eliminate facultative intracellular organisms [1]. Damaged/senescent proteins and degraded organelles traffic to autophagosomes which migrate along tubulin tracks to a perinuclear location and, there, undergo degradation following lysosomal fusion. Dysregulation of autophagy allows pathogens to evade host defenses as well as promoting the development of malignancy [9]. Autophagy disrupting agents including chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) limit proliferation of RCC cell lines [9]. CQ inhibits autophagy by preventing fusion of autophagic vacuoles with degradative lysosomal acidic vesicular organelles [9], thereby inhibiting generation of amino acids, nucleotides, anaplerotic substrates, limiting cell proliferation and tumor growth.
SCREENS FOR AUTOPHAGY INHIBITORS, HIGH CONTENT IMAGING
Autophagy is recognized as a critical regulator in a variety of disease processes. Due to the prominence and influence of autophagic processes in disease, detection and screening for modulatory compounds and genes are an important component of RCC treatment research. Some of the common autophagy detection methods as well as screens for autophagic-flux-altering agents are discussed below. Although not restricted for use in RCC, these assays are useful in studying potentially useful agents against this form of cancer.
Flux Assays
There are several assays used to monitor the process of autophagy. A simple fluorescent dye (CytoID) allows quantification of autophagic flux. An RNAi screen performed using this dye revealed two novel autophagy modulators [10]. Another method of evaluating autophagic flux is the GFP-LC3-RFP construct, which forms a ratiometric readout, GFP disappears while RFP remains [11]. Determining the influence of the downregulation or overexpression of specific proteins in evaluating autophagic flux is to examine cell lines stably expressing eGFP-LC3 [12]. Analyzing levels of eGFP-LC3, free eGFP (generated by autophagic degradation), and endogenous LC3 yields information about autophagy-modulating compounds and autophagic flux [12]. An alternative assay engineers peptides to act as intracellular sensors specific for both LC3A and LC3B [13]. These sensors are valuable in decoding biological functions of central autophagosomal biogenesis. To screen compounds that affect the rate of autophagy, reporter systems based on induced expression of GFP-p62/SQSTM1, GFP-NBR1 or GFP-LC3B indicate that GFP-p62/SQSTM1 performed the best out of the three methods [14].
In addition, additional classical methods have been developed to monitor autophagic flux that do not rely on the use of cell reporters. For example, lysosomal inhibitors and the analysis of endogenous LC3B-II can be used to monitor autophagic flux via Western Blot of the protein. Flux induction is determined by a difference in LC3B-II levels (or SQSTM1/p62) following treatment [15], [16]. Enzyme-linked immunosorbent assays (ELISAs) have also been used to monitor autophagy following cytosolic and membrane fragmentation. Two LC3 antibodies are utilized to monitor changes in autophagosomal membrane-associated LC3-II levels compared to the LC3-I levels free within the cytosol [17]. This comparison provides insight as to the extent of autophagic flux within the cells.
Genetics / Screens
An siRNA screen identified modulators of autophagic flux by monitoring the fluorescence of GFP-SQSTM1 (an autophagy receptor) as well as its association with LAMP2-positive lysosomal vesicles (lysosomal-associated membrane protein 2) [18]. An image-based RNAi screen identifies autophagy regulators utilizing constitutive autophagy marker proteins. This screen allows for the monitoring of both early and late stages of autophagy [19].
A high-throughput screen (HTS) can be used to identify small molecules involved in autophagy. A specific HTS screen performed in yeast was able to identify autophagy modulators involved in key steps of autophagic flux [20]. In addition, a high-content, image-based, genome-wide siRNA screen, identified 141 genes required for autophagy [11]. Other interesting screens have been identified [21], [22].
Nucleus-related autophagic processes (nucleophagy) have not been extensively studied. High Content screening, coupled with Analysis (HCA), has been implemented to identify the effects of small molecules on nuclear morphology and LC3 location [23]. Image-based features from an LC3 antibody were used to identify changes induced by compound treatments. This method may be useful in instances where autophagy needs to be assessed under conditions where validated reporters are not available.
MITOPHAGY AND MITOCHONDRIAL TRANSFER
The role of mitochondrial mutations in renal cell cancer has been documented for many years, and appears to most commonly occur in non-clear cell variants [24–27]. Mitochondrial transfer between mammalian cells was first described several years ago with substantial validation in multiple types of cancer [28–34]. Genetic provision of recombinant mitochondrial fluorescent proteins in addition to dyes staining mitochondria have been utilized to image intercellular trafficking of mitochondria. Tumor-associated mitochondria harboring genetic mutations can also be tracked after transfer to endothelial cells, with the mutant gene products also serving as the foundation for development of therapeutic vaccines in murine models (RENCA) of RCC [35]. Recently, means to lineage trace cells based on acquired mitochondrial mutations have been developed[36].
CYTOSKELETON AND AUTOPHAGY
General Role of Cytoskeleton in Autophagy
The importance of the actin cytoskeleton in autophagy was realized more than two decades ago, based on observations that disruption of the actin cytoskeleton by actin-depolymerizing agents (cytochalasin D, latrunculin B) leads to impairment in starvation-induced autophagosome formation in cells [37]. Similarly, stabilization of actin filaments by phalloidin also inhibits autophagolysosome formation. This leads to increased intracellular accumulation of many proteins including myosin in hepatocytes, further exhibiting the importance of dynamic control of actin polymerization in autophagy-mediated protein degradation in cells [38]. Consistent with these initial findings, several recent studies have identified regulators of actin cytoskeletal assembly and actin cytoskeleton-related motor proteins that are important for various temporal aspects of autophagy. For example, Arp (actin-related protein) 2/3 complex, a major actin-nucleating protein, is localized in the autophagosome and required not only for its proper formation but also for transport and delivery subsequent to detachment. This is not surprising given that vesicle transport is powered by the propulsive force of Arp2/3-nucleated actin filaments [39–43]. The Arp2/3 complex is activated by a set of factors collectively known as nucleation-promoting factors (NPFs) that include proteins belonging to the WASP (Wiskott Aldrich Syndrome Proteins) family (example: N-WASP [neural WASP- the ubiquitously expressed form of WASP], WAVE (WASP-associated verprolin homology protein], WHAMM [WASP homology associated with actin, membranes and microtubules], WASH [WAS protein family homolog] complex) and JMY (junction-mediated and regulatory protein). Co-localization of WASH complexes with various markers found in the autophagosome (Atg5, SQSTM1/p62 and LC3-II) but not the autolysosome initially suggested that the role of the WASH complex was restricted to the early phases of autophagy [44] [45, 46] [47]. Increased autophagy in mouse embryos deficient in WASH implies an inhibitory role of WASP in autophagy. At the molecular level, WASH interacts with Beclin1, inhibiting the ubiquitination of Beclin1, subsequent inactivation of Vps34 activity and, as a result, suppression of autophagy [44]. However, WASH is also required for lysosomal recycling and efficient autophagic degradation [47], suggesting that WASH may both promote and inhibit specific aspects of autophagy. Other NPFs, such as WHAMM (interacting with PI3P lipids) and JMY (interacts with LC3II) also localize and participate in autophagosome formation and maturation. WHAMM is presumably the molecular mediator of the early stages of omegasome formation and phagophore expansion while JMY plays an active role in the maturation of autophagosomes subsequent to LC3-II recruitment [39, 42]. Lack of WHAMM and JMY genes in plants likely explains the indispensable role of other WASP family members such as WAVE in plant autophagy [48]. Based on the evidence of the involvement of Spire, another actin nucleating protein, in vesicle trafficking [49], it is possible that other nucleating factors besides the Arp2/3 complex could also play a role in autophagy.
Actin-binding proteins involved in barbed-end capping (CapZ) and severing/depolymerization (e.g. cofilin) are required for proper shaping of autophagosomes [50]. Profilin 1, an evolutionarily ancient actin-binding protein overexpressed in ccRCC and its associated endothelium (see below) has been linked to autophagy. Additionally, fusion of autophagosomes to lysosomes requires the involvement of HDAC6 (a ubiquitin-binding deacetylase)-mediated recruitment of the F-actin stabilizing protein cortactin to the fusion site [51]. Various classes of myosin motor proteins (myosin-I, -II and -VI) participate in trafficking of autophagy-specific proteins (such as Atg9) from the trans-Golgi network to promote autophagosome initiation and maturation, as well as in the lysosomal-autophagosomal fusion process [52] [53] [54] [55]. Actin remodeling in cells depends on the actions of several Rho-family small GTPases (Rho, Rac and Cdc42) and kinases activated downstream of Rho (such as ROCK) as they play critical role in activating NPFs (e.g. WASP family proteins), nucleation factors (e.g. formin) and myosin. Some of these signaling proteins (RhoA and Rac1) also have regulatory functions in early autophagosome formation during starvation-induced autophagy in cells [56]. Interestingly, this study demonstrated opposing effects of Rho and Rac in autophagy with RhoA/ROCK stimulating and Rac1 inhibiting autophagy, respectively. Given that Rac1 is a key activator of the Arp2/3-mediated actin assembly process, it is not intuitively obvious why Rac1 inhibits autophagy. However, as these findings are based on overexpression of constitutively active or dominant negative variants of GTPases (which are known to suppress the cellular levels of other GTPases), it would be important to further validate these findings in endogenous expression settings.
Microtubules and their motor proteins are important in autophagosome formation (requires labile microtubules) and long-distance transport of autophagosomes (requires stable microtubules) during autophagy [57] [58]. Among the two classes of microtubule motor proteins [kinesins (+end-directed) and dyneins (-end directed)], dynein is critical for centripetal movement of autophagosomes prior to their lysosomal fusion [59]. There is also evidence of the involvement of kinesins in autophagosome maturation and maintenance of lysosomal homeostasis [60] [61].
The Cytoskeleton and Autophagy in the Renal Cancer and Renal Cancer Endothelial Context
Autophagy is critical to perivascular podocyte health. As podocytes do not regenerate, some reparative mechanism to address cellular injury and to eliminate protein aggregates, such as autophagy, is required [62]. Dysregulation of autophagy is a mechanism that promotes podocyte injury. This is supported by the study of genetic mutations in a number of cytoskeletal genes including CD2AP, ACTN4, MYO1E, INF2, ARHGDIA [63]. The mammalian target of rapamycin (mTOR) pathway plays a key role in autophagy regulation. mTORC1-activated autophagy is important for cytoprotective effect in podocytes [64]. Na+/H+ exchanger-1 (NHE-1) facilitates cytoskeleton rearrangement, whereas activation of NHE-1 leads to activation of autophagy and cytoprotective effects in podocytes and cytoskeletal damage leads to NHE-1 downregulation and reduced autophagy [62]. SIRT6, a histone modifying protein, plays an important role in the maintenance of the actin cytoskeleton and promotes autophagy in podocytes. Accordingly, SIRT6 deletion exacerbates podocyte injury and proteinuria in mouse models of nephropathy [65]. Alpha(α)-actinin is a major actin cytoskeletal cross-linking protein whose mutation at K255E (K256E in the mouse) results in hereditary focal segmental glomerulosclerosis (FSGS) in humans. This mutation leads to podocyte injury through cytoskeletal disturbance, ER stress and stimulation of autophagy, further exemplifying the critical importance of proper function of cytoskeletal proteins in autophagy and podocyte health [66]. Kidney-derived c-kit+ progenitor/stem cells are involved in the regeneration of tubules of the kidney by targeting through modulation of the podocyte cytoskeleton α-actinin-4. Importantly, these c-kit+ progenitor/stem cells aided in autophagosome formation [67]. Co-localization of autophagy markers (AQP2 and LAMP1) with actin cytoskeleton-associated proteins (e.g. ERM [ezrin-radixin-moesin], cortactin) and autophagy-induced downregulation of several actin-binding proteins was found in a rat model of hypokalemia-induced nephropathy [68]. In diabetic nephropathy, dysfunction of glomerular endothelial cells is promoted by the activation of RhoA/ROCK signaling, actin cytoskeletal disturbance and impaired autophagy downstream of the action of AGE (advanced glycation products), which can be ameliorated by salvionalic acid, conferring a renoprotective effect [69]. Furthermore, renal fibrosis secondary to injury is promoted by increased apoptosis and autophagy partly due to microtubule destabilization as a result of reduced expressions of α-tubulin N-acetyltransferase and HDAC6 (an α-tubulin deacetylase).These effects are mitigated by genetic ablation of C/EBP homologous protein (CHOP) [70]. Collectively, these findings demonstrate a close connection between actin and the microtubule-associated cytoskeleton, autophagy and renal heath.
Dysregulation of actin-regulatory proteins also contributes to malignant progression of tumors. Proteomic studies have demonstrated upregulation of cytoskeletal proteins in RCC [71]. Notably, there is a compelling association between dysregulation of the actin-binding protein Profilin1 (Pfn1 – a major actin nucleotide exchange factor and promoter of actin polymerization), clinicopathological features of advanced tumors, and poor patient prognosis in RCC. Pfn1 is overexpressed in RCC and increases with stage and evidence of metastasis. Higher Pfn1 expression in the tumor as well as the associated endothelium is found in patients with higher grade tumors. Lower overall survival as well as diminished disease-free survival of clear cell RCC patients is associated with increased Pfn1 expression [72–75]. Pfn1 expression is also detected at a higher level in serum and urine samples of RCC patients, further suggesting Pfn1 may prove useful as a novel prognostic biomarker in RCC. There is already evidence of Pfn1-dependent modulation of autophagy in cancer cells albeit in a context-specific manner. In pancreatic cancer cells, Pfn1 suppresses autophagy and sensitizes these cells to radiation-induced apoptosis while in multiple myeloma cells, Pfn1 appears to stimulate autophagy through its binding to Beclin1 complex. [76, 77]. Another recent study correlated reduced expression of Pfn1 with sirtuin activator-induced stimulation of autophagic death of glioblastoma cells [78]. In addition to Pfn1, cofilin, another key regulator of actin dynamics implicated in autophagy [79, 80], is upregulated in RCC [74]. Whether upregulation of any of these actin-binding proteins promotes tumor progression and therapeutic resistance in RCC through autophagy modulation remains unclear and should be pursued in future studies.
THERAPEUTIC TARGETING OF AUTOPHAGY IN TUMOR-ASSOCIATED VASCULAR ENDOTHELIAL CELLS (VEC)
Autophagy represents a cytoprotective process in normal vascular endothelial cells (VEC) involving lysosome-mediated removal or recycling of defective proteins, lipids and sub-cellular organelles [81]. This homeostatic mechanism is accentuated under pathologic environmental conditions associated with cellular stress, including those inherent within the tumor microenvironment (TME) [81–83]. VEC-intrinsic autophagy appears crucial to neoangiogenesis based on studies performed in endothelial-specific Atg7−/− mice [84], supporting the concept that interventional strategies targeting autophagy in tumor-associated VECs may mediate substantial therapeutic efficacy [81]. Such targeted therapy may be achieved by 3 means: i.) application of autophagy inhibitors such as (hydroxy)chloroquine to neutralize the acidic pH of lysosomes and thereby limiting the autophagic process in endothelial cells within the TME, ii.) delivery of agents that remove environmental stress and “normalize” the TME [85, 86] leading to a reduced need for VEC-intrinsic autophagy and iii.) specific vaccination against autophagy-associated proteins to promote specific humoral (i.e. neutralizing/blocking antibodies) or CD8+ T cells that can interrupt VEC-intrinsic autophagy or remove/recondition pro-tumor, autophagic VECs.
Therapeutic utility of (hydroxy)chloroquine has been extensively evaluated in the context of solid vascularized forms of cancer, including renal cell carcinoma [1, 87–89]. A few clinical trials involve combination treatment of (hydroxyl)chloroquine with anti-cancer agents for treatment of RCC patients, among other forms of cancer (Table 1) [1, 90]. For instance, clinical trials combining mTOR inhibitors and hydroxychloroquine are currently being assessed in patients with advanced-stage ccRCC [91]. Translational studies report enhanced anti-RCC activity associated with hydroxychloroquine administration combined with everolimus [88] or sunitinib [9].
Table 1.
Clinical Trials of Autophagy Inhibition in Patients with Renal Cancer
| 1 | Terminated | Study of Hydroxychloroquine Before Surgery in Patients With Primary Renal Cell Carcinoma | Renal Cell Carcinoma | Hydroxychloroquine (HC) |
|---|---|---|---|---|
| 2 | Active, not recruiting | Study of Hydroxychloroquine and Aldesleukin in Renal Cell Carcinoma Patients (RCC) | Metastatic Renal Cell Carcinoma | HC and Interleukin 2 |
| 3 | Active, not recruiting | Autophagy Inhibition to Augment mTOR Inhibition: A Phase I/II Trial of RAD001 and Hydroxychloroquine in Patients With Previously Treated Renal Cell Carcinoma | Metastatic Clear Cell Renal Cell Carcinoma | HC and RAD001 |
| 4 | Active, not recruiting | Akt Inhibitor MK2206 and Hydroxychloroquine in Treating Patients With Advanced Solid Tumors, Melanoma, Prostate or Kidney Cancer | Adult Solid Neoplasm Hormone-Resistant Prostate Carcinoma Recurrent Melanoma (and 8 others) |
HC and MK2206 |
TME/vascular normalizing agents, such as metformin [92–95], have also recently been shown to inhibit autophagy in endothelial cells via intrinsic activation of the Hedgehog signaling pathway [96].
Therapeutic vaccine targeting of autophagy-associated protein antigens overexpressed by tumor cells or stromal cells (including VECs) in the TME has not been extensively studied. However, vaccination using a DNA-based vaccine encoding the SQSTM1 (p62) is broadly protective/therapeutic against tumors in mice and dogs, with treatment anti-tumor efficacy associated with immune infiltrates in the TME and with fibrotic tumor encapsulation [97–99]. The latter biologic outcome is consistent with observations from studies in which endothelial cell autophagy is inhibited, leading to the endothelial-to-mesenchymal transition (EMT) and the promotion of tissue fibrosis [100]. To the best of our knowledge, no studies have yet been reported evaluating vaccines targeting autophagy-associated proteins for the promotion of specific CD8+ T cell responses to enable targeting of tumor cells or tumor-associated VECs.
As an alternative to vaccine targeting of autophagy-specific gene products, translational models and early phase clinical trials of peptide- and gene-based vaccines targeting tumor-associated blood vessel antigens (TBVA; such as DLK1, DLK2, ENG, EphA2, HBB, NRP1, PDGFRα, RGS5, TEM1, VEGFR1, VEGFR2, amongst others) have demonstrated safety, as well as, T cell-dependent anti-angiogenic activity and anti-tumor efficacy [101–111]. Increased inflammation of tumors treated with vascular normalization (VN)-promoting agents has also been linked to the local development of tertiary lymphoid structures/organs that may serve as sites of local T cell (cross)priming that fuel expansion in the therapeutic CD8+ T cell repertoire over time on treatment [112]. Interestingly, the anti-tumor efficacy of VN-promoting autophagy inhibitors and genetic vaccines targeting the TBVA Delta-like homologue-1 (DLK1) in a murine RCC (RENCA) tumor model was tied to increased NOTCH1 signaling in tumor-associated VECs [113]. This is consistent with therapy-associated interruption of autophagy-associated degradation of the signaling Notch intracellular domain [113] in tumor-associated VECs and the coordinate maturation/senescence of tumor blood vessels [108, 114]. These results foreshadow the possible utility of tumor VEC-targeted NOTCH1 agonists as therapeutic modalities to promote/sustain VN and therapeutic immune cell infiltration/function [108, 115]. Recent studies also support therapeutic synergy when combining treatment with VN-promoting agents and modern immunotherapies, including immune checkpoint inhibitors (ICI), mTOR inhibitors, vaccines, and adoptive cell therapies [115–122].
Targeting autophagy-specific proteins to elicit immune responses holds great potential for development of future treatments, especially in combination with other therapies. Understanding the interaction between autophagy and immunity is important for further progression of research in the development of more effective RCC interventional therapies.
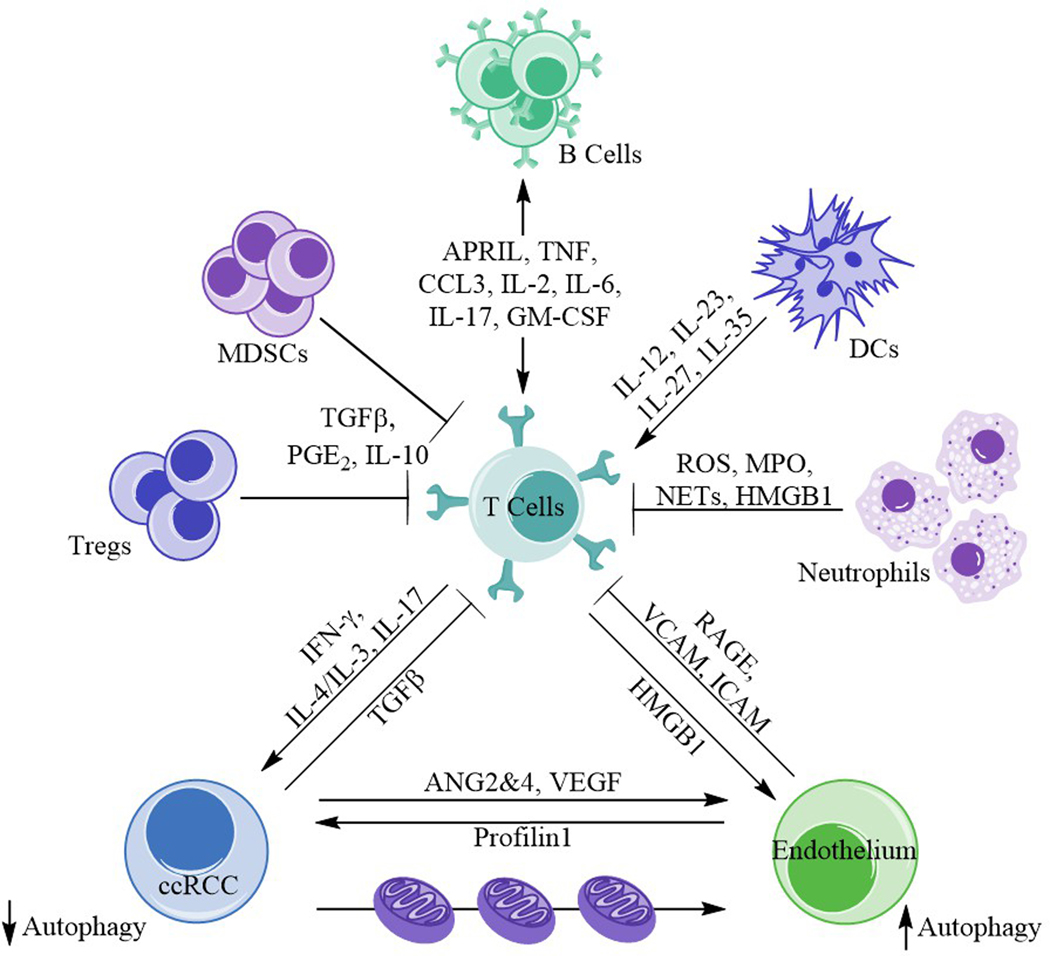
AUTOPHAGY AND IMMUNITY (Figure 2)
Figure 2. Ménage à trois in renal cancer biology.
Immune cells (T cells), endothelium, and the emergent ccRCC are involved in a complex relationship, influencing both the tumor biology and microenvironment. T cells interact, through various mechanisms and molecules, with a number of other immune cells including T regulatory cells, neutrophils, myeloid derived suppressor cells (MDSCs), dendritic cells (DCs), and B cells. The affected T cells and cancer cells interact, expressing IFN-γ, IL-4/IL-3, IL-17, and TGFβ. T cell and endothelium interaction is governed primarily with RAGE, VCAM, and ICAM upregulation subsequent to HMGB1, IL-1, TNF, and IL-4/IL-13 release. ccRCC and the endothelium interact through release of angiopoietins 2 and 4 (ANG2&4), VEGF, and profilin1. There is also the potential of mitochondrial swap between these two cell types in the setting of renal cancer. Autophagy is diminished in the tumor but enhanced in endothelial cells.
Xenophagy
Autophagy plays a crucial role in numerous processes concerning immunity and tolerance, such as antigen presentation and cross-presentation, infection and inflammation, and T cell selection in the thymus. [1, 123, 124]. Autophagy is also important for immune cells to respond to activation stimuli, as it provides metabolic substrates necessary for the shift from quiescence to activation [125, 126]. Furthermore, the autophagic machinery constitutes the first line of defense against intracellular invaders, contained in phagosomes or ubiquitinated in the cytosol, which are sequestered in autophagosomes for degradation in lysosomes in a process termed xenophagy. Adaptor proteins such as p62/SQTM1 and NDP52 bridge ubiquitinated microbes to LC3-containing isolation membranes. Through various mechanisms, various microorganisms can induce xenophagy in host cells, including Salmonella typhimurium [127], Group A Streptococcus [128], Mycobacterium tuberculosis [129] and Herpes simplex virus type 1 [130]. Not surprisingly, some pathogens have evolved mechanisms to suppress and escape xenophagy through inhibition of autophagosome formation, fusion with lysosomes or enzymatic lysosomal degradation. Pathogens delivered and cleared by the kidney may indeed utilize autophagy with altered autophagy promoting changes in function and susceptibility to cancer. Human and murine macrophages infected with Mycobacterium tuberculosis upregulate expression of miR-33, targeting several effector components of the autophagic machinery such as Atg5 and the lysosomal-associated membrane protein 1 (LAMP-1) gene, limiting not only microbial clearance but also fatty acid degradation in lysosomes. This creates a lipid-enriched environment capable of sustaining microbial survival and growth [131]. The bacteria Legionella pneumophila prevents formation of autophagosomes in infected cells via its product RavZ, which uncouples lipidated LC3 from PE and prevents their re-conjugation [132]. RavZ mediates deubiquitination of Salmonella-containing vesicles in a model of co-infection with Salmonella typhimurium and Legionella pneumophila [133]. Viruses can also regulate xenophagic activity in infected cells. Human cytomegalovirus infection limits autophagosome formation through phosphorylation of mTOR, which in turn inhibits autophagy [134]. The Herpes simplex virus type 1 product γ34.5 causes autophagosome accumulation without cargo degradation by binding Beclin-1 in infected dendritic cells [135]. Furthermore, there is increasing evidence linking evasion of xenophagic clearance of Helicobacter pylori and Epstein-Barr virus with gastric tumorigenesis [136, 137]. A role for specific pathogens in ccRCC carcinogenesis has not been demonstrated.
LC3-associated Phagocytosis
Another intersection between the autophagic machinery and the immune response is the process known as LC3-associated phagocytosis (LAP). LAP occurs in phagocytic cells through recognition of cargo carried in phagosomes by PRRs (pattern recognition receptors). It is an autophagosome-independent pathway and therefore does not recruit initial molecules of the ULK1 complex, indispensable for canonical activation of autophagy [138]. It does, however, require participation of molecules responsible for LC3 conjugation with PE, which will bind to phagosomes to form LAPosomes, engaging the autophagic machinery and ensuring rapid and efficient degradation of cargo [125]. LAP is less inflammatory than traditional phagocytosis and therefore pivotal in the clearance of dead cells (efferocytosis) to avoid autoimmunity. Cells undergoing apoptosis, necrosis and necroptosis are efficiently disposed of by macrophages through LAP, with a shift towards secretion of anti-inflammatory cytokines whereas LAP-deficient macrophages fail to degrade cargo in phagosomes and produce higher levels of IL-1β and IL-6 [138]. In the context of cancer, LAP can shape TAM polarization promoting an anti-inflammatory, M2 phenotype in vivo, following uptake of dead tumor cells in the tumor microenvironment, while LAP-deficient TAMs promote anti-tumor responses that are dependent on regulation of T cell function via type I IFN signaling [139].
Autophagy as a Cytoprotective Tool
It is well established that several types of human tumors, including renal cell carcinoma, make use of the autophagic machinery in order to survive chemotherapy and immune-mediated cellular stress [1]. Autophagy serves as a cytoprotective mechanism limiting cells involved in immunosurveillance. Both natural killer (NK) cells and T cells induce “cell-mediated autophagy” in tumor cells following direct contact in vitro, rendering them resistant to immune and radiation-induced death [2]. Accordingly, hypoxic breast cancer cells transport NK-derived granzyme B to autophagosomes for subsequent degradation in lysosomes [140]. Additionally, autophagy-deficient tumor cells are more efficiently eliminated by NK cells than their autophagy-competent counterparts in vivo. This phenomenon has been further elucidated by the findings that stabilization of HIF2α in VHL-mutated RCC cells – which are in a persistent state of “faux hypoxia” – leads to upregulation of inositol 1,4,5-triphosphate receptor type 1 (ITPR1), necessary for NK-induced cell-mediated autophagy and resistance to granzyme B [141]. In vivo silencing of ITPR1 promotes tumor regression mediated in part by NK cell immunosurveillance and lysis. Toll-like receptors and other PRRs are expressed by cells of the kidney parenchyma such as renal tubular epithelial cells (RTEC) [142, 143]. TLR4 engagement by LPS, both in vitro and in vivo, leads to enhanced cytoprotective autophagy in RTEC in a model of septic acute kidney injury (AKI). Kidneys of ATG7KO mice injected IP with LPS present with less LC3-II and more severe tubular injury than wild type mice. However, the direct mechanism linking autophagy and protection from kidney injury remains unclear [144]. A similar effect is observed in female mice injected with arsenic, whereby estrogen-mediated downregulation of autophagy via a STAT3/SOCS3 pathway worsens the effects of AKI [145].
The cytoprotective effects of autophagy are intended to protect the cell, however, in some instances, these mechanisms contribute to progression/resistance to treatment. Cellular resistance to mTOR inhibition as a consequence of cytoprotective autophagic mechanisms occurs in RCC.
mTOR AND AUTOPHAGY
mTOR is a serine/threonine kinase of the phosphoinositide kinase-related protein kinase (PIKK) family involved in cell survival, proliferation, differentiation, metabolism and autophagy. It is the catalytic subunit of two distinct complexes, mTORC1 and mTORC2. mTORC1 (mTOR, RAPTOR, PRAS40, mLST8 and DEPTOR) is classically activated by growth factors, chemokines, nutrients and cell energy status via PI3K/AKT signaling. It is alternatively activated by these same stimuli via Ras/Raf/MEK/ERK, WNT and LKB1/AMPK. mTORC1 activation promotes protein synthesis due to phosphorylation of multiple ribosomal components and induces glycolytic flux by increasing transcription and translation of HIF1α [146]. In addition, it negatively regulates autophagy by direct phosphorylation and suppression of the ULK1/Atg13/FIP200 complex, required for initiation of autophagy. In contrast, mTORC2 regulates cell survival, metabolism and spatial control of cell growth via cytoskeletal actin fibers (see above) but plays no regulatory role for autophagy [146].
The first mTOR inhibitor was isolated from bacteria found in the soil of Rapa Nui island, commonly known as Easter Island. Rapamycin is a highly selective allosteric inhibitor of the mTORC1 complex [147]. Several more water-soluble analogues with improved pharmacodynamics soon followed. Two of these “rapalogs,” temsirolimus and everolimus, have been FDA approved for treatment of patients with metastatic renal cancer based on improved overall survival compared with interferon alpha [148, 149]. Dual mTOR inhibitors target the functional domain of mTOR and suppress both the mTORC1 and mTORC2 pathways with or without co-inhibition of PI3K [146].
Resistance to mTOR inhibition has been attributed in part to the induction of cytoprotective autophagy [150, 151]. Consistent with this effect, increased ULK expression is observed in the tissue of RCC patients following mTOR therapy [152]. Multiple groups have demonstrated synergistic antiproliferative activity on RCC cell lines when temsirolimus or everolimus is combined with the autophagy inhibitors chloroquine or SAR405, particularly when the autophagy inhibitors are administered first [8, 88, 153–157]. Even greater antiproliferative effects have been reported when adding autophagy inhibition to the dual mTOR inhibitors, AZD-2014 and NVP-BEZ235 (Zheng 2014, Li 2012). A recent clinical trial studied the combination of oral everolimus plus oral hydroxychloroquine (HCQ) in 33 heavily pre-treated metastatic RCC patients, demonstrating excellent tolerability and 67% disease control (SD+PR) [91]. Similarly, Lotze and Appleman, working with the Cytokine Working Group, have identified a fourfold increase in progression-free survival in patients treated with high dose Interleukin 2 and HCQ (Lotze MT, et al; submitted). In summary, treatment with mTOR inhibitors induces autophagy, protecting RCC cells. Addition of autophagy inhibition therefore functions synergistically to enhance cell death, largely through promoting apoptotic and limiting necroptotic pathways.
Although autophagy can play a cytoprotective role, it can also promote cell death under certain conditions. Interestingly, several recent reports have demonstrated enhanced apoptosis mediated by induction of prolonged autophagy. Sinomenine promoted apoptosis in RCC by enhancing autophagy through suppression of the mTOR pathway [158]. Similarly, resveratrol treatment suppressed mTOR, leading to induction of autophagy and promotion of apoptosis in RCC cells [159]. Further, the dual mTOR inhibitor PP242 in combination with curcurmin induced autophagy-mediated RCC cell death, which was abrogated by autophagy inhibition [160]. Thus, induction of autophagy may contribute to cell survival or cell death in renal cancer cells, largely by enabling apoptosis. It will be critical to better understand this delicate balance when developing future therapeutics.
One study examined the impact of the autophagy inhibitor, hydroxychloroquine (HQ) on the mTOR pathway. Decreased expression of phospho-S6, a key down-stream readout for mTOR activity, suggests that HQ itself behaves as an mTOR inhibitor. Interestingly, this function is specific to HQ and not seen with other autophagy inhibitors [161].
mTOR inhibition is a common treatment for patients with advanced RCC and impacts many cellular functions. Inhibiting this pathway modulates autophagy as well as angiogenesis. RCC is recognized as one of the most vascular tumors as a result of faux hypoxia, making an understanding of angiogenic processes in relation to autophagy important in future research.
ANTIANGIOGENESIS AND AUTOPHAGY
Vascular endothelial growth factor (VEGF) stimulates angiogenesis [162] and is necessary for survival of endothelial cells, which carry out autophagy and eventually apoptosis when it is not available [163]. A possible relationship between angiogenesis and autophagy has already been noted outside the kidney [164]. While VEGF promotes angiogenesis by increasing tube length, number of branch points and migration of bovine aortic endothelial cells (ECs), inhibition of autophagy with 3-methyladenine (3-MA) or ATG5 knockdown reduces these increases brought on by VEGF.
Although autophagy inhibition reduces angiogenesis in bovine aortic ECs, hinting at a direct relationship between autophagy and angiogenesis, the relationship between the two entities may be more complex. For instance, in human umbilical vein endothelial cells (HUVECs), Decorin, an extracellular matrix proteoglycan, promotes autophagy by increasing the number of autophagosomes and the expression and colocalization of autophagy markers LC3 and Beclin 1 [165]. On the other hand, it decreases angiogenesis by reducing tube length and migration. Likewise, in the context of glioblastoma, bevacizumab, a monoclonal antibody to VEGF and an approved treatment for RCC [166], increases autophagosomes and the expression of autophagy markers LC3 and IRF1, indicating that an anti-angiogenic therapy promotes autophagy [167]. Thus, whether or not autophagy and angiogenesis are directly or inversely related may depend on context.
In the context of kidney cancer, therapeutic agents influence various processes including autophagy and angiogenesis, which in turn can determine drug efficacy. For instance, temsirolimus, an inhibitor of mammalian target of rapamycin (mTOR) and a therapeutic option for advanced kidney cancer [148], induces autophagy in kidney cancer cells in vitro, while reducing their angiogenic support in vivo [168]. Decreased efficacy of sunitinib, a tyrosine kinase inhibitor targeting VEGFR-mediating signaling and a frequently-used treatment option for patients with advanced kidney cancer [169], has been attributed to its accumulation in lysosomes and limited autophagic degradation [170]. In addition to the above examples, new therapeutic targets of kidney cancer can also include both autophagy and angiogenesis. High mobility group box 1 protein (HMGB1) and its receptor for advanced glycation end products (RAGE) signaling increase expression of LC3 and Beclin-1, as well as VEGF [171]. Thus, therapies simultaneously addressing angiogenesis and autophagy aspects of kidney cancer have shown promise and should be developed in the future.
TYROSINE KINASE INHIBITORS AND AUTOPHAGY
VEGF expression is induced by HIF-mediated transcription. The loss of VHL function in RCC leads to over-abundance of HIF and overexpression of VEGF [172]. Clinical anti-tumor activity in metastatic RCC has been demonstrated with a neutralizing anti-VEGF monoclonal antibody (bevacizumab) [173] and with small-molecule tyrosine kinase inhibitors (TKIs) that inhibit the VEGF receptor family members and other kinases. Among these agents, axitinib, cabozantinib, lenvatinib (in combination with everolimus) pazopanib, sorafenib and sunitinib are FDA-approved for patients with advanced RCC [174–179].
Anti-angiogenic agents such as the VEGF receptor TKIs limit tumor access to oxygen and nutrients and can thus lead to autophagy as a survival mechanism. Inhibition of autophagy may enhance the anti-tumor activity of these anti-angiogenic agents [180]. The effect of these agents on autophagic pathways has been investigated in several model systems. Sunitinib accumulates in lysosomes and increases autophagy in cell culture models [181–183]. In a murine model of pancreatic neuroendocrine tumor, the anti-tumor activity of sunitinib was enhanced with the addition of hydroxychloroquine [182]. Enhanced anti-tumor activity of sunitinib with hydroxychloroquine was also seen in preclinical models of clear cell ovarian cancer and lung adenocarcinoma [183, 184]. Sunitinib exposure led to incomplete autophagy and lysosome-dependent necrosis in bladder cancer cell lines [185]. Inhibition of autophagy with hydroxychloroquine enhanced the inhibitory effect of bevacizumab in hepatoma xenografts [186]. Taken together, these pre-clinical data suggest a rationale for combining VEGF-targeted agents with inhibitors of autophagy in RCC. However, the findings also indicate that the pleiomorphic cellular effects of VEGFR TKIs on autophagic mechanisms and lysosomes seen in cell culture likely has more complicated biological effects in vivo [180].
A phase I study of sunitinib plus hydroxychloroquine in patients with advanced solid tumors found that dose escalation of hydroxychloroquine to pharmacodynamically adequate doses was limited by toxicity [187]. Sunitinib metabolites accumulated at greater levels with the co-administration of HCQ, suggesting that greater exposure to these active species may explain the greater degree of sunitinib-related toxicity. Individual patients with non-small cell lung cancer, adrenocortical carcinoma and malignant peripheral nerve sheath tumors had tumor shrinkage that did not meet RECIST criteria for partial response.
HGF AND AUTOPHAGY
Hepatocyte growth factor (HGF) is a cytokine secreted by mesenchymal cells, acting upon epithelial cells and endothelial cells primarily promoting regeneration and wound healing. It can also promote T cell recruitment into tissues as a potent chemokine-like molecule. Its receptor c-Met (also termed HGFR) and resultant downstream signaling promotes tumor invasiveness in RCC and other cancers. The gene is transcribed, translated, and the protein is secreted by apoptotic cells [188, 189]. In the kidney, HGF can ameliorate podocyte injury and proteinuria in diabetic nephropathy. HGF significantly decreases apoptosis, oxidative stress, and autophagy impairment in podocytes of diabetic patients [190]. Once the HGF receptor is blocked by SU11274, the beneficial effects of HGF disappear. Moreover, HGF significantly suppresses HG (High glucose)-stimulated glycogen synthase kinase 3beta (GSK3B) activity [190]. HGF prevents HG-induced podocyte injury via an autophagy-promoting mechanism, which involves GSK3β inhibition [190]. Exogenous HGF increases autophagy and suppresses apoptosis, while neutralizing HGF yields the opposite effect. Various methods targeting podocyte survival such as antioxidant, anti-apoptosis and pro-autophagy strategies have been used for treatment of individuals with diabetic neuropathy [191–193]. HGF not only has reno-trophic and anti-fibrotic effects, but functions as an anti-apoptotic and anti-oxidant factor in kidney and other tissues [194–196].
Met is the tyrosine kinase receptor for HGF. The HGF/Met pathway promotes repair in various diseases through its anti-apoptotic and anti-inflammatory activity [196]. Cobalt chloride (CoCl2), a chemical mimic of hypoxia, can induce cardiac damage, which results in loss of cell viability by: a) caspase-dependent apoptosis and b) enhanced autophagy [197]. Blocking apoptosis with either the caspase inhibitor benzyloxycarbonyl-VAD-fuoromethylketone or preventing autophagosome formation with 3-methyladenine prevented loss of cell viability. This suggests that both processes contribute to cardiomyoblast injury [197], and a dual role of autophagy-promoted survival as well as autophagy-enabled cell death are replete in the literature.
Exogenous HGF induces Parkin, a marker of mitochondrial autophagy (mitophagy), promoting increased clearance of injured mitochondria [198], and in turn mitochondrial biogenesis. HGF could increase neonatal rat ventricular myocyte (NRVM) autophagy at early stages of hypoxia and inhibit myocyte apoptosis [198]. The adaptive autophagy was related to enhanced clearance of damaged mitochondria.
In clear cell RCC, HGF promotes invasion together with hypoxia [192]. Through HGF-driven invasion including β-catenin degradation, VHL loss generates faux hypoxia. Hypoxia also increases HGF-driven invasiveness by papillary RCC cells. Loss of VHL signaling involves three parallel pathways: 1) Hypoxia-induced reactive oxygen species production and suppressed DUSP2 expression, leading to increased MAPK cascade activation (Mitogen-activated protein kinase); 2) Reactive oxygen species -induced diacylglycerol production by phosphatase-2A activity, thereby suppressing HGF-induced Akt activation; 3) A profound shift from HGF-enhanced, proliferation-oriented metabolism to autophagy-dependent invasion and suppression of proliferation [192]. Synergy of HGF and faux hypoxia from VHL loss promotes enhanced angiogenesis and illustrates the plasticity of invasive and proliferative tumor cell states, and provides signaling profiles by which they may be predicted (Figure 1) [192]. Increased HGF is a poor prognostic finding in patients with RCC [199–203] and may enhance HMGB1/SDF1-CXCL12 signaling through upregulation of the CXCR4 receptor [204–210]. CXCL12 production in the TME is also associated with the recruitment of immunosuppressive immune cell populations such as myeloid-derived suppressor cells (MDSC) and Treg, that potentiate tumor progression [211].
References
- 1.Lotze MT, Maranchie J, and Appleman L, Inhibiting autophagy: a novel approach for the treatment of renal cell carcinoma. Cancer J, 2013. 19(4): p. 341–7. [DOI] [PubMed] [Google Scholar]
- 2.Buchser WJ, et al. , Cell-mediated autophagy promotes cancer cell survival. Cancer Res, 2012. 72(12): p. 2970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckardt KU, et al. , Hypoxia-inducible transcription factors and their role in renal disease. Semin Nephrol, 2007. 27(3): p. 363–72. [DOI] [PubMed] [Google Scholar]
- 4.Bouhamdani N, et al. , Quantitative proteomics to study a small molecule targeting the loss of von Hippel-Lindau in renal cell carcinomas. Int J Cancer, 2017. 141(4): p. 778–790. [DOI] [PubMed] [Google Scholar]
- 5.Maranchie JK, et al. , The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell, 2002. 1(3): p. 247–55. [DOI] [PubMed] [Google Scholar]
- 6.Schodel J, et al. , Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer. Eur Urol, 2016. 69(4): p. 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, et al. , Autophagy defects and related genetic variations in renal cell carcinoma with eosinophilic cytoplasmic inclusions. Sci Rep, 2018. 8(1): p. 9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singla M. and Bhattacharyya S, Autophagy as a potential therapeutic target during epithelial to mesenchymal transition in renal cell carcinoma: An in vitro study. Biomed Pharmacother, 2017. 94: p. 332–340. [DOI] [PubMed] [Google Scholar]
- 9.Li ML, et al. , Chloroquine potentiates the anticancer effect of sunitinib on renal cell carcinoma by inhibiting autophagy and inducing apoptosis. Oncol Lett, 2018. 15(3): p. 2839–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo S, et al. , A rapid and high content assay that measures cyto-ID-stained autophagic compartments and estimates autophagy flux with potential clinical applications. Autophagy, 2015. 11(3): p. 560–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orvedahl A, et al. , Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature, 2011. 480(7375): p. 113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz-Braceras S. and Escalante R, Analysis of Relevant Parameters for Autophagic Flux Using HeLa Cells Expressing EGFP-LC3. Methods Mol Biol, 2016. 1449: p. 313–29. [DOI] [PubMed] [Google Scholar]
- 13.Stolz A, et al. , Fluorescence-based ATG8 sensors monitor localization and function of LC3/GABARAP proteins. EMBO J, 2017. 36(4): p. 549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen KB, et al. , A reporter cell system to monitor autophagy based on p62/SQSTM1. Autophagy, 2010. 6(6): p. 784–93. [DOI] [PubMed] [Google Scholar]
- 15.Chittaranjan S, Bortnik S, and Gorski SM, Monitoring Autophagic Flux by Using Lysosomal Inhibitors and Western Blotting of Endogenous MAP1LC3B. Cold Spring Harb Protoc, 2015. 2015(8): p. 743–50. [DOI] [PubMed] [Google Scholar]
- 16.Jiang P. and Mizushima N, LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods, 2015. 75: p. 13–8. [DOI] [PubMed] [Google Scholar]
- 17.Oh SH, et al. , Quantification of autophagy flux using LC3 ELISA. Anal Biochem, 2017. 530: p. 57–67. [DOI] [PubMed] [Google Scholar]
- 18.Hale CM, et al. , Identification of modulators of autophagic flux in an image-based high content siRNA screen. Autophagy, 2016. 12(4): p. 713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung J. and Behrends C, Protocol for Establishing a Multiplex Image-based Autophagy RNAi Screen in Cell Cultures . Bio Protoc, 2017. 7(17): p. 27982478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra P, et al. , Discovery of pan autophagy inhibitors through a high-throughput screen highlights macroautophagy as an evolutionarily conserved process across 3 eukaryotic kingdoms. Autophagy, 2017. 13(9): p. 1556–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peppard JV, et al. , Identifying Small Molecules which Inhibit Autophagy: a Phenotypic Screen Using Image-Based High-Content Cell Analysis. Curr Chem Genom Transl Med, 2014. 8(Suppl 1): p. 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoji-Kawata S, [Identification of a candidate therapeutic autophagy-inducing peptide]. Seikagaku, 2015. 87(4): p. 481–4. [PubMed] [Google Scholar]
- 23.Kolla L, et al. , High content screen for identifying small-molecule LC3B-localization modulators in a renal cancer cell line. Sci Data, 2018. 5: p. 180116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangkhathat S, et al. , Renal cell carcinoma in a pediatric patient with an inherited mitochondrial mutation. Pediatr Surg Int, 2005. 21(9): p. 745–8. [DOI] [PubMed] [Google Scholar]
- 25.Klomp JA, et al. , Birt-Hogg-Dube renal tumors are genetically distinct from other renal neoplasias and are associated with up-regulation of mitochondrial gene expression. BMC Med Genomics, 2010. 3: p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang M, et al. , Mitochondrial DNA mutations distinguish bilateral multifocal renal oncocytomas from familial Birt-Hogg-Dube tumors. Mod Pathol, 2015. 28(11): p. 1458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandhi S, et al. , Heteroplasmic shifts in tumor mitochondrial genomes reveal tissue-specific signals of relaxed and positive selection. Hum Mol Genet, 2017. 26(15): p. 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lou E, et al. , Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One, 2012. 7(3): p. e33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge MV, Dong L, and Neuzil J, Mitochondrial DNA in Tumor Initiation, Progression, and Metastasis: Role of Horizontal mtDNA Transfer. Cancer Res, 2015. 75(16): p. 3203–8. [DOI] [PubMed] [Google Scholar]
- 30.Berridge MV, et al. , Horizontal transfer of mitochondria between mammalian cells: beyond co-culture approaches. Curr Opin Genet Dev, 2016. 38: p. 75–82. [DOI] [PubMed] [Google Scholar]
- 31.Ratajczak MZ and Ratajczak J, Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin Transl Med, 2016. 5(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berridge MV and Neuzil J, The mobility of mitochondria: Intercellular trafficking in health and disease. Clin Exp Pharmacol Physiol, 2017. 44 Suppl 1: p. 15–20. [DOI] [PubMed] [Google Scholar]
- 33.Dong LF, et al. , Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griessinger E, et al. , Mitochondrial Transfer in the Leukemia Microenvironment. Trends Cancer, 2017. 3(12): p. 828–839. [DOI] [PubMed] [Google Scholar]
- 35.Pierini S, et al. , A Tumor Mitochondria Vaccine Protects against Experimental Renal Cell Carcinoma. J Immunol, 2015. 195(8): p. 4020–7. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig LS, et al. , Lineage Tracing in Humans Enabled by Mitochondrial Mutations and Single-Cell Genomics. Cell, 2019. 176(6): p. 1325–1339 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aplin A, et al. , Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. Journal of Cellular Physiology, 1992. 152(3): p. 458–466. [DOI] [PubMed] [Google Scholar]
- 38.Ueno T, et al. , Phalloidin-induced accumulation of myosin in rat hepatocytes is caused by suppression of autolysosome formation. European Journal of Biochemistry, 1990. 190(1): p. 63–69. [DOI] [PubMed] [Google Scholar]
- 39.Kast DJ and Dominguez R, WHAMM links actin assembly via the Arp2/3 complex to autophagy. Autophagy, 2015. 11(9): p. 1702–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kast DJ and Dominguez R, The Cytoskeleton-Autophagy Connection. Curr Biol, 2017. 27(8): p. R318–r326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kast DJ, et al. , WHAMM Directs the Arp2/3 Complex to the ER for Autophagosome Biogenesis through an Actin Comet Tail Mechanism. Curr Biol, 2015. 25(13): p. 1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutts AS and La Thangue NB, Actin nucleation by WH2 domains at the autophagosome. Nat Commun, 2015. 6: p. 7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coutts AS and La Thangue NB, Regulation of actin nucleation and autophagosome formation. Cell Mol Life Sci, 2016. 73(17): p. 3249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia P, et al. , WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. Embo j, 2013. 32(20): p. 2685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zavodszky E, et al. , Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun, 2014. 5: p. 3828. [DOI] [PMC free article] [PubMed]
- 46.Zavodszky E, Seaman MN, and Rubinsztein DC, VPS35 Parkinson mutation impairs autophagy via WASH. Cell Cycle, 2014. 13(14): p. 2155–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King JS, et al. , WASH is required for lysosomal recycling and efficient autophagic and phagocytic digestion. Mol Biol Cell, 2013. 24(17): p. 2714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P, et al. , Arabidopsis NAP1 Regulates the Formation of Autophagosomes. Curr Biol, 2016. 26(15): p. 2060–2069. [DOI] [PubMed] [Google Scholar]
- 49.Kerkhoff E, et al. , The Spir actin organizers are involved in vesicle transport processes. Curr Biol, 2001. 11(24): p. 1963–8. [DOI] [PubMed] [Google Scholar]
- 50.Mi N, et al. , CapZ regulates autophagosomal membrane shaping by promoting actin assembly inside the isolation membrane. Nat Cell Biol, 2015. 17(9): p. 1112–23. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, et al. , HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. Embo j, 2010. 29(5): p. 969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandstaetter H, et al. , Loss of functional MYO1C/myosin 1c, a motor protein involved in lipid raft trafficking, disrupts autophagosome-lysosome fusion. Autophagy, 2014. 10(12): p. 2310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tumbarello DA, et al. , Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nat Cell Biol, 2012. 14(10): p. 1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang HW, et al. , Atg1-mediated myosin II activation regulates autophagosome formation during starvation-induced autophagy. Embo j, 2011. 30(4): p. 636–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruppa AJ, Kendrick-Jones J, and Buss F, Myosins, Actin and Autophagy. Traffic, 2016. 17(8): p. 878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguilera MO, Berón W, and Colombo MI, The actin cytoskeleton participates in the early events of autophagosome formation upon starvation induced autophagy. Autophagy, 2012. 8(11): p. 1590–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geeraert C, et al. , Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem, 2010. 285(31): p. 24184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kochl R, et al. , Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic, 2006. 7(2): p. 129–45. [DOI] [PubMed] [Google Scholar]
- 59.Kimura S, Noda T, and Yoshimori T, Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct, 2008. 33(1): p. 109–22. [DOI] [PubMed] [Google Scholar]
- 60.Maday S. and Holzbaur EL, Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell, 2014. 30(1): p. 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du W, et al. , Kinesin 1 Drives Autolysosome Tubulation. Dev Cell, 2016. 37(4): p. 326–336. [DOI] [PubMed] [Google Scholar]
- 62.Feng Z, et al. , Na+/H+ exchanger-1 reduces podocyte injury caused by endoplasmic reticulum stress via autophagy activation. Laboratory Investigation, 2014. 94: p. 439–454. [DOI] [PubMed] [Google Scholar]
- 63.Akchurin O. and Reidy KJ, Genetic causes of proteinuria and nephrotic syndrome: Impact on podocyte pathobiology. Pediatric Nephrology, 2015. 30(2): p. 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang YL, et al. , The cytoprotective role of autophagy in puromycin aminonucleoside treated human podocytes. Biochemical and Biophysical Research Communications, 2014. 443(2): p. 628–634. [DOI] [PubMed] [Google Scholar]
- 65.Liu M, et al. , Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nature Communications, 2017. 8(413): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yee A, et al. , Proteostasis as a therapeutic target in glomerular injury associated with mutant American Journal of Physiology-Renal Physiology, 2018. 315(4): p. F954–966. [DOI] [PubMed] [Google Scholar]
- 67.Rangel EB, et al. , Kidney-derived c-kit+ progenitor/ stem cells contribute to podocyte recovery in a model of acute proteinuria. Scientific Reports, 2018. 8(1473): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khositseth S, et al. , Autophagic degradation of aquaporin-2 is an early event in hypokalemia-induced nephrogenic diabetes insipidus. Scientific Reports, 2015. 5(18311): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou B, et al. , Salvianolic Acid A Protects Against Diabetic Nephropathy through Ameliorating Glomerular Endothelial Dysfunction via Inhibiting AGE-RAGE Signaling. Cell Physiol Biochem, 2017. 44(6): p. 2378–2394. [DOI] [PubMed] [Google Scholar]
- 70.Noh MR, et al. , Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim Biophys Acta Mol Basis Dis, 2018. 1864(5 Pt A): p. 1634–1641. [DOI] [PubMed] [Google Scholar]
- 71.Zhang N, et al. , Quantitative Global Proteome and Lysine Succinylome Analyses Reveal the Effects of Energy Metabolism in Renal Cell Carcinoma. Proteomics, 2018. 18(19): p. e1800001. [DOI] [PubMed] [Google Scholar]
- 72.Karamchandani JR, et al. , Profilin-1 expression is associated with high grade and stage and decreased disease-free survival in renal cell carcinoma. Hum Pathol, 2015. 46(5): p. 673–80. [DOI] [PubMed] [Google Scholar]
- 73.Masui O, et al. , Quantitative proteomic analysis in metastatic renal cell carcinoma reveals a unique set of proteins with potential prognostic significance. Mol Cell Proteomics, 2013. 12(1): p. 132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neely BA, et al. , Proteotranscriptomic Analysis Reveals Stage Specific Changes in the Molecular Landscape of Clear-Cell Renal Cell Carcinoma. PLoS One, 2016. 11(4): p. e0154074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minamida S, et al. , Profilin 1 overexpression in renal cell carcinoma. Int J Urol, 2011. 18(1): p. 63–71. [DOI] [PubMed] [Google Scholar]
- 76.Cheng H, et al. , Profilin1 sensitizes pancreatic cancer cells to irradiation by inducing apoptosis and reducing autophagy. Curr Mol Med, 2013. 13(8): p. 1368–75. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen DKH, Thombre R, and Wang J, Autophagy as a common pathway in amyotrophic lateral sclerosis. Neurosci Lett, 2018. [DOI] [PMC free article] [PubMed]
- 78.Yao ZQ, et al. , A novel small-molecule activator of Sirtuin-1 induces autophagic cell death/mitophagy as a potential therapeutic strategy in glioblastoma. Cell Death Dis, 2018. 9(7): p. 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang HJ, Chen SF, and Lo WY, Identification of Cofilin-1 Induces G0/G1 Arrest and Autophagy in Angiotensin-(1–7)-treated Human Aortic Endothelial Cells from iTRAQ Quantitative Proteomics. Sci Rep, 2016. 6: p. 35372. [DOI] [PMC free article] [PubMed]
- 80.Zhu JS, et al. , Cucurbitacin B induces cell cycle arrest, apoptosis and autophagy associated with G actin reduction and persistent activation of cofilin in Jurkat cells. Pharmacology, 2012. 89(5–6): p. 348–6. [DOI] [PubMed] [Google Scholar]
- 81.Schaaf MB, et al. , Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ, 2019. [DOI] [PMC free article] [PubMed]
- 82.Nussenzweig SC, Verma S, and Finkel T, The role of autophagy in vascular biology. Circ Res, 2015. 116(3): p. 480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torisu T, et al. , Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat Med, 2013. 19(10): p. 1281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhuang SF, et al. , Atg7 Regulates Brain Angiogenesis via NF-kappaB-Dependent IL-6 Production. Int J Mol Sci, 2017. 18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carmeliet P. and Jain RK, Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov, 2011. 10(6): p. 417–27. [DOI] [PubMed] [Google Scholar]
- 86.Jain RK, Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science, 2005. 307(5706): p. 58–62. [DOI] [PubMed] [Google Scholar]
- 87.Manic G, et al. , Chloroquine and hydroxychloroquine for cancer therapy. Mol Cell Oncol, 2014. 1(1): p. e29911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grimaldi A, et al. , Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells. Cancer Biol Ther, 2015. 16(4): p. 567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levy JMM, Towers CG, and Thorburn A, Targeting autophagy in cancer. Nat Rev Cancer, 2017. 17(9): p. 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang X, et al. , Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res, 2012. 72(11): p. 2791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haas NB, et al. , Autophagy Inhibition to Augment mTOR Inhibition: a Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma. Clin Cancer Res, 2019. [DOI] [PMC free article] [PubMed]
- 92.Han J, et al. , Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS One, 2018. 13(3): p. e0193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Y, et al. , Metformin inhibits esophageal squamous cell carcinoma-induced angiogenesis by suppressing JAK/STAT3 signaling pathway. Oncotarget, 2017. 8(43): p. 74673–74687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kannarkatt J, et al. , Metformin and Angiogenesis in Cancer - Revisited. Oncology, 2016. 91(4): p. 179–184. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, et al. , Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1alpha/VEGF secretion axis. Oncotarget, 2015. 6(42): p. 44579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niu C, et al. , Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy, 2019: p. 1–28. [DOI] [PMC free article] [PubMed]
- 97.Venanzi F, et al. , Broad-spectrum anti-tumor and anti-metastatic DNA vaccine based on p62-encoding vector. Oncotarget, 2013. 4(10): p. 1829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabai V, et al. , Pilot study of p62 DNA vaccine in dogs with mammary tumors. Oncotarget, 2014. 5(24): p. 12803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gabai VL and Shifrin VI, Feasibility analysis of p62 (SQSTM1)-encoding DNA vaccine as a novel cancer immunotherapy. Int Rev Immunol, 2014. 33(5): p. 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh KK, et al. , The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J Biol Chem, 2015. 290(5): p. 2547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wada S, et al. , Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res, 2005. 65(11): p. 4939–46. [DOI] [PubMed] [Google Scholar]
- 102.Dong Y, et al. , Identification of H-2Db-specific CD8+ T-cell epitopes from mouse VEGFR2 that can inhibit angiogenesis and tumor growth. J Immunother, 2006. 29(1): p. 32–40. [DOI] [PubMed] [Google Scholar]
- 103.Ishizaki H, et al. , Inhibition of tumor growth with antiangiogenic cancer vaccine using epitope peptides derived from human vascular endothelial growth factor receptor 1. Clin Cancer Res, 2006. 12(19): p. 5841–9. [DOI] [PubMed] [Google Scholar]
- 104.Seavey MM, et al. , An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol, 2009. 182(9): p. 5537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chi N, et al. , Update on vaccine development for renal cell cancer. Open Access J Urol, 2010. 2: p. 125–41. [DOI] [PMC free article] [PubMed]
- 106.Wood LM, et al. , Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother, 2011. 60(7): p. 931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao X, et al. , Vaccines targeting tumor blood vessel antigens promote CD8(+) T cell-dependent tumor eradication or dormancy in HLA-A2 transgenic mice. J Immunol, 2012. 188(4): p. 1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chi Sabins N, et al. , DLK1: a novel target for immunotherapeutic remodeling of the tumor blood vasculature. Mol Ther, 2013. 21(10): p. 1958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okuyama R, et al. , Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology, 2013. 2(11): p. e27010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Facciponte JG, et al. , Tumor endothelial marker 1-specific DNA vaccination targets tumor vasculature. J Clin Invest, 2014. 124(4): p. 1497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fabian KP, et al. , Therapeutic efficacy of combined vaccination against tumor pericyte-associated antigens DLK1 and DLK2 in mice. Oncoimmunology, 2017. 6(3): p. e1290035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johansson-Percival A, et al. , De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol, 2017. 18(11): p. 1207–1217. [DOI] [PubMed] [Google Scholar]
- 113.Jia Z, et al. , Autophagy eliminates cytoplasmic beta-catenin and NICD to promote the cardiac differentiation of P19CL6 cells. Cell Signal, 2014. 26(11): p. 2299–305. [DOI] [PubMed] [Google Scholar]
- 114.Maes H, et al. , Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell, 2014. 26(2): p. 190–206. [DOI] [PubMed] [Google Scholar]
- 115.Wieland E, et al. , Endothelial Notch1 Activity Facilitates Metastasis. Cancer Cell, 2017. 31(3): p. 355–367. [DOI] [PubMed] [Google Scholar]
- 116.Nair S, et al. , Synergy between tumor immunotherapy and antiangiogenic therapy. Blood, 2003. 102(3): p. 964–71. [DOI] [PubMed] [Google Scholar]
- 117.Shrimali RK, et al. , Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res, 2010. 70(15): p. 6171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okamoto I, et al. , Clinical phase I study of elpamotide, a peptide vaccine for vascular endothelial growth factor receptor 2, in patients with advanced solid tumors. Cancer Sci, 2012. 103(12): p. 2135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang Y, et al. , Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res, 2013. 73(10): p. 2943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farsaci B, et al. , Immune consequences of decreasing tumor vasculature with antiangiogenic tyrosine kinase inhibitors in combination with therapeutic vaccines. Cancer Immunol Res, 2014. 2(11): p. 1090–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stanton MJ, et al. , Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance. Cancer Res, 2013. 73(1): p. 160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tarhini AA, Tawbi H, and Storkus WJ, Vaccine therapy + dasatinib for the treatment of patients with stage IIIB-IV melanoma. Melanoma Manag, 2016. 3(4): p. 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cadwell K, Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol, 2016. 16(11): p. 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang D, et al. , PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev, 2012. 249(1): p. 158–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heckmann BL, et al. , LC3-Associated Phagocytosis and Inflammation. J Mol Biol, 2017. 429(23): p. 3561–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pearce EL and Pearce EJ, Metabolic pathways in immune cell activation and quiescence. Immunity, 2013. 38(4): p. 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng YT, et al. , The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol, 2009. 183(9): p. 5909–16. [DOI] [PubMed] [Google Scholar]
- 128.Nozawa T, et al. , The intracellular microbial sensor NLRP4 directs Rho-actin signaling to facilitate Group A Streptococcus-containing autophagosome-like vacuole formation. Autophagy, 2017. 13(11): p. 1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sakowski ET, et al. , Ubiquilin 1 Promotes IFN-gamma-Induced Xenophagy of Mycobacterium tuberculosis. PLoS Pathog, 2015. 11(7): p. e1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Talloczy Z, Virgin H.W.t., and Levine B, PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy, 2006. 2(1): p. 24–9. [DOI] [PubMed] [Google Scholar]
- 131.Ouimet M, et al. , Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol, 2016. 17(6): p. 677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choy A, et al. , The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science, 2012. 338(6110): p. 1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kubori T, et al. , Legionella RavZ Plays a Role in Preventing Ubiquitin Recruitment to Bacteria-Containing Vacuoles . Front Cell Infect Microbiol, 2017. 7: p. 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chaumorcel M, et al. , Human cytomegalovirus controls a new autophagy-dependent cellular antiviral defense mechanism. Autophagy, 2008. 4(1): p. 46–53. [DOI] [PubMed] [Google Scholar]
- 135.Gobeil PA and Leib DA, Herpes simplex virus gamma34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. MBio, 2012. 3(5): p. e00267–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L, et al. , Reduced lysosomal clearance of autophagosomes promotes survival and colonization of Helicobacter pylori. J Pathol, 2018. 244(4): p. 432–444. [DOI] [PubMed] [Google Scholar]
- 137.Zhang L, et al. , Xenophagy in Helicobacter pylori- and Epstein-Barr virus-induced gastric cancer. J Pathol, 2014. 233(2): p. 103–12. [DOI] [PubMed] [Google Scholar]
- 138.Martinez J, et al. , Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A, 2011. 108(42): p. 17396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cunha LD, et al. , LC3-Associated Phagocytosis in Myeloid Cells Promotes Tumor Immune Tolerance. Cell, 2018. 175(2): p. 429–441 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baginska J, et al. , Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A, 2013. 110(43): p. 17450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Messai Y, et al. , ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Cancer Res, 2014. 74(23): p. 6820–32. [DOI] [PubMed] [Google Scholar]
- 142.Leventhal JS, He JC, and Ross MJ, Autophagy and immune response in kidneys. Semin Nephrol, 2014. 34(1): p. 53–61. [DOI] [PubMed] [Google Scholar]
- 143.Leventhal JS and Schroppel B, Toll-like receptors in transplantation: sensing and reacting to injury. Kidney Int, 2012. 81(9): p. 826–32. [DOI] [PubMed] [Google Scholar]
- 144.Leventhal JS, et al. , Autophagy Limits Endotoxemic Acute Kidney Injury and Alters Renal Tubular Epithelial Cell Cytokine Expression. PLoS One, 2016. 11(3): p. e0150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kimura A, et al. , Exaggerated arsenic nephrotoxicity in female mice through estrogen-dependent impairments in the autophagic flux. Toxicology, 2016. 339: p. 9–18. [DOI] [PubMed]
- 146.Chiarini F, et al. , Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci, 2015. 36(2): p. 124–35. [DOI] [PubMed] [Google Scholar]
- 147.Li J, Kim SG, and Blenis J, Rapamycin: one drug, many effects. Cell Metab, 2014. 19(3): p. 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hudes G, et al. , Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med, 2007. 356(22): p. 2271–81. [DOI] [PubMed] [Google Scholar]
- 149.Kapoor A. and Figlin RA, Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer, 2009. 115(16): p. 3618–30. [DOI] [PubMed] [Google Scholar]
- 150.Bray K, et al. , Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLoS One, 2012. 7(7): p. e41831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y, et al. , MTOR inhibition attenuates DNA damage and apoptosis through autophagy-mediated suppression of CREB1. Autophagy, 2013. 9(12): p. 2069–86. [DOI] [PubMed] [Google Scholar]
- 152.Nishikawa M, et al. , UNC-51-like kinase 1 expression in radical nephrectomy specimens as a predicting factor of progression-free survival in patients with metastatic renal cell carcinoma treated with mammalian target of rapamycin inhibitors. Urol Oncol, 2015. 33(12): p. 506 e1–7. [DOI] [PubMed] [Google Scholar]
- 153.Zeng Y, et al. , Attenuation of everolimus-induced cytotoxicity by a protective autophagic pathway involving ERK activation in renal cell carcinoma cells. Drug Des Devel Ther, 2018. 12: p. 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ronan B, et al. , A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol, 2014. 10(12): p. 1013–9. [DOI] [PubMed] [Google Scholar]
- 155.Pasquier B, SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells. Autophagy, 2015. 11(4): p. 725–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng B, et al. , Pre-clinical evaluation of AZD-2014, a novel mTORC1/2 dual inhibitor, against renal cell carcinoma. Cancer Lett, 2015. 357(2): p. 468–75. [DOI] [PubMed] [Google Scholar]
- 157.Li H, et al. , Inhibition of autophagy enhances apoptosis induced by the PI3K/AKT/mTor inhibitor NVP-BEZ235 in renal cell carcinoma cells. Cell Biochem Funct, 2013. 31(5): p. 427–33. [DOI] [PubMed] [Google Scholar]
- 158.Deng F, et al. , The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother, 2018. 97: p. 1269–1274. [DOI] [PubMed]
- 159.Liu Q, et al. , Resveratrol-mediated apoptosis in renal cell carcinoma via the p53/AMPactivated protein kinase/mammalian target of rapamycin autophagy signaling pathway. Mol Med Rep, 2018. 17(1): p. 502–508. [DOI] [PubMed] [Google Scholar]
- 160.Seo SU, et al. , mTORC1/2 inhibitor and curcumin induce apoptosis through lysosomal membrane permeabilization-mediated autophagy. Oncogene, 2018. 37(38): p. 5205–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee HO, et al. , Hydroxychloroquine Destabilizes Phospho-S6 in Human Renal Carcinoma Cells. PLoS One, 2015. 10(7): p. e0131464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferrara N, et al. , Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev, 1992. 13(1): p. 18–32. [DOI] [PubMed] [Google Scholar]
- 163.Domigan CK, et al. , Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J Cell Sci, 2015. 128(12): p. 2236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Du J, et al. , Role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol, 2012. 302(2): p. C383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Buraschi S, et al. , Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A, 2013. 110(28): p. E2582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vredenburgh JJ, et al. , Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol, 2007. 25(30): p. 4722–9. [DOI] [PubMed] [Google Scholar]
- 167.Liang J, et al. , Interferon-regulatory factor-1 (IRF1) regulates bevacizumab induced autophagy. Oncotarget, 2015. 6(31): p. 31479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang H, et al. , A comparison of Ku0063794, a dual mTORC1 and mTORC2 inhibitor, and temsirolimus in preclinical renal cell carcinoma models. PLoS One, 2013. 8(1): p. e54918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Motzer RJ, et al. , Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol, 2009. 27(22): p. 3584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giuliano S, et al. , Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux. Autophagy, 2015. 11(10): p. 1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu CZ, et al. , HMGB1/RAGE axis mediates the apoptosis, invasion, autophagy, and angiogenesis of the renal cell carcinoma. Onco Targets Ther, 2018. 11: p. 4501–4510. [DOI] [PMC free article] [PubMed]
- 172.Wiesener MS, et al. , Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer Res, 2001. 61(13): p. 5215–22. [PubMed] [Google Scholar]
- 173.Yang JC, et al. , A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med, 2003. 349(5): p. 427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Motzer RJ, et al. , Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol, 2006. 24(1): p. 16–24. [DOI] [PubMed] [Google Scholar]
- 175.Sternberg CN, et al. , Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol, 2010. 28(6): p. 1061–8. [DOI] [PubMed] [Google Scholar]
- 176.Choueiri TK, et al. , Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med, 2015. 373(19): p. 1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Motzer RJ, et al. , Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol, 2013. 14(6): p. 552–62. [DOI] [PubMed] [Google Scholar]
- 178.Escudier B, et al. , Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med, 2007. 356(2): p. 125–34. [DOI] [PubMed] [Google Scholar]
- 179.Motzer RJ, et al. , Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol, 2015. 16(15): p. 1473–1482. [DOI] [PubMed] [Google Scholar]
- 180.Liu J, et al. , Autophagy, a double-edged sword in anti-angiogenesis therapy. Med Oncol, 2016. 33(1): p. 10. [DOI] [PubMed] [Google Scholar]
- 181.Gotink KJ, et al. , Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res, 2011. 17(23): p. 7337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wiedmer T, et al. , Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism. Mol Cancer Ther, 2017. 16(11): p. 2502–2515. [DOI] [PubMed] [Google Scholar]
- 183.DeVorkin L, et al. , Autophagy Inhibition Enhances Sunitinib Efficacy in Clear Cell Ovarian Carcinoma. Mol Cancer Res, 2017. 15(3): p. 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eng CH, et al. , Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc Natl Acad Sci U S A, 2016. 113(1): p. 182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Santoni M, et al. , Pazopanib and sunitinib trigger autophagic and non-autophagic death of bladder tumour cells. Br J Cancer, 2013. 109(4): p. 1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guo XL, et al. , Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J Mol Med (Berl), 2013. 91(4): p. 473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Melnyk N, et al. , CTEP #8342 autophagy modulation with antiangiogenic therapy: A phase I trial of sunitinib (Su) and hydroxychloroquine (HCQ). J. Clin. Oncol, 2013. 31 Suppl: p. Abstract 2553. [Google Scholar]
- 188.Vogel S, et al. , Necrotic cell-derived high mobility group box 1 attracts antigen-presenting cells but inhibits hepatocyte growth factor-mediated tropism of mesenchymal stem cells for apoptotic cell death. Cell Death Differ, 2015. 22(7): p. 1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vogel S, et al. , Activated platelets interfere with recruitment of mesenchymal stem cells to apoptotic cardiac cells via high mobility group box 1/Toll-like receptor 4-mediated down-regulation of hepatocyte growth factor receptor MET. J Biol Chem, 2014. 289(16): p. 11068–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang C, et al. , HGF alleviates high glucose-induced injury in podocytes by GSK3beta inhibition and autophagy restoration. Biochim Biophys Acta, 2016. 1863(11): p. 2690–2699. [DOI] [PubMed] [Google Scholar]
- 191.Dong C, et al. , Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis. Exp Cell Res, 2015. 337(2): p. 146–59. [DOI] [PubMed] [Google Scholar]
- 192.Lee YH, Morrison BL, and Bottaro DP, Synergistic signaling of tumor cell invasiveness by hepatocyte growth factor and hypoxia. J Biol Chem, 2014. 289(30): p. 20448–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Khazim K, et al. , The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol, 2013. 305(5): p. F691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ogaly HA, et al. , Hepatocyte Growth Factor Mediates the Antifibrogenic Action of Ocimum bacilicum Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Molecules, 2015. 20(8): p. 13518–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arechederra M, et al. , Met signaling in cardiomyocytes is required for normal cardiac function in adult mice. Biochim Biophys Acta, 2013. 1832(12): p. 2204–15. [DOI] [PubMed] [Google Scholar]
- 196.Ishibashi H, et al. , Hepatocyte growth factor/c-met promotes proliferation, suppresses apoptosis, and improves matrix metabolism in rabbit nucleus pulposus cells in vitro. J Orthop Res, 2016. 34(4): p. 709–16. [DOI] [PubMed] [Google Scholar]
- 197.Gallo S, et al. , Agonist antibodies activating the Met receptor protect cardiomyoblasts from cobalt chloride-induced apoptosis and autophagy. Cell Death Dis, 2014. 5: p. e1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Y, et al. , Exogenous HGF Prevents Cardiomyocytes from Apoptosis after Hypoxia via Up-Regulating Cell Autophagy. Cell Physiol Biochem, 2016. 38(6): p. 2401–13. [DOI] [PubMed] [Google Scholar]
- 199.Macher-Goeppinger S, et al. , MET expression and copy number status in clear-cell renal cell carcinoma: prognostic value and potential predictive marker. Oncotarget, 2017. 8(1): p. 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Polimeno M, et al. , Regulatory T cells, interleukin (IL)-6, IL-8, vascular endothelial growth factor (VEGF), CXCL10, CXCL11, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as surrogate markers of host immunity in patients with renal cell carcinoma. BJU Int, 2013. 112(5): p. 686–96. [DOI] [PubMed] [Google Scholar]
- 201.Tran HT, et al. , Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol, 2012. 13(8): p. 827–37. [DOI] [PubMed] [Google Scholar]
- 202.Tanimoto S, et al. , Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. J Med Invest, 2008. 55(1–2): p. 106–11. [DOI] [PubMed] [Google Scholar]
- 203.Choueiri TK, et al. , Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J Clin Oncol, 2017. 35(26): p. 2993–3001. [DOI] [PubMed] [Google Scholar]
- 204.Son BR, et al. , Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells, 2006. 24(5): p. 1254–64. [DOI] [PubMed] [Google Scholar]
- 205.Maroni P, et al. , HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappaB. Carcinogenesis, 2007. 28(2): p. 267–79. [DOI] [PubMed] [Google Scholar]
- 206.Majka M, et al. , SDF-1 alone and in co-operation with HGF regulates biology of human cervical carcinoma cells. Folia Histochem Cytobiol, 2006. 44(3): p. 155–64. [PubMed] [Google Scholar]
- 207.Zia MK, et al. , The expression of the von Hippel-Lindau gene product and its impact on invasiveness of human breast cancer cells. Int J Mol Med, 2007. 20(4): p. 605–11. [PubMed] [Google Scholar]
- 208.Esencay M, Newcomb EW, and Zagzag D, HGF upregulates CXCR4 expression in gliomas via NF-kappaB: implications for glioma cell migration. J Neurooncol, 2010. 99(1): p. 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huang S, et al. , HGF-induced PKCzeta activation increases functional CXCR4 expression in human breast cancer cells. PLoS One, 2012. 7(1): p. e29124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Qian D, et al. , Bone Marrow-Derived Mesenchymal Stem Cells Repair Necrotic Pancreatic Tissue and Promote Angiogenesis by Secreting Cellular Growth Factors Involved in the SDF-1 alpha /CXCR4 Axis in Rats. Stem Cells Int, 2015. 2015: p. 306836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zeng Y, et al. , Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J, 2019: p. fj201802067RR. [DOI] [PMC free article] [PubMed]