Abstract
Background:
As the burden of opioid use disorder (OUD) increases in the United States, manifold federal and state initiatives have sought to increase access to treatment for OUD, which includes behavioral and pharmaceutical treatment modalities. Although the evidence base for outpatient treatment for OUD—including medications for opioid use disorder—is substantial, few studies have examined the risk factors for fatality during treatment for OUD.
Methods:
Treatment Episode Data Set-Discharges (TEDS-D) data were used to evaluate correlates of death during outpatient treatment for OUD in 2016. To determine the correlates of mortality during an outpatient treatment for OUD, we constructed a pooled logistic regression model, stratified by use of medication for opioid use disorder (MOUD), to control for the duration of time in treatment and to identify the independent characteristics that may lead to differences in the odds of mortality during treatment.
Findings:
1861 (0.8%) of 235,745 outpatient treatment episodes for OUD included in our analysis resulted in fatality. Many factors correlated with death during treatment were similar for individuals who did and did not receive MOUD. However, non-White race was only significantly associated with decreases in fatality in non-MOUD treatment episodes. Male sex and reported intravenous drug use at admission were associated with fatality only for treatment episodes that did not involve MOUD.
Conclusions:
In this national study of outpatient treatment episodes for OUD, we found differences in age, sex, region, drug use history, treatment setting, and treatment history significantly affected the risk of death during treatment. As more people become engaged with treatment, facilities should work toward delivering optimal treatment for all patients regardless of personal characteristics.
Keywords: Medications for opioid use disorder, Opioid use disorder, Fatality, Outpatient treatment
1. Introduction
The rise of opioid use over the past decade has risen in parallel with staggering rates of fatal overdose in the United States (Dart et al., 2015; Rudd, 2016). Years of life lost from opioid overdose has increased from 165,000 years of life lost in 1999 to 830,700 years of life lost in 2016 (Mokdad et al., 2018). The National Epidemiologic Survey on Alcohol and Related Conditions-III found that opioid use disorder (OUD) prevalence among adults has more than doubled, increasing from 1.4% in 2002 to 2.9% in 2013 (Saha, Kerridge, & Goldstein, 2016). As the burden of OUD has increased, so has the need for OUD treatment (Alderks, 2017; Gomes, Tadrous, Mamdani, Paterson, & Juurlink, 2018; Jones, Campopiano, Baldwin, & McCance-Katz, 2015; Stein et al., 2018). According to the 2016 National Survey on Drug Use and Health, approximately 2.1 million people, about 1% of all people living in the United States 12 years or older, meet the diagnostic criteria for an OUD and could benefit from treatment (Ahrnsbrak, Bose, Hedden, Lipari, & Park-Lee, 2017; Schuckit, 2016). However, treatment engagement for OUD is low due to factors related to lack of insurance coverage, limited access to treatment services, and stigma (Wu, Zhu, & Swartz, 2016). In order to address low treatment utilization, the federal government has provided significant funding to expand access to treatment for OUD (Davis, 2018; U.S Department of Health and Human Services, 2018; Wickramatilake et al., 2017). In addition to increased funding for treatment centers, new federal legislation lifts some of the barriers for covering treatment costs through Medicaid and Medicare (Congressional Budget Office, 2018, Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act, 2018; Davis, 2018; Meinhofer & Witman, 2018).
OUD treatment options and duration of care are variegated and diverse (Veilleux, Colvin, Anderson, York, & Heinz, 2010). Over the past two decades, a growing body of literature has established effective, evidence-based treatments for OUD that include both pharmaco- and behavioral modalities (Carroll & Weiss, 2017; Schuckit, 2016; Tsui, Burt, Thiede, & Glick, 2018). The use of medications for opioid use disorder (MOUD), the gold-standard outpatient treatment strategy involving the use of medications such as buprenorphine and methadone (National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, & Committee on Medication-Assisted Treatment for Opioid Use Disorder, 2019), has not only been found to help patients manage their symptoms of OUD, but has led to decreases in fatal and non-fatal overdose, as well as recurrent opioid use (Connery, 2015; Hawk, Vaca, & D’Onofrio, 2015; Larochelle et al., 2018). Psychosocial treatments for OUD such as counseling services and community support groups have also been shown to help people manage OUD, though they have only been found to be effective at reducing mortality when used in conjunction with MOUD (Kampman & Jarvis, 2015; Pierce et al., 2016). Research on treatment for OUD has largely focused on retention and other short-term treatment outcomes (Connery, 2015; Schuckit, 2016; Sokol, LaVertu, Morrill, Albanese, & Schuman-Olivier, 2018). Though there is no single metric that captures treatment success, abstinence from non-medical and illicit opioid use during and after treatment, treatment retention, severity of withdrawal symptoms, and reduction in mortality have been broadly used to assess treatment success (Amato et al., 2005; Veilleux et al., 2010). As both treatment access and burden of OUD increase, it is imperative that we understand the risk factors not only for unsuccessful treatment, but also mortality during treatment.
There is significant scholarship discussing various modalities, characteristics, and other aspects of outpatient treatment of OUD (Bisaga et al., 2018; McCarty et al., 2014; Mennis, Stahler, El Magd, & Baron, 2019; Naeger, Mutter, Ali, Mark, & Hughey, 2016). Many studies have also examined the risk factors for fatality during treatment (Clausen, Anchersen, & Waal, 2008; Cornish, Macleod, Strang, Vickerman, & Hickman, 2010; Degenhardt et al., 2011, 2009; McCowan, Kidd, & Fahey, 2009; Merrall, Bird, & Hutchinson, 2012; Pierce et al., 2016); however, few studies have assessed fatality during treatment in the United States since the beginning of the third wave of the opioid overdose epidemic involving synthetic opioids, primarily fentanyl and related analogs. According to the Treatment Episode Data Set - Discharges (TEDS-D), a dataset comprised of discharge records from publicly funded drug treatment facilities across the US, about 1% of all people admitted to a treatment facility for OUD die during inpatient treatment. The rate of death during treatment is similar among those who are receiving outpatient treatment for OUD (Center for Behavioral Health Statistics and Quality; Substance Abuse and Mental Health Services Administration, 2018). The objective of this study is to assess the sociodemographic and treatment characteristics that are associated with fatality during outpatient treatment for OUD.
2. Methods and materials
2.1. Study design
In order to assess correlates of death during outpatient treatment for OUD, we examined data from the TEDS-D. TEDS-D, administered by the Substance Abuse and Mental Health Services Administration (SAMHSA), documents detailed information about sociodemographics, drug use history, and treatment received for individuals who were discharged from state or federally funded treatment facilities in 2016 (Treatment Episode Data Set-Discharges (TEDS-D), 2016). Data are collected at the state-level by substance use agencies and are subsequently sent to the federal government for compilation into a deidentified nationwide dataset (Substance Abuse and Mental Health Services Administration, 2014). As TEDS-D is publicly available and only contains non-identifiable data, IRB approval was not necessary for the conduct of this study.
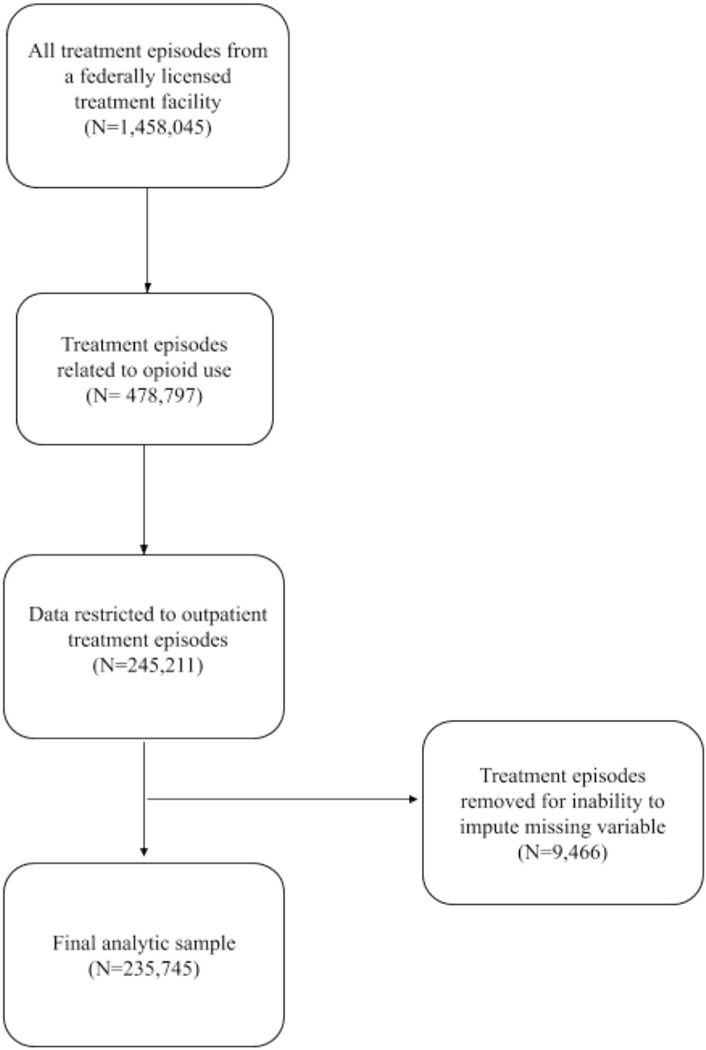
To capture those who received outpatient treatment for OUD, data from the TEDS-D 2016 were restricted to include treatment episodes in an outpatient facility which the primary substance used was listed as “heroin”, “non-prescription methadone”, and “other opioid or synthetics” (Fig. 1). Each treatment was classified as either being intensive or non-intensive. Intensive treatments were defined as clients receiving treatment services, including MOUD and behavioral services for two or more hours per day for three or more days per week, and non-intensive treatment was broadly defined as ambulatory treatment services including pharmacological, individual, family, and group support services (Substance Use and Mental Health Services Administration, 2018).
Fig. 1.
Selection of 2016 treatment discharges from publicly funded treatment facilities included in final analytic sample.
2.2. Measures
The primary outcome for this analysis was death, defined as all-cause mortality, during outpatient treatment for OUD. In TEDS-D, substance use treatment facilities can report one of seven different reasons for discharge including: transfer to another facility, termination by the facility, incarceration, leaving against medical advice, other, unknown, death, and treatment completion. In this analysis, we aggregated these outcomes such that a binary variable was created, representing fatality experienced during treatment (yes vs. no).
Demographic characteristics assessed included age, race, ethnicity, education level, employment status, and housing status. Race was categorized as White, Black or other (which includes Alaskan Native, American Indian, Asian or Pacific Islander, Native Hawaiian or Pacific Islander, or people identifying as multiracial), due to sample size limitations. Housing status was reported as homeless, independent, or dependent, which SAMHSA defined as living in a supervised setting, such as a residential institution or group home (Substance Use and Mental Health Services Administration, 2018). Additionally, we examined intravenous drug use at admission, primary drug reported at treatment admission, and previous treatment admission, presented as a binary variable abstracted from state records. These demographic and treatment setting characteristics were selected for analysis based on literature demonstrating their significance in predicting treatment outcomes (Marcovitz, McHugh, Volpe, Votaw, & Connery, 2016; Stahler, Mennis, & DuCette, 2016). Length of treatment was defined categorically based on information available in the TEDS-D dataset. Time was broken into the following increments: 1–30 days, 31–45 days, 46–60 days, 61–90 days, 91–120 days, 121–180 days, 181–365 days and >365 days.
2.3. Statistical analysis
Nine variables, including sex, race, ethnicity, employment status, living arrangement, MOUD use, education, history of arrests, and previous treatment episode contained a substantial amount of missing data. In order to account for missing data, multiple imputation was conducted using a validated R package, Multiple Imputation using Chained Equations (MICE) (van Buuren & Groothuis-Oudshoorn, 2011; Zhongheng Zhang, 2016). As shown in Fig. 1, a small proportion (3.9%) of treatment episodes could not be imputed and were excluded from the analysis. All statistical analyses were performed using the imputed dataset.
Initial exploratory data analysis revealed substantial differences between MOUD and non-MOUD treatment episodes. To control for differences, we stratified the analytic sample by MOUD use. Chi-square tests were performed to assess differences in demographic and treatment setting characteristics by MOUD use. Descriptive statistics were created for the demographic, substance use, and treatment facility characteristics. We performed chi-square tests to measure the association of potential correlates of experiencing death during a treatment episode. In order to control for length of treatment, which was captured categorically in the TEDS-D database, we constructed bivariable and multivariable pooled logistic regressions using RStudio in lieu of performing a traditional time-to-event analysis. In instances where a time interval is provided, and time to event is not available, pooled logistic regression provides a robust estimate of the conditional odds for experiencing an outcome given a specific time interval (Ngwa et al., 2016). All potential correlates of death were included in the final model. Given that MOUD is usually long-term treatment, and there may be fundamental differences in characteristics in treatment episodes of longer duration and correlates of fatality on a longer timeline, a sensitivity analysis was conducted with data restricted to treatment duration of less than a year (Bhatraju et al., 2017; Zhang, Friedmann, & Gerstein, 2003). For all statistics, two-sided P-values were used, and significance was considered at p ≤ 0.05.
3. Results
There were 235,745 outpatient treatment episodes for OUD that were included in our analysis (see Fig. 1). Imputation was necessary for 19,134 (8.1%) observations. Variables that needed to be imputed included the following: sex, race, ethnicity, education, employment, living arrangement, arrests and use of MOUD. Of the full analytic sample, 1861 (0.8%) resulted in a fatality during treatment. A total of 130,735 (55.5%) treatment episodes did not involve MOUD and 105,010 (44.5%) involved MOUD. Once the sample was limited to only treatment episodes that lasted less than a year, there were a total of 202,943 outpatient treatment episodes for OUD, of which 965 (0.5%) resulted in fatality during treatment. Of treatment episodes that lasted less than a year, 122,947 (60.6%) did not involve MOUD and 79,996 (39.4%) treatment episodes involved MOUD.
Among those who did not receive MOUD, the majority of treatment episodes were among people between the ages of 18–34 (65.5%), males (57.9%), people who identified as White (82.2%), and non-Hispanic (91.4%). Approximately 69% of those not receiving MOUD had previously received OUD treatment, 62.1% reported heroin as their primary drug of use, and 67.7% received treatment at a non-intensive facility. Similarly, a majority of treatment episodes involving MOUD were among males (58.4%), people who identified as White (69.8%) and non-Hispanic (82.5%), people who reported a previous treatment episode (76.7%) and heroin as their primary drug of use (80.3%), and received non-intensive treatment (84.5%). Differences between treatment episodes that involved MOUD and did not involve MOUD can be found in Table 1. Compared to treatment episodes that did not involve MOUD, those that involved MOUD were more likely to be older, female, have had a previous treatment episode, report heroin and intravenous drug use at admission, received treatment at a non-intensive treatment center, and had longer treatment episodes. Additionally, treatment episodes that involved MOUD were more likely to involve people who were Black or another racial background, Hispanic, and lived in the Northeast.
Table 1.
Comparison of demographic and treatment setting characteristics of those receiving opioid use disorder outpatient treatment at a publicly finding facility in 2016 by MOUD receipt.
| Characteristic | Non-MOUFE. P.D Total (n = 130,735) n (%) | MOUD Total (n = 105,010) n (%) | P-value |
|---|---|---|---|
| Age | <0.001 | ||
| 18–34 | 85,686 (65.5) | 52,204 (49.7) | |
| 35–54 | 39,562 (30.3) | 42,035 (40.0) | |
| 55 or older | 5487 (4.2) | 10,771 (10.3) | |
| Sex | 0.029 | ||
| Female | 55,004 (42.1) | 43,712 (41.6) | |
| Male | 75,731 (57.9) | 61,298 (58.4) | |
| Race | <0.001 | ||
| White | 107,422 (82.2) | 73,284 (69.8) | |
| Black | 11,053 (8.4) | 14,233 (13.5) | |
| Other | 12,269 (9.4) | 17,493 (16.6) | |
| Ethnicity | <0.001 | ||
| Non-Hispanic | 119,546 (91.4) | 86,587 (82.5) | |
| Hispanic | 11,189 (8.6) | 18,423 (17.5) | |
| Region | <0.001 | ||
| Northeast | 45,780 (35.1) | 50,727 (48.3) | |
| Midwest | 26,166 (20.0) | 13,911 (13.2) | |
| South | 39,855 (30.4) | 12,348 (11.8) | |
| West | 18,934 (14.5) | 28,024 (26.7) | |
| Education | <0.001 | ||
| College graduate | 6338 (4.8) | 4872 (4.6) | |
| Some college | 27,423 (21.0) | 20,506 (19.6) | |
| Less than college | 96,974 (74.2) | 79,632 (75.8) | |
| Employment | <0.001 | ||
| Full time | 21,720 (16.6) | 15,537 (14.8) | |
| Part time | 11,137 (8.5) | 8669 (8.3) | |
| Unemployed | 97,878 (74.9) | 80,804 (76.9) | |
| Living arrangement | <0.001 | ||
| Dependent living | 23,658 (18.1) | 14,170 (13.5) | |
| Homeless | 9341 (7.1) | 8117 (7.7) | |
| Independent living | 97,736 (74.8) | 82,723 (78.8) | |
| Previous treatment episode | <0.001 | ||
| No | 40,518 (31.0) | 24,425 (23.3) | |
| Yes | 90,217 (69.0) | 80,585 (76.7) | |
| Previous arrests | <0.001 | ||
| None | 121,004 (92.6) | 99,843 (95.1) | |
| One or more | 9731 (7.4) | 5167 (4.9) | |
| Intravenous drug use reported at admission | <0.001 | ||
| No | 65,231 (49.9) | 45,542 (43.4) | |
| Yes | 65,504 (50.1) | 59,468 (56.6) | |
| Primary substance reported at admission | <0.001 | ||
| Other opioid | 49,558 (37.9) | 20,666 (19.7) | |
| Heroin | 81,117 (62.1) | 84,344 (80.3) | |
| Number of substances reported at admission | <0.001 | ||
| One | 41,685 (31.9) | 43,994 (41.2) | |
| Two | 45,489 (34.8) | 38,193 (35.8) | |
| Three | 43,561 (33.3) | 24,522 (23.0) | |
| Outpatient treatment setting | <0.001 | ||
| Intensive | 42,202 (32.2) | 8890 (8.5) | |
| Non-intensive | 88,533 (67.7) | 96,120 (91.5) | |
| Length of treatment | <0.001 | ||
| 1–30 days | 48,080 (36.8) | 24,752 (23.2) | |
| 31–90 days | 36,154 (27.6) | 21,721 (20.3) | |
| 91–120 days | 11,290 (8.6) | 7253 (6.8) | |
| 121–365 days | 27,423(21.0) | 27,291 (25.6) | |
| >365 days | 7788 (5.6) | 25,692 (24.1) |
Of the 130,735 outpatient treatment episodes that did not involve MOUD, 561 (0.4%) resulted in a fatality. The percentage of treatment episodes resulting in a fatality did not meaningfully change once episodes longer than a year in duration were removed from the sample. Shown in Table 2, bivariable analysis found that experiencing fatality during treatment among those not receiving MOUD was associated with being older than 34, male, having a previous treatment episode, reporting intravenous drug and heroin use at admission, and receiving treatment in a non-intensive facility.
Table 2.
Demographic and treatment setting characteristics of outpatient treatment episodes for opioid use disorder stratified by MOUD use at publicly funded treatment facilities in 2016.
| Characteristic | Non-MOUD |
MOUD |
||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 130,735) n (%) | Non-fatality during treatment (n = 130,174) n (%) | Fatality during treatment (n = 561) n (%) | P-value | Total (n = 100,334) n (%) | Non-fatality during treatment (n = 99,034) n (%) | Fatality during treatment (n = 1300) n (%) | P-value | |
| Age | <0.001 | <0.001 | ||||||
| 18–34 | 85,686 (65.5) | 85,362 (65.8) | 324 (57.7) | 49,904 (49.7) | 49,697 (50.2) | 207 (15.9) | ||
| 35–54 | 39,562 (30.3) | 39,372 (30.0) | 190 (33.9) | 40,183 (40.0) | 39,556 (39.9) | 627 (48.2) | ||
| 55 or older | 5487 (4.2) | 5440 (4.2) | 47 (8.4) | 10,247 (10.2) | 9781 (9.9) | 466 (35.8) | ||
| Sex | <0.001 | <0.001 | ||||||
| Female | 55,004 (42.1) | 54,822 (42.1) | 182 (32.4) | 41,691 (41.6) | 41,205 (41.6) | 486 (37.3) | ||
| Male | 75,731 (57.9) | 75,352 (57.9) | 379 (67.6) | 58,643 (58.4) | 57,829 (58.4) | 814 (62.6) | ||
| Race | <0.001 | |||||||
| White | 107,422 (82.2) | 106,941 (82.2) | 481 (85.7) | 70,356 (70.1) | 69,616 (70.3) | 740 (56.9) | ||
| Black | 11,053 (8.4) | 11,013 (8.4) | 40 (7.1) | 13,407 (13.4) | 13,125 (13.3) | 282 (21.7) | ||
| Other | 12,260 (9.4) | 12,220 (9.4) | 40 (7.1) | 0.072 | 6571 (16.5) | 16,293 (16.4) | 278 (21.3) | |
| Ethnicity | 0.819 | <0.001 | ||||||
| Non-Hispanic | 119,546 (91.4) | 119,031 (91.4) | 515 (91.8) | 82,496 (82.2) | 81,503 (82.3) | 993 (76.4) | ||
| Hispanic | 11,189 (8.6) | 11,143 (8.6) | 46 (8.2) | 17,838 (17.8) | 17,531 (17.7) | 307 (23.6) | ||
| Region | <0.001 | <0.001 | ||||||
| Northeast | 45,70 (35.0) | 45,531 (35.0) | 248 (44.4) | 49,390 (49.2) | 48,734 (49.2) | 656 (50.4) | ||
| Midwest | 26,166 (20.0) | 26,019 (20.0) | 147 (26.2) | 12,031 (12.0) | 11,910 (12.0) | 122 (9.3) | ||
| South | 39,855 (30.5) | 39,757 (30.5) | 98 (17.5) | 11,345 (11.3) | 11,236 (11.3) | 109 (8.3) | ||
| West | 18,934 (14.5) | 18,867 (14.5) | 67 (11.9) | 27,567 (27.5) | 27,154 (27.4) | 413 (31.7) | ||
| Education | 0.002 | 0.008 | ||||||
| College graduate | 6338 (4.8) | 6302 (4.8) | 36 (6.4) | 4610 (4.6) | 4543 (4.6) | 67 (5.2) | ||
| Some college | 27,423 (21.0) | 27,278 (21.0) | 145 (25.8) | 19,621 (19.6) | 19,410 (19.6) | 211 (16.2) | ||
| Less than college | 96,974 (74.2) | 96,594 (74.2) | 380 (67.7) | 76,103 (75.8) | 75,081 (75.8) | 1022 (78.6) | ||
| Employment | 0.734 | <0.001 | ||||||
| Full time | 21,720 (16.6) | 21,621 (16.6) | 99 (17.7) | 15,043 (15.0) | 14,919 (15.0) | 124 (9.5) | ||
| Part time | 11,137 (8.5) | 11,087 (8.5) | 50 (8.9) | 8294 (8.3) | 8232 (8.3) | 62 (4.8) | ||
| Unemployed | 97,878 (74.9) | 97,466 (74.9) | 412 (73.4) | 76,997 (76.7) | 75,883 (76.6) | 1115 (85.7) | ||
| Living arrangement | 0.032 | 0.055 | ||||||
| Dependent living | 23,658 (18.1) | 23,565 (18.1) | 93 (16.6) | 135,576(13.6) | 13,423 (13.6) | 153 (11.8) | ||
| Homeless | 9341 (7.1) | 9316 (7.2) | 26 (4.6) | 7665 (7.6) | 7578 (7.6) | 87 (6.7) | ||
| Independent living | 97,736 (74.8) | 97,294 (74.7) | 442 (78.8) | 79,093 (78.8) | 78,033 (78.8) | 1060 (81.5) | ||
| Previous treatment episode | <0.001 | <0.001 | ||||||
| No | 40,518 (31.0) | 40,393 (31.0) | 125 (22.2) | 23,635 (23.6) | 23,418 (23.6) | 217 (16.7) | ||
| Yes | 90,217 (69.0) | 89,781 (69.0) | 436 (77.7) | 76,699 (76.4) | 75,616 (76.4) | 1083 (83.3) | ||
| Previous arrests | 0.277 | 0.074 | ||||||
| None | 121,004 (92.6) | 120,492 (92.6) | 512 (91.2) | 95,452 (95.1) | 94,201 (95.1) | 1251 (96.2) | ||
| One or more | 9731 (7.4) | 9682 (7.4) | 49 (8.7) | 4882 (4.9) | 4833 (4.9) | 49 (3.8) | ||
| Intravenous drug use reported at admission | <0.001 | 0.950 | ||||||
| No | 65,231(49.9) | 65,027 (50.0) | 204 (37.3) | 43,500 (43.4) | 42,938 (43.5) | 562 (43.2) | ||
| Yes | 65,504 (50.1) | 65,147 (50.0) | 357 (63.6) | 56,834 (56.6) | 56,096 (56.6) | 738 (56.8) | ||
| Primary substance reported at admission | <0.001 | <0.001 | ||||||
| Other opioid | 49,558 (37.9) | 49,416 (38.0) | 142 (25.3) | 19,686 (19.6) | 19,478 (19.7) | 208 (16.0) | ||
| Heroin | 81,177 (62.1) | 80,758 (62.0) | 419 (74.7) | 80,648 (80.4) | 79, 556 (80.1) | 1092 (84.0) | ||
| Number of substances reported at admission | 0.042 | <0.001 | ||||||
| One | 41,685 (31.9) | 41,520 (31.9) | 165 (29.4) | 41,198 (41.1) | 40,585 (41.0) | 613 (46.2) | ||
| Two | 45,489 (34.8) | 45,308 (34.8) | 181 (32.3) | 36,128 (36.0) | 35,653 (36.0) | 475 (36.5) | ||
| Three | 43,561 (33.3) | 43,346 (33.3) | 215 (38.3) | 23,008 (22.9) | 22,796 (23.0) | 212 (16.3) | ||
| Outpatient treatment setting | <0.001 | <0.001 | ||||||
| Intensive | 42,202 (32.3) | 42,081 (32.3) | 121 (21.6) | 8604 (8.6) | 8604 (8.7) | 40 (3.1) | ||
| Non-intensive | 88,533 (67.7) | 88,093 (67.7) | 440 (78.4) | 90,430 (91.4) | 90,430 (91.3) | 1260 (96.9) | ||
| Length of treatment | <0.001 | <0.001 | ||||||
| 1–30 days | 48,080 (36.8) | 47,948 (36.8) | 132 (23.5) | 23,444 (23.4) | 23,344 (23.6) | 100 (7.7) | ||
| 31–90 days | 36,154 (27.7) | 36,003 (27.6) | 151 (26.9) | 20,440 (20.4) | 20,346 (20.5) | 94 (7.2) | ||
| 91–120 days | 11,290 (8.6) | 11,248 (8.6) | 42 (7.5) | 6786 (6.8) | 6747 (6.8) | 39 (3.0) | ||
| 121–365 days | 27,423 (21.0) | 27,274 (20.9) | 149 (26.6) | 25,679 (25.6) | 25,453 (25.7) | 226 (17.4) | ||
| >365 days | 7788 (6.0) | 7701 (5.9) | 87 (15.5) | 22,985 (23.9) | 23,144 (23.3) | 841 (64.7) | ||
Of the 100,334 treatment episodes that involved MOUD, 1300 (1.3%) resulted in a fatality during treatment. When limited to treatment episodes that lasted longer than a year, 0.6% resulted in fatality. Initial bivariable analysis treatment episodes involving MOUD found many of the same associations with those that did not involve MOUD, except that in the subset of treatment episodes that involved MOUD, fatality was also associated with reporting a non-White race and Hispanic ethnicity.
Multivariable analysis of characteristics of treatment episodes and their association with mortality experienced during a treatment episode are presented in Table 3. Among treatment episodes not involving MOUD, people age 34–54 (adjusted odds ratio [AOR],1.43; 95% confidence interval [CI], 1.19–1.72) and those older than 55 years of age (AOR, 2.77; 95% CI, 1.994–3.86) had higher odds of mortality compared to those 18–34 years of age. Compared to females, males had higher odds of fatality (AOR, 1.42; 95% CI, 1.18–1.70). Non-white race was associated with a decrease in odds of fatality. Compared to treatment episodes among White patients, Black patients (AOR, 0.68, 95% CI 0.47–0.96) and patients identifying as another race (AOR, 0.65; 95% CI 0.45–0.93) had lower odds of fatality. Compared to dependent living, living independently was associated with increased odds of fatality during treatment (AOR, 1.27; 95% CI, 1.01–1.32). Those who reported intravenous drug use (AOR, 1.53; 95% CI, 1.18–1.97) or heroin use (AOR, 1.43; 95% CI, 1.14–1.80) at admission had higher odds of fatality compared to those who did not.
Table 3.
Factors associated with fatality during outpatient opioid use disorder treatment at publicly funded treatment facilities in 2016.
| Characteristic | Non-MOUD |
MOUD |
||
|---|---|---|---|---|
| Unadjusted odds OR (95% CI) | Adjusted odds OR (95% CI) | Unadjusted odds OR (95% CI) | Adjusted odds OR (95% CI) | |
| Age 18–34 |
Reference | Reference | Reference | Reference |
| 35–54 | 1.27 (1.06–1.52) | 1.43 (1.19–1.72) | 3.81 (3.25–4.46) | 3.60 (3.07–4.23) |
| 55 or older | 2.28 (1.67–3.09) | 2.77 (1.99–3.85) | 11.44 (9.70–13.49) | 10.34 (8.64–12.36) |
| Sex Female |
Reference | Reference | Reference | Reference |
| Male | 1.52 (1.27–1.81) | 1.42 (1.18–1.70) | 1.20 (1.07–1.33) | 0.96 (0.85–1.07) |
| Race White |
Reference | Reference | Reference | Reference |
| Black | 0.81 (0.58–1.11) | 0.67 (0.47–0.96) | 2.01 (1.75–2.32) | 1.00 (0.85–1.17) |
| Other | 0.73 (0.53–1.01) | 0.65 (0.45–0.93) | 1.60 (1.40–1.84) | 1.03 (0.84–1.23) |
| Ethnicity Non-Hispanic |
Reference | Reference | Reference | Reference |
| Hispanic | 0.95 (0.71–1.29) | 0.99 (0.70–1.39) | 1.44 (1.26–1.63) | 1.03 (0.85–1.24) |
| Region Northeast |
Reference | Reference | Reference | Reference |
| Midwest | 1.03 (0.84–1.26) | 1.16 (0.94–1.43) | 0.76 (0.62–0.92) | 0.74 (0.60–0.90) |
| South | 0.45 (0.37–0.57) | 0.53 (0.42–0.67) | 0.72 (0.59–0.88) | 0.74 (0.60–0.92) |
| West | 0.65 (0.50–0.85) | 0.70 (0.53–0.93) | 1.13 (1.00–1.28) | 0.88 (0.77–1.01) |
| Education College graduate |
Reference | Reference | Reference | Reference |
| Some college | 0.93 (0.64–1.34) | 0.88 (0.61–1.28) | 0.73 (0.56–0.97) | 0.80 (0.61–1.06) |
| Less than college | 0.69 (0.49–0.97) | 0.69 (0.48–0.96) | 0.93 (0.71–1.18) | 0.91 (0.71–1.17) |
| Employment Full time |
Reference | Reference | Reference | Reference |
| Part time | 0.98 (0.70–1.38) | 1.07 (0.76–1.50) | 0.91 (0.67–1.23) | 0.94 (0.69–1.28) |
| Unemployed | 0.92 (0.74–1.15) | 1.04 (0.83–1.30) | 1.77 (1.47–2.13) | 1.35 (1.11–1.64) |
| Living arrangement Dependent living |
Reference | Reference | Reference | Reference |
| Homeless | 0.71 (0.46–1.09) | 0.70 (0.45–1.09) | 1.01 (0.77–1.31) | 0.84 (0.64–1.10) |
| Independent living | 1.15 (0.92–1.44) | 1.27 (1.01–1.60) | 1.19 (1.00–1.41) | 1.12 (0.94–1.33) |
| Previous treatment episode No |
Reference | Reference | Reference | Reference |
| Yes | 1.57 (1.28–1.92) | 1.18 (0.96–1.46) | 1.54 (1.33–1.79) | 1.40 (1.21–1.63) |
| Previous arrests None |
Reference | Reference | Reference | Reference |
| One or more | 1.19 (0.89–1.60) | 1.18 (0.87–1.58) | 0.76 (0.57–1.02) | 1.00 (0.75–1.34) |
| Intravenous drug use reported at admission No |
Reference | Reference | Reference | Reference |
| Yes | 1.75 (1.47–2.07) | 1.54 (1.26–1.88) | 1.01 (0.90–1.12) | 1.12 (0.98–1.27) |
| Primary substance reported at admission Other opioid |
Reference | Reference | Reference | Reference |
| Heroin | 1.80 (1.49–2.18) | 1.43 (1.15–1.80) | 1.29 (1.11–1.49) | 0.95 (0.80–1.12) |
| Number of substances reported at admission One |
Reference | Reference | Reference | Reference |
| Two | 1.01 (0.81–1.24) | 0.97 (0.79–1.20) | 0.88 (0.78–0.99) | 1.08 (0.86–1.35) |
| Three | 1.24 (1.02–1.53) | 1.15 (0.93–1.41) | 0.62 (0.53–0.72) | 0.88 (0.66–1.18) |
| Outpatient treatment setting Intensive |
Reference | Reference | Reference | Reference |
| Non-intensive | 1.74 (1.42–2.12) | 1.84 (1.50–2.26) | 3.00 (2.18–4.11) | 2.44 (1.77–3.35) |
Among treatment episodes involving MOUD, being 34–54 years of age (AOR 3.60; 95% CI, 3.07–4.23) or older than 55 years old (AOR 10.34; 95% CI, 8.64–12.36) was associated with higher odds of mortality than those 18–34 years of age. Among those who used MOUD, sex was not associated with fatality. Compared to treatment provided to white-identifying patients, being Black (AOR, 1.00, 95% CI, 0.85–1.17) or another race (AOR 1.03, 95% CI 0.84–1.23) was not associated with higher odds of mortality among treatments involving MOUD. Unemployment (AOR 1.35; 95% CI, 1.11–1.64) was associated with increased odds of mortality. Compared to receiving treatment in the Northeast, receiving treatment in the Midwest (AOR 0.74; 95% CI, 0.60–0.90) and the South (AOR, 0.74; 95% CI 0.60–0.92) resulted in lower odds of death. Unlike those who did not receive MOUD, heroin use was not associated with higher odds of fatality (AOR, 0.95; 95% CI, 0.80–1.12). Reporting intravenous drug use at admission also was not associated with fatality. Additionally, having previously been in treatment was associated with an increased odds of fatality among those who were treated with MOUD (AOR, 1.40; 95% CI, 1.21–1.63). Finally, compared to treatment in intensive facilities, receiving treatment in a non-intensive facility was associated with a two-fold increase in the odds of fatality (AOR 2.44; 95% CI, 1.77–3.35).
For both treatment episodes that did and did not involve MOUD, limiting the sample to treatment episodes that lasted for less than a year resulted in some changes in the magnitude and association of observed estimates, shown in Table 4. Among non-MOUD treatment less than a year in duration, compared to being 18–34, being 55 or older was still associated with fatality in treatment (AOR, 2.50; 95% CI, 1.72–3.61), but being 35–54 years of age was not associated with increased odds of fatality (AOR, 1.18; 95% CI, 0.96–1.45). Male sex and receiving treatment in the South and West compared to the Northeast were associated with decreased odds of fatality with treatment. Reporting intravenous drug and heroin use at admission and having a previous treatment episode resulted in significant increases in the odds of fatality in treatment. Compared to receiving treatment in an intensive setting, receiving treatment in a non-intensive setting resulted in a 67% increase in the odds of experiencing fatality in treatment (AOR, 1.67; 95% CI, 1.35–2.07).
Table 4.
Factors associated with fatality during outpatient opioid use disorder treatment within a year of treatment at a publicly funded treatment facilities in 2016.
| Characteristic | Non-MOUD |
MOUD |
||
|---|---|---|---|---|
| Unadjusted odds OR (95% CI) | Adjusted odds OR (95% CI) | Unadjusted odds OR (95% CI) | Adjusted odds OR (95% CI) | |
| Age | Reference | Reference | Reference | Reference |
| 18–34 | Reference | Reference | Reference | Reference |
| 35–54 | 1.03 (0.84–1.26) | 1.18 (0.96–1.45) | 2.17 (1.73–2.74) | 2.20 (1.73–2.78) |
| 55 or older | 1.92 (1.35–2.72) | 2.50 (1.72–3.61) | 7.37 (5.79–9.40) | 7.42 (5.68–9.67) |
| Sex Female |
Reference | Reference | Reference | Reference |
| Male | 1.60 (1.31–1.94) | 1.49 (1.22–1.82) | 1.24 (1.02–1.50) | 1.12 (0.91–1.36) |
| Race White |
Reference | Reference | Reference | Reference |
| Black | 0.69 (0.47–1.00) | 0.60 (0.40–0.90) | 1.52 (1.19–1.94) | 0.75 (0.57–1.00) |
| Other | 0.63 (0.44–0.92) | 0.58 (0.38–0.87) | 0.86 (0.65–1.13) | 0.62 (0.43–0.89) |
| Ethnicity Non-Hispanic |
Reference | Reference | Reference | Reference |
| Hispanic | 0.90 (0.64–1.26) | 0.99 (0.67–1.44) | 0.99 (0.77–1.27) | 0.99 (0.71–1.37) |
| Region Northeast |
Reference | Reference | Reference | Reference |
| Midwest | 0.98 (0.79–1.22) | 1.12 (0.88–1.39) | 0.92 (0.68–1.25) | 0.89 (0.66–1.22) |
| South | 0.40 (0.31–0.52) | 0.48 (0.38–0.64) | 1.03 (0.77–1.37) | 1.03 (0.76–1.40) |
| West | 0.56 (0.41–0.77) | 0.60 (0.44–0.83) | 1.13 (0.91–1.40) | 0.97 (0.77–1.23) |
| Education College graduate |
Reference | Reference | Reference | Reference |
| Some college | 1.00(0.67–1.50) | 0.95 (0.63–1.43) | 0.65 (0.43–1.00) | 0.72 (0.47–1.10) |
| Less than college | 0.70 (0.48–1.02) | 0.68 (0.47–1.00) | 0.74 (0.50–1.08) | 0.77 (0.53–1.14) |
| Employment Full time |
Reference | Reference | Reference | Reference |
| Part time | 0.90 (0.62–1.30) | 0.98 (0.68–1.42) | 1,26 (0.77–2.05) | 1.31 (0.81–2.13) |
| Unemployed | 0.82 (0.65–1.04) | 0.94 (0.75–1.20) | 1.90 (1.37–2.63) | 1.64 (1.17–2.30) |
| Living arrangement Dependent living |
Reference | Reference | Reference | Reference |
| Homeless | 0.72 (0.45–1.12) | 0.77 (0.47–1.17) | 1.16 (0.77–1.77) | 1.00 (0.66–1.53) |
| Independent living | 1.06 (0.84–1.35) | 1.17 (0.91–1.50) | 1.21 (0.91–1.61) | 1.14 (0.84–1.52) |
| Previous treatment episode No |
Reference | Reference | Reference | Reference |
| Yes | 1.56 (1.26–1.94) | 1.14 (0.90–1.42) | 1.58 (1.24–2.02) | 1.57 (1.22–2.01) |
| Previous arrests None |
Reference | Reference | Reference | Reference |
| One or more | 1.21 (0.87–1.66) | 1.17 (0.85–1.61) | 0.76 (0.47–1.21) | 0.92 (0.57–1.48) |
| Intravenous drug use reported at admission No |
Reference | Reference | Reference | Reference |
| Yes | 1.81 (1.49–2.18) | 1.52 (1.22–1.90) | 0.95 (0.79–1.15) | 1.02 (0.82–1.27) |
| Primary substance reported at admission Other opioid |
Reference | Reference | Reference | Reference |
| Heroin | 1.91 (1.54–2.36) | 1.48 (1.15–1.90) | 1.07 (0.85–1.35) | 0.97 (0.73–1.27) |
| Number of substances reported at admission One |
Reference | Reference | Reference | Reference |
| Two | 1.03 (0.82–1.30) | 1.00 (0.79–1.26) | 0.96 (0.78–1.18) | 1.04 (0.85–1.28) |
| Three | 1.27 (1.02–1.59) | 1.14 (0.91–1.43) | 0.71 (0.56–0.92) | 0.84 (0.68–1.16) |
| Outpatient treatment setting Intensive |
Reference | Reference | Reference | Reference |
| Non-intensive | 1.56 (1.26–1.92) | 1.67 (1.35–2.07) | 1.55 (1.08–2.22) | 1.40 (0.97–2.02) |
For MOUD treatment of less than a year of duration, age and having had previous treatment episode for OUD remained associated with increased odds of fatality during treatment. Compared to treatment episodes belonging to people classified as White, those belonging to people classified as belonging to another race had decreased odds of fatality (AOR, 0.62; 95%CI, 0.43–0.89). In this model, receiving treatment at a non-intensive treatment was not associated with increased odds of fatality during treatment (AOR, 1.40; 95% CI, 0.97–2.02).
4. Discussion
Overall, a little <1% of outpatient treatments in publicly funded treatment facilities ended in death. Our analysis demonstrated a wide array of treatment and demographic characteristics associated with increased odds of fatality. For many of the correlates of death during treatment, similar associations were found for those who did and did not receive MOUD despite the fact that the characteristics of the treatment groups varied significantly. However, non-White race was only significantly associated with decreased odds of fatality in non-MOUD treatment that lasted less than a year. Male sex and reporting intravenous drug use at admission were associated with fatality only for non-MOUD treatment. Another important difference observed was the magnitude of the association with receiving treatment in a non-intensive treatment setting. Most MOUD was given in non-intensive treatment settings; among treatment episodes involving MOUD, receiving treatment in a non-intensive setting was associated with a two to three-fold increase in odds of experiencing fatality compared to receiving treatment intensive facilities. Receiving treatment at a non-intensive treatment setting only resulted in a one to two-fold increase in odds of experiencing fatality for non-MOUD treatment.
Demographic and treatment characteristics varied considerably between treatment episodes that did and did not involve MOUD. Those receiving MOUD were more likely to belong to racial and ethnic minority groups, which is contrast with previous literature that has demonstrated reduced MOUD availability in communities of color (Hansen, Siegel, Wanderling, & DiRocco, 2016; Lagisetty, Ross, Bohnert, Clay, & Maust, 2019). However, as TEDS-D does not include those receiving office-based buprenorphine, it is possible that a large proportion of people prescribed buprenorphine (many of whom may be white), are not captured in the TEDS-D data (Hadland et al., 2017; Stein et al., 2018). Additionally, the decreased odds of experiencing fatality among people of color may reflect the fact that people belonging to racial and ethnic minorities may be less likely to access any kind of treatment, not just MOUD (Hansen et al., 2016; Liebling et al., 2016). There was also significant geographic variation for those receiving MOUD. Findings from this study are consistent with previous scholarship that has found that the Northeast has greater MOUD capacity and engagement than other regions in the US (Hand, Short, & Abatemarco, 2017; Jones et al., 2015). Our finding that age was associated with both MOUD use and fatality during treatment is in contrast to much of the literature. In this sample, treatment episodes involving older adults were associated with fatality across both MOUD and non-MOUD use regardless of treatment duration. Previous research has yielded mixed results as to whether age is associated with treatment success and adherence or is a risk factor for mortality in treatment; while some studies have found that aging is protective against overdose (Boscarino et al., 2010; Satre, Mertens, Areán, & Weisner, 2004; Stahler et al., 2016), others have found the contrary (Merrall et al., 2012; Pierce, Bird, Hickman, & Millar, 2015). Deaths in this sample are due to all causes, and therefore, older adults might be subjected to age-related comorbidity and fatality. Further, the increased odds of fatality in treatment we observed may reflect the rise in overdose rates seen in older adults over the past few years (Gomes et al., 2018; Rudd, 2016).
It is important to note that MOUD has overall been found to reduce overdose mortality and prevent recurrent illicit opioid use (Larochelle et al., 2018; Ma et al., 2018; Otiashvili et al., 2013), yet a larger percentage of the fatalities were experienced by those whose treatment involved MOUD. This could be attributed to a number of factors. A majority of MOUD episodes took place in non-intensive treatment settings, which may offer decreased client engagement and supervision than intensive treatment settings (Center for Substance Abuse Treatment, 2006). According to the guidelines set by the American Society of Addiction Medicine (ASAM), patients with active drug or alcohol use, as well as those with co-occurring psychological disorders, may require a higher level of care due to medical instability and lack of oversight of care (Kampman & Jarvis, 2015). The TEDS-D data set does not include information about OUD severity or co-occurring mental health conditions, so we are unable to adjust for this in our study models. MOUD has been found to be most successful when used in conjunction with psychological interventions and social support, like family or group-based therapy, which may more likely to be found in intensive treatment settings (Duber et al., 2018; Kermack, Flannery, Tofighi, McNeely, & Lee, 2017; Veilleux et al., 2010). Though TEDS-D does not record details of what occurs in each treatment episode, based on the definition of intensive treatment, it is likely that those receiving MOUD at an intensive setting may also be receiving psychosocial therapy; however, it is unclear whether those in non-intensive settings are receiving combined pharmacological and behavioral therapies (McCarty et al., 2014). Regardless, MOUD has been shown to have benefit on its own with and without behavioral counseling. While behavioral counseling may be beneficial in combination with MOUD, to minimize barriers to MOUD, it should not be a requirement (Amato, Minozzi, Davoli, & Vecchi, 2011; Fiellin et al., 2006).
Treatment episodes belonging to people with higher risk drug use characteristics (e.g., injection drug use) were more like to experience mortality during treatment. In non-MOUD treatment, reporting heroin use and intravenous drug use at admission was positively associated with mortality. Consistent with our findings, previous research has found that those who injected drugs were more likely to experience higher rates of fatality (Mathers et al., 2013; Pierce et al., 2016). This may be explained by the increased likelihood of experiencing either a fatal or non-fatal overdose after abrupt treatment discontinuation among those who inject drugs compared to other methods of drug administration (Liebling, Green, Hadland, & Marshall, 2018; Mitra, Wood, Nguyen, Kerr, & DeBeck, 2015; Smolina et al., 2018). Similarly, when compared to non-medical use of a prescription opioids, heroin use is associated with increased likelihood of overdose (Compton, Jones, & Baldwin, 2016; Rudd, 2016; Seth, Scholl, Rudd, & Bacon, 2018). It follows that those treatment episodes belonging to people with these higher risk drug use practices would also be more likely to experience mortality during treatment. As the presence of fentanyl contamination in the heroin supply and overall fentanyl use has become more widespread (Colon-Berezin, 2019), there has been increased risk of fentanyl exposure among individuals who use heroin (Kenney, Anderson, Conti, Bailey, & Stein, 2018). Treatment facilities should offer harm reduction strategies, such as fentanyl test strips and naloxone distribution and training, to mitigate the risk of overdose and overdose death in the event of return to injection drug use (Goldman et al., 2019; Krieger et al., 2018; Latkin, Dayton, Davey-Rothwell, & Tobin, 2019).
4.1. Limitations
This study has a number of limitations. Due to differences in how discharge forms are administered state-by-state, treatment and demographic characteristics such as insurance status, primary source of payment for treatment, days waiting to enter treatment, and attending a self-help group in the days preceding admission or following a discharge, were not captured consistently across all states and thus could not be examined in this national study due to large amounts of missing data. Additionally, behavioral covariates were only captured at admission and were not updated throughout treatment. Characteristics of treatment episodes that involved MOUD may be less accurate than those belonging to other treatment episodes due to the fact those treatment episodes were generally longer in duration and characteristics may have changed over time. It should also be noted that MOUD use, as well as a number of characteristics that significantly predicted fatality in treatment that required imputation such as sex, race, and ethnicity, did require multiple imputation.
In discussing implications of MOUD use and fatality during treatment, it is pivotal to assess differences in types of treatment provided. There are inherent differences between buprenorphine and methadone use in treatment as well as these patient populations and mortality in treatment (Kimber, Larney, Hickman, Randall, & Degenhardt, 2015). Being able to distinguish which type of treatment a patient receives would provide greater insight into correlates of fatality experienced during treatment. As cause of death was not reported in this dataset, there is no way to know whether fatalities during treatment were related to overdose, substance use, or other causes generally. Other large cohort studies that have assessed mortality among those seeking treatment for opioid use disorder found that overdose accounted for up to 50% of fatalities (Degenhardt et al., 2009; Pierce et al., 2015). Similarly, one recent population-based study in Massachusetts examining the effect of MOUD on mortality after a non-fatal opioid overdose, approximately 46% of observed deaths were attributed to unintentional opioid overdose (Larochelle, Stopka, Xuan, Liebschutz, & Walley, 2019). Significant underreporting of opioid overdose deaths has been noted in vital statistics data (Horon, Singal, Fowler, & Sharfstein, 2018; Lowder, Ray, Huynh, Ballew, & Watson, 2018; Slavova et al., 2019). One enhanced surveillance technique found nearly doubles the number of opioid overdoses than those identified by death certificates alone (Horon et al., 2018). Given this underreporting, many researchers also examine all-cause mortality in addition to opioid overdose deaths as a primary endpoint (Larochelle et al., 2019; Leece et al., 2019). Improving vital statistics data collection is key not only to improve opioid overdose surveillance but also our public health response (Horon et al., 2018).
Methods to control for the association of duration of therapy and mortality were limited due to the way that time was captured in this data set (i.e., as a categorical variables). Nonetheless, we used pooled logistic regression to control for the risk of mortality across these discrete time frames. Records within TEDS-D are at the treatment episode level, which means that multiple observations may come from the same individual and may have resulted in the over-estimation of the standard errors of the observed estimates. We tried to adjust for this by including previous treatment as a covariate in the model; however, we acknowledge that without being able to cluster by the individual, there will be residual bias. Finally, TEDS-D does not include records from private or office-based treatment; therefore, the results of this study cannot be generalized to these settings.
5. Conclusions
In this national study of outpatient treatment for OUD, we found a number of demographic and treatment setting characteristics that were associated with fatality while undergoing OUD treatment; these include age, race, sex, introvenous drug use and treatment setting. Despite the fact that MOUD is the most effective treatment for opioid use disorder, it was involved in less than half of treatment episodes. As treatment capacity expands, policy makers and those operating facilities should ensure optimal treatment and safety for all patients regardless of their drug use behaviors and demographic characteristics, and work to increase access and uptake of MOUD. Datasets with more granularity about treatment provided and individual outcomes should be used to get a better understanding of other factors that may contribute to fatality.
Acknowledgements
We would like to thank Yu Li, Maxwell Krieger, and Sarah Bessey for their support.
Funding
This work is supported by the Center for Biomedical Research Excellence (COBRE) on Opioids and Overdose at Rhode Island Hospital (funded by the National Institute of General Medical Sciences, Award: P20-GM125507; Principal Investigator: Josiah D. Rich, MD).
Footnotes
Declaration of competing interest
The authors do not have any interests to declare.
References
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, & Park-Lee E. (2017). Key substance use and mental health indicators in the United States. Retrieved from Substance Abuse and Mental Health Services Administration website https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf.
- Alderks CE (2017). Trends in the use of methadone, buprenorphine, and extendedrelease naltrexone at substance abuse treatment facilities: 2003–2015 (update). Retrieved from Substance Abuse and Mental Health Services Administration website https://www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.html. [PubMed]
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, & Mattick RP (2005). An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment, 28(4), 321–329. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M, & Vecchi S. (2011). Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database of Systematic Reviews, 9, CD005031. [DOI] [PubMed] [Google Scholar]
- Bhatraju EP, Grossman E, Tofighi B, McNeely J, DiRocco D, Flannery M, ... Lee JD (2017). Public sector low threshold office-based buprenorphine treatment: Outcomes at year 7. Addiction Science & Clinical Practice, 12(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Mannelli P, Yu M, Nangia N, Graham CE, Tompkins DA, ... Sullivan MA (2018). Outpatient transition to extended-release injectable naltrexone for patients with opioid use disorder: A phase 3 randomized trial. Drug and Alcohol Dependence, 187, 171–178. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, & Stewart WF (2010). Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction, 105(10), 1776–1782. [DOI] [PubMed] [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K. (2011). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, Articles, 45(3), 1–67. [Google Scholar]
- Carroll KM, & Weiss RD (2017). The role of behavioral interventions in buprenorphine maintenance treatment: A review. The American Journal of Psychiatry, 174(8), 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, & Substance Abuse and Mental Health Services Administration (2018). 2016 TEDS annual report. Retrieved from https://www.samhsa.gov/data/sites/default/files/2016_Treatment_Episode_Data_Set_Annual_Revised.pdf.
- Center for Substance Abuse Treatment (2006). Chapter 4. Services in Intensive Outpatient Treatment Programs. Substance Abuse and Mental Health Services Administration (US). [PubMed] [Google Scholar]
- Clausen T, Anchersen K, & Waal H. (2008). Mortality prior to, during and after opioid maintenance treatment (OMT): A national prospective cross-registry study. Drug and Alcohol Dependence, 94(1–3), 151–157. [DOI] [PubMed] [Google Scholar]
- Colon-Berezin C. (2019). Overdose deaths involving fentanyl and fentanyl analogs — New York City, 2000–2017. MMWR. Morbidity and Mortality Weekly Report, 68 10.15585/mmwr.mm6802a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Jones CM, & Baldwin GT (2016). Relationship between nonmedical prescription-opioid use and heroin use. The New England Journal of Medicine, 374(2), 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congressional Budget Office (2018). Estimated direct spending and revenue effects of H.R. 6, substance use-disorder prevention that promotes opioid recovery and treatment (SUPPORT) for patients and communities act. Retrieved from https://www.cbo.gov/system/files?file=2018-09/hr6ConferenceSept27.pdf. [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry, 23(2), 63–75. [DOI] [PubMed] [Google Scholar]
- Cornish R, Macleod J, Strang J, Vickerman P, & Hickman M. (2010). Risk of death during and after opiate substitution treatment in primary care: Prospective observational study in UK General Practice Research Database. BMJ, 341, c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, & Green JL (2015). Trends in opioid analgesic abuse and mortality in the United States. The New England Journal of Medicine, 372(3), 241–248. [DOI] [PubMed] [Google Scholar]
- Davis CS (2018). The SUPPORT for patients and communities act - what will it mean for the opioid-overdose crisis? The New England Journal of Medicine. 10.1056/NEJMp1813961. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, & McLaren J. (2011). Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction, 106(1), 32–51. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Randall D, Hall W, Law M, Butler T, & Burns L. (2009). Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug and Alcohol Dependence, 105(1–2), 9–15. [DOI] [PubMed] [Google Scholar]
- Duber HC, Barata IA, Cioè-Peña E, Liang SY, Ketcham E, Macias-Konstantopoulos W, ... Whiteside LK (2018). Identification, management, and transition of care for patients with opioid use disorder in the emergency department. Annals of Emergency Medicine, 72(4), 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, & Schottenfeld RS (2006). Counseling plus buprenorphine–naloxone maintenance therapy for opioid dependence. The New England Journal of Medicine, 355(4), 365–374. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Waye KM, Periera KA, Krieger MS, Yedinak JL, & Marshall BDL (2019). Perspectives on rapid fentanyl test strips as a harm reduction practice among young adults who use drugs: A qualitative study. Harm Reduction Journal, 16(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Tadrous M, Mamdani MM, Paterson JM, & Juurlink DN (2018). The burden of opioid-related mortality in the United States. JAMA Network Open, 1(2), e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, & Larochelle MR (2017). Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001–2014. JAMA Pediatrics, 171(8), 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand DJ, Short VL, & Abatemarco DJ (2017). Substance use, treatment, and demographic characteristics of pregnant women entering treatment for opioid use disorder differ by United States census region. Journal of Substance Abuse Treatment, 76, 58–63. [DOI] [PubMed] [Google Scholar]
- Hansen H, Siegel C, Wanderling J, & DiRocco D. (2016). Buprenorphine and methadone treatment for opioid dependence by income, ethnicity and race of neighborhoods in New York City. Drug and Alcohol Dependence, 164, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk KF, Vaca FE, & D’Onofrio G. (2015). Reducing fatal opioid overdose: Prevention, treatment and harm reduction strategies. The Yale Journal of Biology and Medicine, 88(3), 235–245. [PMC free article] [PubMed] [Google Scholar]
- Horon IL, Singal P, Fowler DR, & Sharfstein JM (2018). Standard death certificates versus enhanced surveillance to identify heroin overdose-related deaths. American Journal of Public Health, 108(6), 777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, & McCance-Katz E. (2015). National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health, 105(8), e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, & Jarvis M. (2015). American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. Journal of Addiction Medicine, 9(5), 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney SR, Anderson BJ, Conti MT, Bailey GL, & Stein MD (2018). Expected and actual fentanyl exposure among persons seeking opioid withdrawal management. Journal of Substance Abuse Treatment, 86, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack A, Flannery M, Tofighi B, McNeely J, & Lee JD (2017). Buprenorphine prescribing practice trends and attitudes among New York providers. Journal of Substance Abuse Treatment, 74, 1–6. [DOI] [PubMed] [Google Scholar]
- Kimber J, Larney S, Hickman M, Randall D, & Degenhardt L. (2015). Mortality risk of opioid substitution therapy with methadone versus buprenorphine: A retrospective cohort study. The Lancet. Psychiatry, 2(10), 901–908. [DOI] [PubMed] [Google Scholar]
- Krieger MS, Goedel WC, Buxton JA, Lysyshyn M, Bernstein E, Sherman SG, & Marshall BDL (2018). Use of rapid fentanyl test strips among young adults who use drugs. The International Journal on Drug Policy. 10.1016/j.drugpo.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty PA, Ross R, Bohnert A, Clay M, & Maust DT (2019). Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 10.1001/jamapsychiatry.2019.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, ... Walley, A. Y. (2018). Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Annals of Internal Medicine, 169(3), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Stopka TJ, Xuan Z, Liebschutz JM, & Walley AY (2019). Medication for opioid use disorder after nonfatal opioid overdose and mortality. Annals of Internal Medicine, 170, 430. 10.7326/l18-0685. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Dayton L, Davey-Rothwell MA, & Tobin KE (2019). Fentanyl and drug overdose: Perceptions of fentanyl risk, overdose risk behaviors, and opportunities for intervention among people who use opioids in Baltimore, USA. Substance use & Misuse (pp. 1–9). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leece P, Chen C, Manson H, Orkin AM, Schwartz B, Juurlink DN, & Gomes T. (2019). One-year mortality after emergency department visit for nonfatal opioid poisoning: A population-based analysis. Annals of Emergency Medicine. 10.1016/j.annemergmed.2019.07.021. [DOI] [PubMed] [Google Scholar]
- Liebling EJ, Green TC, Hadland SE, & Marshall BDL (2018). Injection drug use and overdose among young adults who use prescription opioids non-medically. Addictive Behaviors, 76, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebling EJ, Yedinak JL, Green TC, Hadland SE, Clark MA, & Marshall BDL (2016). Access to substance use treatment among young adults who use prescription opioids non-medically. Substance Abuse Treatment, Prevention, and Policy, 11(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowder EM, Ray BR, Huynh P, Ballew A, & Watson DP (2018). Identifying unreported opioid deaths through toxicology data and vital records linkage: Case study in Marion County, Indiana, 2011–2016. American Journal of Public Health, 108, 1682–1687. 10.2105/ajph.2018.304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Bao Y-P, Wang R-J, Su M-F, Liu M-X, Li J-Q, ... Lu L. (2018). Effects of medication-assisted treatment on mortality among opioids users: A systematic review and meta-analysis. Molecular Psychiatry. 10.1038/s41380-0180094-5. [DOI] [PubMed] [Google Scholar]
- Marcovitz DE, McHugh RK, Volpe J, Votaw V, & Connery HS (2016). Predictors of early dropout in outpatient buprenorphine/naloxone treatment. The American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions, 25(6), 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, & Hickman M. (2013). Mortality among people who inject drugs: A systematic review and meta-analysis. Bulletin of the World Health Organization, 91(2), 102–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D, Braude L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, & Delphin-Rittmon ME (2014). Substance abuse intensive outpatient programs: Assessing the evidence. Psychiatric Services, 65(6), 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan C, Kidd B, & Fahey T. (2009). Factors associated with mortality in Scottish patients receiving methadone in primary care: Retrospective cohort study. BMJ, 338, b2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhofer A, & Witman AE (2018). The role of health insurance on treatment for opioid use disorders: Evidence from the affordable care act Medicaid expansion. Journal of Health Economics, 60, 177–197. [DOI] [PubMed] [Google Scholar]
- Mennis J, Stahler GJ, El Magd SA, & Baron DA (2019). How long does it take to complete outpatient substance use disorder treatment? Disparities among Blacks, Hispanics, and Whites in the US. Addictive Behaviors, 93, 158–165. [DOI] [PubMed] [Google Scholar]
- Merrall ELC, Bird SM, & Hutchinson SJ (2012). Mortality of those who attended drug services in Scotland 1996–2006: Record-linkage study. The International Journal on Drug Policy, 23(1), 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra G, Wood E, Nguyen P, Kerr T, & DeBeck K. (2015). Drug use patterns predict risk of non-fatal overdose among street-involved youth in a Canadian setting. Drug and Alcohol Dependence, 153, 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, ... Murray, C. J. L. (2018). The state of US health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA: The Journal of the American Medical Association, 319(14), 1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeger S, Mutter R, Ali MM, Mark T, & Hughey L. (2016). Post-discharge treatment engagement among patients with an opioid-use disorder. Journal of Substance Abuse Treatment, 69, 64–71. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, & Committee on Medication-Assisted Treatment for Opioid Use Disorder. (2019). Medications for opioid use disorder save lives. National Academies Press. [PubMed] [Google Scholar]
- Ngwa JS, Cabral HJ, Cheng DM, Pencina MJ, Gagnon DR, LaValley MP, & Cupples LA (2016). A comparison of time dependent Cox regression, pooled logistic regression and cross sectional pooling with simulations and an application to the Framingham heart study. BMC Medical Research Methodology, 16(1), 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, & Woody GE (2013). Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior–outcomes of a randomized trial. Drug and Alcohol Dependence, 133(2), 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Bird SM, Hickman M, Marsden J, Dunn G, Jones A, & Millar T. (2016). Impact of treatment for opioid dependence on fatal drug-related poisoning: A national cohort study in England. Addiction, 111(2), 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Bird SM, Hickman M, & Millar T. (2015). National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005–2009. Drug and Alcohol Dependence, 146, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA (2016). Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR. Morbidity and Mortality Weekly Report, 65, 50–51. Retrieved from https://www.cdc.gov/mmwr/volumes/65/wr/mm655051e1.htm. [DOI] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, & Goldstein RB (2016). Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. The Journal of Clinical, 76(6), 772–780. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555044/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Areán JR, & Weisner PA (2004). Five-year alcohol and drug treatment outcomes of older adults versus middle-aged and younger adults in a managed care program. Addiction, 99(10), 1286–1297. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (2016). Treatment of opioid-use disorders. The New England Journal of Medicine, 375(4), 357–368. [DOI] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, & Bacon S. (2018). Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. MMWR. Morbidity and Mortality Weekly Report, 67(12), 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavova S, Delcher C, Buchanich JM, Bunn TL, Goldberger BA, & Costich JF (2019). Methodological complexities in quantifying rates of fatal opioid-related overdose. Current Epidemiology Reports, 6(2), 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolina K, Crabtree A, Chong M, Zhao B, Park M, Mill C, & Schütz CG (2018). Patterns and history of prescription drug use among opioid-related drug overdose cases in British Columbia, Canada, 2015–2016. Drug and Alcohol Dependence. 10.1016/j.drugalcdep.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Sokol R, LaVertu AE, Morrill D, Albanese C, & Schuman-Olivier Z. (2018). Group-based treatment of opioid use disorder with buprenorphine: A systematic review. Journal of Substance Abuse Treatment, 84, 78–87. [DOI] [PubMed] [Google Scholar]
- Stahler GJ, Mennis J, & DuCette JP (2016). Residential and outpatient treatment completion for substance use disorders in the U.S.: Moderation analysis by demographics and drug of choice. Addictive Behaviors, 58, 129–135. [DOI] [PubMed] [Google Scholar]
- Stein BD, Dick AW, Sorbero M, Gordon AJ, Burns RM, Leslie DL, & Pacula RL (2018). A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Substance Abuse: Official Publication of the Association for Medical Education and Research in Substance Abuse, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2014). Treatment Episode Data Set-Discharges (TEDS-D-2014-DS0001) [Data set]. Retrieved from https://www.datafiles.samhsa.gov/study-series/treatment-episode-data-set-discharges-teds-d-nid13520. [Google Scholar]
- Substance Use and Mental Health Services Administration (2018). 2016 TEDS-D Codebook. Retrieved from https://wwwdasis.samhsa.gov/dasis2/teds_pubs/TEDS/Discharges/TEDS_D_2016/2016_teds_d_codebook.pdf.
- Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act (2018). [Google Scholar]
- Treatment Episode Data Set-Discharges (TEDS-D). (2016). [Data set]. Rockville, MD. [Google Scholar]
- Tsui JI, Burt R, Thiede H, & Glick SN (2018). Utilization of buprenorphine and methadone among opioid users who inject drugs. Substance Abuse: Official Publication of the Association for Medical Education and Research in Substance Abuse, 39(1), 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S Department of Health and Human Services (2018). Strategy to combat opioid abuse, misuse, and overdose: A framework based on the five point strategy. Retrieved from https://www.hhs.gov/opioids/sites/default/files/2018-09/opioid-fivepoint-strategy20180917-508compliant.pdf.
- Veilleux JC, Colvin PJ, Anderson J, York C, & Heinz AJ (2010). A review of opioid dependence treatment: Pharmacological and psychosocial interventions to treat opioid addiction. Clinical Psychology Review, 30(2), 155–166. [DOI] [PubMed] [Google Scholar]
- Wickramatilake S, Zur J, Mulvaney-Day N, Klimo MC, Selmi E, & Harwood H. (2017). How states are tackling the opioid crisis. Public Health Reports, 132(2), 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-T, Zhu H, & Swartz MS (2016). Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence, 169, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. (2016). Multiple imputation with multivariate imputation by chained equation (MICE) package. Annals of Translational Medicine, 4(2), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Friedmann PD, & Gerstein DR (2003). Does retention matter? Treatment duration and improvement in drug use. Addiction, 98(5), 673–684. [DOI] [PubMed] [Google Scholar]