ABSTRACT
Despite recent advances in cancer therapeutics, pancreatic ductal adenocarcinoma (PDAC) remains a lethal disease with a 5-year overall survival of only 10%. Since either at or within a few months of diagnosis, most patients with PDAC will present with metastatic disease, a more individualized approach to select patients who may benefit from more aggressive therapy has been suggested. Although studies have reported improved survival in PDAC and isolated pulmonary metastasis (ISP) compared to extrapulmonary metastases, such findings remain controversial. Furthermore, the added benefit of pulmonary metastasectomy and other lung-directed therapies remains unclear. In this review, we discuss the metastatic pattern of PDAC, evaluate the available evidence in the literature for improved survival in PDAC and ISP, evaluate the evidence for the added benefit of pulmonary metastasectomy and other lung-directed therapies, identify prognostic factors for survival, discuss the biological basis for the reported improved survival and identify areas for further research.
KEYWORDS: Pancreatic ductal adenocarcinoma, isolated pulmonary metastases, pulmonary metastasectomy, Lung resection, survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) represents approximately 3.2% of all new cancer cases but is responsible for 7.8% of cancer deaths in the United States.1,2 An estimated 62,210 new cases of pancreatic cancer and 49,830 deaths are projected for 20223. Though pancreatic cancer is the eleventh most common cancer in the United States, it is the fourth leading cause of cancer-related mortality and is projected to become the second by 2030.1,3,4 Despite recent advances in cancer therapeutics with targeted agents and immunotherapy, pancreatic adenocarcinoma remains a lethal disease with a 5-year survival of only 10% from 2010 to 2016.2 An important factor contributing to the high mortality is the lack of validated screening techniques, with a majority of patients presenting with advanced disease not amenable to curative resection.5 Over 50% of the patients at diagnosis have distant metastases with a 5-year survival rate of 2.9%.2 Even among the 20% of patients who present with resectable disease, the majority develop recurrent disease within a few months, and only about 28% survive for 5 years following curative resection and adjuvant chemotherapy.6 Another factor contributing to the poor prognosis in pancreatic adenocarcinoma is the relative chemoresistant nature of the disease.7
Following curative resection for pancreatic adenocarcinoma, approximately half of recurrences occur at distant sites.6 Thus, either at or within a few months of diagnosis, most patients with pancreatic adenocarcinoma will present with stage IV disease. The incidence of lung only metastases at the initial diagnosis or upon recurrence of PDAC has not been well defined. The incidence rates ranging from 4.76 to 12.3% have been reported.8,9 The standard therapeutic approach for both de novo and recurrent metastatic pancreatic adenocarcinoma typically involves palliative chemotherapy with a very dismal prognosis.10,11 However, especially with recurrence after pancreatectomy, there are reports that patients with isolated pulmonary metastases (IPM) may have relatively longer survival than those with metastases to other sites.12,13 Furthermore, previous studies reported survival benefit of pulmonary metastasectomy in selected patients with lung only oligo-metastatic PDAC after curative pancreatectomy.12,14 This therapeutic approach is similar to the practice in other solid tumors such as colorectal cancer where resection of pulmonary metastases in well-selected patients resulted in improved survival.15,16 A recent metanalysis by Guerra et al.13 reported improved disease-free survival (DFS) and overall survival (OS) in patients with PDAC with isolated lung metastases compared to patients with single organ metastases to the liver and those with locoregional relapse. However, due to methodology limitations of the included studies, it is unclear if the reported improved survival was due to the use of lung-directed therapy or not.13 Furthermore, the significant heterogeneity of the studies in the metanalysis may affect the validity of a single summary survival estimate, prompting this review of the current evidence.
In this review, we discuss (1) the metastatic pattern of PDAC, (2) evaluate the available evidence in the literature for survival in patients with PDAC with IPM, (3) evaluate the evidence for the added benefit of pulmonary metastasectomy and other forms of lung-directed therapies, (4) identify prognostic factors for survival, (5) discuss the possible biological basis for the reported improved survival in this subset of patients with PDAC and (6) identify areas for further research.
Methods
We conducted a search of original articles and case reports in PubMed, EMBASE, Scopus Web of Knowledge with the search performed in January 2022 using the medical subject heading (MeSH) terms “pancreatic cancer”, “pancreatic adenocarcinoma”; “isolated pulmonary metastases”, or “Isolated lung metastases” and “pancreatic cancer “or “pancreatic adenocarcinoma” and “pulmonary metastasectomy” or and “pancreatic cancer “or “pancreatic adenocarcinoma” and “lung resection”. All retrieved abstracts were reviewed for relevance. Furthermore, we conducted a manual search by using the references of the selected articles to identify additional publications. We also reviewed published abstracts of society meetings including the American Society of Clinical Oncology. Articles were then selected based on the relevance to the topic of interest. Articles published in languages other than English were excluded. Additionally, publications that do not report separate survival data for post-pancreatectomy patients with PDAC and metachronous IPM were excluded.
Results
A total of 22 publications were selected. Fourteen articles addressed the issue of survival in patients with metachronous IPM following pancreatectomy for PDAC compared to patients with metastases to other organs (Table 1). Four of the 14 publications also reported findings in patients with PDAC and synchronous IPM (Table 2). Eight publications reported the benefit or not of lung-directed therapy: mainly pulmonary metastasectomy (Table 3). Most studies were retrospective cohort studies while a few were case series or case reports. None of the selected studies was a randomized controlled trial.
Table 1.
Studies reporting survival for PDAC and metachronous isolated lung metastases.
| Author | Year | Median age (years) | Sample size | Median Survival after recurrence (months) | Median overall survival (months) |
|---|---|---|---|---|---|
| Zheng et al.17 Meyers et al.18 Gotfried and Kozuch9 Kruger et al.19 Jones et al.6 Sahin et al.20 Liu et al.21 Guerra et al.13 * Wangjam et al.22 Watanabe et al.23 Nakagawa et al.24 ** Ariake et al.25 Liu et al.26 * Downs-Canner et al.12 |
2017 2014 2011 2016 2019 2018 2019 2020 2015 2017 2018 2017 2020 2016 |
67 68 53 69 66 66 62 NR NR 70 68 67 NR 66 |
24 15 3 27 52 47 22 286 24 11 22 25 79 49 |
20 14 36 31.3 15 15 NR NR 8.5 17 NR NR 83 NR |
36 32 NR 46.4 33 NR 11.8 34.7 27.8 36 NR 31.9 120 35.6 |
NR: Not Reported. *Metanalysis: data included are for PDAC and IPM after pancreatectomy.
**Study reported 5-year overall survival of about 50%.
Table 2.
Studies reporting survival for PDAC with synchronous isolated lung metastases.
| Author | Year | Median age (years) | Sample size | Median overall survival (months) |
|---|---|---|---|---|
| Liu et al.26 Kruger et al.19 Downs-Canner et al.12* Guerra et al.13 * |
2020 2016 2016 2020 |
NR 69 66 NR |
740 13 96 799 |
6 22.8 10.1 7.3 |
NR: Not Reported. *Studies may also include some patients who had initial chemoradiation for unresected PDAC and later developed Isolated pulmonary metastases.
Table 3.
Studies evaluating impact of pulmonary metastasectomy and other lung directed therapies.
| Author | Year | Median age (years) | Sample size | Median Survival after Lung recurrence with Lung Directed Therapy | Median OS with Chemotherapy (Months) |
Median OS with Lung Directed Therapy (Months) |
p-value |
|---|---|---|---|---|---|---|---|
| Downs-Canner et al.12 * Robinson et al.14 Thomas et al.27 Arnaoutakis et al.28 Tagawa et al.29 Kurahara et al.30 Kim et al.31 Groot et al.27 Lovecek et al.32 |
2015 2016 2012 2011 2017 2019 2019 2019 2017 |
NR 61.5 61.7 31 72 72 NR 68 NR |
41 16 7 9 10 33 23 96 3 |
NR NR NR 18.6 38.5 NR 36.5 35.0 12 |
33.8 NR NR 23 NR 16.4 NR 34.2 NR |
67.5 28 92.3 51 66.2 36.5 NR 68.9 NR |
0.006 NR NR 0.04 NR 0.025 0.01** <0.001*** NR |
NR: Not Reported. *Study included patient who had lung resection, SBRT and other lung directed therapies. **Lung resection compared with palliative chemotherapy. ***Lung resection compared to palliative chemotherapy and or radiotherapy.
Patterns of metastases in pancreatic cancer
Although the pattern of metastatic disease at presentation generally does not influence therapeutic decisions in patients with synchronous metastatic pancreatic cancer, some studies have reported on the metastatic pattern at presentation. Liu et al.26 in an analysis of patients with PDAC using the surveillance, epidemiology, and end results (SEER) database from 2010 to 2014 reported synchronous metastases limited to a single organ in 81% of subjects. Of the patients with single organ synchronous metastases,10.1% had isolated lung metastases.26 Oweira et al.33, in another retrospective review of SEERs database from 2010 to 2013, reported single organ metastases in 66.3% of subjects with synchronous metastatic PDAC. Liver and lung only metastases were reported in 76% and 19.9% respectively.
Jones et al.6 in a secondary analysis of the ESPAC-4 trial reported metachronous oligometastases to the lung in 10.9% of all recurrences. Other retrospective series have reported incidences as high as 13% for IPM in patients who developed recurrence after pancreatectomy for PDAC.34 According to Katz et al.35, the most common organ for first recurrence in the first 3 years after diagnosis was liver, accounting for about 46%, while the most common organ for recurrence after 3 and 5 years following pancreatectomy was lung accounting for 56% and 86% of recurrences respectively. Downs-Canner et al.12 found that the time to development of lung metastases was not significantly different between patients with lung first and only, or those with synchronous lung and intrabdominal, or patients with intrabdominal before lung metastases. In summary, the findings from these studies suggest that metastasis to the lungs in patient with resected PDAC is typically a later event when compared to other sites.
Metachronous pulmonary recurrence after pancreatectomy (Table 1)
Reports from two retrospective series suggested significantly improved DFS and OS in patients with recurrent lung only metastases that were treated with palliative chemotherapy without specific lung directed therapy, compared to liver, peritoneal, lymphatic, or local recurrence. Meyers et al.18 reported on 70 patients who developed recurrent disease following pancreatectomy for PDAC and noted 14 months survival from the time of recurrence in patients with IPM compared to 6 and 4 months for patients with liver first metastases, and other site recurrence respectively. The median OS in patients with IPM was 32 months, compared to 17 and 20 months for liver first and other site recurrences, respectively. Lung as first site of recurrence and treatment with chemotherapy was determined to be a predictor of improved post recurrence survival and OS .18 In another study including 232 patients who had a relapse following pancreatectomy, 24 patients had lung only recurrence.17. These patients had longer survival after recurrence and OS of 20 and 36 months respectively compared to 5 and 10 months in patients with liver recurrence. The corresponding five-year OS was 27% for patients with lung only metastases compared to 1.27% and 7.76% for liver and local recurrence, respectively. Patients with lung only metastases at recurrence when compared to those with liver and local recurrences also have a longer disease-free interval, measured from primary resection of the pancreatic cancer to the time of recurrence (DFS1), of 15 months vs. 5 and 8 months, respectively. Non-lung recurrence was a significant independent predictor of poorer prognosis.17
Improved OS for patients with PDAC with isolated pulmonary metastases was also confirmed in another retrospective study by Kruger et al.19 who reported median OS of 25.5 months and 9.4 months for PDAC patients with IPM and unselected patients with metastatic disease respectively. A majority (84%) of these patients underwent palliative chemotherapy mostly with single agent regimens (56%), five patients had only supportive care and one patient had palliative radiation to the lung metastasis. None had pulmonary metastasectomy.19
Gotfried and Kozuch9 reported three cases with prolonged survival ranging from 32 to 44 months following lung only recurrence after primary treatment of pancreatic adenocarcinoma with either definitive chemo(radio)therapy or pancreatectomy and adjuvant chemotherapy. One patient in the series survived 32 months without any treatment after developing progression of his disease with lung only metastasis while others received subsequent line(s) of chemotherapy.9 In a recent metanalysis including 1199 patients with IPM of which 286 had lung only recurrence post-pancreatectomy, Guerra et al.13 reported an OS of 34.7 months for lung only recurrence. Sahin et al.20 in a retrospective cohort study including 197 patients with recurrent disease either in the liver (102 patients) or lung (47 patients) after an initial pancreatectomy for PDAC reported a significantly improved time from recurrence to death (15 vs. 9 months; p = .02) and a 2-year OS rate (24.9% vs. 17.5%) for patients with lung only metastases compared to those with liver metastases. Patients with IPM were more likely to have positive lymph nodes at their initial pancreatectomy.20 In a secondary analysis of ESPAC-4 trial including 730 patients, Jones et al.6 identified 52 patients with lung only recurrence and reported a significantly better OS (33.4 vs. 24.3 months, p = .001) and disease free survival after therapy for recurrence (DFS2) (15 vs. 8.4 months) for patients with IPM compared to patients with liver metastases.
Other retrospective case-control studies have reported improved median OS and DFS2 ranging from 11.8 to 33.3 months and 11 to 12.4 months respectively for patients with metachronous IPM compared to 5.0 to 14.0 months and 3.5 months in those with PDAC and liver metastases.21,23,36–38 Watanabe et al.23, in addition to the findings of improved OS in patients with lung only recurrence, reported increasing incidence of IPM in the latter half of the study period that correlated with the increased use of adjuvant chemotherapy. Nakagawa et al.24 also reported a significantly higher 5-year OS for patients with PDAC and lung only metastases when compared to recurrence in other organs and noted the use of adjuvant chemotherapy may have led to alteration in the recurrence pattern and contributed to improved survival as all patients with significant long-term survival received adjuvant chemotherapy. The latter two studies suggested that the increased use of adjuvant chemotherapy may have resulted in the increased likelihood of developing lung only recurrence but the underlying mechanism for the observation is unclear. Ariake et al.25 reported a significantly higher 5-year overall survival for patients with first recurrence in the lung after pancreatectomy compared to those patients with first recurrence in the peritoneum (20.4 vs. 0%, p < .001) or liver (20.4 vs. 10.2%).
Synchronous isolated pulmonary metastases (Table 2)
Studies addressing the impact of IPM at presentation on survival in PDAC are rare. In an analysis of the SEER Database, Liu et al.26 reported 1- year and median survival of 19.46% and 6 months respectively for patients with synchronous isolated lung metastases compared to 13.87% and 4 months for patients with synchronous isolated liver metastases. In a retrospective study of 40 patients with IPM, Jones et al.19 reported on 13 patients who had synchronous isolated pulmonary metastases at the time of initial diagnosis of PDAC and reported a median OS of 22.8 months which is comparable to patients with PDAC and metachronous IPM. Downs-Canner et al.12 reported on 174 patients with pulmonary metastases from PDAC and noted no significant difference in OS between patients with lung first, intrabdominal, or synchronous lung and abdominal metastases, among subjects who did not undergo pancreatic resection. The study by Guerra et al.13 reported a median survival of 7.3 months in pooled analysis of 799 patient with unresected pancreatic cancer and isolated lung metastases. It is however unclear if the last two publications included patients with an initial diagnosis of a locally advanced and unresected PDAC who later developed IPM. Given the limitations of the available studies, it is unknown if patients with PDAC and synchronous IPM enjoy longer OS compared to patients with metastases to other organs.
Evidence for and against improved overall survival with pulmonary metastasectomy and other lung-directed therapies(Table 3)
Downs-Canner et al.12 reported on 41 patients with available full treatment data, who completed initial pancreatectomy and had metastases to the lung first. They noted a significantly better median OS for patients who had either surgical resection or stereotactic radiosurgery as the treatment of lung metastases, compared to those treated with chemotherapy only (67.5 vs. 33.8 months, p = .006) or observation (67.5 vs. 29.9 months, p = .008). There was a non-significant trend toward a benefit in survival measured from the time of first recurrence for patients who received palliative chemotherapy compared to no treatment (18.9 months vs. 11.5 months, p-value 0.69) but no overall survival benefit.12
Groot et al.27 reported a significant difference in DFS1(52.4 vs. 7.6 months; p = .007), DFS2 (not reached vs. 6 months, p = .023) and median OS (92.3 vs. 32.5 months; p = .024) for patients with IPM who had resection of their lung metastases compared to those with liver metastases treated with hepatic resection. Another study of 31 patients with PDAC and isolated pulmonary metastases compared the outcome in patients who underwent pulmonary metastasectomy with patients who did not. The authors reported a significant improvement in median cumulative survival (51 vs. 23 months, p = .04), a trend toward improved median post-relapse survival (18.5 vs.7.5 months) and a non-significant increase in 2-year post-relapse survival (40% vs. 27%, p = .2).28 Patients who underwent pulmonary metastasectomy were a highly selected group with a relatively longer DFS1, had a favorable response to chemotherapy and a better ECOG performance status. Thus, though the observed improved median cumulative survival may represent the impact of pulmonary metastasectomy, it may also derive from selection bias.28 In a larger retrospective series from the same institution, which included some of the subjects in the earlier study, a significantly longer median post-recurrence survival of 35 months after pulmonary metastasectomy compared to 20.2 months for chemoradiotherapy and 8.1 months for best supportive care was reported. The median OS for patients who had lung resection was 68.9 months.34 Patients who had pulmonary metastasectomy had significantly lower median CA 19–9 compared to those who received best supportive care, but no significant difference compared to patients who had chemoradiotherapy. Additionally, they were less often symptomatic at recurrence, less likely to have≥5 lung lesions and less likely to have bilateral lung lesions.
Robinson et al.14 reported on pulmonary metastasectomy for suspicious isolated lung lesions following primary treatment for pancreaticobiliary adenocarcinoma. Sixteen of the included 29 patients had confirmation of metastases from PDAC while 12 patients had lung primaries and one had a cryptococcus infection. The median post-metastasectomy OS for the 16 patients with metastatic lesions (15 pancreatic adenocarcinoma and 1 cholangiocarcinoma) was 28 months. For patients whose new pulmonary lesions were unrelated to the initial primary (12 lung primaries), the median OS was 78 months. In addition to demonstrating a prolonged survival after pulmonary metastasectomy for patients with pancreaticobiliary cancer and IPM, the authors noted that consideration for resection of suspected isolated lung metastases in patients who underwent primary therapy for pancreaticobiliary cancer may reduce the risk of missing an unrelated but curable lung primary. Another retrospective study examined 20 patients with metachronous pulmonary metastases out of a population of 159 patients with metastatic PDAC.32 Patients with IPM were observed to have a longer OS compared to those with pulmonary and extrapulmonary metastases, and to those with non-pulmonary metastases. Of these, 2 patients with isolated pulmonary metastases who underwent lung resection were still disease free at an average of 12 months after resection.
Wangjam et al.22 reported 28 patients who had an isolated pulmonary recurrence after pancreatectomy and noted no significant difference in time from recurrence to death with or without treatment including lung directed therapy (resection and radiation) and chemotherapy (HR = 0.71, 95% CI = 0.27–1.92, p = .510). Tagawa et al.29 in a retrospective review of patients with IPM from pancreaticobiliary cancer reported DFS1 and DFS2 of 31.6 and 37.7 months respectively in four patients with pancreatic cancer who underwent pulmonary metastasectomy. Kurahara et al.30 reported on 33 patients who developed metachronous isolated lung metastases following resection of PDAC. Seven patients underwent resection of their lung metastases while 18 patients had palliative chemotherapy and 8 patients had supportive care. Median survival was significantly longer in patients who had pulmonary resection compared to those who received palliative chemotherapy or supportive care (36.5 vs.16.4 vs. 5.2, p = .0025). Of note, the patients who underwent pulmonary metastasectomy in this study only had a single metastatic lung lesion.30 In a retrospective series which included 197 patients with an isolated pancreatic cancer recurrence in either the liver, lung, or pancreas, Kim et al.31 reported significantly improved median survival after recurrence in patients who underwent resection of the recurrence compared to those who did not (23.5 vs. 12 months, HR: 0.58, p = .014). Patients with IPM who underwent pulmonary metastasectomy had a significantly longer median survival after recurrence compared to those who did not. (36.5 vs. 9.5 months; p = .010).
A systematic review which included 79 patients with PDAC and metachronous IPM who underwent resection of lung metastases from 11 studies reported an estimate of 120.0 ± 6.32 months and 83.0 ± 24.84 months for OS and survival after metastasectomy, respectively. The authors reported that longer duration from initial surgery to recurrence (DFS1), lower TNM stage at diagnosis, and a single lung lesion were factors that correlated with a better survival, although only a DFS1 > 36 months reached statistical significance.26 The same study analyzed the SEERs database on 21 patients with PDAC and synchronous IPM and reported no significant difference but a trend to better OS in patients who underwent simultaneous resection of both primary and lung metastases compared to those who underwent resection of the primary tumor only. The authors also noted a significant benefit of resection of the primary tumor with a median OS of 14 months with pancreatic resection compared to 10 months without. It is relevant to mention that only a small proportion of patients in this study underwent resection of the primary tumor or simultaneous resection of both the primary and the pulmonary metastases. Similarly, a population-based study reported no benefit in overall survival for surgery for distant metastatic lesion(s) in patients with PDAC with synchronous isolated pulmonary or distant lymph nodal metastases.33
Although there has been an increased interest in the use of non-surgical modalities such as stereotactic body radiation therapy (SBRT) or cryotherapy in the management of patients with solid tumor oligometastases in general, no current study has addressed the utility of these modalities in patients with PDAC and isolated lung metastases in a dedicated manner.
Exploring predictors of long-term survival in patients with isolated pulmonary metastatic disease (Table 4)
Table 4.
Prognostic factors of pancreatic adenocarcinoma with isolated pulmonary metastases and novel molecular candidates.
| Prognostic Factor | Evidence | References |
|---|---|---|
| Disease-free survival (DFS1) between pancreatectomy and recurrence of cancer |
|
Thomas et al.27 Groot et al.34 Kruger et al.19 |
| Laterality |
|
Kruger et al.19 |
| Number of lung lesions |
|
Kruger et al.19 Groot et al.34 |
| Size of lesions |
|
Kruger et al.19 |
| Carbohydrate Antigen 19–9 (CA 19–9) |
|
Groot et al.34 Chen et al.36 Haas et al.42 |
| Circulating DNA (ctDNA) and exosome DNA (exoDNA) |
|
Pietrasz et al.50 Toledano-Fonseca et al.47 Bernard et al.52 |
| MicroRNAs |
|
Ohuchida et al.53 Yu et al.55 Greither et al.54 Namkung et al.56 |
*Metastatic PDAC including IPM. **After surgical resection ***All stages of PDAC.
IPM: isolated pulmonary metastases; PDAC: Pancreatic Ductal adenocarcinoma; MAF: mutant allele fraction; PFS: Progression Free Survival.
Although certain clinicopathological features including DFS1, number and size of lung metastases, and laterality, have been reported as prognostic, no clinicopathological features or biomarkers have been conclusively validated as prognostic factor for survival in patients with PDAC and isolated pulmonary metastases. Available low-level evidence points to the importance of patient selection in the application of lung-directed therapy for patients with isolated pulmonary metastases.28 Thus there is a need for a validated tool to predict which patients with IPM will benefit from lung-directed therapy. Similar to the findings in other solid tumors, disease free survival after initial pancreatectomy to the development of locoregional or metastatic recurrence (DFS1) has been identified by multiple studies as a predictor of longer overall survival in patients with resected PDAC who developed single organ recurrence. The DFS1 can in turn be applied to select patients who may benefit from lung directed therapies.26,27,39–41. Varying DFS1, from greater than 10 months to greater than 36 months, has been reported to be associated with improved OS in patients with IPM after pancreatectomy for PDAC. Thomas et al.27 reported that DFS1 greater than 20 months is predictive of long-term survival in patients with isolated pulmonary metastases as well as those with isolated hepatic metastases. In another retrospective series, a 16-month cutoff was identified as prognostic for longer OS (66.3 vs. 22.2 months for>16 and </ = 16 months respectively. p < .001).34 Kruger et al.19 on the other hand found no prognostic significance on OS for DFS1 greater than either 9 months or 20 months. Other clinicopathological prognostic factors including laterality, size of the largest lesion, and the number of lesions have been investigated.19,26,34 Unilateral pulmonary metastases (compared to bilateral) and the number of lung lesions<10 were significantly associated with better OS while the size of the lesion was not.19 Groot et al.34 reported that in addition to DFS1 of less 16 months, the absence of symptoms and fewer than five lung lesions were prognostic for longer post-recurrence survival. There was a non-significant trend for worse survival in patients with bilateral lung recurrence.
Prognostic biomarkers that predict a longer survival in non-selected patients with metastatic pancreatic cancer have been reported in multiple studies.42,43 CA 19–9 has been investigated as a prognostic factor for time to progression (TTP) and OS in patients with locally advanced and metastatic PDAC with log [CA 19–9] and a 25% decline cutoff during chemotherapy reported as independent predictors of TTP and OS respectively.42 Song et al.43 developed a scoring system using pretreatment CA 19–9 and neutrophil-to-lymphocyte ratio (NLR) at cutoffs of 626 U/mL and 3.75 respectively. The scoring system performs significantly better as a predictor of survival compared to CA 19–9 or NLR alone. Ueno et al.44 proposed a prognostic index that classifies patients with metastatic PDAC into three prognostic group by combining CA 19–9, performance status and C-reactive protein. The resulting prognostic group classification demonstrated significant difference in OS. Other authors have proposed even more complex nomograms incorporating log CA 19–9, performance status, liver metastases, ANC, and albumin as prognostic factors for OS in metastatic pancreatic cancer.45 In patients with PDAC and metachronous IPM, multiple retrospective studies have identified the prognostic importance of different cutoffs of CA 19–9.14,34,36 In a series of patients with PDAC and metachronous IPM who underwent pulmonary metastasectomy, Robinson et al.14 reported that the CA 19–9 level prior to lung resection significantly predict overall survival.14 In another study, a CA 19–9 cutoff of 100 U/ml at recurrence was identified as a prognostic factor for post recurrence survival.34 Chen et al.36 reported that CA 19–9 greater than 185 U/ml at diagnosis of recurrence was predictive of poorer outcome. Given the widely varied CA 19–9 cutoffs in these studies, it appeared all that can be inferred is that higher CA 19–9 level at the time of recurrence predicts shorter survival.
Circulating tumor DNA (ctDNA) and other types of liquid biopsies (circulating tumor cells, exosome DNA, miRNAs) are currently being investigated for clinical utility in various stages of pancreatic cancer.46–48 Trials in locally advanced and metastatic PDAC reported that 50 to 100% of patients have ctDNA and the presence of ctDNA has been associated with a worse OS.49 In patients with locally advanced and metastatic PDAC, Pietrasz et al.50, after a median follow up of 34.2 months, reported an OS of 6.5 months vs.19 months in patients with positive and negative ctDNA respectively. Of note, in the same study, no significant correlation was found between the presence of ctDNA and the number of metastatic sites. Another study evaluating the prognostic utilities of ctDNA also reported significantly lower plasma ctDNA levels in patients with non-hepatic metastases, including patients with isolated lung metastases compared to those with hepatic metastases.47
Exosomes, which are circulating microvesicles consisting of bilayer lipid membranes surrounding high molecular weight nucleic acid materials, have been identified as a source of high-quality tumor DNA for next generation sequencing analysis.51 Bernard et al.52 investigated the prognostic utility of exosome DNA (exo DNA) and ctDNA in metastatic PDAC and reported a KRAS mutation detection rate of 61% and 53% respectively. The KRAS mutant allele fraction (MAF) in exoDNA and in ctDNA were correlated with disease burden. Patients with hepatic metastases had a significantly greater KRAS MAF in both exoDNA and ctDNA compared to patients with isolated pulmonary metastases. The authors further noted that a KRAS MAF of 5% in exoDNA was a significant independent predictor of PFS and OS. Although any detection of ctDNA was not an independent predictor of PFS and OS, the combination of both a positive ctDNA and a KRAS MAF of 5% in exoDNA at baseline significantly correlated with poorer OS. The presence of ctDNA in patients with a CA19–9 > 300 U/mL at baseline prior to any therapy correlated with a poorer OS.52 The abilities of ctDNA and exoDNA techniques to predict longer survival in patients with metastatic PDAC should be harnessed to determine which patients with PDAC and IPM may benefit from a more aggressive therapeutic approach.
MicroRNAs have also been investigated for clinical utility in the diagnosis, prognosis, and therapy in pancreatic adenocarcinoma. Various patterns of miRNAs expression have been reported to be associated with a better response to gemcitabine (miR-142-5p and miR-320c), a better survival (miR-200c, miR-142-5p, and miR-204) or poor survival (miR-155, miR-203, miR-210, miR-222, miR-21, and miR-196a-2) after surgical resection.53–55 In a study which included 104 patients who underwent pancreatectomy most of whom developed distant recurrence on follow-up, Namkung et al.56 reported multiple miRNA molecular subtypes with significant difference in PFS and OS. No difference in clinicopathological characteristics were noted between these molecular subtypes. The authors reported six out of nineteen tested miRNAs (miR-574-5p, miR-1244, miR-145-star, miR-328, miR-26b-star, and miR-4321) were independently prognostic of PFS and OS.56 Furthermore, investigations into microRNAs expressed in oligometastases in multiple solid tumors including PDAC have reported unique patterns of expression associated with a clinical oligometastatic state.57,58 Uppal et al.57 identified microRNAs encoded on 14q32 including miR-127-5p, miR-544a, and miR-655-3p as mediators of an oligometastatic phenotype in solid tumors. These microRNAs are involved in the regulations of multiple metastatic pathways. Currently, no microRNAs patterns have been identified to be specifically associated with oligometastatic spread in PDAC. An investigation of microRNA subtyping and survival in patients with PDAC and isolated pulmonary metastases is desirable.
In addition to CA 19–9, the investigation of the clinical utility of newer prognostic markers such as ctDNA, exoDNA and micro-RNAs specifically in patients with PDAC with IPM may help identify a cohort of these patients that may benefit from lung directed therapies. Additionally clinical trials involving oligometastatic patients with PDAC, including patients with isolated pulmonary metastases should incorporate these novel prognostic markers.46,59
Proposed mechanistic theories for improved survival in patients with PDAC and isolated pulmonary metastases (Table 5)
Table 5.
Mechanistic theories for improved survival in pancreatic adenocarcinoma with isolated pulmonary metastases.
| Mechanistic Factor | Theories | References |
|---|---|---|
| Limited anatomic spread bypassing the liver and systemic dissemination |
|
Kamisawa et al.8 Leach60 |
| Genetic heterogeneity and mutational subclones |
|
Yachida et al.61 |
| Organ-specific driver mutations |
|
Yachida et al.61 |
| Mutations in tumor suppressor genes |
|
Vitellius et al.63 Arnaoutakis et al.28 Arnaoutakis et al.28 Embuscado et al.65 Tascilar et al.66 Blackford et al.68 |
Two possible explanations for the observed improved OS in patients with PDAC and IPM includes the limited anatomic pattern of metastatic spread and an underlying favorable molecular biology. In a study of 130 autopsy cases of patients with known PDAC, Kamisawa et al.8 reported on 16 patients with pulmonary metastases but without hepatic involvement. Retroperitoneal invasion and lymph node involvement was confirmed in most of these patients and most had PDAC located in the body and tail of the pancreas. Furthermore, eleven of the sixteen patients received radiation therapy and the authors noted portal vein obstruction and the development of collateral venous circulation in six of eight patients examined. They suggested that pulmonary metastasis was due to an unusual pattern of spread. The tumor could metastasize either retrogradely through the lymphatics via the tracheobronchial glands, through the involvement of lymph nodes at the venous angle or spread through the portal system collateralization without preceding hepatic involvement. The authors proposed that the preponderance of radiation therapy including the route for metastatic spread may explain the unusual pattern of spread and improved survival noted in this group.8 Other authors, suggested the possibility of a limited lymphatic spread to the lung via the mediastinal lymph nodes without a more widespread systemic dissemination.60
Pancreatic cancer has a unique molecular biology. Previous studies have identified multiple genetic alterations including KRAS, CDKN2A, p53, and SMAD4 that are regarded as founder mutations in PDAC. Other studies have reported different subclones with acquired mutations that promote metastatic spread.61,62 Furthermore, genetic mutations that promotes different patterns of metastatic spread have been identified. Previous work by Yachida et al.61 suggested the existence of geographically distinct subclones that may be capable of metastasizing to different organs such as the peritoneum, lung, and liver. These proposed genetic subclones would have distinct mutational profiles which are part of the genetic diversity of the primary tumor. However, no consistent genetic mutational pattern of these metastatic subclones was identified in the study. The same study reported genetic heterogeneity of cells from the primary tumor compared to cells of the metastatic lesions and noted that all metastatic lesions in the same organ possessed additional unique driver mutations that were not present in the metastases of other organs. Specifically, the authors reported rearrangements/amplifications in MYC and CCNE1 cancer genes in multiple pulmonary metastatic lesions that were not present in abdominal metastases. Furthermore, lung lesions were reported to be even further clonally evolved. Such additional driver mutations may explain the differential behavior of metastases to the lung compared to other organ sites.61
In a limited study of 7 patients with PDAC and isolated pulmonary metastases, Vitellius et al.63 reported a median survival of 57 months for patients with isolated pulmonary metastases compared to 25.3 months for those with metastases to other sites and noted absence of mutations in CDK2NA and SMAD4 tumor-suppressor genes in subjects with IPM. The authors proposed that the lack of mutations in these tumor suppressor genes may be linked to the observed improved OS. The relatively low proportion of patients with PDAC and isolated pulmonary metastases demonstrating loss of SMAD4(DPC4) via somatic genetic inactivation was also reported in an earlier study.28 Mutations in CDK2NA and SMAD4(DPC4) Tumor-suppressor genes have been identified as one of the most common genetic aberrations in PDAC,64 and higher prevalence of genetic inactivation of DPC4 was noted in metastatic PDAC compared to resectable disease.65 Furthermore, loss or inactivation of SMAD4(DPC4) has been associated with more aggressive tumor, development of widespread metastases and poor prognosis.66–68 Thus, the preservation of CDK2NA and SMAD4 tumor suppressor pathways in isolated pulmonary metastases from PDAC may be an important underlying biologic basis for improved survival (Figure 1).
Figure 1.
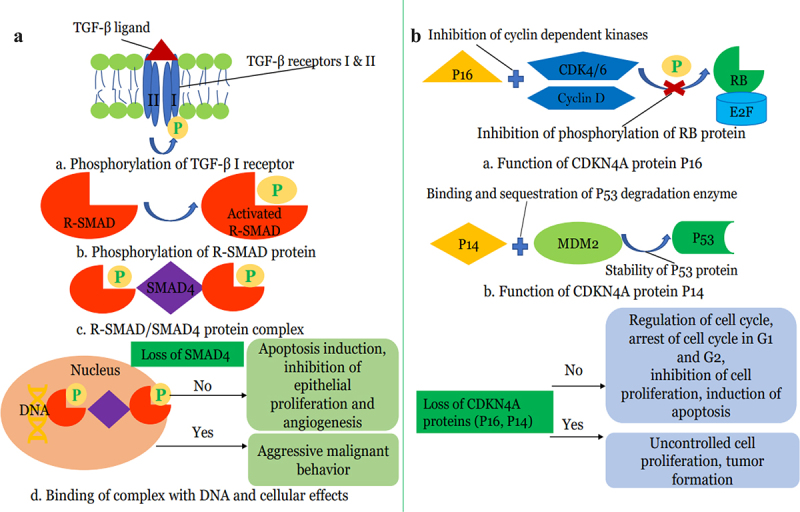
(a): Role of SMAD4 tumor suppressor gene in Pancreatic Cancer a) TGF-β ligand binds to TGF-β transmembrane serine-threonine kinase receptor leading to activation of TGF-β2 receptors. TGF-β2 receptor then phosphorylates TGF-β1 receptor. b). TGF-β2 receptors then phosphorylate receptor-activated SMAD (R-SMAD) proteins such as SMAD2 and SMAD3 which leads to activation of R-SMAD proteins. c). Activated R-SMAD proteins bind SMAD4 protein in the cytosol and form R-SMAD/SMAD4 complex which translocates to the nucleus. d). R-SMAD/SMAD4 complex binds to specific regions on DNA and controls the gene expression and regulates protein synthesis. Wild-type SMAD4 or no loss of SMAD4 protein promotes apoptosis, inhibits epithelial proliferation, and inhibits angiogenesis. SMAD-4 proteins reduce the expression of VEGF (vascular endothelial growth factor) and hence inhibit angiogenesis. Loss of SMAD4 results in unchecked cellular proliferation of pancreatic tissue along with the increased vascular invasion. Loss of SMAD4 results in increased EMT (epithelial to mesenchymal transition) which promotes malignant behavior. (b): Role of CDK4NA tumor suppressor gene in Pancreatic Cancer a). P16 protein, a tumor suppressor protein of the CDK4NA gene, binds cyclin-dependent kinases such as CDK4/6 and Cyclin D. This binding prevents the phosphorylation of RB protein. b). P14, another tumor suppressor protein, binds E3 ubiquitin ligase MDM2 protein and prevents degradation of P53 protein by MDM2 and hence stabilizes P53 protein. Both RB and P53 proteins regulate the cell cycle and promote apoptosis. Loss of the CDK4NA gene results in loss of these tumor suppressor proteins with subsequent uncontrolled cellular proliferation and hence aggressive pancreatic cancer biology.
Chou et al.69 reported a 2.1% rate of HER2 amplification in PDAC and noted an unusual pattern of metastases to the lung and not to the liver. HER2 amplification and or expression has been associated with poor OS raising the possibility of biological diversity among patients with PDAC and isolated pulmonary metastases.70
Discussion
Pancreatic adenocarcinoma remains a very grave disease with a poor prognosis despite some therapeutic advances over the last decade. Despite the introduction of more effective chemotherapeutic regimens, most individuals with metastatic PDAC die within 5 years of diagnosis.2,11,71,72 A subset of patients with metastatic PDAC, specifically those with IPM, appear to have a prolonged overall survival when compared to those with metastases to other organs such as the liver. However, evidence for improved survival in this subset of patients is limited to observational studies with a small number of patients and to case series.6,9,12,13,17–19,73 The improved survival in patients with PDAC and isolated pulmonary metastases has been mainly observed in patients who had a pancreatectomy after diagnosis of PDAC and subsequently developed metachronous isolated pulmonary recurrences. The relatively prolonged survival observed in patients with PDAC and isolated pulmonary metastases post-pancreatectomy were also reported in patients who did not undergo curative lung-directed therapy.18,19 There are significantly fewer studies addressing survival in patients with PDAC and synchronous IPM and the reported findings have been mixed.13,19,26 Thus at this time, it is unclear if patients with PDAC and IPM at diagnosis have longer survival compared to patients with metastases to other organs and prospective studies to address this gap are needed.
While no clinicopathological factor or biomarker has been validated for prediction of prolonged survival or benefit from aggressive lung directed therapy in patients with PDAC and isolated pulmonary metastases, factors such as disease-free survival from initial pancreatectomy (DFS1> or <20 months), laterality (unilateral vs. bilateral), and number of pulmonary lesions (< or>10), and CA 19–9 level at recurrence have been identified as possible candidates.19,27,40,41 Newer techniques such as ctDNA, exoDNA, microRNAs are currently being investigated as prognostic and predictive biomarkers for metastatic PDAC and may have significant potential in identifying patients with PDAC and isolated pulmonary metastases who may benefit from lung directed therapy.47,52,53,56. Thus, future clinical trials involving patients with PDAC and IPM should evaluate multiple candidate clinicopathological and molecular biomarkers.46,59
The biologic basis of oligometastatic status in solid tumors is not fully understood but recent studies have reported the role of microRNAs involved in multiple metastatic pathways in the development of the oligometastatic phenotypes in solid tumors including pancreatic adenocarcinoma.57,58 Other studies have identified a possible underlying molecular basis for an improved survival in some patients with PDAC and isolated pulmonary metastases. The absence of mutations in tumor suppressor genes such as CDK2NA and SMAD4(DPC4), which are common genetic aberrations in pancreatic cancer, have also been suggested.28,63 However, there appears to be tumoral genetic diversity among patients with PDAC and IPM and further investigation to identify other possible molecular subtypes is warranted.70 An improved understanding of the molecular mechanism underpinning the development of a pulmonary oligometastatic state in PDAC will lead to better identification of patients for clinical trials and for a targeted therapeutic approach.57
Current guideline recommendations for the management of metastatic pancreatic cancer patients are anchored on palliative chemotherapy in those with a reasonable performance status.11,71,72 Improved survival has been reported in patients with PDAC and isolated pulmonary metastases who received either single or multiagent chemotherapy compared to those who did not receive any treatment.12,18 However, optimization of the use of more effective chemotherapy regimens such gemcitabine with nanoparticle albumin bound paclitaxel and modified folinic acid, 5-fluorouracil, irinotecan and oxaliplatin (mFOLFIRINOX) in this subset of patients with PDAC is urgently needed.
The increasing recognition that a small proportion of patients with metastatic PDAC may achieve a reasonably extended survival has prompted the debate about the applicability of the oligometastatic management principle in other solid tumors such as colon cancer.28,59,74 Generally, the management of oligometastatic solid tumors has involved perioperative chemotherapy and simultaneous or staged resection of the primary and metastatic lesions in selected patients.75,76 The application of a comparable management paradigm in selected patients with PDAC and hepatic oligometastases resulted in a 5-year overall survival of 8.1% whereas no long-term survival has been reported with palliative chemotherapy only.74 Furthermore, the technical advances and improved safety of local ablatives techniques such as radiofrequency ablation have made these modalities an acceptable approach either alone or in combination with resection for selected patients with oligometastatic solid tumor.59,77 Given the reported longer median overall survival of patients with isolated metachronous pulmonary recurrences from PDAC, and safety and minimal morbidity of pulmonary metastasectomy and ablative techniques, the application of these modalities may not be an unreasonable consideration in selected patients.28 However, at this time, given the uncontrolled nature of the available studies including highly selected patients, it is unclear if the reported benefit of pulmonary metastasectomy and other lung directed therapies is a true benefit of the procedure(s) or a manifestation of selection bias. Criteria that have been utilized in the selection of patients for pulmonary metastasectomy in PDAC include the ability to tolerate a pulmonary resection, a long duration from pancreatectomy to first relapse (DFS1), an isolated and stable disease course, and a favorable response to chemotherapy. The latter three selection criteria were considered to be indicative of “good biology”.28 The lack of high-level evidence for pulmonary metastasectomy is not limited to pulmonary oligometastases in PDAC. In patients with colorectal cancer and limited pulmonary metastases, pulmonary metastasectomy has been a common practice despite a lack of level 1 evidence for its benefit on survival.78,79. As noted for PDAC with isolated pulmonary metastases, it is unclear if the observed prolongation of OS in patients with colon cancer with lung only metastases who underwent pulmonary metastasectomy is due to selection bias or a benefit of lung resection.78 A multicenter randomized clinical trial (PulMiCC) designed to test the effectiveness of pulmonary metastasectomy patients with colorectal cancer and lung oligometastases failed to accrue and was discontinued.80 However, the analysis of the available limited data revealed a much higher 5-year survival for the matched controls than previously believed (29% vs.<5%) and comparable to the intervention arm, which further raises the question of selection bias in earlier uncontrolled studies.80 Similarly, the application of pulmonary metastasectomy in PDAC with isolated pulmonary metastases currently lack supportive high level evidence. Thus, the procedure should not be routinely offered to all patients with PDAC with isolated pulmonary metastases. Additionally, there is a need for trials specifically investigating the role of non-surgical ablative techniques such as SBRT and cryotherapy in the management of patients with PDAC and lung only metastases.
Conclusion
Patients with resected PDAC and metachronous IPM may represent a subset of patients with metastatic PDAC with a favorable prognosis. The underlying mechanism for the observed improved survival is yet to be fully determined but may be related to specific genetic aberrations/signatures. Furthermore, the true benefit of pulmonary resection and other lung directed therapy is unknown. Further studies are necessary to elucidate the underlying molecular mechanism for the improved survival in patient with PDAC and lung only metastases compared to PDAC with metastases to other sites and to determine the role of pulmonary resection and other lung directed therapies. Such trials should include known molecular, laboratory, and clinicopathological prognostic markers to help identify which of these patients might benefit from lung directed therapy.
Biographies
Orimisan Samuel Adekolujo MD, MBA, FACP is an Assistant Professor of Medicine at Michigan State University and Medical Oncologist at Karmanos Cancer Institute at McLaren Flint, MI, USA. He completed his Oncology Fellowship at Michigan State University. Dr. Adekolujo’s research interest is in gastrointestinal malignancy.
Ahsan Wahab MD, MPH was affiliated with Prattville Baptist Hospital, Prattville, Alabama at the time of the manuscript writing and is now a fellow at Department of Supportive Oncology, Levine Cancer Institute, Charlotte, NC. He holds an MPH degree in Epidemiology from the University of Alabama at Birmingham. His main area of interest is gastrointestinal Oncology.
Maxwell Akanbi MD, PhD, is a Chief Resident in Internal Medicine at McLaren Flint, Michigan and incoming Fellow, Hematology and Oncology Fellowship Program at the Michigan State University. He holds a Masters in Clinical Investigation and PhD in Health Services and Outcomes Research from Northwestern University, Chicago, USA. Maxwell’s research interests include gastrointestinal and thoracic oncology, HIV associated malignancies, and health disparities.
Dr. Tolutope Oyasiji MD, MHSA, MRCSI, FACS is a board-certified Surgical Oncologist and a Clinical Associate Professor of Surgery at the Department of Surgery, Michigan State University. He is also the Associate Medical Director for surgical oncology within the Karmanos Cancer Network. He completed his general surgery residency at Yale-New Haven Hospital followed by a complex general surgical oncology fellowship at Roswell Park Comprehensive Cancer Center. He is a fellow of the American College of Surgeons and Society of Surgical Oncology. His research interests include gastrointestinal malignancies, neuroendocrine tumors, population sciences, and outcomes research.
Dr. Borys Hrinczenko MD, PhD, FACP is a Professor of Medicine in the Division of Hematology and Oncology at Michigan State University. He received his medical degree from SUNY Downstate in New York and completed an internal medicine internship and residency at the Mayo Clinic in Rochester, MN, followed by a fellowship in hematology and medical oncology at the National Institutes Health in Bethesda, Maryland. He has pursued an academic medicine career and his research has focused on the development of novel therapeutic strategies and biomarkers for lung and other cancers. He has been a principal investigator on more than 50 cancer clinical trials and has more than 100 publications, abstracts, and presentations.
Dr. Olatunji Alese MD is an Associate Professor in the Department of Hematology and Medical Oncology, Emory University, Atlanta, GA. He is also the Associate Medical Director, Ambulatory Infusion Center at the Winship Cancer Institute. He is a member of numerous professional societies and the gastrointestinal (GI) committees of multiple national cooperative oncology groups. He previously served on the Advisory Group of ASCO International Affairs Committee and is currently a member of the ASCO Annual Meeting Education Committee. His research focuses on GI malignancies, developmental therapeutics, and health disparities. He has over 70 peer-reviewed publications and numerous conference presentations.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Author contribution statement
OS Adekolujo: Article conception, writing of first draft, and worked on subsequent drafts
A Wahab: Article conception, developed figure and tables, and worked on manuscript drafts
MO Akanbi: Article conception, developed figure and worked on manuscripts drafts
T Oyasiji: Article conception and worked on manuscript drafts
B Hrinczenko: Article conception and worked on manuscript drafts
OB Alese: Article conception and worked on manuscript drafts
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Siegel RL, Miller KD, Jemal A.. 2020. Cancer statistics, 2020. CA Cancer J Clin. 70(1):7–12. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer I . Surveillance, epidemiology, and end results program: cancer stat facts: pancreatic cancer. 2020.
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Rahib L, Wehner MR, Matrisian LM, Nead KT. 2021. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizrahi JD, Surana R, Valle JW, Shroff RT. 2020. Pancreatic cancer. Lancet (London, England). 395:2008–2020. [DOI] [PubMed] [Google Scholar]
- 6.Jones RP, Psarelli EE, Jackson R, Ghaneh P, Halloran CM, Palmer DH, Campbell F, Valle JW, Faluyi O, O’reilly DA, et al. 2019. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg. 154:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. 2019. Chemoresistance in pancreatic canceancreatic cancer. Int J Mol Sci. 20:4504. doi: 10.3390/ijms20184504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. 1995. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 11:345–349. [DOI] [PubMed] [Google Scholar]
- 9.Gotfried JI, Kozuch PS. Case report: long-term survival in patients with initial lung-only metastasis from pancreatic adenocarcinoma. J Gastrointest Cancer. 2012;43(1):S50–5. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. 2013. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. 2011. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 12.Downs-Canner S, Zenati M, Boone BA, Varley PR, Steve J, Hogg ME, Zureikat A, Zeh HJ, Lee KK. 2015. The indolent nature of pulmonary metastases from ductal adenocarcinoma of the pancreas. J Surg Oncol. 112:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra F, Barucca V, Coletta D. 2020. Metastases or primary recurrence to the lung is related to improved survival of pancreatic cancer as compared to other sites of dissemination. Results of a systematic review with meta-analysis. Eur J Surg Oncol. 46:1789–1794. [DOI] [PubMed] [Google Scholar]
- 14.Robinson LAMD, Tanvetyanon TMD, Springett GMD, Fontaine JMD, Toloza EMD, Hodul PMD, Pimiento JMMD, Malafa MMD. 2016. Pulmonary metastasectomy for suspected pancreaticobiliary cancer. J Thorac Cardiovasc Surg. 152:75–82. [DOI] [PubMed] [Google Scholar]
- 15.Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. 2010. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 59:1383–1388. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S, et al. 2004. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 78:238–244. [DOI] [PubMed] [Google Scholar]
- 17.Zheng B, Ohuchida K, Yan Z, Okumura T, Ohtsuka T, Nakamura M. 2017. Primary recurrence in the lung is related to favorable prognosis in patients with pancreatic cancer and postoperative recurrence. World J Surg. 41:2858–2866. [DOI] [PubMed] [Google Scholar]
- 18.Meyers MO, Meszoely IM, Hoffman JP, Watson JC, Ross E, Eisenberg BL. 2004. Is reporting of recurrence data important in pancreatic cancer? Ann Surg Oncol. 11:304–309. [DOI] [PubMed] [Google Scholar]
- 19.Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Michl M, Kleespies A, Angele MK, Hartwig W, et al. 2016. Isolated pulmonary metastases define a favorable subgroup in metastatic pancreatic cancer. Pancreatology: Official Journal of the International Association of Pancreatology (IAP) [Et Al]. 16:593–598. [DOI] [PubMed] [Google Scholar]
- 20.Sahin IH, Elias H, Chou JF, Capanu M, O’reilly EM. 2018. Pancreatic adenocarcinoma: insights into patterns of recurrence and disease behavior. BMC Cancer. 18 769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Hsueh C, Chen L, Yeh H, Tsang C. 2019. Lung metastases in patients with stage IV pancreatic cancer: prevalence, risk factors, and survival impact. Journal of Clinical Medicine. 8(9):1402. doi: 10.3390/jcm8091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wangjam T, Zhang Z, Zhou XC, Lyer L, Faisal F, Soares KC, Fishman E, Hruban RH, Herman JM, Laheru D, et al. 2015. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget. 6:36903–36910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe Y, Nishihara K, Niina Y, Kudo Y, Kurata K, Okayama T, Fujii A, Wakamatsu S, Abe Y, Nakano T.. 2017. Patients with lung recurrence after curative resection for pancreatic ductal adenocarcinoma have a better prognosis than those with recurrence at other sites. Journal of the Pancreas. 18(1):54–61. https://www.primescholars.com/articles/patients-with-lung-recurrence-after-curative-resection-forpancreatic-ductal-adenocarcinoma-have-a-better-prognosis-than--99102.html . [Google Scholar]
- 24.Nakagawa K, Akahori T, Nishiwada S, Nagai M, Nakamura K, Tanaka T, Tamamoto T, Ohbayashi C, Hasegawa M, Kichikawa K, et al. 2018. Prognostic factors for actual long-term survival in the era of multidisciplinary treatment for pancreatic ductal adenocarcinoma. Langenbeck’s Archives of Surgery. 403:693–700. [DOI] [PubMed] [Google Scholar]
- 25.Ariake K, Motoi F, Ohtsuka H, Fukase K, Masuda K, Mizuma M, Hayashi H, Nakagawa K, Morikawa T, Maeda S, et al. 2017. Predictive risk factors for peritoneal recurrence after pancreatic cancer resection and strategies for its prevention. Surg Today. 47:1434–1442. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Zhang R, Michalski CW, Liu B, Liao Q, Kleeff J. 2020. Surgery for synchronous and metachronous single-organ metastasis of pancreatic cancer: a SEER database analysis and systematic literature review. Sci Rep. 10 4444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas R, Truty M, Nogueras-Gonzalez G, Fleming J, Vauthey J-N, Pisters P, Lee J, Rice D, Hofstetter W, Wolff R, et al. 2012. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. Journal of Gastrointestinal Surgery. 16:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnaoutakis G, Rangachari D, Laheru D, Iacobuzio-Donahue C, Hruban R, Herman J, Edil B, Pawlik T, Schulick R, Cameron J, et al. 2011. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. Journal of Gastrointestinal Surgery. 15:1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagawa T, Ito K, Fukuzawa K, Okamoto T, Yoshimura A, Kawasaki T, Masuda T, Iwaki K, Terashi T, Okamoto M, et al. 2017. Surgical resection for pulmonary metastasis from pancreatic and biliary tract cancer. Anticancer Res. 37:1413–1416. [DOI] [PubMed] [Google Scholar]
- 30.Kurahara H, Maemura K, Mataki Y, Tanoue K, Iino S, Kawasaki Y, Idichi T, Arigami T, Mori S, Shinden Y, et al. 2020. Lung recurrence and its therapeutic strategy in patients with pancreatic cancer. Pancreatology: Official Journal of the International Association of Pancreatology (IAP) [Et Al]. 20:89–94. [DOI] [PubMed] [Google Scholar]
- 31.Kim YI, Song KB, Lee YJ, Park KM, Hwang DW, Lee JH, Shin SH, Kwon JW, Ro JS, Kim SC. 2019. Management of isolated recurrence after surgery for pancreatic adenocarcinoma. Br J Surg. 106:898–909. [DOI] [PubMed] [Google Scholar]
- 32.Lovecek M, Skalicky P, Chudacek J, Szkorupa M, Svebisova H, Lemstrova R, Ehrmann J, Melichar B, Yogeswara T, Klos D, et al. 2017. Different clinical presentations of metachronous pulmonary metastases after resection of pancreatic ductal adenocarcinoma: retrospective study and review of the literature. World J Gastroenterol. 23:6420–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schöb O, Giryes A, Decker M, Abdel-Rahman O. 2017. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol. 23:1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groot VP, Blair AB, Gemenetzis G, Ding D, Burkhart RA, van Oosten AF, Molenaar IQ, Cameron JL, Weiss MJ, Yang SC, et al. 2019. Isolated pulmonary recurrence after resection of pancreatic cancer: the effect of patient factors and treatment modalities on survival. HPB (Oxford). 21:998–1008. [DOI] [PubMed] [Google Scholar]
- 35.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, et al. 2009. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 16:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K-K, Singla S, Papavasiliou P, Arrangoiz R, Gaughan JP, Hoffman JP. 2013. Patterns of recurrence and outcomes in pancreatic cancer. JCO. 31:234. [Google Scholar]
- 37.Decoster C, Gilabert M, Autret A, Turrini O, Oziel-Taieb S, Poizat F, Giovannini M, Viens P, Iovanna J, Raoul JL. 2016. Heterogeneity of metastatic pancreatic adenocarcinoma: lung metastasis show better prognosis than liver metastasis-a case control study. Oncotarget. 7:45649–45655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Itchins M, Arena J, Nahm C, Pavlakis N, Clarke S, Gill A, Samra SJ, Mittal A. 2017. Patterns and determinants of recurrence for pancreatic ductal adenocarcinoma after resection. Journal of the Pancreas. 18(6):458–464. https://www.primescholars.com/articles/patterns-and-determinants-of-recurrence-for-pancreatic-ductal-adenocarcinoma-after-resection-99172.html [Google Scholar]
- 39.Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. 2013. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 20:572–579. [DOI] [PubMed] [Google Scholar]
- 40.Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, Friess H, Biichler MW. 2007. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 245:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura A, Itasaka S, Takaori K, Kawaguchi Y, Shibuya K, Yoshimura M, Matsuo Y, Mizowaki T, Uemoto S, Hiraoka M. 2014. Radiotherapy for patients with isolated local recurrence of primary resected pancreatic cancer. Strahlentherapie und Onkologie. 190:485–490. [DOI] [PubMed] [Google Scholar]
- 42.Haas M, Heinemann V, Kullmann F, Laubender R, Klose C, Bruns C, Holdenrieder S, Modest D, Schulz C, Boeck S. 2013. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol. 139:681–689. [DOI] [PubMed] [Google Scholar]
- 43.Song JY, Chen MQ, Guo JH, Lian SF, Xu BH. 2018. Combined pretreatment serum CA19-9 and neutrophil-to-lymphocyte ratio as a potential prognostic factor in metastatic pancreatic cancer patients. Medicine. 97:e9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueno H, Okada S, Okusaka T, Ikeda M. 2000. Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology. 59:296–301. [DOI] [PubMed] [Google Scholar]
- 45.Hang J, Wu L, Zhu L, Sun Z, Wang G, Pan J, Zheng S, Xu K, Du J, Jiang H. 2018. Prediction of overall survival for metastatic pancreatic cancer: development and validation of a prognostic nomogram with data from open clinical trial and real-world study. Cancer Med. 7:2974–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heredia-Soto V, Rodríguez-Salas N, Feliu J. Liquid biopsy in pancreatic cancer: are we ready to apply it in the clinical practice? Cancers 2021; 13:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toledano-Fonseca M, Cano MT, Inga E, Rodríguez-Alonso R, Gómez-España MA, Guil-Luna S, Mena-Osuna R, de la Haba-Rodríguez JR, Rodríguez-Ariza A, Aranda E. 2020. Circulating cell-free DNA-Based liquid biopsy markers for the non-invasive prognosis and monitoring of metastatic pancreatic cancer. Cancers. 12:1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MK, Woo SM, Park B, Yoon K-A, Kim Y-H, Joo J, Lee WJ, Han S-S, Park S-J, Kong S-Y. 2018. Prognostic implications of multiplex detection of KRAS mutations in cell-free DNA from patients with pancreatic ductal adenocarcinoma. Clinical Chemistry (Baltimore, Md). 64:726–734. [DOI] [PubMed] [Google Scholar]
- 49.Buscail E, Maulat C, Muscari F, Chiche L, Cordelier P, Dabernat S, Alix-Panabières C, Buscail L. 2019. Liquid biopsy approach for pancreatic ductal adenocarcinoma. Cancers. 11:852. 10.3390/cancers11060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrasz D, Pécuchet N, Garlan F, Didelot A, Dubreuil O, Doat S, Imbert-Bismut F, Karoui M, Vaillant J-C, Taly V, et al. 2017. Plasma circulating tumor DNA in pancreatic cancer patients is a prognostic marker. Clinical Cancer Research. 23:116–123. [DOI] [PubMed] [Google Scholar]
- 51.San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, Ehli EA, Davies GE, Petersen JL, Li D, et al. 2016. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 27:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, Stephens BM, Huang J, Semaan A, Guerrero PA, et al. 2019. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology (New York, NY 1943). 156:108–18.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohuchida K, Mizumoto K, Kayashima T, Fujita H, Moriyama T, Ohtsuka T, Ueda J, Nagai E, Hashizume M, Tanaka M. 2011. MicroRNA expression as a predictive marker for gemcitabine response after surgical resection of pancreatic cancer. Ann Surg Oncol. 18:2381–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greither T, Grochola LF, Udelnow A, Lautenschläger C, Würl P, Taubert H. 2010. Elevated expression of microRnas 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. International Journal of Cancer. 126:73–80. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, Nakata K, Tanaka M. 2010. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Namkung J, Kwon W, Choi Y, Yi SG, Han S, Kang MJ, Kim SW, Park T, Jang JY. 2016. Molecular subtypes of pancreatic cancer based on miRNA expression profiles have independent prognostic value. J Gastroenterol Hepatol. 31:1160–1167. [DOI] [PubMed] [Google Scholar]
- 57.Uppal A, Wightman SC, Mallon S, Oshima G, Pitroda SP, Zhang Q, Huang X, Darga TE, Huang L, Andrade J, et al. 2015. 14q32-encoded microRnas mediate an oligometastatic phenotype. Oncotarget. 6:3540–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lussier YA, Xing HR, Salama JK, Khodarev NN, Huang Y, Zhang Q, Khan SA, Yang X, Hasselle MD, Darga TE, et al. 2011. MicroRNA expression characterizes oligometastasis(es). Plos One. 6:e28650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niesen W, Primavesi F, Gasteiger S, Neoptolemos J, Hackert T, Stättner S. 2019. Surgical and local therapeutic concepts of oligometastatic pancreatic cancer in the era of effective chemotherapy. European Surgery. 51:153–164. [Google Scholar]
- 60.Leach WB. 1950. Carcinoma of the pancreas; a clinical and pathologic analysis of 39 autopsied cases. Am J Pathol. 26:333–347. [PMC free article] [PubMed] [Google Scholar]
- 61.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. 2010. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 467:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ischenko I, Petrenko O, Hayman MJ. 2014. Analysis of the tumor-initiating and metastatic capacity of PDX1-positive cells from the adult pancreas.111(9):3466–3471. doi: 10.1073/pnas.1319911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitellius C, Griveaux O, Morvant B, Pedrono E, Venara A, Ingster O, Baize N, Dincuff E, Rousselet MC, Guardiola P, et al. 2020. Impact of driver mutations on the evolution of isolated metachronous lung metastasis of pancreatic ductal adenocarcinoma. Molecular Diagnosis & Therapy. 24:443–449. [DOI] [PubMed] [Google Scholar]
- 64.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. 2012. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 491:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Embuscado EE, Laheru D, Ricci F, Yun KJ, de Boom Witzel S, Seigel A, Flickinger K, Hidalgo M, Bova GS, Iacobuzio-Donahue CA. 2005. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biology & Therapy. 4:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, et al. 2001. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 7:4115–4121. [PubMed] [Google Scholar]
- 67.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P, et al. 2009. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 27:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Eshleman JR, et al. 2009. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 15:4674–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou A, Waddell N, Cowley MJ, Gill AJ, Chang DK, Patch AM, Nones K, Wu J, Pinese M, Johns AL, et al. 2013. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han SH, Ryu KH, Kwon AY. 2021. The prognostic impact of HER2 genetic and protein expression in pancreatic carcinoma-HER2 protein and gene in pancreatic cancer. Diagnostics (Basel).11(4):653 doi: 10.3390/diagnostics11040653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP. 2019.. Chemotherapy for pancreatic cancer. Presse Med. 48(3 pt 2):e159–74. doi: 10.1016/j.lpm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, et al. 2015. Nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 107. dju413. Print 2015 Feb. 10.1093/jnci/dju413 [DOI] [PubMed] [Google Scholar]
- 73.Kitasato Y, Nakayama M, Akasu G, Yoshitomi M, Mikagi K, Maruyama Y, Kawahara R, Ishikawa H, Hisaka T, Yasunaga M, et al. 2012. Metastatic pulmonary adenocarcinoma 13 years after curative resection for pancreatic cancer: report of a case and review of Japanese literature. JOP: Journal of the Pancreas. 13:296–300. [PubMed] [Google Scholar]
- 74.Hackert T, Niesen W, Hinz U, Tjaden C, Strobel O, Ulrich A, Michalski CW, Büchler MW. 2017. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 43:358–363. [DOI] [PubMed] [Google Scholar]
- 75.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, Barbas AS, Abdalla EK, Choti MA, Vauthey JN, et al. 2007. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 14:3481–3491. [DOI] [PubMed] [Google Scholar]
- 76.Primavesi F, Stättner S, Jäger T, Göbel G, Presl J, Tomanová K, Buchner S, Maglione M, Resch T, Hutter J, et al. 2019. Progressive oncological surgery is associated with increased curative resection rates and improved survival in metastatic colorectal cancer. Cancers. 11:218. 10.3390/cancers11020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Evrard S, Poston G, Kissmeyer-Nielsen P, Diallo A, Desolneux G, Brouste V, Lalet C, Mortensen F, Stättner S, Fenwick S, et al. 2014. Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. Plos One. 9:e114404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Treasure T. 2014. Pulmonary metastasectomy for colorectal cancer: recent reports prompt a review of the available evidence. Curr Colorectal Cancer Rep. 10:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakawa T. 2021. Past, present, and future perspectives of pulmonary metastasectomy for patients with advanced colorectal cancer. Surg Today. 51:204–211. [DOI] [PubMed] [Google Scholar]
- 80.Treasure T, Farewell V, Macbeth F, Monson K, Williams NR, Brew-Graves C, Lees B, Grigg O, Fallowfield L, Cctg P. 2019. Pulmonary metastasectomy versus continued active monitoring in colorectal cancer (PulMicc): a multicentre randomised clinical trial. Trials. 20 718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


