Abstract
Achondroplasia causes narrowing of the foramen magnum and the spinal canal leading to increased mortality due to cervicomedullary compression in infants and significant morbidity due to spinal stenosis later in adulthood. Vosoritide is a C-natriuretic peptide analogue that has been shown to improve endochondral ossification in children with achondroplasia. The objective of this trial is to evaluate the safety of vosoritide and whether vosoritide can improve the growth of the foramen magnum and spinal canal in children that may require decompression surgery. An Achondroplasia Foramen Magnum Score will be used to identify infants at risk of requiring decompression surgery. This is a 2-year open label randomized controlled trial of vosoritide in infants with achondroplasia ages 0 to ≤12 months. Approximately 20 infants will be randomized 1:1 to either open label once daily subcutaneous vosoritide combined with standard of care or standard of care alone. The primary and secondary aims of the study are to evaluate the safety and efficacy of vosoritide in children with cervicomedullary compression at risk of requiring decompression surgery. The trial will be carried out in specialized skeletal dysplasia treatment centers with well established multidisciplinary care pathways and standardized approaches to the neurosurgical management of cervicomedually compression. After 2 years, infants randomized to standard of care alone will be eligible to switch to vosoritide plus standard of care for an additional 3 years. This pioneering trial hopes to address the important question as to whether treatment with vosoritide at an early age in infants at risk of requiring cervicomedullary decompression surgery is safe, and can improve growth at the foramen magnum and spinal canal alleviating stenosis. This in turn may reduce compression of surrounding structures including the neuraxis and spinal cord, which could alleviate future morbidity and mortality.
Trial registrations: ClinicalTrials.gov, NCT04554940; EudraCT number, 2020-001055-40
Keywords: Clinical trials, skeletal dysplasia, precision therapy, genetics, achondroplasia
Background
Achondroplasia, the most common form of disproportionate short stature, is a rare condition, with a prevalence of between 1/15,000 (www.orpha.net) and 1/25,000. 1 The condition is caused by a gain-of-function pathogenic variant in the fibroblast growth factor receptor 3 gene (FGFR3) that constitutively activates the mitogen-activated protein kinase (MAPK) via the p38 and extracellular signal-regulated kinase (ERK) pathways in chondrocytes, which inhibits endochondral ossification. 2 Achondroplasia is de novo in the majority of cases (80%), and the most common variant is the p.(Gly380Arg) substitution in the region encoding the transmembrane domain of FGFR3. 3 FGFR3 is one of many physiological regulators of linear bone growth and normally functions as an inhibitor, acting negatively on both proliferation and terminal differentiation of chondrocytes, which are integral to endochondral bone formation, the principal means for long-bone growth. 4
In infancy and early childhood, the most important medical challenge driving morbidity and mortality is narrowing of the foramen magnum, which has been implicated in an excess of sudden deaths in children with achondroplasia under age 4 years.5,6 The foramen magnum is narrow in all individuals with achondroplasia but individuals with signs and symptoms of cervicomedullary compression at the level of the foramen magnum tend to have critically stenosed foramen magnum dimensions. 7 The foramen magnum lies completely within the occipital bone and its margins form and grow by endochondral ossification and are uniformly affected in achondroplasia. Pathological foramen magnum stenosis, as seen in achondroplasia, is postulated to be secondary to hypertrophy of the occipital rim, overgrowth of the opisthion and abnormal position and premature closure of skull base synchondroses. 8 Stenosis of the foramen magnum has the potential to compromise the neural and vascular structures passing through this region and poses significant medical risks to all children born with this condition. Compression of vital brainstem structures including central respiratory centers, cranial nerve nuclei and cranial nerves can occur resulting in disordered breathing. Centrally mediated apnea, a recognized complication of foramen magnum stenosis, is a likely contributory factor to the increased risk of sudden unexpected death in infancy, which is reported to be as high as 7.5% in infants with achondroplasia.6,9
The base of the skull surrounding the foramen magnum has several synchondroses and these close at an earlier age in children with achondroplasia. 10 Unlike long bones, the foramen magnum attains 70%–80% of its final transverse and sagittal diameter by 1 year of age and, although it continues to grow into adulthood, the rate is much slower. In average-stature children, the entire extent of growth of the foramen magnum is around 1.5 cm in the transverse diameter and 2.4 cm in the sagittal diameter. 7 In infants with achondroplasia, the growth of the foramen magnum is significantly slower. The foramen magnum is small at birth and grows at a significantly slower rate during the first 2 years, particularly in the transverse diameter, as compared with average-statured children.7,11–13 Increasing the growth rate of the foramen magnum in infancy could therefore be a crucial advance in the clinical management of children with achondroplasia, but the challenges of identifying those infants at greatest risk and initiating treatment as soon after birth as possible and at least before 2 years of age remain formidable.
Later in life, medical complications associated with the thoraco-lumbar spine tend to be more prominent than those associated with the cervical spine. 14 Short pedicles, ligamentous thickening and kyphotic deformity compromise the caliber of the spinal canal resulting in reduced space for the spinal cord and the cauda equina. 15 Exaggerated lumbar lordosis, anterior vertebral body wedging, and thickening of the pedicles and laminae are additional factors that mean that the spinal canal is one-third to one-half of the size of the canal in an individual of average stature. These features collectively contribute to a narrowed spinal canal as well as adjacent spinal foramina resulting in a clinical picture of myelopathy and radiculopathy.
Spinal growth is the product of more than 130 growth plates. 16 The neurocentral synchondroses in the spine are located at the junction of the pedicle and the vertebral body and are important in the growth of the vertebral body and the posterior arch. In average stature children, the neurocentral synchondroses fuse at approximately 9 years of age, and by 5 years of age the spinal canal has already grown to approximately 95% of its final size. 16 In children with achondroplasia the final size of the spinal canal is achieved even earlier. Therefore, similarly to the foramen magnum, increasing the growth rate of the spine in infancy could be another crucial step forward in the clinical management of achondroplasia potentially benefitting these individuals throughout their adult lives as well.
Current standard of care
Achondroplasia typically presents in the last trimester or at birth, so recognition by neonatal and general pediatricians of early complications is important. Despite efforts to publish expert consensus driven clinical guidelines, there is a lack of alignment surrounding the need for and optimal timing and choice of screening modality for cervicomedullary compression in infants with achondroplasia.8,15,17,18 The American Academy of Pediatrics recommends either CT or MRI to evaluate changes at the foramen magnum in all infants with achondroplasia. 15 However, more recently best practice guidance based on expert consensus recommended that MRI scans should be reserved for those infants with either an abnormal detailed clinical neurological history and examination (performed every 2 months for the first year of life), or polysomnography abnormalities suggestive of foramen magnum stenosis. 18 Abnormal neurological manifestations of foramen magnum stenosis include hypotonia, motor delay, feeding, and sleep disorders, and clinical features of myelopathy such as hyper-reflexia and ankle clonus, which when present strongly predict the need for decompression surgery in the infant population.9,19 Neurological sequelae, however, are a late manifestation of high cervical spinal cord compression and considerable experience of what is “normal” in achondroplasia is required to determine what constitutes clinically significant findings. Studies examining the predictive value of polysomnography or cardiorespiratory sleep study parameters in the identification of foramen magnum stenosis in children with achondroplasia suggest low screening sensitivity.20,21 Neurosurgical opinion regarding the need for surgery in the face of foramen magnum stenosis in achondroplasia remains inconsistent and practice varies significantly between different centers, with rates of cervicomedullary decompression ranging from 4.6% to 43%.22,23 While the outcome of neurosurgical intervention is generally favorable, severe complications, such as cerebrospinal fluid leakage, vascular injury, and worsening neurological function, are well recognized. 24
Having overcome the risks of surgery associated with foramen magnum stenosis and cervicomedullary compression, as children with achondroplasia reach adolescence and adulthood, they become at risk of symptomatic spinal canal stenosis (particularly in the lumbar spine), which can lead to significant morbidity throughout adult life.4,14 By 20 years of age, approximately 20% have abnormal neurological signs. Back and leg pain occurs in up to 80% by the sixth decade of life. 14 Worsening stenosis of the canal and foramina results in sensory dysfunction, radicular pain, neurogenic claudication, bladder dysfunction, and, in severe cases, fecal incontinence. About one-third require specialist laminectomy surgery for symptomatic spinal canal stenosis. 4
Recently, an Achondroplasia Foramen Magnum Score (AFMS) has been proposed based on magnetic resonance imaging (MRI) of the cervicomedullary junction. 25 The advantage of such a scoring system is that it relies on the progressive effect of the decreased foramen magnum size on the neuraxis as opposed to measurement of the dimensions of the foramen magnum. The advantage of such a scale is that it is easily determined via objective MRI findings, is age independent and does not require normalization. Further details of the scale are shown in Table 1. This trial takes advantage of this easily qualifiable scoring system in order to evaluate the progression of the score overtime and compare the intervention and control groups.
Table 1.
Achondroplasia foramen magnum score (AFMS).
| AFM risk score | MRI determined classification |
|---|---|
| AFMS 0 | Normal cranio-cervical junction (CCJ) |
| AFMS 1 | Narrowed cranio-cervical junction with cerebrospinal fluid (CSF) surrounding the cord |
| AFMS 2 | Loss of CSF signal surrounding the cord at the CCJ without cord remodeling |
| AFMS 2a | Loss of CSF signal posterior to the cord |
| AFMS 2b | Loss of CSF signal posteriorly and anteriorly |
| AFMS 2c | Loss of CSF signal posteriorly, anteriorly and at the sides |
| AFMS 3 | Remodeling of the cord at the CCJ |
| AFMS 3a | CSF signal remains visible at the CCJ |
| AFMS 3b | No CSF signal is visible at the CCJ |
| AFMS 4 | High T2 signal in the cord at the CCJ |
| AFMS 4a | CSF signal remains visible at the CCJ |
| AFMS 4b | No CSF signal is visible at the CCJ |
C-natriuretic peptide and vosoritide
The binding of C-type natriuretic peptide (CNP) to its receptor, natriuretic peptide receptor 2 (NPR2), inhibits FGFR3 downstream signaling at the level of Raf-1 and is a potent stimulator of endochondral ossification. 26 CNP overexpression in a transgenic mouse model is associated with skeletal overgrowth, including of the axial and appendicular skeleton, craniofacial bones, and foramen magnum. 27 Similarly, overexpression of CNP in the liver leading to increased serum CNP concentrations as well as continuous CNP infusions also rescue the craniofacial abnormalities, skeletal, and foramen magnum abnormalities of an achondroplasia mouse model.28,29 Furthermore, pathogenic variants that result in overexpression of CNP in humans are also associated with enhanced skeletal growth, manifest by abnormally tall stature, supporting the hypothesis that systemic therapy with CNP should help ameliorate the skeletal phenotype of achondroplasia including at the foramen magnum.30–32
Vosoritide is a recombinant CNP analogue that has been engineered to resist degradation by neutral endopeptidase, allowing for a longer half-life and an impact on endochondral ossification. Like CNP, vosoritide activates NPR2 signaling with subsequent inhibition of FGFR3 downstream signaling, leading to the promotion of chondrocyte proliferation and differentiation, and subsequent increased endochondral bone formation.
Vosoritide administration restored impaired bone growth in a mouse model of achondroplasia and improved bone growth in wild-type monkeys. 33 These promising animal data led to an open-label, phase 2 study of vosoritide in children aged 5 to 14 years with achondroplasia. 34 This study showed that vosoritide was generally well tolerated at the doses tested and led to increases in annualized growth velocity (AGV) that were maintained for up to 42 months. This study was followed by a pivotal, phase 3, randomized, placebo-controlled, double-blinded study of vosoritide in 121 children aged 5 to 18 years with achondroplasia, where 60 participants were administered vosoritide and 61 a matching placebo for 52 weeks. 35 This study confirmed the effectiveness of vosoritide through increasing AGV in the treated group as compared to the placebo group, with similar side effect profiles observed in treated and placebo groups.
Since most of the growth of the foramen magnum is achieved in the first 2 years of life, vosoritide might be of benefit in young children with achondroplasia, by increasing the growth of the foramen magnum, through its stimulatory effects on endochondral ossification. Vosoritide therapy may consequently reduce the risk of cervico-medullary compression, the morbidity and mortality associated with cervicomedullary compression, and the need for surgical decompression. Similarly, since the growth of the spinal canal is completed by 9 years of age, increased growth of the spinal canal during the first years of life, may also significantly reduce spinal morbidity later in life. The primary aim of this study is to evaluate the safety of daily subcutaneous vosoritide injections in infants and young children with achondroplasia who are at increased risk of requiring surgical intervention for cervicomedullary compression. The study will also explore the efficacy of vosoritide in preventing the need for decompression surgery and contribute to the understanding of the natural history of moderate foramen magnum stenosis (AFMS grades 2 and 3).
Trial design and patient eligibility criteria
This is a stratified, randomized, controlled, open-label clinical study to investigate the safety of vosoritide treatment in infants and young children with achondroplasia who are at risk of requiring cervicomedullary decompression surgery. Patients aged 0 to ≤12 months who have achondroplasia confirmed by genetic testing and who meet the study eligibility criteria will be able to enroll directly into the study. While the management of children with achondroplasia may vary among clinical centers worldwide, this study will utilize an achondroplasia foramen magnum stenosis risk score using MRI criteria to identify eligible children. 25 Patient selection for entry into the study will be at specialized skeletal dysplasia units that routinely perform MRI in infants with achondroplasia as per standard of care. Confirmation of patient eligibility for the study will be performed through an independent blinded central read of the MRI confirming that the patient has an AFMS of AFMS2 defined as, “narrowing of the foramen magnum with loss of cerebrospinal fluid space surrounding the cord” or AFMS3 defined as, “narrowing of the foramen magnum with flattening of the cervical cord without T2 signal change,” 25 thereby including patients who are not in immediate need for foramen magnum decompression but nevertheless remain at risk of requiring it. A full list of all inclusion and exclusion eligibility criteria are shown in Table 2.
Table 2.
Inclusion criteria.
| Inclusion criteria |
|---|
| 1. Parent(s) or guardian(s) willing and able to provide signed informed consent after the nature of the study has been explained and prior to performance of any research related procedure. |
| 2. Have ACH, documented by genetic testing. |
| 3. Are willing and able to perform all study procedures as physically possible. |
| 4. Age 0 to ≤12 months, at study entry (Day 1). |
| 5. Parent(s) or caregiver(s) are willing to administer daily injections to the patient and complete the required training. |
| 6. Have evidence of cervicomedullary compression that “may” require surgical intervention defined as: ○ Baseline MRI assessment from central blinded evaluation showing at least one of the following findings: • Narrowing of the foramen magnum with loss of cerebrospinal fluid space surrounding the cord. • Narrowing of the foramen magnum with flattening of the cervical cord without T2 signal change. ○ Supported by (but not required for eligibility) the following findings: • Baseline physical examination • Gross or fine motor developmental milestone delay compared to expected for ACH (e.g. lifting head when lying on stomach). • Abnormal reflex (e.g. brisk reflex/abnormal clonus for age). • Weakness (e.g. opisthotonus). • Baseline sleep study Sleep apnea with a primary central component (e.g. not secondary to obstructive sleep apnea). |
| Exclusion criteria |
| 1. Have hypochondroplasia or short-stature condition other than achondroplasia (e.g. trisomy 21, pseudoachondroplasia, etc.). |
| 2. Have cervicomedullary compression that either does not require surgical intervention (e.g. foramen magnum narrowing with preservation of the cerebrospinal fluid space) or does require immediate surgical intervention (e.g. narrowing of the foramen magnum with cervical cord signal change). |
| 3. Have any of the following: ○ Untreated congenital hypothyroidism or maternal history of hyperthyroidism. ○ Insulin-requiring neonatal diabetes mellitus. ○ Autoimmune inflammatory disease. ○ Inflammatory bowel disease. ○ Autonomic neuropathy. |
| 4. Have a history of any of the following: ○ Renal insufficiency. ○ Chronic anemia. ○ Baseline systolic blood pressure below age and gender specified normal range or recurrent symptomatic hypotension (defined as episodes of low blood pressure generally accompanied by symptoms [e.g. pallor, cyanosis, irritability, poor feeding]). ○ Cardiac or vascular disease, including the following: • Cardiac dysfunction (abnormal echocardiogram determined to be clinically significant by investigator and medical monitor) at screening. • Hypertrophic cardiomyopathy. • Pulmonary hypertension. • Clinically significant structural heart disease or valvular insufficiency (associated with symptoms or requiring intervention). • Clinically significant cerebrovascular disease. • Aortic insufficiency or other clinically significant valvular dysfunction. • Clinically significant atrial or ventricular arrhythmias. |
| 5. Have a clinically significant finding or arrhythmia that indicates abnormal cardiac function or conduction or QTc-Frederica ≥450 ms on screening ECG. |
| 6. Current treatment with antihypertensive medications, ACE inhibitors, angiotensin II receptor blockers, diuretics, beta-blockers, calcium-channel blockers, cardiac glycosides, systemic anticholinergic agents, any medication that may impair or enhance compensatory tachycardia, drugs known to alter renal function that is expected to continue for the duration of the study. |
| 7. Require any other investigational product prior to completion of the study period. |
| 8. Have received another investigational product or investigational medical device within 30 days prior to Screening. |
| 9. Have used any other investigational product or investigational medical device for the treatment of ACH or short stature at any time. |
| 10. Require current chronic therapy with antihypertensive medication or any medication that, in the Investigator’s judgment, may compromise the safety or ability of the patient to participate in this clinical study. |
| 11. Have been treated with growth hormone, insulin-like growth factor 1, or anabolic steroids in the 6 months prior to screening, or long-term treatment (>3 months) at any time. |
| 12. Have had regular long-term treatment (>1 month) with oral corticosteroids (low-dose ongoing inhaled steroid for asthma, or intranasal steroids, are acceptable) prior to screening. |
| 13. Have ever had prior cervicomedullary decompression surgery. |
| 14. Have had a fracture of the long bones or spine within 6 months prior to screening. |
| 15. Have aspartate aminotransferase (AST) or alanine aminotransferase (ALT) or total bilirubin >1.5× the upper limit of normal at Screening (except for patients with a known history of Gilbert’s syndrome or transient indirect hyperbilirubinemia). |
| 16. Have current malignancy, history of malignancy, or currently under work-up for suspected malignancy. |
| 17. Have known hypersensitivity to vosoritide or its excipients. |
| 18. Have a history of hip surgery or clinically significant hip abnormality in the 30 days prior to screening. |
| 19. Have a condition or circumstance that, in the view of the Investigator, places the patient at high risk for poor treatment compliance or for not completing the study. |
| 20. Have any concurrent disease or condition that, in the view of the Investigator, would interfere with study participation or safety evaluations, for any reason. While vitamin D deficiency (Vitamin D concentration <37.5 nmol/L or <15 ng/ml) is not an exclusion criterion, it should be immediately treated with oral supplementation with 2000 IU daily and the value repeated 4 weeks to confirm normalization of serum concentrations. Once achieved daily maintenance dose is recommended. If there is evidence of severe cervicomedullary compression, that “does” in the opinion of the Investigator require surgical intervention then the child is not eligible to enroll. For example, the presence of abnormal MRI T2 signal intensity at and immediately above and below the cervicomedullary junction should be considered high risk for requiring surgery and the child would not be a candidate for this trial. |
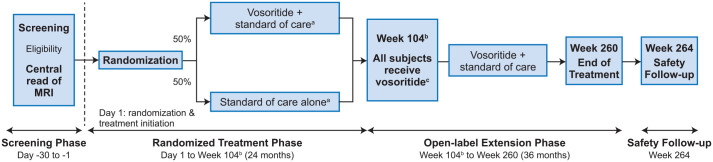
Approximately 20 patients will be enrolled into the trial, stratified based on age (0 to ≤ 6 months, > 6 months to ≤ 12 months), and randomized 1:1 by a centralized IXRS randomization to either open label, once daily, subcutaneous vosoritide combined with standard of care (vosoritide + standard of care) or standard of care alone. After 24 months of randomized treatment, all patients may be eligible to complete an additional 36 months on-study receiving open-label vosoritide treatment + standard of care. Patients will be enrolled at up to four clinical centers worldwide. The overall study design is presented in Figure 1.
Figure 1.
Study design.
This is a stratified, randomized, controlled, open-label clinical study to investigate the safety of vosoritide treatment in infants and young children with achondroplasia at risk of requiring cervicomedullary decompression surgery. Approximately 20 patients, aged 0 to ≤12 months who have documented achondroplasia confirmed by genetic testing and who meet the study eligibility criteria will be eligible to enroll. Enrolled patients will be stratified based on age (0 to ≤6 months, >6 months to ≤12 months) and will be randomized 1:1 to either open-label, once daily, subcutaneous vosoritide combined with standard of care (vosoritide + standard of care) or standard of care alone. After 104 weeks of randomized treatment, all patients will be eligible to complete an additional 156 weeks on-study receiving open-label vosoritide treatment + standard of care. All patients will complete a safety follow-up visit approximately 4 weeks after the end of treatment.
The primary objective is to assess the safety of daily subcutaneous vosoritide + standard of care versus standard of care alone when administered to infants and young children with achondroplasia and signs and symptoms of cervicomedullary compression. The secondary objective of the study is to evaluate the efficacy of vosoritide in children who are at risk of requiring cervicomedullary decompression surgery.
Patients randomized to receive vosoritide will be administered the dose determined to be appropriate for their current age as identified in the ongoing Phase 2 randomized, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vosoritide in infants and young children with ACH, aged 0 to <60 months (111-206; NCT03583697). The 111–206 study determined the appropriate dose to achieve adequate exposure is age dependent with patients under 24 months of age requiring 30 µg/kg/day and those older than 24 months of age requiring 15 µg/kg/day. Therefore reaching 24 months of age is the criterion to de-escalate the dose. The de-escalation will occur on the visit immediately preceding a patient reaching 24 months of age.
Safety outcome assessments
Safety in this study will be determined from evaluation of Adverse Events, Serious Adverse Events, clinical laboratory assessments (chemistry, hematology), vital signs measurements (heart rate, blood pressure, respiratory rate, and temperature), physical examinations, ECGs, echocardiograms, and concomitant medications.
Any abnormal laboratory test results determined to be clinically significant by the Investigator will be repeated (at the Investigator’s discretion) along with any necessary specialist consultation, until the cause of the abnormality is determined, the value returns to baseline or to within normal limits, or the Investigator determines that the abnormal value is no longer clinically significant.
For patients who have not previously had genetic testing confirming diagnosis of achondroplasia, molecular genetic diagnosis to identify the FGFR3 pathogenic variant (p.(Gly390Arg), p.(Gly346Glu), p.(Gly375Cys), or “other”) will be performed locally, within the screening period. If patients had previous genetic testing, patients must have a report from a certified laboratory with the study specific mutation documented.
Vital signs will include seated or supine systolic blood pressure and diastolic blood pressure measured in mmHg, heart rate in beats per minute, respiration rate in breaths per minute, and temperature in degrees Celsius.
Complete physical examinations will include major body systems, including assessment of general appearance, cardiovascular function, dermatologic, head, eyes, ears, nose, and throat, lymphatic, respiratory, gastrointestinal, musculoskeletal, and neurological and genitourinary.
A standard 12-lead ECG will be performed and evaluated locally and will include assessment of heart rate, rhythm, intervals, axis, conduction defects, and anatomic abnormalities. If clinically significant abnormalities are noted, the Investigator or designee is required to report it as an Adverse Event and assess whether it is appropriate for the patient to continue in the study.
Cardiac anatomy and function will be evaluated by a standard two-dimensional Doppler echocardiogram by a local cardiologist. Echocardiograms will provide information regarding cardiac anatomy and function prior to enrollment and at the end of the study.
Data monitoring committee
In addition to safety monitoring by BioMarin personnel, an independent Data Monitoring Committee will act as an advisory body and will monitor the safety of patients in the study. If a study patient requires cervicomedullary decompression surgery, the committee will be informed and provided all available information for review. The Data Monitoring Committee will make recommendations for stopping or continuing the study on an individual patient level or on a study level according to the pre-specified criteria. The Data Monitoring Committee may also make endorsements for dose adjustments if needed.
Efficacy outcome assessments
Signs and symptoms of foramen magnum stenosis leading to cervicomedullary compression will be assessed and monitored during this study using a combination of data from MRI, neurological examinations, polysomnography, and condition specific developmental milestone acquisition.
Magnetic resonance imaging assessments
MRI assessments will be performed to evaluate the effect of vosoritide on the the skull and the brain (including volumes of the face, sinuses, calvarium, brain, and ventricles; and dimensions of the foramen magnum and relations to surrounding structures) as well as the whole spinal cord (including relations to the spinal canal). MRI will be carried out at screening, then biannually at weeks 26, 52, 78, and 104 and annually thereafter.
The MRI assessment procedures will be performed under general anesthesia, with the head in a neutral position and according to pre-defined parameters as shown in Table 3 (Brainstem and Skull) and Table 4 (Thoracolumbar Spine). Independent, blinded expert review of all MRI assessments will be performed to standardize eligibility assessment and interpretation of any changes observed during the study. Any decisions on whether surgery is required will remain the responsibility of the investigators and neurosurgeons at each participating site, who will be blinded to the AFMS assigned to their patients by the independent reviewer.
Table 3.
MRI parameters for the brainstem and skull.
| Parameter | Description |
|---|---|
| Superior extent | Vertex of skull (one slice above skull) |
| Inferior extent | Cervical C4 (ensure the bottom of the chin is included in the image volume) |
| Sequences | Scout |
| Sagittal 3D T1-weighted image (T1WI) | |
| Sagittal 3D T2-weighted image (T2WI) | |
| Axial and coronal reconstructions of the Sagittal T1 and T2 | |
| Plane | Sagittal (T2 and T1) |
| Reconstruction (MPR) plane | Axial and coronal |
| Slice thickness | 1.2 mm or less for 3D Sagittal |
| 1 mm for reconstruction scans | |
| Interslice gap (Spacing) | 0 mm |
| Matrix | 256 × 256 highly recommended |
| FOV | 20–25 cm required |
| NEX | 1–2 |
| Number of slices | Sagittal T1 and T2 should have the same number of slices.Follow up timepoint must be consistent with screening. |
Table 4.
MRI parameters for the thoracolumbar spine.
| Parameter | Description |
|---|---|
| Superior extent | Vertex of T11 (one slice above T11). Bony detail should bevisible from T-11 |
| Inferior extent | Infracoccygeal (one slice below the tip of the coccyx) |
| Sequences | Scout |
| Sagittal T1-weighted image (T1WI) | |
| Sagittal T2-weighted image (T2WI) | |
| Axial T1-weighted image (T1WI) | |
| Axial T2-weighted image (T2WI) | |
| Axial T2 gradient echo | |
| Plane | Sagittal and axial |
| Slice thickness | 3 mm |
| Interslice gap (spacing) | 0 mm |
| Matrix | 256 × 256 highly recommended |
| FOV | 20–25 cm required |
| NEX | 1–2 |
| Number of slices | Follow up timepoint must be consistent with screening. |
Neurological examination
To evaluate the effect of vosoritide on neurological signs and symptoms of cervicomedullary compression, complete neurological examinations will be performed at all visits, including assessment of lower cranial nerves, sensory and motor examinations, spinal reflexes, co-ordination, and gait.
Age-specific developmental milestone acquisition
To assess the effect of vosoritide on age-specific developmental milestones, Bayley-III scores will be used according to age throughout the course of the study. The Bayley-III is a performance-based outcome assessment for use in children from 1 to 42 months. It is individually administered by the trained clinician to the patient/child. Scales include Cognitive subscale, Receptive and Expressive subscales, and Gross and Fine Motor subscales. The two language scales make up a composite Language Scale score and the Gross and Fine Motor subscales yield a composite Motor Scale score. The scales have clinical and research utility as a diagnostic assessment for young children with varied disorders and disabilities and reflect current professional standards for early childhood assessment. 36
Sleep study
Polysomnography will be used to assess the presence and severity of sleep-disordered breathing by measurement of blood oxygen saturation, pulse rate, and airflow during overnight monitoring. Assessment of episodes of sleep apnea will include the number of episodes of apnea and hypopnea per hour (Apnea/Hypopnea Index).
Anthropometric measurements
Anthropometric measurements will be taken during study visits with each measurement taken in triplicate and conducted using standardized techniques using the same equipment across all study sites.
Statistical analysis
Approximately 20 patients aged 0 months to ≤12 months at study entry will participate in this study. No formal sample size calculations were performed but the number of patients is considered appropriate to evaluate the safety and efficacy of vosoritide in this at-risk population.
The statistical analysis plan will provide additional details on the planned statistical analysis. Unless otherwise stated, all analyses will be performed using SAS v. 9.4. Because the completeness of the data affects the integrity and accuracy of the interim and final study analysis, every effort will be made to ensure complete, accurate, and timely data collection and, therefore, avoid missing data. Missing data will not be imputed in any of the summaries, with the exception of missing dates for medications or adverse events. Any patient who prematurely discontinues study drug will be encouraged to continue to participate in the study assessments for the remaining duration of the study, as long as in the judgment of the Investigator such continued participation would not detrimentally affect the health, safety, or welfare of the patient.
Efficacy and safety data will be summarized descriptively by study treatment allocation; no confirmatory statistical testing will be performed and differences between treatment arms will be provided with 95% confidence intervals. Summaries of the continuous data will include the number of patients with assessments, mean, SD, median, 25th and 25th percentile, minimum and maximum, and 95% confidence limits. The frequency and incidence will be presented for categorical data.
Safety analyses
The safety analysis will be performed on the Safety Population. The MedDRA dictionary will be used to assign system organ class and preferred term classification to events and diseases, based on the original terms entered on the electronic case report form (eCRF).
The incidence of AEs will be summarized by system organ class, preferred term and study treatment allocation. All AEs, including SAEs and AEs that lead to permanent discontinuation from the study and from the study treatment, will be listed. Events of Interest such as hypersensitivity reactions, injection site reactions and symptomatic hypotension, and the percentage of patients who report these AEs will be presented.
Clinical laboratory data will be summarized by the type of laboratory test. For each clinical laboratory test, descriptive statistics will be provided on baseline as well as all subsequent visits. Shift tables from baseline to worst post-baseline value based on the Common Terminology Criteria Grading (Normal—Grade 5) will be generated.
All other safety measures including vital signs, physical examination, ECG, echocardiograms, and concomitant medication data will also be summarized descriptively. All safety results will be listed.
Efficacy analysis
The efficacy analysis will be performed on the Final Analysis Set (FAS) consisting of all randomized consented patients. Analyses to evaluate efficacy endpoints include:
Frequency of surgical cervicomedullary decompression over the course of the study.
Change in clinical signs and symptoms (including neurological assessment) every 6 months.
Change in MRI measurement of area of foramen magnum and antero-posterior (AP) diameter, brain stem, and spinal cord volume and ratio of area of spinal cord to foramen magnum every 6 months.
Change in MRI measurement of area of spinal canal, transverse and AP diameter, spinal cord volume and ratio of area of spinal cord to spinal canal every 6 months.
Change in developmental skills (Bayley-III) every 6 months.
Change in status of sleep apnea including central and obstructive components (sleep study) every 6 months.
Change in anthropometric measurements every 6 months.
Descriptive summaries at each visit, including change from baseline (defined as date of randomization), will be provided by study treatment allocation and strata in which patients are enrolled.
Conclusion
Abnormal development and growth of the foramen magnum in infants and young children with achondroplasia, leading to compression of the brainstem and other vital structures passing through the cranio-cervical junction, is the most important factor leading to increased mortality and morbidity in these children in the first 5 years of life. Similarly, impaired growth of the spinal canal results in spinal canal stenosis and cord compression leading to significant morbidity later in adulthood. It follows that one of the key goals of any precision therapy aimed at improving the impaired endochondral ossification that lies at the source of this condition, is to improve and restore the growth rate of the foramen magnum and spinal canal.
The recent advent of vosoritide, combined with its safety profile, and promising effects on long bone growth in children with achondroplasia aged 5 to 18 years, allows us to hypothesize whether treatment of infants and young children with this therapy might be safe and have a positive effect on growth of the foramen magnum and spinal canal, decreasing consequent medical complications.
It is hoped that the underlying rationale, design and methods of this pioneering clinical trial will begin the journey to answering this key question, with potential life-saving or health-improving benefits for young children born with achondroplasia. It will also serve as a benchmark against which other potential therapies for achondroplasia (currently in early clinical development) can be assessed. 37
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_1049732320931430 for Rationale, design, and methods of a randomized, controlled, open-label clinical trial with open-label extension to investigate the safety of vosoritide in infants, and young children with achondroplasia at risk of requiring cervicomedullary decompression surgery by Ravi Savarirayan, Melita Irving, Wirginia Maixner, Dominic Thompson, Amaka C Offiah, Daniel JA Connolly, Ashok Raghavan, James Powell, Marcin Kronhardt, George Jeha, Sajda Ghani, Elena Fisheleva and Jonathan RS Day in Science Progress
Author biographies
Ravi Savarirayan is clinical geneticist and scientist committed to bringing new therapies to patients affected with rare bone diseases through innovative clinical trials.
Melita Irving clinical geneticist and scientist committed to bringing new therapies to patients affected with rare bone diseases through innovative clinical trials.
Wirginia Maixner is a neurosurgeon with expertise in rare diseases and achondroplasia.
Dominic Thompson is a neurosurgeon working at Great Ormond Street and involved in the multidisciplinary management of children with achondroplasia.
Amaka C Offiah is a radiologist with expertise in the diagnosis of skeletal dysplasia.
Daniel JA Connolly is a clinican involved with the diagnosis and management of rare diseases including achondroplasia.
Ashok Raghavan is a clinican involved with the diagnosis and management of rare diseases including achondroplasia.
James Powell is an employees of BioMarin Inc., responsible for trial design, clinical operations, medical oversight and execution of clinical trials of vosoritide.
Marcin Kronhardt is an employees of BioMarin Inc., responsible for trial design, clinical operations, medical oversight and execution of clinical trials of vosoritide.
George Jeha is an employees of BioMarin Inc., responsible for trial design, clinical operations, medical oversight and execution of clinical trials of vosoritide.
Sajda Ghani is an employees of BioMarin Inc., responsible for trial design, clinical operations, medical oversight and execution of clinical trials of vosoritide.
Elena Fisheleva is an employees of BioMarin Inc., responsible for trial design, clinical operations, medical oversight and execution of clinical trials of vosoritide.
Jonathan RS Day is an employees of BioMarin Inc., responsible for trial design, clinical operations, medical oversight and execution of clinical trials of vosoritide.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Received honoraria from BioMarin Pharma.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funded by BioMarin Pharmaceutical.
Ethical approval for this study was obtained from:
•United Kingdom—Yorkshire & The Humber—Sheffield Research Ethics Committee (20/YH/0182) and the Health Research Authority (282694).
•Australia: The Royal Children’s Hospital Human Research Ethics Committee (HREC/64322/RCHM-2020).
Written informed consent was obtained from parents/caregivers of all subjects prior to enrolment into the study, including information that anonymized results arising from the study would be published in a peer-reviewed scientific journal.
ClinicalTrials.gov, NCT04554940; EudraCT number, 2020-001055-40
ORCID iD: Ravi Savarirayan https://orcid.org/0000-0002-5105-8427
References
- 1. Wynn J, King TM, Gambello MJ, et al. Mortality in achondroplasia study: a 42-year follow-up. Am J Med Genet A 2007; 143A(21): 2502–2511. [DOI] [PubMed] [Google Scholar]
- 2. Foldynova-Trantirkova S, Wilcox WR, Krejci P. Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum Mutat 2012; 33(1): 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellus GA, Hefferon TW, Ortiz de Luna RI, et al. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet 1995; 56(2): 368–373. [PMC free article] [PubMed] [Google Scholar]
- 4. Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet 2007; 370(9582): 162–172. [DOI] [PubMed] [Google Scholar]
- 5. Pauli RM, Scott CI, Wassman ER, Jr, et al. Apnea and sudden unexpected death in infants with achondroplasia. J Pediatr 1984; 104(3): 342–348. [DOI] [PubMed] [Google Scholar]
- 6. Hecht JT, Francomano CA, Horton WA, et al. Mortality in achondroplasia. Am J Hum Genet 1987; 41(3): 454–464. [PMC free article] [PubMed] [Google Scholar]
- 7. Hecht JT, Nelson FW, Butler IJ, et al. Computerized tomography of the foramen magnum: achondroplastic values compared to normal standards. Am J Med Genet 1985; 20(2): 355–360. [DOI] [PubMed] [Google Scholar]
- 8. Cheung MS, Alves I, Hagenäs L, et al. Meeting report from the achondroplasia foramen magnum workshop, Salzburg, Austria, 22 June 2019. Bone2019; 127: 499–502. [DOI] [PubMed] [Google Scholar]
- 9. Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis 2019; 14(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsushita T, Wilcox WR, Chan YY, et al. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum Mol Genet 2009; 18(2): 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vital JM, Beguiristain JL, Algara C, et al. The neurocentral vertebral cartilage: anatomy, physiology and physiopathology. Surg Radiol Anat 1989; 11(4): 323–328. [DOI] [PubMed] [Google Scholar]
- 12. Hecht JT, Horton WA, Redi CS, et al. Growth of the foramen magnum in achondroplasia. Am J Med Genet 1989; 32(4): 528–535. [DOI] [PubMed] [Google Scholar]
- 13. Coin CG, Malkasian DR. Foramen magnum. In: Newton TH, Potts DG. (eds.) Radiology of the Skull and Brain. The Skull, Vol. 1. St Louis, MO: Mosby, 1971, pp.275–286. [Google Scholar]
- 14. Wright MJ, Irving MD. Clinical management of achondroplasia. Arch Dis Child 2012; 97(2): 129–134. [DOI] [PubMed] [Google Scholar]
- 15. Trotter TL, Hall JG; American Academy of Pediatrics Committee on Genetics. Health supervision for children with achondroplasia. Pediatrics 2005; 116(3): 771–783. [DOI] [PubMed] [Google Scholar]
- 16. Canavese F, Dimeglio A. Normal and abnormal spine and thoracic cage development. World J Orthop 2013; 4(4): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubota T, Adachi M, Kitaoka T, et al. Clinical practice guidelines for achondroplasia. Clin Pediatr Endocrinol 2020; 29(1): 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White KK, Bompadre V, Goldberg MJ, et al. Best practices in the evaluation and treatment of foramen magnum stenosis in achondroplasia during infancy. Am J Med Genet A 2016; 170A(1): 42–51. [DOI] [PubMed] [Google Scholar]
- 19. Ruiz-Garcia M, Tovar-Baudin A, Del Castillo-Ruiz V, et al. Early detection of neurological manifestations in achondroplasia. Childs Nerv Syst 1997; 13(4): 208–213. [DOI] [PubMed] [Google Scholar]
- 20. Mogayzel PJ, Jr, Carroll JL, Loughlin GM, et al. Sleep-disordered breathing in children with achondroplasia. J Pediatr 1998; 132(4): 667–671. [DOI] [PubMed] [Google Scholar]
- 21. White KK, Parnell SE, Kifle Y, et al. Is there a correlation between sleep disordered breathing and foramen magnum stenosis in children with achondroplasia? Am J Med Genet A 2016; 170A(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 22. Nadel JL, Wilkinson DA, Garton HJL, et al. Screening and surgery for foramen magnum stenosis in children with achondroplasia: a large, national database analysis. J Neurosurg Pediatr 2018; 23(3): 374–380. [DOI] [PubMed] [Google Scholar]
- 23. Sanders VR, Sheldon SH, Charrow J. Cervical spinal cord compression in infants with achondroplasia: should neuroimaging be routine? Genet Med 2019; 21(2): 459–463. [DOI] [PubMed] [Google Scholar]
- 24. Ho NC, Guarnieri M, Brant LJ, et al. Living with achondroplasia: quality of life evaluation following cervico-medullary decompression. Am J Med Genet A 2004; 131(2): 163–167. [DOI] [PubMed] [Google Scholar]
- 25. Cheung MS, Irving M, Cocca A, et al. Achondroplasia foramen magnum score: screening infants for stenosis. Arch Dis Child 2021; 106(2): 180–184. [DOI] [PubMed] [Google Scholar]
- 26. Yasoda A, Nakao K. Translational research of C-type natriuretic peptide (CNP) into skeletal dysplasias. Endocr J 2010; 57(8): 659–666. [DOI] [PubMed] [Google Scholar]
- 27. Kake T, Kitamura H, Adachi Y, et al. Chronically elevated plasma C-type natriuretic peptide level stimulates skeletal growth in transgenic mice. Am J Physiol Endocrinol Metab 2009; 297(6): E1339–E1348. [DOI] [PubMed] [Google Scholar]
- 28. Yamanaka S, Nakao K, Koyama N, et al. Circulatory CNP rescues craniofacial hypoplasia in achondroplasia. J Dent Res 2017; 96(13): 1526–1534. [DOI] [PubMed] [Google Scholar]
- 29. Yasoda A, Kitamura H, Fujii T, et al. Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology 2009; 150(7): 3138–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bocciardi R, Giorda R, Buttgereit J, et al. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat 2007; 28(7): 724–731. [DOI] [PubMed] [Google Scholar]
- 31. Bocciardi R, Ravazzolo R. C-type natriuretic peptide and overgrowth. Endocr Dev 2009; 14: 61–66. [DOI] [PubMed] [Google Scholar]
- 32. Moncla A, Missirian C, Cacciagli P, et al. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum Mutat 2007; 28(12): 1183–1188. [DOI] [PubMed] [Google Scholar]
- 33. Lorget F, Kaci N, Peng J, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet 2012; 91(6): 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Savarirayan R, Irving M, Bacino CA, et al. C-type natriuretic peptide analogue therapy in children with achondroplasia. N Engl J Med 2019; 381(1): 25–35. [DOI] [PubMed] [Google Scholar]
- 35. Savarirayan R, Tofts L, Irving M, et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomized, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet 2020; 396: 684–692. [DOI] [PubMed] [Google Scholar]
- 36. Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt. [Google Scholar]
- 37. Legeai-Mallet L, Savarirayan R. Novel therapeutic approaches for the treatment of achondroplasia. Bone 2020; 141: 115579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_1049732320931430 for Rationale, design, and methods of a randomized, controlled, open-label clinical trial with open-label extension to investigate the safety of vosoritide in infants, and young children with achondroplasia at risk of requiring cervicomedullary decompression surgery by Ravi Savarirayan, Melita Irving, Wirginia Maixner, Dominic Thompson, Amaka C Offiah, Daniel JA Connolly, Ashok Raghavan, James Powell, Marcin Kronhardt, George Jeha, Sajda Ghani, Elena Fisheleva and Jonathan RS Day in Science Progress