Abstract
Integrin Alpha v Beta 6 is expressed primarily in solid epithelial tumors, such as cholangiocarcinoma, pancreatic cancer, and colorectal cancer. It has been considered a potential and promising molecular marker for the early diagnosis and treatment of cancer. Cholangiocarcinoma and pancreatic ductal adenocarcinoma share genetic, histological, and pathophysiological similarities due to the shared embryonic origin of the bile duct and pancreas. These cancers share numerous clinicopathological characteristics, including growth pattern, poor response to conventional radiotherapy and chemotherapy, and poor prognosis. This review focuses on the role of integrin Alpha v Beta 6 in cancer progression. It addition, it reviews how the marker can be used in molecular imaging and therapeutic targets. We propose further research explorations and questions that need to be addressed. We conclude that integrin Alpha v Beta 6 may serve as a potential biomarker for cancer disease progression and prognosis.
Keywords: integrin αvβ6, cholangiocarcinoma, pancreatic ductal adenocarcinoma, imaging, therapeutic target, biomarker
Introduction
Cholangiocarcinoma (CCA) is an aggressive malignant tumor that originates in bile duct epithelium. It is a rare cancer that is classified based on its anatomic location within the bile duct tree, as follows: intrahepatic, perihilar, or distal CCA. Perihilar CCA (PHC) is the most common CCA type, with an annual incidence of approximately 2/100 000 in western countries. 1 The three CCA subtypes have different etiologies, risk factors, prognoses, and clinical therapeutic management. 2 CCA is highly fibroproliferative, supported by a dense tumor microenvironment (TME), and has significant genetic heterogeneity, which contributes to its therapeutic resistance. Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies, accounting for 80% to 90% of all pancreatic cancer cases. Despite its low incidence, PDAC is the seventh leading cause of global cancer mortality. 3 PDAC prognosis is poor due to its complicated and multifactorial nature and overlapping symptoms. The biliary tract and pancreas share a common embryonic origin 4 ; therefore, preneoplastic and neoplastic lesions exhibit similar molecular, histological, pathophysiological, and clinicopathological features. 5 In addition, these features contribute to the lack of reliable biomarkers and detection methods for early diagnosis. 6 Thus, the mortality-to-morbidity ratio of pancreatic cancer has not significantly changed in the past few decades. 7 The PDAC 5-year survival rate is less than 5%. 8
CCA and PDAC treatment options are limited due to the following reasons. In the early disease stage, nerve infiltration, peripheral tissue, and distant metastasis occur; and in the late disease stage, effective non-surgical treatment remains elusive. When feasible, hepatectomy is the preferred treatment for CCA, followed by systemic chemotherapy with capecitabine, 9 but only 30% of cases can be completely removed by surgery. Surgical intervention is classified into two types. One is extended hemi-hepatectomy combined with extrahepatic bile duct resection, which requires strict preoperative evaluation and patients to be in good condition. The other is minimal-invasive resection which has poor effect.10,11 In other cases, systemic chemotherapy with gemcitabine and cisplatin is usually the first-line treatment option, but the prognosis is still poor. 12 Whether neoadjuvant chemotherapy can improve the survival rate is still unclear. Liver transplantation (LT) can avoid an R1 resection and an inadequate future liver remnant (FLR), but the actual benefits of LT still need more evidence to support. In patients with very early iCCA, upfront LT may be beneficial, while for patients with late unresectable tumor, neoadjuvant chemoradiation is needed. 13
Targeted therapy, which can be used alone or in combination with chemotherapy, has been utilized as a second-line treatment to enhance patient survival. Many potential molecular targets have been discovered in recent years, including fibroblast growth factor receptor (FGFR), 14 epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), 15 metabolic regulators such as isocitrate dehydrogenase 1 and 2 (IDH1/2), BRAF, transcription factor Fos-like antigen-1 (FOSL1). 16
In addition, immunotherapy methods are also being studied. CCA is considered as “immune cold” due to its low and medium tumor mutation load. 17 Furthermore, the TME promotes immune escape and inhibits immune responses. In this case, immune checkpoint inhibitors against programmed cell death 1 (PD-1),18,19 programmed cell death ligand 1 (PD-L1), 20 cytotoxic T-lymphocyte antigen-4 (CTLA-4) and new therapeutic methods such as tumor vaccines 21 and chimeric antigen receptor (CAR) T-cells 22 can enhance antitumor activity.23–25
Chemotherapy and surgery are the main treatment options for PDAC. However, only 20% of patients were eligible for surgery at the time of diagnosis. 7 Most PDAC patients have distant metastasis at the time of diagnosis, and major surgery to remove the main primary lesion is unlikely to improve the prognosis. At the same time, patients must be healthy enough to undergo this big operation and recuperate from it. 26
In the past decade, two new combination therapies have become the first-line treatment for patients with advanced PDAC. The first is FOLFIRINOX, which stands for 5-fluorouracil (5-FU), leucovorin, irinotecan and oxaliplatin. The second is a combination of gemcitabine and an albumin nanoparticle conjugate of paclitaxel (nab-paclitaxel).27,28 Patients having surgery usually undergo adjuvant chemotherapy after surgery to eradicate PDAC cells that escape from the original tumor during resection. However, because many patients are unable to bear adjuvant treatment due to surgical complications, systemic preoperative (neoadjuvant) therapy is currently used.29,30 Studies have shown that neoadjuvant chemoradiotherapy increases overall survival (OS) when compared to postoperative adjuvant therapy. 31
Despite the progress of surgical methods and the emergence of various chemotherapy regimens, the poor prognosis of PDAC has not improved in the past decades, and there is an urgent need to explore new therapeutic strategies. Therefore, targeted therapy and immunotherapy came into being. The realization of targeted therapy for PDAC patients can be summarized into three methods. First, dysregulated oncogenes such as KRAS, NRG1 and NTRK and related molecules can be suppressed. Second, inactivated tumor suppressors or regulatory related molecules such as TP53, CDKN2A, and Smad4 can be reactivated. Finally, genes such as KDM6A and BRCA can maintain the structural stability and physiological functions of normal chromosomes, and have defects in some PDAC patients, so they can be used as potential targets to precisely eliminate these abnormal tumor cells. 32 In addition, immunotherapies such as CAR-T,33–36 antibody drug conjugates,37,38 and immune checkpoint inhibitors also show the potential to accurately target tumors. Due to the complex immunosuppressive TME of PDAC, tumors are protected from effective cytotoxic immune responses. Therefore, the therapeutic utility of immunotherapy as a PDAC treatment is still limited. 39
The future direction of precision oncology in CCA and PDAC will still focus on target genes and related signaling pathways, and its research will also aid in the identification of curable patient subgroups. Targeted therapy will certainly provide diverse therapeutic strategies for CCA and PDAC and improve their poor prognosis.
Targeted agents for FGFR2 fusions and IDH1 mutations have been developed. In addition, EGFR inhibitors have been combined with first-line chemotherapeutics. However, the treatment results have been poor. Research on CCA-related mutations within EGFR and BRAF has been conducted 40 ; however, the prevalence of these mutations in CCA is extremely low, which highlights the need for identification of novel therapies and therapeutic targets. There are ongoing trials investigating immune checkpoint inhibitor (ICI) monotherapy. So far, the results prove limited efficacy; however, in mismatch repair (MMR)-deficient and instable microsatellite (MSI)-high CCA, ICIs have proven effective. 41 Studies have suggested a link between BRCA1/2 mutations (BRCAm) and BTC response to ICI therapy. It is hypothesized that, in addition to BRCA, other DNA damage repair (DDR) pathway genes found in BTC may impact therapy response and should be further investigated. 42
Furthermore, the search for new biomarkers continues as they could potentially serve as markers for early cancer diagnosis, which could improve patient survival rates. Carbohydrate antigen 19-9 (CA-199) and carcino-embryonic antigen (CEA) are used as clinical markers for monitoring PDAC and CCA prognosis in patients following treatment. However, the markers are expressed in benign conditions such as pancreatitis and cholangitis; and increased expression is not observed in early disease. These markers, therefore, have low sensitivity and specificity.43,44 Prostate stem cell antigen (PSCA) expression is highly associated with PDAC, and labeled probes targeting PSCA on the surface of pancreatic cancer cells have been used for targeted imaging; however, studies of PSCA in CCA have been conducted. 45 Mucin genes 4 (MUC4) is overexpressed in the majority of pancreatic cancers, and the use of this marker would allow for the distinction between pancreatitis and pancreatic cancer. However, MUC4 is also overexpressed in pancreatic cysts. Recently, MUC4 has been identified as a novel tumor-associated antigen for pancreatic cancer immunotherapy.46,47 The expression of cytokeratin 7 (CK7) and cytokeratin 19 (CK19) is elevated in cancer, and their expression could be used to predict ICC prognosis. 48 Integrin Alpha v Beta 6 (αvβ6) is not expressed in islet, acinar or hepatocellular carcinoma (HCC) cells, but is expressed at low to moderate levels in the normal pancreatic duct and biliary duct epithelial cells, 49 as well as significantly in PDAC and CCA.50,51 Consequently, integrin αvβ6 represents a potential therapeutic target.
Integrins are a family of cell adhesion molecules composed of two non-covalently linked heterodimer subunits, α and β. Integrins mediate cell-cell and cell-extracellular matrix adhesion as well as regulate multiple signaling pathways that promote tumor cell proliferation, migration, survival, differentiation, invasion, and metastasis.52,53 Integrin αvβ6 was first reported in the 1990s. Among them, the αv subunit is encoded by the gene CD51, 54 which is located at 2q31-q32, while integrin β6 (ITGB6) subunit has been shown to be encoded by beta gene located at 2q24-q31. 55 The αv subunit binds to β1, β3, β5, β6 and β8 subunits, whereas the β6 subunit only binds to the αv subunit, which is highly specific. 52 Subunits αv and β6 contain three domains: extracellular domain, transmembrane domain and intracellular domain. The extracellular and transmembrane domains participate in the activation, adhesion and epithelial-mesenchymal transition (EMT) of transforming growth factor-β (TGF-β) through 11 amino acids at the carboxyl terminus, while the cytoplasmic domain affects proliferation, matrix metalloproteinases (MMPs) production, migration and survival.56–58
Unlike the large majority of integrins, αvβ6 is rarely expressed in healthy adult epithelial cells; however, it is upregulated during embryogenesis, tissue repair, and carcinogenesis. 59 Integrin αvβ6 is overexpressed in a variety of cancers, including cholangiocarcinoma, 50 and breast, 58 gastric, 60 pancreatic, 61 colorectal,62,63 ovarian, 64 and endometrial cancers. 65
It has been found that elevated integrin αvβ6 expression is associated with a poor cancer prognosis, which may be related to EMT, tumor cell invasion and increased metastatic potential. At present, for digestive system epithelial cancer, a large number of studies focus on the expression and role of integrin αvβ6 in colorectal cancer.66–68 However, some studies have found that almost all PDAC patients have integrins αvβ6 positive expression, 69 and some studies have shown that because integrin αvβ6 expression was significantly up-regulated in CCA tissues but not in adjacent non-tumor tissues or liver derived tumors, it can be used as an immunohistochemical marker for the diagnosis and differential diagnosis of CCA.50,70 Therefore, although limited research has been done on it, integrins αvβ6 may serve as potential molecular markers for the differentiation, diagnosis, treatment and prognosis of CCA and PDAC. These features will be reviewed in this essay.
Integrin αvβ6 Signaling Pathway in Promoting CCA and PDAC Progression
Integrin αvβ6 and TGF-β1
Recent studies on malignant tumors indicate that transforming growth factor-β1 (TGF-β1) may play an essential role in tumor genesis and development by promoting tumor angiogenesis, invasion, EMT, and immune escape.71–73
TGF-β receptor activation induces signal transduction via the formation of Smad complexes that are translocated to the nucleus, as well as via non-Smad pathways, including Erk 1/2, RAS and MAPK. 57 Smad-dependent signaling pathways can suppress tumors by inhibiting cell cycle activation, promoting epithelial cell apoptosis, and maintaining genomic integrity. 74 Alternatively, Smad4 mutations have been identified in PDAC and various types of CCA.75,76 TGF-β pathway mutations were discovered in greater than 50% of the patients with PDAC. Therefore, tumor inhibition is frequently lost due to the inactivation on Smad4-dependent TGF-β signaling.76–78
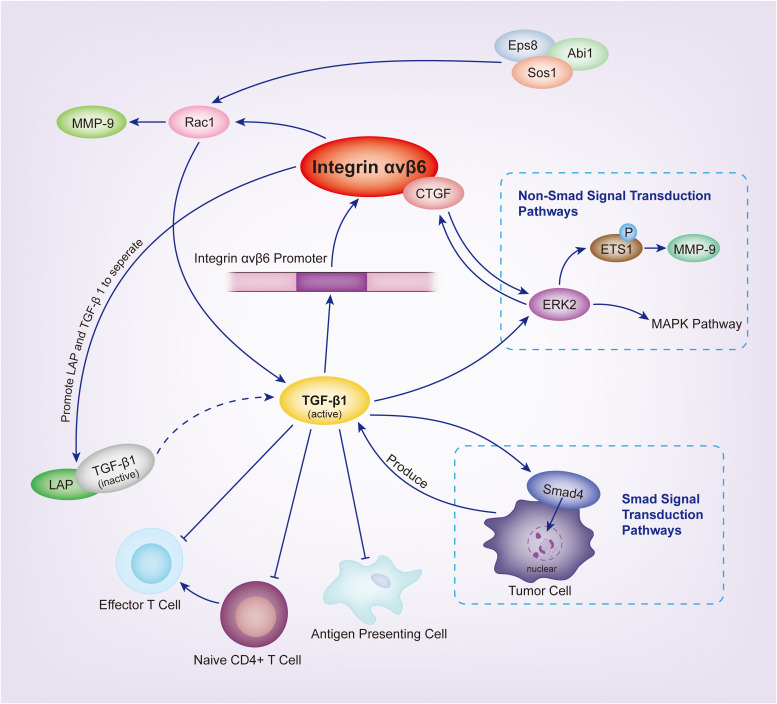
TGF-β1 binds to TGF-β1 LAP and forms an inactive complex. LAP-β1–binding to integrin αvβ6 participates in TGF-β1 maturation and activation. Activated TGF-β1 plays key roles in many activation pathways in vivo. 79 TGF-β1 also plays a vital role in the maintenance of integrin αvβ6 expression in epithelial cells and activates integrin αvβ6 transcription in the regulatory region of promoters, thereby increasing the integrin αvβ6 expression. 80 Integrin αvβ6 and TGF-β1 have a balanced relationship, and when this balance is disrupted, many diseases can occur. 81
Tumors evade the host immune response by secreting immunosuppressive cytokines into the TME, such as TGF-β1. Thepmalee 82 detected the TGF-β1 ligand in dendritic cells (DCs) supernatant and bile duct cancer cells. Other studies 83 have shown that TGF-β1 drives cell migration in CCA by inducing a transition from epithelial to mesenchymal cell phenotype without affecting cell proliferation; thus, increased TGF-β1 gene expression is associated with a poor prognosis of CCA. 84 Blocking the TGF-β1 receptor on DCs, with specific neutralizing antibodies, increases the cytolytic ability of DC-activated effector T cells. 82 Connective tissue growth factor (CTGF) is a cell-matrix protein that mediates cell-matrix interactions through various integrin receptor subtypes. CTGF and the integrin αvβ6 protein are highly expressed in the ductal reaction of human liver cirrhosis and cholangiocarcinoma. A study by Pi 85 discovered that integrin αvβ6 could bind to CTGF, mediate oval cell adhesion to CTGF and fibronectin matrices, and promote TGF-β1 activation in vitro.. 86 These results suggest that TGF-β1 is crucial for tumor evasion, and integrin αvβ6 may influence the TGF-β1 pathway to promote tumor progression.
The interaction between integrin αvβ6 and TGF-β1 merits additional research. An important research question could address how the balance between integrin αvβ6 and TGF-β1 and their expression could be maintained in Smad-deficient PDAC and CCA. In addition, research should focus on whether enhancing the Smad-dependent TGF-β1 signaling pathway in Smad-maintained PDAC and CCA inhibits tumor formation?
Integrin αvβ6 and Rac1
Rac1, a member of the small GTPase Rho family, is highly expressed in numerous cancer cell lines and is involved in various cellular processes, such as cytoskeletal recombination and gene transcription.87,88 Furthermore, Rac1 regulates several downstream effector molecules associated with tumor aggressiveness, such as MMP-9 and uPA, making it a central regulator of tumor malignancy.89,90
Tod 91 discovered that Eps8 overexpression promotes integrin αvβ6-dependent tumor invasion while inhibiting integrin αvβ6-dependent TGF-β1 activation in PDAC. The study revealed an inverse relationship between tumor cell migration expression and TGF-β1 activation, and this function could be affected by the presence or absence of Eps8. Eps8 regulates tumor invasiveness by Rac1 activation, which inhibits stress fiber formation and conformational alterations in the TGF-β1-LAP complex, thereby antagonizing the Rho pathway. The Eps8/Abi1/Sos1 tricomplex was discovered to be a crucial regulator of integrin αvβ6-dependent tumor cell function, acting as a molecular switch that alters the balance between Rac1 and Rho activation, thereby skewing cell function toward a pro-migration (RAC1-dependent) or pro-TGF -β1 activation (Rho-dependent) phenotype.
MMP-9 is the main collagenase in keratinocyte, and it is required for many biological processes, such as wound healing. 92 It can digest the cell surface protein of the extracellular domain structure, thereby reducing the number of cell adhesions and increasing cell motility. 93 Recent studies have established a link between MMP-9 and tumor invasion, metastasis, and angiogenesis. 94 Yang 95 observed high αvβ6 and MMP-9 reactively in invasive tumors at 73.7% and 76.5%, respectively. αvβ6 expression and MMP-9 secretion are enhanced in high cell densities. Since integrin β6 only forms a heterodimer with integrin αv, detecting the mRNA or protein β6 subunit will provide dimer information. 96 Li 70 demonstrated that integrin β6 promotes tumor migration and invasion by activating Rac1 and upregulating MMP-9 expression in bile duct cancer cells, thereby establishing the integrin β6/Rac1/MMP-9 pathway.
According to these findings, integrin αvβ6 can promote tumor cell migration via the Eps8 and MMP-9-involving Rac1 pathway.
Integrin αvβ6 and ERK2
Mitogen-activated protein kinase cascades, which include ERK, JNK and P38, are critical intracellular signaling pathways that control a wide range of cellular functions. 97 Ahmed 56 demonstrated that the cytoplasmic domain of integrin β6 directly binds to ERK2, thereby increasing MAP kinase activity. According to Li, 69 integrin β6 significantly promotes pancreatic cancer cell proliferation and invasion and induces ETS1 phosphorylation in an ERK-dependent manner, resulting in MMP-9 upregulation. The absence of ERK2 binding sites in the cytoplasmic domain of integrin β6 affects density-dependent expression of integrin β6 and inhibits integrin β6-mediated MMP-9 secretion, thereby inhibiting tumor growth. 98 It is well established that the MAP kinase pathway promotes cancer growth in vivo. It plays a vital role in tumor metastasis, and integrin αvβ6 promotes MAP kinase pathway activation. 99 Song 100 inhibited integrin αvβ6-mediated extracellular matrix degradation by inhibiting ERK activation, disrupted integrin αvβ6 internalization, and reduced cancer cell migration to fibronectin. 101 Additionally, Li 69 demonstrated that silencing integrin αvβ6 with small interfering RNA significantly inhibited the growth of pancreatic xenograft tumors in vivo, possibly via the ERK2 pathway.
EMT is considered a crucial process in tumor progression 102 and is closely associated with the systemic invasiveness of pancreatic tumors. 103 In non-Smad signaling responses, TGF-β activates the ERK–MAPK and other pathways to induce EMT.102,104,105 Meanwhile, the ERK/MAPK signaling pathway is essential for chemical and immune resistance in tumor cells. 106 CCA and PDAC are resistant to chemo- and radiotherapy and are prone to metastasis. Targeting integrin αvβ6 on ERK2 may provide a novel treatment option for CCA and PDAC.
Integrinβ6 and PODXL
PODXL2, a newly discovered member of the CD34 family, is a type I transmembrane sialic acid protein that functions as an L-selectin ligand. 107 The role of PODXL2 in cancer is unclear, and its regulatory pathway has not been identified. PODXL1, an additional member of the CD34 family member, is overexpressed in several cancers, including pancreatic cancer.108,109 It promotes cancer cell invasion by activating EMT in PDAC cells via the C5aR/C5a axis. 110 According to Soejima, 111 integrin β6 gene ITGB6 knock out significantly downregulates PODXL2 expression, which is significantly correlated with integrin β6 expression in CCA. Decreased PODXL2 expression inhibits the formation of intrahepatic cholangiocarcinoma (ICC) cells. It is further suggested that PODXL2 may be necessary to maintain the clonogenic potential of bile duct cancer cells. Currently, the relationship between integrin and PODXL is unknown. Studying the role of PODXL in the integrin β6 pathway may be a potential targeted therapy for CCA and PDAC (Figure 1).
Figure 1.
Interaction between integrin αvβ6 and the TGF-β1, ERK2, RAC1 signaling pathways.
Application of Integrin αvβ6 in Diagnosis, Treatment, and Prognosis of CCA and PDAC
Integrin αvβ6 as a Biomarker
As early as 2004, Sipos 112 studied gastrointestinal pancreatic cancer and found that all these cancer types overexpressed integrin αvβ6. The strongest expression was observed in PDAC and was not expressed in HCC and pancreatic neuroendocrine tumors. In gastrointestinal pancreatic cancer, integrin αvβ6 expression is limited to tumor cells. Steiger 113 conducted integrin αvβ6 immunohistochemical analysis on a large number of PDAC samples (383 primary tumors, 7 lymph nodes, 8 distant metastases, and 34 pancreatic intraepithelial neoplasia [PanIN]). The study found that integrin αvβ6 is highly expressed in primary PDAC foci (88%), metastatic foci (∼100%), and PanIN (57%). According to Patsenker et al, 50 integrin αvβ6 is largely expressed in hilar cholangiocarcinoma (HCCA, 87%) and ICC (88%), with similar expression intensity in the two types of CCA. In contrast, integrin αvβ6 expression has been observed in HCC. Integrin αvβ6 immunohistochemical analysis distinguishes CCA and HCC with high specificity. This contradicts Franken's 114 finding that HCCA expresses αvβ6 more significantly than ICC; but, the difference in integrin αvβ6 expression between HCCA and ICC was too low to distinguish. Soejima 115 discovered that integrin αvβ6 was highly expressed in majority of the CCA cells, including ICC, and αvβ6 expression was higher in non-peripheral and periductal infiltration or intraductal growth types compared to peripheral and mass formation types. Integrin αvβ6 expression is closely related to ICC subtypes.
In terms of traditional imaging modalities, HCC and ICC overlap and are hard to distinguish; however, their treatment options and prognoses are different. Primary HCC treatment is liver transplantation, which is generally contraindicated in patients with ICC. 116 The distinction between HCC and HCCA is critical for patient treatment selection and prognosis. Integrin αvβ6 may be a molecular marker of epithelial malignancy. However, different results were reported in the above studies, providing a direction for future research. The following questions should be addressed. What is the reason for the difference between the different experiments? What is the relationship between the expression quantity in HCC, HCCA, and ICC?
Integrin αvβ6 as Imaging Point in Vivo
Carpenter et al used 4-[18F]fluorobenzoyl A20FMDV2 to quantify integrin αvβ6 117 in positron emission tomography (PET); however, the drug's low tumor uptake rate and metabolic instability limit its application. Nakamoto 118 developed a PET radiopharmaceutical preparation 18F-FP-R01-Mg-F2, which showed rapid, distinct, and specific uptake of tumors in cell and animal models. Localized lymph node metastases can be detected retroperitoneally, as can distant metastases in organs with low physiological intake, such as the lungs and liver. Hausner created a novel tracer by combining polydiethyl glycogen (PEG) and 18F-A20FMDV2, 119 and increased tumor uptake was observed. In addition, Hausner created a copper strain-free tracer [18F] FBA-C6-adibon3-PEg7-A20FMDV2, its uptake was observed in the gallbladder and gastrointestinal tract in animal studies. 120 This is undoubtedly a research direction for the treatment development of CCA lesions Further research would need to prove how the contrast agent binds CCA cells and exploit the mechanism, thus reducing the occurrence of false-positive development. In addition, researchers would need to understand how to noninvasively enrich the contrast agent in the bile duct and ensure that the body eliminates the agent in a specific time to reduce the occurrence of false-negative development.
Kimura additionally designed several highly stable cysteine binding peptides with high integrin αvβ6 affinity, that were labeled with 64Cu 121 or 18F. 122 Copper-labeled peptide 64Cu-NoTA-S02 showed promising in vivo imaging properties. 18F allows for quick peptide elimination in vivo and has improved dynamic characteristics. Since linear peptides are rapidly metabolized in vivo, their clinical potential is limited. 123 At present, cyclized peptides have been proposed as a practical modification approach for improving peptide stability in vivo. 124 XunFeng et al created a novel 68Ga labeled cyclic peptide that targets the RGDLATL sequence based on integrin αvβ6. The imaging agent is simple to prepare and has good pharmacokinetic and dosiological characteristics; but, it has a low tumor uptake rate. 125
In addition, Tummers 126 used IRDye800CW in conjunction with cysteine peptide R01-MG to guide the intraoperative near-infrared fluorescence imaging (NIRF) of PDAC. Liu 127 designed a new molecular probe, cy7.5-RGD /MSNs, to bind CCA tissue and label it fluorescently with ICG. These specialized imaging materials are critical for determining the intraoperative resection range of cancer focal margin and cancer treatment. Gao 128 synthesized a near-infrared phthalocyanine dye marker, dye-SA-B-HK, that specifically binds to integrin αvβ6 by streptavidin biotin chemistry, and once bound, cells can be viewed using optical imaging techniques. This can guide the anatomic location and complete surgical removal of in-situ pancreatic cancer lesions. Furthermore, under certain wavelength light irradiation, Dye-SA-B-HK demonstrated clear antitumor effects in vitro and in vivo. This suggests that we can create multifunctional molecular imaging agents with radioisotope and near-infrared phthalocyanine dye dual-labeling. This imaging agent enables the detection of primary and metastatic tumor foci using whole-body PET or SPECT scans, followed by optically image-guided tumor surgery and tumor-targeted phototherapy (PT).
At present, contrast agent research allows for non-invasive and accurate localization of PDAC. However, only PET and SPECT have been developed,94,123 and contrast agents for ultrasound, x-ray and other imaging approaches have not yet been developed. The application of targeted integrin αvβ6 in optical imaging and localization of CCA is also rare. Several studies have shown that integrin αvβ6 is upregulated in ICC and HCCA, but not in HCC.50,70,129 Therefore, PET tracers can be used to differentiate cholangiocarcinoma from benign disease and cholangiocarcinoma from HCC prior to surgery, which is extremely important for improving treatment accuracy. Based on the tissue and embryo homology of CCA and PDAC, applying the PDAC imaging technology to CCA should be further investigated.
Integrin αvβ6 as a Therapeutic Target
It has been reported 130 that the ITGB6 gene is required for Ras oncogene-dependent PDAC cell growth, allowing tumor cell proliferation, migration, and invasion. Integrin αvβ6 blocking antibody therapy can inhibit tumor growth in animal models, suggesting integrin αvβ6 inhibition may serve as a potential cancer therapy. 130 Integrin αvβ6 is a novel therapeutic target for CCA and PDAC, due to its unique expression and essential role in epithelial tumors. A significant risk factor for CCA is biliary fibrosis. By demonstrating that integrin αvβ6 was upregulated in biliary fibrosis, Wang 49 showed that integrin αvβ6 plays a role in fibrosis response, and integrin αvβ6 or TGF-β inhibition prevents the development of biliary fibrosis. Liang 131 constructed integrin αvβ6-targeted immune liposomes and discovered that they could effectively inhibit tumor growth and induce apoptosis of cancer cells with tumor specificity and targeting. Soejima 111 knocked out the integrin β6 gene, ITGB6, in two CCA cell lines (with significantly high and low integrin αvβ6 expression). The results showed that, in both cell lines, cell migration, invasion, wound healing, colony formation, PODXL2 expression, and healthy cell cycle decreased. These results suggest that integrin β6 could serve as a therapeutic target or diagnostic marker. Eberlein 132 found that the therapeutic antibody, 264RAD, can target integrin αvβ6 activity and exert antitumor effects by decreasing expression in a dose-dependent manner, as well as significantly inhibit p-ERK in cancer cells. 264RAD delays tumor cell invasion and diffusion by inhibiting TGF-β activation and MMP-9 secretion. E-cadherin is overexpressed in regions of decreased avβ6 expression, suggesting that integrin αvβ6 effectively regulates different cancer pathways. Reader 130 applied 264RAD to PDAC animal studies. Tumors in KDC mice treated with 264RAD had significantly decreased proliferation ability (Ki67), tumor growth signal (pERK), vascular density (Endomucin), and TGF-β signal (nuclear Smad4), as well as decreased the expression of phosphorylated Smad3 and αSMA, and collagen deposition.
Since the discovery of the highly targeted integrin αvβ6 capsid protein A20FMDV2 in 2007, 133 many studies have been conducted on the efficient and low toxic transport of the capsid in foot-and-mouth disease. Tumor clearance was achieved by binding tesirine to A20FMDV2, which was selectively delivered to mouse pancreatic cancer foci. 134 Adenovirus 5 (Ad5) is a common tool in oncolytic virus therapy, and targeted therapy with αvβ6 integrin-binding peptide (A20) modified adenovirus AD5-3 δ-A20 T eliminates the off-target effect of Ad5 alone, in the liver and spleen, and significantly reduces pancreatic cancer progression. 135 CAR-T cell therapy is one of the most popular immunotherapies for hematological malignancies; but, it has not yet been approved for clinical use in solid tumors. Since IL-8 is overexpressed in TME, Whilding 136 injected mice with A20FMDV2-CXCR2-CAR cells, co-expressed by A20FMDV2 and the IL-8 receptor, which increased the number of T cells migrating to TME and improved the antitumor activity and tumor toxicity of CAR therapy. In addition, the tumor load was significantly reduced in mice.136,137 Despite the fact that there are many targeted studies of integrin αvβ6 using A20FMDV2, there are no clinical studies to support these therapies, including the popular CAR-T therapies. The high affinity of A20FMDV2 to integrin αvβ6 appears to be a breakthrough indication for integrin αvβ6-targeted therapy. However, several research questions need to be addressed. How can these therapies be made safe and efficient? Can these technologies be applied clinically for the treatment of bile duct cancer and PDAC?
Hezel 138 blocked TGF-β1 and integrin αvβ6 expression in KRASPDAC mouse models and found that it accelerated disease progression in Smad4-expressing tumors. The tumor inhibition function of integrin αvβ6 was lost in tumors with homozygomorphic deletion of Smad4. This suggests that blocking TGF-β1 or integrin αvβ6 can reduce tumor cell migration but increase proliferation, accelerating disease progression. Targeting integrin αvβ6 may not always have an inhibitory effect on all tumors, and widespread use of TGF-β1 inhibitors in an unselected population of patients with PDAC may have negative consequences, especially in cancers with intact TGF-β/SMAD4 signaling pathways, and may accelerate disease progression. It is necessary and urgent to conduct in-depth and diverse studies on the integrin αvβ6 pathway in CCA and PDAC in order to identify downstream genes for more precise targeting, as well as to avoid unwanted tumor therapy results.
Moreover, epithelial-derived malignancies encompass more than just CCA and PDAC. Chernaya 139 found that integrin αvβ6 expression was up to 18-fold higher in papillary thyroid carcinoma when compared to that in normal tissues. This suggests that integrin αvβ6 is not limited to cholangiocarcinoma and may have similar implications for other epithelial cancers. As a result, more research is required to ascertain whether there are more precise markers of integrin αvβ6 in the CCA pathway (Table 1).
Table 1.
Studies on Integrin αvβ6 as Imaging Point and Therapeutic Target.
| Peptide/drug | Pharmaceutical | Binding materials | Characteristics summary | References |
|---|---|---|---|---|
| A20FMDV2 | [18F]FBA-PEG28-A20FMDV2; [18F]FBA-(PEG28)2-A20FMDV2; [18F]-FBAA20FMDV2-PEG28; [18F]-FBA-PEG28-A20FMDV2-PEG28 |
18F;PEG 28 | PEG increases biological half-life, metabolic stability, and tumor absorption. When compared to just one PEG, the addition of two PEGs inhibited drug clearance. Experiments show that adding one PEG 28 to the N-terminal and one PEG 28 to the C-terminal produces optimal results. | 119 , 140 |
| Ad-3Δ-A20T | Oncolytic adenovirus AdΔΔ | Ad5-3d-a20t retains the replication function of the virus, even in the presence of gemcitabine. It can effectively kill cancer cells, inhibit tumor growth, and weaken the ability of cancer cells to bind with red blood cells and blood factors. It also has a high binding affinity for PDAC. | 141 | |
| Ad-3Δ-A20T | 125I;oncolytic adenovirus AdΔΔ | The addition of 125I does not necessitate gene modification or significantly reduce viral biological activity. It directly radioactively labels the virus. 125I shows biological distribution, elimination rate, and off-target effect rate of the adenovirus. | 135 | |
| PDC SG3299 | Tesirine | SG3299 can cure PDAC with an optimal dosage and dosing regimen. The presence of pancreatic stellate cells has no effect on SG3299 cytotoxicity. SG3299 significantly reduces the spherogenesis of PDACPDX cells. | 134 | |
| Knottins (Cystine forms peptides) 121 | 99m Tc-SAAC-S02 | 99mTc | Rapid tumor targeting with renal clearance. High blood and liver uptake and retention rates. Low miss rate. | 142 |
| 18F-FP-R01 and 18F-FP-S02 | 18F | 18F-FP-R01 intake was low in non-target tissues and healthy tissues, and moderate in the kidney. 18F-FP-R01 outperforms 18F-FP-S02 in terms of tumor affinity and plasma stability. | 122 | |
| R01-MG-IRDye800 | IRDye800 | Tumor-specific targeting and a high rate of renal clearance. High tumor background ratio and a clear correlation were observed between fluorescence signal and the PDAC histopathology. | 126 | |
| 18F-FP-R01-MG-F2 | 18F | Tumor accumulation is faster, higher, and stable. Uptake of 18F-FP-R01-Mg-F2 in retroperitoneal lymph node metastasis was reported. Distant metastases are visible in organs with low physiological intake, such as the lungs and liver. Knottin may be able to meet the need for accurate cancer spread assessment. | 143 , 118 | |
| 177Lu-DOTA-integrin αvβ6 knottin | 177Lu | It is a high-affinity tracer for PDAC with high tumor accumulation and moderate, rapidly declining renal uptake. | 96 | |
| HK (TP H2009.1) | 99m Tc-HHK | 99m Tc | With rapid tumor accumulation, the radiotracer showed maximum tumor uptake at 0.5h after injection. The tumor can be seen with high contrast but it has a low uptake rate. It has high sensitivity and accuracy in identifying and localizing small metastatic liver lesions. | 123 |
| Dye-SA-B-HK | IRDye700 | Dye-SA-B-HK has a high receptor-binding affinity, indicating that dye-SA-B-HK could be enriched in tumors in large and specific quantities. It can be used as photodynamic therapy (PDT) to necrotize cancer cells and reduce tumor proliferation. | 128 | |
| 99m Tc-HYNIC-cHK | 99m Tc | In vivo, cyclic HK peptide (cHK) had similar biological distribution characteristics to linear HK peptide but significantly improved metabolic stability and rapid tumor accumulation in vivo. The binding affinity of cHK to integrin αvβ6 was slightly lower than compared to that of the HK peptide, which might be attributed to peptide sequence shortening and conformational constraints. | 144 | |
| Cycratide | 68Ga-cycratide | 68Ga | It is easy to prepare and has good pharmacokinetic and biological characteristics. Cleared by renal and bladder pathways, the blood and surrounding abdominal organs have a low background, it can be sensitively detected in pancreatic neoplasms, and is internally stable. | 125 |
| IsoDGR | 99mTc-3PisoDGR | 99m Tc | IsoDGR has the ability to target both αvβ6 and α5β1. This strategy avoids the tedious labor and timely preparation. It avoids the interaction of two different targeted molecules. High tumor uptake. | 145 |
| αvβ6-BP | [64Cu]Cu DOTA-EB-αvβ6-BP([64Cu]1) and [64Cu]Cu DOTA-IP-αvβ6-BP([ 64Cu] 2) |
64Cu;Albumin binding moirty (ABM) (EB、IP) | Rapid tumor uptake, increased circulation time, decreased renal uptake, rapid clearance, and increased tumor accumulation. [64Cu]2 is stable in serum and improves tumor visualization. | 146 |
| Avebehexin | Ga-68-avebehexin | 68Ga | Polymerization has no effect on the αvβ6 integrin-selective peptide C (FRGDLAFp(NMe)K), and the TRAP-conjugated monomer 68Ga-Avebehexin can achieve highly sensitive PET imaging as well as therapeutic effects. Despite the fact that tumor intake is low, it is still higher than in all other organs, except the kidney. | 147 |
| Trivehexin | Ga-68-Trivehexin | 68Ga | 68Ga-trivehexin showed significant selectivity to integrin αvβ6 over other RGD-binding integrins. With the exception of the kidney, nonspecific uptake is almost completely eliminated. More specific and sensitive metastatic cancer diagnosis. | 148 |
Integrin αvβ6 Combined with Drugs
Statins, also known as hydroxy-3-methylglutaryl (HMG-CoA) reductase inhibitors, are commonly used as lipid-lowering agents to inhibit cholesterol biosynthesis. 149 In addition to lowering blood lipids, statins are involved in a variety of physiological processes. In cancer, simvastatin inhibits PC3 micrometastasis in prostate cancer by inhibiting integrin αvβ3 activity, 150 and it also reduces tumor cell adhesion in human peritoneal mesenchymal cells by decreasing vCAM-1 and integrin β1 expression. 151 Studies on lovastatin have shown that it induces cytoskeletal changes and, as a result, regulates adhesion, motility, and proteolysis. 151
Recent studies have focused on the use of statins to treat CCA.149,152,153 Simvastatin inhibits cholangiocarcinoma proliferation by inhibiting Rac1 activity 154 or downregulating E2F-1/TS.153,154 Yang et al155,156 showed that lovastatin inhibits ICC proliferation and aids in the treatment of gefitinib-resistant CCA. In general, statins may have potential proliferative inhibitory and therapeutic effects on cholangiocarcinoma via interaction with the integrin pathway.
Gemcitabine is used as a baseline treatment for PDAC and CCA; however, due to its treatability and chemotherapeutic resistance, gemcitabine is ineffective when used alone. 157 Based on this fact and the poor response to ICIS treatment, there is no more effective treatment for PDAC patients. It is urgent to continue studying the drug mechanism and developing more effective projects to overcome drug resistance. 158 Reader 130 administered a combination of gemcitabine and 264RAD (an integrin αvβ6 inhibitor) to tumor-bearing mice. The combination treatment resulted in significantly smaller or even complete tumor disappearance. However, the specific mechanism has yet to be clarified. Studies 159 have focused on explaining the mechanism by which silencing ADP Ribosylation Factor 6 (ARF6) reduces gemcitabine resistance in pancreatic cancer. ARF6 is located downstream of the Kras/Erk signaling pathway and has been shown to induce lipid peroxidation. Because reactive oxygen species (ROS) can promote gemcitabine resistance in pancreatic cancer, 160 inhibiting ARF6 can improve gemcitabine sensitivity. The 264D antibody can block pErk growth signals. Therefore, the integrin αvβ6 inhibitor may increase the sensitivity of gemcitabine via the Erk pathway. Although integrin αvβ6 has been shown to be part of an effective cancer treatment, it has also been demonstrated that integrin αvβ6 participates in HCC resistance to cisplatin via ERK/MAPK signaling. 161 However, due to the promising results of Reader's trial, if we continue to further study the mechanism and combined application of integrin αvβ6 and various chemotherapeutic drugs or other targeted drugs, we may be able to solve the problem of poor treatment effect caused by PDAC resistance to chemotherapeutic drugs and translate the research result into clinical application to bring good news to PDAC patients.
Integrin αvβ6 as a Prognostic Factor
Integrin αvβ6 is known to promote epithelial cancer cell proliferation, migration, and invasion via multiple pathways. Through experiments and data analysis, Li 70 discovered that integrin β6 increased Rac1-GTPase, resulting in MMP-9 upregulation and F-actin polymerization, which promoted tumor invasion. The study results concluded that increased integrin β6 inhibition in CCA is related to lymph node metastasis and distant metastasis. Through extensive statistical analyses on HCCA, Sun 161 discovered that integrin αvβ6 expression is significantly correlated with tumor differentiation and lymph node metastasis. Reader 130 examined 491 PDAC cases and found that the survival rate of patients with PDAC correlates with the expression of integrin αvβ6; the higher the integrin αvβ6 expression, the lower the survival rate of patients.
When cancer metastasizes, the prognosis of patients is often poor. Reader 130 detected the expression level of integrin αvβ6 in six patients with primary PDAC tumor tissues with corresponding lung, colon, or liver metastases, and concluded that integrin αvβ6 was strongly expressed in both primary and metastatic sites. In addition, the expression intensity in 70% of the metastatic samples was similar to that of the primary tumor, suggesting that non-invasive screening of cancer metastasis can be accomplished using appropriate imaging methods. It provides us with an additional prognosis method, even though there is still a false-negative rate.
Integrin αvβ6 is a potential biomarker for cancer diagnosis and tumor metastasis detection. In the future, the range of integrin αvβ6 expression corresponding to different grades of CCA and PDAC can be obtained by sequencing analysis of a large number of clinical cases, guiding clinical diagnosis and prognosis.
Discussion
CCA and PDAC are difficult to diagnose and treat, and have extremely low survival rates. Therefore, advances are required to improve cancer diagnosis, treatment, and prognosis. Integrin αvβ6 is specific to epithelial malignant tumors, such as CCA and PDAC, which share the same histopathological origin and similar manifestations. It plays a vital role in the occurrence, development, and proliferation of cancer. The higher the integrin αvβ6 expression, the worse the cancer prognosis. These results suggest that regulating integrin αvβ6 or its upstream and downstream genes may help to reduce the progression of cancer. The development of molecular imaging agents that work in conjunction with integrin αvβ6 can allow for effective visualization of anatomic location, thus guiding diagnosis and surgery. In addition, integrin αvβ6 inhibitors can be used in combination with Gemcitabine and other drugs to enhance cancer treatment. The expression level of integrin αvβ6 can also influence prognosis.
In addition, integrin αvβ6 expression is increased in a variety of fibrotic diseases, including idiopathic pulmonary, liver, and renal fibrosis; limiting its specificity as a prognostic marker. 143 However, the properties of integrin αvβ6 can be exploited for imaging, through imaging technology such as PET, to evaluate its expression (and possible cancer stage). It can be integrated with the expression levels of other molecular markers for a more comprehensive assessment, precisely estimating cancer prognosis and excluding other disease effects. In addition, bile duct fibrosis can lead to carcinogenesis, and studies have shown that integrin αvβ6 inactivation effectively inhibits ductal reaction in vivo, inhibiting the progression of biliary fibrosis and tumorigenesis. Therefore, the use of targeted agents may be beneficial for cancer prevention. 162
Furthermore, several questions remain unsolved, including the integrin αvβ6 mutation rate, the impact of other gene mutations on integrin αvβ6 expression, and the efficacy of targeted agents when other gene mutations or integrin αvβ6 mutation occur. Understanding these associations may aid in the identification of high-risk patient populations and the development of individualized treatment methods for patients with these specific gene mutations. Further exploration is still needed to address the remaining questions.
Further exploration into the relationship between integrin αvβ6 and cancer, and additional drugs like lovastatin and gemcitabine that act on integrin αvβ6, as well as adjuvant imaging, and targeted therapy molecules like A20FMDV2, are important directions for treating CCA and PDAC with integrin αvβ6. Further research is also needed to determine how to avoid tumor proliferation when TGF-β and integrin αvβ6 are blocked in homozygous Smad tumors.
Various studies on the effective CCA and PDAC diagnosis and treatment are ongoing; however, the mechanisms are still not fully clarified. Studies on combination therapy with currently recommended methods have not been reported; however, since integrin αvβ6 is now known to have promising expression and imaging characteristics, we believe that research in this area will increase continuously over the next five years. Finally, the advantages and drawbacks of integrin αvβ6 will be presented in greater detail.
This review has some limitations. Due to the article's unique starting point, which begins with two related cancers, and the focus of integrin αvβ6 research on these two cancers differs, parts of the article focus more on one cancer. In addition, due to the focus of this review on the molecular marker integrin αvβ6, other ongoing treatment studies for PDAC and CCA were only briefly reviewed. In addition, we have included only extensively studied imaging agents, but emerging and less extensively researched image agents are not be included.
Abbreviations
- ARF6
ADP ribosylation factor 6
- BRCAm
BRCA1/2 mutations
- BTC
Biliary tract cancer
- CA19-9
Carbohydrate antigen 19-9
- CAR-T
Chimeric antigen receptor T-Cell
- CCA
Cholangiocarcinoma
- CEA
Carcino-embryonic antigen
- CK7
Cytokeratin 7
- CK19
Cytokeratin 19
- CTGF
Connective tissue growth factor
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
- DCs
Dendritic cells
- DDR
DNA damage repair
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-Mesenchymal Transition
- Eps8
Epidermal growth factor receptor pathway substrate 8
- ERK
Extracellular regulated protein kinases
- FGFR
fibroblast growth factor receptor
- FLR
Future liver remnant
- FOSL1
Fos-like antigen-1
- HCC
Hepatocellular carcinoma
- HCCA
Hilarcholangiocarcinoma
- HER2
Human epidermal growth factor receptor 2
- (HMG)-CoA
Hydroxy-3-methylglutaryl
- ICC
Intrahepatic cholangiocarcinoma
- ICI
immune checkpoint inhibitor
- IDH
Isocitrate dehydrogenase
- ITGB6
Integrin β6
- LT
Liver transplanation
- MAPK
Mitogen-activated protein kinase
- MMP-9
Matrix metalloprotein-9
- MMR
Mismatch repair
- MSI
instable microsatellite
- MUC4
Mucin genes 4
- NIRF
near-infrared fluorescence imaging
- OS
Overall survival
- PanIN
pancreatic intraepithelial neoplasia
- PD-1
Programmed cell death 1
- PDAC
Pancreatic ductal adenocarcinoma
- PD-L1
Programmed cell death ligand 1
- PDT
Photodynamic therapy
- PET
Positron emission tomography
- PHC
Perhilar cholangiocarcinoma
- PODXL2
Podocalyxin like 2
- PSCA
Prostate stem cell antigen
- PT
Phototherapy
- RAC1
Ras-related C3 botulinum toxin substrate 1
- SPECT
Single-photon emission computed tomography
- TGF-β
Transforming growth factor-β
- TME
Tumor microenvironment
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key R&D Program of China, Science and Technology Program of Guangzhou, China, National Natural Science Foundation of China, National High Technology Research and Development Program of China, Science and technology projects funded by Guangdong Province (grant numbers 2016YFC0106500, 201604020144, 12026602, 81627805, U1401254, 2012AA021105, 2017ZC0110).
ORCID iD: Yunyu Lian https://orcid.org/0000-0002-7122-8956
References
- 1.Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99(2):315‐335. doi: 10.1016/j.suc.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 2.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261‐280. doi: 10.1038/nrgastro.2016.51 [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Gray H. Anatomy of the Human Body. Am J Med Sci. 1919;157(5). doi: 10.1097/00000441-191905000-00011 [DOI] [Google Scholar]
- 5.Zaccari P, Cardinale V, Severi C, et al. Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas. World J Gastroenterol. 2019;25(31):4343‐4359. doi: 10.3748/wjg.v25.i31.4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157‐188. doi: 10.1146/annurev.pathmechdis.3.121806.154305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607‐620. doi: 10.1016/S0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 9.Rizzo A, Brandi G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: reflections on a standard of care. Expert Rev Gastroenterol Hepatol. 2021;15(5):483‐485. doi: 10.1080/17474124.2021.1864325 [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):364‐377. doi: 10.1016/j.jhep.2019.11.020 [DOI] [PubMed] [Google Scholar]
- 11.Levi Sandri GB, Spoletini G, Mascianà G, et al. The role of minimally invasive surgery in the treatment of cholangiocarcinoma. Eur J Surg Oncol. 2017;43(9):1617‐1621. doi: 10.1016/j.ejso.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 12.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469‐474. doi: 10.1038/sj.bjc.6605779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64(4):1178‐1188. doi: 10.1002/hep.28744 [DOI] [PubMed] [Google Scholar]
- 14.Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7(3):252‐263. doi: 10.1158/2159-8290.CD-16-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idris R, Chaijaroenkul W, Na-Bangchang K. Molecular targets and signaling pathways in cholangiocarcinoma: a systematic review. Asian Pac J Cancer Prev. 2023;24(3):741‐751. Published 2023 Mar 1. doi: 10.31557/APJCP.2023.24.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallejo A, Erice O, Entrialgo-Cadierno R, et al. FOSL1 promotes cholangiocarcinoma via transcriptional effectors that could be therapeutically targeted. J Hepatol. 2021;75(2):363‐376. doi: 10.1016/j.jhep.2021.03.028 [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Shen W. Latest evidence on immunotherapy for cholangiocarcinoma. Oncol Lett. 2020;20(6):381. doi: 10.3892/ol.2020.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suriyo T, Fuangthong M, Artpradit C, et al. Inhibition of T-cell-mediated immune response via the PD-1/ PD-L1 axis in cholangiocarcinoma cells. Eur J Pharmacol. 2021;897:173960. doi: 10.1016/j.ejphar.2021.173960 [DOI] [PubMed] [Google Scholar]
- 19.Ye Z, Zhang Y, Chen J, Wang X, Hong Y, Zhao Q. First-line PD-1 inhibitors combination therapy for patients with advanced cholangiocarcinoma: a retrospective real-world study [published online ahead of print, 2023 May 26]. Int Immunopharmacol. 2023;120:110344. doi:10.1016/j.intimp.2023.110344. [DOI] [PubMed] [Google Scholar]
- 20.Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer [published correction appears in Sci Rep. 2020 Dec 3;10(1):21552]. Sci Rep. 2020;10(1):12348. Published 2020 Jul 23. doi:10.1038/s41598-020-69366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aruga A, Takeshita N, Kotera Y, et al. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med. 2014;12:61. Published 2014 Mar 7. doi:10.1186/1479-5876-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Feng K, Liu Y, et al. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res. 2018;24(6):1277‐1286. doi: 10.1158/1078-0432.CCR-17-0432 [DOI] [PubMed] [Google Scholar]
- 23.Du J, Lv X, Zhang Z, Huang Z, Zhang E. Revisiting targeted therapy and immunotherapy for advanced cholangiocarcinoma. Front Immunol. 2023;14:1142690. Published 2023 Mar 1. doi:10.3389/fimmu.2023.1142690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heij L, Bednarsch J, Tan X, et al. Expression of checkpoint molecules in the tumor microenvironment of intrahepatic cholangiocarcinoma: implications for immune checkpoint blockade therapy. Cells. 2023;12(6):851. Published 2023 Mar 9. doi:10.3390/cells12060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vatankhah F, Salimi N, Khalaji A, Baradaran B. Immune checkpoints and their promising prospect in cholangiocarcinoma treatment in combination with other therapeutic approaches. Int Immunopharmacol. 2023;114:109526. doi: 10.1016/j.intimp.2022.109526 [DOI] [PubMed] [Google Scholar]
- 26.Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, Maitra A. Pancreatic cancer: advances and challenges. Cell. 2023;186(8):1729‐1754. doi: 10.1016/j.cell.2023.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX Versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817‐1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 28.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691‐1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011‐1024. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 30.Park W, Chawla A, O'Reilly EM. Pancreatic cancer: a review [published correction appears in JAMA. 2021 Nov 23;326(20):2081]. JAMA. 2021;326(9):851‐862. doi: 10.1001/jama.2021.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mavros MN, Moris D, Karanicolas PJ, et al. Clinical trials of systemic chemotherapy for resectable pancreatic cancer: a review. JAMA Surg. 2021;156(7):663‐672. doi: 10.1001/jamasurg.2021.0149 [DOI] [PubMed] [Google Scholar]
- 32.Qian Y, Gong Y, Fan Z, et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2020;13(1):130. Published 2020 Oct 2. doi:10.1186/s13045-020-00958-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin L, Tao H, Karachi A, et al. CXCR1- Or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019;10(1):4016. Published 2019 Sep 5. doi:10.1038/s41467-019-11869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran E, Robbins PF, Lu YC, et al. T-Cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255‐2262. doi: 10.1056/NEJMoa1609279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raj D, Yang MH, Rodgers D, et al. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut. 2019;68(6):1052‐1064. doi: 10.1136/gutjnl-2018-316595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishigaki T, Takahashi T, Serada S, et al. Anti-glypican-1 antibody-drug conjugate is a potential therapy against pancreatic cancer. Br J Cancer. 2020;122(9):1333‐1341. doi: 10.1038/s41416-020-0781-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43‐66. doi:10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 38.Sinn M, Bahra M, Liersch T, et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol. 2017;35(29):3330‐3337. doi: 10.1200/JCO.2017.72.6463 [DOI] [PubMed] [Google Scholar]
- 39.Ullman NA, Burchard PR, Dunne RF, Linehan DC. Immunologic strategies in pancreatic cancer: making cold tumors hot. J Clin Oncol. 2022;40(24):2789‐2805. doi: 10.1200/JCO.21.02616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng KC, Guo YL, Liu Y, et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10(1):4. doi: 10.1186/s13045-016-0378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricci AD, Rizzo A, Brandi G. Immunotherapy in biliary tract cancer: worthy of a second look. Cancer Control. 2020;27(3):1073274820948047. doi: 10.1177/1073274820948047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricci AD, Rizzo A, Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora’s box? ESMO Open. 2020;5(5):e001042. doi: 10.1136/esmoopen-2020-001042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.AlMasri SS, Zenati MS, Desilva A, et al. Encouraging long-term survival following autophagy inhibition using neoadjuvant hydroxychloroquine and gemcitabine for high-risk patients with resectable pancreatic carcinoma. Cancer Med. 2021;10(20):7233‐7241. doi: 10.1002/cam4.4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarasiuk A, Mackiewicz T, Małecka-Panas E, Fichna J. Biomarkers for early detection of pancreatic cancer – miRNAs as a potential diagnostic and therapeutic tool? Cancer Biol Ther. 2021;22(5-6):347‐356. doi: 10.1080/15384047.2021.1941584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zettlitz KA, Tsai WK, Knowles SM, et al. Dual-modality immuno-PET and near-infrared fluorescence imaging of pancreatic cancer using an anti-prostate stem cell antigen Cys-diabody. J Nucl Med. 2018;59(9):1398‐1405. doi: 10.2967/jnumed.117.207332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, You L, Dai M, Zhao Y. Mucins in pancreatic cancer: a well-established but promising family for diagnosis, prognosis and therapy. J Cell Mol Med. 2020;24(18):10279‐10289. doi: 10.1111/jcmm.15684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etekpo A, Alghawalby A, Alghawalby M, et al. Differences in MUC4 expression in pancreatic cancers and pancreatic cysts in Egypt. J Carcinog Mutagen. 2018;9(1):1000312. doi: 10.4172/2157-2518.1000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu LZ, Yang LX, Zheng BH, et al. CK7/CK19 index: a potential prognostic factor for postoperative intrahepatic cholangiocarcinoma patients. J Surg Oncol. 2018;117(7):1531‐1539. doi: 10.1002/jso.25027 [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Dolinski BM, Kikuchi N, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46(5):1404‐1412. doi: 10.1002/hep.21849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patsenker E, Wilkens L, Banz V, et al. The alphavbeta6 integrin is a highly specific immunohistochemical marker for cholangiocarcinoma. J Hepatol. 2010;52(3):362‐369. doi: 10.1016/j.jhep.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 51.Hezel AF, Deshpande V, Zimmerman SM, et al. TGF-β and αvβ6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 2012;72(18):4840‐4845. doi: 10.1158/0008-5472.CAN-12-0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- 53.Thomas GJ, Nyström ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35(1):1‐10. doi: 10.1111/j.1600-0714.2005.00374.x [DOI] [PubMed] [Google Scholar]
- 54.Fernández-Ruiz E, Sánchez-Madrid F. Regional localization of the human integrin beta 6 gene (ITGB6) to chromosome 2q24-q31. Genomics. 1994;21(3):638‐640. doi: 10.1006/geno.1994.1325 [DOI] [PubMed] [Google Scholar]
- 55.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215. doi: 10.1186/gb-2007-8-5-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed N, Niu J, Dorahy DJ, et al. Direct integrin alphavbeta6-ERK binding: implications for tumour growth. Oncogene. 2002;21(9):1370‐1380. doi: 10.1038/sj.onc.1205286 [DOI] [PubMed] [Google Scholar]
- 57.Thomas GJ, Hart IR, Speight PM, Marshall JF. Binding of TGF-beta1 latency-associated peptide (LAP) to alpha(v)beta6 integrin modulates behaviour of squamous carcinoma cells. Br J Cancer. 2002;87(8):859‐867. doi: 10.1038/sj.bjc.6600545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen MD, Thomas GJ, Clark S, et al. Altered microenvironment promotes progression of preinvasive breast cancer: myoepithelial expression of αvβ6 integrin in DCIS identifies high-risk patients and predicts recurrence. Clin Cancer Res. 2014;20(2):344‐357. doi: 10.1158/1078-0432.CCR-13-1504 [DOI] [PubMed] [Google Scholar]
- 59.Busk M, Pytela R, Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992;267(9):5790‐5796. doi: 10.1016/S0021-9258(18)42622-1 [DOI] [PubMed] [Google Scholar]
- 60.Zhao R, Liu XQ, Wu XP, et al. Vascular endothelial growth factor (VEGF) enhances gastric carcinoma invasiveness via integrin alpha(v)beta6. Cancer Lett. 2010;287(2):150‐156. doi: 10.1016/j.canlet.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 61.Niu W, Liu X, Zhang Z, et al. Effects of alphavbeta6 gene silencing by RNA interference in PANC-1 pancreatic carcinoma cells. Anticancer Res. 2010;30(1):135‐142. [PubMed] [Google Scholar]
- 62.Zhao-Yang Z, Ke-sen X, Qing-Si H, et al. Signaling and regulatory mechanisms of integrin alphavbeta6 on the apoptosis of colon cancer cells. Cancer Lett. 2008;266(2):209‐215. doi: 10.1016/j.canlet.2008.02.054 [DOI] [PubMed] [Google Scholar]
- 63.Wang B, Wang W, Niu W, et al. SDF-1/CXCR4 axis promotes directional migration of colorectal cancer cells through upregulation of integrin αvβ6. Carcinogenesis. 2014;35(2):282‐291. doi: 10.1093/carcin/bgt331 [DOI] [PubMed] [Google Scholar]
- 64.Ahmed N, Pansino F, Baker M, Rice G, Quinn M. Association between alphavbeta6 integrin expression, elevated p42/44 kDa MAPK, and plasminogen-dependent matrix degradation in ovarian cancer. J Cell Biochem. 2002;84(4):675‐686. doi: 10.1002/jcb.10080 [DOI] [PubMed] [Google Scholar]
- 65.Hecht JL, Dolinski BM, Gardner HA, Violette SM, Weinreb PH. Overexpression of the alphavbeta6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol. 2008;16(6):543‐547. doi: 10.1097/PAI.0b013e31816bc5ee [DOI] [PubMed] [Google Scholar]
- 66.Wang B, Wang S, Wang W, et al. Hyperglycemia promotes liver metastasis of colorectal cancer via upregulation of integrin ανβ6. Med Sci Monit. 2021;27:e930921. Published 2021 Aug 19. doi:10.12659/MSM.930921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Busenhart P, Montalban-Arques A, Katkeviciute E, et al. Inhibition of integrin αvβ6 sparks T-cell antitumor response and enhances immune checkpoint blockade therapy in colorectal cancer. J Immunother Cancer. 2022;10(2):e003465. doi: 10.1136/jitc-2021-003465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang B, Li L, Miao R, et al. Expression of interleukin-6 and integrin ανβ6 in colon cancer: association with clinical outcomes and prognostic implications. Cancer Invest. 2019;37(3):174‐184. doi: 10.1080/07357907.2019.1597103 [DOI] [PubMed] [Google Scholar]
- 69.Li Z, Lin P, Gao C, et al. Integrin β6 acts as an unfavorable prognostic indicator and promotes cellular malignant behaviors via ERK-ETS1 pathway in pancreatic ductal adenocarcinoma (PDAC). Tumour Biol. 2016;37(4):5117‐5131. doi: 10.1007/s13277-015-4353-7 [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Biswas S, Liang B, et al. Integrin β6 serves as an immunohistochemical marker for lymph node metastasis and promotes cell invasiveness in cholangiocarcinoma. Sci Rep. 2016;6:30081. Published 2016 Jul 21. doi:10.1038/srep30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore-Smith LD, Isayeva T, Lee JH, Frost A, Ponnazhagan S. Silencing of TGF-β1 in tumor cells impacts MMP-9 in tumor microenvironment. Sci Rep. 2017;7(1):8678. Published 2017 Aug 17. doi:10.1038/s41598-017-09062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hao Y, Baker D, Ten Dijke P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11):2767. doi: 10.3390/ijms20112767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924‐940. doi: 10.1016/j.immuni.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu R, Xu M, Fu Y, et al. Transforming growth factor-β1 and lysophosphatidic acid activate integrin β6 gene promoter in Hep-3B cells. Oncol Lett. 2018;16(1):439‐446. doi: 10.3892/ol.2018.8672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghannad F, Nica D, Fulle MI, et al. Absence of alphavbeta6 integrin is linked to initiation and progression of periodontal disease. Am J Pathol. 2008;172(5):1271‐1286. doi: 10.2353/ajpath.2008.071068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14(6):1423‐1431. doi: 10.1080/21645515.2018.1431598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato Y, Harada K, Itatsu K, et al. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177(1):141‐152. doi: 10.2353/ajpath.2010.090747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Ma L, He Q, Zhang S, Zhang C, Jia W. TGF-β1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol Res. 2015;48(1):26. doi: 10.1186/s40659-015-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pi L, Robinson PM, Jorgensen M, et al. Connective tissue growth factor and integrin αvβ6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology. 2015;61(2):678‐691. doi: 10.1002/hep.27425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinreb PH, Simon KJ, Rayhorn P, et al. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279(17):17875‐17887. doi: 10.1074/jbc.M312103200 [DOI] [PubMed] [Google Scholar]
- 81.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118(16):3573‐3584. doi: 10.1242/jcs.02554 [DOI] [PubMed] [Google Scholar]
- 82.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117‐129. doi: 10.1038/ng1001-117 [DOI] [PubMed] [Google Scholar]
- 83.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003‐1010. doi: 10.1038/ng.3375 [DOI] [PubMed] [Google Scholar]
- 84.Ahmed S, Bradshaw AD, Gera S, Dewan MZ, Xu R. The TGF-β/Smad4 signaling pathway in pancreatic carcinogenesis and its clinical significance. J Clin Med. 2017;6(1):5. doi: 10.3390/jcm6010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagner M, Kleeff J, Lopez ME, Bockman I, Massaqué J, Korc M. Transfection of the type I TGF-beta receptor restores TGF-beta responsiveness in pancreatic cancer. Int J Cancer. 1998;78(2):255–260. [DOI] [PubMed] [Google Scholar]
- 86.Lin X, Feng XH. Abrogation of transforming growth factor-beta signaling in pancreatic cancer. World J Surg. 2005;29(3):312‐316. doi: 10.1007/s00268-004-7824-3 [DOI] [PubMed] [Google Scholar]
- 87.Cadamuro M, Nardo G, Indraccolo S, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58(3):1042‐1053. doi: 10.1002/hep.26384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang WH, Lan HY, Huang CH, et al. RAC1 Activation mediates Twist1-induced cancer cell migration. Nat Cell Biol. 2012;14(4):366‐374. doi: 10.1038/ncb2455 [DOI] [PubMed] [Google Scholar]
- 89.Santibáñez JF, Kocić J, Fabra A, Cano A, Quintanilla M. Rac1 modulates TGF-beta1-mediated epithelial cell plasticity and MMP9 production in transformed keratinocytes. FEBS Lett. 2010;584(11):2305‐2310. doi: 10.1016/j.febslet.2010.03.042 [DOI] [PubMed] [Google Scholar]
- 90.Han Q, Leng J, Bian D, et al. Rac1-MKK3-p38-MAPKAPK2 pathway promotes urokinase plasminogen activator mRNA stability in invasive breast cancer cells. J Biol Chem. 2002;277(50):48379‐48385. doi: 10.1074/jbc.M209542200 [DOI] [PubMed] [Google Scholar]
- 91.Tod J, Hanley CJ, Morgan MR, et al. Pro-migratory and TGF-β-activating functions of αvβ6 integrin in pancreatic cancer are differentially regulated via an Eps8-dependent GTPase switch. J Pathol. 2017;243(1):37‐50. doi: 10.1002/path.4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mohan R, Chintala SK, Jung JC, et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277(3):2065‐2072. doi: 10.1074/jbc.M107611200 [DOI] [PubMed] [Google Scholar]
- 93.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448‐464. doi: 10.1002/path.1400 [DOI] [PubMed] [Google Scholar]
- 94.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16‐27. doi: 10.1111/j.1742-4658.2010.07919.x [DOI] [PubMed] [Google Scholar]
- 95.Yang GY, Guo S, Dong CY, et al. Integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression. World J Gastroenterol. 2015;21(24):7457‐7467. doi: 10.3748/wjg.v21.i24.7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sachindra S, Hellberg T, Exner S, et al. SPECT/CT imaging, biodistribution and radiation dosimetry of a 177Lu-DOTA-integrin αvβ6 cystine knot peptide in a pancreatic cancer xenograft model. Front Oncol. 2021;11:684713. doi: 10.3389/fonc.2021.684713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: the TGFβ and MAPK pathways in cancer progression. Cell Biosci. 2011;1:42. doi: 10.1186/2045-3701-1-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gu X, Niu J, Dorahy DJ, Scott R, Agrez MV. Integrin alpha(v)beta6-associated ERK2 mediates MMP-9 secretion in colon cancer cells. Br J Cancer. 2002;87(3):348‐351. doi: 10.1038/sj.bjc.6600480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mansour SJ, Matten WT, Hermann AS, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265(5174):966‐970. doi: 10.1126/science.8052857 [DOI] [PubMed] [Google Scholar]
- 100.Liu S, Wang J, Niu W, et al. The β6-integrin-ERK/MAP kinase pathway contributes to chemo resistance in colon cancer. Cancer Lett. 2013;328(2):325‐334. doi: 10.1016/j.canlet.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 101.Wang J, Zhang Z, Xu K, et al. Suppression of integrin alphaupsilonbeta6 by RNA interference in colon cancer cells inhibits extracellular matrix degradation through the MAPK pathway. Int J Cancer. 2008;123(6):1311‐1317. doi: 10.1002/ijc.23656 [DOI] [PubMed] [Google Scholar]
- 102.Fuxe J, Karlsson MC. TGF-β-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol. 2012;22(5-6):455‐461. doi: 10.1016/j.semcancer.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 103.Beuran M, Negoi I, Paun S, et al. The epithelial to mesenchymal transition in pancreatic cancer: a systematic review. Pancreatology. 2015;15(3):217‐225. doi: 10.1016/j.pan.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 104.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740‐746. doi: 10.1016/j.ceb.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 105.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973‐981. doi: 10.1083/jcb.200601018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salaroglio IC, Mungo E, Gazzano E, Kopecka J, Riganti C. ERK Is a pivotal player of chemo-immune-resistance in cancer. Int J Mol Sci. 2019;20(10):2505. doi: 10.3390/ijms20102505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kerr SC, Fieger CB, Snapp KR, Rosen SD. Endoglycan, a member of the CD34 family of sialomucins, is a ligand for the vascular selectins. J Immunol. Baltimore, MD. 2008;181(2):1480‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ney JT, Zhou H, Sipos B, et al. Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum Pathol. 2007;38(2):359‐364. doi: 10.1016/j.humpath.2006.08.025 [DOI] [PubMed] [Google Scholar]
- 109.Heby M, Elebro J, Nodin B, Jirström K, Eberhard J. Prognostic and predictive significance of podocalyxin-like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin Pathol. 2015;15:10. doi: 10.1186/s12907-015-0009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saito K, Iioka H, Maruyama S, Sumardika IW, Sakaguchi M, Kondo E. PODXL1 Promotes metastasis of the pancreatic ductal adenocarcinoma by activating the C5aR/C5a axis from the tumor microenvironment. Neoplasia. 2019;21(12):1121‐1132. doi: 10.1016/j.neo.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soejima Y, Takeuchi M, Miyamoto N, Sawabe M, Fukusato T. ITGB6-knockout Suppresses cholangiocarcinoma cell migration and invasion with declining PODXL2 expression. Int J Mol Sci. 2021;22(12):6303. doi: 10.3390/ijms22126303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sipos B, Hahn D, Carceller A, et al. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45(3):226‐236. doi: 10.1111/j.1365-2559.2004.01919.x [DOI] [PubMed] [Google Scholar]
- 113.Steiger K, Schlitter AM, Weichert W, Esposito I, Wester HJ, Notni J. Perspective of αvβ6-integrin imaging for clinical management of pancreatic carcinoma and its precursor lesions. Mol Imaging. 2017;16:1536012117709384. doi: 10.1177/1536012117709384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franken LC, Vuijk FA, Soer EC, et al. Expression of integrin ανβ6 differentiates perihilar cholangiocarcinoma (PHC) from benign disease mimicking PHC. Eur J Surg Oncol. 2021;47(3(B)):628‐634. doi: 10.1016/j.ejso.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 115.Soejima Y, Inoue M, Takahashi Y, Uozaki H, Sawabe M, Fukusato T. Integrins ανβ6, α6β4 and α3β1 are down-regulated in cholangiolocellular carcinoma but not cholangiocarcinoma. Hepatol Res. 2014;44(14):E320‐E334. doi: 10.1111/hepr.12312 [DOI] [PubMed] [Google Scholar]
- 116.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14(4):203‐217. doi: 10.1038/nrgastro.2016.193 [DOI] [PubMed] [Google Scholar]
- 117.Carpenter RD, Hausner SH, Sutcliffe JL. Copper-free click for PET: rapid 1,3-dipolar cycloadditions with a fluorine-18 cyclooctyne. ACS Med Chem Lett. 2011;2(12):885‐889. doi: 10.1021/ml200187j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakamoto R, Ferri V, Duan H, et al. Pilot-phase PET/CT study targeting integrin αvβ6 in pancreatic cancer patients using the cystine-knot peptide-based 18F-FP-R01-MG-F2. Eur J Nucl Med Mol Imaging. 2022;50(1):184‐193. doi: 10.1007/s00259-021-05595-7. Advance online publication. . [DOI] [PubMed] [Google Scholar]
- 119.Hausner SH, Abbey CK, Bold RJ, et al. Targeted in vivo imaging of integrin alphavbeta6 with an improved radiotracer and its relevance in a pancreatic tumor model. Cancer Res. 2009;69(14):5843‐5850. doi: 10.1158/0008-5472.CAN-08-4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hausner SH, Carpenter RD, Bauer N, Sutcliffe JL. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl Med Biol. 2013;40(2):233‐239. doi: 10.1016/j.nucmedbio.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 121.Kimura RH, Teed R, Hackel BJ, et al. Pharmacokinetically stabilized cystine knot peptides that bind alpha-v-beta-6 integrin with single-digit nanomolar affinities for detection of pancreatic cancer. Clin Cancer Res. 2012;18(3):839‐849. doi: 10.1158/1078-0432.CCR-11-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hackel BJ, Kimura RH, Miao Z, et al. 18F-fluorobenzoate-labeled Cystine knot peptides for PET imaging of integrin αvβ6. J Nucl Med. 2013;54(7):1101‐1105. doi: 10.2967/jnumed.112.110759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z, Liu H, Ma T, et al. Integrin αvβ₆-targeted SPECT imaging for pancreatic cancer detection. J Nucl Med. 2014;55(6):989‐994. doi: 10.2967/jnumed.113.132969 [DOI] [PubMed] [Google Scholar]
- 124.Liu S. Radiolabeled cyclic RGD peptide bioconjugates as radiotracers targeting multiple integrins. Bioconjug Chem. 2015;26(8):1413‐1438. doi: 10.1021/acs.bioconjchem.5b00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng X, Wang Y, Lu D, et al. Clinical translation of a 68ga-labeled integrin αvβ6-targeting cyclic radiotracer for PET imaging of pancreatic cancer. J Nucl Med. 2020;61(10):1461‐1467. doi: 10.2967/jnumed.119.237347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tummers WS, Kimura RH, Abou-Elkacem L, et al. Development and preclinical validation of a cysteine knottin peptide targeting integrin αvβ6 for near-infrared fluorescent-guided surgery in pancreatic cancer. Clin Cancer Res. 2018;24(7):1667‐1676. doi: 10.1158/1078-0432.CCR-17-2491 [DOI] [PubMed] [Google Scholar]
- 127.Liu B. Application of Intraoperative Navigation Technology Based on Fluorescent Tracer in Hepatobiliary Malignant Tumor Surgery. Chinese People’s Liberation Army General Hospital; 2019. [Google Scholar]
- 128.Gao D, Gao L, Zhang C, et al. A near-infrared phthalocyanine dye-labeled agent for integrin αvβ6-targeted theranostics of pancreatic cancer. Biomaterials. 2015;53:229‐238. doi: 10.1016/j.biomaterials.2015.02.093 [DOI] [PubMed] [Google Scholar]
- 129.Soejima Y, Takeuchi M, Akashi T, Sawabe M, Fukusato T. Β4 and β6 integrin expression is associated with the subclassification and clinicopathological features of intrahepatic cholangiocarcinoma. Int J Mol Sci. 2018;19(4):1004. doi: 10.3390/ijms19041004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reader CS, Vallath S, Steele CW, et al. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol. 2019;249(3):332‐342. doi: 10.1002/path.5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liang B. Integrin αvβ6-targeted immunoliposomes: a novel approach for tumor-specific drug delivery and therapy in colon carcinoma. Shandong University; 2017. [Google Scholar]
- 132.Eberlein C, Kendrew J, McDaid K, et al. A human monoclonal antibody 264 rad targeting αvβ6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32(37):4406‐4416. doi: 10.1038/onc.2012.460 [DOI] [PubMed] [Google Scholar]
- 133.Hausner SH, DiCara D, Marik J, Marshall JF, Sutcliffe JL. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin alphavbeta6 expression with positron emission tomography. Cancer Res. 2007;67(16):7833‐7840. doi: 10.1158/0008-5472.CAN-07-1026 [DOI] [PubMed] [Google Scholar]
- 134.Moore KM, Desai A, Delgado BL, et al. Integrin αvβ6-specific therapy for pancreatic cancer developed from foot-and-mouth-disease virus. Theranostics. 2020;10(7):2930‐2942. doi: 10.7150/thno.38702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stella Man YK, Foster J, Carapuça E, et al. Systemic delivery and SPECT/CT in vivo imaging of 125I-labelled oncolytic adenoviral mutants in models of pancreatic cancer. Sci Rep. 2019;9(1):12840. doi: 10.1038/s41598-019-49150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whilding LM, Halim L, Draper B, et al. CAR T-cells targeting the integrin αvβ6 and co-expressing the chemokine receptor CXCR2 demonstrate enhanced homing and efficacy against several solid malignancies. Cancers (Basel). 2019;11(5):674. doi: 10.3390/cancers11050674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meecham A, Marshall J. Harnessing the power of foot-and-mouth-disease virus for targeting integrin alpha-v beta-6 for the therapy of cancer. Expert Opin Drug Discov. 2021;16(7):737‐744. doi: 10.1080/17460441.2021.1878143 [DOI] [PubMed] [Google Scholar]
- 138.Allen MD, Marshall JF, Jones JL. Αvβ6 expression in myoepithelial cells: a novel marker for predicting DCIS progression with therapeutic potential. Cancer Res. 2014;74(21):5942‐5947. doi: 10.1158/0008-5472.CAN-14-1841 [DOI] [PubMed] [Google Scholar]
- 139.Chernaya G, Mikhno N, Khabalova T, et al. The expression profile of integrin receptors and osteopontin in thyroid malignancies varies depending on the tumor progression rate and presence of BRAF V600E mutation. Surg Oncol. 2018;27(4):702‐708. doi: 10.1016/j.suronc.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 140.Hausner SH, Bauer N, Hu LY, Knight LM, Sutcliffe JL. The effect of bi-terminal pegylation of an integrin αvβ₆-targeted ¹⁸F peptide on pharmacokinetics and tumor uptake. J Nucl Med. 2015;56(5):784‐790. doi: 10.2967/jnumed.114.150680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Man YKS, Davies JA, Coughlan L, et al. The novel oncolytic adenoviral mutant Ad5-3Δ-A20 T retargeted to αvβ6 integrins efficiently eliminates pancreatic cancer cells. Mol Cancer Ther. 2018;17(2):575‐587. doi: 10.1158/1535-7163.MCT-17-0671 [DOI] [PubMed] [Google Scholar]
- 142.Zhu X, Li J, Hong Y, et al. 99mTc-labeled Cystine knot peptide targeting integrin αvβ6 for tumor SPECT imaging. Mol Pharm. 2014;11(4):1208‐1217. doi: 10.1021/mp400683q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kimura RH, Wang L, Shen B, et al. Evaluation of integrin αvβ6 cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis. Nat Commun. 2019;10(1):4673. doi: 10.1038/s41467-019-11863-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu H, Gao L, Yu X, et al. Small-animal SPECT/CT imaging of cancer xenografts and pulmonary fibrosis using a 99mTc-labeled integrin αvβ6-targeting cyclic peptide with improved in vivo stability. Biophys Rep. 2018;4(5):254‐264. doi: 10.1007/s41048-018-0071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao H, Gao H, Luo C, et al. An integrin-αvβ6/α5β1-Bitargeted probe for the SPECT imaging of pancreatic adenocarcinoma in preclinical and primary clinical studies. Bioconjug Chem. 2021;32(7):1298‐1305. doi: 10.1021/acs.bioconjchem.1c00296 [DOI] [PubMed] [Google Scholar]
- 146.Davis RA, Hausner SH, Harris R, Sutcliffe JL. A comparison of evans blue and 4-(p-iodophenyl)butyryl albumin binding moieties on an integrin αvβ6 binding peptide. Pharmaceutics. 2022;14(4):745. doi: 10.3390/pharmaceutics14040745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Notni J, Reich D, Maltsev OV, et al. In vivo PET imaging of the cancer integrin αvβ6 using 68ga-labeled cyclic RGD nonapeptides. J Nucl Med. 2017;58(4):671‐677. doi: 10.2967/jnumed.116.182824 [DOI] [PubMed] [Google Scholar]
- 148.Quigley NG, Steiger K, Hoberück S, et al. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the “Cancer integrin” αvβ6 with Ga-68-Trivehexin. Eur J Nucl Med Mol Imaging. 2022;49(4):1136‐1147. doi: 10.1007/s00259-021-05559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41(6):554‐567. doi: 10.1016/j.ctrv.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 150.Al-Husein B, Goc A, Somanath PR. Suppression of interactions between prostate tumor cell-surface integrin and endothelial ICAM-1 by simvastatin inhibits micrometastasis. J Cell Physiol. 2013;228(11):2139‐2148. doi: 10.1002/jcp.24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wagner BJ, Löb S, Lindau D, et al. Simvastatin reduces tumor cell adhesion to human peritoneal mesothelial cells by decreased expression of VCAM-1 and β1 integrin. Int J Oncol. 2011;39(6):1593‐1600. doi: 10.3892/ijo.2011.1167 [DOI] [PubMed] [Google Scholar]
- 152.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering—are they clinically relevant? Eur Heart J. 2003;24(3):225‐248. doi: 10.1016/s0195-668x(02)00419-0 [DOI] [PubMed] [Google Scholar]
- 153.Cai JP, Chen W, Hou X, Liang LJ, Hao XY, Yin XY. Simvastatin enhances the chemotherapeutic efficacy of S-1 against bile duct cancer: E2F-1/TS downregulation might be the mechanism. Anti-Cancer Drugs. 2013;24(10):1020‐1029. doi: 10.1097/CAD.0b013e328364f935 [DOI] [PubMed] [Google Scholar]
- 154.Miller T, Yang F, Wise CE, et al. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig Liver Dis. 2011;43(5):395‐403. doi: 10.1016/j.dld.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang SH, Lin HY, Changou CA, et al. Integrin β3 and LKB1 are independently involved in the inhibition of proliferation by lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(1):362‐373. doi: 10.18632/oncotarget.6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang SH, Lin HY, Chang VH, et al. Lovastatin overcomes gefitinib resistance through TNF-α signaling in human cholangiocarcinomas with different LKB1 statuses in vitro and in vivo. Oncotarget. 2015;6(27):23857‐23873. doi: 10.18632/oncotarget.4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang X, Ma Y, Wang T, et al. Targeting UBE2 T potentiates gemcitabine efficacy in pancreatic cancer by regulating pyrimidine metabolism and replication stress. Gastroenterology. 2023;164(7):1232‐1247. doi: 10.1053/j.gastro.2023.02.025 [DOI] [PubMed] [Google Scholar]
- 158.Hsu SK, Jadhao M, Liao WT, Chang WT, Hung CT, Chiu CC. Culprits of PDAC resistance to gemcitabine and immune checkpoint inhibitor: tumour microenvironment components. Front Mol Biosci. 2022;9:1020888. doi: 10.3389/fmolb.2022.1020888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ye Z, Hu Q, Zhuo Q, et al. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am J Cancer Res. 2020;10(4):1182‐1193. [PMC free article] [PubMed] [Google Scholar]
- 160.Meng Q, Shi S, Liang C, et al. Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3β/Snail signaling. Oncogene. 2018;37(44):5843‐5857. doi: 10.1038/s41388-018-0392-z [DOI] [PubMed] [Google Scholar]
- 161.Sun Q, Dong X, Shang Y, Sun F, Niu J, Li F. Integrin αvβ6 predicts poor prognosis and promotes resistance to cisplatin in hilar cholangiocarcinoma. Pathol Res Pract. 2020;216(7):153022. doi: 10.1016/j.prp.2020.153022 [DOI] [PubMed] [Google Scholar]
- 162.Peng ZW, Ikenaga N, Liu SB, et al. Integrin αvβ6 critically regulates hepatic progenitor cell function and promotes ductular reaction, fibrosis, and tumorigenesis. Hepatology. 2016;63(1):217‐232. doi: 10.1002/hep.28274 [DOI] [PMC free article] [PubMed] [Google Scholar]