Abstract
The cytoplasmic domain of an envelope transmembrane glycoprotein (gp30) of bovine leukemia virus (BLV) has two overlapping copies of the (YXXL)2 motif. The N-terminal motif has been implicated in in vitro signal transduction pathways from the external to the intracellular compartment and is also involved in infection and maintenance of high viral loads in sheep that have been experimentally infected with BLV. To determine the role of YXXL sequences in the replication of BLV in vitro, we changed the tyrosine or leucine residues of the N-terminal motif in an infectious molecular clone of BLV, pBLV-IF, to alanine to produce mutated proviruses designated Y487A, L490A, Y498A, L501A, and Y487/498A. Transient transfection of African green monkey kidney COS-1 cells with proviral DNAs that encoded wild-type and mutant sequences revealed that all of the mutated proviral DNAs synthesized mature envelope proteins and released virus particles into the growth medium. However, serial passages of fetal lamb kidney (FLK) cells, which are sensitive to infection with BLV, after transient transfection revealed that mutation of a second tyrosine residue in the N-terminal motif completely prevented the propagation of the virus. Similarly, Y498A and Y487/498A mutant BLV that was produced by the stably transfected COS-1 cells exhibited significantly reduced levels of cell-free virion-mediated transmission. Analysis of the protein compositions of mutant viruses demonstrated that lower levels of envelope protein were incorporated by two of the mutant virions than by wild-type and other mutant virions. Furthermore, a mutation of a second tyrosine residue decreased the specific binding of BLV particles to FLK cells and the capacity for viral penetration. Our data indicate that the YXXL sequences play critical roles in both viral entry and the incorporation of viral envelope protein into the virion during the life cycle of BLV.
Retroviral envelope (Env) proteins perform multiple functions that are critical for both viral replication and pathogenicity and serve as principle targets of humoral and cellular immune responses (31). They are synthesized as glycosylated polyproteins that are proteolytically processed by host enzymes into surface (SU) and transmembrane (TM) subunits during passage through the Golgi apparatus, and they are selectively incorporated into budding virions. The SU protein is anchored to the virion membrane by association with the TM protein, and it appears to mediate both binding to receptors and determination of the host range of the virus. The TM protein has three distinct domains: extracellular, membrane-spanning, and cytoplasmic domains. The extracellular domain binds covalently or noncovalently to the SU protein; at its N terminus it contains a stretch of about 20 hydrophobic amino acids, which is designated as the fusion peptide (5, 6, 21, 39, 65) and which contributes to oligomerization of Env proteins (16, 48, 64, 69). The hydrophobic membrane-spanning domain anchors the Env protein in the cell and viral membrane (4, 47).
The cytoplasmic tail of the TM protein is processed still further in murine leukemia virus (27, 29, 35), Mason-Pfizer monkey virus (M-PMV) (61), and equine infectious anemia virus (52). The cleavage event is catalyzed by a virus-encoded protease and seems to occur only after the fully assembled virus particle has been released from the cell (12, 29, 36, 43, 52, 57, 61). In addition to such processing, the cytoplasmic domain is naturally truncated in a number of cases. Thus, propagation of equine infectious anemia virus (52), simian immunodeficiency viruses (SIVs) (10, 11, 19, 37, 38, 45), and human immunodeficiency virus type 1 (HIV-1) (59), under some conditions, selects for mutants with a termination codon in the coding region for the cytoplasmic domain. It seems that natural selection favors an extended cytoplasmic domain in some situations and a much shorter one in others and, furthermore, that in some systems it favors synthesis of an extended form and subsequent removal of the extension by proteolysis. The reasons for the existence of these different forms are, however, unknown. It seems likely that an understanding of these phenomena might provide some insight into the function(s) of the cytoplasmic domain itself. Indeed, introduction of a deletion or a site-directed mutation into the corresponding coding region has been shown to cause changes in viral infectivity, the host range of the virus, the membrane fusion activity, and the level of incorporation of Env protein into virus particles for HIV-1, murine leukemia virus, M-PMV, and SIV (7, 13, 20, 25, 34, 49, 50, 53, 70). It seems likely, therefore, that the cytoplasmic domain contributes to the regulation of the viral life cycle and viral pathogenicity.
Bovine leukemia virus (BLV), an oncovirus related to human T-cell leukemia virus types 1 and 2, causes enzootic bovine leukosis, a disease characterized by a very extended course that often involves persistent lymphocytosis and culminates in B-cell lymphoma (9). Under experimental conditions, sheep can easily be infected with BLV and some sheep develop B-cell leukemia/lymphoma at higher frequencies and after a shorter latency period than cattle (1, 14). The Env protein of BLV is synthesized as a 72-kDa precursor that is cleaved to yield a 51-kDa SU protein (gp51) and a 30-kDa TM protein (gp30). gp51 determines the cellular tropism of the virus, whereas gp30 is responsible for anchoring the complex into the membrane and mediates virus-cell fusion (9, 42). The 58-amino-acid cytoplasmic domain of gp30 contains two overlapping copies of the (YXXL/I)2 motif (where X corresponds to a variable residue) (51). This motif, designated the immunoreceptor tyrosine-based activation motif, is found as a pair of YXXL/I sequences that are separated by seven or eight variable amino acids, and it contains all the structural information necessary for signal transduction after the stimulation of T- and B-cell receptors (18, 67). Studies with chimeric proteins in which the cytoplasmic domain of CD8-α was replaced by that of BLV gp30 have shown that the N-terminal (YXXL/I)2 motif participates in induction of the activation of B cells (3). In addition to such activity, the two tyrosine residues in the motif also appear to be involved in infection and the maintenance of high viral loads in sheep that have been experimentally infected with BLV (70). However, the mechanism whereby the YXXL sequences of the cytoplasmic domain control or mediate infection and viral propagation in vivo remains unclear.
The present study was designed to determine the role in viral infectivity of the YXXL sequences of gp30. We recently established a line of cells that is stably transfected with an infectious full-length molecular clone of BLV, designated pBLV-IF, that produced virus in sufficient quantities for subsequent infection experiments (32). In this study, we changed either a tyrosine or a leucine residue to an alanine residue in the YXXL sequences encoded by pBLV-IF by site-directed mutagenesis and we then established stable transfectants that produced the corresponding modified virus. We report the effects of these mutations on viral propagation in vitro, the incorporation of Env protein into virions, and the entry of viruses into host cells.
MATERIALS AND METHODS
Plasmids and site-directed mutagenesis.
The pBLV-IF plasmid contains a full-length BLV provirus with two copies of the long terminal repeat (LTR) and encodes infectious BLV (32). To generate the mutant proviral DNAs designated Y487A, L490A, Y498A, and L501A, we introduced site-directed mutations into pBLV-IF by following the instructions in the manual provided with the ExSite PCR-based site-directed mutagenesis kit from Stratagene (La Jolla, Calif.) (66), as shown in Fig. 1. The numbering of nucleotides (nt) corresponds to that of the complete sequence of BLV reported by Sagata et al. (55). In brief, 2.3-kb XhoI-XhoI (nt 4553 to 6888) fragments containing BLV env sequences were excised from pBLV-IF and subcloned into pBluescript II KS(−) (Stratagene) to yield pKS-env. A tyrosine or leucine codon was changed to an alanine codon by PCR with pKS-env as a template and the following primers: Y487m, 5′-TAGCAAGGCCTGCGCATCAGAATCGGG-3′, and Y487a, 5′-CCATCTGCACCAGAGATCTAC-3′, for Y487A; L490m, 5′-GCAGATGGTAGCGCGGCCTGATAATC-3′, and L490a, 5′-ACCAGAGATCTACTCTCA-3′, for L490A; Y498m, 5′-GGGGGAGAGGTGGCTAGCGATCTCTGGTGC-3′, and Y498a, 5′-GTCAAACCCGATTAGATCAAC-3′, for Y498A; and L501m, 5′-TTTGACGGGGGACGCGTGAGAGTAGATC-3′, and L501a, 5′-CCCGATTACATCAACCTCCGA-3′, for L501A. The proviral DNA encoding a protein with two mutated tyrosine residues, Y487/498A, was also obtained by PCR with the Y487A template and the Y498m and Y498a primers. In all mutageneses, altered nucleotides are underlined. The primers were designed to introduce the desired amino acid residue(s), as well as to introduce or to disrupt the recognition site of a restriction endonuclease. The XhoI-XhoI fragments including mutated env sequences were used to replace the corresponding region of pBLV-IF. To verify the presence of the desired mutations and the absence of errors due to the use of Taq DNA polymerase, all of the mutated plasmids were sequenced by the dideoxy chain-termination method (56) with a BcaBEST dideoxy sequencing kit (Takara, Otsu, Japan).
FIG. 1.
Relevant sequences of BLV mutants. The amino acid sequence of the cytoplasmic tail of wild-type gp30 is shown at the top, and the locations of the two (YXXL)2 motifs are indicated. The cytoplasmic tail contains three repeats of the YXXL sequence. These repeats may be arranged as two overlapping (YXXL)2 motifs, denoted 1 and 2. The positions of substitutions by alanine of leucine and tyrosine residues described in this study are indicated under the wild-type sequence. Amino acids identical to those in the latter sequence are indicated by dashes. The residues in gp30 are numbered according to reference 55.
The structures of pBLTRCAT, pBLVLTR(U3)-neo, and pSV-β-galactosidase (Promega, Madison, Wis.) have been described previously (32).
Cells and transfections.
African green monkey kidney COS-1 cells and fetal lamb kidney FLK cells, the latter of which are permissive with respect to infection by BLV, were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin, and streptomycin. The FLK/BLV cell line, which is persistently infected with BLV, was also used as a positive control in Western blotting analysis.
For assays of chloramphenicol acetyltransferase (CAT) activity, COS-1 cells (5 × 105) were plated in 10-cm-diameter dishes the day before transfection, and they were transfected with 5 μg of wild-type or mutant proviral DNA, together with 8 μg of pBLTRCAT and 2 μg of pSV-β-galactosidase, by the DEAE-dextran method (58). For transfections for other assays, COS-1 and FLK cells (5 × 106) were transfected with 50 μg of either wild-type or mutant proviral DNAs by electroporation in a cuvette with a 0.4-cm electrode gap and a Gene Pulser II (Bio-Rad Inc., Hercules, Calif.) at 260 V and 975 μF.
Cells that constitutively produced mutant BLV were established by stable transfection with mutant proviral DNAs as described previously (32). In brief, COS-1 cells (5 × 106) were cotransfected with 50 μg of mutant proviral DNA that had been linearized with NotI and 5 μg of pBLVLTR(U3)-neo that had been linearized with EcoRI by electroporation, as described above, and stable transfectants that were resistant to the antibiotic G418 (1 mg/ml) were selected.
Assay of viral entry into cells.
To monitor the adsorption to and penetration of FLK cells by BLV, we performed separate experiments at 4 and 37°C. Trypsin was used to eliminate virus particles that had adhered to but not penetrated cell surfaces. Viruses produced by FLK/BLV cells were concentrated as described above, and then serial 5-fold dilutions of virus (including reverse transcriptase [RT] activity of 100,000 cpm) were made in RPMI 1640 medium that contained 4 μg of Polybrene/ml. A portion of the suspension of viruses was also heated at 60°C for 1 h as a negative control. Viruses produced by COS-1 cells that had been stably transfected with wild-type or mutant proviral DNA were suspended (RT units, 10,000 cpm) in RPMI 1640 medium that contained 4 μg of Polybrene/ml. FLK cells that had been plated at 3 × 105 cells per 3.5-cm-diameter dish 1 day previously were incubated with 200-μl aliquots of the suspension of viruses at 4 or 37°C for 2 h. Then cells were washed five times with ice-cold RPMI 1640 medium. In experiments designed to estimate viral penetration, after the first wash with RPMI 1640, cells were treated with 0.25% trypsin for 5 min at room temperature. Cells were harvested and washed once with 1 ml of ice-cold RPMI 1640 medium by mixing for 5 s on a vortex mixer. Cell pellets were collected by centrifugation at 700 × g for 30 s and lysed in buffer that contained 2% sodium dodecyl sulfate (SDS) and 2 mM phenylmethylsulfonyl fluoride. Entire cell lysates after treatment with trypsin and one-quarter of cell lysates without such treatment were subjected to Western blotting analysis to monitor levels of p24 protein.
Other procedures.
Assays for CAT activity, RT activity, and formation of syncytia, Western blot analysis, immunofluorescence (IF) microscopy, inoculation of cell-free virus, and serial passage of cells after transfection with proviral DNA were performed as described previously (32).
RESULTS
Three YXXL sequences in the gp30 transmembrane protein of the BLV infectious molecular clone pBLV-IF and their biological relevance.
In our efforts to clarify the functional role of the YXXL sequences in the life cycle of BLV, we used the infectious molecular clone pBLV-IF, which has great potential utility for molecular genetic studies and for characterization of the biological properties of BLV. First, to verify the presence of YXXL sequences in gp30 encoded by pBLV-IF, we determined the nucleotide sequence of the env gene for gp30 that corresponded to the cytoplasmic domain and compared the sequence with the previously published sequences obtained from nine variants of BLV (42, 55, 70). The comparison of sequences revealed the existence of three copies of the YXXL sequences in pBLV-IF and in all nine variants (data not shown). In addition, the sequence between nt 6211 and 6368 of pBLV-IF was identical to that in the Japanese variant λ-BLV (55).
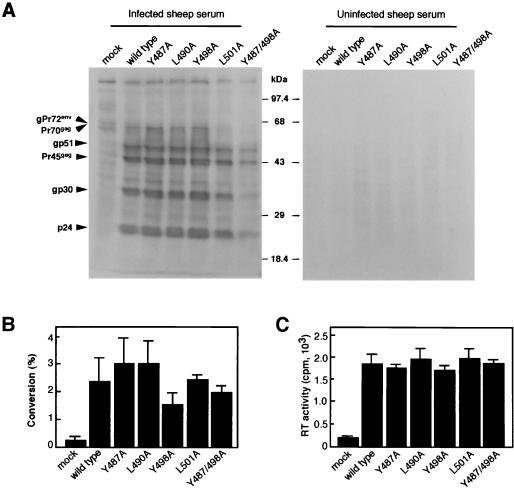
It has been reported that, among the three copies of the YXXL sequence found in gp30 of BLV, the two N-terminal YXXL sequences are essential for signal transduction in cultured cells and that the third sequence is not necessary for this activity. Therefore, we separately mutated the codons for tyrosine or leucine residues at positions 487, 490, 498, 501, and 487 plus 498 to the codon for an alanine residue(s) to obtain mutant proviral DNAs (Fig. 1). In order to examine expression of cell-associated viral proteins and production of viral particles by the various mutant proviruses, we transiently transfected COS-1 cells, which release virus and strongly express BLV antigens after transfection with pBLV-IF (32). Sixty hours after transfection, we performed Western blotting, CAT, and RT analyses (Fig. 2). Bands corresponding to the structural proteins of cell-associated BLV, such as p24, gp30, Pr45gag, gp51, Pr70gag, and gPr72env, were detected specifically in the analyses of all cells that had been transfected with mutant proviral DNA by Western blotting with serum from a BLV-infected sheep (Fig. 2A). The molecular masses of these proteins were indistinguishable from those of proteins detected in analyses of COS-1 cells that had been transfected with wild-type proviral DNA, which served as positive controls. By contrast, no specific bands were detected in the analysis with a control serum from an uninfected sheep (Fig. 2A). Next, to examine expression of trans-activational Tax proteins, we transfected COS-1 cells with either wild-type or mutant proviral DNA together with the reporter plasmid pBLTRCAT, which harbored the LTR sequences of BLV upstream of a gene for CAT, and pSV-β-galactosidase for normalization of the efficiency of transfection. The CAT assay indicated that cells transfected with all mutant proviral DNAs synthesized a functional Tax transactivator at levels similar to those obtained with wild-type proviral DNA (Fig. 2B). Moreover, we examined whether virus particles were released into the growth medium of COS-1 cells that had been transfected with either mutant proviral DNAs or wild-type DNA by assays of RT activity. As shown in Fig. 2C, similar levels of RT activity were detected in growth media from all transfectants. Together, these results demonstrate that none of the five mutations in the YXXL sequences affected the synthesis, processing, and expression of viral proteins and the production of viral particles after transfection.
FIG. 2.
Biological features of mutant forms of BLV after transient transfection of COS-1 cells with mutant proviral DNAs. Sixty hours after transfection of COS-1 cells with either wild-type or mutant proviral DNA, Western blotting, CAT, and RT analyses were performed. (A) Expression of cell-associated BLV structural proteins. Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel, and then the proteins in the gel were electrophoretically transferred to a polyvinylidene difluoride membrane filter (Immobilon; Millipore, Bedford, Mass.). For detection of viral structural proteins, the membrane was incubated in buffer that contained either serum from a BLV-infected sheep or control sheep serum. After washing, the filter was incubated with rabbit antibodies against sheep immunoglobulin (Ig) G (Cappel, Cochranville, Pa.) as the second antibody and incubated with horseradish peroxidase-conjugated antibodies raised against rabbit immunoglobulin (Amersham) as the third antibody. Positions of the protein markers with molecular masses and of the BLV structural proteins are indicated. Molecular masses are expressed in kilodaltons. (B) Expression of functional transactivation Tax protein. CAT activities of cells transfected with pBLTRCAT, which harbored the LTR sequences of BLV upstream of a gene for CAT, wild-type or mutant proviral DNA, and pSV-β-galactosidase were determined. (C) RT activity of a concentrated preparation of viruses that was released into growth medium. Ten milliliters of the growth medium from each culture of transfectants was concentrated by ultracentrifugation, and the virus pellet was suspended in 50 μl of serum-free RPMI 1640 medium. Ten microliters of each concentrated preparation of virus was then used for the RT assay. Each column and error bar represent the mean ± standard error of results from three independent experiments (B and C).
Serial passage of FLK cells after transient transfection with YXXL mutant proviral DNAs.
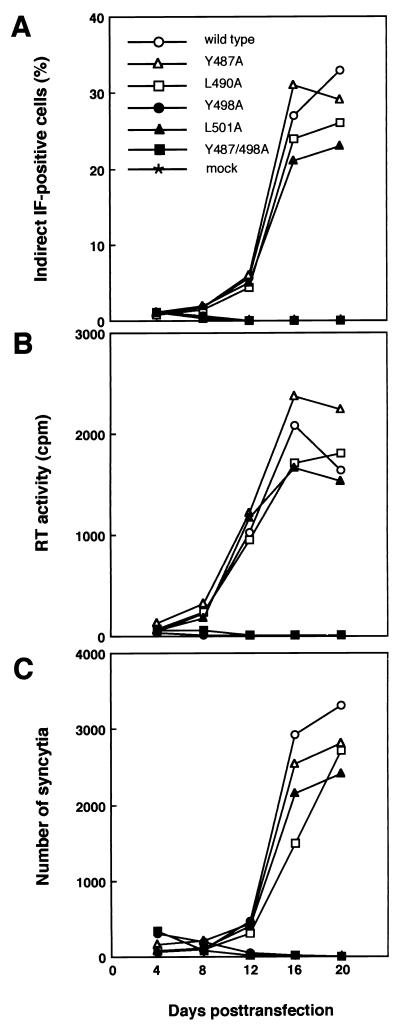
We showed recently that serial passage of FLK cells after transient transfection with wild-type pBLV-IF results in the propagation of BLV with sequentially increasing percentages of BLV antigen-positive cells and progressively increasing numbers of syncytia (32). To investigate whether the mutant proviral DNA encoded infectious viruses, we transfected FLK cells with wild-type proviral DNA or mutant proviral DNA and then monitored the kinetics of viral replication by serially determining the percentages of cells that expressed BLV antigens, as well as by monitoring the RT activity of concentrated viruses from the growth media and the formation of syncytia in cells passaged at 4-day intervals (Fig. 3). The efficiency of transfection seemed unaffected, with approximately 0.8 to 1.1% of cells being positive for BLV antigens in all cases 4 days after transfection (Fig. 3A). In FLK cells transfected with Y487A, L490A, or L501A mutant proviral DNA, the percentage of cells that expressed viral antigens increased with the number of passages and approximately 20 to 30% of cells expressed viral antigens within 20 days after transfection. Similarly, all proviral DNAs caused an increase in the formation of syncytia and the production of virus that appeared to spread rapidly though the culture, as indicated by the increase in RT activity in the concentrated growth media (Fig. 3B and C). The kinetics of replication of the mutant viruses were indistinguishable from that of the wild type in terms of the increase in the percentage of BLV antigen-positive cells, the RT activity of the concentrated growth medium, and the number of syncytia. By contrast, no replication of Y498A and Y487/498A mutant viruses was detected for 20 days after transfection by any of the three assays (Fig. 3). Thus, it appeared that replacement by alanine of Y498 in the second YXXL sequence of gp30 dramatically decreased the efficiency of both primary infection and secondary infection.
FIG. 3.
Propagation of mutant BLV in FLK cells after transient transfection with mutant proviral DNAs. Twenty-four hours after transfection with wild-type or mutant proviral DNA, cells were replated at 2.5 × 105 cells per 10-cm-diameter dish and serially passaged every 4 days in the presence of 4 μg of Polybrene/ml. Cultured cells were serially passaged at 4-day intervals. Aliquots of passaged cells were monitored for replication of virus at the indicated times by indirect IF microscopy of fixed cell smears by using serum from a BLV-infected sheep (A), RT assay of a concentrated preparation of the virus that had been released into the growth medium (B), and monitoring formation of syncytia (C). The data represent those of a single experiment reproduced in triplicate with similar results.
Establishment of stable transfectants that harbored YXXL mutant proviral DNA.
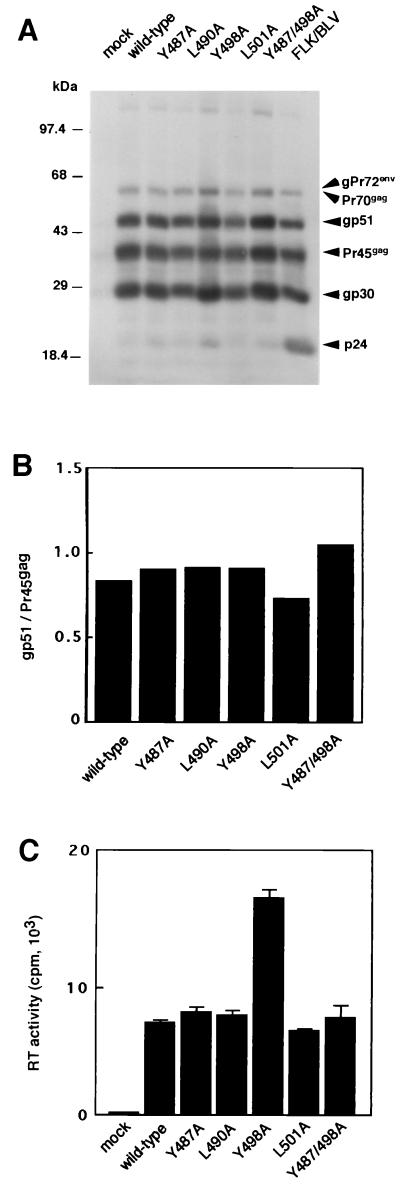
During serial passages of transient transfectants generated as described above, BLV can spread by both cell-to-cell and cell-free transmission. To characterize the biological functions of YXXL sequences in gp30 in viral transmission, it is essential to establish stable transfectants that can produce sufficient quantities of mutant BLV for subsequent infection experiments. Therefore, we transfected COS-1 cells with linearized pBLV-IF mutant DNA and pBLVLTR(U3)-neo, which contained the LTR-U3 region of BLV cloned upstream of the neomycin resistance gene, and then cultured cells in the presence of G418 as described previously (32). After G418-resistant colonies had grown up, we individually expanded several colonies within each series of transfectants and examined the expression of cell-associated BLV antigens by Western blotting (Fig. 4A). Bands corresponding to the structural proteins of cell-associated BLV, such as p24, gp30, Pr45gag, gp51, Pr70gag, and gPr72env, were detected specifically in the analyses of five mutant proviral DNA-transfected lines by Western blotting with serum from a BLV-infected sheep. By contrast, no specific bands were detected in the analysis with a control serum from an uninfected sheep (data not shown). An RT assay revealed that five lines of transfectants that harbored mutant BLV released significant amounts of BLV into the growth medium (Fig. 4C).
FIG. 4.
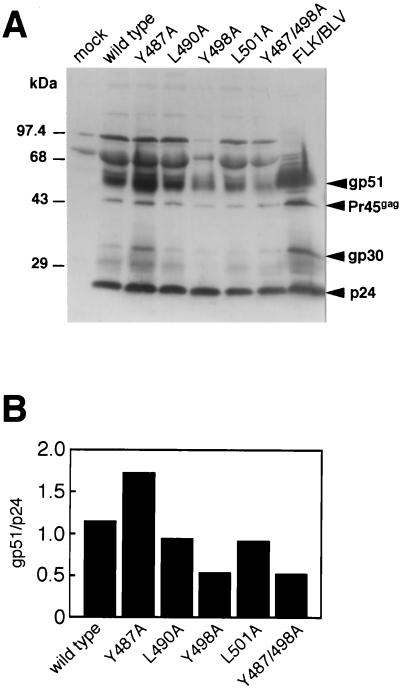
Establishment of cell clones that produced mutant BLV. COS-1 cells were transfected with linearized wild-type or mutant proviral DNA in combination with pBLVLTR(U3)-neo. Twenty-four hours after transfection, cells were replated and cultured in the presence of 1 mg of G418/ml. From 14 to 28 days after transfection, several colonies within each series of transfectants were individually expanded, the expression of BLV structural proteins was confirmed by Western blotting with serum from a BLV-infected sheep, and a typical clone for each mutant was then selected. Expression of BLV, as detected by Western blotting with serum collected from a BLV-infected sheep and subsequently treated with rabbit antibodies against sheep immunoglobulin G as the second antibody and horseradish peroxidase-conjugated antibodies raised against rabbit immunoglobulin as the third antibody (A), and production of viral particles, as detected by the RT assay (C), by typical clones are shown (C). Each column and error bar represent the mean ± standard error of results from three independent experiments. (B) For quantification, the intensities of bands (A) were determined with a Bio-Image densitometer and software (Millipore). Levels of gp51 were normalized for incorporated Pr45gag levels.
Transmission of YXXL mutant BLV by cell-free infection.
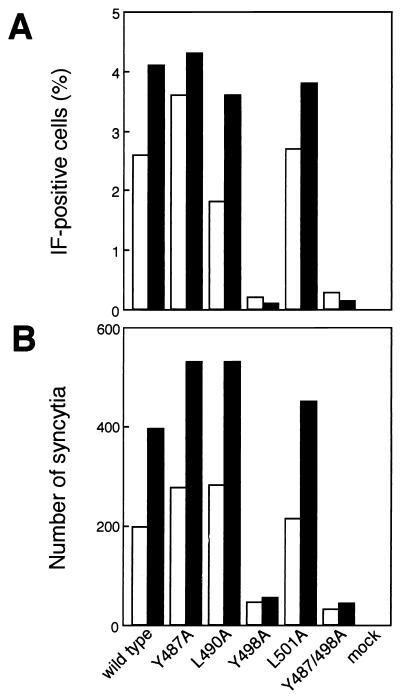
We investigated the effects of mutations in YXXL sequences in gp30 on cell-free infection. Supernatants from cultures of COS-1 cells that had been stably transfected with either wild-type proviral DNA or mutant proviral DNA were concentrated by ultracentrifugation, and the RT activity of the viral suspension in serum-free RPMI 1640 was measured. An equivalent number of RT units of virus was inoculated into FLK cells, which are highly susceptible to cell-free infection of BLV (32). Three and 5 days after inoculation, we examined the success of infection by indirect IF microscopy, using serum from a BLV-infected sheep, and by monitoring the formation of syncytia. As shown in Fig. 5A, among FLK cells that had been inoculated with wild-type BLV, Y487A, L490A, or L501A mutant BLV 3 days previously, approximately 1.8 to 3.6% of cells were positive for BLV antigens. Five days after inoculation, the percentage of positive cells was even higher (3.6 to 4.3%). By contrast, very few of the FLK cells that had been inoculated with Y498A or Y487/498A mutant BLV were positive for BLV antigens 3 and 5 days after infection. Similarly, the numbers of syncytia induced by inoculation with Y498A or Y487/498A mutant BLV were approximately 4 to 8 times lower than those of syncytia induced by inoculation with wild-type, Y487A, L490A, or L501A mutant BLV (Fig. 5B). Thus, the tyrosine residue at position 498 in the second YXXL sequence in gp30 appears to be essential for successful cell-free transmission of BLV.
FIG. 5.
Transmission of mutant BLV by cell-free inoculation. To concentrate virus particles, growth medium was clarified by centrifugation at 2,000 × g for 10 min at 4°C and then the supernatants were filtered (pore diameter, 0.45 μm). The filtered supernatant was layered on a cushion of 10% glycerol and centrifuged at 20,000 rpm in an SW28 rotor (Beckman, Palo Alto, Calif.) for 2 h at 4°C. Pelleted materials were resuspended in serum-free RPMI 1640 medium. A 200-μl aliquot of a concentrated preparation of virus (RT units, 10,000 cpm) was used to inoculate FLK cells (105 cells/3.5-cm-diameter dish) by incubation at 37°C for 2 h in the presence of 4 μg of Polybrene/ml. Cells were then fed 1.5 ml of complete medium that contained 4 μg of Polybrene/ml after removal of viruses by aspiration. Twenty-four hours after inoculation, cells were replated in four 3.5-cm-diameter dishes and then the expression of BLV (A) and the formation of syncytia (B) were monitored at three (open columns) and five (shaded columns) days after inoculation. The expression of BLV was detected by indirect IF microscopy of fixed cell smears by using serum from a BLV-infected sheep and subsequently fluorescein isothiocyanate-conjugated rabbit antibodies against the F(ab′)2 fragment of sheep immunoglobulin G (Cappel). Each column represents the mean of results from two independent experiments.
Effects of YXXL mutations on the assembly of Env protein into viral particles.
The cytoplasmic domain of the transmembrane glycoprotein gp30 is involved in virus assembly, participating both in the intracellular transport of the glycoprotein and in incorporation of the glycoprotein into virions, as well as in entry in the life cycle of the virus (15, 47, 54, 60). Therefore, we examined whether the YXXL mutant BLV supported the incorporation of the Env protein gp51 into virions. Before assessing the level of virion-associated Env proteins in mutant BLV, we first analyzed the levels of cell-associated Env proteins in COS-1 cells that had been stably transfected with each mutant proviral DNA. The film of the Western blot shown in Fig. 4A was scanned with a Bio-Image densitometer, and then the relative intensities of bands that corresponded to structural proteins Pr45gag and gp51 were quantified (Fig. 4B). The ratios of intensities of the bands of gp51 and Pr45gag were similar for each of the five mutant proviral DNAs and the wild-type provirus. We next analyzed concentrated virus particles that had been released from COS-1 cells that had been stably transfected with each of the five mutant proviruses by Western blotting, using serum from a BLV-infected sheep (Fig. 6A). As in the cases of wild-type virions and virions from FLK/BLV cells that had been productively infected with BLV, which served as positive controls, bands corresponding to structural proteins, such as p24, gp30, Pr45gag, and gp51, were readily detected in the analyses of all five mutant virions. However, the relative level of gp51 incorporated into each preparation of virus varied (Fig. 6B). The ratios of intensities of the bands of the gp51 and p24 proteins in virions produced by L490A or L501A mutants were approximately the same as those in virions produced by the wild-type construct. The gp51 protein of the Y487A mutant was packaged 1.5 times more efficiently than that of the wild type. By contrast, virions produced by Y498A and Y487/498A mutants contained lower relative levels of gp51, namely, 45% of the wild-type level. It appeared, therefore, that mutation of the tyrosine residue at position 498 in the second YXXL sequence in gp30 reduced the incorporation of gp51 into virus particles without affecting the synthesis and processing of Env proteins in COS-1 transfectants. Our findings also suggest that this phenomenon might be closely related to the dramatically decreased infectivity that was observed after mutation of the tyrosine residue at position 498 in the YXXL sequences.
FIG. 6.
Incorporation of mutant Env proteins into virions. (A) Concentrated preparations of virus particles (including RT activity of 20,000 cpm) that had been released from COS-1 cells stably transfected with wild-type or mutant proviral DNA were fractionated by SDS-10% polyacrylamide gel electrophoresis and then subjected to Western blotting with serum from a BLV-infected sheep followed by rabbit antibodies against sheep immunoglobulin G as the second antibody and horseradish peroxidase-conjugated antibodies raised against rabbit immunoglobulin as the third antibody. Viruses released from FLK/BLV cells, which are productively infected with BLV, were used as a positive control. Positions of marker proteins with molecular masses and of BLV structural proteins are indicated. Molecular masses are expressed in kilodaltons. (B) For quantification, the intensities of bands on the film shown in panel A were determined with a Bio-Image densitometer and software (Millipore). Levels of gp51 were normalized for incorporated p24 levels.
Effects of YXXL mutations on the adsorption to and penetration of FLK cells by BLV.
We investigated whether mutations in the YXXL sequences had any effect on adsorption to and penetration of permissive FLK cells by the virus. After incubation with BLV at 37°C (for viral penetration) or at 4°C (for viral adsorption), cells were washed to remove unbound virus before treatment with trypsin. Control cells were not treated with trypsin. Cells were then lysed, and levels of p24 protein were measured by Western blotting with a monoclonal antibody against p24 (2). At 4°C adsorption of virus occurs without subsequent penetration, whereas both viral adsorption to and penetration of permissive cells are possible at 37°C (40, 68). Treatment with trypsin after the virus has been allowed to interact with cells eliminates virus particles that have adsorbed to but not penetrated the cell surface (28, 30). In the absence of trypsin treatment, the amount of p24 protein reflects the amounts of both virus that has adsorbed to the cell surface and virus that has already entered cells.
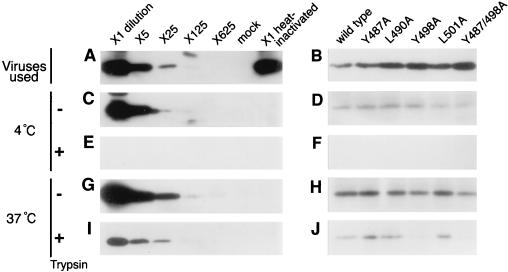
To confirm the validity of the assay that we used to assess viral adsorption to cells, we diluted virus that had been prepared from growth medium of FLK/BLV cells (RT units, 100,000 cpm) from 1:5 to 1:625 in RPMI 1640 medium (Fig. 7A) and then incubated the diluted virus with FLK cells at 4°C. Nonspecific adsorption of the virus was measured by using FLK cells that had been incubated with heat-inactivated virus. As shown in Fig. 7C, in the absence of treatment with trypsin after virus-cell interaction, the amount of p24 detected depended on the amount of virus in the original undiluted preparation and in preparations diluted as much as 1:125. Consistent with the hypothesis that trypsin eliminates virus particles that have adhered to the cell surface, no p24 was detected when cells were treated with trypsin after they had been exposed to viruses (Fig. 7E). To examine whether adsorbed viruses subsequently entered cells, we incubated diluted preparations of viruses, as described above, with FLK cells at 37°C. In contrast to the results obtained at 4°C, the amount of p24 detected depended on the amount of virus in undiluted preparations, even at dilutions as high as 1:125 in the cases of both untreated and trypsin-treated cells (Fig. 7I and G). The amounts of p24 detected in cells that had been treated with trypsin were approximately 1% of those in trypsin-untreated cells, which reflected viruses that had penetrated cells.
FIG. 7.
Assay of entry of mutant BLV into cells. Viruses produced by FLK/BLV cells (RT units, 100,000 cpm) were diluted 1:5 to 1:626 with RPMI 1640 medium that contained 4 μg of Polybrene/ml or were heated at 60°C for 1 h (A, C, E, G, and I). Viruses produced by COS-1 cells that had been stably transfected with wild-type or mutant proviral DNA (RT units, 10,000 cpm) were prepared in RPMI 1640 medium that contained 4 μg of Polybrene/ml (B, D, F, H, and J). A 200-μl aliquot of a suspension of viruses was incubated with FLK cells (3 × 105) in a 3.5-cm-diameter dish at 4°C (C, D, E, and F) or at 37°C (G, H, I, and J) for 2 h. After virus-cell interaction, cells were washed five times with ice-cold RPMI 1640 medium. Aliquots were subsequently treated with 0.25% trypsin for 5 min at room temperature (E, F, I, and J). Cells were harvested and washed once with 1 ml of ice-cold RPMI 1640 medium by mixing for 5 s on a vortex mixer. Cells were pelleted by centrifugation at 700 × g for 1 min and lysed in phosphate-buffered saline that contained 2% SDS and 2 mM phenylmethylsulfonyl fluoride. A 10-μl aliquot of the viral suspension (A and B), one-quarter of cell lysates prepared without treatment with trypsin (C, D, G, and H), and the entire amounts of cell lysates obtained after treatment with trypsin (E, F, I, and J) were fractionated by SDS-10% polyacrylamide gel electrophoresis and then subjected to Western blotting with a monoclonal antibody against p24 (2) and subsequently incubated with horseradish peroxidase-conjugated sheep antibodies against mouse immunoglobulin (Amersham).
To analyze effects of YXXL mutations on viral adsorption, we incubated viruses prepared from growth medium of COS-1 cells that had been stably transfected with wild-type provirus DNA or with mutant proviral DNA (RT units, 10,000 cpm) with FLK cells at 4°C. As shown in Fig. 7D, when the virus-cell suspension was not treated with trypsin, the amounts of p24 detected for all five mutant viruses were similar to that for wild-type BLV (Fig. 7D). When the virus-cell suspension was treated with trypsin, bands corresponding to p24 were completely lost in all cases (Fig. 7F). Furthermore, to analyze the effects of YXXL mutations on the entry of viruses into cells, we incubated viruses prepared from the growth medium of COS-1 cells that had been stably transfected with wild-type or mutant proviral DNA (RT units, 10,000 cpm) with FLK cells at 37°C. As shown in Fig. 7H, lower levels of p24 that became associated with cells were detected for Y498A and Y487/498A mutant viruses without trypsin treatment after the virus-cell interactions than for the wild-type virus and the Y487A, L490A, and L501A mutants. Likewise, when the virus-cell suspension was treated with trypsin, the intensity of the band of p24 was markedly lower for Y498A and Y487/498A mutant viruses than for the wild-type virus and the three other mutants. Together, these results indicate that mutation of the tyrosine residue at position 498 in the second YXXL sequence of gp30 reduced the potential for both specific binding of the virus to the cell surface and entry into cells.
DISCUSSION
Our results lead to two major conclusions. First, the present study revealed that replacement of tyrosine by an alanine residue at position 498 in the second YXXL sequence caused a significant reduction in the infectivity of BLV in both cell-to-cell and cell-free infection. This result strongly supports the results of Willems et al. (70), who reported that the tyrosine residue in the second YXXL sequence is essential for successful infection by BLV in vivo. Second, our results also demonstrated that this mutation affects the efficiency of entry into cells during an early stage of the virus’s life cycle and the incorporation of Env proteins into virions during the late stage. Thus, residue Y498 within the YXXL sequence seems likely to play a critical role in the replication of BLV.
Western blotting of cell-associated viral proteins and virus particles revealed that although all of the mutant gp30 glycoproteins were synthesized, processed, and expressed similarly to the wild-type glycoprotein, gp51, they were incorporated less efficiently into the Y498A and Y487/498A mutant particles than they were into particles of the wild-type virus and the Y487A, L490A, and L501A mutants. This result suggests that the cytoplasmic tail of gp30 regulates the level of incorporation of gp51 into viral particles. It is still unclear how mutations in the cytoplasmic domain of BLV might affect incorporation of viral Env proteins. However, there are at least four possibilities. First, it has been postulated that interaction of specific sequences in TM protein with Gag proteins is a necessary step for incorporation of Env proteins into viral particles (24, 25, 44). Such interactions in retroviruses are supported by the results of studies of chemical cross-linking of TM protein and matrix (MA) protein in Rous sarcoma virus (26), the influence of MA mutations on cleavage of the M-PMV cytoplasmic domain (8), and the inefficient incorporation of Env proteins in HIV-1 with a mutated MA protein (20, 71). Thus, the replacement of tyrosine by alanine at residue 498 in the second YXXL sequence might result in a less stable association between gp30 and Gag protein, which might play an important role in the incorporation of gp51 into the virion. A second possibility is that the conformation of gp30 that is required for efficient incorporation of Env proteins is disrupted by the mutation of residue Y498. This possibility is supported by the following observation: removal of 104 amino acids from the carboxyl terminus of gp41 of HIV-1 resulted in a mutant virus with significantly reduced incorporation of Env proteins compared with wild-type virions. However, the surface expression and the surface anchorage of the gp120 Env protein of HIV-1 were unaffected by this truncation (72). Spies et al. have demonstrated that a truncated TM protein of SIV formed more stable SDS-resistant oligomers than did the full-length TM protein, suggesting that truncation of the cytoplasmic domain of the SIV Env protein might affect the conformation of the external domain of this TM protein (62). These results also imply a third possibility, that the association between SU and TM proteins on the outside of the membrane is affected by the mutations in the cytoplasmic domain of BLV. A fourth possibility is that the cytoplasmic domains of viral glycoproteins are utilized by the virus as a signal for the incorporation of these glycoproteins into virions, therefore providing a mechanism for the exclusion of cell surface proteins (44). For full clarification of the role of YXXL sequences in the cytoplasmic domain of gp30 in the incorporation of gp51 into virions, the biochemical characterization of the mutant forms of BLV, i.e., the study of interactions between gp30 and Gag protein and of the structure of gp30 in Y498A and Y487/497A mutant BLV, is essential.
In the present study, we found results indicating that mutant virions Y498A and Y487/497A, which have significantly reduced incorporation of gp51 compared to wild-type virions, were noninfectious in FLK cells, which are permissive with respect to BLV infection. Similarly, previous studies have suggested that the cytoplasmic domain of TM protein might make an important contribution to viral morphogenesis and infectivity: some mutants of HIV-1, SIV, and M-PMV with truncations of the cytoplasmic domain of TM protein exhibit defective infectivity that is associated with inefficient incorporation of Env proteins (7, 15, 20, 34, 71). Thus, there appeared to be a correlation between viral infectivity and incorporation of Env proteins into virions. Furthermore, it appears that the reduced infectivity of the Y498A and Y487/498A mutant forms of BLV might be the result of substitution of alanine for tyrosine at residue 498 in the second YXXL sequence in the cytoplasmic tail of gp30 and its effects on some step(s) after adsorption and/or binding of the virus to cells. Therefore, in addition to SU protein, TM protein is responsible for successful infection after entry of the virus into a cell.
The YXXL sequences of gp30 appear to have multiple functions and to be involved in efficient replication of the virus and development of leukemogenesis. This hypothesis is supported by the fact that the three YXXL sequences in gp30 are completely conserved in all of the eight strains reported to date (42, 55, 70). The potential signal transduction activity of these sequences (3) also suggests that Env proteins might function as transducers that mediate abnormal signals that induce tumorigenesis. It is of interest, in this context, that latent membrane protein 2A of Epstein-Barr virus, which also induces human B-cell tumors, has a copy of the (YXXL)2 motif. The immunoreceptor tyrosine-based activation motif in latent membrane protein 2A blocks signal transduction via B-cell receptors as a result of constitutive phosphorylation of tyrosine residues within the motif and an association with Syk tyrosine kinase (22, 23). Moreover, the present study and the results reported by Willems et al. (70) demonstrate the important roles of these sequences for successful infection of BLV. Recently, we obtained evidence suggesting that these sequences might be involved in the cell surface expression and fusogenic capacity of Env proteins (33), and several reports have indicated that a YXXL sequence might be recognized as a tyrosine-containing internalization motif and might regulate the transport of proteins that contain this sequence (17, 41, 46, 63). A full understanding of the roles of YXXL sequences might clarify some of the biological properties of BLV and the mechanism by which leukemogenesis is induced by BLV.
ACKNOWLEDGMENTS
We thank Makio Iwashima (Mitsubishi Kasei Institute of Life Science) and Yoshiyuki Nagai (University of Tokyo) for helpful discussions and Shin-nosuke Takeshima (RIKEN) for kind help with preparation of the manuscript.
This study was supported by Special Coordination Funds for the Promotion of Science and Technology from the Science and Technology Agency of the Japanese government.
REFERENCES
- 1.Aida Y, Miyasaka M, Okada K, Onuma M, Kogure S, Suzuki M, Minoprio P, Levy D, Ikawa Y. Further phenotypic characterization of target cells for bovine leukemia virus experimental infection in sheep. Am J Vet Res. 1989;50:1946–1951. [PubMed] [Google Scholar]
- 2.Aida Y, Onuma M, Tsukiyama K, Ogawa Y, Fujieda T, Mikami T, Izawa H. Monoclonal antibodies define antigenic regions on the major internal protein p24 of bovine leukemia virus (BLV) Arch Virol. 1987;94:315–321. doi: 10.1007/BF01310725. [DOI] [PubMed] [Google Scholar]
- 3.Beaufils P, Choquet D, Mamoun R Z, Malissen B. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. doi: 10.1002/j.1460-2075.1993.tb06205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman P W, Nunes W M, Haffar O K. Expression of membrane-associated and secreted variants of gp160 of human immunodeficiency virus type 1 in vitro and in continuous cell lines. J Virol. 1988;62:3135–3142. doi: 10.1128/jvi.62.9.3135-3142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch M L, Earl P L, Fargnoli K, Picciafuoco S, Giombini F, Wong-Staal F, Franchini G. Identification of the fusion peptide of primate immunodeficiency viruses. Science. 1989;244:694–697. doi: 10.1126/science.2541505. [DOI] [PubMed] [Google Scholar]
- 6.Brasseur R. Differentiation of lipid-associating helices by use of three-dimensional molecular hydrophobicity potential calculations. J Biol Chem. 1991;266:16120–16127. [PubMed] [Google Scholar]
- 7.Brody B A, Rhee S S, Hunter E. Postassembly cleavage of a retroviral glycoprotein cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. J Virol. 1994;68:4620–4627. doi: 10.1128/jvi.68.7.4620-4627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brody B A, Rhee S S, Sommerfelt M A, Hunter E. A viral protease-mediated cleavage of the transmembrane glycoprotein of Mason-Pfizer monkey virus can be suppressed by mutations within the matrix protein. Proc Natl Acad Sci USA. 1992;89:3443–3447. doi: 10.1073/pnas.89.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burny A, Cleuter Y, Kettmann R, Mammerickx M, Marbaix G, Portetelle D, Van den Broeke A, Willems L, Thomas R. Bovine leukemia: facts and hypotheses derived from the study of an infectious cancer. Adv Vet Sci Comp Med. 1988;32:149–170. doi: 10.1016/b978-0-12-039232-2.50010-4. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti L, Emerman M, Tiollais P, Sonigo P. The cytoplasmic domain of simian immunodeficiency virus transmembrane protein modulates infectivity. J Virol. 1989;63:4395–4403. doi: 10.1128/jvi.63.10.4395-4403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarti L, Guyader M, Alizon M, Daniel M D, Desrosiers R C, Tiollais P, Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987;328:543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- 12.Crawford S, Goff S P. A deletion mutation in the 5′ part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985;53:899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denesvre C, Carrington C, Corbin A, Takeuchi Y, Cosset F L, Schulz T, Sitbon M, Sonigo P. TM domain swapping of murine leukemia virus and human T-cell leukemia virus envelopes confers different infectious abilities despite similar incorporation into virions. J Virol. 1996;70:4380–4386. doi: 10.1128/jvi.70.7.4380-4386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djilali S, Parodi A L, Levy D, Cockerell G L. Development of leukemia and lymphosarcoma induced by bovine leukemia virus in sheep: a hematopathological study. Leukemia. 1987;1:777–781. [PubMed] [Google Scholar]
- 15.Dubay J W, Roberts S J, Hahn B H, Hunter E. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan M A, Carruth L M, Rowell J F, Yu X, Siliciano R F. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol. 1996;70:6547–6556. doi: 10.1128/jvi.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaswinkel H, Reth M. Dual role of the tyrosine activation motif of the Ig-alpha protein during signal transduction via the B cell antigen receptor. EMBO J. 1994;13:83–89. doi: 10.1002/j.1460-2075.1994.tb06237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchini G, Gurgo C, Guo H G, Gallo R C, Collalti E, Fargnoli K A, Hall L F, Wong-Stall F, Reitz M J., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987;328:539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- 20.Freed E O, Martin M A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freed E O, Myers D J, Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci USA. 1990;87:4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fruehling S, Lee S K, Herrold R, Frech B, Laux G, Kremmer E, Grasser F A, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 24.Gabuzda D, Olshevsky U, Bertani P, Haseltine W A, Sodroski J. Identification of membrane anchorage domains of the HIV-1 gp160 envelope glycoprotein precursor. J Acquired Immune Defic Syndr. 1991;4:34–40. [PubMed] [Google Scholar]
- 25.Gabuzda D H, Lever A, Terwilliger E, Sodroski J. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1992;66:3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebhardt A, Bosch J V, Ziemiecki A, Friis R R. Rous sarcoma virus p19 and gp35 can be chemically crosslinked to high molecular weight complexes. An insight into virus assembly. J Mol Biol. 1984;174:297–317. doi: 10.1016/0022-2836(84)90340-1. [DOI] [PubMed] [Google Scholar]
- 27.Green N, Shinnick T M, Witte O, Ponticelli A, Sutcliffe J G, Lerner R A. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc Natl Acad Sci USA. 1981;78:6023–6027. doi: 10.1073/pnas.78.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harouse J M, Bhat S, Spitalnik S L, Laughlin M, Stefano K, Silberberg D H, Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991;253:320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- 29.Henderson L E, Sowder R, Copeland T D, Smythers G, Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984;52:492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himathongkham S, Luciw P A. Restriction of HIV-1 (subtype B) replication at the entry step in rhesus macaque cells. Virology. 1996;219:485–488. doi: 10.1006/viro.1996.0276. [DOI] [PubMed] [Google Scholar]
- 31.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 32.Inabe K, Ikuta K, Aida Y. Transmission and propagation in cell culture of virus produced by cells transfected with an infectious molecular clone of bovine leukemia virus. Virology. 1998;245:53–64. doi: 10.1006/viro.1998.9140. [DOI] [PubMed] [Google Scholar]
- 33.Inabe, K., S. Tajima, and Y. Aida. Unpublished data.
- 34.Johnston P B, Dubay J W, Hunter E. Truncations of the simian immunodeficiency virus transmembrane protein confer expanded virus host range by removing a block to virus entry into cells. J Virol. 1993;67:3077–3086. doi: 10.1128/jvi.67.6.3077-3086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karshin W L, Arcement L J, Naso R B, Arlinghaus R B. Common precursor for Rauscher leukemia virus gp69/71, p15(E), and p12(E) J Virol. 1977;23:787–798. doi: 10.1128/jvi.23.3.787-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katoh I, Yoshinaka Y, Rein A, Shibuya M, Odaka T, Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from “immature” to “mature” core form and for virus infectivity. Virology. 1985;145:280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- 37.Kodama T, Burns D P, Kestler III H W, Daniel M D, Desrosiers R C. Molecular changes associated with replication of simian immunodeficiency virus in human cells. J Med Primatol. 1990;19:431–437. [PubMed] [Google Scholar]
- 38.Kodama T, Wooley D P, Naidu Y M, Kestler III H W, Daniel M D, Li Y, Desrosiers R C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989;63:4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 40.Krichbaum-Stenger K, Poiesz B J, Keller P, Ehrlich G, Gavalchin J, Davis B H, Moore J L. Specific adsorption of HTLV-I to various target human animal cells. Blood. 1987;70:1303–1311. [PubMed] [Google Scholar]
- 41.LaBranche C C, Sauter M M, Haggarty B S, Vance P J, Romano J, Hart T K, Bugelski P J, Marsh M, Hoxie J A. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J Virol. 1995;69:5217–5227. doi: 10.1128/jvi.69.9.5217-5227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamoun R Z, Morisson M, Rebeyrotte N, Busetta B, Couez D, Kettmann R, Hospital M, Guillemain B. Sequence variability of bovine leukemia virus env gene and its relevance to the structure and antigenicity of the glycoproteins. J Virol. 1990;64:4180–4188. doi: 10.1128/jvi.64.9.4180-4188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menendez A L, Gotte D, Oroszlan S. Moloney murine leukemia virus protease: bacterial expression and characterization of the purified enzyme. Virology. 1993;196:557–563. doi: 10.1006/viro.1993.1511. [DOI] [PubMed] [Google Scholar]
- 44.Metsikko K, Garoff H. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J Virol. 1990;64:4678–4683. doi: 10.1128/jvi.64.10.4678-4683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naidu Y M, Kestler III H W, Li Y, Butler C V, Silva D P, Schmidt D K, Troup C D, Sehgal P K, Sonigo P, Daniel M D, Desrosiers R C. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988;62:4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno H, Stewart J, Fournier M C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 47.Perez L G, Davis G L, Hunter E. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J Virol. 1987;61:2981–2988. doi: 10.1128/jvi.61.10.2981-2988.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M K, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 52.Rice N R, Henderson L E, Sowder R C, Copeland T D, Oroszlan S, Edwards J F. Synthesis and processing of the transmembrane envelope protein of equine infectious anemia virus. J Virol. 1990;64:3770–3778. doi: 10.1128/jvi.64.8.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ritter G, Jr, Mulligan M J, Lydy S L, Compans R W. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology. 1993;197:255–264. doi: 10.1006/viro.1993.1586. [DOI] [PubMed] [Google Scholar]
- 54.Rose J K, Bergmann J E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983;34:513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- 55.Sagata N, Yasunaga T, Tsuzuku-Kawamura J, Ohishi K, Ogawa Y, Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci USA. 1985;82:677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schultz A, Rein A. Maturation of murine leukemia virus env proteins in the absence of other viral proteins. Virology. 1985;145:335–339. doi: 10.1016/0042-6822(85)90168-0. [DOI] [PubMed] [Google Scholar]
- 58.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu H, Hasebe F, Tsuchie H, Morikawa S, Ushijima H, Kitamura T. Analysis of a human immunodeficiency virus type 1 isolate carrying a truncated transmembrane glycoprotein. Virology. 1992;189:534–546. doi: 10.1016/0042-6822(92)90577-c. [DOI] [PubMed] [Google Scholar]
- 60.Simpson D A, Lamb R. Alternations to influenza virus hemagglutinin cytoplasmic tail modulate virus infectivity. J Virol. 1992;66:790–803. doi: 10.1128/jvi.66.2.790-803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sommerfelt M A, Petteway S R, Jr, Dreyer G B, Hunter E. Effect of retroviral proteinase inhibitors on Mason-Pfizer monkey virus maturation and transmembrane glycoprotein cleavage. J Virol. 1992;66:4220–4227. doi: 10.1128/jvi.66.7.4220-4227.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spies C P, Ritter G, Jr, Mulligan M J, Compans R W. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein alters the conformation of the external domain. J Virol. 1994;68:585–591. doi: 10.1128/jvi.68.2.585-591.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 64.Tucker S P, Srinivas R V, Compans R W. Molecular domains involved in oligomerization of the Friend murine leukemia virus envelope glycoprotein. Virology. 1991;185:710–720. doi: 10.1016/0042-6822(91)90542-j. [DOI] [PubMed] [Google Scholar]
- 65.Voneche V, Portetelle D, Kettmann R, Willems L, Limbach K, Paoletti E, Ruysschaert J M, Burny A, Brasseur R. Fusogenic segments of bovine leukemia virus and simian immunodeficiency virus are interchangeable and mediate fusion by means of oblique insertion in the lipid bilayer of their target cells. Proc Natl Acad Sci USA. 1992;89:3810–3814. doi: 10.1073/pnas.89.9.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiner M P, Costa G L, Schoettlin W, Cline J, Mathur E, Bauer J C. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 1994;151:119–123. doi: 10.1016/0378-1119(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 67.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 68.Weiss R. Experimental biology and assay. In: Weiss P, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 209–260. [Google Scholar]
- 69.Wild C, Dubay J W, Greenwell T, Baird T, Jr, Oas T G, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willems L, Gatot J S, Mammerickx M, Portetelle D, Burny A, Kerkhofs P, Kettmann R. The YXXL signalling motifs of the bovine leukemia virus transmembrane protein are required for in vivo infection and maintenance of high viral loads. J Virol. 1995;69:4137–4141. doi: 10.1128/jvi.69.7.4137-4141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu X, Yuan X, Matsuda Z, Lee T H, Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu X, Yuan X, McLane M F, Lee T-H, Essex M. Mutations in the cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein impair the incorporation of Env proteins into mature virions. J Virol. 1993;67:213–221. doi: 10.1128/jvi.67.1.213-221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]