Abstract
Context
Kaihoujian Throat Spray children's type (KHJSC) is a Chinese medicine prescription for treating pediatric acute pharyngitis and tonsillitis (APT). However, its relevant mechanisms remain unclear.
Objective
To investigate the pharmacological effects of KHJSC on APT in vitro and in vivo, and explore the possible mechanism and target proteins.
Materials and methods
The antiviral and antibacterial effects in vitro were evaluated by IC50 and MICs. Thirty-six Japanese white rabbits were averagely divided into control group, model group, amoxicillin group and 3 dose groups of KHJSC (720, 540 and 360 μL/kg/d). The model rabbits were injected with β-hemolytic Streptococcus solution into the tonsils for 2 consecutive days. KHJSC treatment started on the third day. The whole blood, serum, tonsil tissues and pharyngeal mucosa tissues were collected for routine blood tests, proteomic, ELISA and other tests on the sixth day.
Results
The IC50 of KHJSC on HCoV-229E, influenza PR8 and Ad3 were 1.99, 1.99 and 4.49 mg/mL, respectively; MICs of MDR-PA, MRSA and β-hemolytic Streptococcus were 350, 350, and 175 mg/mL. KHJSC markedly decreased the number of white blood cells, lymphocytes, neutrophils, and the level of IL-1β, IL-5, IL-6, IL-18, TNF-α and MCP-1; increased the content of IL-2 and IFN-γ. Proteomic analysis and ELISA revealed that PI3K-Akt signaling pathway, NF-κB signaling pathway and Toll-like receptor signaling pathway were the potential mechanisms of KHJSC against APT.
Discussion and conclusion
These results provided the reference and scientific basis for the application of KHJSC in clinic and further mechanisms study.
Keywords: Traditional Chinese medicine, β-hemolytic Streptococcus, Antibacterial, Antiviral, TMT quantitative proteomic
1. Introduction
Pediatric APT, which are characterized by inflammation of the posterior pharynx and tonsils, are the most common and frequently-occurring respiratory diseases in children, especially in early school-aged children [1,2]. The main clinical manifestations are sore throat, fever, cough, sputum, and difficulty swallowing. The disease progresses rapidly and may cause acute edema of the throat, leading to dysphagia, dyspnea, and even suffocation. The quality of life of the children is seriously jeopardized, and if not treated in time, it is likely to endanger the children's lives [2]. Infection with pathogenic microorganisms is the main factor for pediatric APT [[3], [4], [5]]. Acute sore throat in up to 75–95% of children is caused by viral infection, mainly respiratory viruses, including influenza virus, adenovirus, coxsackie virus, and parainfluenza virus, while 15–30% of cases are caused by bacteria, involving Streptococcus and Staphylococcus [[6], [7], [8]]. It is worth noting that β-hemolytic Group A Streptococcus, a kind of Streptococcus, can trigger more severe symptoms and complications, such as postinfection systemic complications, acute rheumatic fever, and poststreptococcal glomerulonephritis [9,10]. At present, there are no specific drugs for the treatment of viral pharyngitis and tonsillitis, while antibiotics are mainly used to prevent complications and secondary infection in bacterial pharyngitis and tonsillitis [11]. However, antibiotic resistance and toxic side effects, especially in children, severely limit prevention and treatment in the clinic [12].
KHJSC is the formula of Miao medicine in China, which is mainly composed of Ba Zhao Jin Long (Ardisia crispa (Thunb.) A.DC.), Shan Dou Gen (Sophorae tonkinensis Gagnep), Chan Tui (Cicadae Periostracum) and Bo He Nao (Menthol). In traditional Chinese medicine (TCM), KHJSC has the functions of clearing heat, removing toxicity, and relieving swelling and pain, which are commonly used in the clinical treatment of APT, herpetic pharyngitis and stomatitis in children. The drug is a liquid spray preparation which can directly act on the oral mucosa, form liquid film in the lesion area, and can be directly absorbed. It has the advantages of quick effect, high curative effect and short course of treatment.
A clinical study demonstrated the efficacy and safety of KHJSC in the treatment of sore throat caused by APT in children [1]. Sun demonstrated that KHJSC could have significant anti-inflammatory, analgesic and antibacterial effects [13,14]. Although KHJSC is widely treated for pediatric APT in clinic, there is a lack of research on animal pharmacology and therapeutic mechanism. Previously, since the dosage and daily use times of the medicine were not clear in the instruction manual of KHJSC, we have conducted an experimental study on the standard dosage in different acute pharyngitis animal models [13]. Then, the optimal dosage and usage of KHJSC were defined and applied to the present experiment. The mechanism of KHJSC in the treatment of APT remains unclear. In the present study, we investigated the antiviral and antibacterial effects of KHJSC in vitro and the effectiveness of KHJSC on APT in young rabbits caused by β-hemolytic Streptococcus infection in vivo, as well as the underlying mechanism.
2. Materials and methods
2.1. Medication
KHJSC was purchased from Guizhou Sanli Pharmaceutical Co., Ltd. (Cat 20200721; Guizhou, China). KHJSC is an oral spray preparation and consist of four herbs, specififically Ardisia crispa, Sophorae tonkinensis, Cicadae Periostracum and Menthol. The instructions and quality inspection report show that each 1 mL liquid of this product is equivalent to 0.7 g of raw drug. Amoxicillin capsules were produced by Kunming Baker Norton Pharmaceutical Co., Ltd. (Cat. 2020.07.24; Kunming, China).
2.2. Cells, viruses and bacteria
Human laryngeal epidermoid carcinoma cells (Hep-2), human embryonic lung fibroblast cells (MRC-5), and human lung carcinoma cells (A549) were purchased from Beijing BeNa Culture Collection (BNCC) Biotechnology Research Institute. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and 1% penicillin streptomycin at 37 °C with 5% CO2.
Influenza A virus (H1N1) strain PR/8/34, human respiratory syncytial virus (RSV), human adenovirus serotype 3 (Ad3), coxsackievirus A6 (CV-A6) and human parainfluenza virus 3 (HPIV-3) were purchased from American Type Culture Collection (ATCC). Human coronavirus 229E (HCoV-229E) was obtained from the Institute of Medical Biotechnology, Chinese Academy of Medical Sciences. All viruses were propagated in the ABSL-2 laboratory, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, and stored at −80 °C.
Multidrug-resistant Pseudomonas aeruginosa (MDR-PA), methicillin-resistant Staphylococcus aureus (MRSA), and β-hemolytic Streptococcus were purchased from ATCC and BNCC, respectively, and were subcultured in the ABSL-2 laboratory, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
2.3. Animals
Thirty-six Japanese White Rabbits, 6 weeks old, 1.5 ± 0.1 kg, 18 males and 18 females, were purchased from Beijing Jinmuyang Experimental Animal Breeding Co., Ltd., and all animal care and operations in the experiment followed the regulations of the National Institutes of Health (NIH) and Beijing Experimental Animal Ethics Committee and were approved by the Animal Ethics Committee of the Institute of Chinese Materia Medica, No. 2020D036.
2.4. UPLC-QE-HF-MS/MS analysis of KHJSC
The UPLC-Q Exactive HF-X (Thermo, Waltham, MA, USA) with ACQUITY UPLC ® HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters, Milford, USA) was carried out for the qualitative analysis and the column temperature was maintained at 40 °C. The mobile phases consisted of phase A (95% water + 5% acetonitrile with 0.1% formic acid) and phase B (47.5% acetonitrile + 47.5% isopropyl alcohol + 5% water with 0.1% formic acid). The positive ion modes liner gradient program was performed as 20% B from 0 to 3 min; 20–35% B from 3 to 4.5 min; 35–100% B from 4.5 to 5 min; 100% B from 5 to 6.3 min; 100-0% B from 6.3 to 6.4 min; 0% B from 6.4 to 8 min. The negative ion modes liner gradient program was performed as 5% B from 0 to 1.5 min; 5–10% B from 1.5 to 2 min; 10–30% B from 2 to 4.5 min; 30–100% B from 4.5 to 5 min; 100% B from 5 to 6.3 min; 100-0% B from 6.3 to 6.4 min; 0% B from 6.4 to 8 min. The injective volume was 3 μL with flow rate of 0.4 mL/min. Mass spectra were recorded in the range of m/z 70–1050 Da with electrospray ionization (ESI) source. The parameter conditions were performed as follows: spray voltage (±), +3.5/-3.5 kV; collision energy, 20–60 eV; capillary temperature, 325 °C; heater temperature, 425 °C; cone gas flow rate, 50 L/h; resolution, 7500 MS2. The sample were dissolved in methanol - acetonitrile (1:1), and the supernatant was extracted as the test solution after a 0.22 μm microporous membrane filtering.
2.5. Cytotoxicity assay
Hep-2 cells, MRC-5 cells and A549 cells were seeded onto 96-well plates at a density of 5 × 104 cells/mL and a volume of 100 μL per well. The media was removed after 24 h, and then the cells were rinsed with PBS. Then, 100 μL/well medium containing 2% serum and various concentrations (350, 175, 87.50, 43.75, 21.88, 10.94, 5.47, 2.73 mg/mL) of KHJSC were applied to culture wells, and untreated cells were added as a control (4 replicate wells for each group). The plates were incubated at 37 °C and 5% CO2 for 72 h. The cytopathic effect was observed under an inverted microscope to determine the maximal noncytotoxic concentration (TC0). The 50% cytotoxicity concentration (TC50) was calculated by the Reed-Muench method.
2.6. Antiviral assay
The cells were cultured in 96-well plates for 24 h and washed three times with PBS. Then, MRC-5 cells and A549 cells were exposed to HCoV-229E and PR8 virus at 100 TCID50, respectively, while Hep-2 cells were treated with 100 TCID50 of RSV, Ad3, CoxA6 and HPIV-3 virus. All the infected cells were incubated at 37 °C with 5% CO2 for 1 h, and then the cells were incubated with serially diluted KHJSC solutions for 72 h. The cytopathic condition was observed for 3 consecutive days. The 50% inhibitory concentration (IC50) and therapeutic index (TI) were calculated according to the Reed-Muench method, TI = TC50/IC50.
2.7. Antibacterial assay
The antibacterial effect of KHJSC in vitro was evaluated by determination of minimum inhibitory concentrations (MICs). MDR-PA, MRSA and β-hemolytic Streptococcus were cultured in nutrient broth media at 37 °C for 18 h. MIC testing of KHJSC was performed by the broth dilution method according to the guidelines of the Clinical and Laboratory Standards Institute. The bacterial concentrations were adjusted to 106 cfu/mL by an electronic turbidimeter (DensiCHEK Plus). Then, the bacterial solution was added to 96-well plates and incubated in liquid medium at a series of concentrations (350, 175, 87.50, 43.75, 21.88, 10.94, 5.47, and 2.73 mg/mL) for 24 h at 37 °C. The MICs of KHJSC against MDR-PA, MRSA and β-hemolytic Streptococcus were determined through the optical density of each well at 600 nm recorded by a microplate reader.
2.8. Animal infection and treatment
Rabbits were randomly divided into 6 groups: Control group, Model group, Amoxicillin group, KHJSC High-dose (720 μL/kg/d, 6 times/d), Medium-dose (540 μL/kg/d, 6 times/d), and Low-dose (360 μL/kg/d, 6 times/d) (n = 6). All rabbits were anesthetized, and then the rabbits’ mouths were opened by sterilized tweezers to expose their bilateral tonsils. Except for the control group, 0.5 mL β-hemolytic Streptococcus solution was injected into the tonsils of rabbits for 2 consecutive days. The rabbits in the control group were injected with nutrient broth under the same conditions.
KHJSC treatment started on the third day and was maintained for 3 consecutive days. The rabbits in the control group and model group were sprayed with distilled water. Blood samples from the marginal auricular vein of rabbits were collected for routine blood tests on the 6th day. After that, the animals were sacrificed by excessive anesthesia, and then the tonsil tissues and pharyngeal mucosa tissues were collected for pathological examination and other tests.
2.9. Histopathological examination of pharyngeal mucosa and tonsil tissues
The pharyngeal mucosa and tonsil tissues were fixed in 4% paraformaldehyde solution for 48 h. The specimens were removed and rinsed with water, dehydrated by gradient ethanol, vitrification with xylene, trimmed, and embedded in paraffin blocks. Paraffin sections were cut and stained with hematoxylin and eosin (HE). The slices were sealed with neutral gum and examined by light microscopy (DMLB-HC, Leica, Germany) to evaluate the histopathological changes in the pharyngeal mucosa and tonsil tissues.
2.10. Cytokine measurement in serum
Blood was obtained from the rabbit marginal auricular vein. The serum was collected after 10 min centrifugation (3500 rpm). The determination of serum IL-1β, IL-2, IL-5, IL-6, IL-18, MCP-1, TNF-α and IFN-γ was performed by ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd.) following the manufacturer's instructions.
2.11. TMT-based proteomic analysis
2.11.1. Protein preparation and extraction
The frozen tonsil tissue samples were put into MP lysis matrix tubes and homogenized in protein lysis buffer (8 M urea +1% SDS with protease inhibitor) for 30 min on ice. Then, the samples were centrifuged at 16000×g for 30 min at 4 °C, and the supernatant was collected. The protein concentrations of the samples were measured by the BCA protein determination method.
2.11.2. Trypsin digestion and TMT labeling
The collected proteins (100 μg) from each sample were digested by trypsin enzyme overnight at 37 °C followed by reductive alkylation to generate the peptides. Then, the peptides were labeled with TMT label reagent (ThermoFisher, 90111, USA) at room temperature for 2 h. Three repeated trials of the control group were labeled with TMT10-126, TMT10-127 N, and TMT10-127C; the model group was labeled with TMT10-128 N, TMT10-128C, and TMT10-129 N; and the KHJSC group was labeled with TMT10-130 N, TMT10-130C, and TMT10-131.
2.11.3. High-pH reversed-phase liquid chromatography analysis (high-pH RPLC)
The TMT-labeled peptides were first separated by a high-PH RPLC. The samples were loaded onto an ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 150 mm, Waters, USA) in phase A (2% acetonitrile in aqueous ammonia, pH 10) and phase B (80% acetonitrile in aqueous ammonia, pH 10) for 48 min. A total of 20 fractions were collected and finally merged into 10 fractions. Then, the fractions were dissolved with mass spectrometry loading buffer (2% acetonitrile + 0.1% formic acid) after vacuum centrifugation and then subjected to mass spectrometry for analysis.
2.11.4. LC‒MS/MS analysis
Each fraction was subjected to mass spectrometry analysis using a reverse-phase C18 column (75 μm × 25 cm, Thermo, USA) with buffer A (2% acetonitrile + 0.1% formic acid) and buffer B (80% acetonitrile + 0.1% formic acid) at a flow rate of 300 nL/min. The eluted peptides were analyzed using Thermo Xcalibur 4.0 software (Thermo, USA). MS data acquisition was performed using a data-dependent top 20 method.
2.11.5. Protein identification and quantitative analysis
Proteome Discoverer™ Software 2.4 was used to analyze the original LC-MS/MS data. The peptide false discovery rate (FDR) was set as ≤ 0.01. The precursor mass tolerance and fragment mass tolerance were 20 ppm and 0.02 DA, respectively. The detection of at least one unique peptide per protein was set as the requirement for protein identification.
The protein quantitative analysis was based on reporter ion peak intensity. Differentially expressed proteins (DEPs) were identified through ratio-fold change as well as P value calculated with a t-test. A protein with a fold change >1.5 or <0.67 and p < 0.05 was regarded as an up- or down-regulated protein. The proteins that satisfied the set thresholds were collected as “Differentially expressed proteins” for further bioinformatics analysis.
2.11.6. Bioinformatics annotation
Functional annotations of DEPs were performed through Gene Ontology (GO) annotation and enrichment analysis (http://www.geneontology.org/). In addition, KEGG pathway enrichment (http://www.genome.jp/kegg/) was used to analyze canonical pathways, diseases and functions, and biological networks associated with the lists of DEPs.
2.12. Enzyme-linked immunosorbent assay (ELISA) analysis
Tonsilla tissues were homogenized in PBS (pH 7.4) by a tissue grinder. The supernatant solution was collected after 20 min of centrifugation (3000 rpm). Samples and reagent were added to 96-well plates according to the manufacturer's instructions. The absorbance (OD value) of each well was measured sequentially at a wavelength of 450 nm. Statistical analysis was performed using the statistical software GraphPad Prime 9 (GraphPad Software, Inc., CA USA).
2.13. Statistical analysis
The statistical analysis of the data was accomplished using independent t-test and one-way analysis of variance (ANOVA) (SPSS 17.0, IBM Corporation, Armonk, NY, USA); p values < 0.05 were considered to be statistically significant. All data are expressed as the mean ± standard deviation (SD) (‾x ± s).
3. Results
3.1. UPLC-QE-HF-MS/MS analysis of KHJSC
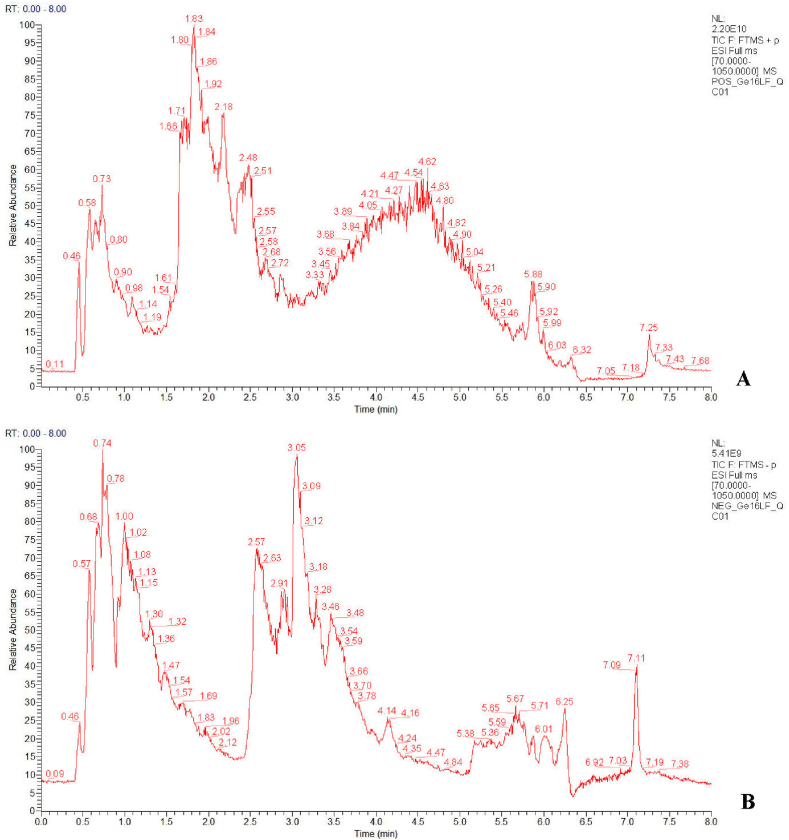
Both positive and negative ion modes were tried for UPLC-QE-HF-MS/MS analysis. The base peak intensity (BPI) chromatogram profile of KHJSC was shown in Fig. 1A and B. There were 67 compounds (1–67) identified in positive ion mode and 76 compounds (68–143) identified in negative ion mode. The identification results of 143 compounds were shown in Supplementary file 1. Among them, narirutin (42), coumarin (49), kaempferide (58), bergenin (82) quercetin (129) and isorhamnetin (137) were considered the main bioactive constituents in Ba Zhao Jin Long. Cytisine (5), matrine (11), sophocarpine (13), trifolirhizin (35), coumarin (49), genistein (55), isocoumarin (80) and quercetin (129) were major active ingredients in Shan Dou Gen. These compounds with multiple pharmacological effects, such as anti-inflammatory, antiviral, antibacterial activities and immunomodulatory [[15], [16], [17]].
Fig. 1.
Representative base peak intensity (BPI) chromatograms of KHJSC in positive (A) and negative (B) ion modes.
3.2. Antiviral activity of KHJSC in vitro
In the present study, the cytotoxic effect of KHJSC was first determined by visualizing the CPE. Following 72 h of treatment, the maximal noncytotoxic concentration (TC0) of KHJSC was 10.94 mg/mL in MRC-5 and A549 cells and 5.47 mg/mL in Hep-2 cells. The 50% cytotoxicity concentration (TC50) for MRC-5, A549 and Hep-2 cells was calculated to be 15.60, 15.60 and 7.80 mg/mL, respectively, by the Reed-Muench method.
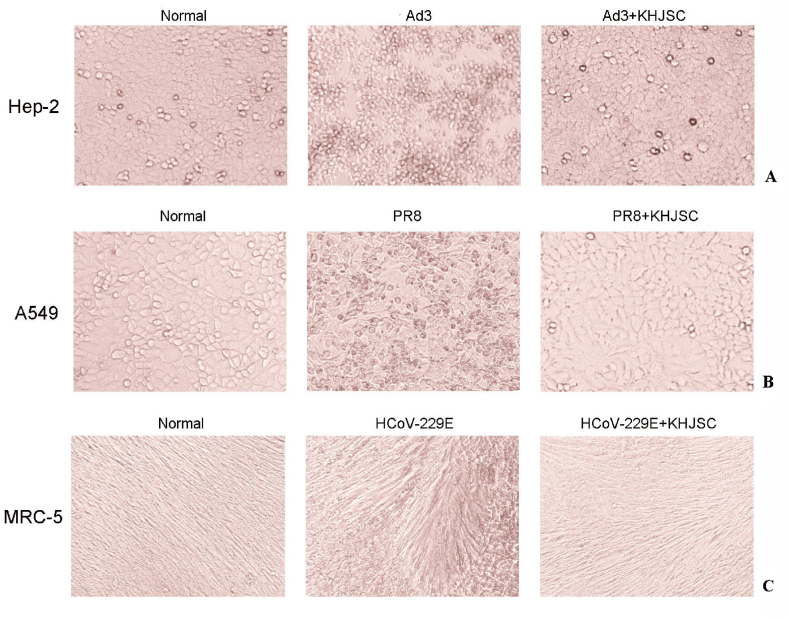
The broad-spectrum antiviral action of KHJSCs on different cells were evaluated by CPE assay. KHJSC significantly improved cell damage induced by Ad3, PR8 and HCoV-229E (Fig. 2A–C). The IC50 values were 1.99, 1.99 and 4.49 mg/mL, and TI values were 7.8, 7.8, 1.75, respectively. However, there were no obvious antiviral effects on RSV, CoxA6 and HPIV-3.
Fig. 2.
Antiviral activity of KHJSC on different cells by CPE assay. Typical CPE characteristics, cell rounding and crimpling were observed in the Hep-2 (A) and A549 cells (B) infected by Ad3 and PR8, fibrous cells break off and curl up in MRC-5 induced by HCoV-229E (C). The cellular damage were significantly alleviated by KHJSC (TC0) treatment group.
3.3. Antibacterial effect of KHJSC in vitro
The in vitro antibacterial effect of KHJSC was evaluated on MDR-PA, MRSA and β-hemolytic Streptococcus. A series of concentrations of KHJSC were added to 96-well plates containing culture medium with different strains. After 24 h, the suppression effect was determined by observing the color and turbidity of the culture medium. KHJSC has obvious inhibitory effects on MDR-PA, MRSA and β-hemolytic Streptococcus, with minimum inhibitory concentrations (MICs) of 350, 350, and 175 mg/mL, respectively.
3.4. Therapeutic effect of KHJSC for APT in rabbits infected by β-hemolytic Streptococcus
To assess the efficacy of KHJSC on APT in children, young Japanese white rabbits (1.4–1.6 kg, 35–42 days) were selected to make the animal model. After 2 consecutive days of infection by β-hemolytic Streptococcus and medicine intervention for 3 days, whole blood, tonsil and pharyngeal tissues were collected on the 6th day for pharmacodynamic evaluation and mechanistic study.
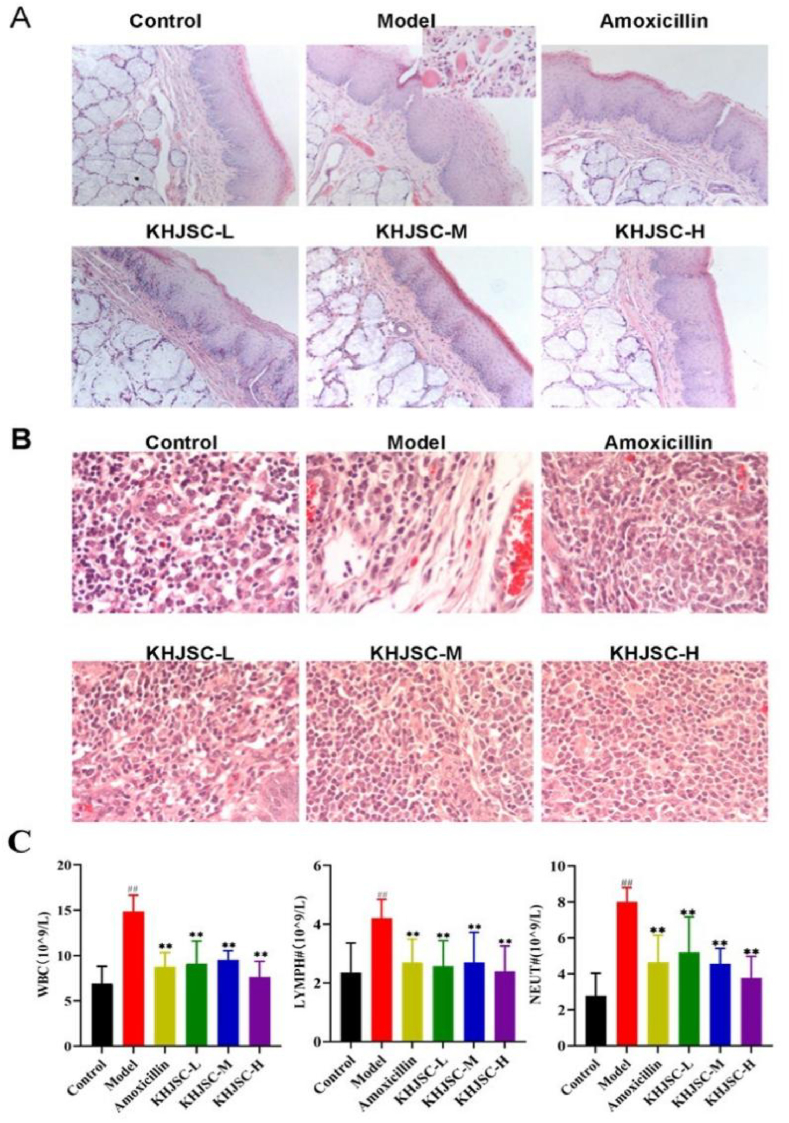
The morphological changes in the bilateral tonsil tissues and mucosal tissues of the pharynx from the young rabbits were first investigated. Compared with the control group, the β-hemolytic-Streptococcus-infected rabbits exhibited severe congestion in the pharynx, redness and swelling in the tonsil tissues, even purple color, and more yellow mucus. The HE staining results revealed that the tonsil tissues of the young rabbits had obvious edema and intravascular congestion and showed diffuse lesions. Abundant apoptosis of capsule cells, cytoplasmic laxity and enlarged and hyperchromatic nuclei were observed in tonsil tissue; lymph nodes were incomplete, and massive inflammatory infiltration was observed in the interstitium, characterized by more eosinophilic leaf nuclei. In addition, the epithelium of the pharyngeal mucosa had different degrees of hyperplasia and degeneration, subcutaneous tissue swelling and obvious inflammatory infiltration. In contrast, after treatment with KHJSC or amoxicillin, the lesions of tonsil tissue and pharyngeal mucosa were significantly ameliorated, as evidenced by a more intact lymph nodule peritoneum, allayed lymphocytes and tissue congestion (Fig. 3A and B).
Fig. 3.
Effect of KHJSC for APT in rabbits infected by β-hemolytic Streptococcus. (A) Pathological changes of pharyngeal tissues (HE 10 × 10); (B) pathological changes of tonsil tissues (HE 10 × 40); (C) levels of WBC, LYMPH, and NEUT in whole blood (n = 6). Data were expressed as the mean ± SD. ##P < 0.01 vs. Control, **P < 0.01 vs. Model.
Routine blood examination showed that compared with the control group, the number of white blood cells (WBCs), lymphocytes (LYMPH) and neutrophils (NEUT) of the rabbits in the model group increased significantly. Both the amoxicillin group and the three KHJSC treatment groups had significantly decreased numbers of WBCs, LYMPH and NEUT (P < 0.01, Fig. 3C).
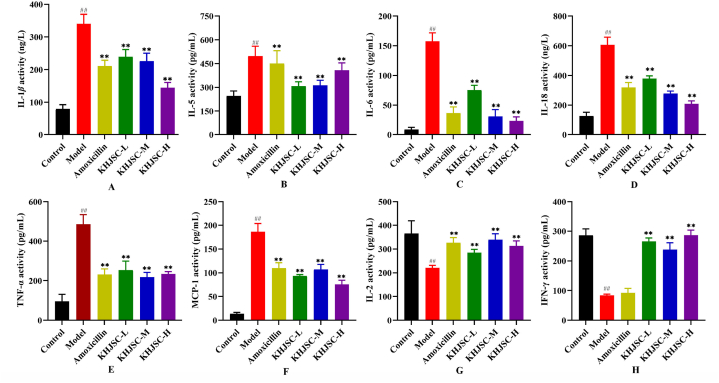
After β-hemolytic Streptococcus infection, the expression of inflammatory factors changed, including the increased expression of proinflammatory factors and the decreased expression of anti-inflammatory factors. As shown in Fig. 4A–H, the expression levels of IL-1β, IL-5, IL-6, IL-18, TNF-α and MCP-1 in the Model group were significantly increased, and IL-2 and IFN-γ were significantly decreased after infection with β-hemolytic Streptococcus compared with the Control group (P < 0.01). Conversely, all three doses of KHJSC significantly regulated the expression levels of inflammatory cytokines in β-hemolytic Streptococcus infection (P < 0.01).
Fig. 4.
The effects of KHJSC on the expression levels of IL-1β(A), IL-5 (B), IL-6 (C), IL-18 (D), TNF-α(E), MCP-1 (F), IL-2 (G) and IFN-γ(H) inflammatory cytokines in rabbits (n = 6). Data were expressed as the mean ± SD. ##P < 0.01 vs. Control, **P < 0.01 vs. Model.
3.5. Effects on protein expression in rabbit tonsil and pharyngeal tissues
3.5.1. Characterization of differentially expressed proteins
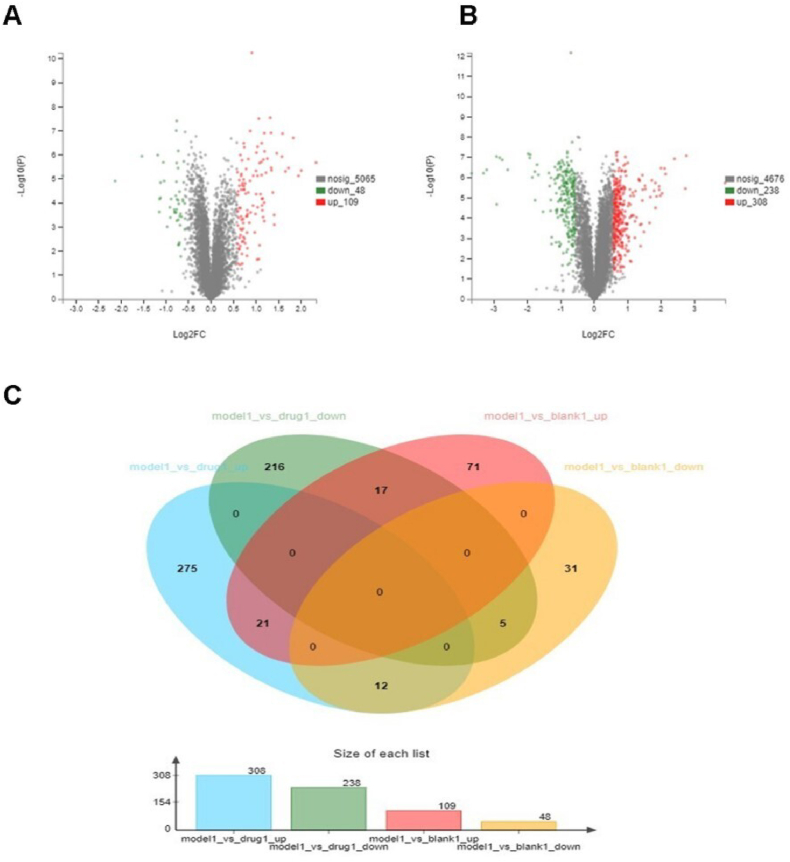
After quantitative proteomic analysis and database searches, a total of 5222 differentially expressed proteins (DEPs) were identified in the 3 sample groups. Based on the criteria of DEPs,157 proteins were identified in the model group compared with the control group, and 109 proteins were significantly upregulated and 48 proteins were significantly downregulated in the model group (Fig. 5A). A total of 546 proteins in the KHJSC group were abnormally expressed versus the Model group. Of these, 238 proteins were significantly downregulated, and 308 proteins were significantly upregulated (Fig. 5B). For further screening of the proteins, the following criteria were used: upregulated proteins in the model group vs. the control group and downregulated proteins in the KHJSC group vs. the model group. Finally, there were twenty-one proteins that matched the requirements. Furthermore, five proteins showed the following characteristics: downregulated proteins in the Model group vs. Control group and upregulated proteins in the KHJSC group vs. Model group (Fig. 5C).
Fig. 5.
Characterization of differentially expressed proteins in 3 sample groups. (A) Volcano plot of differentially expressed proteins in Model group vs. Control group. Green dots showed downregulated proteins, red dots showed upregulated proteins, and the gray dots showed proteins with no statistically significant difference between the Control and Model rabbits; (B) Volcano plot of differentially expressed proteins in Model group vs. KHJSC group. Green dots showed downregulated proteins, red dots showed upregulated proteins, and the gray dots showed proteins with no statistically significant difference between the Model and KHJSC group; (C) The common differentially expressed proteins in Model group vs. Control groups and Model group vs. KHJSC. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5.2. GO annotation analysis
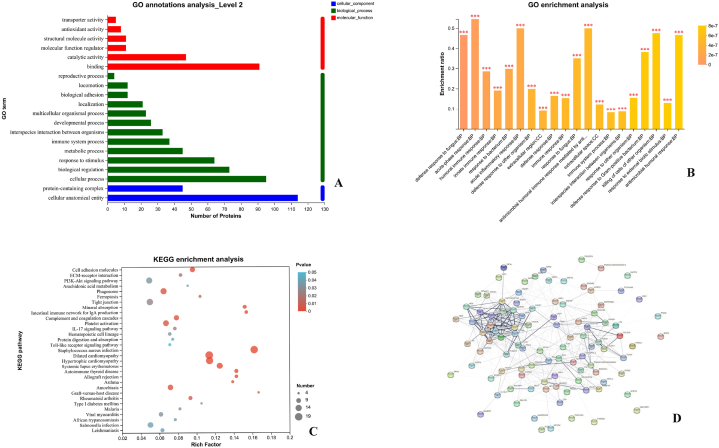
As shown in Fig. 6A, biological process annotation revealed that the DEPs in acute pharyngitis rabbits were mainly associated with cellular process (60.51%), biological regulation (46.50%), response to stimulus (40.76%), metabolic process (28.66%), and immune system process (23.57%). Gene Ontology analysis for cellular component annotation indicated that the DEPs were mainly related to cellular anatomical entities (72.61%) and protein-containing complexes (28.66%), while molecular function annotation showed that the DEPs were involved in molecular function analysis. These DEPs were mainly involved in binding (57.96%), catalytic activity (29.94%), molecular function regulators (7.01%), structural molecule activity (7.01%) and antioxidant activity (5.10%). The number of DEPs in the GO secondary annotation analysis (Level 2) was shown in Table 1.
Fig. 6.
(A) Bioinformatics analysis for GO annotation of differentially expressed proteins in acute pharyngitis rabbits; (B) Bioinformatics analysis for GO enrichment of differentially expressed proteins in acute pharyngitis rabbits; (C) Bioinformatics analysis for KEGG pathway enrichment of differentially expressed proteins in acute pharyngitis rabbits; (D) Protein-protein interactions among differentially expressed proteins analyzed using the String database.
Table 1.
The number of differentially expressed proteins in GO secondary annotations analysis (Level 2).
| Term Type | GO Term | Control/Model number of DEPs | Model/KHJSC number of DEPs |
|---|---|---|---|
| biological process | immune system process | 37 | 54 |
| biological regulation | 73 | 237 | |
| metabolic process | 45 | 230 | |
| locomotion | 12 | 17 | |
| reproductive process | 4 | 12 | |
| cellular process | 95 | 366 | |
| developmental process | 26 | 98 | |
| multicellular organismal process | 23 | 73 | |
| interspecies interaction between organisms | 33 | 38 | |
| growth | 1 | 5 | |
| rhythmic process | 1 | 4 | |
| localization | 21 | 128 | |
| biological adhesion | 12 | 19 | |
| behavior | 1 | 9 | |
| detoxification | 2 | 7 | |
| signaling | – | 9 | |
| biomineralization | 1 | 4 | |
| response to stimulus | 64 | 146 | |
| cellular component | protein-containing complex | 45 | 174 |
| cellular anatomical entity | 114 | 428 | |
| other organism part | 1 | – | |
| molecular function | translation regulator activity | 1 | 4 |
| transcription regulator activity | 2 | 8 | |
| structural molecule activity | 11 | 27 | |
| cargo receptor activity | – | 2 | |
| molecular carrier activity | – | 1 | |
| antioxidant activity | 8 | 8 | |
| transporter activity | 5 | 50 | |
| molecular function regulator | 11 | 45 | |
| binding | 91 | 327 | |
| protein tag | – | 1 | |
| molecular transducer activity | 3 | 5 | |
| catalytic activity | 47 | 232 |
3.5.3. GO enrichment analysis
The DEPs were performed GO enrichment analysis to annotate the functions according to cellular component, molecular function and biological process. The results of the control group and model group showed that these DEPs have calcium ion binding, antioxidant activity, Toll-like receptor 4 binding, and Toll-like receptor binding molecular functions (MFs). They were distributed in cellular components (CCs), such as the extracellular region, extracellular space, nucleosome, and DNA packaging complex. The main biological processes (BPs) included the acute inflammatory response and antimicrobial humoral response (Fig. 6B).
3.5.4. KEGG pathway enrichment analysis
KEGG pathway enrichment analysis showed that the DEPs in acute pharyngitis rabbits were mainly involved in PI3K-Akt signaling pathway, Toll-like receptor signaling pathway, and NF-kappa B signaling pathway (P < 0.05), as shown in Fig. 6C.
3.5.5. Protein‒protein interaction analysis
As shown in Fig. 6D, the DEPs were mainly associated with the immune system, and these proteins included P50117 (Protein S100-A9), O77791 (Protein S100-A12), G1ST17 (Peptidoglycan-recognition protein), P16973 (Lysozyme C), P23108 (Immunoglobulin J chain) and W8CXL2 (Toll like receptor 3). Moreover, these DEPs were located at key nodes of the protein interaction network, which indicated that the pathogenesis of acute pharyngitis caused by β-hemolytic Streptococcus may involve antibacterial and anti-inflammatory effects on the immune system.
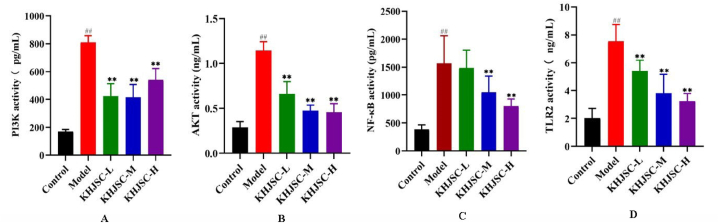
3.6. Validation of DEPs
To verify the results of our analysis, the expression of the candidate DEPs PI3K, AKT, NF-κB and TLR2 were detected by ELISA kit. DEP expression levels were entirely consistent with the results of the proteomics analysis. As shown in Fig. 7A–D, compared to the control group, all target DEPs were up-regulated in the model group, but down-regulated in the KHJSC group (p < 0.01).
Fig. 7.
The Elisa analysis demonstrated the expression levels of PI3K (A), AKT (B), NF-κB (C) and TLR2 (D) in the Control, Model, KHJSC groups. Data were expressed as the mean ± SD. ##P < 0.01 vs. Control, **P < 0.01 vs. Model.
4. Discussion
APT are common and frequently-occurring respiratory diseases in children. The clinical manifestations are acute edema of the throat, dyspnea, and even suffocation. Although most of the pathogens are viral, antibiotics are still the most frequent prescription for pediatric APT treatment. It was reported that approximately 50% of antibiotics were prescribed to children who suffered nonbacterial infectious diseases [18,19]. Inappropriate antibiotic abuse and misuse will result in many adverse events and drug toxicity due to children's special physiological condition and higher rates of bacterial resistance. Therefore, the rational use of antibiotics and the development of broad-spectrum drugs are important in developing better strategies for pediatric APT treatment.
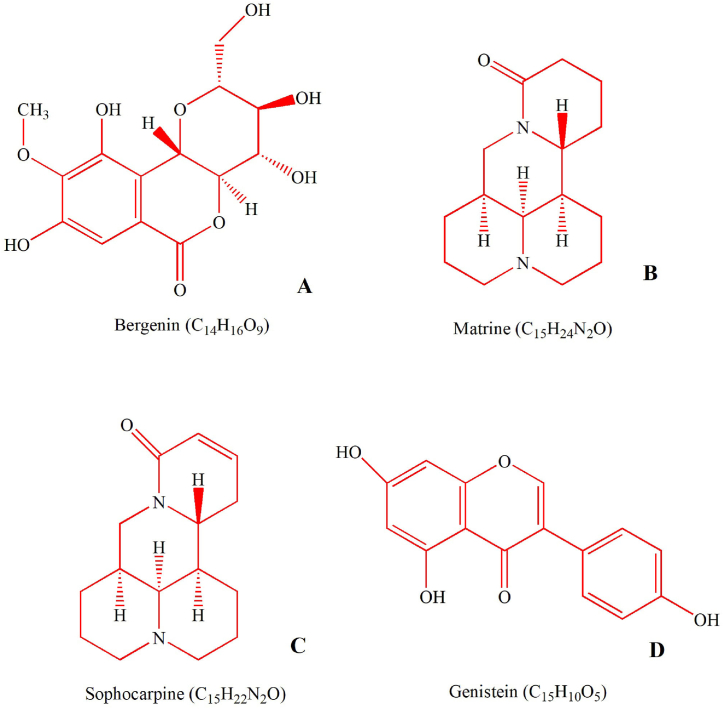
According to the theory of TCM, “heat leads to swelling”. Heat and poison accumulation leads to blood stasis and meat decay and eventually turns into abscess and putrid blood. The heat poison knot in the throat causes tonsillitis and acute chronic pharyngitis [20]. KHJSC is a prescription of TCM with obvious effects of clearing heat and removing toxin, relieving swelling and pain, and it is widely used in clinical practice for pediatric acute or chronic pharyngitis, tonsillitis, sore throat, stomatitis and swollen gums. The main bioactive constituents of KHJSC were identified by UPLC-MS/MS, and the structure were shown in Fig. 8A–D. Bergenin is the main chemical component of Ba Zhao Jin Long, which has good antitussive and anti-inflammatory effects [21]. Shan Dou Gen is a TCM plant that is widely used to treat sore throat, swollen gums and tumours [22,23]. Matrine, sophocarpine and genistein are the major active ingredients of Shan Dou Gen. Among them, Matrine can produce anti-inflammatory and analgesic effects by inhibiting the release of the inflammatory mediator histamine. Sophocarpine and genistein are obvious against acute inflammation caused by bacteria [24].
Fig. 8.
The chemical structure and formula of Bergeninum (A), Matrine (B), Sophocarpine (C) and Genistein (D).
Previous studies have shown that KHJSC has immediate analgesic efficacy in treating sore throat caused by APT in children [1]. The in vitro experiments in this study indicated that KHJSC demonstrates broad-spectrum antiviral activity. Cell pathological changes induced by respiratory tract viruses, including influenza viruses, parainfluenza viruses and coronaviruses, which are common pathogenic pathogens of APT, were evidently inhibited by KHJSC in a certain range of concentrations. The broad-spectrum antibacterial action of KHJSC was investigated by determination of MIC through the broth dilution method in vitro. The results showed that KHJSC has obvious inhibitory effects on MDR-PA, MRSA and β-hemolytic Streptococcus. The in vivo experiments were performed in young rabbits whose pharynx was injected with β-hemolytic Streptococcus [25]. The rabbit treatment with KHJSC significantly ameliorated pathologic changes in the bilateral tonsil and mucosal tissues and alleviated the levels of WBC, LYMPH, NEUT, and inflammatory cytokines compared to the model group.
Furthermore, the potential mechanism of KHJSC in treating APT infected by β-hemolytic Streptococcus was revealed through TMT labeling proteomics analysis. KEGG enrichment analysis showed that the primary DEPs affected by KHJSC are involved in PI3K-Akt signaling pathway, NF-κB signaling pathway and Toll-like receptor signaling pathway. The PI3K-Akt signaling pathway is involved in acute and chronic inflammatory processes, including cell metabolism, cell regulation and apoptosis [[26], [27], [28]]. The PI3K-Akt signaling pathway is activated by a variety of cellular stimuli or toxic insults to regulate essential cellular functions such as transcription, translation, proliferation, growth and survival [[29], [30], [31], [32]]. PI3K catalyzes the generation of PIP3 on the cell membrane, which acts as the second messenger to help activate Akt. Once activated, Akt can participate in cell apoptosis, protein synthesis and metabolism through phosphorylation, thus controlling key cellular processes [33]. When pathogenic microorganisms invade an organism, the PI3K-Akt signaling pathway is activated. The PI3K-Akt signaling pathway enhances the translation of interferon-stimulated genes through the regulation of downstream mTOR and p70S6K [[34], [35], [36]], and it also upregulates the production of inflammatory cytokines through the activation of NF-κB. This initiates the mRNA expression and transcription of inflammatory factors such as TNF-α, IL-1β, and IL-6 [29,37,38], which triggers the immune stress response. In the Toll-like receptor signaling pathway, TLR2 and TLR4 play a central role in antibacterial infection. The difference is that TLR4 is the primary recognition receptor for LPS, and TLR2 is the primary recognition receptor for Gram-positive bacterial lipoproteins [[39], [40], [41], [42]]. TLR2 recognizes not only the ligands of peptidoglycan and phosphopeptides of Gram-positive bacteria but also the lipoproteins and lipopeptides of certain bacteria and mycoplasma [[43], [44], [45]]. In the Toll-like receptor signaling pathway, TLR2 regulates the downstream PI3K-Akt signaling pathway and NF-κB signaling pathways. TLR2 is activated by the specific receptor of β-hemolytic Streptococcus and becomes overexpressed, leading to an increase in the expression of phosphatidylinositol-4,5-bisphosphate 3-kinase (U3KM40) and nonspecific serine/threonine protein kinase (G1SD70), which in turn affects the expression of β-NFKB inhibitor (G1TL53) and nuclear factor kappa B subunit 2 (G1SPK2), resulting in the release of large amounts of IL-6, IL-12, IL-1β and TNF-α inflammatory factors and ultimately triggering an immunomodulatory response. At the same time, a large number of inflammatory factors in turn activate the NF-κB pathway and can trigger inflammation and injury by regulating the expression of chemokines such as MCP-1, recruiting large numbers of macrophages and neutrophils [46]. The detection results of target proteins and inflammatory cytokines show that KHJSC could reduce the levels of TLR2, PI3K, AKT and NF-κB, thus inhibiting the expression of inflammatory mediators and severe inflammatory reaction in blood and tissues, and alleviating tissue edema and vascular stasis at the lesion site.
5. Conclusion
In summary, KHJSC is widely used as a prescription drug in pediatrics for the treatment of APT,and showed obvious clinical efficacy. It can significantly relieve the pharyngeal and tonsil swelling, pain and other symptoms in children. However, the exact dosage is not specified in the instructions, as well as its therapeutic mechanism is still unclear. In our previous study, the dosage of KHJSC was determined and applied to this experiment. In this article, we further verified the antibacterial and antiviral effects of KHJSC in vitro and confirmed its protective effect against APT induced by β-hemolytic Streptococcus in a rabbit model as shown by inhibiting the release of inflammatory cytokines, decreasing the number of white blood cells, lymphocytes, neutrophils, alleviating pathological damage of pharyngeal and tonsil tissues. TMT labeling proteomics analysis indicated that mechanism of KHJSC is related to PI3K-Akt, NF-κB and Toll-like receptor signaling pathway. These results provided the reference and scientific basis for the application of KHJSC in clinic and further mechanisms study. In brief, these results not only provided evidence for the treatment of acute sore throat with Chinese herbal medicine, but also provided reference and scientific basis for the application of KHJSC in clinic and further mechanisms study. We will also pay more attention to the active ingredients in KHJSC and study on the further pharmacology action and mechanism of ingredients on APT.
Author contribution statement
Bo Pang; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ronghua Zhao; Bo Peng; Yingli Xu; Lirun Zhou: Performed the experiments.
Lei Bao; Zihan Geng; Shuran Li: Contributed reagents, materials, analysis tools or data.
Shanshan Guo; Xiaolan Cui: Conceived and designed the experiments.
Jing Sun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Ethics statement
Animal Ethics Committee of the Institute of Chinese Materia Medica, No. 2020D036.
Funding statement
This study was supported by the National Key R&D Program of China [2018YFC1708100], [2018YFC1708105] and the National Natural Science Foundation of China (NO. 82104500).
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We would like thank all authors have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17802.
Contributor Information
Shanshan Guo, Email: ssguo@icmm.ac.cn.
Xiaolan Cui, Email: xlcui@icmm.ac.cn.
Jing Sun, Email: jingziaiwei@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ma Y.N., Zhong C.L., Hu S.Y., Cai Q.H., Guo S.X. Evaluation on immediate analgesic efficacy and safety of Kai-Hou-Jian spray (children's type) in treating sore throat caused by acute pharyngitis and tonsillitis in children: study protocol for a randomized controlled trial. Trials. 2021;22:216. doi: 10.1186/s13063-021-05148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavez D., Pérez R., Cofré J., Rodríguez J. Recommendations for diagnosis and etiological treatment of acute streptococcal pharyngotonsilitis in pediatrics. Rev Chilena Infectol. 2019;36:69–77. doi: 10.4067/S0716-10182019000100069. [DOI] [PubMed] [Google Scholar]
- 3.Altamimi S., Khalil A., Khalaiwi K.A., Milner R.A., Pusic M.V., Al Othman M.A. Cochrane Database Syst Rev; 2012. Short-term Late-Generation Antibiotics versus Longer Term Penicillin for Acute Streptococcal Pharyngitis in Children. [DOI] [PubMed] [Google Scholar]
- 4.Espadas Macia D., Flor Macian E.M., Borras R., Poujois Gisbert S., Munoz Bonet J.I. Streptococcus pyogenes infection in paediatrics: from pharyngotonsillitis to invasive infections. Anales de pediatria. 2018;88:75–81. doi: 10.1016/j.anpedi.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Ughasoro M.D., Akpeh J.O., Echendu N., Mgbachi N.G., Okpala S., Amah L., Okolo O.H., Udem N. The profile of microorganisms that associate with acute tonsillitis in children and their antibiotics sensitivity pattern in Nigeria. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-99570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook I. Microbiology of common infections in the upper respiratory tract. Prim Care. 1998;25:633–648. doi: 10.1016/s0095-4543(15)30006-3. [DOI] [PubMed] [Google Scholar]
- 7.Evsikova M.M., Radtsig E.Y., Varavina M.A. The role of group A β-hemolytic streptococcus in the etiology of acute inflammatory pathology of the pharynx in children and adolescents. Vestn. Otorinolaringol. 2020;85:22–24. doi: 10.17116/otorino20208501122. [DOI] [PubMed] [Google Scholar]
- 8.Wi D., Choi S.H. Positive rate of tests for group a Streptococcus and viral features in children with acute pharyngitis. Children. 2021;8:599. doi: 10.3390/children8070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soderholm A.T., Barnett T.C., Sweet M.J., Walker M.J. Group A streptococcal pharyngitis: immune responses involved in bacterial clearance and GAS-associated immunopathologies. J. Leukoc. Biol. 2018;103:193–213. doi: 10.1189/jlb.4MR0617-227RR. [DOI] [PubMed] [Google Scholar]
- 10.Windfuhr J.P., Toepfner N., Steffen G., Waldfahrer F., Berner R. Clinical practice guideline: tonsillitis I. Diagnostics and nonsurgical management. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:973–987. doi: 10.1007/s00405-015-3872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meskina E.R., Stashko T.V. How to reduce the antibacterial load in the treatment of acute tonsillitis and pharyngitis? Possible tactics and practical approaches. Vestn. Otorinolaringol. 2020;85:90–99. doi: 10.17116/otorino20208506190. [DOI] [PubMed] [Google Scholar]
- 12.Altamimi S., Khalil A., Khalaiwi K.A., Milner R., Pusic M.V., Al Othman M.A. Cochrane Database Syst Rev; 2009. Short versus Standard Duration Antibiotic Therapy for Acute Streptococcal Pharyngitis in Children; p. CD004872. [DOI] [PubMed] [Google Scholar]
- 13.Jing S., Yingjie G., Xin M., Yujing S., Ronghua Z., Shanshan G., Rong-mei Y., Xiu D., Changbiao W., Xiaolan C. Usage and dosage of kaihoujian throat spray (for children) in treatment of acute pharyngitis and acute tonsillitis. Chin J Exp Tradit Med Form. 2019;25(10):33–40. [Google Scholar]
- 14.Jing S., Ronghua Z., Xin M., Shanshan G., Yingjie G., Rongmei Y., Xiu D., Changbiao W., Xiaolan C. Study on the usage and dosage of kaihoujian spray in the treatment of acute pharyngitis. World Chin Med. 2019;14(3):577–580. [Google Scholar]
- 15.Zhang S.N., Li X.Z., Tan L.Y., Zhu K.Y. A review of pharmacological and toxicological effects of Sophora tonkinensis with bioinformatics prediction. Am. J. Chin. Med. 2021;49(2):359–389. doi: 10.1142/S0192415X21500178. [DOI] [PubMed] [Google Scholar]
- 16.Pan Q.M., Li Y.H., Hua J., Huang F.P., Wang H.S., Liang D. Antiviral matrine-type alkaloids from the rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015;78(7):1683–1688. doi: 10.1021/acs.jnatprod.5b00325. [DOI] [PubMed] [Google Scholar]
- 17.Chang L., Tingting F., Xiongwei L., Jingxin D., Hui S., Jie P., Ying Z. Transcriptome analysis and identification of related genes involved in secondary metabolism biosynthesis in Ardisia crispa. Chin. Tradit. Herb. Drugs. 2021;52(5):1434–1447. [Google Scholar]
- 18.Chiappini E., Regoli M., Bonsignori F., Sollai S., Parretti A., Galli L., de Martino M. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin. Therapeut. 2011;33:48–58. doi: 10.1016/j.clinthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Kettunen S., Lantto U., Koivunen P., Tapiainen T., Uhari M., Renko M. Risk factors for periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome: a case-control study. Eur. J. Pediatr. 2018;177:1201–1206. doi: 10.1007/s00431-018-3175-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Xie Y.M., Li G.X., Gao Y., Zhao Y.C., Tang J.J., Yao X.Y., Li M. Advantages and problems of traditional Chinese medicine in treatment of acute pharyngitis. China J. Chin. Mater. Med. 2017;42:3819–3825. doi: 10.19540/j.cnki.cjcmm.20170901.002. [DOI] [PubMed] [Google Scholar]
- 21.JH Z., S. H, Z. Y. LY Z., Z. H, D. X. XW L., TT F. Simultaneous determination of four constituents in kaihoujian throat spray (for children) by QAMS. Chin. Tradit. Pat. Med. 2022;44(6):1760–1765. [Google Scholar]
- 22.Dang Z., Liu C., He Q., Feng T., Quan W., Dong X., Zhou Y. Mechanism of Sophora tonkinensis Gagnep regulating leukocyte transendothelial migration pathway in improving acute pharyngitis based on network pharmacology. Chin. Pharmacol. Bull. 2023;39(2):348–356. [Google Scholar]
- 23.Li H., Yuan D., Liu Y. Research progress on chemical constituents in plants of Euchresta J. Benn and their biological activities. Chin. Tradit. Herb. Drugs. 2014;45(25):3486–3493. [Google Scholar]
- 24.Du G., Zuo Y., Pan W., Li X., Tian H., Wu Q., Du Y. Simultaneous determination of four constituents in Kaihoujian Spray by GC-FID. Chin. Tradit. Pat. Med. 2017;39(1):94–97. [Google Scholar]
- 25.Xiao C., Huang Y., Wei Q., Liu Y., Ji Q., Li K., Bao G. Comparative proteomic analysis reveals complex responses to Bordetella bronchiseptica infections in the spleen of rabbits. Proteomics. 2020;20 doi: 10.1002/pmic.202000117. [DOI] [PubMed] [Google Scholar]
- 26.Kwon D.H., Park C., Lee H., Hong S.H., Kim G.Y., Cha H.J., Kim S., Kim H.S., Hwang H.J., Choi Y.H. Ethanol extract of Chondracanthus tenellus (Harvey) Hommersand attenuates lipopolysaccharide-induced inflammatory and oxidative response by blocking the NF-κB, MAPKs, and PI3K/Akt signaling pathways. Asian Pac. J. Trop. Biomed. 2021;11:450–459. [Google Scholar]
- 27.Wang X., Han Y., Liu J., Zhang Y., Cheng K., Guo J., Guo Q., Liu S., Sun H., Hua Y., Zhang G., Xu S., Guo F., Yang Z. Exosomes play an important role in the progression of plasma cell mastitis via the PI3K-Akt-mTOR signaling pathway. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/4312016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W., Yuan G., Wang Q. Vitamin D attenuates lipopolysaccharide-induced inflammatory response in endothelial cells through inhibition of PI3K/Akt/NF-κB signaling pathway. Pharmazie. 2019;74:412–417. doi: 10.1691/ph.2019.9373. [DOI] [PubMed] [Google Scholar]
- 29.Blanco J., Cameirao C., López M.C., Muñoz-Barroso I. Phosphatidylinositol-3-kinase-Akt pathway in negative-stranded RNA virus infection: a minireview. Arch. Virol. 2020;165:2165–2176. doi: 10.1007/s00705-020-04740-1. [DOI] [PubMed] [Google Scholar]
- 30.Dunn E.F., Connor J.H. HijAkt: the PI3K/Akt pathway in virus replication and pathogenesis. Prog Mol Biol Transl Sci. 2012;106:223–250. doi: 10.1016/B978-0-12-396456-4.00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z., Zou X., Feng P., Zhan H., Xiong D., Lang J. Inhibition of the PI3K/AKT signaling pathway or overexpression of Beclin1 blocks reinfection of Streptococcus pneumoniae after infection of influenza A virus in severe community-acquired pneumonia. Inflammation. 2019;42(5):1741–1753. doi: 10.1007/s10753-019-01035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y., Su R., Gu Q., Hu Y., Yang H. PI3K/AKT-mediated autophagy inhibition facilitates mast cell activation to enhance severe inflammatory lung injury in influenza A virus- and secondary Staphylococcus aureus-infected mice. Antivir. Res. 2023;209 doi: 10.1016/j.antiviral.2022.105502. [DOI] [PubMed] [Google Scholar]
- 33.XY H., C. N. Sl Y., RH W., W. MF A study on the mechanism of Jiegeng plus Gancao on treating pharyngitis based on network pharmacology. Clin J Chin Med. 2021;13(7):1–7. [Google Scholar]
- 34.Kaur S., Katsoulidis E., Platanias L.C. Akt and mRNA translation by interferons. Cell Cycle. 2008;7(14):2112–2116. doi: 10.4161/cc.7.14.6258. [DOI] [PubMed] [Google Scholar]
- 35.Kaur S., Sassano A., Dolniak B., Joshi S., Majchrzak-Kita B., Baker D.P., Hay N., Fish E.N., Platanias L.C. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl. Acad. Sci. U.S.A. 2008;105(12):4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur S., Sassano A., Joseph A.M., Majchrzak-Kita B., Eklund E.A., Verma A., Brachmann S.M., Fish E.N., Platanias L.C. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J. Immunol. 2008;181(10):7316–7323. doi: 10.4049/jimmunol.181.10.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas K.W., Monick M.M., Staber J.M., Yarovinsky T., Carter A.B., Hunninghake G.W. Respiratory syncytial virus inhibits apoptosis and induces NF-kappa B activity through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 2002;277:492–501. doi: 10.1074/jbc.M108107200. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin M.S., Kim S.E., Heo J.Y., Lee M.E., Kim H.M., Paik S.G., Lee H., Lee J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Thompson J.M., Iwasaki A. Toll-like receptors regulation of viral infection and disease - ScienceDirect. Adv. Drug Deliv. Rev. 2008;60:786–794. doi: 10.1016/j.addr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller J.F., Carrouel F., Colomb E., Durand S.H., Baudouin C., Msika P., Bleicher F., Vincent C., Staquet M.J., Farges J.C. Toll-like receptor 2 activation by lipoteichoic acid induces differential production of pro-inflammatory cytokines in human odontoblasts, dental pulp fibroblasts and immature dendritic cells. Immunobiology. 2010;215:53–59. doi: 10.1016/j.imbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Meng J., Lien E., Golenbock D.T. MD-2-mediated ionic interactions between lipid A and TLR4 are essential for receptor activation. J. Biol. Chem. 2010;285:8695–8702. doi: 10.1074/jbc.M109.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cen X., Liu S., Cheng K. The role of toll-like receptor in inflammation and tumor immunity. Front. Pharmacol. 2018;9:878. doi: 10.3389/fphar.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O"Neill L.A.J., Bryant C.E., Doyle S.L. Therapeutic targeting of toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol. Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int. Immunopharm. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brigelius-Flohé R., Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.