Abstract
Islet cell transplantation (ITx) is an effective therapeutic approach for selected patients with type 1 diabetes with hypoglycemia unawareness and severe hypoglycemia events. In organ transplantation, human leukocyte antigen (HLA) mismatching between donor and recipient negatively impacts transplant outcomes. We aimed to determine whether HLA matching has an impact on islet allograft survival. Forty-eight patients were followed up after islet transplantation at our institution from 2000 to 2020 in a retrospective cohort. Patients underwent intrahepatic ITx or laparoscopic omental approach. Immunosuppression was dependent upon the protocol. We analyzed HLA data restricted to A, B, and DR loci on allograft survival using survival and subsequent multivariable analyses. Patients were aged 42.8 ± 8.4 years, and 64.3% were female. Diabetes duration was 28.6 ± 11.6 years. Patients matching all three HLA loci presented longer graft survival (P = 0.030). Patients with ≥1 HLA-B matching had longer graft survival compared with zero matching (P = 0.025). The number of HLA-B matching was positively associated with time of graft survival (Spearman’s rho = 0.590; P = 0.034). Analyses adjusted for confounders showed that ≥1 matching for HLA-B decreased the risk of allograft failure (P = 0.009). Our data suggest that HLA-B matching between recipients and donors improved islet allograft survival. Matching all three HLA loci (A, B, and DR) was also associated with prolonged islet allograft survival. Prospective studies and a larger sample size are warranted to validate our findings.
Keywords: islet transplantation, type 1 diabetes, human leukocyte antigens, islet survival
Introduction
Islet cell transplantation (ITx) is an effective therapeutic approach for selected patients with type 1 diabetes affected by hypoglycemia unawareness and severe hypoglycemia events 1 . ITx may be considered among the safest of all transplantation procedures compared with solid organs 2 , demonstrating improved glycemic control, decrease in the incidence of hypoglycemic events, and reduction of daily insulin dose, even in the absence of insulin independence, accompanied by improvement in quality of life3–6. Despite all the benefits reported, the prevention of islet rejection remains still a challenge in this field.
The human leukocyte antigen (HLA) system, which encodes the major histocompatibility complex, is known as an important factor for donor selection in solid organ transplantation 7 . For kidney transplant, the association between HLA matching and graft survival is well established8–10. On the other hand, the impact of HLA matching on pancreas transplant outcomes is still conflicting11,12. Most of the studies were limited by the small sample size or incomplete data due to earlier eras of pancreas transplantation 13 , but a positive role of HLA-B matching in preventing acute rejection in pancreas recipients has been shown 14 . The level of HLA mismatches correlates with the strength of the immune system response, and HLA typing is used to estimate the immunological risk of donor recognition 15 .
Islet transplantation recipients often receive more than one islet infusion from different donors, and this procedure is responsible for an exposure of the recipients to multiple HLA mismatches. The role of HLA matching on ITx outcomes has not been formally evaluated, and currently, HLA matching is not a criterion for islet donor selection. In this study, we aimed to evaluate the impact of HLA matching on graft survival in patients after ITx.
Materials and Methods
A retrospective cohort study was conducted on 48 patients with type 1 diabetes who underwent ITx at our institution between 2000 and 2020. Patients were followed-up for 6.4 ± 5.8 years. Forty-five patients underwent intrahepatic ITx and three patients received allogeneic islets via a surgical laparoscopic omental approach with islets distributed on the omentum surface. Induction and maintenance of immunosuppression were according to the clinical transplant protocol (Table 1). Protocol procedures were approved by the University of Miami Health Research Ethics Board. All patients provided informed consent and were enrolled in different research protocols (Table 1). Clinical, demographic, and transplant-related characteristics including the number of islet infusions, islet equivalents (IEQs) transplanted, glucagon-like peptide-1 (GLP-1) receptor agonist or dipeptidyl peptidase 4 inhibitor (DPP4i) use, and immunosuppressant drugs were recorded. HLA serological antigen typing was performed and matching calculations were based on the level of HLA-A, HLA-B, and HLA-DR antigen specificities.
Table 1.
Chronic Immunosuppression Protocols in Clinical Islet Transplantation.
| Period | Research protocol | Type of transplant | N | Induction | Immunosuppression maintenance |
|---|---|---|---|---|---|
| 2000 | NCT00315614 | ITA + CD34+BMC | 6 | Daclizumab, etanercept | SIR, TAC |
| 2001–2003 | NCT00306098 | ITA | 16 | Daclizumab, infliximab | SIR, TAC |
| 2002–2004 | NCT01309022 NCT00306098 | ITA | 5 | Daclizumab | SIR, TAC |
| 2005–2006 | NCT00315627 | ITA | 3 | Campath, etanercept | SIR, TAC |
| 2005–2007 | NCT00306098 | ITA | 4 | Daclizumab, etanercept, exenatide | SIR, TAC |
| 2005–2007 | NCT00315614 | ITA + CD34+BMC | 3 | Campath, etanercept | SIR, TAC |
| 2008–2011 |
NCT00434811 NCT00464555 |
ITA (CIT) | 8 | ATG, etanercept | SIR, TAC |
| 2015–2017 | NCT02213003 | ITA (omentum) | 3 | ATG, etanercept | TAC, MMF |
ATG: rabbit anti-thymocyte globulin; BMC: bone marrow cell; CD34+BMC: CD34-enriched bone marrow cell; CIT: clinical islet transplantation consortium; ITA: islet transplant alone; MMF: mycophenolate mofetil; SIR: sirolimus; TAC: tacrolimus.
We evaluated the impact of HLA donor and recipient matching on islet graft survival. Islet graft failure was defined as either a fasting C-peptide ≤0.10 ng/ml (in the absence of hypoglycemia) on two consecutive measurements obtained on different days or a stimulated C-peptide ≤0.3 ng/ml.
Statistical Analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation. The Shapiro–Wilk test was used for normality assessment; asymmetrically distributed continuous variables were expressed as median and interquartile range (25th–75th); and categorical variables were expressed as absolute and relative frequencies. For between-group comparisons, Student’s t-test was used for symmetrically distributed variables; Mann–Whitney U test for asymmetrically distributed variables; and Pearson chi-square or Fisher’s exact test for categorical variables. Survival rates were estimated by Kaplan–Meier curves, while log-rank, Breslow, and Tarone-Ware tests were used to compare time with outcome (graft failure) between patients according to the number of HLA matching. Survival time was defined as the time from the first transplant to the date of graft failure. Patients lost to follow-up were censored either at outcome event or at the last recorded visit. Pearson and Spearman correlation tests were performed to identify correlation between number of matches or number of mismatches and time of graft survival. Multiple linear regression was used to identify a model that predicts the time of graft survival. Multivariable analysis was performed using the Cox proportional hazards model, considering graft failure as the dependent variable while the covariates were defined by either the clinical relevance or statistical significance. Values of P < 0.05 (two-tailed) were significant, and all data were analyzed on SPSS version 22.0 (SPSS Inc, Chicago, IL, USA).
Results
Patients were aged 42.8 ± 8.4 years, and 64.3% (n = 36) of the recipients were female. Mean body mass index of recipients was 23.6 ± 2.7 kg/m2 and type 1 diabetes duration was 28.5 ± 11.6 years. First infusion of islet transplantation was done in 85.4% (n = 41) of the patients during the 2000 decade, while 14.6% (n = 7) occurred in the 2010 decade.
Induction immunosuppression consisted of anti-interleukin 2 (IL2) blockade with either daclizumab or basiliximab and anti-tumor necrosis factor (TNF) blockade with either infliximab or etanercept (Edmonton-like protocol) in 65% (n = 31) of patients of our cohort. Third-five percent (n = 17) of patients received T-cell depletion as immunosuppressive induction. Maintenance immunosuppression consisted of a dual combination strategy with sirolimus, tacrolimus, or mycophenolate mofetil (MMF). Autoantibodies glutamic acid decarboxylase (GAD) and islet antigen 2 (IA2) were present in 50% and 37.5% of the recipients, respectively. Only two patients developed donor-specific antibodies (DSA) anti-HLA while on immunosuppression, making unfeasible further analysis of this parameter.
Graft failure occurred in 18 patients (37.5%). Seven out of the 18 patients (38.9%) developed graft failure within the first year after transplant. HLA data analyzed were restricted to A, B, and DR loci. HLA-A matching was present in 28 out of the 48 patients (58%), HLA-B matching was present in 16 patients (33%), and HLA-DR matching was present in 27 patients (56%). HLA-DR3 or HLA-DR4 matching was present in 16 patients (33.3%), and it represents 60% of the HLA-DR matching patients. Thirty-four out of the 48 recipients were HLA-DR3 or HLA-DR4 (70.8%), while 3 (6%) of the donors were HLA-DR3, and 22 (45%) were HLA-DR4.
Islet graft survival in recipients with HLA-DR3 or HLA-DR4 alleles was not different from those recipients who were not HLA-DR3 or HLA-DR4 positive [χ2 = 0.270 (df = 1); P = 0.870].
We analyzed the impact of recipient and donor matching for HLA-A, HLA-B, and HLA-DR loci combined or independently, while data are reported as either 0 (for no matching) or ≥1 matching.
HLA-A, HLA-B, and HLA-DR Matching
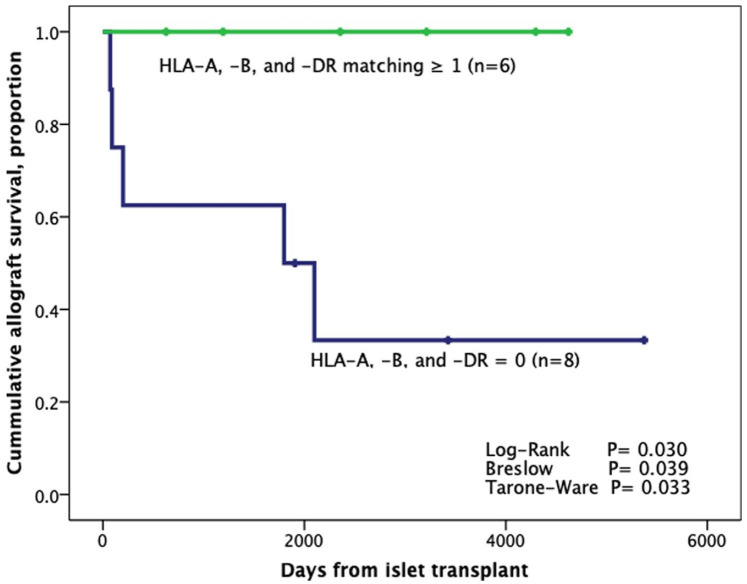
Time of graft survival between patients who presented matching for all three HLA loci (HLA-A, HLA-B, and HLA-DR; n = 6) in comparison with patients with zero matching (n = 8) showed that patients with HLA-A, HLA-B, and HLA-DR combined matching have longer graft survival. (P = 0.030; Fig. 1).
Figure 1.
Kaplan–Meier analysis from the first transplant according to the combined (all three) HLA-A, HLA-B, and HLA-DR matching (n = 14). HLA: human leukocyte antigen.
HLA Matching for Specific Locus
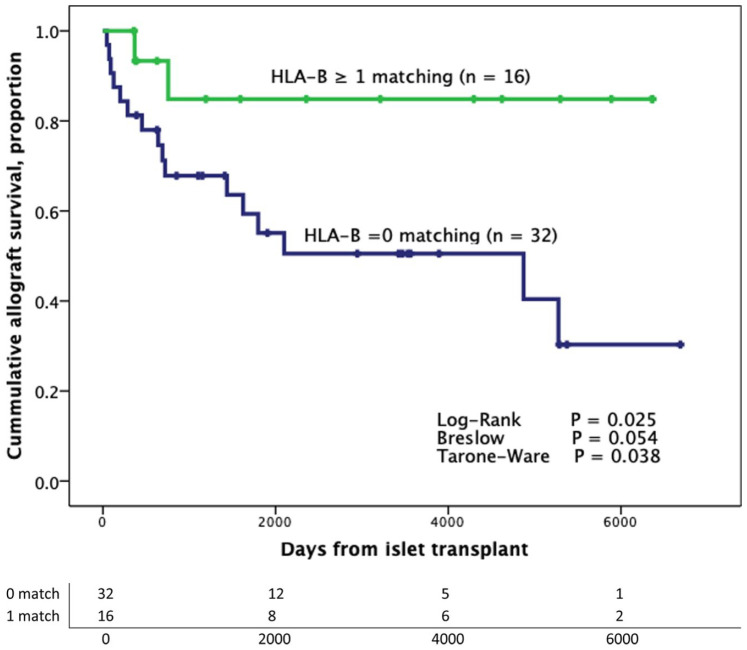
Analyses of graft survival according to matching for specific locus showed that patients with no HLA-B matching (zero matching) developed graft failure at 9.6 ± 1.4 years compared with 15.2 ± 1.6 years in those who had ≥1 HLA-B matching (log-rank P = 0.025; Breslow P = 0.054; Tarone-Ware P = 0.038) (Fig. 2). Clinical and transplant characteristics of patients according to the presence of HLA-B matching are described in Table 2.
Figure 2.
Kaplan–Meier analysis from the first transplant according to the presence of HLA-B. Patients were censored at the time of graft failure (n = 48). HLA: human leukocyte antigen.
Table 2.
Clinical and Transplant Characteristics According to the Presence of HLA-B Matching.
| HLA-B | HLA-B | P value* | |
|---|---|---|---|
| 0 matching (n = 32) | ≥1 matching (n = 16) | ||
| Female sex, n (%) | 23 (71.9) | 9 (56.2) | 0.279 |
| Age, years | 44.8 ± 8.2 | 38.8 ± 7.6 | 0.019 |
| BMI, kg/m2 | 23.3 ± 2.4 | 24.1 ± 3.1 | 0.339 |
| Duration of diabetes | 28.7 ± 10.8 | 26.0 ± 13.1 | 0.312 |
| Number of donors | 2 (1–3) | 2 (1–3) | 0.297 |
| Infused islet per kg (1,000), IEQ | 12.83 (8.1–16.5) | 12.72 (9.6–15.4) | 0.999 |
| ∆ Time (1st and 2nd infusion), months | 5 (1–8) | 1 (1) | 0.039 |
| ∆ Time (1st and last infusion), months | 20 (15–51) | 33 (22–41) | 0.686 |
| Edmonton-like protocol immunosuppression induction, n (%) | 17 (53.1) | 14 (87.5) | 0.019 |
| T-cell depletion immunosuppression induction, n (%) | 15 (46.9) | 2 (12.5) | 0.019 |
| GLP1 RA and/or DPP4i use, n (%) | 15 (46.9) | 9 (56.2) | 0.540 |
| GAD65 autoantibodies, n (%) | 11 (45.8) | 9 (56.2) | 0.519 |
| IA2 autoantibodies, n (%) | 9 (41.7) | 6 (31.2) | 0.505 |
Data are shown as mean and standard deviation, median and interquartile interval, or percentages. DPP4i: dipeptidyl peptidase 4 inhibitor; GAD65: glutamic acid decarboxylase 65-kilodalton isoform; GLP-1 RA: glucagon-like peptide-1 receptor agonist; HLA: human leukocyte antigen; IA2: islet antigen 2; IEQ: islet equivalent; BMI: body mass index.
P value between groups and are according to χ2 test, T-test, or Mann–Whitney U test as appropriate. Italicized values show a significant P value <0.05.
Univariate analyses were performed to identify possible confounders that could influence allograft survival independently of the HLA-B matching (Table 3). Recipient age, GLP-1 RA and/or DPP4i use, BMI, number of donors, IEQs infused, delta time between the first and second infusion, immunosuppression induction protocol, and GAD65/IA2 positivity were variables selected for this analysis. There was no statistical significance for any of the variables investigated. The Cox proportional-hazards model was performed to identify the best combined predictive effect, and variables were selected based on clinical relevance. The following were included in this model: immunosuppression induction protocol, GLP-1 RA use, number of donors, GAD65/IA2 positivity, and IEQs per kg infused. This analysis showed that ≥1 matching for HLA-B decreased the risk of graft failure compared with the zero matching group—hazard ratio (HR): 0.107; 95% confidence interval (CI): 0.022–0.508; P = 0.005.
Table 3.
Univariate Analysis: OR (CI) and Significance Tests Considering Graft Failure as the Dependent Variable.
| Univariate OR (95% CI) | P value | |
|---|---|---|
| Recipients age | 1.026 (0.956–1.101) | 0.478 |
| GLP-1 RA use | 1.000 (0.311–3.218) | 0.999 |
| Number of donors | 0.912 (0.481–1.728) | 0.777 |
| T-cell depletion a | 2.676 (0.711–10.0.72) | 0.145 |
| BMI | 0.889 (0.715–1.131) | 0.362 |
| IEQ/kg infused | 1.000 (1.0–1.0) | 0.767 |
| ∆ Time (1st and 2nd infusion) | 0.905 (0.757–1.082) | 0.275 |
| GAD 65/AI2 positivity | 1.417 (0.374–5.365) | 0.608 |
BMI, body mass index; CI, confidence interval; GAD65: glutamic acid decarboxylase 65-kilodalton isoform; GLP-1 RA: glucagon-like peptide-1 receptor agonist; DPP4i: dipeptidyl peptidase 4 inhibitor; IEQ/kg, islet equivalent per kilogram infused; OR, odds ratio.
T-cell depletion as immunosuppression induction protocol.
Six out of the 32 patients (12.5%) with zero matching developed graft failure within 1 year after transplant. The impact of HLA-B matching on early (<1 year) versus late (≥1 year) graft failure was evaluated. There was no association between early graft failure and the absence of HLA-B matching (P = 0.398).
It was also observed that the number of HLA-B matching has a moderate positive correlation with time of graft survival (Spearman’s rho = 0.590; P = 0.034).
Then, further analyses were performed, adjusting for confounders. A multiple linear regression model showed that the number of HLA-B matching predicts the time of allograft survival, adjusting for number of IEQ/kg infused and immunosuppression protocol [F(3,9) = 8.041, P = 0.006; R2 = 0.728).
HLA-B mismatch, on the other hand, presented a number ranging from 0 to 7 and, as expected, is positively correlated with the number of donors (Spearman’s rho = 0.925; P < 0.0001). The number of HLA-B mismatch was negatively correlated with time of graft survival (Spearman’s rho = −0.775; P < 0.0001) in patients who presented allograft failure (n = 18).
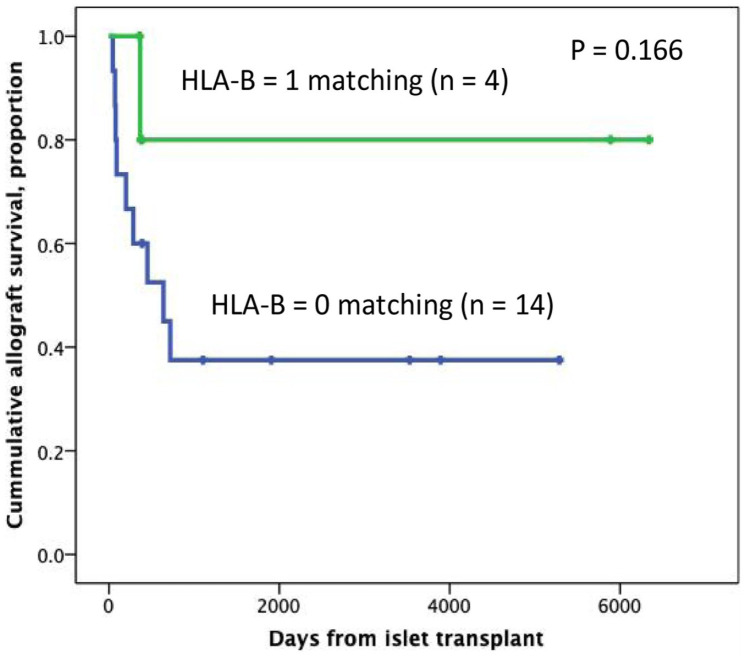
A subanalysis of the patients who received islets from a single donor was performed (n = 18), resulting in 22.2% (n = 4) of the patients matching for HLA-B, while 77.8% had zero matching (n = 14). Fig. 3 shows the analysis of the time of graft survival according to HLA-B matching in this subgroup of patients.
Figure 3.
Kaplan–Meier analysis from the first transplant according to the presence of HLA-B. Subgroup analysis considering patients who received from a single donor. Patients were censored at the time of graft failure (n = 18). HLA: human leukocyte antigen.
No significant findings were observed in the analyses of time of graft survival for locus-specific HLA-A matching (log-rank P = 0.965) or HLA-DR matching (log-rank P = 0.955). HLA-A, HLA-B, and HLA-DR loci combinations were also evaluated (HLA-A and HLA-B; HLA-A and HLA-DR; HLA-B and HLA-DR). There were no significant differences in graft survival for any locus combination between recipients and donors (data not shown).
Discussion
In this study, we aimed to determine whether HLA matching has any impact on graft survival in patients who received islet transplant. HLA-B matching showed an association with islet graft survival and conferred protection for allograft failure, after adjustment for immunosuppression induction protocol, GLP-1 RA use, number of donors, GAD65/IA2 positivity, and number of IEQ/kg infused. In this same context, patients who matched for all three HLA loci (A, B, and DR) also presented a longer graft survival.
Human HLA genes are located on chromosome 6 and encode for three major class I alleles (HLA-A, HLA-B, and HLA-C) and three major class II alleles (HLA-DR, HLA-DQ, and HLA-DP). The contribution of the HLA genotype on genetic risk for T1D is well determined 16 .
The highest risk HLA-DR3/DR4 DQ8 genotype has been shown to be highly associated with beta-cell autoimmunity. Indeed, the study-specific estimates for the association between the HLA class II DR3/DR4 genotype and type 1 diabetes showed a general odds ratio of approximately 16, demonstrating the importance of this genetic region for T1D 17 . However, these genes cannot completely explain the association between type 1 diabetes and the MHC region. In experimental models, MHC class I–mediated events, principally involving HLA-B*39, contributed to the etiology of type 1 diabetes 18 .
The effect of HLA compatibility on solid organ transplantation outcomes has been the focus of several studies spanning more than a decade, but there is no conclusive evidence of this effect in the ITx field 19 . In 2009, Vantyghem et al followed up a small cohort of 14 patients who received ITx (two to three infusions) for up to 39 months after transplant. In this study, there were no significant differences in the number of HLA mismatches in patients with optimal versus suboptimal primary graft function 20 . Studies that analyzed the role of HLA matching on pancreas transplantation outcomes reported controversial results. Rudolph et al studied 1,219 pancreas transplants performed at the University of Minnesota and observed a correlation between the number of HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ mismatches and acute rejection. However, HLA matching did not affect patient or graft survival rates 14 . Similarly, Lo et al analyzed the significance of HLA matching on the outcome of simultaneous pancreas-kidney transplantation in a cohort of 297 patients and found that the degree of HLA mismatching was associated with an increased risk for acute rejection at 1 year, but did not affect short-term patient or graft survival rates 21 . In this same context, Berney et al found a 2.6 times higher relative risk of acute rejection among patients with multiple HLA mismatches, although without evidence that HLA matching was associated with improved kidney or pancreas survival 12 . Other authors reported that pancreas after kidney and pancreas transplant alone recipients had better outcomes if the donor and recipient shared at least one matching on the HLA-A and/or HLA-B loci 11 . On the other hand, Mittal et al reported that neither degree of HLA mismatch (0–4 vs 5–6) nor DR mismatch (0, 1, or 2) was associated with pancreas graft failure in a cohort of 433 pancreas transplant recipients 22 .
In our study, two patients developed donor-specific antibodies and we did not perform analyses considering this parameter. A previous study observed that pretransplant DSAs and de novo development of post-transplant DSAs were not associated with reduced graft survival and reduced graft function 23 .
Our results seem to be in accordance with the proposed interactive effect of the HLA class I and the HLA class II regions on the regulation of the immune response 24 . In our study, matching for HLA-B is associated with prolonged graft survival. Therefore, the number of B matching predicts the time of graft survival, after adjustment for IEQ/kg infused, GLP-1 RA use, number of donors, GAD65/IA2 positivity, and immunosuppressive induction protocol. These covariates are listed as important factors for favorable outcomes in islet transplantation 25 . In the subset analysis of the time of graft survival in patients who received islets from a single donor, only 4 patients had ≥1 HLA-B matching and 14 had no HLA-B matching (P = 0.166). This analysis is not statistically significant probably due to the small sample size.
In our study, patients who had ≥1 HLA-B matching also received the second infusion in a shorter time interval in comparison with patients with zero HLA-B matching. However, there was no association between delta time of infusions and graft survival. This parameter was previously analyzed by Forbes et al who showed that shorter time interval between islet transplants was significantly associated with greater insulin dose reduction, but not associated with graft survival or any other metabolic measurement 26 .
Polymorphisms of class I HLA-B gene are associated with T1D and are considered one of the major genetic determinants of T1D likewise polymorphisms of class II HLA genes encoding DQ, DR, and, less frequently, DP 17 . The most significantly protective T1D-associated class I allele is B*57:01 27 . The observed HLA-B association may also be explained by the strong linkage disequilibrium in the HLA region with nearby causal gene(s) in some populations 28 . Historically, a greater impact of HLA-A, HLA-B, and HLA-DR antigens has been observed in renal graft rejection 29 , with a larger effect for B and DR matching 30 . More recently, a meta-analysis evaluated HLA-A, HLA-B, and HLA-DR mismatches and graft failure in renal transplantation; after adjustment, each incremental increase of HLA-DR mismatch was significantly associated with 12% higher risk of overall graft failure (HR: 1.12; 95% CI: 1.05–1.21; P = 0.002) 31 .
Another study in renal transplantation analyzed 106,019 recipients from the Scientific Registry of Transplant Recipients database and observed that HLA-B and HLA-DR mismatches were all significantly associated with worse graft survival outcomes 32 .
Taking into account the role of class I and class II region in the immunological system and the previous studies reporting an association between HLA matching with graft outcomes, we believe that the evaluation for HLA matching should be considered an important factor for donor eligibility in islet transplantation.
Hopefully, in the near future, the careful selection of stem cell islet donors, aiming at HLA matching with the recipient, will be a reality.
The results of this study, however, carry limitations associated with the retrospective design, the small sample size, and the restriction of HLA matching to A, B, and DR loci, but not DQ locus.
Our data suggest that patients who presented matching for HLA-B showed improved islet allograft survival. Also, matching all three HLA loci (A, B, and DR) also conferred longer islet allograft survival when compared with zero matching, suggesting that HLA matching assessment should be considered for islet transplantation. To validate our findings, the analysis of HLA matching in islet transplantation is worth assessing in prospective studies on a larger number of patients.
Acknowledgments
The authors are grateful to the members of the Current Good Manufacturing Practices (cGMP) Human Cell Processing Facility, the preclinical Human Immunology and Immunogenetics Program, the Clinical Cell Transplant Program (CCTP) at the Diabetes Research Institute (DRI), and the University of Miami Clinical and Translational Science Institute (CTSI) for their support of this work.
Footnotes
Authors’ Contributions: Joana R N Lemos, David A Baidal, Raffaella Poggioli, Virginia Fuenmayor, and Rodolfo Alejandro participated in the writing of the manuscript. Joana R N Lemos participated in data analysis. Joana R N Lemos, Virginia Fuenmayor, Carmen Chavez, and Ana Alvarez participated in data collection. David A Baidal, Camillo Ricordi, and Rodolfo Alejandro participated in the research design.
Availability of Data and Materials: The data that support the findings of this study are available on request from the corresponding author Rodolfo Alejandro.
Ethical Approval: Protocol procedures were approved by the University of Miami Health Research Ethics Board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: All patients provided informed consent and were enrolled in different research protocols.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (NIH) grants R01 DK55347, R01 DK056953, R01 DK025802, DK070460, R01DK55347, U42 RR016603, M01RR16587, and UL1TR000460; the Miami Clinical and Translational Science Institute (CTSI) from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities; the Juvenile Diabetes Research Foundation International 4-200-946, 4-2004-361, 17-2012-361, 3-SRA-2017-347-M-B; the State of Florida; and the Diabetes Research Institute Foundation.
ORCID iD: Rodolfo Alejandro  https://orcid.org/0000-0003-4387-5360
https://orcid.org/0000-0003-4387-5360
References
- 1. Gorn L, Faradji RN, Messinger S, Monroy K, Baidal D, Froud T, Mastrototaro J, Ricordi C, Alejandro R. Impact of islet transplantation on glycemic control as evidenced by a continuous glucose monitoring system. J Diabetes Sci Technol. 2008;2:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shapiro AMJ, Ricordi C. Islet cell transplantation procedure and surgical technique. In: Kirk AD, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, Webber SA, editors. Textbook of organ transplantation. Hoboken, NJ: John Wiley; 2014, p. 1314–33. [Google Scholar]
- 3. Poggioli R, Faradji RN, Ponte G, Betancourt A, Messinger S, Baidal DA, Froud T, Ricordi C, Alejandro R. Quality of life after islet transplantation. Am J Transplant. 2006;6(2):371–8. [DOI] [PubMed] [Google Scholar]
- 4. Brennan DC, Kopetskie HA, Sayre PH, Alejandro R, Cagliero E, Shapiro AM, Goldstein JS, DesMarais MR, Booher S, Bianchine PJ. Long-term follow-up of the Edmonton protocol of islet transplantation in the united states. Am J Transplant. 2016;16(2):509–17. [DOI] [PubMed] [Google Scholar]
- 5. Foster ED, Bridges ND, Feurer ID, Eggerman TL, Hunsicker LG, Alejandro R; Clinical Islet Transplantation Consortium. Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41(5):1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertuzzi F, De Carlis L, Marazzi M, Rampoldi AG, Bonomo M, Antonioli B, Tosca MC, Galuzzi M, Lauterio A, Fava D, Dorighet P, et al. Long-term effect of islet transplantation on glycemic variability. Cell Transplant. 2018;27(5):840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bray RA, Gebel HM. Strategies for human leukocyte antigen antibody detection. Curr Opin Organ Transplant. 2009;14(4):392–7. [DOI] [PubMed] [Google Scholar]
- 8. Roberts JP, Wolfe RA, Bragg-Gresham JL, Rush SH, Wynn JJ, Distant DA, Ashby VB, Held PJ, Port FK. Effect of changing the priority for HLA matching on the rates and outcomes of kidney transplantation in minority groups. N Engl J Med. 2004;350(6):545–51. [DOI] [PubMed] [Google Scholar]
- 9. Su X, Zenios SA, Chakkera H, Milford EL, Chertow GM. Diminishing significance of HLA matching in kidney transplantation. Am J Transplant. 2004;4(9):1501–8. [DOI] [PubMed] [Google Scholar]
- 10. Süsal C, Opelz G. Impact of HLA matching and HLA antibodies in organ transplantation: a collaborative transplant study view. Methods Mol Biol. 2012;882:267–77. [DOI] [PubMed] [Google Scholar]
- 11. Gruessner AC, Sutherland DE, Gruessner RW. Matching in pancreas transplantation—a registry analysis. Transplant Proc. 2001;33(1–2):1665–6. [DOI] [PubMed] [Google Scholar]
- 12. Berney T, Malaise J, Morel P, Toso C, Demuylder-Mischler S, Majno P, Bühler LH, Mentha G; Euro-SPK Study Group. Impact of HLA matching on the outcome of simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 2005;20(Suppl 2):ii48–53, ii62. [DOI] [PubMed] [Google Scholar]
- 13. Choo SY. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med J. 2007;48(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudolph EN, Dunn TB, Mauer D, Noreen H, Sutherland DER, Kandaswamy R, Finger EB. HLA-A, -B, -C, -DR, and -DQ matching in pancreas transplantation: effect on graft rejection and survival. Am J Transplant. 2016;16(8):2401–12. [DOI] [PubMed] [Google Scholar]
- 15. Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The importance of HLA assessment in “Off-the-shelf” Allogeneic mesenchymal stem cells based-therapies. Int J Mol Sci. 2019;20(22):5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nerup J, Platz P, Andersen OO, Christy M, Lyngsoe J, Poulsen JE, Ryder LP, Nielsen LS, Thomsen M, Svejgaard A. HL-A antigens and diabetes mellitus. Lancet. 1974;2(7885):864–6. [DOI] [PubMed] [Google Scholar]
- 17. Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11(6):533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450(7171):887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broeders N, Racapé J, Hamade A, Massart A, Hoang AD, Mikhalski D, Le Moine A, Vereerstraeten P. A new HLA allocation procedure of kidneys from deceased donors in the current era of immunosuppression. Transplant Proc. 2015;47(2):267–74. [DOI] [PubMed] [Google Scholar]
- 20. Vantyghem MC, Kerr-Conte J, Arnalsteen L, Sergent G, Defrance F, Gmyr V, Declerck N, Raverdy V, Vandewalle B, Pigny P, Noel C, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care. 2009;32(8):1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo A, Stratta RJ, Alloway RR, Hodge EE; PIVOT Study Group. A multicenter analysis of the significance of hla matching on outcomes after kidney-pancreas transplantation. Transplant Proc. 2005;37(2):1289–90. [DOI] [PubMed] [Google Scholar]
- 22. Mittal S, Page SL, Friend PJ, Sharples EJ, Fuggle SV. De novo donor-specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant. 2014;14(7):1664–71. [DOI] [PubMed] [Google Scholar]
- 23. Chaigne B, Geneugelijk K, Bédat B, Ahmed MA, Hönger G, De Seigneux S, Demuylder-Mischler S, Berney T, Spierings E, Ferrari-Lacraz S, Villard J, et al. Immunogenicity of anti-HLA antibodies in pancreas and islet transplantation. Cell Transplant. 2016;25(11):2041–50. [DOI] [PubMed] [Google Scholar]
- 24. Bućin D. Specific immune tolerance related to disparity in MHC class I region. Med Hypotheses. 1995;44(2):132–6. [DOI] [PubMed] [Google Scholar]
- 25. Hering BJ, Ballou CM, Bellin MD, Payne EH, Kandeel F, Witkowski P, Alejandro R, Rickels MR, Barton FB. Factors associated with favourable 5 year outcomes in islet transplant alone recipients with type 1 diabetes complicated by severe hypoglycaemia in the Collaborative Islet Transplant Registry. Diabetologia. 2023;66:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forbes S, Flatt AJ, Bennett D, Crookston R, Pimkova M, Birtles L, Pernet A, Wood RC, Burling K, Barker P, Counter C, et al. The impact of islet mass, number of transplants, and time between transplants on graft function in a national islet transplant program. Am J Transplant. 2022;22(1):154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baschal EE, Baker PR, Eyring KR, Siebert JC, Jasinski JM, Eisenbarth GS. The HLA-B 3906 allele imparts a high risk of diabetes only on specific HLA-DR/DQ haplotypes. Diabetologia. 2011;54(7):1702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park L. Population-specific long-range linkage disequilibrium in the human genome and its influence on identifying common disease variants. Sci Rep. 2019;9(1):11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Opelz G. Correlation of HLA matching with kidney graft survival in patients with or without cyclosporine treatment. Transplantation. 1985;40(3):240–3. [DOI] [PubMed] [Google Scholar]
- 30. Dewar PJ, Wilkinson R, Elliott RW, Ward MK, Kerr DN, Kenward DH, Proud G, Taylor RM. Superiority of B locus matching over other HLA matching in renal graft survival. Br Med J. 1982;284(6318):779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi X, Lv J, Han W, Zhong X, Xie X, Su B, Ding J. What is the impact of human leukocyte antigen mismatching on graft survival and mortality in renal transplantation? a meta-analysis of 23 cohort studies involving 486,608 recipients. BMC Nephrol. 2018;19(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Massie AB, Leanza J, Fahmy LM, Chow EK, Desai NM, Luo X, King EA, Bowring MG, Segev DL. A risk index for living donor kidney transplantation. Am J Transplant. 2016;16(7):2077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]