ABSTRACT
This study aimed to evaluate the efficacy of combining immune checkpoint inhibitors (ICIs) and anti-angiogenic agents in treating lung cancer patients with bone metastases (BMs), as it is unclear whether this combination is effective for this condition. Non-small cell lung cancer patients with BMs receiving ICIs were divided into experimental and control groups based on anti-angiogenic treatment. Progression-free survival (PFS) and overall survival (OS) were evaluated using the Kaplan-Meier method, with log-rank test for comparisons. Prognostic factors were determined by univariate and multivariate Cox regression analyses. The study included 95 patients. The experimental group (n = 42) had a higher disease control rate (DCR) (90.5% vs. 68.6%, p = .009), objective response rate (ORR) (35.7% vs. 24.5%, p = .235), and longer median bone PFS (14.3 months vs. 8.3 months, p = .011) for bone metastasis. However, there were no significant differences in overall DCR (92.8% vs. 86.7%, p = .339), ORR (64.3% vs. 62.3%, p = .839), and PFS (12.4 months vs. 11.6 months, p = 0.383) between the 2 groups. The experimental group had a lower incidence of skeleton-related events (SREs) (28.6% vs. 35.8%, p = .425), and SRE patients had shorter PFS (7.7 vs. 14.3 months, p < .001) and OS (12.1 vs. 19.0 months, p = .028). Anti-angiogenic therapy (HR = 0.55, p = .012) and SRE (HR = 2.93, p < .001) were identified as independent prognostic factors for bone metastatic PFS. Adverse events were slightly higher in the experimental group (29.3% vs. 18.9%, p = .238), but not statistically significant. The combination of ICIs and anti-angiogenic agents leads to a significant PFS for BMs and potentially decreases SRE.
KEYWORDS: Non-small cell lung cancer, bone metastases, immune checkpoint inhibitors, anti-angiogenic agents, efficacy
Introduction
Lung cancer has a high incidence of morbidity and mortality, with a significant number of patients already having distant metastases at diagnosis.1 Bone metastasis occurs in approximately 30–40% of advanced non-small cell lung cancer (NSCLC) cases, second only to brain metastasis.2,3 The spine is the most common site of bone metastasis, followed by the ribs, pelvis, limbs, and sternum.4,5 Patients with bone metastases (BMs) often suffer from complications, with 30–50% developing skeleton-related events (SREs) during treatment, such as pathological fractures, spinal cord compression, skeletal surgery, or bone palliative radiotherapy. These complications significantly impact their quality of life and subsequent treatment strategies.6–8
Immune checkpoint inhibitors (ICIs) inhibit the programmed death receptor 1/Programmed death ligand 1 (PD-1/PD-L1) or cytotoxic T lymphocyte-associated protein 4 (CTLA-4) pathways, releasing the inhibitory effect on T lymphocytes and exerting antitumor effects.9 Previous studies have demonstrated that ICIs significantly prolong progression-free survival (PFS) and overall survival (OS) of NSCLC patients, providing hope for those with advanced lung cancer.10–14 Anti-angiogenic agents primarily inhibit neovascularization and normalize tumor blood vessels, playing an anti-tumor role. Additionally, they can modify the immunosuppressive state of the tumor microenvironment and enhance immune cell tumor-killing activity.15,16 Clinical studies have shown that combining ICIs with anti-angiogenic agents improves the therapeutic effect of NSCLC.17 However, it is unclear whether anti-angiogenic agents enhance the efficacy of ICIs in treating BMs.
Previous studies have demonstrated that malignant BMs are highly vascularized, which creates a favorable environment for their growth.18 Moreover, the bone has a unique immune microenvironment that differs from other organs, and studies have shown that bone is particularly immunocompromised.19 Anti-angiogenic agents can alleviate hypoxia conditions and downregulate the expression of vascular endothelial growth factor, which may improve the immunosuppressed state of BMs. Therefore, they have the potential to enhance the efficacy of immunotherapy. This retrospective study was conducted to evaluate the effectiveness of combining ICIs and anti-angiogenic agents in treating BMs in NSCLC.
Patients & methods
Patient and study design
This retrospective study included lung cancer patients with BMs who received combined immunotherapy and chemotherapy for at least 2 cycles at the First Affiliated Hospital of Guangzhou Medical University from January 2018 to June 2021. Patient information, including demographics, clinical characteristics, treatment regimens, and outcomes, was collected through the electronic medical record system. Patients with incomplete information were excluded. The study was followed up until September 2022. Patients were divided into experimental and control groups based on whether they received anti-angiogenic treatment or not. During the data collection, we observed that patients who received ICIs alone were rarely treated with anti-angiogenic agents at the outset. To mitigate this bias, we focused our investigation on evaluating the impact of anti-angiogenic drugs on BMs, thereby excluding patients who exclusively received ICIs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. Written informed consent was waived due to the nature of the retrospective study.
Efficacy evaluation
The efficacy of primary lung and bone metastasis lesions was assessed using imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography/computed tomography (PET/CT), and emission computed tomography (ECT). Immunotherapy efficacy was evaluated according to Response Evaluation Criteria In Solid Tumors (version 1.1),20 while the efficacy of BMs was evaluated using MD Anderson Cancer Center criteria.21 The objective response rate (ORR) was calculated as the percentage of patients with CR or PR, while disease control rate (DCR) was calculated as the percentage of patients with CR, PR, or SD. PFS for overall efficacy and bone metastatic PFS (bPFS) were calculated from the first ICIs administration to disease progression, death, or last follow-up on October 1, 2022. OS was estimated from the ICIs start date until death from any cause or last follow-up. Patients who had not reached the endpoint event at the study cutoff or were lost to follow-up were considered censored.
Statistical analysis
Jamovi (version 2.3.13) was used for statistical analysis. The independent sample t-test was used for normally distributed continuous variables, while the independent sample rank sum test was used for non-normally distributed continuous variables. Categorical variables were expressed as frequencies (%), and differences between groups were compared using the chi-square test (χ2) or Fisher’s exact test. Kaplan-Meier method was used to estimate PFS and OS, and Log-rank tests were performed to compare differences between groups. Cox regression was utilized to identify variables associated with PFS, with variables having p < .05 and clinical significance included in multivariate analysis to identify PFS-independent prognostic factors. All tests were two-tailed, and p < .05 was considered statistically significant.
Results
Patient characteristics
The study included 95 patients, with 42 in the experimental group and 53 in the control group. The clinical characteristics of all patients are presented in Table 1. The majority of the overall population was younger than 65 years (67.4%), and 74.7% were male. Among them, most had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0–1, a smoking history, and were diagnosed with lung adenocarcinoma, mainly at stage IV B. The proportion of patients with EGFR mutation-positive tumors was 16.8%, and PD-L1 expression was detected in 50.5% of patients before immunotherapy. In addition to BMs, most patients had other distant metastases, including brain and liver metastases. ICIs were administered as first-line treatment in 52.7% of patients and as second-line or beyond therapy in 43.2% of patients. There were no significant clinical differences between the experimental and control groups.
Table 1.
Clinical characteristics between the experimental group and control group.
| Characteristic | All (N = 95) | ICIs (n = 53) | ICIs + Anti-angiogenic agents (n = 42) | p |
|---|---|---|---|---|
| Sex, Male, n (%) | 71 (74.7%) | 31 (74.7%) | 40 (73.8%) | .853 |
| Age, ≤65, n (%) | 64 (67.4%) | 35 (66.0%) | 29 (69.0%) | .756 |
| Smoking, Yes, n (%) | 45 (47.4%) | 29 (54.7%) | 16 (38.1%) | .107 |
| ECOG PS | .463 | |||
| 0–1 | 84 (88.4%) | 48 (90.6%) | 36 (85.7%) | |
| ≥2 | 11 (11.6%) | 5 (9.4%) | 6 (14.3%) | |
| Histology | .285 | |||
| Adenocarcinoma | 73 (76.8%) | 39 (73.6%) | 34 (81.0%) | |
| Squamous carcinoma | 15 (15.8%) | 11 (20.8%) | 4 (9.5%) | |
| Other | 7 (7.4%) | 3 (5.7%) | 4 (9.5%) | |
| Clinical stage | .152 | |||
| IVa | 11 (11.6%) | 9 (17.0%) | 3 (7.1%) | |
| IVb | 84 (88.4%) | 44 (83.0%) | 39 (92.9%) | |
| Treatment line | .106 | |||
| 1 | 54 (56.8%) | 34 (64.2%) | 20 (47.6%) | |
| ≥2 | 41 (43.2%) | 19 (35.8%) | 22 (52.4%) | |
| EGFR status, Yes, n (%) | 16 (16.8%) | 7 (13.2%) | 9 (21.4%) | .288 |
| PD-L1 expression | .936 | |||
| <1% | 18 (18.9%) | 11 (20.8%) | 7 (16.7%) | |
| 1–49% | 13 (13.7%) | 7 (13.2%) | 6 (14.3%) | |
| ≥50% | 17 (17.9%) | 10 18.9%) | 7 (16.7%) | |
| NA | 47 (49.5%) | 25 (47.2%) | 22 (52.4%) | |
| Other distant metastases, Yes, n (%) | 71 (74.7%) | 39 (73.6%) | 32 (76.2%) | .772 |
| Pleura, Yes, n (%) | 52 (54.7%) | 33 (62.3%) | 19 (45.2%) | .098 |
| Brain metastases, Yes, n (%) | 24 (25.3%) | 12 (22.6%) | 12 (28.6%) | .509 |
| Liver metastases, Yes, n (%) | 21 (22.1%) | 10 (18.9%) | 11 (26.2%) | .393 |
| Adrenal metastases, Yes, n (%) | 10 (10.5%) | 4 (7.5%) | 6 (14.3%) | .288 |
ECOG PS, Eastern Cooperation Oncology Group performance status; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death-ligand 1; ICIs, immune checkpoint inhibitors.
Status of bone metastases
Figure 1 displays the sites and number of BMs for all patients. The spine was the most common site of BMs, followed by the ribs, pelvis, limb, and sternum. Among spinal metastases, the thoracic vertebra (67.4%) was the most common, while the cervical and sacrococcygeal vertebrae were the least common. The majority of patients (73.7%) had two or more bone lesions, while only 26.3% had one.
Figure 1.
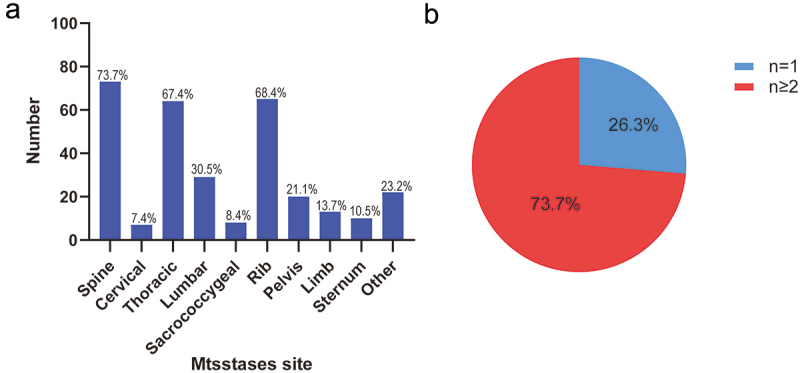
Sites (A) and number of bone metastases (B) in all patients (n = 95).
Efficacy and prognosis
Figure 2a illustrates the evaluation of bone metastasis efficacy, with the experimental group demonstrating a higher DCR (90.5% vs. 68.6%, p = .009), ORR (35.7% vs. 24.5%, p = .235), and longer median bone PFS (mbPFS) (14.3 months vs. 8.3 months, p = .011) compared to the control group (Figure 2c). However, in the overall efficacy evaluation, there was no significant difference between the experimental and control groups, with a DCR of 92.8% vs. 86.7% (p = .339), ORR of 64.2% vs. 62.2% (p = .839) (Figure 2b), and mPFS of 12.4 months vs. 11.6 months (p = .383) (Figure 2d). The analysis of overall survival revealed that the mOS of the control group was slightly longer than the experimental group (19.2 months vs. 17.5 months) (Figure 2e), but the difference was not statistically significant (p = .932). These findings suggest that the combination of ICI and anti-angiogenic drugs (such as bisphosphonates and RANK-ligand inhibitors) slightly prolonged PFS but showed no significant impact on OS among patients with BM.
Figure 2.
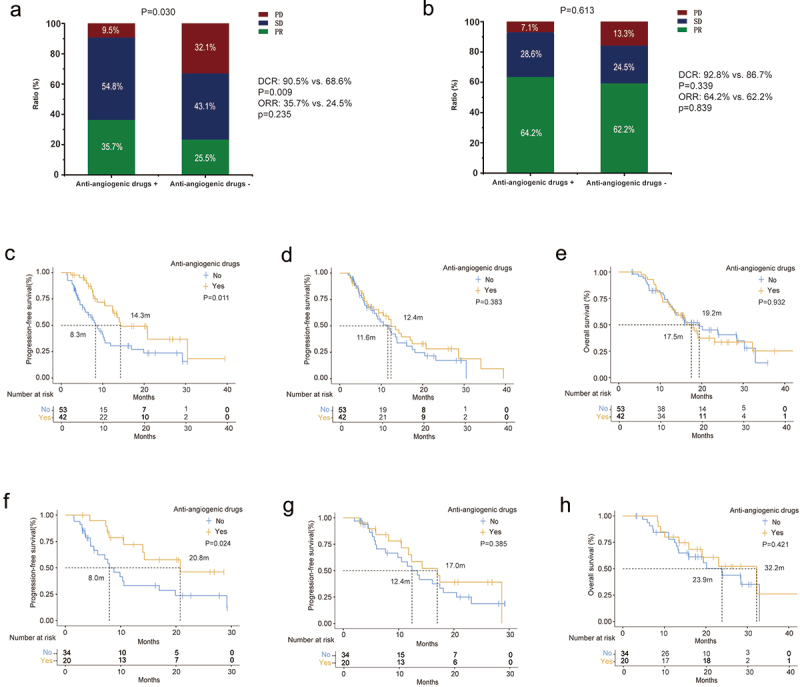
Bone metastases and overall efficacy and survival characteristics of two group patients. Response rate of BMs (a) and overall efficacy (b). PFS of BMs (c) and overall efficacy (d), and OS (e) of two group patients. PFS of BMs (f) and overall efficacy (g), and OS (h) of two group patients receiving first-line therapy.
To account for the potential impact of treatment line on outcomes, we included 54 patients who received ICIs as first-line treatment, with 20 in the experimental group and 34 in the control group. In this subgroup analysis, the experimental group still demonstrated a significantly longer mbPFS compared to the control group (20.8 months vs. 8.0 months, p = .024) (Figure 2f), and a slightly better overall efficacy with a mPFS of 17.0 months vs. 12.4 months (p = .385) (Figure 2g). While the experimental group had a longer OS than the control group (32.2 months vs. 23.9 months, p = .421) (Figure 2h), the difference was not statistically significant.
Efficacy and prognosis of skeleton-related events (SREs)
As shown in Table 2, 31 patients (32.6%) experienced at least one SRE, including pathological fracture (12.6%), spinal cord compression (3.2%), bone surgery (6.3%), and bone radiation therapy (10.5%). Of these, 22 patients (23.2%) developed an SRE after ICIs treatment. Compared to the control group, the experimental group had a slightly lower incidence of SRE (28.6% vs. 35.8%, p = .425), but the difference was not statistically significant. In the bone metastasis efficacy and survival analysis, patients who experienced at least one SRE had a significantly shorter PFS of BMs (7.7 months vs. 14.3 months, p < .001) and OS (12.1 months vs. 19.0 months, p = .028) (Figure 3).
Table 2.
Comparison of SRE between the two groups.
| Type of SRE | All (N = 95) | ICIs (n = 53) | ICIs + Antiangiogenic agents (n = 42) | p |
|---|---|---|---|---|
| All SRE | 31 (32.6%) | 19 (35.8%) | 12 (28.6%) | .425 |
| Pathological fractures | 12 (12.6%) | 8 (15.1%) | 4 (9.5%) | .417 |
| Spinal cord compression | 3 (3.2%) | 1 (1.9%) | 2 (4.8%) | .426 |
| Surgery | 6 (6.3%) | 4 (7.5%) | 2 (4.8%) | .579 |
| Palliative bone radiotherapy | 10 (10.5%) | 6 (11.3%) | 4 (9.5%) | .777 |
| SRE after ICIs | 22 (23.2%) | 15 (28.3%) | 7 (16.7%) | .182 |
SRE, skeletal-related event; ICIs, immune checkpoint inhibitors.
Figure 3.
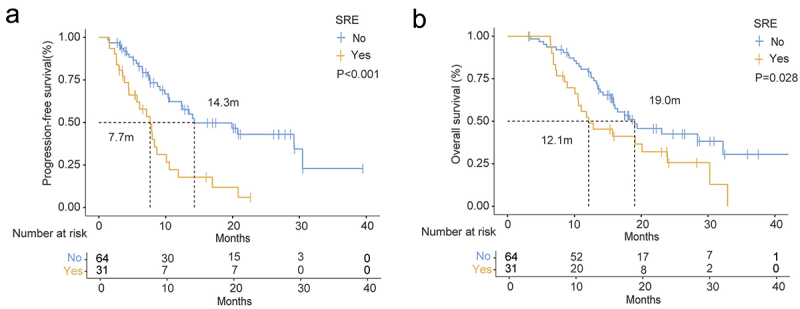
Bone metastases efficacy and survival characteristics of patients with or without SRE. (a) PFS of BMs and OS (b) of two group patients.
Univariate and multivariate analyses
The univariate analysis encompassed clinical variables including the utilization of bone targeting agents, which may have an effect on BMs and overall PFS. More than half of the patients received bone targeting agents (bisphosphonates and RANK-ligand inhibitors), and the use of other bone agents was comparable in the two groups (Table 3). In the univariate analysis, ECOG PS score (HR = 2.70, p < .021), squamous cell carcinoma (HR = 1.89, p = .059), anti-angiogenic therapy (HR = 0.49, p = .012), SRE (HR = 2.93, p < .001), and pleural metastases (HR = 2.05, p = .014) exhibited significant correlations with PFS of bone metastasis lesions. These factors, along with those deemed clinically significant, were included in the multivariate analysis. The results revealed that anti-angiogenic therapy (HR = 0.55, p = .047) and SRE (HR = 2.93, p = .002) remained as independent prognostic factors for PFS of bone metastasis lesions.
Table 3.
Univariate and multivariate analysis of bPFS in all patients.
| Characteristic | n (%) | Univariable |
Multivariable |
||
|---|---|---|---|---|---|
| HR(95%CI) | p | HR(95%CI) | p | ||
| Sex(Female VS. Male) | 24 (25.3) | 1.31 (0.71–2.43) | .392 | ||
| Age (>65 VS. ≤65) | 31 (32.6) | 1.02 (0.57–1.84) | .947 | ||
| Smoking, Yes, n (%) | 45 (47.4) | 1.24 (0.72–2.14) | .444 | ||
| ECOG PS (≥2 VS.0–1) | 11 (11.6) | 2.70 (1.16–6.30) | .021 | 2.07 (0.86–4.96) | .103 |
| Clinical stage (IVb VS. IVa) | 83 (87.4) | 1.20 (0.51–2.81) | .679 | ||
| Pathology | |||||
| Adenocarcinoma | 73 (76.8) | - | |||
| Squamous | 15 (15.8) | 1.89 (0.98–3.64) | .059 | 1.59 (0.81–3.15) | .179 |
| Other | 7 (7.4) | 0.89 (0.27–2.90) | .847 | 1.91 (0.54–6.74) | .311 |
| EGFR, Yes, n (%) | 16 (16.8) | 1.64 (0.79–3.41) | .185 | ||
| KRAS, Yes, n (%) | 11 (11.6) | 0.42 (0.15–1.17) | .098 | ||
| PD-L1 expression | |||||
| <1% | 18 (18.9) | - | |||
| 1–49% | 13 (13.7) | 1.14 (0.44–2.98) | .783 | ||
| ≥50% | 17 (17.9) | 0.60 (0.22–1.63) | .316 | ||
| NA | 47 (49.5) | 1.10 (0.55–2.21) | .795 | ||
| anti-angiogenic agents, Yes, n (%) | 42 (44.2) | 0.49 (0.28–0.86) | .013 | 0.55 (0.30–0.99) | .047 |
| Line (≥2 VS.1) | 41 (43.2) | 1.11 (0.63–1.94) | .719 | ||
| Bone targeting agents, Yes, n (%) | 55 (57.9) | 0.86 (0.49–1.49) | .583 | 0.64 (0.34–1.20) | .165 |
| Number (≥2 VS.1) | 70 (73.7) | 0.99 (0.54–1.84) | .984 | ||
| SRE, Yes, n (%) | 31 (32.6) | 2.93 (1.67–5.17) | <.001 | 2.93 (1.50–5.70) | .002 |
| Other metastases, Yes, n (%) | 71 (74.7) | 1.22 (0.65–2.28) | .544 | ||
| Brain, Yes, n (%) | 24 (25.3) | 1.45 (0.80–2.65) | .223 | ||
| Liver, Yes, n (%) | 21 (22.1) | 1.41 (0.75–2.66) | .286 | ||
| Adrenal glands, Yes, n (%) | 10 (10.5) | 0.60 (0.22–1.68) | .331 | ||
| Pleura, Yes, n (%) | 52 (54.7) | 2.05 (1.16–3.63) | .014 | 1.54 (0.83–2.86) | .174 |
bPFS, progression-free survival of bone metastases lesions; ECOG PS, Eastern Cooperation Oncology Group performance status; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog; PD-L1, programmed cell death-ligand 1; SRE, skeletal-related event.
Adverse events (AEs)
We recorded and evaluated severe AEs ≥ Grade 3 after different treatments in this study, as presented in Table 4. These included dermatitis (5.3%), embolism (3.2%), hemoptysis (4.3%), diarrhea (2.1%), hypohepatia (1.1%), bone marrow suppression (3.2%), immune pneumonia (2.1%), immune myocarditis (1.1%), immune-related myositis (1.1%), immune-related ophthalmia (1.1%), and hypothyroidism (2.1%). The probability of severe AEs in the experimental group was slightly higher than that in the control group (29.3% vs. 18.9%, p = .238), particularly in hemoptysis (7.3% vs. 1.9%, p = .196) and severe diarrhea (4.9% vs. 0%, p = .104), but the differences were not statistically significant.
Table 4.
Comparison of severe AEs between the two groups.
| Type of AEs | All (N = 95) | ICIs (n = 53) | ICIs + Antiangiogenic agents (n = 42) | p |
|---|---|---|---|---|
| All severe AEs | 22 (23.4%) | 10 (18.9%) | 12 (29.3%) | .238 |
| Dermatitis | 5 (5.3%) | 2 (3.8%) | 3 (7.3%) | .448 |
| Embolism | 3 (3.2%) | 1 (1.9%) | 2 (4.9%) | .413 |
| Hemoptysis | 4 (4.3%) | 1 (1.9%) | 3 (7.3%) | .196 |
| Diarrhoea | 2 (2.1%) | 0 (0.0%) | 2 (4.9%) | .104 |
| Hypohepatia | 1 (1.1%) | 1 (1.9%) | 0 (0.0%) | .377 |
| Bone marrow suppression | 3 (3.2%) | 1 (1.9%) | 2 (4.9%) | .413 |
| Immune pneumonia | 2 (2.1%) | 1 (1.9%) | 1 (2.4%) | .854 |
| Immune myocarditis | 1 (1.1%) | 0 (0.0%) | 1 (2.4%) | .253 |
| Immune-related myositis | 1 (1.1%) | 1 (1.9%) | 0 (0.0%) | .377 |
| Immune-related ophthalmia | 1 (1.1%) | 1 (1.9%) | 0 (0.0%) | .377 |
| Hypothyroidism | 2 (2.1%) | 2 (3.8%) | 0 (0.0%) | .209 |
AEs, adverse events; ICIs, immune checkpoint inhibitors.
Discussion
Currently, ICIs are widely used in advanced lung cancer, particularly in NSCLC. Compared to no anti-angiogenic agents, combining immunotherapy with anti-angiogenic therapy has shown certain benefits for advanced lung cancer patients.17 In patients with distant metastases, the combination of ICIs and anti-angiogenic therapy has demonstrated good efficacy in those with brain metastases.22 However, the benefit of combining ICIs and anti-angiogenic therapy in patients with BMs is currently unknown. Our findings suggest that the combination of ICIs with anti-angiogenic agents may significantly prolong the PFS of BMs lesions and reduce the incidence of SRE in BMs. Furthermore, our study shows that after achieving stabilization of BMs with anti-angiogenic agents at an early stage, these lesions can remain stable for an extended period of time.
Previous studies have demonstrated the effectiveness of anti-angiogenic therapy in treating BMs during the period when chemotherapy was the primary treatment. Takaaki et al. found that the combination of chemotherapy and bevacizumab resulted in a median time to progression (TTP) of BMs of 13.7 months with an ORR of 23%, compared to a TTP of 4.3 months and an ORR of 0% without the combination.23 Nowadays, immunotherapy is widely used in advanced lung cancer patients, but most studies have suggested that bone metastasis is a negative factor in immunotherapy.24–26 Furthermore, ICIs alone are not effective for BMs and do not provide long-lasting benefits. A study of 48 patients with malignant BMs treated with ICIs alone found that the ORR of BMs was only 23%, the DCR was 69%, and the mPFS was only 6 months.27 Our study also revealed that the DCR and mPFS of BMs in the control group were only 67.9% and 8.3 months, respectively, significantly lower than those in the experimental group (DCR: 90.5%, mPFS: 14.3 months). Furthermore, our results showed a lower incidence of SRE in the experimental group, although this was not statistically significant. Further analysis revealed that patients who developed SRE had worse treatment outcomes and prognoses. Finally, both univariate and multivariate analyses demonstrated that anti-angiogenic therapy was an independent protective factor for BMs. These results suggest that the combination of immunotherapy and anti-angiogenic therapy can improve the efficacy of BMs.
The role of anti-angiogenic agents in the immunotherapy of BMs is still not fully understood. Bone is an organ that is rich in blood vessels, particularly in the axial bone of the spine, ribs, and other parts of the body where there is a rich blood supply and slow blood flow, making it easier for tumor cells to metastasize and proliferate.4 A previous study has shown that BMs in malignant tumors contain a more complex and richer vascular system than normal bone marrow and surrounding bone tissue.18 These findings suggest that angiogenesis plays an important role in the occurrence and development of BMs in malignant tumors. Additionally, as bone is the primary site of hematopoiesis production, the skeletal immune microenvironment is continually in a state of suppression to prevent immune cells from destroying new blood cells.28,29 Previous studies have shown that anti-angiogenic agents can not only inhibit tumor angiogenesis and normalize tumor vasculature but also alter the tumor microenvironment and reverse its immunosuppressive state, thereby enhancing the anti-tumor effects of the body’s immune cells.15,16 Therefore, we speculate that based on the combination of anti-angiogenic agents, ICIs may further alleviate the immunosuppressive state of BMs and enable immune cells to more effectively kill tumor cells.
In our analysis of overall therapeutic efficacy, we found that the ORR and DCR in the experimental group were slightly improved, and the mPFS was also slightly prolonged compared to the control group (12.4 m VS 11.6 m, p = .383), which was consistent with previous studies.30–32 However, there was no significant difference in survival between the two groups in our study, which may be due to the smaller sample size and the fact that more patients in the experimental group had received second-line and above treatments. It is worth noting that the ORR and DCR of overall efficacy were better than those of BMs in both groups, and the overall mPFS was longer than the mPFS of BMs lesions in the control group, while the overall mPFS was shorter than those of BMs lesions in the experimental group. This indicates that BMs are still less sensitive to drug treatment than other metastases. It also suggests that after obtaining stabilization of BMs with anti-angiogenic agents at an early stage, BMs can remain stable for a long time in the future even if the tumor in the primary lesion progresses during subsequent treatment. Finally, regarding adverse events, although the experimental group had slightly higher severe AEs such as thrombosis, hemoptysis, and diarrhea, we found that these adverse reactions could be alleviated by symptomatic treatment.
Although there was no significant increase in overall PFS, the increase in bPFS may have a positive impact on patients’ quality of life by reducing a series of bone-related events brought about by the progression of BM and reducing pain. Moreover, there is evidence to suggest that anti-angiogenic agents may enhance the efficacy of ICI. Previous studies have demonstrated that malignant BM are highly vascularized, which creates a favorable environment for their growth. Moreover, the bone has a unique immune microenvironment that differs from other organs, and studies have shown that bone is particularly immunocompromised. Anti-angiogenic agents can alleviate hypoxia conditions and downregulate the expression of VEGF, which may improve the immunosuppressed state of BM. Therefore, they have the potential to enhance the efficacy of immunotherapy. Therefore, we recommend considering the addition of anti-angiogenic agents in the early treatment stage for lung cancer patients with BM, as this may result in stable control of BM. Future studies may consider exploring the immune microenvironment of BM to elucidate the mechanism underlying the efficacy.
To the best of our knowledge, this study is the first to investigate the effect of combining ICIs with anti-angiogenic agents on the efficacy of BMs. Our findings suggest that combining these therapies in patients with BMs is necessary and that adverse effects can be managed. However, our study has several limitations. First, as a retrospective study, some clinical data may be missing or incomplete, which could limit the interpretation of the results. Second, the efficacy of BMs was evaluated using the MD Anderson criteria, which do not accurately measure the size of BMs lesions, and empirical judgment by clinical and imaging physicians may introduce bias. Finally, due to the small sample size, future studies with larger sample sizes or relevant prospective studies are needed to validate our findings.
Conclusions
In summary, our study suggests that the combination of ICIs with anti-angiogenic agents can significantly prolong the PFS of BMs lesions, and that after obtaining stabilization of BMs with anti-angiogenic agents at an early stage, BMs lesions can remain stable for a long time. Furthermore, the incidence of SRE could be decreased with the combined treatment, although adverse events might increase, they could be managed and controlled with symptomatic treatment.
Acknowledgments
We thank Dr. Bin Qiao for editing this manuscript.
Funding Statement
This study was supported by grants from State Key Laboratory of Respiratory Disease-The Open Project [No. SKLRD-OP-202011], the Beijing Xisike Clinical Oncology Research Foundation [No. Y-HS202102-0118], and the Wu Jieping Medical Foundation [No. 320.6750.2021-17-7].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical disclosure
Institutional review board/ethics committee approval was obtained from the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, Guangdong, China). Individual consent for this retrospective analysis was waived.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. A Cancer J Clin. 2021;71(3):209–8. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57(2):229–32. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Kuchuk M, Addison CL, Clemons M, Kuchuk I, Wheatley-Price P. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol. 2013;2(1):22–9. doi: 10.1016/j.jbo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y, Zhang J, Zhou Z, Liu D, Zhu H, Wen J, Xu X, Chen T, Fan M. Metastasis patterns and prognosis of octogenarians with NSCLC: a population-based study. Aging Dis. 2020;11(1):82–92. doi: 10.14336/AD.2019.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 7.Sun JM, Ahn JS, Lee S, Kim JA, Lee J, Park YH, Park HC, Ahn MJ, Ahn YC, Park K. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71(1):89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Decroisette C, Monnet I, Berard H, Quere G, Le Caer H, Bota S, Audigier-Valette C, Geriniere L, Vernejoux JM, Chouaid C, et al. Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol. 2011;6(3):576–82. doi: 10.1097/JTO.0b013e318206a1e3. [DOI] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 14.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 15.Heine A, Held SA, Bringmann A, Holderried TA, Brossart P. Immunomodulatory effects of anti-angiogenic drugs. Leukemia. 2011;25(6):899–905. doi: 10.1038/leu.2011.24. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, Gao Y, Li K. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Disease. 2020;11(5):309. doi: 10.1038/s41419-020-2511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol. 2021;12:689132. doi: 10.3389/fimmu.2021.689132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez-Macgregor M, Aviles-Salas A, Green D, Fuentes-Alburo A, Gómez-Ruiz C, Aguayo A. Angiogenesis in the bone marrow of patients with breast cancer. Clin Cancer Res. 2005;11(15):5396–400. doi: 10.1158/1078-0432.CCR-04-2420. [DOI] [PubMed] [Google Scholar]
- 19.Del Conte A, De Carlo E, Bertoli E, Stanzione B, Revelant A, Bertola M, Spina M, Bearz A. Bone metastasis and immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): microenvironment and possible clinical implications. Int J Mol Sci. 2022;23(12):6832. doi: 10.3390/ijms23126832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22(14):2942–53. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 22.Fang L, Zhao W, Ye B, Chen D. Combination of immune checkpoint inhibitors and anti-angiogenic agents in brain metastases from non-small cell lung cancer. Front Oncol. 2021;11:670313. doi: 10.3389/fonc.2021.670313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokito T, Shukuya T, Akamatsu H, Taira T, Ono A, Kenmotsu H, Naito T, Murakami H, Takahashi T, Endo M, et al. Efficacy of bevacizumab-containing chemotherapy for non-squamous non-small cell lung cancer with bone metastases. Cancer Chemother Pharmacol. 2013;71(6):1493–8. doi: 10.1007/s00280-013-2148-3. [DOI] [PubMed] [Google Scholar]
- 24.Landi L, D’Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A, Passaro A, Signorelli D, Gelsomino F, Galetta D, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019;7(1):316. doi: 10.1186/s40425-019-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Wang L, Chen S, Zhou F, Zhao J, Zhao W, Su C. Adverse impact of bone metastases on clinical outcomes of patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2020;11(10):2812–9. doi: 10.1111/1759-7714.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin A, Zhao S, Miah A, Wei L, Patel S, Johns A, Grogan M, Bertino EM, He K, Shields PG, et al. Bone metastases, skeletal-related events, and survival in patients with metastatic non–small cell lung cancer treated with immune checkpoint inhibitors. J Natl Compr Cancer Netw. 2021;19(8):915–21. doi: 10.6004/jnccn.2020.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gefard-Gontier E, Markich R, Zysman M, Veillon R, Daste A, Domblides C, Sionneau B, Gross-Goupil M, Lefort F, Prey S, et al. Evolution of bone metastases in patients receiving at least three months of checkpoint inhibitors. Cancer Immunol Immunother. 2022;71(11):2609–18. doi: 10.1007/s00262-022-03180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baschuk N, Rautela J, Parker BS. Bone specific immunity and its impact on metastasis. Bonekey Rep. 2015;4:665. doi: 10.1038/bonekey.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Huang Y, Hu Z, Zhao M, Li M, Bi G, Zheng Y, Liang J, Lu T, Jiang W, et al. Landscape and dynamics of single tumor and immune cells in early and advanced-stage lung adenocarcinoma. Clin Transl Med. 2021;11(3):e350. doi: 10.1002/ctm2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 31.Huang D, Cui P, Huang Z, Wu Z, Tao H, Zhang S, Xiang R, Hu Y. Anti-PD-1/L1 plus anti-angiogenesis therapy as second-line or later treatment in advanced lung adenocarcinoma. J Cancer Res Clin Oncol. 2021;147(3):881–91. doi: 10.1007/s00432-020-03380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Ji M, Jiang Y, Yin R, Wang Z, Li H, Wang S, He K, Ma Y, Wang Z, et al. A cohort study of the efficacy and safety of immune checkpoint inhibitors plus anlotinib versus immune checkpoint inhibitors alone as the treatment of advanced non-small cell lung cancer in the real world. Transl Lung Cancer Res. 2022;11(6):1051–68. doi: 10.21037/tlcr-22-350. [DOI] [PMC free article] [PubMed] [Google Scholar]


